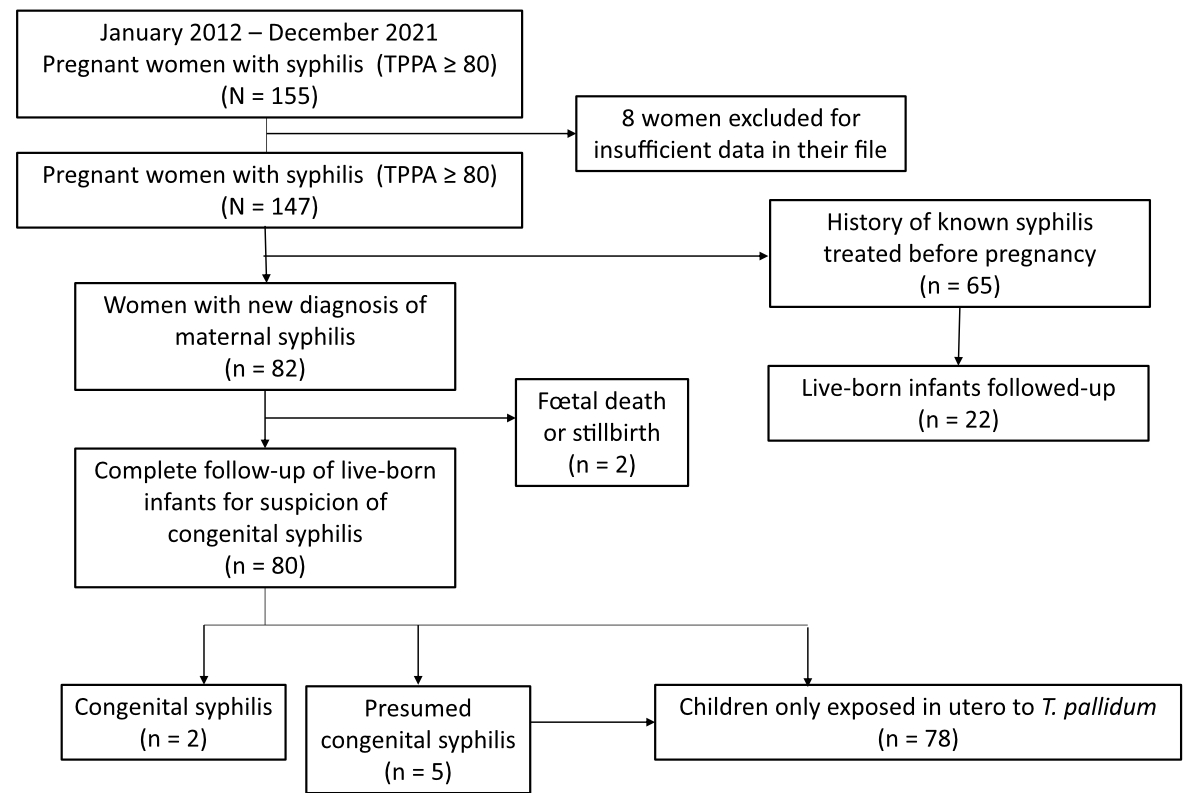
Figure 1 Study population.
DOI: https://doi.org/https://doi.org/10.57187/s.3678
Congenital syphilis is a rare complication of syphilis, an infection caused by the spirochete Treponema pallidum (T. pallidum) that can be transmitted vertically at any time during pregnancy from mother to child. The estimated risk of transplacental transmission is up to 80% in untreated mothers [1]. This disease can have serious consequences on the foetus (miscarriage, stillbirth, premature birth, perinatal death, malformations, intrauterine growth retardation, etc) and can also be responsible for life-long health sequelae in the child (neurological and mental deficits, bone deformities, etc) [2]. However, congenital syphilis is easily preventable by prenatal screening and adequate early treatment of maternal syphilis. Therefore, it is universally recommended to screen for syphilis in pregnant women, regardless of their risk-taking behaviours [1].
Since 2007, eradication of congenital syphilis has been one of the World Health Organization’s goals, through the reduction of the prevalence of syphilis in pregnant women and the prevention of vertical transmission [1, 3]. Despite this, resurgence of syphilis has been described worldwide since the 2000s [4], in high-income countries such as the United States [5, 6], China [7, 8], Japan [9] and New Zealand [10], but also in middle-income countries such as Brazil [11], resulting in an increase in syphilis among reproductive aged women. This higher incidence can subsequently lead to an increase in congenital syphilis cases.
Following the global trend, the incidence of syphilis in Switzerland has been increasing over the past two decades, with the most affected age group being individuals between 25 and 44 years old [12]. In Switzerland, notification of T. pallidum is mandatory. Coverage of antenatal screening for syphilis is high and the case rate of congenital syphilis is low, with an average incidence of 1.6 cases per year over the last ten years [13]; however there are still reported cases of congenital syphilis, particularly in vulnerable populations [14, 15]. These populations, such as undocumented migrants, remain under the radar in our current health systems. As we know, they are often not covered by health insurance so pregnant women from this population may not undergo the recommended screening. Several studies have identified the role of immigration status, the lack of medical insurance, substance use issues and sexual or domestic violence in cases of congenital syphilis [8, 10, 11, 14, 16–19]. Thus, congenital syphilis seems to be associated with particular maternal characteristics, such as specific sociodemographic and cultural features. This may lead to difficulties in accessing the healthcare system, lack of antenatal care and no adequate timely treatment of syphilis during pregnancy [20].
In this study, we evaluated cases of syphilis among pregnant women identified by screening over a 10-year period in Switzerland and subsequent congenital syphilis, to identify maternal risk profiles and enhance prevention strategies. We also compared characteristics of women diagnosed with syphilis in early pregnancy (1st trimester) versus late pregnancy (2nd and 3rd trimesters), and assessed risk factors for premature birth among women diagnosed with syphilis during their pregnancy.
A multicentre case series of pregnant women who screened positive for syphilis during their pregnancy and their newborns was conducted in Switzerland in major hospitals (Geneva, Lausanne, Zurich, Bern, Basel, Saint Gallen, Valais, Lugano), including all university hospitals, over a 10-year recruitment period, from 1 January 2012 to 31 December 2021. The study protocol was approved by the Swiss Association of Research Ethics Committees (study number 2022-01296) and registered on ClinicalTrials.gov (ID NCT05975502).
All pregnant women with a positive screen for syphilis (serology using T. pallidum haemagglutination assay [TPHA] / T. pallidum particle agglutination assay [TPPA] ≥1:80) and all newborns exposed to T. pallidum in utero and/or congenitally infected and with a positive syphilis serology at birth, were investigated. Included women had either given their hospital’s general research consent or reuse of their health-related personal data was possible by virtue of the Swiss ethics law, article 34.
Patients were excluded if a document in their medical file attested a refusal of consent to reuse of their data. False-positive syphilis test results (low reactive non-treponemal tests or treponemal tests, with negative IgG immunoblot test for T. pallidum, on two repeated sera with at least a 1-month interval [21]) were also excluded.
All pregnant women and newborns with a positive TPHA/TPPA were selected using the medical laboratory software of dermatology, gynaecology and paediatric wards of all hospitals.
Data were collected from medical and laboratory patient records, and included sociodemographic characteristics (such as age, country of origin, couple status, presence of the partner, Swiss healthcare insurance, employment status, number of children); pregnancy data (weeks of pregnancy at first antenatal consultation, number of prenatal care visits, substances used during pregnancy, co-infection by other sexually transmitted diseases [HIV, Chlamydia trachomatis, Neisseria gonorrhoea, hepatitis B or C virus, Herpes simplex virus]); syphilis evaluation (time of diagnosis of syphilis during pregnancy, stage of disease in pregnancy, maternal and infantʼs subsequent therapy and syphilis serology results (Enzyme-Linked Immuno Sorbent Assay [ELISA], Venereal Disease Research Laboratory test [VDRL] / rapid plasma reagin [RPR], TPHA/TPPA, Immunoglobulins G [IgG] and M [IgM]). Follow-up data was collected for children (weight-bearing, motor and mental development, syphilis serology results) to the age of 6 years whenever available at the date of inclusion and with a follow-up implemented for more recent cases.
Maternal syphilis and congenital syphilis were defined according to the 2020 European guideline on the management of syphilis [22] and the CDC guideline [23]. A syphilis serological scar was defined as a positive treponemal test and a negative non-treponemal test and a history of appropriate treatment for syphilis [22].
Infants of positive mothers who did not meet congenital syphilis criteria were considered to have been “exposed in utero to T. pallidum and not infected”.
The secondary outcome was premature birth, defined as a birth before 37 weeks of gestation.
All data were recorded in an online case report form using Research Electronic Data Capture (REDCap) software, and analysed using R software, version 4.0.3.
We used a convenient sample of all pregnant women who screened positive for syphilis during their pregnancy between 1 January 2012 and 31 December 2021 in the reference laboratories in Switzerland following the case definition. With a total of 147 women and 20 premature births, we only reported univariate logistic regression models.
Continuous variables were described by the mean ± standard deviation, the median and interquartile range (IQR). Categorical variables were described by their counts and relative percentages (including missing or unknown status). We compared continuous variables between the group of women who screened positive for syphilis during late pregnancy (2nd or 3rd trimester) and those who screened positive during early pregnancy (1st trimester) using Mann-Whitney nonparametric tests (and after exclusion of missing or unknown status); categorical variables were compared between both groups using either chi-square test or Fisher’s exact test, depending on applicability criteria (and after exclusion of missing or unknown status). We quantified the association between each variable and time of syphilis diagnosis during the pregnancy by performing univariate logistic regression models on complete cases (after exclusion of missing variables). Finally, we explored the variables associated with the likelihood of premature birth by performing univariate logistic regression models. We reported odds ratios (ORs) along with their corresponding 95% confidence intervals (95% CI). P-values <0.05 were considered statistically significant.
From 1 December 2012 to 31 December 2021, a total of 147 pregnant women with a positive syphilis serology and 102 children exposed in utero to T. pallidum and with a positive syphilis serology at birth were studied (figure 1).

Figure 1 Study population.
Characteristics of mothers are reported in table 1.
Table 1Characteristics of 147 mothers diagnosed with syphilis during pregnancy.
| n | % | |||
| Maternal country of origin (n = 147) | Latin America | 47 | 34% | |
| Africa | 32 | 23% | ||
| Asia | 20 | 14% | ||
| Europe | 40 | 29% | ||
| Switzerland | 10 | 7% | ||
| Oceania | 1 | 0.7% | ||
| Unknown | 7 | 5% | ||
| Marital status (n = 147) | Married or common-law union | 108 | 74% | |
| Single or separated / divorced or widowed | 37 | 25% | ||
| Unknown | 2 | 1% | ||
| Swiss healthcare insurance (n = 147) | Yes | 106 | 72% | |
| No | 36 | 25% | ||
| Unknown | 5 | 3% | ||
| Employment status (n = 147) | Yes | 72 | 49% | |
| No | 55 | 37% | ||
| Unknown | 20 | 14% | ||
| Number of pregnancies (including the current pregnancy) (n = 147) | One | 25 | 17% | |
| Two | 41 | 28% | ||
| Three | 26 | 18% | ||
| Four or more | 52 | 35% | ||
| Unknown | 3 | 2% | ||
| Parity (including the current birth) (n = 147) | Zero | 3 | 2% | |
| One | 59 | 40% | ||
| Two | 36 | 25% | ||
| Three | 30 | 20% | ||
| Four or more | 17 | 12% | ||
| Unknown | 2 | 1% | ||
| Previous syphilis diagnosis (n = 147) | Yes | 65 | 44% | |
| No | 60 | 41% | ||
| Unknown | 22 | 15% | ||
| Type of syphilis diagnosed (n = 147) | Primary syphilis | 1 | 0.7% | |
| Secondary syphilis | 1 | 0.7% | ||
| Early latent syphilis | 4 | 3% | ||
| Late latent syphilis | 9 | 6% | ||
| Syphilis of undetermined duration | 63 | 43% | ||
| Serological scar | 69 | 47% | ||
| Unknown | 0 | 0% | ||
| Timing of screening in pregnancy (n = 147) | First trimester | 79 | 54% | |
| Second trimester | 42 | 29% | ||
| Third trimester | 19 | 13% | ||
| Unknown | 7 | 5% | ||
| Syphilis treated in pregnancy (n = 147) | Yes | 80 | 54% | |
| No (97% were serological scars / 3% were syphilis of undetermined duration) | 67 | 46% | ||
| Syphilis treatment (n = 80) | Benzathine penicillin, 1 injection | 6 | 8% | |
| Benzathine penicillin, 3 injections | 72 | 90% | ||
| Ceftriaxone | 1 | 1% | ||
| Other drugs (Penicillin G) | 1 | 1% | ||
| Co-infection with other sexually transmitted infections in pregnancy (n = 147) | No | 112 | 76% | |
| Yes | 35 | 24% | ||
| HIV | 6 | 4% | ||
| Chlamydia trachomatis | 6 | 4% | ||
| Neisseria gonorrhoeae | 0 | 0% | ||
| Hepatitis B virus | 25 | 17% | ||
| Hepatitis C virus | 3 | 2% | ||
| HSV | 1 | 0.7% | ||
| Unknown | 0 | 0% | ||
| Reported use of substances during pregnancy (n = 147) | No | 113 | 77% | |
| Yes | 28 | 19% | ||
| Alcohol | 4 | 3% | ||
| Tobacco | 23 | 16% | ||
| Intravenous drugs | 2 | 1% | ||
| Smoked drugs | 5 | 3% | ||
| Unknown | 6 | 4% | ||
| Premature birth (n = 147) | No | 82 | 55.8% | |
| Yes | 20 | 13.6% | ||
| Unknown | 45 | 30.6% | ||
| Median (IQR) | ||||
| Maternal age | 33 (29–37) | |||
| Weeks of pregnancy at first antenatal consultation | 10 (8–14.5) | |||
| Number of prenatal care visits | 8 (5–11) | |||
| Gestational age at delivery | 39 (37.25–40) | |||
The median age of pregnant women with a positive syphilis serology was 33 years (IQR 29–37).
They were mainly from outside Switzerland: 34% (47/147) from Latin America (16% from Brazil); 23% (32/147) from Africa; 14% (20/147) from Asia (5% from Mongolia) and 29% (40/147) from Europe (8% from Portugal). Only 7% (10/147) were born in Switzerland.
Mothers were single in 25% of cases (37/147). About one quarter of mothers (25% or 36/147) had no health insurance, and the number of pregnant women without insurance was higher in women diagnosed in the second or third trimester than in those diagnosed in the first trimester (OR 0.41 [95% CI 0.19–0.89], p = 0.024) (available in table S1 in the appendix).
Half of the pregnant women (72/147) had an employment status, the most frequently reported of which was cleaning (38% of cases or 27/72); 37% (55/147) had no employment status and employment status was unknown for 14% (20/147) of women.
The median number of pregnancies per woman was 3, and more than one third (35% or 52/147) of mothers had had 4 or more pregnancies. Median parity was 2, with 12% (17/147) of mothers having had 4 or more children.
Median weeks of amenorrhoea at first antenatal consultation was 10 (IQR 8–14.5) and the median number of prenatal care visits was 8 (IQR 5–11).
A history of treated syphilis prior to the pregnancy was known in 44% (65/147) of the pregnant women (corresponding to a serological scar), with new syphilis identified in the remaining 56% (82/147). Among these newly diagnosed syphilis cases, 77% (63/82) were syphilis of undetermined duration, 11% (9/82) were late latent syphilis and 5% (4/82) were early latent syphilis. Only one primary and one secondary syphilis were diagnosed.
Syphilis screening was done in the first trimester in 54% (79/147) of pregnancies, in the second trimester in 29% (42/147) and in the third trimester in 13% (19/147).
Substance use was reported by 19% (28/147) of women, of whom 5% (7/147) declared drug use (smoked or intravenous drugs), 3% (4/147) alcohol and 16% (23/147) tobacco.
24% (35/147) of mothers with syphilis presented with a concomitant sexually transmitted infection: 4% (6/147) were living with HIV, 4% (6/147) had Chlamydia trachomatis, 1% (25/147) had hepatitis B (mostly healed), 2% (3/147) had hepatitis C and 0.7% (1/147) had herpes simplex virus.
46% (67/147) of mothers were not treated for syphilis during their pregnancy; among the untreated mothers, 97% (65/67) had a serological scar and 3% (2/67) had syphilis of undetermined duration. Among the 54% (80/147) of mothers treated during pregnancy, 83% (66/80) were correctly treated (1 injection of benzathine penicillin G for early syphilis <1 year or 3 injections for late syphilis >1 year, as recommended by the European guideline [22]) to prevent vertical transmission. Among incorrectly treated syphilis cases, 5 of 6 early syphilis cases were treated with 3 injections of (overtreated), 5 late syphilis cases were treated with 1 injection of BPG, another late syphilis was treated with 1 injection of ceftriaxone and 1 with 1 injection of penicillin G which resulted in a congenital syphilis.
Syphilis diagnosed in the second or third trimester was associated with a late first antenatal consultation (defined as a first consultation occurring after more than 13 weeks of pregnancy; p <0.001) (table 2).
Table 2Comparison of women who screened positive for syphilis during early vs late pregnancy (n = 140; 7 missing data points).
| Early pregnancy (1st trimester) (n = 79) | Late pregnancy (2nd or 3rd trimester) (n = 61) | p-value | |||
| Maternal age (mean ± SD, median: interquartile range) (n = 140) | 32.8 ± 6.0, 34: 29–37 | 32.6 ± 6.5, 33: 28–37 | 0.287* | ||
| Maternal country of origin (n = 140) | 0.233** | ||||
| Latin America | 28 (35.4%) | 18 (29.5%) | |||
| Africa | 17 (21.5%) | 13 (21.3%) | |||
| Asia | 7 (8.9%) | 13 (21.3%) | |||
| Europe | 23 (29.1%) | 15 (24.6%) | |||
| Oceania | 0 (0%) | 1 (1.6%) | |||
| Unknown | 4 (5.1%) | 1 (1.6%) | |||
| Marital status (n = 140) | 0.632*** | ||||
| Married or common-law union | 56 (70.9%) | 46 (75.4%) | |||
| Single or separated / divorced or widowed | 22 (27.8%) | 15 (24.6%) | |||
| Unknown | 1 (1.3%) | 0 (0%) | |||
| Swiss healthcare insurance (n = 140) | 0.022*** | ||||
| Yes | 14 (17.7%) | 22 (36.1%) | |||
| No | 61 (77.2%) | 39 (63.9%) | |||
| Unknown | 4 (5.1%) | 0 (0%) | |||
| First antenatal visit (n = 140) | |||||
| <13 weeks | 49 (62%) | 17 (27.9%) | <0.001* | ||
| ≥13 weeks | 1 (1.3%) | 27 (44.3%) | |||
| Unknown | 29 (36.7%) | 17 (27.9%) | |||
| Employment status (n = 140) | 0.083*** | ||||
| Yes | 44 (55.7%) | 26 (42.6%) | |||
| No | 25 (31.6%) | 28 (45.9%) | |||
| Unknown | 10 (12.7%) | 7 (11.5%) | |||
| Number of pregnancies (including the current pregnancy) (n = 140) | 0.448*** | ||||
| One | 17 (21.5%) | 7 (11.5%) | |||
| Two | 20 (25.3%) | 19 (31.2%) | |||
| Three | 13 (16.5%) | 12 (19.7%) | |||
| Four or more | 29 (36.7%) | 23 (37.7%) | |||
| Unknown | 0 (0%) | 0 (0%) | |||
| Parity (including current birth) (n = 140) | 0.391*** | ||||
| ≤2 children | 54 (68.3%) | 38 (62.3%) | |||
| ≥3 children | 24 (30.4%) | 23 (37.7%) | |||
| Unknown | 1 (1.3%) | 0 (0%) | |||
| Co-infection with other sexually transmitted infections in pregnancy (n = 140) | 0.879*** | ||||
| No | 60 (76.0%) | 47 (77.0%) | |||
| Yes | 19 (24.0%) | 14 (23.0%) | |||
| HIV | 3 (3.8%) | 1 (1.6%) | |||
| Chlamydia trachomatis | 2 (2.5%) | 4 (6.6%) | |||
| Neisseria gonorrhoeae | 0 (0%) | 0 (0%) | |||
| Hepatitis B virus | 14 (17.7%) | 11 (18.0%) | |||
| Hepatitis C virus | 0 (0%) | 3 (4.9%) | |||
| HSV | 1 (1.3%) | 0 (0%) | |||
| Unknown | 0 (0%) | 0 (0%) | |||
| Reported use of substances during pregnancy (n = 140) | 0.668*** | ||||
| No | 62 (80.5%) | 46 (79.3%) | |||
| Yes | 15 (19.5%) | 12 (20.7%) | |||
| Alcohol | 2 | 2 | |||
| Tobacco | 13 | 9 | |||
| Intravenous drugs | 1 | 1 | |||
| Smoked drugs | 3 | 2 | |||
| Unknown | – | – | |||
| Premature birth (n = 140) | 0.180*** | ||||
| No | 50 (63.3%) | 28 (45.9%) | |||
| Yes | 9 (11.4%) | 10 (16.4%) | |||
| Unknown | 20 (25.3%) | 23 (37.7%) | |||
* Mann-Whitney nonparametric test; ** Fisher’s exact test; *** Chi-square test; all tests excluded missing data.
Among the 102 babies born to syphilis-seropositive women, only 2 (2%) were diagnosed with confirmed congenital syphilis, while the others were considered exposed but not infected by syphilis. Five cases were initially presumed to be congenital syphilis but the diagnosis was ultimately retracted.
There were 49% girls. Median birthweight was 3.1 kg (IQR 2.6–3.5), with 18% of infants weighing <2.5 kg and median height was 49 cm (IQR 47–51)].
Median gestational age at delivery was 39 weeks of amenorrhoea (IQR 37.25–40). Preterm delivery rate (<37 weeks of gestation) was 18%. Ten children (9.8%) were born very preterm (<32 weeks of gestation). Prematurity was observed in newborns exposed to mothers with syphilis of undetermined duration (70%), mothers with a serological scar (25%) and with late latent syphilis (5%).
Intrauterine growth retardation was observed in 9.8% (10/102) of the children.
The two cases of congenital syphilis are described in table 3. The first case of congenital syphilis occurred in a mother from Brazil who was diagnosed with syphilis of undetermined duration in the first trimester, and treated during her pregnancy but with an inappropriate treatment of 3 injections of aqueous penicillin G; the outcome was a recurrence as it was benzathine penicillin that was required. This case presented with cutaneous signs, periostitis of long bones, fever, anaemia, thrombocytopenia and intrauterine growth retardation. The second case was diagnosed in a mother who immigrated from Angola during her pregnancy, and with a positive primary syphilis screen in the third trimester. This case presented with cutaneous signs, pancreatic steatosis, osteitis, thrombocytopenia, elevated liver enzymes and bilirubin. Both cases had a positive syphilis serology at birth (with an 8-fold increase in rapid plasma reagin within 3 months after birth for the first child); the second case also had inflammatory cerebrospinal fluid with a positive non-treponemal test.
Intravenous aqueous penicillin G (150,000 U/kg) was administered in 31% (32/102) of the exposed infants for 1 or 2 days for 30 children corresponding to the time to obtain the syphilis laboratory results. The duration of this antibiotherapy was, respectively, 12 and 10 days for the two confirmed congenital syphilis cases (table 3).
Table 3Characteristics of the two congenital syphilis cases.
| 1 | 2 | |
| Sex | M | M |
| Origin | Brazil | Angola |
| Birth weight (kg) | 2.1 | 2.9 |
| Gestational age (weeks) | 37 | 38 |
| Pregnancy stage at diagnosis | 1st trimester | 3rd trimester |
| Maternal treatment during pregnancy | Penicillin G, 3 injections | Benzathine penicillin G, 1 injection |
| Type of maternal syphilis | Undetermined duration | Primary syphilis |
| Congenital syphilis diagnosis: serologic tests and other investigations | Symptoms, 8-fold increase in rapid plasma reagin within 3 months after birth | Symptoms, cerebrospinal fluid positive |
| Congenital syphilis diagnosis: Clinical features | Cutaneous signs, fever, anaemia, thrombocytopenia, intrauterine growth retardation | Cutaneous signs, thrombocytopenia, elevated liver enzymes and bilirubin, pancreatic steatosis |
| Long bone X-ray | Periostitis of long bones | Osteitis |
| Cerebrospinal fluid | White blood cells 20/mm3 | White blood cells 15/mm3, proteins 1.02 g/l, non-treponemal tests positive |
| Treatment | Penicillin G IV 50,000 IU/kg, 12 days | Penicillin G IV 50,000 IU/kg, 10 days |
| Outcome | Normal psychomotor development; severe growth retardation | Normal psychomotor development |
We compared the maternal characteristics between premature (n = 20) and at-term births (n = 82) and we did not find any statistically significant differences (all p-values >0.05 [table 4]).
Table 4Risk factors for premature birth among pregnant women infected by syphilis (complete case analysis, univariate logistic regression models).
| Odds ratio | 95% confidence interval | p-value | ||
| Period of diagnosis (n = 97) | 0.734 | |||
| Early pregnancy (ref) | 1.00 | – | ||
| Late pregnancy | 1.275 | 0.314–5.173 | ||
| Maternal age (n = 98) | 1.057 | 0.972–1.150 | 0.193 | |
| Maternal country of origin (n = 95) | 0.8175 | |||
| Europe | 1.00 | – | – | |
| Latin America | 0.89 | 0.25–3.20 | 0.854 | |
| Africa | 0.95 | 0.22–4.16 | 0.946 | |
| Asia | 1.52 | 0.33–6.96 | 0.590 | |
| Marital status (n = 100) | 0.148 | |||
| Married or common-law union | 1.00 | – | ||
| Single or separated / divorced or widowed | 2.140 | 0.762–6.010 | ||
| Swiss healthcare insurance (n = 97) | 0.781 | |||
| Yes | 1.00 | – | ||
| No | 0.850 | 0.270–2.675 | ||
| Employment status (n = 86) | 0.752 | |||
| Yes | 0.848 | 0.305–2.356 | ||
| No | 1.00 | |||
| Number of pregnancies (including the current pregnancy) (n = 101) | 0.707 | |||
| One | 1.00 | – | – | |
| Two | 0.739 | 0.203–2.695 | 0.647 | |
| Three | 0.354 | 0.062–2.017 | 0.242 | |
| Four or more | 0.680 | 0.187–2.466 | 0.557 | |
| Parity (including the current birth) (n = 100) | 0.830 | |||
| ≤2 children | 1.00 | – | ||
| ≥3 children | 0.890 | 0.307–2.581 | ||
| Co-infection with other sexually transmitted infections in pregnancy (n = 102) | 0.110 | |||
| No | 1.00 | – | ||
| Yes | 2.405 | 0.820–7.054 | ||
| Reported use of substances during pregnancy (n = 96) | 0.802 | |||
| No | 1.00 | – | ||
| Yes | 0.840 | 0.215–3.278 | ||
Over a 10-year period, we reported 82 mothers newly diagnosed with syphilis during pregnancy, and only 2 confirmed cases of congenital syphilis. Fortunately, high coverage of antenatal screening has kept congenital syphilis rates low in Switzerland in recent years [24].
Our study identified several potential factors that could contribute to women’s risk of syphilis during pregnancy such as the lack of medical insurance, the use of drugs, concomitant other sexually transmitted infections and lack of early prenatal care. We also noted that among women with syphilis during pregnancy there was a high number of pregnancies and children per woman, higher than the Swiss national average, which was calculated as 1.52 children per woman in 2021 [25]. Most of the syphilis-positive pregnant women in our study were not from Switzerland, but mainly from Latin America, where the prevalence of syphilis is much higher than in Switzerland and no antenatal testing exists. A lot of them have an immigrant background, linked to the high immigration rate in Switzerland [26], with difficulties accessing health facilities and often a low rate of healthcare insurance [19]. Our two cases of congenital syphilis mirror findings from the Netherlands where reported cases of congenital syphilis are due to delayed or non screening of pregnant women and late or inadequate treatment [27].
All these socioeconomic factors may contribute to barriers hampering timely and adequate prenatal screening and care, leading to missed diagnosis and treatment of syphilis in pregnancy. Ensuring that all pregnant women, even the most socially underserved women, have access to prenatal care and healthcare services (for example for free in cases of illegal immigration) is crucial. When risk factors and features indicating an elevated maternal risk profile are identified, focused preventive measures during pregnancy should be implemented. In our cohort, only half of the women were screened for syphilis during the first trimester. To maintain congenital syphilis at a low incidence, screening of syphilis during the first trimester of pregnancy should be mandatory. As congenital syphilis has also been described as a result of late maternal infection after screening was performed or re-infection following treatment of maternal syphilis [24, 28], syphilis serology should also be repeated during pregnancy. When maternal risk factors are identified (living in a community with high syphilis morbidity or at risk of syphilis acquisition during pregnancy [drug use, sexually transmitted infections during pregnancy, multiple partners, a new partner, partner with sexually transmitted infections]), closer monitoring with repeated serologies should be done at least at the beginning of the third trimester (28 weeks of amenorrhoea) and again at delivery, as recommended by the European guideline [22] and the CDC [23]. A study in Florida and Louisiana has shown that a universal repeated third trimester screen effectively prevented most congenital syphilis cases [29]. Adequate surveillance and reporting systems are also necessary to monitor the prevalence of syphilis and congenital syphilis cases as underreporting or incomplete data can hinder the assessment of the problem and the development of effective interventions. A proactive sentinel network could be developed with tools that could send electronic alerts to the various specialists involved (biologist, gynaecologist, paediatrician, infectious diseases specialist, dermatologist) when a syphilis serology comes back positive, in order to track, treat and follow these pregnant women. Moreover, ensuring that the partners of pregnant women with syphilis are notified and treated is essential to prevent reinfection and transmission to the unborn child. This can be challenging, especially when individuals are not willing to disclose their infection or when partners are difficult to reach in vulnerable or illegal populations.
We observed in our cohort a low birthweight with 18% (95% CI 10.8–26.4%) of infants weighing <2.5 kg, a higher rate than in the general population (6% in Switzerland in 2022 [30]). A high rate of intrauterine growth retardation and of prematurity (18%; 95% CI 11.6–27.6%) was also seen, which is higher than in the general population (6% of children were born preterm in Switzerland in 2022 [30]). This may be related to syphilis but also to other confounding factors such as tobacco or drug use, alcohol, multiple pregnancies, other sexually transmitted infections, a late maternal age, late or no health care during pregnancy or environmental factors such as domestic violence, lack of social support, etc. These risk factors are also more common in economically disadvantaged populations.
Despite a reported high rate of screening, our findings highlighted some inadequacies in the management of syphilis-positive pregnant women [31]. Of those who received treatment, 18% (14/80) were not treated as recommended by the European guideline. Among them, 5 of 6 cases of early syphilis were treated with three injections instead of the recommended one. This possibly reflects a precautionary approach to treatment, whereas insufficient dosages are of greater concern. One of the congenital syphilis cases was due to an inadequate treatment and illustrates well that a lack of knowledge in the treatment of pregnant women with syphilis can have serious consequences. Regular errors in penicillin formulations and dosages are still observed in many countries. In addition, a regular shortage of benzathine penicillin has been reported in many European countries [32] which sometimes leads to the use of alternative treatments that do not cross the foetoplacental barrier.
While our study highlights characteristics of a vulnerable population that could benefit from a targeted preventive intervention, there are several limitations. First, syphilis cases in pregnant women in this study only partially represent cases reported in Switzerland (62%, 147/236) during the 10-year period as some pregnancy screening was performed in private practices. However positive syphilis serologies are most of the time referred to university hospitals to be handled, so it is likely that cases not reported in this study correspond to syphilis serological scars. Second, follow-up data in children was limited, leading to potential missed congenital syphilis revealed after the neonatal period. Third, we did not obtain data from a few women who could have been at high risk of syphilis (8 women or 5.8%) due to the retrospective collection of data. Lastly, again owing to retrospective collection of data from medical files, some information is missing such as women’s educational level, which was poorly documented (unknown in 71% of cases), male partner syphilis status, which was not obtained from the mother’s file. The rate of reinfection during pregnancy was also not evaluated. Therefore, some risk factors may have been overlooked.
Women who had a positive syphilis screening test in pregnancy presented several health determinants, such as late antenatal consultation and preterm delivery, especially in vulnerable population groups, which deserve more targeted prevention. Such determinants should be identified to improve surveillance and healthcare. Moreover, we highlight the importance of better treatment knowledge for infrequent diseases.
Data will be shared on an open data repository on Yareta / University of Geneva.
With contributions of the Clinical Research Center, Geneva University Hospitals and Faculty of Medicine, Geneva (data manager Laurent Brodier and statistical advice Angèle Gayet-Ageron).
We would like to thank all the biologists who participated in the identification of the cases, and especially Alexis Dumoulin from the Laboratory of Bacteriology in Valais.
This work was supported by the Swiss National Science Foundation (An interdisciplinary project: searching for an integrated model to explain never-ending infectious diseases) (grant number CRSII5-186394).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. World Health Organization. The global elimination of congenital syphilis: rationale and strategy for action. Geneva, Switzerland: WHO, 2007. Available at: http://apps.who.int/iris/bitstream/10665/43782/1/9789241595858_eng.pdf. Accessed 29 November 2023.
2. De Santis M, De Luca C, Mappa I, Spagnuolo T, Licameli A, Straface G, et al. Syphilis Infection during pregnancy: fetal risks and clinical management. Infect Dis Obstet Gynecol. 2012;2012:430585. doi: https://doi.org/10.1155/2012/430585
3. World Health Organisation - Governance guidance for the validation of elimination of mother-to-child transmission of HIV and syphilis - 2020 Jun - Available at: https://www.who.int/publications-detail-redirect/governance-guidance-for-validation-of-emtct-syphilis-hiv
4. Worls Health Organization. The global health observatory. Data on syphilis. Available at: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/data-on-syphilis
5. Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2021. National Overview of STDs, 2021. Available at: https://www.cdc.gov/std/statistics/2021/overview.htm#Syphilis
6. Centers for Disease Control and Prevention. U.S. Syphilis Cases in Newborns Continue to Increase: A 10-Times Increase Over a Decade. 2023,11. Available at: https://www.cdc.gov/nchhstp/newsroom/2023/syphilis-cases-in-newborns.html
7. Dai Y, Zhai G, Zhang S, Chen C, Li Z, Shi W. The Clinical Characteristics and Serological Outcomes of Infants With Confirmed or Suspected Congenital Syphilis in Shanghai, China: A Hospital-Based Study. Front Pediatr. 2022 Feb;10:802071. doi: https://doi.org/10.3389/fped.2022.802071
8. Wang Y, Wu M, Gong X, Zhao L, Zhao J, Zhu C, et al. Risk Factors for Congenital Syphilis Transmitted from Mother to Infant - Suzhou, China, 2011-2014. MMWR Morb Mortal Wkly Rep. 2019 Mar;68(10):247–50. doi: https://doi.org/10.15585/mmwr.mm6810a4
9. Kanai M, Arima Y, Shimada T, Hori N, Yamagishi T, Sunagawa T, et al. Increase in congenital syphilis cases and challenges in prevention in Japan, 2016-2017. Sex Health. 2021 May;18(2):197–9. doi: https://doi.org/10.1071/SH21004
10. Gilmour LS, Best EJ, Duncanson MJ, Wheeler BJ, Sherwood J, Thirkell CE, et al. High Incidence of Congenital Syphilis in New Zealand: A New Zealand Pediatric Surveillance Unit Study. Pediatr Infect Dis J. 2022 Jan;41(1):66–71. doi: https://doi.org/10.1097/INF.0000000000003233
11. Heringer AL, Kawa H, Fonseca SC, Brignol SM, Zarpellon LA, Reis AC. [Inequalities in congenital syphilis trends in the city of Niterói, Brazil, 2007-2016Desigualdades en la tendencia de sífilis congénita en la ciudad de Niterói, Brasil, 2007-2016]. Rev Panam Salud Publica. 2020 Feb;44:e3.
12. Office fédéral de la santé publique - Bulletin 45/2022 Available at: https://www.bag.admin.ch/bag/fr/home/das-bag/publikationen/periodika/bag-bulletin.html
13. Office fédéral de la santé publique OFSP. Chiffres Maladies Infectieuses 2023. Available at: https://www.bag.admin.ch/bag/fr/home/zahlen-und-statistiken/zahlen-zu-infektionskrankheiten.html
14. Wolff H, Epiney M, Lourenco AP, Costanza MC, Delieutraz-Marchand J, Andreoli N, et al. Undocumented migrants lack access to pregnancy care and prevention. BMC Public Health. 2008 Mar;8(1):93. doi: https://doi.org/10.1186/1471-2458-8-93
15. Meyer Sauteur PM, Trück J, Bosshard PP, Tomaske M, Morán Cadenas F, Lautenschlager S, et al. Congenital syphilis in Switzerland: gone, forgotten, on the return. Swiss Med Wkly. 2012 Jan;141(102):w13325. doi: https://doi.org/10.4414/smw.2012.13325
16. Nicolay N, Gallay A, Michel A, Nicolau J, Desenclos JC, Semaille C. Reported cases of congenital syphilis in the French national hospital database. Euro Surveill. 2008 Dec;13(50):19062. doi: https://doi.org/10.2807/ese.13.50.19062-en
17. Chan EY, Smullin C, Clavijo S, Papp-Green M, Park E, Nelson M, et al. A qualitative assessment of structural barriers to prenatal care and congenital syphilis prevention in Kern County, California. PLoS One. 2021 Apr;16(4):e0249419. doi: https://doi.org/10.1371/journal.pone.0249419
18. Tridapalli E, Capretti MG, Sambri V, Marangoni A, Moroni A, D’Antuono A, et al. Prenatal syphilis infection is a possible cause of preterm delivery among immigrant women from eastern Europe. Sex Transm Infect. 2007 Apr;83(2):102–5. doi: https://doi.org/10.1136/sti.2006.021352
19. Wolff H, Stalder H, Epiney M, Walder A, Irion O, Morabia A. Health care and illegality: a survey of undocumented pregnant immigrants in Geneva. Soc Sci Med. 2005 May;60(9):2149–54. doi: https://doi.org/10.1016/j.socscimed.2004.12.007
20. Trepka MJ, Bloom SA, Zhang G, Kim S, Nobles RE. Inadequate syphilis screening among women with prenatal care in a community with a high syphilis incidence. Sex Transm Dis. 2006 Nov;33(11):670–4. doi: https://doi.org/10.1097/01.olq.0000216032.52731.ea
21. Peeling RW, Mabey D, Kamb ML, Chen XS, Radolf JD, Benzaken AS. Syphilis. Nat Rev Dis Primers. 2017 Oct;3(1):17073. doi: https://doi.org/10.1038/nrdp.2017.73
22. Janier M, Unemo M, Dupin N, Tiplica GS, Potočnik M, Patel R. 2020 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2021 Mar;35(3):574–88. doi: https://doi.org/10.1111/jdv.16946
23. Centers for Disease Control and Prevention. Sexually Transmitted Infections Treatment Guidelines, 2021. Available at: https://www.cdc.gov/std/treatment-guidelines/screening-recommendations.htm
24. Scherler G, Tomaske M, Cannizzaro V, Steppacher A, Zucol F, Theiler M, et al. Congenital syphilis in Switzerland: a retrospective cohort study, 2010 to 2019. Swiss Med Wkly. 2023 Nov;153(11):40121. doi: https://doi.org/10.57187/smw.2023.40121
25. Office fédéral de la statistique. Comparaisons internationales. Available at: https://www.bfs.admin.ch/bfs/fr/home/statistiques/population/familles/comparaisons-internationales.html
26. David A, Posfay-Barbe KM, Aguiar Nogueira C, Toutous Trellu L. Congenital syphilis in Switzerland: a marker of inequality? A mini-review. Front Public Health. 2023 Sep;11:1265725. doi: https://doi.org/10.3389/fpubh.2023.1265725
27. Visser M, van der Ploeg CP, Smit C, Hukkelhoven CW, Abbink F, van Benthem BH, et al. Evaluating progress towards triple elimination of mother-to-child transmission of HIV, syphilis and hepatitis B in the Netherlands. BMC Public Health. 2019 Mar;19(1):353. doi: https://doi.org/10.1186/s12889-019-6668-6
28. Keuning MW, Kamp GA, Schonenberg-Meinema D, Dorigo-Zetsma JW, van Zuiden JM, Pajkrt D. Congenital syphilis, the great imitator-case report and review. Lancet Infect Dis. 2020 Jul;20(7):e173–9. doi: https://doi.org/10.1016/S1473-3099(20)30268-1
29. Matthias JM, Rahman MM, Newman DR, Peterman TA. Effectiveness of Prenatal Screening and Treatment to Prevent Congenital Syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017 Aug;44(8):498–502. doi: https://doi.org/10.1097/OLQ.0000000000000638
30.Office fédéral de la statistique. Santé des nouveaux-nés. Available at: https://www.bfs.admin.ch/bfs/fr/home/statistiques/sante/etat-sante/sante-nouveau-nes.html
31. Aebi-Popp K, Kahlert C, Rauch A, Mosimann B, Baud D, Low N, et al. Heterogeneity in testing practices for infections during pregnancy: national survey across Switzerland. Swiss Med Wkly. 2016 Jul;146:w14325. doi: https://doi.org/10.4414/smw.2016.14325
32. European Center for Disease Prevention and Control. Syphilis and congenital syphilis in Europe - A review of epidemiological trends (2007–2018) and options for response. 2019. Available at: https://www.ecdc.europa.eu/en/publications-data/syphilis-and-congenital-syphilis-europe-review-epidemiological-trends-2007-2018
Table S1Association (univariate analyses) between women who screened positive for syphilis during early vs late pregnancy and maternal characteristics (complete case analyses, unknown status excluded).
| Odds ratio | 95% CI | p-value | ||
| Maternal age, in years | 0.99 | 0.94–1.05 | 0.808 | |
| Maternal country of origin | Europe (= ref) | 0.248 | ||
| Africa | 1.17 | 0.44–3.10 | 0.748 | |
| Asia | 2.85 | 0.92–8.78 | 0.068 | |
| Latin America | 0.99 | 0.41–2.38 | 0.974 | |
| Oceania | – | – | – | |
| Marital status | Married or common-law union (= ref) | 0.632 | ||
| Single or separated / divorced or widowed | 0.83 | 0.39–1.78 | ||
| Swiss healthcare insurance | Yes (= ref) | 0.024 | ||
| No | 0.41 | 0.19–0.89 | ||
| First antenatal visit | <13 weeks (= ref) | <0.001 | ||
| ≥13 weeks | 77.82 | 9.81–617.21 | ||
| Employment status | Yes (= ref) | 0.084 | ||
| No | 0.53 | 0.26–1.09 | ||
| Number of pregnancies (including the current pregnancy) | One (= ref) | 0.4605 | ||
| Two | 2.31 | 0.78–6.80 | 0.130 | |
| Three | 2.24 | 0.69–7.29 | 0.180 | |
| Four or more | 1.93 | 0.68–5.43 | 0.215 | |
| Parity (including the current birth) | ≤2 children (= ref) | 0.392 | ||
| ≥3 children | 1.36 | 0.67–2.76 | ||
| Co-infection with other sexually transmitted infections in pregnancy | No (= ref) | 0.879 | ||
| Yes | 0.94 | 0.43–2.07 | ||
| Reported use of substances during pregnancy | No (= ref) | 0.862 | ||
| Yes | 1.08 | 0.46–2.52 | ||
| Premature birth | No (= ref) | 0.185 | ||
| Yes | 1.98 | 0.72–5.46 | ||