Hereditary alpha-tryptasemia – a potential cause of severe anaphylactic reactions
and a modifier of mast cell diseases
DOI: https://doi.org/https://doi.org/10.57187/s.3679
Axel Rüfera,
Gerhard Müllnerb,
Oliver Fuchsc,
Wolfgang R. Sperrde,
Gregor Hoermannef
a Department
of Hematology, Centre of Excellence of the European Competence Network on
Mastocytosis (ECNM), Luzerner Kantonsspital, Lucerne, and University of Lucerne, Lucerne,
Switzerland
b Allergology
and Immunology, Medbase Kriens Mattenhof, Kriens, Switzerland
c Department
of Allergology, Centre of Excellence of ECNM, Luzerner Kantonsspital, Luzern,
and University of Berne, Berne, Switzerland
d Division
of Hematology and Hemostaseology, Department of Internal Medicine I, Centre of
Excellence of ECNM, Medical University of Vienna, Vienna, Austria
e Ludwig
Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna,
Austria
f MLL
Munich Leukaemia Laboratory, Reference Centre of the ECNM, Munich, Germany
Summary
Hereditary alpha-tryptasemia
(HAT) is an autosomal dominant genetic trait affecting 4% to 6% of the general
population. Hereditary alpha-tryptasemia is caused by an excess of alpha
tryptase encoding TPSAB1 copy numbers on one parenteral allele, most
often duplications or triplications, leading to elevated levels of basal serum
tryptase. There might be a gene dosage effect between the number of additional TPSAB1
copies, the level of basal serum tryptase and the severity of clinical symptoms,
including atopic, cutaneous, gastrointestinal, musculoskeletal, autonomic and
neuropsychiatric manifestations. Hereditary alpha-tryptasemia is a potential risk
factor for severe anaphylactic reactions. The prevalence of hereditary
alpha-tryptasemia is higher in patients with systemic mastocytosis. In the
diagnostic workup of patients with anaphylactic reactions and symptoms of mast
cell mediator release after measurement of basal serum tryptase, it is
therefore essential to screen for both the KIT D816V activating point
mutation and hereditary alpha-tryptasemia by droplet digital polymerase chain
reaction. Such a diagnostic approach can identify patients with hereditary
alpha-tryptasemia, which may allow the avoidance of further diagnostic workup
with bone marrow examination. Moreover, it can identify patients at high risk of
anaphylactic reactions. So far, no targeted therapy for hereditary
alpha-tryptasemia is available. Treatment for symptom control consists of H1-
and H2-blockers, leukotriene antagonists and cromoglicic acid. Urticaria and
anaphylaxis are especially successfully treated with the monoclonal
anti-IgE-antibody omalizumab in patients with hereditary alpha-tryptasemia. H1-blockers
and steroids are sufficient in emergencies. As hereditary alpha-tryptasemia is
a hereditary condition, first-degree relatives with anaphylactic reactions or
symptoms of mast cell mediator release should be tested for hereditary
alpha-tryptasemia after measurement of basal serum tryptase.
A case illustrating the clinical problem
A 53-year-old
gentleman was referred for an allergological evaluation. He had a history of a
severe anaphylactic reaction grade III, according to H.L. Mueller, with rapid
onset of paresthesias, dyspnoea, nausea and vomiting, low blood pressure, and
somnolence but no urticaria after a bee sting. Notably, he reported a similar
reaction that had occurred more than a decade ago after a wasp sting. A month
after the recent event, total immunoglobulin (Ig) E was normal, skin tests
revealed no significant sensitisation to bee venom and an elevation of specific
IgG against bee venom and wasp venom was documented. A venom immunotherapy with
bee venom in an ultra-rush procedure was commenced. Basal serum tryptase was largely
increased, with a value of 194 µg/l (normal range: <11.4 µg/l), and
repeatedly measured with basal serum tryptase-values >125 µg/l without signs
of mastocytosis in the skin. Other aetiologies associated with an elevated basal
serum tryptase, such as renal failure, increased body mass, chronic
inflammatory diseases or chronic infections, were excluded.
Diagnostic
workup to further explore the elevated basal serum tryptase, including
molecular genetic testing and bone marrow investigation, revealed a 5% infiltration
of the bone marrow with neoplastic, spindle-shaped, CD25- and CD2-positive mast
cells. The activating D816V point mutation in KIT was detected by
digital droplet polymerase chain reaction (PCR). There was a normal bone
density on the DXA scan, no evidence of hepato-/splenomegaly on ultrasound, and
no other B- or C-findings. According to the 2017 WHO Classification, the
diagnosis of indolent systemic mastocytosis was made based on the presence of
four minor diagnostic criteria. Venom immunotherapy was continued with the
recommendation of a life-long implementation, and an H1-blocker therapy was
started. The patient was given an emergency kit with an H1-blocker, steroids
and a self-injectable adrenalin injector and trained in its use.
Interestingly,
the daughter of this patient also suffered from venom allergy,
albeit to wasp venom. In combination with an elevated basal serum tryptase of 31.6
µg/l, a diagnostic workup was performed in the absence of mastocytosis in the
skin. Bone marrow examination revealed normal haematopoiesis with no evidence of
an increase in mast cells, and no D816V point mutation in KIT was
detected by digital droplet PCR. Therefore, systemic mastocytosis was ruled
out.
Since the
cause of the elevated basal serum tryptase was unclear, a quantitative digital
droplet PCR for genotyping of the TPSAB1/TPSB2 gene locus was
performed. This analysis revealed a hereditary alpha-tryptasemia (HAT) with documentation
of a total of three copies of sequences encoding alpha and beta tryptase each,
compatible with the diagnosis of hereditary alpha-tryptasemia with a
triplication of the alpha tryptase encoding TPSAB1 gene (one copy of the
beta tryptase encoding sequence on each TPSB2 allele, one copy of the beta
tryptase encoding sequence on one TPSAB1 allele and three copies of the alpha
tryptase encoding sequence on the other TPSAB1 allele). These additional
germline copies of the TPSAB1 gene encoding the alpha isoform lead to the
increased constitutive release of tryptase and, thus, to elevated basal serum
tryptase levels.
Therefore,
the father’s case was again reviewed, and molecular genetic testing revealed
the presence of four copies of alpha tryptase and two copies of beta tryptase,
confirming the additional diagnosis of hereditary alpha-tryptasemia. Therefore,
the definitive diagnosis in this case was systemic mastocytosis and hereditary
alpha-tryptasemia. The subtype of systemic mastocytosis had been initially diagnosed
as indolent systemic mastocytosis based on the basal serum tryptase level of ≥125
µg/l. When correcting the initial basal serum tryptase of 194 µg/l to
account for the presence of an alpha tryptase gene triplication (i.e. division
of basal serum tryptase by 1 plus the extra copy numbers of the alpha tryptase
gene, in this case, 194 µg/l divided by 3 for two extra copies), the hereditary
alpha-tryptasemia-corrected basal serum tryptase was 65 µg/l. Therefore, bone
marrow mastocytosis was diagnosed according to the 2022 WHO Classification [1,
2]. Despite ongoing venom immunotherapy and usage of an H1-blocker, there was
another anaphylactic reaction after a bee sting, and the dose of venom
immunotherapy was subsequently doubled.
Serum tryptase – genetic
background and clinical symptoms
About 99%
of serum tryptases derive from mast cells, and about 1% originate from
basophils [3]. Elevation of BST (≥11.4 µg/l) is a
typical finding in patients with hereditary alpha-tryptasemia, where basal
serum tryptase is usually ≥8 µg/l. However, this is not
specific to that condition. In many other conditions, basal serum tryptase can
be elevated, such as in systemic mastocytosis, chronic kidney disease, chronic
spontaneous urticaria, and myeloid malignancies, such as chronic myelomonocytic
leukaemia, chronic eosinophilic leukaemia, acute myeloid leukaemia or
myelodysplastic neoplasms [4–10]. Therefore, in patients with an elevated basal
serum tryptase, hereditary alpha-tryptasemia, systemic mastocytosis, renal
failure and myeloid malignancies must be considered in the differential
diagnosis, occurring either as a separate entity or in combination.
Hereditary
alpha-tryptasemia is an autosomal dominant genetic trait found in 4% to 6% of
the general population. Its prevalence is higher in patients with idiopathic
anaphylaxis. There are four tryptase genes on chromosome 16p13.3. TPSB2
encodes beta tryptase isoforms, TPSAB1 encodes alpha tryptase or beta
tryptase isoforms, TPSG1 encodes gamma tryptase, and TPSD1 encodes
delta tryptase [11]. The fifth tryptase isoform, epsilon tryptase, is encoded
by the serine protease 22 (PRSS22) gene [11]. Only alpha- and beta-tryptases
are secreted in relevant amounts and are measured as serum tryptase [11]. Alpha-
and beta-tryptase are produced as protryptase monomeric precursors, which can
be either constitutively secreted or further processed with the formation of
mature tryptase tetramers [12]. The role of mature tryptases is poorly
understood; it has been reported that they are involved in tissue repair,
vascular permeability, chemotaxis of neutrophils and eosinophils, and
thrombolysis [13–16]. The ELISA-based immunoassays used to analyse serum
tryptase levels can detect the protryptase monomeric precursors and mature
tryptase forms [17].
Tryptases
encoded by TPSB2 (beta) and TPSAB1 (alpha or beta) are inherited
as a haplotype from the parents. Each parent passes on one allele from the TPSB2
gene and one allele from the TPSAB1 gene to their offspring. In hereditary
alpha-tryptasemia, there is an excess of alpha tryptase encoding TPSAB1 copy
numbers on one allele (figure 1). Furthermore, an overactive promoter has been
identified in hereditary alpha-tryptasemia [18], leading to increased alpha
tryptase synthesis and secretion and, thus, increased basal serum tryptase. In hereditary
alpha-tryptasemia, one additional TPSAB1 copy increases basal serum
tryptase by approximately 9.5 µg/l [19].
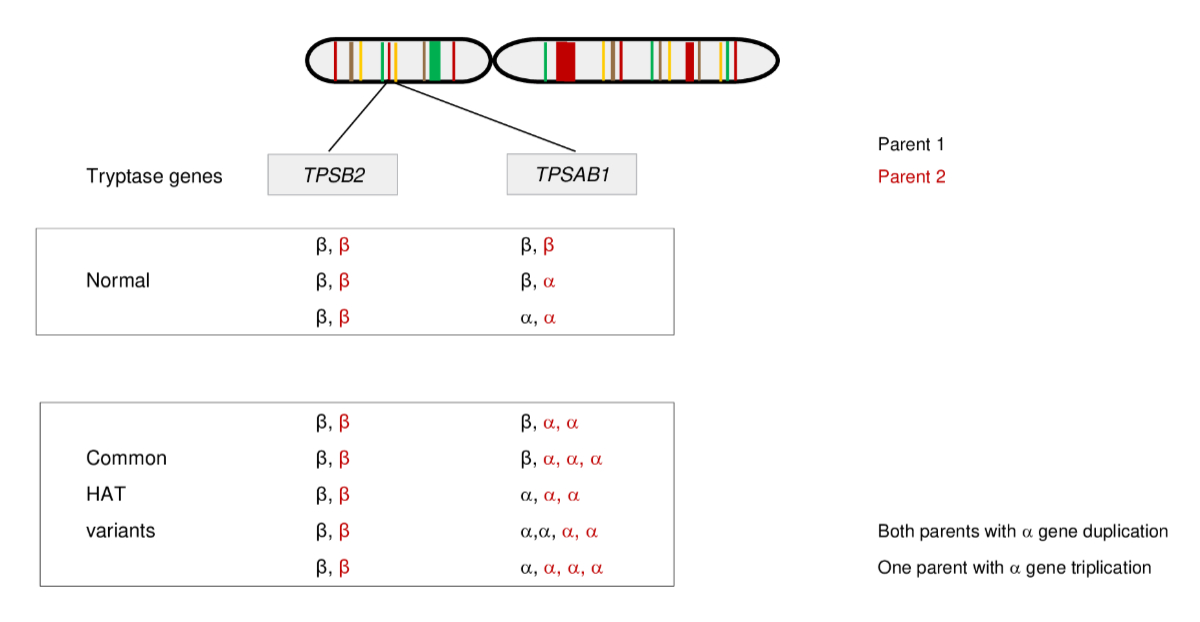
Figure 1Patterns of inheritance in alpha-tryptasemia (HAT) on chromosome 16p13.3 with
different parenteral haplotypes. Alpha-tryptasemia is present when there is an
excess of alpha tryptase encoding TPSAB1 copy numbers on one allele. An
increased copy number can be inherited by one or by both parents. Common alpha-tryptasemia
variants include alpha tryptase duplications or triplications. Adapted from
Luskin KT, White AA, Lyons JJ. The genetic basis and clinical impact of
hereditary alpha-tryptasemia. J Allergy Clin Immunol Pract. 2021;9(6):2235–42. https://doi.org/10.1016/j.jaip.2021.03.005, with permission from Elsevier.
Furthermore,
a gene-dose effect between the TPSAB1 copy number and the severity of
symptoms is frequently recorded, meaning that the more copies of the alpha
tryptase encoding gene are present, the higher the basal serum tryptase level
and the more symptoms may occur in affected patients [20]. However, isolated
studies found no correlation between the frequency of clinical manifestations
and the copy number [21].
The risk of
anaphylaxis in hereditary alpha-tryptasemia is clearly increased, but symptoms
in patients with hereditary alpha-tryptasemia are variably expressed. These
symptoms can manifest at any age, but patients can also be completely
asymptomatic. There is little evidence of the correlation between hereditary
alpha-tryptasemia and clinical symptoms. In one study, one-third of healthy
adults with hereditary alpha-tryptasemia had severe symptoms, one-third had
mild to moderate symptoms, and one-third displayed no difference from healthy
volunteers [20].
The reason
for the phenotypic heterogeneity is unclear, but coinheritance of other genetic
variants might play a role. In addition to allergic conditions, nonallergic
diseases are also associated with hereditary alpha-tryptasemia. Females with hereditary
alpha-tryptasemia are more often symptomatic, and although this is an inherited
condition, the onset of symptoms is usually after puberty [22].
There is a
variety of potential clinical symptoms in patients with hereditary
alpha-tryptasemia. Most of these symptoms are consistent with mast cell
mediator release through activation and degranulation [23]. Most commonly,
there is urticaria, flushing and/or pruritus, gastroesophageal reflux disease,
irritable bowel syndrome-like symptoms and/or diarrhoea, allergic rhinitis
and/or asthma, arthralgias, fibromyalgia and/or headaches as well as
neuropsychiatric manifestations [20].
Anaphylaxis
is significantly modified by hereditary alpha-tryptasemia, increasing both in incidence
and severity [13]. Triggers of anaphylaxis include insect stings, food, drugs,
radiocontrast media and specific immunotherapy; in idiopathic anaphylaxis, no
specific trigger can be identified [24]. Tryptase genotyping for hereditary
alpha-tryptasemia and KIT D816V for systemic mastocytosis should be
considered clinically when evaluating individuals with a history (or are at
risk) of severe anaphylaxis [13].
Hereditary
alpha-tryptasemia is observed at an increased prevalence in systemic
mastocytosis but is also more frequent in non-clonal mast cell disorders. In systemic
mastocytosis, there is an increase in mast cell mediator symptoms if patients also
have hereditary alpha-tryptasemia [25]. In those patients, hereditary
alpha-tryptasemia leads to a doubling of the prevalence of anaphylaxis [13]. Modification
of clinical symptoms in patients with systemic mastocytosis in that context is
clearly present, although it remains unclear whether hereditary
alpha-tryptasemia can independently cause mast cell activation. A recent study
confirmed the association between hereditary alpha-tryptasemia and anaphylaxis
in systemic mastocytosis but found no increased risk of developing anaphylaxis
during the course of the disease in patients with systemic mastocytosis in whom
anaphylaxis has not been part of the presenting symptoms [26]. Patients with both
hereditary alpha-tryptasemia and systemic mastocytosis have higher serum tryptase
levels independent of the mast cell burden and a significantly lower KIT
D816V variant allele frequency [25, 26].
In
contrast, most patients with hereditary alpha-tryptasemia have no clonal mast
cell disease [27]. It is important to realise that a diagnosis of hereditary
alpha-tryptasemia does not rule out a clonal mast cell disease such as systemic
mastocytosis. Clinical warning signs, such as severe anaphylaxis, especially
due to Hymenoptera stings (e.g. bees, bumblebees, wasps, hornets), typical skin
lesions or signs of end-organ involvement, should prompt a further diagnostic
workup.
Hereditary alpha-tryptasemia
– diagnostic workup
In patients
with anaphylactic reactions or symptoms of mast cell mediator release, the
first diagnostic step is the measurement of basal serum tryptase, which can be
done inside or outside a hospital. In addition to a complete blood count to
look for monocytosis or eosinophilia, clinical examination is essential,
especially to evaluate for typical skin lesions and hepato-/splenomegaly.
The normal
value for basal serum tryptase in asymptomatic controls, including hereditary
alpha-tryptasemia-positive individuals, is ≤15 µg/l and a basal serum tryptase
level >20 µg/l is a minor criterion for the diagnosis of systemic
mastocytosis [1, 2, 28]. The presence of hereditary alpha-tryptasemia is
unlikely if the basal serum tryptase is <8 µg/l and can be almost excluded
if the basal serum tryptase is <6.5 µg/l [8].
In
contrast, the presence of systemic mastocytosis cannot be reliably excluded
solely based on a normal basal serum tryptase. In symptomatic patients with basal
serum tryptase ≥8 µg/l, both hereditary alpha-tryptasemia and systemic
mastocytosis should be considered as differential diagnoses. The diagnostic
workup for hereditary alpha-tryptasemia is straightforward as it is diagnosed
by genotyping TPSAB1/TPSB2 by droplet digital PCR (e.g. from a sample
of peripheral blood) in specialised laboratories. The diagnostic workup for systemic
mastocytosis is more complex, but highly sensitive PCR-based techniques
reliably detect the KIT D816V mutation in the peripheral blood in most
cases [29, 30]. Thus, hereditary alpha-tryptasemia genotyping and KIT
D816V testing from peripheral blood with a highly sensitive assay should be
performed together with an evaluation of clinical red flags such as mastocytosis
in the skin as reasonable steps for further workup [6].
In patients
with neither the KIT D816V mutation in the peripheral blood nor mastocytosis
in the skin or other red flags for systemic mastocytosis, one can generally avoid
invasive and expensive further workup for systemic mastocytosis with a bone
marrow examination if basal serum tryptase is only slightly elevated or its elevation
can be attributed to another cause, especially in the presence of hereditary
alpha-tryptasemia. In this scenario, even in the absence of hereditary
alpha-tryptasemia, a basal serum tryptase of <15 µg/l is not an indication
for bone marrow examination, and even a basal serum tryptase <20 µg/l may be
acceptable [29, 31].
However, in
patients with anaphylaxis with bone marrow mastocytosis, the basal serum
tryptase can well be <15 µg/l. Therefore, it is important to consider the
severity of symptoms when deciding on further workup with a bone marrow examination
[32].
In
individuals with hereditary alpha-tryptasemia and neither the KIT D816V
mutation in the peripheral blood nor mastocytosis in the skin, even higher levels
of basal serum tryptase are considered normal for the given genotype and are
generally also not an indication for bone marrow examination to evaluate for systemic
mastocytosis. One approach is to correct basal serum tryptase for the hereditary
alpha-tryptasemia genotype, as suggested by the WHO Classification for the
diagnostic minor criterion of 20 µg/l by division of basal serum tryptase by 1
plus the extra copy numbers of the alpha tryptase gene [1, 2]. Another approach
is the usage of genotype-specific reference intervals for basal serum tryptase
in individuals with hereditary alpha-tryptasemia – these are available via an
online calculator tool (https://bst-calculater.niaid.nih.gov/) [18]. For most
patients, both approaches lead to similar results [8]. For all other patients, a
full workup for clonal mast cell disease is advisable.
Again, as
there are patients with bone marrow mastocytosis without the KIT D816V
mutation, one should consider the severity of symptoms in the decision regarding
the workup for clonal mast cell disease.
The
proposed algorithm for the diagnostic workup of patients with symptoms of mast
cell mediator release and anaphylaxis, considering clinical symptoms, basal
serum tryptase, KIT D816V mutational status, genotyping for hereditary
alpha-tryptasemia and presence or absence of mastocytosis in the skin is
depicted in figure 2.
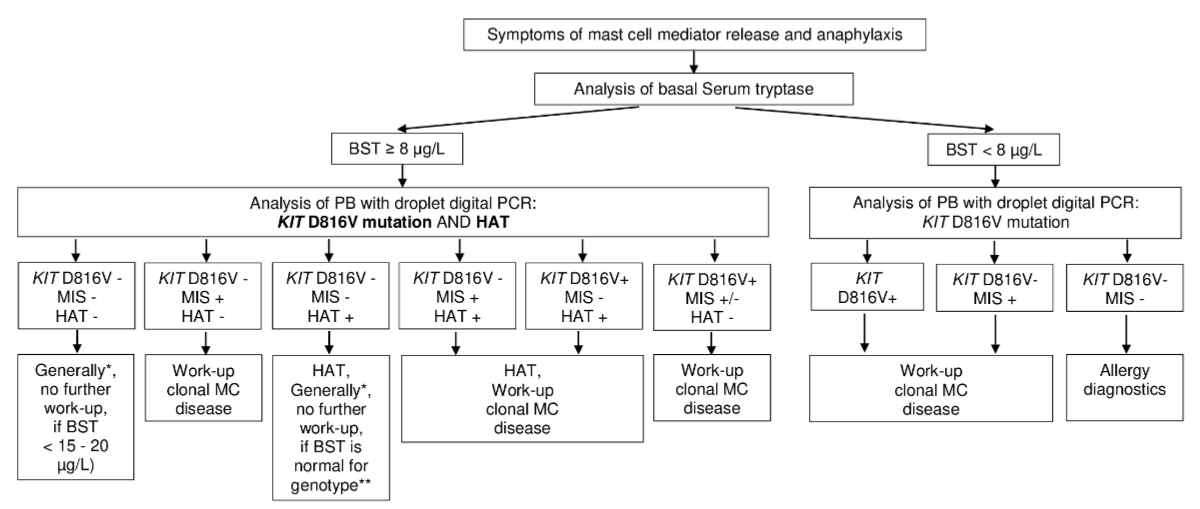
Figure 2Diagnostic algorithm in parents with symptoms of mast cell mediator release and
anaphylaxis – hereditary alpha-tryptasemia (HAT), systematic mastocytosis,
combination of hereditary alpha-tryptasemia and systematic mastocytosis. The
first step in the diagnostic workup of patients with symptoms of mast cell
mediator release and anaphylaxis is the measurement of the basal serum tryptase
level. The second step is the screening for the KIT D816V activating
point mutation and for hereditary alpha tryptasemia with droplet digital polymerase
chain reaction (PCR) from peripheral blood (PB). * Individual decision, as
there are patients with bone marrow mastocytosis with negative results of KIT
D816V mutation – consider also severity of symptoms and rare conditions
(VIPoma, and others). ** Online calculator tool (https://bst-calculater.niaid.nih.gov/)
for genotype specific reference intervals or basal serum tryptase (BST)
correction by division by 1 plus the extra copy numbers of the alpha tryptase
gene. MC: mast cells; MIS: mastocytosis in the skin; +: positive; –: negative.
Such a
diagnostic approach will ensure completeness of the diagnosis and avoidance of
missing patients with the combination of hereditary alpha-tryptasemia and systemic
mastocytosis and, therefore, a higher risk of anaphylaxis compared to the
occurrence of one disease entity alone.
Hereditary alpha-tryptasemia
– treatment options and open questions
There is
still an ongoing discussion about whether hereditary alpha-tryptasemia represents
a disease itself or is instead a risk or aggravating factor for other diseases.
Asymptomatic individuals with hereditary alpha-tryptasemia should currently not
be considered patients, and thus, no therapy is indicated [33]. Symptoms can
become manifest at any age, usually after puberty. Symptoms can begin or get
aggravated after an infection. In addition, non-steroidal antirheumatic drugs,
contrast medium or emotional stress can trigger symptoms.
Presently,
there is no therapy targeting the genetic origin. Treatment of hereditary
alpha-tryptasemia is, therefore, non-specific but symptom-based. Avoidance of
known triggers is always the first step. The therapeutic approach has to be
directed against the individual symptoms and consists of H1- and H2-blockers,
leukotriene antagonists and cromoglicic acid for mast cell stabilisation. If
severe anaphylactic reactions occur, an emergency kit with an H1-blocker,
steroids and a self-injectable adrenalin injector should be provided. In cases
of Hymenoptera venom anaphylaxis, venom immunotherapy should be implemented. It
remains an open question how long venom immunotherapy should be performed in
patients with hereditary alpha-tryptasemia without a clonal mast cell disease.
In patients
with gastrointestinal, cardiac or neurological symptoms, the appropriate
specialists should be consulted, and an interprofessional approach should be
taken. In patients with urticaria, pruritus, nonallergic asthma or abdominal
pain, treatment with the monoclonal anti-IgE-antibody omalizumab – although not
approved for those indications in Switzerland – can achieve good therapeutic
results [34]. This approach is supported by a retrospective study showing improvements
in urticaria and anaphylaxis in patients treated with omalizumab [35]. Whether a
specific drug or intervention is more suitable for a given symptom than others also
remains an open question. Studies using antitryptase antibodies are underway,
but no clinical data are yet available.
Conclusion
Hereditary
alpha-tryptasemia is a major cause of elevated serum tryptase levels and a
relevant risk factor for severe anaphylactic reactions.
The
prevalence of hereditary alpha-tryptasemia is increased in patients with systemic
mastocytosis, and these patients are at high risk of severe mast cell mediator
symptoms and anaphylaxis. Therefore, it is crucial in the diagnostic workup to
screen for both hereditary alpha-tryptasemia and systemic mastocytosis in
patients with anaphylactic reactions or symptoms of mast cell mediator release.
With such a screening approach, one can avoid further diagnostic workup,
including bone marrow examination, in some patients with hereditary
alpha-tryptasemia, and one can identify patients at high risk of anaphylactic
reactions due to the occurrence of both hereditary alpha-tryptasemia and systemic
mastocytosis. This is highly clinically relevant regarding the patient’s information,
the avoidance of triggers of mast cell mediator release if possible, and the
creation of an individual, symptom-based treatment plan.
As hereditary
alpha-tryptasemia is a hereditary condition, first-degree relatives with anaphylactic
reactions or symptoms of mast cell mediator release should be tested for hereditary
alpha-tryptasemia after measurement of basal serum tryptase.
Informed consent
Written informed consent was obtained from the patient for the publication of this
article.
Axel Rüfer
Department
of Hematology
Centre of Excellence of the European Competence Network on
Mastocytosis (ECNM)
Luzerner Kantonsspital (LUKS)
Spitalstrasse
CH-6000 Lucerne
axel.ruefer[at]luks.ch
References
1. Valent P, Akin C, Hartmann K, Alvarez-Twose I, Brockow K, Hermine O, et al. Updated
Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal.
Hemasphere. 2021. 13;5(11):e646. doi: 10.1097/HS9.0000000000000646.
2. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition
of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid
and Histiocytic/Dendritic Neoplasms. Leukemia. 2022 Jul;36(7):1703–19. 10.1038/s41375-022-01613-1
3. Weiler CR, Austen KF, Akin C, Barkoff MS, Bernstein JA, Bonadonna P, et al. AAAAI
Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS)
diagnosis and management. J Allergy Clin Immunol. 2019 Oct;144(4):883–96. doi: https://doi.org/10.1016/j.jaci.2019.08.023
4. Sperr WR, Jordan JH, Fiegl M, Escribano L, Bellas C, Dirnhofer S, et al. Serum tryptase
levels in patients with mastocytosis: correlation with mast cell burden and implication
for defining the category of disease. Int Arch Allergy Immunol. 2002 Jun;128(2):136–41.
doi: https://doi.org/10.1159/000059404
5. Sirvent AE, González C, Enríquez R, Fernández J, Millán I, Barber X, et al. Serum
tryptase levels and markers of renal dysfunction in a population with chronic kidney
disease. J Nephrol. 2010;23(3):282–90.
6. Sperr WR, Jordan JH, Baghestanian M, Kiener HP, Samorapoompichit P, Semper H, et al. Expression
of mast cell tryptase by myeloblasts in a group of patients with acute myeloid leukemia.
Blood. 2001 Oct;98(7):2200–9. doi: https://doi.org/10.1182/blood.V98.7.2200
7. Siles R, Xu M, Hsieh FH. The utility of serum tryptase as a marker in chronic spontaneous
urticaria. Acta Derm Venereol. 2013 May;93(3):354–5. doi: https://doi.org/10.2340/00015555-1486
8. Lyons JJ, Greiner G, Hoermann G, Metcalfe DD. Incorporating Tryptase Genotyping Into
the Workup and Diagnosis of Mast Cell Diseases and Reactions. J Allergy Clin Immunol
Pract. 2022 Aug;10(8):1964–73. doi: https://doi.org/10.1016/j.jaip.2022.05.003
9. Sperr WR, Stehberger B, Wimazal F, Baghestanian M, Schwartz LB, Kundi M, et al. Serum
tryptase measurements in patients with myelodysplastic syndromes. Leuk Lymphoma. 2002 May;43(5):1097–105.
doi: https://doi.org/10.1080/10428190290021470
10. Sperr WR, El-Samahi A, Kundi M, Girschikofsky M, Winkler S, Lutz D, et al. Elevated
tryptase levels selectively cluster in myeloid neoplasms: a novel diagnostic approach
and screen marker in clinical haematology. Eur J Clin Invest. 2009 Oct;39(10):914–23.
doi: https://doi.org/10.1111/j.1365-2362.2009.02184.x
11. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features.
Immunol Allergy Clin North Am. 2018 Aug;38(3):483–95. doi: https://doi.org/10.1016/j.iac.2018.04.003
12. Schwartz LB, Min HK, Ren S, Xia HZ, Hu J, Zhao W, et al. Tryptase precursors are preferentially
and spontaneously released, whereas mature tryptase is retained by HMC-1 cells, Mono-Mac-6
cells, and human skin-derived mast cells. J Immunol. 2003 Jun;170(11):5667–73. doi: https://doi.org/10.4049/jimmunol.170.11.5667
13. Lyons JJ, Chovanec J, O’Connell MP, Liu Y, Šelb J, Zanotti R, et al. Heritable risk
for severe anaphylaxis associated with increased α-tryptase-encoding germline copy
number at TPSAB1. J Allergy Clin Immunol. 2021 Feb;147(2):622–32. doi: https://doi.org/10.1016/j.jaci.2020.06.035
14. Zhang H, Zeng X, He S. Evaluation on potential contributions of protease activated
receptors related mediators in allergic inflammation. Mediators Inflamm. 2014;2014:829068.
doi: https://doi.org/10.1155/2014/829068
15. Ong MS, Tergaonkar V. When alpha meets beta, mast cells get hyper. J Exp Med. 2019 Oct;216(10):2229–30.
doi: https://doi.org/10.1084/jem.20191169
16. Melo FR, Wallerman O, Paivandy A, Calounova G, Gustafson AM, Sabari BR, et al. Tryptase-catalyzed
core histone truncation: A novel epigenetic regulatory mechanism in mast cells. J
Allergy Clin Immunol. 2017 Aug;140(2):474–85. doi: https://doi.org/10.1016/j.jaci.2016.11.044
17. Gotlib J, Horny HP, Valent P. Mast cells and mastocytosis. In: Hoffman R, Benz JE,
editors. Hematology. Basic Principles and Practice. 7th ed. Elsevier; 2018. pp. 1170–86.
18. Chovanec J, Tunc I, Hughes J, Halstead J, Mateja A, Liu Y, et al. Genetically defined
individual reference ranges for tryptase limit unnecessary procedures and unmask myeloid
neoplasms. Blood Adv. 2023 May;7(9):1796–810. doi: https://doi.org/10.1182/bloodadvances.2022007936
19. O’Connell MP, Lyons JJ. Resolving the genetics of human tryptases: implications for
health, disease, and clinical use as a biomarker. Curr Opin Allergy Clin Immunol.
2022 Apr;22(2):143–52. doi: https://doi.org/10.1097/ACI.0000000000000813
20. Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase
identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat
Genet. 2016 Dec;48(12):1564–9. doi: https://doi.org/10.1038/ng.3696
21. Robey RC, Wilcock A, Bonin H, Beaman G, Myers B, Grattan C, et al. Hereditary Alpha-Tryptasemia:
UK Prevalence and Variability in Disease Expression. J Allergy Clin Immunol Pract.
2020;8(10):3549–56. doi: https://doi.org/10.1016/j.jaip.2020.05.057
22. Glover SC, Carter MC, Korošec P, Bonadonna P, Schwartz LB, Milner JD, et al. Clinical
relevance of inherited genetic differences in human tryptases: hereditary alpha-tryptasemia
and beyond. Ann Allergy Asthma Immunol. 2021 Dec;127(6):638–47. doi: https://doi.org/10.1016/j.anai.2021.08.009
23. Atiakshin D, Buchwalow I, Samoilova V, Tiemann M. Tryptase as a polyfunctional component
of mast cells. Histochem Cell Biol. 2018 May;149(5):461–77. doi: https://doi.org/10.1007/s00418-018-1659-8
24. Shaker MS, Wallace DV, Golden DB, Oppenheimer J, Bernstein JA, Campbell RL, et al.;
Collaborators; Chief Editors; Workgroup Contributors; Joint Task Force on Practice
Parameters Reviewers. Anaphylaxis-a 2020 practice parameter update, systematic review,
and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis.
J Allergy Clin Immunol. 2020 Apr;145(4):1082–123. doi: https://doi.org/10.1016/j.jaci.2020.01.017
25. Greiner G, Sprinzl B, Górska A, Ratzinger F, Gurbisz M, Witzeneder N, et al. Hereditary
α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in
mastocytosis. Blood. 2021 Jan;137(2):238–47. doi: https://doi.org/10.1182/blood.2020006157
26. González-de-Olano D, Navarro-Navarro P, Muñoz-González JI, Sánchez-Muñoz L, Henriques A,
de-Andrés-Martín A, et al. Clinical impact of the TPSAB1 genotype in mast cell diseases:
A REMA study in a cohort of 959 individuals. Allergy. 2024 Mar;79(3):711–23. doi: https://doi.org/10.1111/all.15911
27. Sabato V, Chovanec J, Faber M, Milner JD, Ebo D, Lyons JJ. First identification of
an inherited TPSAB1 quintuplication in a patient with clonal mast cell disease. J
Clin Immunol. 2018 May;38(4):457–9. doi: https://doi.org/10.1007/s10875-018-0506-y
28. Valent P, Hoermann G, Bonadonna P, Hartmann K, Sperr WR, Broesby-Olsen S, et al. The
Normal Range of Baseline Tryptase Should Be 1 to 15 ng/mL and Covers Healthy Individuals
With HαT. J Allergy Clin Immunol Pract. 2023 Oct;11(10):3010–20. doi: https://doi.org/10.1016/j.jaip.2023.08.008
29. Hoermann G, Sotlar K, Jawhar M, Kristensen T, Bachelot G, Nedoszytko B, et al. Standards
of Genetic Testing in the Diagnosis and Prognostication of Systemic Mastocytosis in
2022: Recommendations of the EU-US Cooperative Group. J Allergy Clin Immunol Pract.
2022 Aug;10(8):1953–63. doi: https://doi.org/10.1016/j.jaip.2022.03.001
30. Sotlar K, George TI, Kluin P, Reiter A, Schwaab J, Panse J, et al. Standards of Pathology
in the Diagnosis of Systemic Mastocytosis: Recommendations of the EU-US Cooperative
Group. J Allergy Clin Immunol Pract. 2022 Aug;10(8):1986–1998.e2. doi: https://doi.org/10.1016/j.jaip.2022.05.036
31. Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, et al. KIT mutation
analysis in mast cell neoplasms: recommendations of the European Competence Network
on Mastocytosis. Leukemia. 2015 Jun;29(6):1223–32. doi: https://doi.org/10.1038/leu.2015.24
32. Zanotti R, Lombardo C, Passalacqua G, Caimmi C, Bonifacio M, De Matteis G, et al. Clonal
mast cell disorders in patients with severe Hymenoptera venom allergy and normal serum
tryptase levels. J Allergy Clin Immunol. 2015 Jul;136(1):135–9. doi: https://doi.org/10.1016/j.jaci.2014.11.035
33. von Bubnoff D, Koch D, Stocker H, Ludwig RJ, Wortmann F, von Bubnoff N. The Clinical
Features of Hereditary Alpha-Tryptasemia—Implications for Interdisciplinary Practice.
Dtsch Arztebl Int. 2024 Apr;121(8):258–64.
34. Mendoza Alvarez LB, Barker R, Nelson C, DiMaggio T, Stone KD, Milner JD, et al. Clinical
response to omalizumab in patients with hereditary α-tryptasemia. Ann Allergy Asthma
Immunol. 2020 Jan;124(1):99–100.e1. doi: https://doi.org/10.1016/j.anai.2019.09.026
35. Giannetti MP, Weller E, Bormans C, Novak P, Hamilton MJ, Castells M. Hereditary alpha-tryptasemia
in 101 patients with mast cell activation-related symptomatology including anaphylaxis.
Ann Allergy Asthma Immunol. 2021 Jun;126(6):655–60. doi: https://doi.org/10.1016/j.anai.2021.01.016

