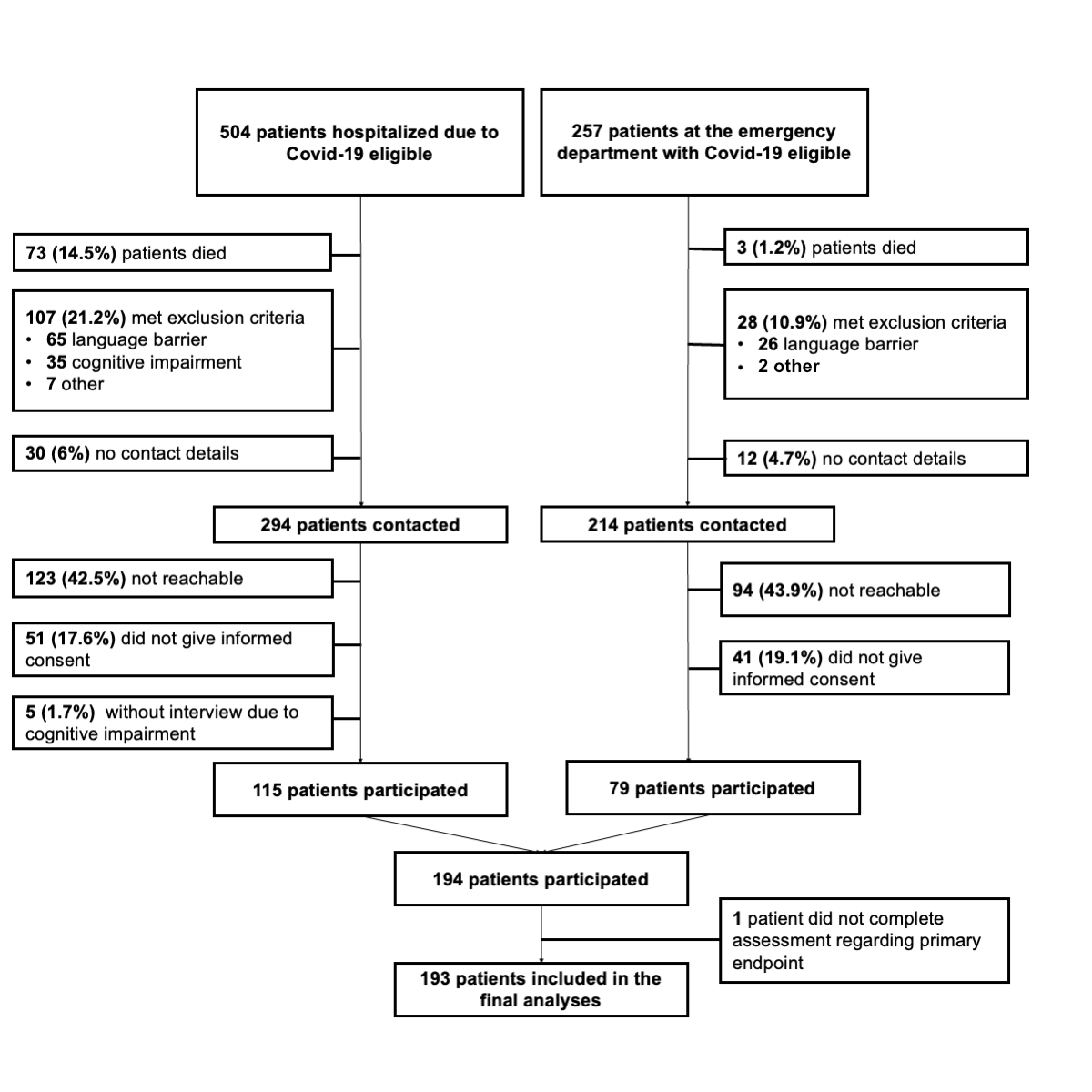
Figure 1Patient flowchart illustrating the recruitment process of the study.
DOI: https://doi.org/https://doi.org/10.57187/s.3634
The COVID-19 pandemic resulted in a surge of patients requiring hospitalisation, which placed immense pressure on healthcare systems and pushed them to their limits. Many physicians and nurses treating patients with COVID-19 experienced physical and emotional exhaustion [1, 2]. The high number of severely ill and dying patients, limited resources and treatment options and the risk of contracting the virus have been cited as common reasons for this exhaustion. Thus, the development of effective vaccines raised hopes that the pandemic would end or hospitalisation rates fall.
In Switzerland, as in many Western countries, vaccination was initially prioritised for people at high risk of developing severe COVID-19 and later, from June 2021, made available to the whole adult general population. However vaccine uptake was hampered due to vaccination hesitancy [3]. In Switzerland, approximately 30% of the adult population chose not to be vaccinated until December 2021 [4].
As a result, in many countries, a division arose in society between vaccinated and non-vaccinated individuals. Contentious sociopolitical discussions frequently raised concerns over increased transmission risks posed by non-vaccinated individuals and dissatisfaction with their perceived lack of contribution to society’s efforts to curb the pandemic and alleviate the strain on the healthcare system. In some cases, this led to moral judgement and criticism directed towards non-vaccinated individuals, potentially driven by personal concerns [5]. A large experimental study assessing 10,740 people in 21 countries showed that vaccinated participants had high antipathy towards non-vaccinated people, perceiving them as untrustworthy and unintelligent [5]. Other studies showed that non-vaccinated people felt judged as immoral by vaccinated people [6, 7].
In an effort to boost vaccination rates and curb the spread of infection, governments of many countries implemented measures aimed at restricting the access of non-vaccinated individuals to social activities and launched campaigns promoting vaccination as a moral obligation — these contributed to social stigma and polarisation [8]. Research suggests that social stigma and polarisation may lead to discrimination and that both have a negative impact on physical and psychological health [9]. This was also shown in people who experienced stigma due to having had COVID-19 [10, 11].
While initial studies evaluated aspects of perceived negative moral judgement or antipathy related to vaccination status, there was no empirical research on the prevalence of and factors associated with perceived healthcare-associated discrimination due to COVID-19 vaccination status. Perceived discrimination in the healthcare setting may be associated with lower satisfaction with care and does not comply with modern ethical standards in medicine; it is therefore important to assess it.
Our aim was to evaluate the association of vaccination status with perceived healthcare-associated discrimination in patients diagnosed with COVID-19 who presented to the emergency department or were hospitalised for inpatient care. We hypothesised that non-vaccinated patients would report higher levels of perceived healthcare-associated discrimination.
We conducted this exploratory, two-centre cohort study at University Hospital Basel and Kantonsspital Aarau, two tertiary teaching hospitals in Switzerland. The study was approved by the local Ethics Committee (Ethics Committee Northwest and Central Switzerland, EKNZ 2022-0123). All participating patients had provided informed consent. This manuscript complies with STROBE guidelines [12].
Consecutive adult patients who presented to the emergency department or were hospitalised due to or with COVID-19 between 1 June and 31 December 2021 in the COVID-specific wards in the internal medicine or intensive care division were eligible for inclusion. The rationale for this specific time frame was that in Switzerland vaccinations were available for the entire adult population from June 2021, and the omicron variant, against which vaccination was known to be much less effective, began to quickly spread in December 2021.
Exclusion criteria were factors resulting in an inability to give informed consent and participate in the study assessment, such as cognitive impairment, serious psychiatric condition (e.g. psychosis), already being included in the study (in case of multiple hospital visits due to COVID-19), death and insufficient knowledge of the local languages (German and French) or English.
As a first step, we screened the medical records of all eligible patients who had presented to the emergency department or had been consecutively admitted for inpatient care at University Hospital Basel or Kantonsspital Aarau with a COVID-19 diagnosis within the predefined time frame. We then contacted eligible patients by phone, informed them about our study and invited them to participate. Screening and telephone interviews took place from 29 July 2022 to 1 September 2022, i.e. 8 to 12 months after their admission to the emergency department or hospitalisation due to or with COVID-19. The average interval between hospitalisation and contact was approximately 9 months.
Patients were informed that the researchers were conducting a study to assess the perceived quality of care during the pandemic, but they were blinded to the primary outcome. We sent a letter including the study information, informed consent form and a postage-paid envelope to those who had agreed to participate. During these structured telephone interviews, we collected sociodemographic characteristics and primary and secondary outcomes, i.e. healthcare-associated discrimination and quality of care referring to their hospital visit with COVID-19 and current psychological distress. We then extracted additional information on comorbidities, known risk factors for severe COVID-19 and acute illness-related factors through a review of medical charts.
We asked patients about their vaccination status at the time of their hospital treatment with COVID-19 and at the present time, i.e. at the time of the telephone interview.
Vaccination status was recorded as “complete” if the patient had completed the initial vaccination protocol (e.g. two doses of an mRNA vaccine), “incomplete” if the patient had received only one dose of a vaccine or “non-vaccinated”. For the analyses, patients with complete and incomplete vaccination were subsumed into one group: “vaccinated”. We assessed sociodemographic characteristics such as age, sex, nationality, education and current employment status. Further, we asked participants if they had any psychological burden (i.e. current psychological comorbidities, psychological counselling since the start of the COVID-19 pandemic, newly prescribed psychoactive drug, psychological comorbidities before COVID-19). Illness-related factors included duration of hospitalisation, stay in an intensive care unit (ICU), intubation and calculated severity of illness using the National Early Warning Score (NEWS) [13]. NEWS is a scoring system that assesses respiratory rate, oxygen saturation, temperature, systolic blood pressure, heart rate and level of consciousness. A score between 0 and 3 is allocated to each item, and a sum score of 7 reflects a high risk for clinical deterioration. We also extracted information from medical charts on relevant comorbidities and calculated the Charlson Comorbidity Index, a measure of comorbidity severity [14].
Our primary outcome was patients’ healthcare-associated discrimination by healthcare workers during their hospital treatment with COVID-19, assessed by the Discrimination in Medical Settings (DMS) Scale [15]. The DMS scale is a patient-rated questionnaire assessing routine experiences of discrimination with healthcare providers. It is based on the Everyday Discrimination Scale [16], a validated and widely used measure to assess self-reported discrimination, which was adapted for use in medical settings by the authors of the DMS scale. Each of the seven items is rated on a 5-point Likert scale and the sum score with a possible range from 7 to 35 points indicates the degree of perceived discrimination with higher values indicating higher perceived discrimination. Items include statements such as “You are treated with less respect than other people.”, “You feel like a doctor or nurse is not listening to what you were saying.” and “You receive poorer service than others.” For the purpose of this study, we dichotomised responses into never vs ever experienced discrimination based on a cut-off of 7 points, similar to previous studies [17, 18].
Secondary outcomes included psychological distress, i.e. symptoms of anxiety and/or depression, assessed using the Hospital Anxiety and Depression Scale (HADS) [19]. HADS is a widely used, validated questionnaire that was specifically designed for patients hospitalised with medical conditions [20]. It consists of two subscales measuring symptoms of anxiety and depression. Further, we investigated different items of patients’ perceived quality of care such as overall satisfaction or trust in the healthcare team rated on a visual analogue scale from 0 to 10. We also asked patients whether and to what extent they now regretted their decision to be or not be vaccinated up until the time of their hospital treatment, and also whether they at some point had felt ashamed, guilty or had had a bad conscience about their decision, all rated on a visual analogue scale from 0 to 10. We evaluated current health-related quality of life at the time of the telephone interview with the internationally established EQ-5D-3L (EuroQol) [21]. The EuroQol index reflects the level of health-related quality of life on a scale from 0 to 1 with higher values indicating higher quality of life. We further asked participants about their self-perceived health status using the EuroQol visual analogue scale, ranging from 0 (worst imaginable health status) to 100 (best imaginable health status) [22].
We used descriptive statistics to present characteristics of the study population. Means ( ± SD) were used for continuous variables, and frequencies for binary or categorical variables. We stratified our population by vaccination status and by perceived discrimination and compared the characteristics of both groups using Student’s t-test for continuous variables and chi-square tests for binary outcomes. To assess the associations of potential risk and protective factors and outcomes, we conducted univariable logistic and linear regression models and multivariable models adjusted for age and sex with (a) vaccination status and (b) discrimination as the independent variable. To achieve a normal distribution, non-normally distributed data of these levels were log-transformed with a base of 10 and categorised by decile.
To address missing data, we used imputed datasets generated through the utilisation of multiple imputations via chained equations. These imputations were computed by incorporating several covariables, such as sociodemographic factors, comorbidities, risk factors for developing severe COVID-19, vital signs as well as the primary endpoint to reduce bias, as previously suggested [23].
All statistical analyses were conducted using Stata 15 (Stata Corp, College Station, Texas, USA).
The study was approved by the local Ethics Committee (Northwest and Central Switzerland, EKNZ 2022-0123).
Between 1 June and 31 December 2021, 504 adult patients hospitalised due to COVID-19 (61% at University Hospital Basel and 39% at Kantonsspital Aarau) and 257 presenting to the Emergency Department were eligible. The study inclusion process is depicted in figure 1. Approximately 15% of hospitalised patients and 1% of those at the emergency department died prior to telephone assessment 6 to 12 months later, 21.2% and 10.9% respectively met exclusion criteria and 6% and 4.7% respectively had no documented contact details. Of the remaining eligible patients, 42.5% and 43.9% were not reachable by phone and 17.6% and 19.1% did not consent. Outcome assessment was not possible in 5 previously hospitalised patients due to cognitive impairment,which was not documented as such in the patient record. The remaining 194 patients, 59% of whom had been hospitalised and 41% treated at the emergency department, participated in the telephone assessment and provided informed consent. One participant did not answer questions regarding the primary endpoint, leaving 193 participants (25.4% of the total initial sample) to include in the analyses.

Figure 1Patient flowchart illustrating the recruitment process of the study.
Table 1 shows detailed information about the sociodemographic and clinical characteristics of the study population stratified for vaccination status. The mean ± SD age of participating patients was 54.6 ± 20.1 years. Patients who were not vaccinated were significantly younger, more often female and had fewer comorbidities compared to vaccinated patients.
Table 1Baseline characteristics stratified by vaccination status.
| Factor | All patients | Vaccinated patients | Unvaccinated patients | Univariable model | Multivariable model, adjusted for age & gender | |||||
| OR (95% CI) | p | OR (95% CI) | p | |||||||
| n | 193 | 80 | 113 | |||||||
| Centre | USB ward | 64 (33.2%) | 35 (43.8%) | 29 (25.7%) | 1 (Ref) | 1 (Ref) | ||||
| USB ED | 40 (20.7%) | 15 (18.8%) | 25 (22.1%) | 2.01 (0.9–4.51) | 0.09 | 1.12 (0.44–2.82) | 0.82 | |||
| KSA ward | 50 (25.9%) | 17 (21.3%) | 31 (27.4%) | 2.34 (1.09–5.03) | 0.03 | 2.57 (1.14–5.82) | 0.02 | |||
| KSA ED | 39 (20.2%) | 13 (16.3%) | 28 (24.8%) | 2.41 (1.05–5.52) | 0.04 | 1.89 (0.77–4.65) | 0.17 | |||
| Sociodemographic factors (at time of hospitalisation) | ||||||||||
| Age (years), mean (SD) | 193 | 54.6 (20.1) | 60.34 (21.84) | 50.47 (17.82) | 0.97 (0.96–0.99) | <0.001 | 0.98 (0.96–0.99)* | 0.006* | ||
| Female sex, n (%) | 193 | 79 (40.9%) | 22 (27.5%) | 57 (50.4%) | 2.68 (1.45–4.96) | <0.01 | 2.27 (1.21–4.28)** | 0.011** | ||
| Nationality, n (%) | Swiss | 193 | 132 (68.4%) | 62 (77.5%) | 70 (61.9%) | Swiss | 1 (Ref) | 1 (Ref) | ||
| German | 10 (5.2%) | 6 (7.5%) | 4 (3.5%) | Non-Swiss | 2.12 (1.11–4.04) | 0.02 | 1.47 (0.73–2.94) | 0.28 | ||
| Kosovan | 10 (5.2%) | 1 (1.3%) | 9 (8.0%) | |||||||
| Italian | 6 (3.1%) | 2 (2.5%) | 4 (3.5%) | |||||||
| Albanian | 2 (1.0%) | 1 (1.3%) | 1 (0.9%) | |||||||
| French | 1 (0.5%) | 1 (1.3%) | 0 (0.0%) | |||||||
| Others | 32 (16.6%) | 7 (8.8%) | 25 (22.1%) | |||||||
| Religious affiliation, n (%) | No religious affiliation | 187 | 62 (32.5%) | 30 (38.0%) | 32 (28.6%) | Christian | 1 (Ref) | 1 (Ref) | ||
| Catholic | 46 (24.1%) | 23 (29.1%) | 23 (20.5%) | Other | 3.82 (1.64–8.91) | <0.01 | 2.56 (1.08–6.05) | 0.03 | ||
| Protestant | 33 (17.3%) | 16 (20.3%) | 17 (15.2%) | |||||||
| Muslim | 30 (15.7%) | 7 (8.9%) | 23 (20.5%) | |||||||
| Jewish | 1 (0.5%) | 1 (1.3%) | 0 (0.0%) | |||||||
| Other | 15 (7.9%) | 1 (1.3%) | 14 (12.5%) | |||||||
| Marital status, n (%) | Married/cohabitation | 192 | 112 (58.3%) | 38 (47.5%) | 74 (66.1%) | Married/cohabitation | 1 (Ref) | 1 (Ref) | ||
| Single | 43 (22.4%) | 23 (28.7%) | 20 (17.9%) | Single/Separated/Widowed | 0.46 (0.26–0.84) | 0.01 | 0.62 (0.47–0.81) | <0.001 | ||
| Separated | 21 (10.9%) | 9 (11.3%) | 12 (10.7%) | |||||||
| Widowed | 16 (8.3%) | 10 (12.5%) | 6 (5.4%) | |||||||
| Has children, n (%) | 188 | 136 (72.3%) | 51 (64.6%) | 85 (78.0%) | 1.94 (1.02–3.71) | 0.04 | 3.47 (1.57–7.66) | <0.001 | ||
| Educational level, n (%) | High school | 186 | 35 (18.8%) | 9 (11.4%) | 26 (24.3%) | High School | 1 (Ref) | 1 (Ref) | ||
| Apprenticeship | 107 (57.5%) | 47 (59.5%) | 60 (56.1%) | Apprenticeship | 0.44 (0.19–1.03) | 0.06 | 0.83 (0.33–2.09) | 0.7 | ||
| College/University | 44 (23.7%) | 23 (29.1%) | 21 (19.6%) | College/University | 0.32 (0.12–0.83) | 0.02 | 0.5 (0.18–1.39) | 0.19 | ||
| Employment status, n (%) | Employed | 192 | 98 (51.0%) | 28 (35.0%) | 70 (62.5%) | Paid work | 1 (Ref) | 1 (Ref) | ||
| Retired | 62 (32.3%) | 39 (48.8%) | 23 (20.5%) | No paid work | 0.32 (0.18–0.59) | <0.001 | 0.75 (0.59–0.95) | 0.02 | ||
| Disability benefits | 13 (6.8%) | 8 (10.0%) | 5 (4.5%) | |||||||
| Unemployed | 9 (4.7%) | 2 (2.5%) | 7 (6.3%) | |||||||
| Homemaker | 4 (2.1%) | 1 (1.3%) | 3 (2.7%) | |||||||
| Other | 6 (3.1%) | 2 (2.5%) | 4 (3.6%) | |||||||
| Known risk factors (at time of hospitalisation) | ||||||||||
| Comorbidities (CCI), mean (SD) | 191 | 2.22 (2.37) | 3.32 (2.61) | 1.46 (1.86) | 0.7 (0.6–0.8) | <0.001 | 0.59 (0.45–0.77) | <0.001 | ||
| Has cardiovascular disease, n (%) | 191 | 68 (35.6%) | 41 (52.6%) | 27 (23.9%) | 0.28 (0.15–0.53) | <0.001 | 0.37 (0.18–0.77) | 0.01 | ||
| Has diabetes, n (%) | 191 | 20 (10.5%) | 13 (16.7%) | 7 (6.2%) | 0.33 (0.13–0.87) | 0.03 | 0.46 (0.16–1.28) | 0.14 | ||
| Is obese (BMI >30), n (%) | 191 | 31 (16.2%) | 13 (16.7%) | 18 (15.9%) | 0.95 (0.43–2.07) | 0.89 | 0.92 (0.41–2.09) | 0.85 | ||
| Has respiratory disease, n (%) | 191 | 34 (17.8%) | 15 (19.2%) | 19 (16.8%) | 0.85 (0.4–1.79) | 0.67 | 0.97 (0.44–2.14) | 0.95 | ||
| Is pregnant, n (%) | 191 | 4 (2.1%) | 0 (0.0%) | 4 (3.5%) | n.a.* | n.a.* | n.a.* | n.a.* | ||
| Is immunosuppressed, n (%) | 191 | 24 (12.6%) | 15 (19.2%) | 9 (8.0%) | 0.36 (0.15–0.88) | 0.03 | 0.36 (0.14–0.92) | 0.03 | ||
| Is aged ≥60 years, n (%) | 193 | 84 (43.5%) | 47 (58.8%) | 37 (32.7%) | 0.34 (0.19–0.62) | <0.001 | 0.58 (0.19–1.79) | 0.35 | ||
| Has malignant disease, n (%) | 191 | 29 (15.2%) | 22 (28.2%) | 7 (6.2%) | 0.17 (0.07–0.42) | <0.001 | 0.24 (0.09–0.62) | <0.001 | ||
| Is a smoker, n (%) | 191 | 20 (10.5%) | 9 (11.5%) | 11 (9.7%) | 0.83 (0.33–2.1) | 0.69 | 0.65 (0.24–1.8) | 0.41 | ||
| Psychological factors, n (%) (at time of telephone interview) | ||||||||||
| Currently has psychological comorbidities | 186 | 26 (14.0%) | 13 (16.5%) | 13 (12.1%) | 1.53 (0.71–3.3) | 0.28 | 1.02 (0.45–2.29) | 0.97 | ||
| Has received psychological treatment since Coronavirus disease | 186 | 23 (12.4%) | 10 (12.7%) | 13 (12.1%) | 0.7 (0.31–1.61) | 0.4 | 0.45 (0.18–1.09) | 0.08 | ||
| Has been newly prescribed a psychoactive drug | 186 | 41 (22.0%) | 18 (22.8%) | 23 (21.5%) | 0.95 (0.4–2.3) | 0.98 | 0.94 (0.37–2.35) | 0.89 | ||
| Had psychological comorbidities before Coronavirus disease | 186 | 35 (18.8%) | 12 (15.2%) | 23 (21.5%) | 0.93 (0.46–1.87) | 0.83 | 0.69 (0.33–1.46) | 0.33 | ||
| Place of care | ||||||||||
| Emergency department | 193 | 81 (41.9%) | 28 (35%) | 53 (47%) | 1 (Ref) | 1 (Ref) | ||||
| Medical ward | 112 (58.1%) | 52 (65%) | 60 (53%) | 0.61 (0.34–1.1) | 0.1 | 0.97 (0.49–1.91) | 0.92 | |||
BMI: body mass index; CCI: Charlson Comorbidity Index; CI: confidence interval; ED: emergency department; KSA: Kantonsspital Aarau; OR: odds ratio; Ref: reference; SD: standard deviation; USB: University Hospital Basel.
* adjusted for sex
** adjusted for age
As a first step, we assessed associations between patients’ characteristics and perceived discrimination (table 2). Patients who felt discriminated against during their hospital treatment were younger (mean ± SD age: 49.4 ± 18.5 vs 57.8 ± 20.5 years; OR: 0.98 [95% CI: 0.96–0.99], p = 0.01) and were more often female (OR: 1.93 [95% CI: 1.07–3.48], p = 0.03). Nationality and psychological factors were not associated with perceived discrimination. Also, there was no difference regarding perceived discrimination between patients hospitalised for COVID-19 and those who were able to return home after being seen in the emergency department.
Table 2Association between patient characteristics and perceived discrimination.
| Factor | All patients | Perceived discrimination: NO | Perceived discrimination: YES | Univariable model | Multivariable model, adjusted for age & sex | ||||||
| OR (95% CI) | OR (95% CI) | ||||||||||
| n | 193 | 118 | 75 | ||||||||
| Centre | USB ward | 193 | 64 (33.2%) | 42 (35.6%) | 22 (29.3%) | 0.51 | 1 (Ref) | 1 (Ref) | |||
| USB ED | 40 (20.7%) | 23 (19.5%) | 17 (22.7%) | 1.41 (0.63–3.18) | 0.41 | 0.78 (0.31–1.94) | 0.59 | ||||
| KSA ward | 50 (25.9%) | 27 (22.9%) | 23 (30.7%) | 1.63 (0.76–3.47) | 0.21 | 1.59 (0.72–3.53) | 0.26 | ||||
| KSA ED | 39 (20.2%) | 26 (22.0%) | 13 (17.3%) | 0.95 (0.41–2.22) | 0.91 | 0.66 (0.26–1.65) | 0.37 | ||||
| Sociodemographic factors (at time of hospitalisation) | |||||||||||
| Age (years), mean (SD) | 193 | 54.56 (20.13) | 57.84 (20.5) | 49.40 (18.5) | <0.01 | 0.98 (0.96–0.99) | 0.01 | 0.98 (0.97–1) * | 0.016* | ||
| Female sex, n (%) | female | 193 | 79 (40.9%) | 41 (34.7%) | 38 (50.7%) | 0.03 | 1.93 (1.07–3.48) | 0.03 | 1.65 (0.9–3.04)** | 0.108** | |
| Nationality, n (%) | Swiss | 193 | 132 (68.4%) | 84 (71.2%) | 48 (64.0%) | 0.54 | Swiss | 1 (Ref) | 1 (Ref) | ||
| German | 10 (5.2%) | 6 (5.1%) | 4 (5.3%) | Non-Swiss | 1.39 (0.75–2.58) | 0.3 | 1.02 (0.53–1.97) | 0.95 | |||
| Kosovan | 10 (5.2%) | 5 (4.2%) | 5 (6.7%) | ||||||||
| Italian | 6 (3.1%) | 2 (1.7%) | 4 (5.3%) | ||||||||
| Albanian | 2 (1.0%) | 2 (1.7%) | 0 (0.0%) | ||||||||
| French | 1 (0.5%) | 1 (0.8%) | 0 (0.0%) | ||||||||
| Others | 34 (16.6%) | 20 (15.3%) | 14 (18.7%) | ||||||||
| Religious affiliation, n (%) | No religious affiliation | 187 | 62 (32.5%) | 30 (38.0%) | 32 (28.6%) | 0.35 | Christian | 1 (Ref) | 1 (Ref) | ||
| Catholic | 46 (24.1%) | 23 (29.1%) | 23 (20.5%) | Other | 0.91 (0.44–1.88) | 0.8 | 0.54 (0.24–1.19) | 0.13 | |||
| Protestant | 33 (17.3%) | 16 (20.3%) | 17 (15.2%) | No religious affiliation | 0.45 (0.22–0.91) | 0.03 | 0.39 (0.19–0.82) | 0.01 | |||
| Muslim | 30 (15.7%) | 7 (8.9%) | 23 (20.5%) | ||||||||
| Other Christian | 4 (2.1%) | 1 (1.3%) | 3 (2.7%) | ||||||||
| Jewish | 1 (0.5%) | 1 (1.3%) | 0 (0.0%) | ||||||||
| Other | 15 (7.9%) | 1 (1.3%) | 14 (12.5%) | ||||||||
| Marital status, n (%) | Married/cohabitation | 192 | 112 (58.3%) | 38 (47.5%) | 74 (66.1%) | 0.29 | Married/cohabitation | 1 (Ref) | 1 (Ref) | ||
| Single | 43 (22.4%) | 23 (28.7%) | 20 (17.9%) | Not in a relationship | 0.82 (0.45–1.47) | 0.5 | 0.9 (0.7–1.16) | 0.42 | |||
| Separated | 21 (10.9%) | 9 (11.3%) | 12 (10.7%) | ||||||||
| Widowed | 16 (8.3%) | 10 (12.5%) | 6 (5.4%) | ||||||||
| Has children, n (%) | 188 | 136 (72.3%) | 83 (73.5%) | 53 (70.7%) | 0.68 | 0.87 (0.45–1.67) | 0.68 | 1.05 (0.51–2.19) | 0.89 | ||
| Educational level, n (%) | High school | 186 | 35 (18.8%) | 19 (17.0%) | 16 (21.6%) | 0.73 | High school | 1 (Ref) | 1 (Ref) | ||
| Apprenticeship | 107 (57.5%) | 66 (58.9%) | 41 (55.4%) | Apprenticeship | 0.74 (0.34–1.59) | 0.44 | 1.1 (0.48–2.54) | 0.82 | |||
| College/University | 44 (23.7%) | 27 (24.1%) | 17 (23.0%) | College/University | 0.75 (0.3–1.84) | 0.53 | 1.13 (0.43–2.95) | 0.81 | |||
| Current employment status, n (%) | Employed | 192 | 98 (51.0%) | 28 (35.0%) | 70 (62.5%) | 0.31 | Paid work | 1 (Ref) | 1 (Ref) | ||
| Retired | 62 (32.3%) | 39 (48.8%) | 23 (20.5%) | No paid work | 0.72 (0.4–1.29) | 0.27 | 1.07 (0.84–1.35) | 0.6 | |||
| Disability benefits | 13 (6.8%) | 8 (10.0%) | 5 (4.5%) | ||||||||
| Unemployed | 9 (4.7%) | 2 (2.5%) | 7 (6.3%) | ||||||||
| Homemaker | 4 (2.1%) | 1 (1.3%) | 3 (2.7%) | ||||||||
| Other | 6 (3.1%) | 2 (2.5%) | 4 (3.6%) | ||||||||
| Known risk factors (at time of hospitalisation) | |||||||||||
| Comorbidity (CCI), mean (SD) | 191 | 2.22 (2.37) | 2.69 (2.51) | 1.49 (1.94) | <0.001 | 0.79 (0.69–0.91) | <0.001 | 0.8 (0.63–1.03) | 0.08 | ||
| Has cardiovascular disease, n (%) | 191 | 68 (35.6%) | 46 (39.7%) | 22 (29.3%) | 0.15 | 0.63 (0.34–1.18) | 0.15 | 1.03 (0.49–2.19) | 0.93 | ||
| Has diabetes, n (%) | 191 | 20 (10.5%) | 15 (12.9%) | 5 (6.7%) | 0.17 | 0.48 (0.17–1.38) | 0.18 | 0.67 (0.22–2.04) | 0.48 | ||
| Is obese (BMI >30), n (%) | 193 | 31 (16.2%) | 16 (13.8%) | 15 (20.0%) | 0.26 | 1.56 (0.72–3.39) | 0.26 | 1.61 (0.73–3.57) | 0.24 | ||
| Has respiratory disease, n (%) | 191 | 34 (17.8%) | 24 (20.7%) | 10 (13.3%) | 0.19 | 0.59 (0.26–1.32) | 0.2 | 0.65 (0.28–1.47) | 0.3 | ||
| Is pregnant, n (%) | 191 | 4 (2.1%) | 1 (0.9%) | 3 (4.0%) | 0.14 | 4.83 (0.49–47.36) | 0.18 | 2.64 (0.26–26.91) | 0.41 | ||
| Is immunosuppressed, n (%) | 191 | 24 (12.6%) | 16 (13.8%) | 8 (10.7%) | 0.52 | 0.75 (0.3–1.84) | 0.53 | 0.83 (0.33–2.12) | 0.7 | ||
| Has neurological disease, n (%) | 191 | 27 (14.1%) | 13 (11.2%) | 14 (18.7%) | 0.15 | 1.82 (0.8–4.12) | 0.15 | 2.67 (1.11–6.43) | 0.03 | ||
| Aged ≥60 years, n (%) | 191 | 84 (43.5%) | 63 (53.4%) | 21 (28.0%) | <0.001 | 0.34 (0.18–0.63) | <0.001 | 0.36 (0.12–1.11) | 0.08 | ||
| Has malignant disease, n (%) | 191 | 29 (15.2%) | 22 (19.0%) | 7 (9.3%) | 0.07 | 0.44 (0.18–1.09) | 0.08 | 0.64 (0.25–1.67) | 0.37 | ||
| Is a smoker, n (%) | 191 | 20 (10.5%) | 13 (11.2%) | 7 (9.3%) | 0.68 | 0.82 (0.31–2.15) | 0.68 | 0.65 (0.24–1.81) | 0.41 | ||
| Psychological factors, n (%) (at time of telephone interview) | |||||||||||
| Currently has psychological comorbidities | 186 | 26 (14.0%) | 13 (11.5%) | 13 (17.8%) | 0.23 | 2.47 (1.17–5.21) | 0.02 | 2.05 (0.95–4.41) | 0.07 | ||
| Has received psychological treatment since Coronavirus disease | 186 | 23 (12.4%) | 15 (13.3%) | 8 (11.0%) | 0.64 | 1.67 (0.72–3.83) | 0.23 | 1.4 (0.59–3.31) | 0.44 | ||
| Has been newly prescribed a psychoactive drug | 186 | 41 (22.0%) | 22 (19.5%) | 19 (26.0%) | 0.29 | 0.8 (0.32–2) | 0.64 | 0.85 (0.33–2.15) | 0.72 | ||
| Had psychological comorbidities before Coronavirus disease | 186 | 35 (18.8%) | 15 (13.3%) | 20 (27.4%) | 0.02 | 1.46 (0.72–2.93) | 0.29 | 1.31 (0.64–2.69) | 0.47 | ||
| Place of care | |||||||||||
| Emergency department | 193 | 81 (41.9%) | 50 (42.4%) | 31 (41.3%) | 1 (Ref) | 1 (Ref) | |||||
| Medical ward | 193 | 112 (58.1%) | 68 (57.6%) | 44 (58.7%) | 0.89 | 1.04 (0.58–1.88) | 0.89 | 1.77 (0.89–3.51) | 0.11 | ||
BMI: body mass index; CCI: Charlson Comorbidity Index; CI: confidence interval; ED: emergency department; KSA: Kantonsspital Aarau; OR: odds ratio; Ref: reference; SD: standard deviation; USB: University Hospital Basel.
* adjusted for sex
** adjusted for age
Non-vaccinated patients (n = 113) had significantly higher scores in the DMS scale compared to vaccinated patients (n = 80) (mean ± SD: 9.54 ± 4.84 vs 7.79 ± 1.85 points). This difference remained significant after adjustment for age and sex (adjusted difference: 1.18 [95% CI: 0.04–2.33 points]). Also, 21 of 80 vaccinated patients felt discriminated against vs 54 of 113 non-vaccinated patients resulting in a 2-fold increased risk in an age- and sex-adjusted model (adjusted OR: 2.09 [95% CI: 1.10–3.99) (table 3).
Table 3Outcomes stratified by vaccination status.
| Outcome | Predictor | ||||||||
| Factor | All patients | Vaccinated patients | Non-vaccinated patients | Univariable model | Multivariable model adjusted for age & sex | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Difference (95% CI) | Adjusted difference (95% CI) | |||||
| n | 193 | 80 | 113 | ||||||
| Primary outcome | |||||||||
| Patient perceived discrimination (at time of hospitalisation) | |||||||||
| Patient perceived discrimination in medical settings (DMS), n (%) | 193 | 75 (38.9%) | 21 (26.3%) | 54 (47.8%) | 2.57 (1.38 – 4.78) | <0.01 | 2.09 (1.1– 3.99) | 0.03 | |
| Patient perceived discrimination in medical settings (DMS), mean (SD) | 193 | 8.81 (3.98) | 7.79 (1.85) | 9.54 (4.84) | 1.75 (0.63 – 2.88) | <0.01 | 1.18 (0.04– 2.33) | 0.04 | |
| Perceived discrimination in medical settings (DMS), log* | 0.14 (0.06 – 0.23)* | 0.001 | 0.1 (0.01– 0.19)* | 0.03 | |||||
| Main secondary outcomes | |||||||||
| Psychological distress (at time of telephone interview) | |||||||||
| Anxiety (HADS-A) score, mean (SD) | 171 | 3.56 (3.37) | 3.71 (3.42) | 3.43 (3.35) | –0.28 (–1.3 – 0.75) | 0.6 | –0.62 (–1.67 – 0.43) | 0.25 | |
| Depression (HADS–D) score, mean (SD) | 171 | 2.94 (3.66) | 3.23 (4.12) | 2.69 (3.23) | –0.54 (–1.65 – 0.57) | 0.34 | –0.47 (–1.62 – 0.68) | 0.42 | |
| Perceived quality of care (VAS 0–10), mean (SD) (at time of hospitalisation) | |||||||||
| I have trust in the physician team | 184 | 8.32 (2.07) | 8.64 (1.67) | 8.08 (2.30) | –0.56 (–1.16 – 0.05) | 0.07 | –0.23 (–0.84 – 0.39) | 0.47 | |
| I have trust in the nursing team | 188 | 8.55 (1.97) | 8.97 (1.28) | 8.25 (2.30) | –0.73 (–1.29 – –0.16) | <0.01 | –0.41 (–0.98 – 0.16) | 0.16 | |
| I feel physicians have high competence to treat COVID-19 | 165 | 7.69 (2.50) | 7.93 (2.45) | 7.52 (2.53) | –0.41 (–1.18 – 0.37) | 0.3 | –0.03 (–0.8 – 0.74) | 0.94 | |
| I feel nurses have high competence to treat COVID-19 | 172 | 8.26 (2.13) | 8.39 (2.05) | 8.17 (2.19) | –0.22 (–0.87 – 0.43) | 0.51 | 0.09 (–0.57 – 0.74) | 0.79 | |
| Physicians were empathic | 171 | 8.43 (5.72) | 8.43 (1.80) | 8.42 (7.38) | –0.01 (–1.76 – 1.75) | 0.99 | 0.1 (–1.74 – 1.94) | 0.92 | |
| Nurses were empathic | 180 | 8.34 (1.98) | 8.66 (1.55) | 8.11 (2.22) | –0.55 (–1.14 – 0.03) | 0.06 | –0.22 (–0.81 – 0.37) | 0.47 | |
| Physicians treated me with respect | 181 | 8.70 (2.18) | 9.20 (1.25) | 8.34 (2.60) | –0.85 (–1.49 – –0.22) | <0.01 | –0.53 (–1.18 – 0.12) | 0.11 | |
| Nurses treated me with respect | 187 | 8.78 (2.00) | 9.30 (1.09) | 8.39 (2.39) | –0.91 (–1.48 – –0.34) | <0.01 | –0.6 (–1.18 – –0.02) | 0.04 | |
| Overall satisfaction with hospital treatment | 187 | 8.09 (2.41) | 8.46 (1.86) | 7.82 (2.71) | –0.65 (–1.35 – 0.06) | 0.07 | –0.3 (–1.01 – 0.41) | 0.41 | |
| I would recommend this hospital to family members and friends | 182 | 8.18 (2.27) | 8.53 (1.83) | 7.93 (2.52) | –0.59 (–1.26 – 0.08) | 0.07 | –0.31 (–0.99 – 0.38) | 0.38 | |
| Comfort with the vaccination decision (VAS 0–10), mean (SD) (at time of telephone interview) | |||||||||
| To what extent do you regret your decision for / against the vaccination? | 184 | 1.10 (2.52) | 0.78 (2.30) | 1.33 (2.66) | 0.55 (–0.19 – 1.29) | 0.14 | 0.5 (–0.27 – 1.28) | 0.2 | |
| To what extent are you ashamed of this decision? | 183 | 0.28 (1.37) | 0.13 (1.13) | 0.39 (1.53) | 0.27 (–0.14 – 0.67) | 0.19 | 0.33 (–0.09 – 0.75) | 0.13 | |
| To what extent do you feel guilty? | 183 | 0.34 (1.63) | 0.13 (1.13) | 0.50 (1.92) | 0.37 (–0.11 – 0.85) | 0.13 | 0.37 –-0.14 – 0.87) | 0.15 | |
| To what extent do you have a bad conscience? | 183 | 0.57 (2.01) | 0.24 (1.50) | 0.83 (2.30) | 0.59 (0 – 1.18) | 0.05 | 0.51 (–0.11 – 1.13) | 0.11 | |
| Further outcomes | |||||||||
| Univariable model | Multivariable model, adjusted for age & sex | ||||||||
| Difference / OR (95% CI) | Adjusted difference / OR (95% CI) | ||||||||
| Acute illness-related factors (at time of hospitalisation) | |||||||||
| Was hospitalised, n (%) | 112 (58.0%) | 52 (65.0%) | 60 (53.1%) | 0.61 (0.34 – 1.1) | 0.1 | 0.94 (0.48 – 1.85) | 0.86 | ||
| Duration of hospitalisation (days), mean (SD) | 112 | 13.19 (21.62) | 16.19 (29.68) | 10.58 (10.16) | –5.61 (–13.7 – 2.48) | 0.17 | –5.44 (–14.38 – 3.49) | 0.23 | |
| Was in ICU, n (%) | 28 (14.5%) | 11 (13.8%) | 17 (15.0%) | 1.11 (0.49 – 2.52) | 0.8 | 1.46 (0.6 – 3.57) | 0.41 | ||
| Duration of ICU stay (days), mean (SD) | 28 | 9.36 (12.14) | 9.45 (12.86) | 9.29 (12.06) | –0.16 (–10 – 9.68) | 0.97 | 4.92 (–7.64 – 17.48) | 0.43 | |
| Was intubated, n (%) | 9 (32%) | 4 (36%) | 5 (29%) | 0.73 (0.15 – 3.65) | 0.701 | 1.17 (0.13 – 10.17) | 0.89 | ||
| Severity of illness (NEWS score), mean (SD) | 184 | 3.07 (2.68) | 2.42 (2.31) | 3.50 (2.82) | 1.07 (0.29 – 1.85) | <0.01 | 1.48 (0.67 – 2.28) | <0.001 | |
| DNR status, n (%) | 26 (19.7%) | 15 (26%) | 11 (15%) | 0.5 (0.21 – 1.19) | 0.12 | 1.23 (0.35 – 4.27) | 0.75 | ||
| Fear of developing severe COVID-19, mean (SD) | 182 | 3.12 (3.51) | 2.91 (3.19) | 3.28 (3.73) | 0.37 (–0.67 – 1.41) | 0.49 | –0.16 (–1.23 – 0.9) | 0.76 | |
| Current health status (at time of telephone interview) | |||||||||
| Health-related quality of life (EuroQol Index), mean (SD) | 189 | 0.86 (0.22) | 0.85 (0.22) | 0.86 (0.23) | 0.02 (–0.05 – 0.08) | 0.61 | 0.01 (–0.05 – 0.08) | 0.69 | |
| Self-perceived health status (EuroQol VAS 0–100), mean (SD) | 190 | 74.47 (20.84) | 72.31 (22.10) | 76.05 (19.83) | 3.73 (–2.3 – 9.77) | 0.22 | 3.65 (–2.68 – 9.98) | 0.26 | |
CI: confidence interval; DMS: Discrimination in Medical Settings Scale; DNR: “Do Not Resuscitate” status; EuroQol: Euro Quality of life; HADS-A: Hospital Anxiety and Depression Scale – Anxiety; HADS-D: Hospital Anxiety and Depression Scale – Depression; ICU: intensive care unit; OR: odds ratio; SD: standard deviation.
* log: log transformed with a base of 10
Vaccinated and non-vaccinated patients did not differ in reported symptoms of anxiety, i.e. mean HADS-Anxiety sum scores (mean ± SD: 3.43 ± 3.35 vs 3.71 ± 3.42, adjusted difference: –0.62 [95% CI: –1.67 – 0.43], p = 0.25). Further, there was no difference in levels of symptoms of depression, i.e. mean HADS-Depression sum scores (mean ± SD: 3.23 ± 4.12 vs 2.69 ± 3.23, adjusted difference: –0.47 [95% CI: –1.62 – 0.68], p = 0.42).
Regarding perceived quality of care, non-vaccinated patients compared to vaccinated patients reported lower levels of trust in the nursing team (mean ± SD: 8.97 ± 1.28 vs 8.25 ± 2.3, difference: –0.73 [95% CI: –1.29 – –0.16], p = 0.01). However, this was no longer significant after adjusting for age and sex: adjusted difference: –0.41 [95% CI: –0.98 – 0.16], p = 0.16). Non-vaccinated patients felt treated with less respect by physicians (mean ± SD: 9.20 ± 1.25 vs 8.34 ± 2.60, difference: –0.85 [95% CI: –1.49 – –0.22], p <0.01) and nurses (mean ± SD: 9.30 ± 1.09 vs 8.39 ± 2.39, difference: –0.91 [95% CI: –1.48 – –0.34], p <0.01]). After adjusting for age and sex in a multivariable model, only the association between vaccination status and the level of the perception of being treated with respect by nurses remained significant (adjusted difference: –0.60 [95% CI: –1.18 – –0.02], p = 0.04). Vaccinated and non-vaccinated patients did not differ in their level of trust in the physician and nursing team, perception of physicians’ and nurses’ high competence to treat COVID-19, how empathic they perceived physicians and nurses to be, their overall satisfaction with the hospital treatment and whether they would recommend the hospital to family members and friends.
There was no difference between vaccinated and non-vaccinated patients regarding their comfort with their vaccination decision, i.e. level of regret regarding their decision, being ashamed of their decision, feeling guilty or having a bad conscience.
Vaccinated and non-vaccinated patients did not differ regarding likelihood and duration of hospital stay and ICU stay, rates of Do Not Resuscitate orders and fear of developing severe COVID-19. The two patient groups also did not differ regarding health-related quality of life and subjective health status.
In this two-centre observational cohort study we found an association between vaccination status and perceived healthcare-associated discrimination in patients who required medical treatment for COVID-19. This finding remained after adjusting for female sex and age, which were also associated with perceived discrimination. Also, non-vaccinated patients felt that they were treated with less respect by nurses compared to patients who were vaccinated. Average levels of psychological distress in our cohort were low and did not differ between vaccinated and non-vaccinated patients.
While some studies have illustrated that infectious diseases, including COVID-19, may be associated with stigma and discrimination during medical care [11, 24], this is – to the best of our knowledge – the first study investigating perceived healthcare-associated discrimination based on COVID-19 vaccination status. Our finding is in line with earlier studies indicating that vaccinated people exhibit discriminatory attitudes towards people who have not received a COVID-19 vaccine [5]. Non-vaccinated people who contract the virus or infect others may also face greater negative attitudes, including lower levels of sympathy, less willingness from others to provide assistance, as well as anger or blame directed towards them [25]. However, these findings were derived from studies recruiting individuals from the general population and may thus not be directly applicable to medical settings as the dynamics between healthcare staff and patients most likely differ to those of personal relationships.
During the pandemic, healthcare workers had to deal with a high number of critically ill patients requiring hospital-based care, affecting their physical and psychological health [26]. Due to their work, frontline workers in healthcare were at increased risk of infection and of developing severe COVID-19 [27]. Several studies have provided evidence that a COVID vaccine could have prevented the need for hospitalisation, or at least severe COVID-19, in many patients and therefore ease the pressure on emergency departments, medical wards and intensive care units [28]. This is why some policymakers argued that moralisation of vaccination and restriction of fundamental human rights may be justified due to the increased risk non-vaccinated people might pose to the healthcare system [29, 30]. Due to a low vaccination rate, Austria was the first country in Europe that made immunisations against SARS-CoV-2 mandatory for the whole adult population [29]. In some cases, the political debate even included consideration of whether to deny medical care, such as intensive care beds, to non-vaccinated patients in case of shortage [31]. This moralisation could have contributed to a mounting political debate and explain why non-vaccinated patients in our study were more likely to have faced healthcare-associated discrimination or at least perceive their care as discriminatory.
According to the literature, perceived discrimination may have a considerable adverse effect on patient outcomes and experiences [9]. A recent study evaluated the association between healthcare-associated discrimination and the perceived quality of care [17]. The authors revealed that patients who experienced discrimination by healthcare staff were twice as likely to perceive the quality of their care as poor. Thus, it might be important for healthcare providers and institutions to promote equitable and inclusive healthcare for all individuals regardless of their vaccination status.
In our study, female sex and younger age were the strongest predictors of perceived healthcare-associated discrimination. In 2020, a report from the United Nations illustrated that 90% of all people have some form of sex bias [32]. Sex-based discrimination has also been described in healthcare. A recent retrospective analysis of 450,000 patients with neuro- and cardiovascular diseases in Switzerland found that women were less likely to be admitted to an ICU than men, despite being more severely ill [33].
Also, a recent literature review looking at patients with chronic pain found that healthcare professionals perceive men as “brave”, whereas women were often judged as being “emotional” [34]. In our study, women were significantly more likely to be non-vaccinated compared to men, which could have contributed to the perceived discrimination in our study. While there is evidence of global variation in the relationship between sex and COVID-19 vaccine acceptance, our finding aligns with a recent Swiss study indicating that women were less likely to receive the vaccine [35].
Literature regarding the association between younger age and perceived discrimination is so far sparse, although some studies suggest that younger patients may be more critical when it comes to evaluating various aspects of the quality of care [36]. During the COVID-19 pandemic, young people often had to make sacrifices to protect older people [37]. With the virus posing a greater risk to the elderly, many young people had to give up social events, travel and other activities to reduce the spread of the virus. One could hypothesise that this might have led to a heightened sensitivity to interactions with healthcare workers and translated into a higher level of perceived discrimination compared to older patients.
From the beginning of the pandemic, healthcare workers increasingly experienced various mental health issues such as depression, insomnia or emotional exhaustion [38]. According to a meta-analysis, being a frontline health worker and being a nurse were among the most significant risk factors [38]. Psychological distress and burnout in healthcare workers may result in negative and cynical attitudes towards patients they care for [39]. During the pandemic, many psychologists and healthcare workers used the term “compassion fatigue” [40, 41]. As vaccine refusal was often considered irresponsible [5], such cynical attitudes might have been pronounced towards non-vaccinated patients. Further, increased personal concern about becoming infected with SARS-CoV-2 was shown to be strongly associated with higher condemnation and moralisation regarding non-vaccinated individuals [6] and thus explain why they perceived healthcare professionals as less respectful.
Interventions to raise awareness of bias and patient-centred communication training might safeguard against discrimination as well as cynicism and thus improve quality of care. To prevent healthcare workers from experiencing strain due to challenging conditions such as staff shortages, it may be helpful to establish a supportive work environment that includes emotional support.
There are a few findings from our study that contrast with previous research.
First, a previous meta-analysis illustrated that perceived discrimination may influence a person’s psychological wellbeing [9]. Nevertheless, the perceived discrimination in non-vaccinated patients of our cohort did not translate into increased levels of anxiety or depression. One reason could be that the healthcare-associated discrimination due to vaccination status in our study was temporary, whereas other people are exposed to long-term discrimination, e.g. ethnicity-based discrimination.
Second, many studies that assessed perceived discrimination in healthcare in various settings found an association between minority ethnic status and perceived healthcare-associated discrimination [17, 42, 43]. In our study however, there was no difference in perceived discrimination among patients from different ethnic backgrounds. This is especially noteworthy as, according to surveys, people of ethnic minority are more likely to be hesitant towards COVID-19 vaccination [44, 45]. However, we excluded patients who did not speak one of the questionnaire languages – German, English or French – possibly leading to selection bias. These patients might have had different experiences with discrimination during their COVID-19 treatment and thus explain the lack of association between ethnicity and perceived discrimination in our study.
This study has several limitations. First, our study was small and did not allow for rigorous statistical adjustment. Also, we did not distinguish between unvaccinated patients who were hospitalised due to symptomatic COVID-19 and asymptomatic patients who tested positive during routine screening on admission. This could have influenced the levels of perceived discrimination reported by patients, as the experiences and contexts of these two groups may differ. Also, we only assessed patients’ perceived discrimination between 8 and 12 months after their hospitalisation, as was done in other studies [46]. Still, we cannot rule out that the reported experiences of patients might have been influenced by other events during this period. Moreover, potential biases may arise from patients feeling discriminated against, particularly among those who are unvaccinated and hospitalised for COVID-19, who may feel guilty due to their vaccination status. This emotional aspect could potentially influence their perception of discrimination more prominently. Similarly, individuals who worry about side effects may also be inclined to perceive discrimination within the healthcare context, potentially affecting the interpretation of our findings.
Finally, the observational design does not allow us to draw any conclusions regarding causalities; rather it permits the generation of hypotheses.
We found an association between vaccination status and perceived healthcare-associated discrimination. Healthcare workers should act in a professional manner regardless of a patient’s vaccination status; in doing so, they might prevent the creation of negative perceptions in patients.
All data will be made available upon request.
Author contributions: Concept and design: Becker, Beck, Hunziker. Acquisition, analysis, or interpretation of data: Becker, Beck, Moser, Lessing, Hunziker. Drafting of the manuscript: Becker, Beck, Moser, Lessing, Hunziker. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Becker, Arpagaus, Beck, Hunziker. Administrative, technical, or material support: Schaefert, Bassetti, Schuetz. Supervision: Hunziker.
S. Hunziker and her research team are supported by the Swiss National Science Foundation (SNSF) (Ref 10001C_192850/1 and 10531C_182422) and the Gottfried Julia Bangerter-Rhyner Foundation (8472/HEG-DSV).
P. Schuetz has received support from the SNF (SNSF Professorship PP00P3_150531), the Forschungsrat of the Kantonsspital Aarau (1410.000.058 and 1410.000.044) and Funds of the Argovia Professorship of the Medical University Clinic (FG 1500000083). P. Schuetz has previously received unrestricted grant money unrelated to this project from Nestlé Health Science and Abbott Nutrition.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Linzer M, Jin JO, Shah P, Stillman M, Brown R, Poplau S, et al. Trends in Clinician Burnout With Associated Mitigating and Aggravating Factors During the COVID-19 Pandemic. JAMA Health Forum. 2022 Nov;3(11):e224163. 10.1001/jamahealthforum.2022.4163
2. Sexton JB, Adair KC, Proulx J, Profit J, Cui X, Bae J, et al. Emotional Exhaustion Among US Health Care Workers Before and During the COVID-19 Pandemic, 2019-2021. JAMA Netw Open. 2022 Sep;5(9):e2232748. 10.1001/jamanetworkopen.2022.32748
3. Aw J, Seng JJ, Seah SS, Low LL. COVID-19 Vaccine Hesitancy-A Scoping Review of Literature in High-Income Countries. Vaccines (Basel). 2021 Aug;9(8):900. 10.3390/vaccines9080900
4. Health FOoP. Geographical distribution - Vaccinated people by canton of residence, Switzerland; Status: 03.07.2023 2023 [Available from: https://www.covid19.admin.ch/en/vaccination/persons/d/geography?geo=CH
5. Bor A, Jørgensen F, Petersen MB. Discriminatory attitudes against unvaccinated people during the pandemic. Nature. 2023 Jan;613(7945):704–11. 10.1038/s41586-022-05607-y
6. Bor A, Jørgensen F, Lindholt MF, Petersen MB. Moralizing the COVID-19 Pandemic: Self-Interest Predicts Moral Condemnation of Other’s Compliance, Distancing, and Vaccination. Polit Psychol. 2022 May.
7. Rosenfeld DL, Tomiyama AJ. Jab my arm, not my morality: perceived moral reproach as a barrier to COVID-19 vaccine uptake. Soc Sci Med. 2022 Feb;294:114699. 10.1016/j.socscimed.2022.114699
8. Bardosh K, de Figueiredo A, Gur-Arie R, Jamrozik E, Doidge J, Lemmens T, et al. The unintended consequences of COVID-19 vaccine policy: why mandates, passports and restrictions may cause more harm than good. BMJ Glob Health. 2022 May;7(5):e008684. 10.1136/bmjgh-2022-008684
9. Schmitt MT, Branscombe NR, Postmes T, Garcia A. The consequences of perceived discrimination for psychological well-being: a meta-analytic review. Psychol Bull. 2014 Jul;140(4):921–48. 10.1037/a0035754
10. Baldassarre A, Giorgi G, Alessio F, Lulli LG, Arcangeli G, Mucci N. Stigma and Discrimination (SAD) at the Time of the SARS-CoV-2 Pandemic. Int J Environ Res Public Health. 2020 Aug;17(17):6341. 10.3390/ijerph17176341
11. Rewerska-Juśko M, Rejdak K. Social Stigma of Patients Suffering from COVID-19: Challenges for Health Care System. Healthcare (Basel). 2022 Feb;10(2):292. 10.3390/healthcare10020292
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct;370(9596):1453–7. 10.1016/S0140-6736(07)61602-X
13. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013 Apr;84(4):465–70. 10.1016/j.resuscitation.2012.12.016
14. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. 10.1016/0021-9681(87)90171-8
15. Peek ME, Nunez-Smith M, Drum M, Lewis TT. Adapting the everyday discrimination scale to medical settings: reliability and validity testing in a sample of African American patients. Ethn Dis. 2011;21(4):502–9.
16. Williams DR, Yan Yu, Jackson JS, Anderson NB. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J Health Psychol. 1997 Jul;2(3):335–51. 10.1177/135910539700200305
17. Benjamins MR, Middleton M. Perceived discrimination in medical settings and perceived quality of care: A population-based study in Chicago. PLoS One. 2019 Apr;14(4):e0215976. 10.1371/journal.pone.0215976
18. Bird ST, Bogart LM. Perceived race-based and socioeconomic status(SES)-based discrimination in interactions with health care providers. Ethn Dis. 2001;11(3):554–63.
19. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983 Jun;67(6):361–70. 10.1111/j.1600-0447.1983.tb09716.x
20. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002 Feb;52(2):69–77. 10.1016/S0022-3999(01)00296-3
21. EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990 Dec;16(3):199–208. 10.1016/0168-8510(90)90421-9
22. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997 Nov;35(11):1095–108. 10.1097/00005650-199711000-00002
23. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009 Jun;338 jun29 1:b2393. 10.1136/bmj.b2393
24. Chew CC, Lim XJ, Chang CT, Rajan P, Nasir N, Low WY. Experiences of social stigma among patients tested positive for COVID-19 and their family members: a qualitative study. BMC Public Health. 2021 Sep;21(1):1623. 10.1186/s12889-021-11679-8
25. Claudy MC, Vijayakumar S, Campbell N. Reckless spreader or blameless victim? How vaccination status affects responses to COVID-19 patients. Soc Sci Med. 2022 Jul;305:115089. 10.1016/j.socscimed.2022.115089
26. Koontalay A, Suksatan W, Prabsangob K, Sadang JM. Healthcare Workers’ Burdens During the COVID-19 Pandemic: A Qualitative Systematic Review. J Multidiscip Healthc. 2021 Oct;14:3015–25. 10.2147/JMDH.S330041
27. Mutambudzi M, Niedwiedz C, Macdonald EB, Leyland A, Mair F, Anderson J, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020 Dec;78(5):307–14. 10.1136/oemed-2020-106731
28. Rahmani K, Shavaleh R, Forouhi M, Disfani HF, Kamandi M, Oskooi RK, et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front Public Health. 2022 Aug;10:873596. 10.3389/fpubh.2022.873596
29. Druml C, Czech H. A pandemic is no private matter: the COVID-19 vaccine mandate in Austria. Lancet Respir Med. 2022 Apr;10(4):322–4. 10.1016/S2213-2600(22)00063-7
30. King J, Ferraz OL, Jones A. Mandatory COVID-19 vaccination and human rights. Lancet. 2022 Jan;399(10321):220–2. 10.1016/S0140-6736(21)02873-7
31. Shaw D. Vaccination status and intensive care unit triage: is it fair to give unvaccinated Covid-19 patients equal priority? Bioethics. 2022 Oct;36(8):883–90. 10.1111/bioe.13069
32. UNDP. 2020 Gender Social Norms Index (GSNI). UNDP. United Nations Development Programme; 2020.
33. Todorov A, Kaufmann F, Arslani K, Haider A, Bengs S, Goliasch G, et al.; Swiss Society of Intensive Care Medicine. Gender differences in the provision of intensive care: a Bayesian approach. Intensive Care Med. 2021 May;47(5):577–87. 10.1007/s00134-021-06393-3
34. Samulowitz A, Gremyr I, Eriksson E, Hensing G. “Brave Men” and “Emotional Women”: A Theory-Guided Literature Review on Gender Bias in Health Care and Gendered Norms towards Patients with Chronic Pain. Pain Res Manag. 2018 Feb;2018:6358624. 10.1155/2018/6358624
35. Reichmuth ML, Heron L, Riou J, Moser A, Hauser A, Low N, et al. Socio-demographic characteristics associated with COVID-19 vaccination uptake in Switzerland: longitudinal analysis of the CoMix study. BMC Public Health. 2023 Aug;23(1):1523. 10.1186/s12889-023-16405-0
36. Batbaatar E, Dorjdagva J, Luvsannyam A, Savino MM, Amenta P. Determinants of patient satisfaction: a systematic review. Perspect Public Health. 2017 Mar;137(2):89–101. 10.1177/1757913916634136
37. Spaccatini F, Giovannelli I, Pacilli MG. “You are stealing our present”: younger people’s ageism towards older people predicts attitude towards age-based COVID-19 restriction measures. J Soc Issues. 2022 Aug;78(4):769–89. 10.1111/josi.12537
38. Chutiyami M, Cheong AM, Salihu D, Bello UM, Ndwiga D, Maharaj R, et al. COVID-19 Pandemic and Overall Mental Health of Healthcare Professionals Globally: A Meta-Review of Systematic Reviews. Front Psychiatry. 2022 Jan;12:804525. 10.3389/fpsyt.2021.804525
39. Delgado N, Bonache H, Betancort M, Morera Y, Harris LT. Understanding the Links between Inferring Mental States, Empathy, and Burnout in Medical Contexts. Healthcare (Basel). 2021 Feb;9(2):158. 10.3390/healthcare9020158
40. Lluch C, Galiana L, Doménech P, Sansó N. The Impact of the COVID-19 Pandemic on Burnout, Compassion Fatigue, and Compassion Satisfaction in Healthcare Personnel: A Systematic Review of the Literature Published during the First Year of the Pandemic. Healthcare (Basel). 2022 Feb;10(2):364. 10.3390/healthcare10020364
41. Fox J, Meisenberg B. The 3-Fold Harms of Compassion Fatigue During COVID-19 Surges. Am J Med. 2022 Aug;135(8):e234–5. 10.1016/j.amjmed.2022.01.023
42. Trivedi AN, Ayanian JZ. Perceived discrimination and use of preventive health services. J Gen Intern Med. 2006 Jun;21(6):553–8. 10.1111/j.1525-1497.2006.00413.x
43. Bazargan M, Cobb S, Assari S. Discrimination and Medical Mistrust in a Racially and Ethnically Diverse Sample of California Adults. Ann Fam Med. 2021;19(1):4–15. 10.1370/afm.2632
44. Razai MS, Osama T, McKechnie DG, Majeed A. Covid-19 vaccine hesitancy among ethnic minority groups. BMJ. 2021 Feb;372(513):n513. 10.1136/bmj.n513
45. Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021 Apr;39(15):2024–34. 10.1016/j.vaccine.2021.02.005
46. Becker C, Beck K, Zumbrunn S, Memma V, Herzog N, Bissmann B, et al. Long COVID 1 year after hospitalisation for COVID-19: a prospective bicentric cohort study. Swiss Med Wkly. 2021 Oct;151(4142):w30091. 10.4414/SMW.2021.w30091