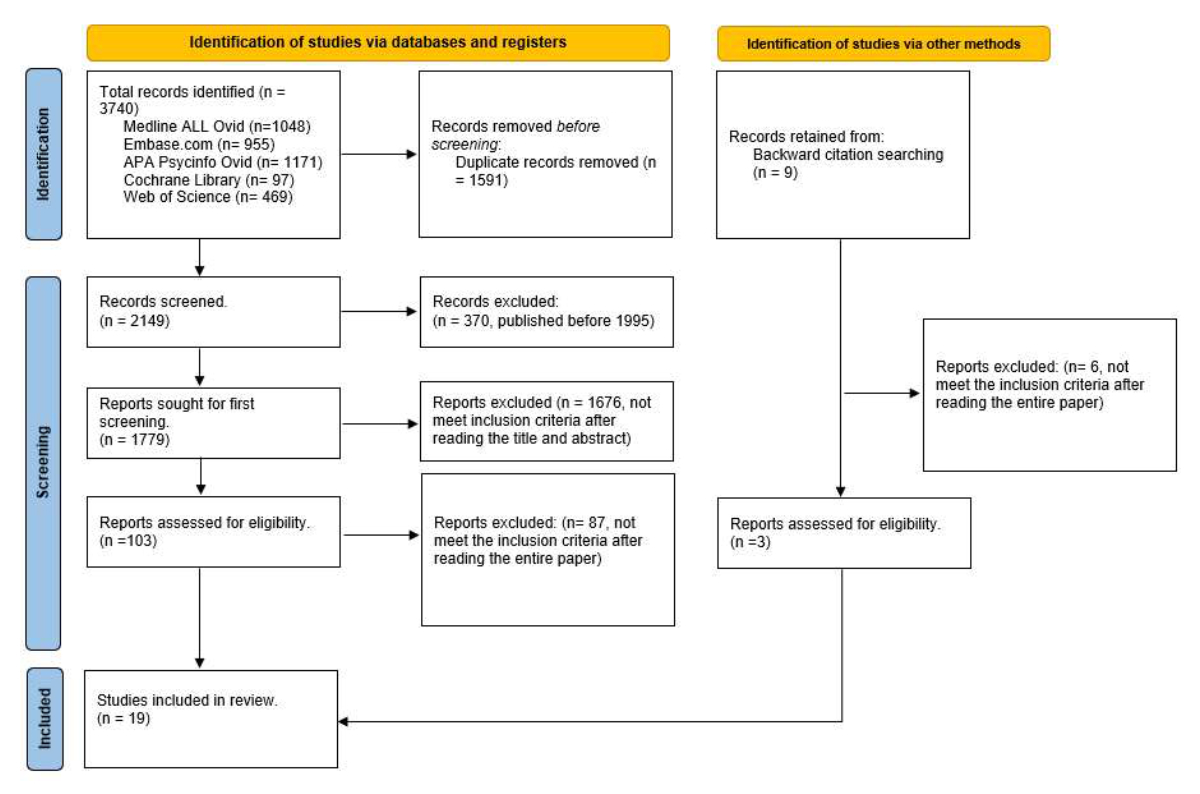
Figure 1PRISMA 2020 Flow diagram. www.prisma-statement.org.
DOI: https://doi.org/https://doi.org/10.57187/s.3684
Depression in the elderly is a complex mood disorder with high comorbidity with both psychiatric and physical diseases [1]. Rates of major depressive disorder in older people range from 5% to 10% in primary care and 37% after intensive care hospitalisation [1, 2]. Older adults with depression are at increased risk for suicide and for impairment in daily functioning [1].
Electroconvulsive therapy (ECT) is a crucial treatment in psychiatric medicine for severe mood and psychotic disorders [3]. In most guidelines for the treatment of depressive disorder, electroconvulsive therapy is considered a first-line treatment for life-threatening severe depressive episodes and when a rapid response is required [4, 5]. In addition, for psychiatric inpatients with severe mood disorders, electroconvulsive therapy may reduce readmissions [6]. The presence of psychotic features, catatonia, high suicide risk and/or food or fluid refusal are indications for the use of electroconvulsive therapy [4, 7, 8]. Indications for first-line use of electroconvulsive therapy include a previous positive response to electroconvulsive therapy and patient preference [4, 7]. With a remission rate of 60–80%, electroconvulsive therapy is the most efficacious treatment for late-life major depression [3, 9]. In fact, electroconvulsive monotherapy has demonstrated superior efficacy compared with pharmacotherapy in the treatment of late-life depression [9, 10]. In addition, the combination of electroconvulsive therapy with medication exceeds the effectiveness of medication alone [10].
When comparing the efficacy of electroconvulsive therapy, ketamine and transcranial magnetic stimulation (TMS), one systematic review and meta-analysis suggests an efficacy advantage of electroconvulsive therapy over ketamine in adults with a major depressive episode [11]. To our knowledge, there are no trials comparing the effectiveness of electroconvulsive therapy and ketamine in late-life depression. Concerning other neuromodulation techniques, electroconvulsive therapy for late-life depression is usually considered the “gold-standard” therapy. Transcranial magnetic stimulation has few cognitive or somatic side effects, but it has not been shown to be as effective as electroconvulsive therapy in the treatment of psychotic depression or treatment-resistant depression in older people [12].
On the other hand, electroconvulsive therapy is a safe and well-tolerated antidepressant treatment for the elderly population [5, 13, 14]. However, some patients and clinicians may be reluctant to use this treatment because of the risks associated with general anaesthesia and cognitive side effects [15, 16].
Previous meta-analyses have identified predictors of good response to electroconvulsive therapy for treating depression, such as the presence of psychotic features, psychomotor retardation, female sex and older age [17, 18]. However, these factors are often interrelated and have been mostly studied in the general population.
Although electroconvulsive therapy is the most effective treatment for severe depression, there is not enough research evidence to make precise recommendations about the place of electroconvulsive therapy in the treatment sequence for depression, particularly in the older population. Therefore, a review of factors predictive of response to electroconvulsive therapy in late-life depression is needed. To our knowledge, no review has focused on the specific factors associated with response to and/or remission following electroconvulsive therapy in the elderly population.
In order to obtain details of original studies on the different factors predictive of the effectiveness of electroconvulsive therapy (as it is currently practised) in elderly patients with depression, we applied the following eligibility criteria:
We conducted a literature search with the collaboration of a medical librarian (J. R. A.) in five bibliographic databases in June 2023: Medline ALL Ovid, Embase.com, APA PsycInfo Ovid, Cochrane Library Wiley and Web of Science Core Collection. All searches were done without language or date restrictions. The detailed search strategies, keywords and index terms are presented in the appendix, supplementary data.
Additional searches were conducted (B. P. M.) to identify possible additional papers by backward citation search, Google Scholar and Google.
In order to acquire an overview of predictors of the effectiveness of electroconvulsive therapy as it is currently applied, we decided to limit our search to papers published between 1995 and June 2023.
The titles and abstracts were first screened for relevance by the first author (B. P. M.). The inclusion of papers, after the first screening, in the review was evaluated separately by two independent researchers (B. P. M. and JP. S.). Disagreements were resolved via consensus (B. P. M., JP. S. and K. L. S.). If no agreement was reached, there was further discussion with a senior researcher (A. v. G.).
We used a data extraction sheet with the following data: (a) study characteristics: year, country and design of the study, diagnostic classification and depression severity scale used; (b) characteristics of the study sample: number of participants, percentage of female participants, mean age of the participants; (c) predictive factor investigated; (d) electroconvulsive therapy related: psychotropic drug modification, position of electrodes, pulse width, duration of seizures, electroconvulsive therapy sessions, number of treatments per week, anaesthetic drugs; (e) main results.
The primary outcome was remission, the secondary outcome was response to electroconvulsive therapy in the elderly population with depression. In the selected studies, remission was defined as a depression scale score equal to or below 7 (for HRSD-17) or 10 (for HRSD-21, HRSD-24 and MADRS). Response was defined as a reduction of at least 50% from the baseline Hamilton Rating Scale for Depression (HRSD) or Montgomery and Åsberg Depression Rating Scale (MADRS) score.
After removal of duplicates and studies published before 1995 (figure 1), the literature search yielded 1779 potentially relevant articles. We excluded 1676 articles after review of titles and/or abstracts. We analysed the full text of the 103 remaining studies; 87 of them did not meet eligibility criteria and were excluded. Furthermore, we found 9 further articles via other methods that could potentially be included in the review. After reading these articles, only 3 of them met the criteria for inclusion. In total, we selected 19 articles (figure 1).

Figure 1PRISMA 2020 Flow diagram. www.prisma-statement.org.
Studies had between 8 and 268 participants. Seventeen studies were age-restricted to the elderly (including only patients older than 50 years old) [19–35] and two other articles included all age populations [36, 37]. Although the latter two trials did not meet the age criterion, they were included because they have the rare advantage of age stratification, permitting comparison of the older population with the younger population.
There was one retrospective study [35] and all the others were prospective. Tables S1–S6 (in the appendix) show the mean age and percentage of women and men in each study. 73.7% of the studies were carried out in Europe. Three studies analysed biological markers, two had data on morphological markers, four on associated symptoms, five on electroconvulsive therapy parameters, two on treatment for the maintenance of remission and three on age.
We found three articles [19–21] that focused on the impact of biomarkers in depression remission and/or response after treatment with electroconvulsive therapy.
No significant relation was found between pre-ECT salivary cortisol values and response or remission [19]. We found one study concluding that moderately elevated levels of CRP at baseline (3 to 10 mg/l), but no other inflammatory markers, were associated with higher remission rates [20].
Carlier et al. aimed to investigate whether higher S100B was associated with favourable treatment outcomes following electroconvulsive therapy and to further explore whether S100B reflects a state marker of depression activity. Patients with S100B levels in the intermediate tertile, that is, between 33 ng/l and 53 ng/l, had higher odds of remission, and were more likely to remit from depression over time, compared with patients in the lowest tertile. However, there was no significant decrease in levels of S100B after electroconvulsive therapy in both remitters and non-remitters [21].
We found no evidence that baseline hippocampal volume, white matter hyperintensity volume or total amyloid burden were predictive of response or remission at 1 and 4 weeks post-ECT, nor of relapse at 4 weeks post-ECT [22].
However, Oudega et al. showed that patients without medial temporal lobe atrophy were three times more likely to remit their depression than patients with moderate or severe medial temporal lobe atrophy [23]. This study found no differences in changes in MADRS scores and white matter hyperintensities or global cortical atrophy [23].
We found one study [24] that analysed the effects of differential response to electroconvulsive therapy in older people with depression using the 3-factor MADRS structure proposed by Suzuki et al. [38], in which factors 1, 2 and 3 represent dysphoria (three items), retardation (four items) and vegetative symptoms (three items), respectively. They concluded that the mean pretreatment score for retardation (factor 2) was significantly lower in responders than in non-responders.
On the other hand, in patients with melancholic depression, the total CORE score (an observational instrument for identifying melancholic depression by assessing psychomotor disturbance [39]) did not predict electroconvulsive therapy outcome [25]. However, another study concluded that the association between age and electroconvulsive therapy efficacy was mediated by psychomotor retardation and, to a lesser extent, by psychotic features [36].
Regarding cognitive functions, poorer performance on the word reading task of the Colour Word Interference Test was associated with a higher likelihood of achieving remission during electroconvulsive therapy in non-demented patients [26]. However, we found no other evidence of significant associations between the outcome of electroconvulsive therapy and cognitive performance parameters at baseline.
Regarding the speed of recovery from disorientation, Magne Bjølseth et al. concluded that a longer post-ictal reorientation time at the first and third treatment sessions predicted a more rapid decline and a lower endpoint on the HRSD17 continuous scores [27].
Several researchers have compared the efficacy of electroconvulsive therapy by right unilateral (RUL ECT) vs bilateral (BL ECT) stimulation. Evidence shows similar results for RUL ECT and BL ECT with more adverse effects in the BL ECT group, such as short-term cognitive impairment [28, 29], whereas improvements in neuropsychological scores were seen in both groups [28].
In another study, elderly patients with major depression were treated with a course of formula-based bifrontal (BF) electroconvulsive therapy or RUL ECT. At the end of the electroconvulsive therapy course, response rates for the BF and RUL groups were 63.9% and 67.6%, respectively. Short-term remission was achieved in 38.9% patients in the BF group and 51.4% patients in the RUL group; however there were no significant differences between the groups [30].
Phase 1 of the large PRIDE study [31] evaluated the efficacy of RUL ultrabrief pulse electroconvulsive therapy combined with venlafaxine for the treatment of geriatric depression. 61.7% of the participants met remission criteria. Among them, the mean decrease in the HAM-D score was 24.7 points. The authors concluded that RUL ultrabrief pulse electroconvulsive therapy, combined with venlafaxine, is a rapidly acting and highly effective treatment option for depressed geriatric patients, with excellent safety and tolerability [31].
From an electrophysiological point of view, a study involving 8 patients with late‐life treatment-resistant depression tested whether the mean and regional frontal cortex theta cordance (TC) were able to differentiate early responders from non-responders [32]. TC is a well‐documented quantitative electroencephalography measure of cerebral energy consumption [40]. Prefrontal cortex TC has been associated with antidepressant response [41]. The study found that, compared with non-responders, early responders exhibited a greater change in TC specifically within the right prefrontal cortex [32].
A Finnish study observed the acute response and outcome in a 1-year follow-up of elderly depressive inpatients with major depressive disorder treated with electroconvulsive therapy and/or antidepressant therapy. The acute response was good in both groups. In this study, electroconvulsive therapy was continued until patients were asymptomatic or had received at least 8 treatments without improvement during the last 2 treatments. However, there was no significant difference in the 1-year rehospitalisation rate with 43% in the electroconvulsive therapy and 38% in the antidepressant group [33].
Phase 2 of the PRIDE study [34] evaluated the efficacy and tolerability of continuation electroconvulsive therapy plus medication compared with medication alone in depressed geriatric patients after a successful course of electroconvulsive therapy (phase 1) [31]. They found that additional electroconvulsive therapy after remission (operationalised as four continuation electroconvulsive treatments followed by further electroconvulsive therapy only as needed) was beneficial in sustaining mood improvement for most patients.
In terms of age, one study compared characteristics and treatment outcomes of adult (up to 59 years), young-old (60 to 74 years) and old-old (75 years or older) patients treated with electroconvulsive therapy for major depression. The authors found that both older groups had shorter index depressive episodes and were less likely to have had inadequate responses to adequate medication trials before electroconvulsive therapy. Despite a higher level of physical illness and cognitive impairment, even the oldest patients with severe major depression tolerate electroconvulsive therapy in a manner similar to younger patients and demonstrate similar or better acute response [37]. This article does not meet the formal inclusion criteria, but it has the rare advantage of age stratification, particularly at older ages.
A team from the Netherlands wondered whether the greater efficacy of electroconvulsive therapy in older depressed people was related to psychomotor disturbance and/or psychotic features. They compared three age groups (under 50, 50–69 and ≥70) and found no significant differences in HAM-D reduction between the three age groups. However, they did examine the mediating effects of symptomatology and found that the association between age and electroconvulsive therapy efficacy was mediated by psychomotor retardation and, to a lesser extent, by psychotic features [36].
Finally, the BrainAge gap (the difference between predicted biological and chronological age) [42] was not a predictor of response to electroconvulsive therapy in late-life depression patients [35].
In this review, we screened 2149 journal articles and selected 19 articles that contained information about the factors predictive of response and/or remission following electroconvulsive therapy in an elderly population with depression. Two meta-analyses had already reviewed the predictors of response to electroconvulsive therapy (ECT) in depression, focusing on the general population [17, 18]. To our knowledge this is the first published attempt to comprehensively describe the different factors predictive of response and/or remission following electroconvulsive therapy in late-life depression. Very few of the screened articles explicitly examined the response to electroconvulsive therapy in depression after the age of 65. Therefore, we had to include studies that investigated the response to electroconvulsive therapy in patients aged 50 and over.
We stratified the predictive factors according to biological [19–21] and morphological markers [22, 23], associated symptoms [24–27], treatment for the maintenance of remission [33, 34] and age [35–37].
The debate about biological markers is wide-ranging. Even if severe subtypes of major depressive disorder are associated with elevated baseline cortisol levels [43], Suijk et al. did not find a significant relationship between pre-electroconvulsive therapy salivary cortisol levels and response or remission [19]. Further studies should focus on the relationship between response and remission following electroconvulsive therapy and variations in cortisol levels following electroconvulsive therapy in the older population.
Moderately elevated levels of CRP and S100B were associated with greater efficacy of electroconvulsive therapy in the treatment of depression [20, 21]. One meta-analysis reported that the increase in S100B correlates with the severity of major depressive disorder [44]. However, another study showed that mildly elevated plasma levels of CRP (above 3.2 mg/l) in later life are associated with higher scores for clinically relevant symptoms of depression [45]. Further research should assess whether the positive relationship between depression severity and electroconvulsive therapy response, but not remission as described by van Diermen et al. [18], can also be mediated by these two biomarkers in late life.
In the elderly, some studies show an association between depression and a decrease in right hippocampal volume [46], and a smaller hippocampal volume predicts poorer outcome with pharmacotherapy treatment [47]. However, Bouckaert et al. [22] did not find an association between hippocampal volume and response or remission following electroconvulsive therapy in late-life depression. One reason for this discrepancy may be the “gold-standard” used as manual segmentation to measure hippocampal volume was used in the former study unlike Oudega et al. [23] who used visual rating of medial temporal atrophy as an approximate stand-in for hippocampal volume.
Bouckaert et al. did not find any relationship between global brain amyloid load and electroconvulsive therapy response in patients with late-life depression [22]. However, one study found an increase in Aβ1-42 in the cerebrospinal fluid after electroconvulsive therapy in patients who responded to treatment, and this increase correlated with the number of electroconvulsive therapy sessions [48]. Consequently, one hypothesis that could be tested in further research is that electroconvulsive therapy has an impact on the risk of developing Alzheimer’s disease in later life in major depressive disorder patients. More generally, treatment resistance in late-life depression has been associated with prodromal dementia, mainly Alzheimer’s disease and vascular dementia [49]. This differential diagnosis should always be considered in cases of non-response to electroconvulsive treatment.
In adults with depression, RUL ECT did not differ from BL electroconvulsive therapy in efficacy and is advantageous in terms of safety and tolerability [50]. It is important to highlight that across the three studies assessing varying efficacy based on electrode position, the average number of electroconvulsive therapy sessions in both the RUL groups and the BL/BF groups was similar (see table S4 in the appendix). These results could be optimised with adjunctive treatment with venlafaxine [31]. This also applies to adults, with several studies agreeing on the similar efficacy of unilateral and bilateral treatment, with the advantage of fewer cognitive side effects with unilateral treatment [28–30].
The post-ictal time to reorientation was a predictor of greater retrograde amnesia effects following electroconvulsive therapy [51, 52]. However, a longer post-ictal reorientation period was associated with a more rapid reduction in the severity of depressive symptoms throughout the course of electroconvulsive therapy [27]. In light of these two findings, cognitive side effects may not necessarily be considered detrimental when seeking an optimal response to electroconvulsive therapy for depressive symptoms.
Studies focusing on adult populations associated psychomotor retardation with a favourable response to electroconvulsive therapy [53, 54]. The results regarding retardation in the elderly were ambiguous. Tominaga et al. concluded that the retardation score of elderly patients with depression was significantly lower in responders to electroconvulsive therapy than in non-responders [24]. Veltman et al. [25] concluded that the psychomotor symptoms were not related to remission or response. However, Heijnen et al. [36] maintained that retardation may be one of the mediators by which elderly people respond better to electroconvulsive therapy compared to younger patients. The results of this study are particularly reliable, since, unlike the others, the researchers systematically stopped benzodiazepines before starting electroconvulsive therapy.
We have taken into account two meta-analyses [17, 18] that analysed articles involving patients aged 18 years or older. These reviews complement the systematic review by van Shaik et al. about the continuation and maintenance of electroconvulsive therapy in elderly patients with depression [14].
Taken together, our results, along with those of van Dierman et al. [18], indicate that age was positively associated with a better response to electroconvulsive therapy. In our review, we found no clear difference in predictive factors between the younger adults and the elderly. This may be because most studies focused on the response to electroconvulsive therapy in the elderly included patients aged from 50 or 55 years. Consequently, the possible differences may be minimised. Further research is needed in the elderly population to identify possible specific factors predictive of response and remission in people aged over 65 years.
The principal study limitation is the age of inclusion. Although the population of geriatric psychiatry is usually defined as being at least 65 years old, we have included articles on subjects aged 50 years or older making sure, however, that the mean age of study participants in the articles was always over 65 years. There is only one article including exclusively patients over 65 years [29]. However, the average age of most of the studies is above 70 years old (table S1 in the appendix). Another limitation of the present review was that participants experiencing various types of depression and undergoing diverse pharmacological treatments were merged together into one analysis. Despite these limitations, we felt compelled to adopt this approach due to the scarcity of existing research and the restricted number of participants across various studies.
Ultimately, even with efforts to separate the factors predictive of response and remission into distinct subgroups (biological markers, radiological markers, correlated symptoms), the groups remained quite heterogeneous, which makes generalisation very challenging. In addition, the small number of studies focusing on this topic makes it particularly difficult to draw valid conclusions.
The thoroughness with which this review was conducted constitutes one of its strengths. Furthermore, this article focuses on a population group in which predictors of good response to electroconvulsive treatment in depression have not yet been studied as a whole.
In conclusion, electroconvulsive therapy is an effective and safe technique for the elderly population. There are already some markers that may help predict response or remission following electroconvulsive therapy in the elderly depressed population; however clinical trials involving larger population samples are needed to draw more reliable conclusions.
We had no funding for this project.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Taylor WD. Clinical practice. Depression in the elderly. N Engl J Med. 2014 Sep;371(13):1228–36. doi: https://doi.org/10.1056/NEJMcp1402180
2. Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, et al.; Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study investigators. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014 May;2(5):369–79. doi: https://doi.org/10.1016/S2213-2600(14)70051-7
3. Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. 2019 Aug;9(1):188. doi: https://doi.org/10.1038/s41398-019-0514-6
4. Rasmussen K. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging (second edition). J ect. 2002;18(1):58-9.
5. Geduldig ET, Kellner CH. Electroconvulsive Therapy in the Elderly: New Findings in Geriatric Depression. Curr Psychiatry Rep. 2016 Apr;18(4):40. doi: https://doi.org/10.1007/s11920-016-0674-5
6. Slade EP, Jahn DR, Regenold WT, Case BG. Association of electroconvulsive therapy with psychiatric readmissions in US hospitals. JAMA Psychiatry. 2017 Aug;74(8):798–804. doi: https://doi.org/10.1001/jamapsychiatry.2017.1378
7. Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, Downar J, et al.; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 4. Neurostimulation Treatments. Can J Psychiatry. 2016 Sep;61(9):561–75. doi: https://doi.org/10.1177/0706743716660033
8. Association AP; American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry. 2000 Apr;157(4 Suppl):1–45.
9. Spaans HP, Sienaert P, Bouckaert F, van den Berg JF, Verwijk E, Kho KH, et al. Speed of remission in elderly patients with depression: electroconvulsive therapy v. medication. Br J Psychiatry. 2015 Jan;206(1):67–71. doi: https://doi.org/10.1192/bjp.bp.114.148213
10. Baba H, Kito S, Nukariya K, Takeshima M, Fujise N, Iga J, et al.; Committee for Treatment Guidelines of Mood Disorders, Japanese Society of Mood Disorders. Guidelines for diagnosis and treatment of depression in older adults: A report from the Japanese Society of mood disorders. Psychiatry Clin Neurosci. 2022 Jun;76(6):222–34. doi: https://doi.org/10.1111/pcn.13349
11. Menon V, Varadharajan N, Faheem A, Andrade C. Ketamine vs Electroconvulsive Therapy for Major Depressive Episode: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2023 Jun;80(6):639–42. doi: https://doi.org/10.1001/jamapsychiatry.2023.0562
12. van Rooij SJ, Riva-Posse P, McDonald WM. The Efficacy and Safety of Neuromodulation Treatments in Late-Life Depression. Curr Treat Options Psychiatry. 2020 Sep;7(3):337–48. doi: https://doi.org/10.1007/s40501-020-00216-w
13. Dominiak M, Antosik-Wójcińska AZ, Wojnar M, Mierzejewski P. Electroconvulsive Therapy and Age: Effectiveness, Safety and Tolerability in the Treatment of Major Depression among Patients under and over 65 Years of Age. Pharmaceuticals (Basel). 2021 Jun;14(6):582. doi: https://doi.org/10.3390/ph14060582
14. van Schaik AM, Comijs HC, Sonnenberg CM, Beekman AT, Sienaert P, Stek ML. Efficacy and safety of continuation and maintenance electroconvulsive therapy in depressed elderly patients: a systematic review. Am J Geriatr Psychiatry. 2012 Jan;20(1):5–17. doi: https://doi.org/10.1097/JGP.0b013e31820dcbf9
15. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010 Sep;68(6):568–77. doi: https://doi.org/10.1016/j.biopsych.2010.06.009
16. Ninke T, Bayerl S, Groene P. [Anesthesia for electroconvulsive therapy]. Anaesthesist. 2021 Apr;70(4):271–9. doi: https://doi.org/10.1007/s00101-020-00831-5
17. Haq AU, Sitzmann AF, Goldman ML, Maixner DF, Mickey BJ. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry. 2015 Oct;76(10):1374–84. doi: https://doi.org/10.4088/JCP.14r09528
18. van Diermen L, van den Ameele S, Kamperman AM, Sabbe BC, Vermeulen T, Schrijvers D, et al. Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. Br J Psychiatry. 2018 Feb;212(2):71–80. doi: https://doi.org/10.1192/bjp.2017.28
19. Suijk DL, Dols A, van Exel E, Stek ML, Veltman E, Bouckaert F, et al. Salivary cortisol as predictor for depression characteristics and remission in electroconvulsive therapy in older persons. World J Biol Psychiatry. 2019 Nov;20(9):683–90. doi: https://doi.org/10.1080/15622975.2018.1433326
20. Carlier A, Berkhof JG, Rozing M, Bouckaert F, Sienaert P, Eikelenboom P, et al. Inflammation and remission in older patients with depression treated with electroconvulsive therapy; findings from the MODECT study.. J Affect Disord. 2019 Sep;256:509–16. doi: https://doi.org/10.1016/j.jad.2019.06.040
21. Carlier A, Boers K, Veerhuis R, Bouckaert F, Sienaert P, Eikelenboom P, et al. S100 calcium-binding protein B in older patients with depression treated with electroconvulsive therapy. Psychoneuroendocrinology. 2019 Dec;110:104414. doi: https://doi.org/10.1016/j.psyneuen.2019.104414
22. Bouckaert F, Emsell L, Vansteelandt K, De Winter FL, Van den Stock J, Obbels J, et al. Electroconvulsive therapy response in late-life depression unaffected by age-related brain changes. J Affect Disord. 2019 May;251:114–20. doi: https://doi.org/10.1016/j.jad.2019.03.055
23. Oudega ML, van Exel E, Wattjes MP, Comijs HC, Scheltens P, Barkhof F, et al. White matter hyperintensities, medial temporal lobe atrophy, cortical atrophy, and response to electroconvulsive therapy in severely depressed elderly patients. J Clin Psychiatry. 2011 Jan;72(1):104–12. doi: https://doi.org/10.4088/JCP.08m04989blu
24. Tominaga K, Okazaki M, Higuchi H, Utagawa I, Nakamura E, Yamaguchi N. Symptom predictors of response to electroconvulsive therapy in older patients with treatment-resistant depression. Int J Gen Med. 2011;4:515–9. doi: https://doi.org/10.2147/IJGM.S21029
25. Veltman EM, de Boer A, Dols A, van Exel E, Stek ML, Sienaert P, et al. Melancholia as Predictor of Electroconvulsive Therapy Outcome in Later Life. J ECT. 2019 Dec;35(4):231–7. doi: https://doi.org/10.1097/YCT.0000000000000579
26. Bjølseth TM, Engedal K, Benth JŠ, Dybedal GS, Gaarden TL, Tanum L. Baseline cognitive function does not predict the treatment outcome of electroconvulsive therapy (ECT) in late-life depression. J Affect Disord. 2015 Oct;185:67–75. doi: https://doi.org/10.1016/j.jad.2015.06.021
27. Magne Bjølseth T, Engedal K, Šaltytė Benth J, Bergsholm P, Strømnes Dybedal G, Lødøen Gaarden T, et al. Speed of recovery from disorientation may predict the treatment outcome of electroconvulsive therapy (ECT) in elderly patients with major depression. J Affect Disord. 2016 Jan;190:178–86. doi: https://doi.org/10.1016/j.jad.2015.10.013
28. Stoppe A, Louzã M, Rosa M, Gil G, Rigonatti S. Fixed high-dose electroconvulsive therapy in the elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006 Jun;22(2):92–9. doi: https://doi.org/10.1097/00124509-200606000-00003
29. Dominiak M, Goetz Z, Antosik-Wojcinska AZ, Swiecicki L. Right unilateral versus bilateral formula-based electroconvulsive therapy in the treatment of major depression in elderly patients: a randomised, open label, pilot controlled trial. Psychogeriatrics. 2021 Mar;21(2):175–84. doi: https://doi.org/10.1111/psyg.12652
30. Bjølseth TM, Engedal K, Benth JŠ, Dybedal GS, Gaarden TL, Tanum L. Clinical efficacy of formula-based bifrontal versus right unilateral electroconvulsive therapy (ECT) in the treatment of major depression among elderly patients: a pragmatic, randomized, assessor-blinded, controlled trial. J Affect Disord. 2015 Apr;175:8–17. doi: https://doi.org/10.1016/j.jad.2014.12.054
31. Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, et al.; CORE/PRIDE Work Group. Right Unilateral Ultrabrief Pulse ECT in Geriatric Depression: Phase 1 of the PRIDE Study. Am J Psychiatry. 2016 Nov;173(11):1101–9. doi: https://doi.org/10.1176/appi.ajp.2016.15081101
32. Ward MJ, Karim HT, Jessen ZF, Ghuman AS, Richardson RM, Reynolds CF 3rd, et al. Association between increased theta cordance and early response to ECT in late-life depression. Int J Geriatr Psychiatry. 2020 Feb;35(2):147–52. doi: https://doi.org/10.1002/gps.5220
33. Huuhka M, Korpisammal L, Haataja R, Leinonen E. One-year outcome of elderly inpatients with major depressive disorder treated with ECT and antidepressants. J ECT. 2004 Sep;20(3):179–85. doi: https://doi.org/10.1097/00124509-200409000-00010
34. Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, et al.; CORE/PRIDE Work Group. A Novel Strategy for Continuation ECT in Geriatric Depression: Phase 2 of the PRIDE Study. Am J Psychiatry. 2016 Nov;173(11):1110–8. doi: https://doi.org/10.1176/appi.ajp.2016.16010118
35. Wagenmakers MJ, Oudega ML, Klaus F, Wing D, Orav G, Han LK, et al. BrainAge of patients with severe late-life depression referred for electroconvulsive therapy. J Affect Disord. 2023 Jun;330:1–6. doi: https://doi.org/10.1016/j.jad.2023.02.047
36. Heijnen WT, Kamperman AM, Tjokrodipo LD, Hoogendijk WJ, van den Broek WW, Birkenhager TK. Influence of age on ECT efficacy in depression and the mediating role of psychomotor retardation and psychotic features. J Psychiatr Res. 2019 Feb;109:41–7. doi: https://doi.org/10.1016/j.jpsychires.2018.11.014
37. Tew JD Jr, Mulsant BH, Haskett RF, Prudic J, Thase ME, Crowe RR, et al. Acute efficacy of ECT in the treatment of major depression in the old-old. Am J Psychiatry. 1999 Dec;156(12):1865–70. doi: https://doi.org/10.1176/ajp.156.12.1865
38. Suzuki A, Aoshima T, Fukasawa T, Yoshida K, Higuchi H, Shimizu T, et al. A three-factor model of the MADRS in major depressive disorder. Depress Anxiety. 2005;21(2):95–7. doi: https://doi.org/10.1002/da.20058
39. Parker G, Hadzi-Pavlovic D, Eyers K. Melancholia: a disorder of movement and mood: a phenomenological and neurobiological review. Cambridge University Press; 1996. doi: https://doi.org/10.1017/CBO9780511759024
40. Leuchter AF, Cook IA, Lufkin RB, Dunkin J, Newton TF, Cummings JL, et al. Cordance: a new method for assessment of cerebral perfusion and metabolism using quantitative electroencephalography. Neuroimage. 1994 Jun;1(3):208–19. doi: https://doi.org/10.1006/nimg.1994.1006
41. Iosifescu DV. Electroencephalography-derived biomarkers of antidepressant response. Harv Rev Psychiatry. 2011;19(3):144–54. doi: https://doi.org/10.3109/10673229.2011.586549
42. Cole JH, Franke K. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci. 2017 Dec;40(12):681–90. doi: https://doi.org/10.1016/j.tins.2017.10.001
43. Nandam LS, Brazel M, Zhou M, Jhaveri DJ. Cortisol and Major Depressive Disorder-Translating Findings From Humans to Animal Models and Back. Front Psychiatry. 2020 Jan;10:974. doi: https://doi.org/10.3389/fpsyt.2019.00974
44. Tural U, Irvin MK, Iosifescu DV. Correlation between S100B and severity of depression in MDD: A meta-analysis. World J Biol Psychiatry. 2022 Jul;23(6):456–63. doi: https://doi.org/10.1080/15622975.2021.2013042
45. de la Torre-Luque A, Ayuso-Mateos JL, Sanchez-Carro Y, de la Fuente J, Lopez-Garcia P. Inflammatory and metabolic disturbances are associated with more severe trajectories of late-life depression. Psychoneuroendocrinology. 2019 Dec;110:104443. doi: https://doi.org/10.1016/j.psyneuen.2019.104443
46. Sawyer K, Corsentino E, Sachs-Ericsson N, Steffens DC. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health. 2012;16(6):753–62. doi: https://doi.org/10.1080/13607863.2012.678478
47. Hsieh MH, McQuoid DR, Levy RM, Payne ME, MacFall JR, Steffens DC. Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatr Psychiatry. 2002 Jun;17(6):519–25. doi: https://doi.org/10.1002/gps.611
48. Kranaster L, Aksay SS, Bumb JM, Janke C, Alonso A, Hoyer C, et al. Electroconvulsive therapy selectively enhances amyloid β 1-42 in the cerebrospinal fluid of patients with major depression: A prospective pilot study. Eur Neuropsychopharmacol. 2016 Dec;26(12):1877–84. doi: https://doi.org/10.1016/j.euroneuro.2016.11.004
49. Mahgoub N, Alexopoulos GS. Amyloid hypothesis: is there a role for antiamyloid treatment in late-life depression? Am J Geriatr Psychiatry. 2016 Mar;24(3):239–47. doi: https://doi.org/10.1016/j.jagp.2015.12.003
50. Dominiak M, Antosik-Wójcińska AZ, Goetz Z, Sikorska O, Stefanowski B, Gorostiza D, et al. Efficacy, safety and tolerability of formula-based unilateral vs bilateral electroconvulsive therapy in the treatment of major depression: A randomized open label controlled trial. J Psychiatr Res. 2021 Jan;133:52–9. doi: https://doi.org/10.1016/j.jpsychires.2020.12.002
51. Sackeim HA, Luber B, Moeller JR, Prudic J, Devanand DP, Nobler MS. Electrophysiological correlates of the adverse cognitive effects of electroconvulsive therapy. J ECT. 2000 Jun;16(2):110–20. doi: https://doi.org/10.1097/00124509-200006000-00003
52. Martin DM, Gálvez V, Loo CK. Predicting Retrograde Autobiographical Memory Changes Following Electroconvulsive Therapy: Relationships between Individual, Treatment, and Early Clinical Factors. Int J Neuropsychopharmacol. 2015 Jun;18(12):pyv067. doi: https://doi.org/10.1093/ijnp/pyv067
53. Buchan H, Johnstone E, McPherson K, Palmer RL, Crow TJ, Brandon S. Who benefits from electroconvulsive therapy? Combined results of the Leicester and Northwick Park trials. Br J Psychiatry. 1992 Mar;160(3):355–9. doi: https://doi.org/10.1192/bjp.160.3.355
54. Hickie I, Parsonage B, Parker G. Prediction of response to electroconvulsive therapy. Preliminary validation of a sign-based typology of depression. Br J Psychiatry. 1990 Jul;157(1):65–71. doi: https://doi.org/10.1192/bjp.157.1.65
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3684.