a Department of Psychosomatics and Psychiatry, University Children's Hospital, University of Zurich, Zurich, Switzerland
b Division of Child and Adolescent Health Psychology, Department of Psychology, University of Zurich, Zurich, Switzerland
c Children's Research Centre, University Children's Hospital Zurich, University of Zurich, Zurich, Switzerland
d University Research Priority Program “ITINERARE – Innovative Therapies in Rare Diseases”, University of Zurich, Zurich, Switzerland
e Institute of Biomedical Ethics and History of Medicine, University of Zurich, Zurich, Switzerland
Introduction
Approximately 7000 rare diseases affect 3.5–5.9% of the world’s population – a total of 263 to 446 million people [1]. Individuals with rare diseases face significant barriers to accessing healthcare, including challenges related to the affordability of care and treatment as well as limited knowledge of healthcare professionals, which may result in feelings of mistrust, frustration, and anger among those affected [2]. Moreover, because rare diseases are characterised by their complex and chronic nature, these individuals typically depend on the healthcare system for their whole lives, making their reliance on it crucial. Addressing the healthcare needs of people suffering from rare diseases involves navigating complicated and interrelated difficulties that cannot be addressed with the usual solutions, requiring comprehensive and individualised approaches. This viewpoint emphasises the need to ensure equitable access to healthcare, focusing on the specific challenges people living with rare diseases face.
The interconnection between health equity and healthcare access in the context of rare diseases
According to the World Health Organization (WHO), the pursuit of health equity is an essential public health goal that is central to realising the fundamental right to health. In line with this, the WHO states that health equity is “achieved when everyone can attain their full potential for health and well-being” [3]. At its core, health equity is the fair and impartial distribution of healthcare resources, opportunities, and outcomes that transcends socioeconomic, ethnic, cultural, and geographical boundaries. This necessitates a global perspective that considers access to healthcare and equity for rare diseases internationally and within each country’s healthcare system.
In the context of rare diseases, disparities evolve because of a lack of specialised expertise, limited training and opportunities for healthcare professionals to acquire knowledge about rare diseases, limited progress in the development of rare disease treatments, and an unequal distribution of resources [4]. Furthermore, inequalities naturally exist among the estimated 7000 rare diseases, such as differences in available resources and collaboration with universities, the pharmaceutical industry, and governmental agencies [5]. These inequalities can impact drug discovery, access to treatment, and public awareness of specific rare diseases. However, the allocation of resources to rare disease treatments, particularly in terms of governmental and philanthropic funding, may also reflect a more complex interplay of factors at the national and global levels.
Health equity and access to healthcare are inextricably linked. Ensuring access to healthcare is necessary to achieve health equity for people living with rare diseases. The WHO defines universal health coverage as ensuring that all individuals have access to the full range of high-quality health services they require, when and where they need them, without incurring financial hardship. This encompasses a comprehensive spectrum of essential health services, from health promotion and prevention to treatment, rehabilitation, and palliative care throughout the entire lifespan [6]. Researchers have approached healthcare access from different angles, often examining singular aspects, such as healthcare utilisation, resource availability, and characteristics of those accessing care [7]. However, quantifying access to healthcare from a universal perspective is an intricate task and often requires surrogate measures that do not adequately capture the multidimensional nature of the construct [8]. Traditionally, such approaches are limited to specific aspects of healthcare access and thus only provide a narrow perspective of its dimensions [9]. To complicate matters, none of the existing measurement scales have undergone rigorous validation, further hindering a holistic understanding of this complex construct. Such shortcomings may obscure and unintentionally exacerbate health inequalities by emphasising particular dimensions of access at the expense of others.
In response to these limitations, various models have emerged that represent the multidimensional nature of this concept, such as the framework proposed by Levesque and colleagues [10]. This model addresses the structural requirements for access and considers the relevant abilities of patients. It includes five structural dimensions of access: accessibility, acceptability, availability and accommodation, affordability, and appropriateness. Notably, within academic discourse, affordability often claims a substantial share of the spotlight [11], while the other dimensions highlighted by Levesque et al. [10] receive comparatively less attention. This imbalance in emphasis may result in the inadequate integration of these less-attended dimensions into individual-level interventions or policy changes, culminating in misaligned healthcare priorities. The challenges that people with rare diseases face are usually even more complex and nuanced, as the knowledge about and awareness of rare diseases is generally low. This underscores the importance of refining and adapting existing models and frameworks to better meet the specific needs of those affected. However, many of these diseases are poorly researched and data are limited, making it challenging to adapt general models to these patients’ specific needs.
The five structural dimensions are complemented by individual abilities, as depicted in figure 1. According to the model by Levesque and colleagues [10], this means that patients need to be actively involved in defining the shortcomings of healthcare and developing approaches that realistically meet patients’ needs. The extent to which these abilities impact healthcare access raises concerns about health equity in society [12]. For example, patients with rare diseases often have deep knowledge about their condition that is not appreciated and utilised by many of their doctors. Some patients even receive ineffective or harmful treatments [2]. This knowledge disparity poses an additional challenge to those affected and highlights the need to incorporate patient knowledge into care. This could increase the chances of better access, as it incorporates patient-centred care, and consequently, it could affect health equity [12]. However, acquiring and utilising such knowledge is not enough. Patients must be empowered to communicate their knowledge effectively to healthcare professionals and ensure that their voice is heard and considered in decision-making processes. In some cases, this may mean negotiating the social roles and power dynamics between patients and their healthcare providers, which is a considerable problem for individuals who are vulnerable and marginalised because of their disease. Even as patients develop health literacy and communication skills, the unconscious biases of providers related to personal characteristics, such as gender and skin colour, can diminish their credibility. This can lead to unequal treatment, misdiagnosis, or inadequate care, perpetuating health inequities by denying certain people with rare diseases access to timely care [13]. It is important to emphasise that individual ability should not be used as a basis for placing the sole responsibility on patients. Instead, the focus should be on providing comprehensive support at all levels and dimensions described in this framework to empower patients.
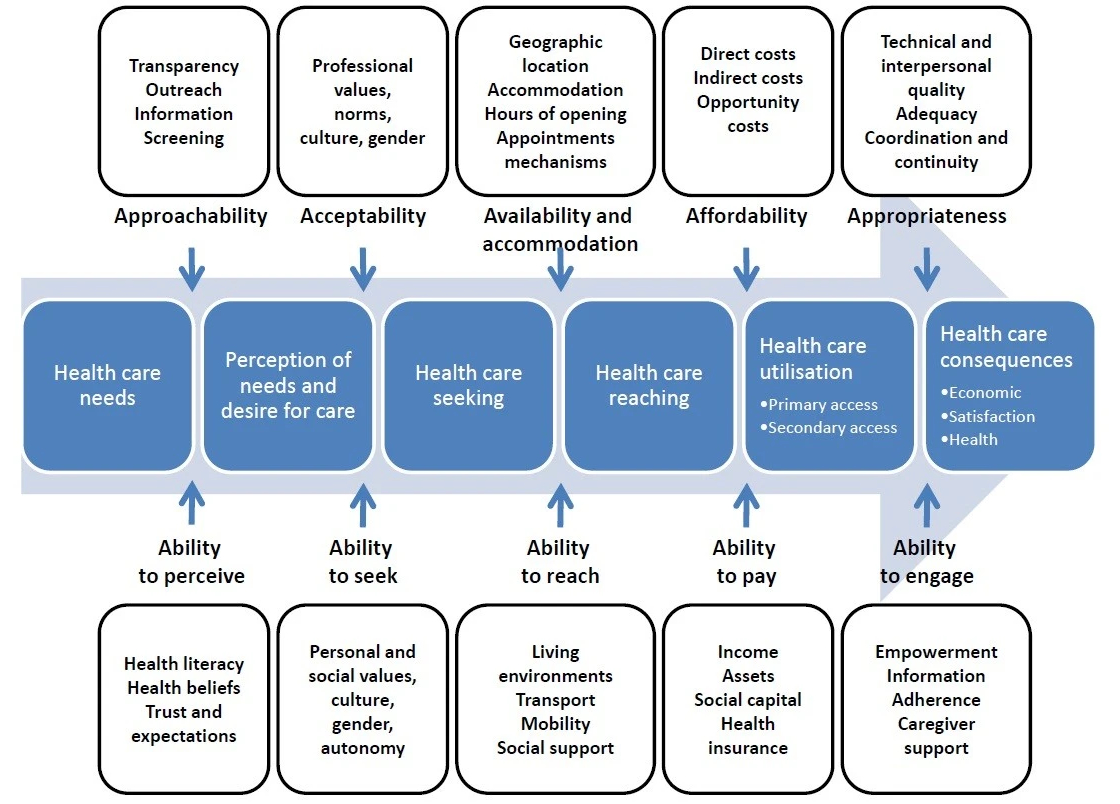
Figure 1A conceptual framework of healthcare access (from: Levesque JF, Harris MF, Russell G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health. 2013;12(1):18. doi: https://doi.org/10.1186/1475-9276-12-18234969841475-9276https://doi.org/10.1186/1475-9276-12-18).
Conclusions and possible implications
This viewpoint underscores the critical need to ensure equitable healthcare access, accounting for its various dimensions within the diverse individual, social, and economic constraints inherent in healthcare systems and people. In addition to creating healthcare structures that facilitate timely, high-quality care, it is imperative to empower individuals to navigate and manage their access effectively. The pursuit of health equity for rare diseases must be approached with careful consideration, as it can inadvertently lead to inequities in the broader population; for instance, a notable emphasis is often placed on financial aspects regarding research and the reimbursement of therapeutic interventions [4]. Even if these therapies successfully reach the market, the resulting individual financial burden or the cost-sharing mechanisms within healthcare systems can be very high. This begs several fundamental questions. First, is it morally defensible to require everyone, including those unafflicted by rare diseases, to bear these costs? Moreover, should we reconsider the allocation of resources within different rare disease groups so that certain conditions receive more funding than others? Finally, in the pursuit of health equity, should rare diseases be prioritised over more common diseases? These queries resonate with the profound words of Paul Farmer: “If access to healthcare is considered a human right, who is considered human enough to have that right?” [14]. Addressing these challenges requires a thoughtful and comprehensive dialogue on the ethical trade-offs and allocation of resources needed to ensure that every person, regardless of their health status, has the opportunity to achieve the highest standard of health and well-being.
Acknowledgement
We thank Tania Manriquez Roa for her very helpful comments on the first version of this article.
Notes
This work was supported by the University Research Priority Program of the University of Zurich (URPP) ITINERARE – Innovative Therapies in Rare Diseases, Switzerland. http://www.itinerare.uzh.ch
Both authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
References
1. Nguengang Wakap S, Lambert DM, Olry A, Rodwell C, Gueydan C, Lanneau V, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020 Feb;28(2):165–73.
2. von der Lippe C, Diesen PS, Feragen KB. Living with a rare disorder: a systematic review of the qualitative literature. Mol Genet Genomic Med. 2017 Nov;5(6):758–73.
3. World Health Organization. Health equity. https://www.who.int/health-topics/health-equity#tab=tab_1)
4. Gaviglio AM, Skinner MW, Lou LJ, Finkel RS, Augustine EF, Goldenberg AJ. Gene-targeted therapies: towards equitable development, diagnosis, and access. Am J Med Genet C Semin Med Genet. 2023 Mar;193(1):56–63.
5. Farooq F, Mogayzel PJ, Lanzkron S, Haywood C, Strouse JJ. Comparison of US Federal and Foundation Funding of Research for Sickle Cell Disease and Cystic Fibrosis and Factors Associated With Research Productivity. JAMA Netw Open. 2020 Mar;3(3):e201737.
6. World Health Organization. Universal health coverage (UHC). https://www.who.int/news-room/fact-sheets/detail/universal-health-coverage-(uhc)
7. Ricketts TC, Goldsmith LJ. Access in health services research: the battle of the frameworks. Nurs Outlook. 2005;53(6):274–80.
8. Souliotis K, Hasardzhiev S, Agapidaki E. A Conceptual Framework of Mapping Access to Health Care across EU Countries: The Patient Access Initiative. Public Health Genomics. 2016;19(3):153–9.
9. Quinn M, Robinson C, Forman J, Krein SL, Rosland AM. Survey Instruments to Assess Patient Experiences With Access and Coordination Across Health Care Settings: Available and Needed Measures. Med Care. 2017 Jul;55(Suppl 7 1 Suppl 7 Suppl 1):S84–91.
10. Levesque JF, Harris MF, Russell G. Patient-centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health. 2013 Mar;12(1):18.
11. Sequeira AR, Mentzakis E, Archangelidi O, Paolucci F. The economic and health impact of rare diseases: A meta-analysis. Health Policy Technol. 2021;10(1):32–44.
12. Halley MC, Halverson CM, Tabor HK, Goldenberg AJ. Rare Disease, Advocacy and Justice: Intersecting Disparities in Research and Clinical Care. Am J Bioeth. 2023 Jul;23(7):17–26.
13. Werner A, Malterud K. It is hard work behaving as a credible patient: encounters between women with chronic pain and their doctors. Soc Sci Med. 2003 Oct;57(8):1409–19. 10.1016/S0277-9536(02)00520-8
14. Farmer P. Pathologies of Power: Health, Human Rights, and the New War on the Poor. University of California Press; 2005.