Pharmacometric in silico studies used to facilitate a national dose standardisation
process in neonatology – application to amikacin
DOI: https://doi.org/https://doi.org/10.57187/s.3632
Verena Gottaabc,
Julia Anna Bielickide,
Paolo Paioniaf,
Chantal Csajkaagh,
Dominic Stefan Brämb,
Christoph Bergerfi,
Elisabeth Gigeri,
Michael Buettcherabj,
Klara M. Posfay-Barbek,
John van den Ankerb,
Marc Pfisterb
a SwissPedDose/SwissPedNet collaboration expert team,
Zürich/Basel/Lausanne, Switzerland
b Pediatric
Pharmacology and Pharmacometrics, University of Basel Children's Hospital,
Basel, Switzerland
c Pediatric
Clinical Pharmacy, University of Basel Children’s Hospital, Basel Switzerland
d Paediatric
Research Centre and Paediatric Infectious Diseases and Vaccinology Division,
University of Basel Children's Hospital, Basel, Switzerland
e Centre
for Neonatal and Paediatric Infection, St George’s University, London, United
Kingdom
f Division
of Infectious Diseaeses, University Children’s Hospital Zurich, Zurich,
Switzerland
g Centre
for Research and Innovation, University Hospital and University of Lausanne,
Lausanne, Switzerland
h School
of Pharmaceutical Sciences, University of Geneva and University of Lausanne,
Geneva/Lausanne, Switzerland
i SwissPedDose,
Zurich, Switzerland
j Paediatric
Infectious Diseases, Lucerne Children's Hospital, Cantonal Hospital Lucerne, and
Faculty of Health Sciences and Medicine, University Lucerne, Lucerne, Switzerland
k General
Pediatrics and Pediatric Infectious Diseases Unit, Department of Woman, Child
and Adolescent, University Hospitals of Geneva and Medical School of Geneva, Geneva,
Switzerland
Summary
BACKGROUND AND AIMS: Pharmacometric in silico approaches are frequently applied
to guide decisions concerning dosage regimes during the development of new
medicines. We aimed to demonstrate how such pharmacometric modelling and
simulation can provide a scientific rationale for optimising drug doses in the
context of the Swiss national dose standardisation project in paediatrics using
amikacin as a case study.
METHODS: Amikacin neonatal dosage is stratified
by post-menstrual age (PMA) and post-natal age (PNA) in Switzerland and many
other countries. Clinical concerns have been raised for the subpopulation of neonates
with a post-menstrual age of 30–35 weeks and a post-natal age of 0–14 days (“subpopulation
of clinical concern”), as potentially oto-/nephrotoxic trough concentrations (Ctrough
>5 mg/l) were observed with a once-daily dose of 15 mg/kg. We applied a two-compartmental
population pharmacokinetic model (amikacin clearance depending on birth weight
and post-natal age) to real-world demographic data from 1563 neonates receiving
anti-infectives (median birth weight 2.3 kg, median post-natal age six days) and
performed pharmacometric dose-exposure simulations to identify extended dosing intervals
that would ensure non-toxic Ctrough (Ctrough <5 mg/l) dosages
in most neonates.
RESULTS: In the subpopulation of clinical
concern, Ctrough <5 mg/l was predicted in 59% versus 79–99% of
cases in all other subpopulations following the current recommendations. Elevated
Ctrough values were associated with a post-natal age of less than
seven days. Simulations showed that extending the dosing interval to ≥36 h in the
subpopulation of clinical concern increased the
frequency of a desirable Ctrough below 5 mg/l to >80%.
CONCLUSION: Pharmacometric in silico studies using high-quality real-world
demographic data can provide a scientific rationale for national paediatric dose
optimisation. This may increase clinical acceptance of fine-tuned standardised dosing
recommendations and support their implementation, including in vulnerable
subpopulations.
Introduction
Neonatal dosing with anti-infective drugs is
highly variable at both national [1, 2]
and international levels [3] and across neonatal
treatment guidelines [4]. In Switzerland,
the Swiss database for dosing medicinal products in paediatrics (SwissPedDose, https://www.swisspeddose.ch/database)
aims to standardise drug dosing in paediatrics at a national level.
Recommendations consider the currently applied dosing regimens, the latest
scientific evidence, clinical experience, and expert opinion [5, 6]. Pharmacometric
modelling and simulation
is a recognised approach to guiding dosage decisions in the development of new
medicines and post-marketing drug optimisation [7,
8]. Modelling may also provide a scientific rationale for neonatal and paediatric
dosing approaches in areas of uncertainty [9–12].
We present a motivational case study to illustrate and discuss the potential
broader usefulness, prerequisites, and implementation in the context of a
national dose standardisation effort.
Motivational case study
In Switzerland, amikacin is a frequently
used aminoglycoside antibiotic with activity against gentamicin-resistant
bacteria [13, 14]. Amikacin is mostly used
in combination with a β-lactam antibiotic as a first-line empirical treatment
for suspected neonatal sepsis [15]. During
the national standardisation process, a dosing approach according to post-menstrual
age (PMA, <30/30–35/35–44 weeks, defined as gestational age plus
chronological post-natal age) and post-natal age (PNA, 0–14/≥14 days) was
proposed by SwissPedDose after a literature review, defining six subpopulations
(table 1) [6]. In the preterm
subpopulation with a post-menstrual age of 30–35 weeks, a 24-hour vs 36-hour
dosing interval was discussed, with the decision of a 24 hour dosing interval disregarding
post-natal age for practical reasons. Clinical concerns regarding this approach
were raised by neonatologists for the preterm subpopulation #3 (table 1)
defined by a post-menstrual age of 30–35 weeks and a post-natal age <14 days
(hereafter referred to as the subpopulation of clinical concern), as elevated trough
concentrations (Ctrough) were observed in a significant proportion
of patients. For this reason, clinicians contacted SwissPedDose and requested an
evaluation of extended dosing intervals such as 36 hours and 48 hours to
mitigate the risk of elevated Ctrough values associated with
potential nephro-/ototoxicity, with a defined safety threshold as Ctrough
<5 mg/l [6, 16].
Table 1Neonatal subpopulations (Popsub),
dosing approach (according to initial SwissPedDose recommendations), and
real-life demographic data from n = 1563 neonates receiving antibiotics
(primary analysis population). n: number of neonates per subpopulation
available (primary analysis population, including those with complete
demographic data with respect to birthweight and postmenstrual age).
Demographic data are given as median [interquartile range].
| Popsub |
Post-menstrual
age (weeks) |
Post-natal
age (days) |
Dosing
approach |
n |
Weight
(kg) |
Post-natal
age (days) |
Birthweight
(kg) |
Post-menstrual
age (weeks) |
| 1 |
<30 |
<14 |
15
mg/kg every 48 hours |
188 |
0.94
[0.74, 1.14] |
4
[2, 7] |
0.98
[0.75, 1.17] |
27.9
[26.1, 29.1] |
| 2 |
<30 |
≥14 |
15
mg/kg every 24 hours |
70 |
0.84
[0.71, 1.01] |
19
[16, 24] |
0.74
[0.64, 0.92] |
28.1
[26.8, 29.1] |
| 3* |
30–35 |
<14 |
15
mg/kg every 24 hours* |
309 |
1.61
[1.34, 1.99] |
4
[2, 7] |
1.66
[1.38, 2.01] |
32.4
[31.3, 33.4] |
| 4 |
30–35 |
≥14 |
15
mg/kg every 24 hours |
96 |
1.29
[1.06, 1.53] |
20
[16, 23] |
1.15
[0.95, 1.41] |
31.9
[30.7, 33.4] |
| 5 |
35–44 |
<14 |
15
mg/kg every 24 hours |
705 |
3.03
[2.64, 3.53] |
4
[2, 8] |
3.04
[2.62, 3.54] |
39.3
[37.4, 40.6] |
| 6 |
35–44 |
≥14 |
15(–20**)
mg/kg every 24 hours |
195 |
2.88
[2.24, 3.51] |
19
[15, 22] |
2.72
[2.20, 3.31] |
40.1
[38.0, 41.7] |
Challenges addressed by pharmacometric in silico studies
To the best of our knowledge, a dosing
approach based on post-menstrual age and post-natal age has not been formally evaluated
for amikacin regarding the suitability to achieve commonly used exposure
targets [13, 17, 18], including safety
targets associated with low risks of oto-/nephrotoxicity [17]. Although post-menstrual
age and post-natal
age have been shown to influence amikacin clearance in various population
pharmacokinetic analyses, none of the reported models provides a description of
clearance based on the combination of post-menstrual age and post-natal age [18, 19].
This is in line with characterisations of kidney function maturation that
determines amikacin clearance: although post-menstrual age and post-natal age are
important determinates of post-natal kidney function compared to other
demographic factors, they have not been used in combination to describe the maturation
of renal amikacin clearance in preterm neonates [20–23].
Pharmacometric in silico studies can
systematically predict pharmacological expectations of amikacin clearance as a
function of birth weight and post-natal age to clinical exposure expectations. They
can be performed (figure 1) leveraging a suitable model combined with a large representative
neonatal demographic dataset (regarding the distribution of and correlation between
weight, birth weight, post-natal age, and post-menstrual age). Various dosing approaches,
including extended dose intervals, can be simulated, and the percentage of neonatal
patients achieving the clinical target of interest derived for each
subpopulation of interest. Model-informed drug development (MIDD) has become a
standard approach in the pharmaceutical and biotech industries and is expected
by health authorities such as the Food and Drug Administration (FDA) and European
Medicines Agency (EMA), particularly in the context of development, approval,
and utilisation of new medicines in paediatrics [24,
25]. In addition, applying a “model-informed dosing” approach to support
efforts to optimise and standardise drug dosing in clinical paediatric practice
has gained interest in other countries [26].
The pharmacometric approach used to support the Swiss neonatal dose standardisation
of amikacin, our motivational case example, will be described in more detail in
the following sections, which may serve as a proof-of-concept for further cases.
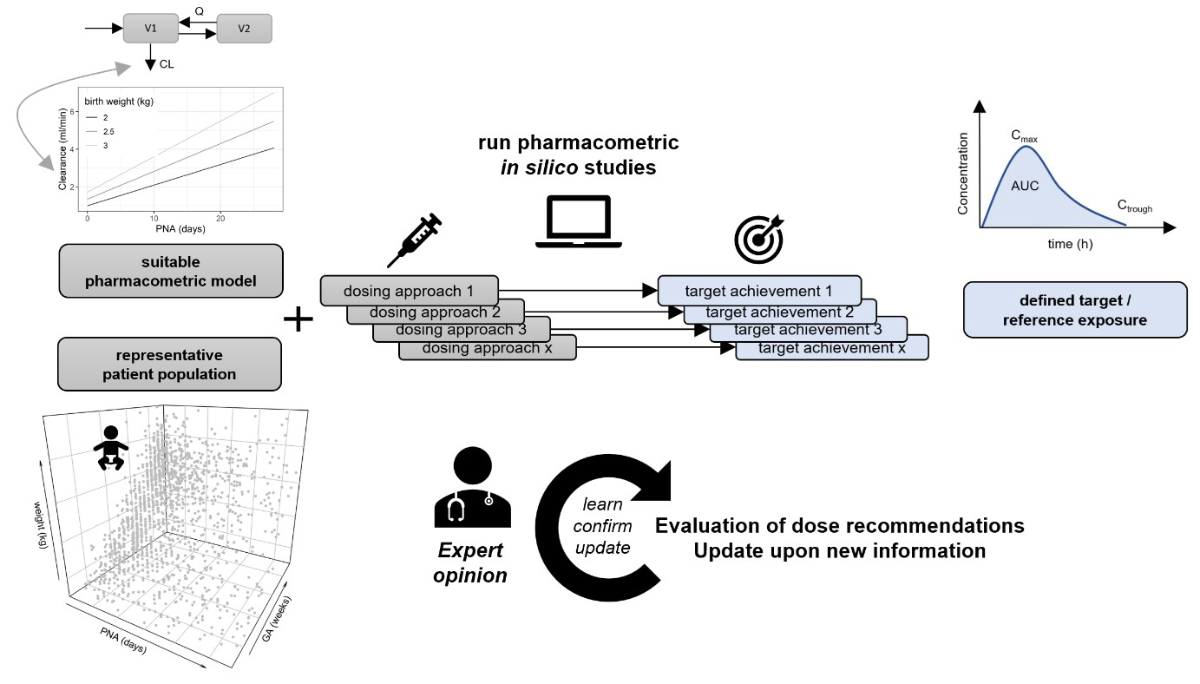
Figure 1Implementation of pharmacometric in silico studies in
neonatal dose standardisation. The general prerequisites for pharmacometric guided
dose
evaluation are (1) the availability of at least one suitable pharmacometric
model for the population of interest (e.g., a population pharmacokinetic or
pharmacokinetic/pharmacodynamic model), (2) the possibility of defining a
representative patient population with relevant covariate information (i.e.,
for the pharmacometric model employed, and considered for different dosing
approaches), and (3) the definition of a quantitative target or reference
outcome (e.g., therapeutic area-under the curve [AUC], trough concentration
[Ctrough], peak concentration [Cmax], or general adult reference exposure). The
illustrated example represents dose-exposure simulations (as shown for the
motivational example of amikacin), but these may be extended to
dose-exposure-response simulations in the case of available pharmacodynamic
models. Proposed in silico studies are not meant to be unidirectional, but are
ideally part of a “learn, confirm, and update” cycle and should be weighted by
expert opinion. PNA: post-natal age; GA: gestational age; CL:
clearance.
Methods
We applied pharmacometric approaches to
assess overall expected exposure target achievement (percentage of neonates with
Ctrough <5 mg/l) under the initially proposed recommended dosage
and to evaluate extended dosing intervals (36 hours and 48 hours instead of 24
hours) to quantify the improvement in target achievement in the neonatal subpopulation
of concern.
As this was a virtual experiment, there was
no clinical trial registration, and the study was deemed exempt from ethics
approval.
Neonatal real-world dataset
Demographic covariate data were taken from the
Antibiotic Resistance and Prescribing in European Children (ARPEC) point
prevalence study [27, 28], an anonymous
population of paediatric and neonatal in-patients treated with antibiotics at 8
A.M. in a one-day cross-sectional international web-based survey. For this
study, only neonates with post-natal age <28 days and weight <5 kg (to
exclude potential erroneous outliers) were included. Post-menstrual age (weeks)
was calculated as gestational age (weeks) + post-natal age (days) / 7. The
total selected neonatal population was stratified into six subpopulations
according to post-menstrual age (<30 weeks, 30–35 weeks, 35–44 weeks) and post-natal
age (0–14 days, and ≥14 days), in line with current dosage recommendation of SwissPedDose
(table 1). A total of 500 individuals were randomly sampled with replacement
from each neonatal subpopulation.
The primary pharmacometric analysis
included only patients with complete information for relevant covariates (post-natal
age, gestational age, weight, and birth weight). Sensitivity analyses were
performed including all patients after imputation of missing covariates (birth weight,
gestational age) as described below.
Pharmacometric model
An extensive externally evaluated and
updated two-compartmental pharmacokinetic model [16]
describing amikacin pharmacokinetics in neonates was used in which typical
pharmacokinetic parameters were set as a function of demographic covariates: typical
clearance (CLtyp) as a function of birth weight and post-natal age, and
the typical central and peripheral volumes of the distribution (V1typ,
V2typ) as a function of weight. The model also accounted for
clearance decreasing during whole-body cooling (therapeutic hypothermia [TH], binary
with 1 = yes,
0 = no) and ibuprofen (IBU, binary with 1 = yes, 0 = no) treatment [16]:
CLtyp [l/h] = CLpop [l/h] × (birthweight [kg]/1.75 kg)1.34 × (1 + 0.22 post-natal age
[days]/2 days) × 0.838IBU × 0.594TH, with CLpop = 0.0495
l/h
V1typ [l] = V1pop [l]
× (weight [kg]/1.75 kg)0.93 = V2typ, with V1pop
= 0.832 l
Inter-compartmental clearance (Qtyp)
was defined as proportional to CLtyp (Qtyp = 0.45 × CLtyp).
The model further described the effects of ibuprofen and therapeutic
hypothermia on amikacin clearance (associated with 16% and 41% decreases in clearance,
respectively). These treatment-associated variables were not used in the
primary analysis, as it was assumed that the population of interest generally
does not receive ibuprofen or therapeutic
hypothermia (i.e., IBU = 0 and TH = 0). The remaining random inter-individual
variability (between-subject variability) was 0.32 (standard deviation on a
log-scale) in clearance, and not quantified for other pharmacokinetic
parameters.
Demographic characteristics of the neonatal
populations on which the model was developed and externally evaluated are
summarised in table S1 in the appendix. Briefly, an initial model [29] was developed
using a total of 874
neonates [30, 31] and externally evaluated
on a total of 239 neonates [32, 33]. The
respective model parameter estimates were similar when re-estimated from
another population of 573 neonates [34].
The structure of the initial model [29] was
further refined by the inclusion of additional therapeutic drug monitoring data
of 56 neonates (the model applied herein), which allowed consideration of the
effect of therapeutic hypothermia on amikacin clearance [16], again yielding almost
identical model parameter estimates as
previously reported [29].
Pharmacometric in
silico studies: dose-exposure simulations
Individual concentration-time profiles were
generated by Monte Carlo simulations, given the covariates and random
inter-individual variability in amikacin clearance. The individual predicted Ctrough
before the third dose was then extracted (the usual sampling time in clinical
practice), and the percentage (%) of neonates with Ctrough <5 mg/l
was calculated for each of the six subpopulations (the goal being at least 80%
with a desirable Ctrough in all neonatal subpopulations).
Individually predicted Ctrough values
were plotted against patient demographics to evaluate their potential correlation
with elevated Ctrough >5 mg/l.
Initially proposed standardised dosing
approach
A total of 500 neonates were simulated for each
of the six neonatal populations to calculate the percentage of neonates with Ctrough
before the third dose with the initially proposed approach.
Evaluation of alternative dosing
intervals
Two additional simulations were performed
for the subpopulation of clinical concern to calculate the percentage of
neonates with Ctrough before the third dose with alternative dosing
intervals of 36 h or 48 h.
Sensitivity analyses
The following sensitivity analyses were
performed: (a) pharmacometric simulations using a dataset with missing
covariates of birth weight and gestational age imputed and obtained by three
methods: (a1) linear regression, and multiple imputation by (a2) predictive
mean matching or (a3) random forest (supplemental methods in the appendix);
(b) pharmacometric simulations, including residual intra-individual variability;
(c) simulations adding hypothetical inter-individual variability in the
distribution (standard deviation = 0.1 for log-transformed parameters) and a
small correlation of 0.3 between individual random effects of the distribution
and clearance; (d) pharmacometric simulations for the same population treated
with therapeutic hypothermia (TH = 1) to decrease the neurological sequelae of perinatal
asphyxia; (e) simulations for the same population receiving ibuprofen (IBU = 1)
treatment for a patent ductus arteriosus.
Software packages
In
silico studies were performed using the software
Simulx (Version 2021R2, Lixoft SAS, a Simulations Plus company). Data
preparation and further statistical computing or figure creation were performed
in R (version 4.2.1, R Foundation for Statistical Computing, https://www.R-project.org/).
Results
Neonatal real-world dataset
From the available demographic ARPEC
database (comprising 3844 patients), 2590 neonates were eligible (post-natal
age <28 days, post-menstrual age <44 weeks, weight <5 kg); among these,
1563 had complete demographic data with respect to gestational age/post-menstrual
age and birth weight and were included in the primary analysis (table 1 and figure
2; overall median [IQR] post-natal age: 6 [3–13 days], weight: 2.3 [1.39, 3.15]
kg). In most other patients with missing covariates (n = 1027, median [IQR] post-natal
age: 8 [4–16], weight: 2.80 [1.80–3.40] kg), both gestational age and birth weight
were missing (n = 918).
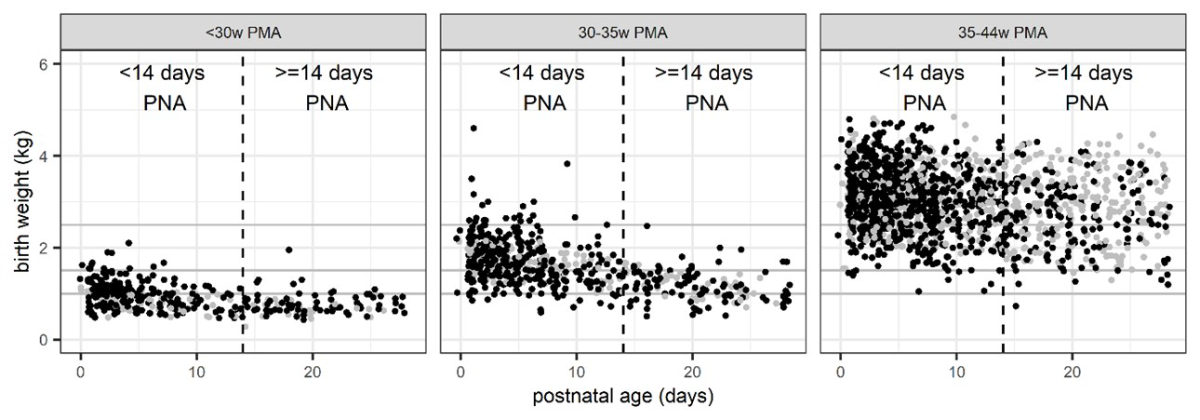
Figure 2Illustration of real-life patient population
demographic data used as a basis for simulations: birth weight versus postnatal
age (PNA) as clearance-relevant covariates depicted by postmenstrual age (PMA)
according to the SwissPedDose recommendations. Post-natal age values are
slightly jittered for easier visual assessment. Black dots: complete
data. Grey dots: imputed data with respect to post-menstrual age and
birth weight (method a1).
Pharmacometric model
Simulated individual pharmacokinetic
parameters for each subgroup are depicted in figure S3 in the appendix. The corresponding
simulated mean
half-lives were 6.6 h and 5.3 h in subpopulations 1 and 3, respectively, and
2.2–3.7 h in the other subpopulations (initial exponential decline), suggesting
that Ctrough sampling before the third dose likely corresponds to a
steady-state measurement in the plasma.
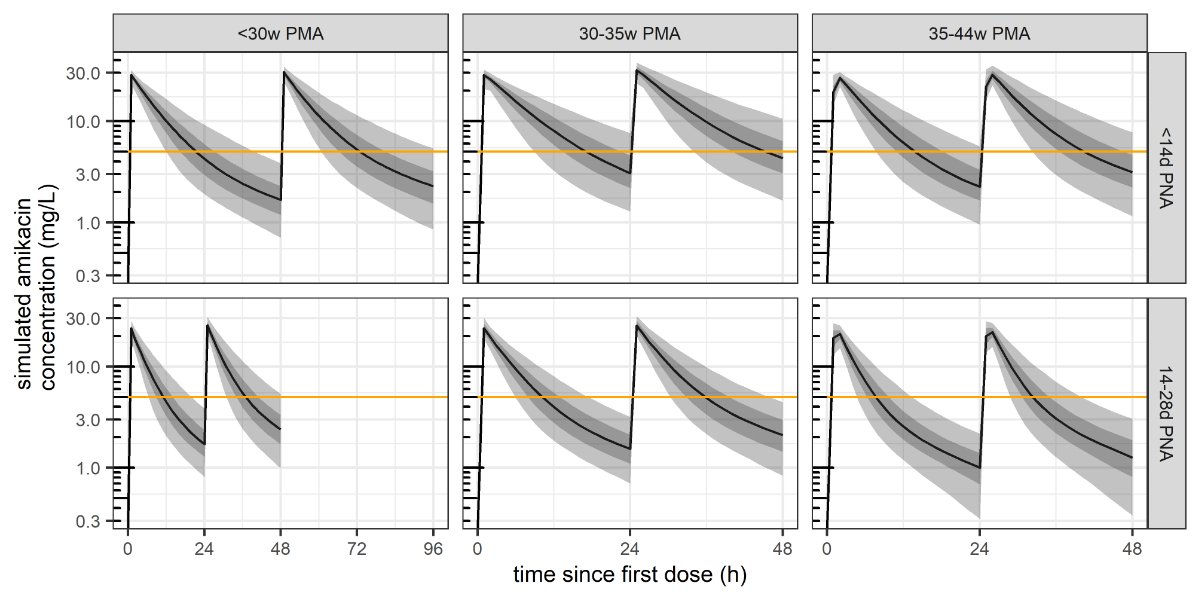
Figure 3Illustration of a simulated amikacin
concentration-time profile distribution for each of the six subpopulations of
the primary analysis (where complete covariate data were available), following
two administrations of the dose according to the initial national SwissPedDose
recommendations. Shaded areas delimit the 50% prediction interval (percentile
25–75, dark grey) and 90% prediction interval (percentile 5–95, light
grey). Orange line: Ctrough target of <5 mg/l. PMA:
post-menstrual age; PNA: post-natal age.
Pharmacometric dose-exposure simulations
The distribution of predicted amikacin exposure
under the initially agreed harmonised dosing approach is shown in figure 3. The
corresponding predicted distribution of Ctrough before the third dose
is shown in figure 4 and table 2.
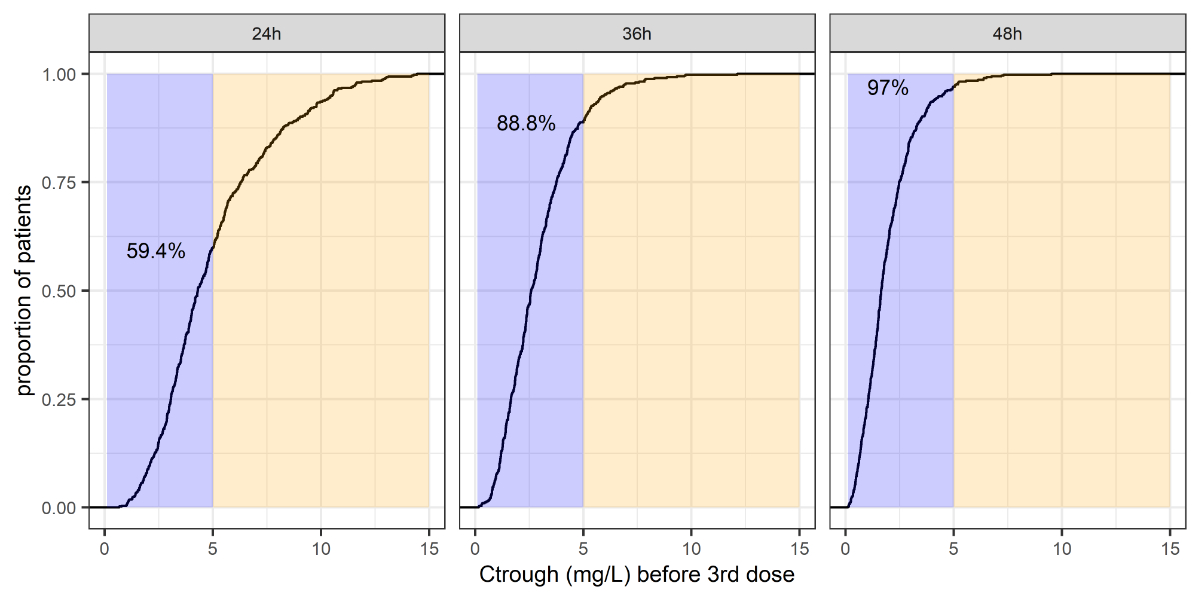
Figure 4Summary of the Ctrough distribution before
the third dose, and the predicted proportion of patients with Ctrough
<5 mg/l (i.e., the violet shaded area, with the proportions summarised by
numbers).
Table 2Predicted percentages with trough <5 mg/l
before the third dose (primary and sensitivity analyses d and e*).
| Popsub |
Post-menstrual
age (weeks) |
Post-natal
age (days) |
Dosing
approach |
Primary
analysis (complete data set) |
Sensitivity
analysis d (therapeutic hypothermia) |
Sensitivity
analysis e (ibuprofen) |
| 1 |
<30 |
<14 |
15
mg/kg every 48 h |
93.4 |
56 |
84 |
| 2 |
<30 |
≥14 |
15
mg/kg every 24 h |
92.6 |
41 |
83 |
| 3** |
30–35 |
<14 |
15
mg/kg every 24 h** |
59.4 |
15 |
45 |
|
|
|
15
mg/kg every 36 h |
88.8 |
46 |
79 |
|
|
|
15
mg/kg every 48 h |
97.0 |
74 |
93 |
| 4 |
30–35 |
≥14 |
15
mg/kg every 24 h |
98.6 |
62 |
89 |
| 5 |
35–44 |
<14 |
15
mg/kg every 24 h |
79.0 |
30 |
66 |
| 6 |
35–44 |
≥14 |
15
mg/kg every 24 h |
99.4 |
88 |
98 |
Initially agreed harmonised dosing
approach
The simulated proportion of neonates with Ctrough
<5 mg/l before the third dose was 59% (95%CI: 55–64%) in the subpopulation of
clinical concern, and 79% (75–82%) to 99% (98–100%) in the other
subpopulations. The correlations between elevated Ctrough and
patient demographics in the subpopulation of clinical concern are depicted in figure
5.
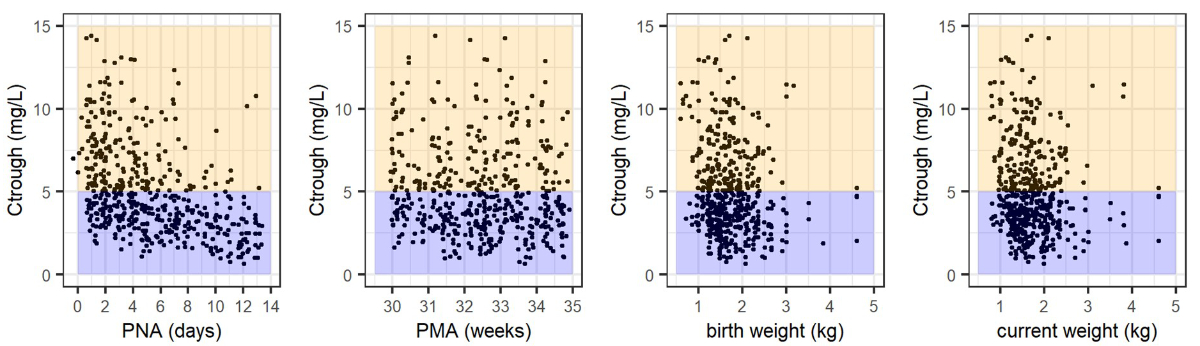
Figure 5Inspection of the predicted Ctrough distribution in
the subpopulation of clinical concern following administration of one dose
every 24 h (preterm neonates post-menstrual age (PMA) 30–35 weeks, post-natal
age (PNA) <14d) according to patient demographics. Violet shaded
area: Ctrough <5 mg/l. Yellow shaded area: Ctrough
≥5 mg/l.
Evaluation of alternative dosing
intervals
When extending the dosing interval in the subpopulation
of clinical concern from 24 h to 36 h or 48 h, the simulated percentages of
neonates with a desirable Ctrough increased to 89% (86–91%) and 97%
(95–98%), respectively.
Sensitivity analyses
The proportion of patients with predicted individual
Ctrough values <5 mg/l before the third dose are summarised in table
S2 (a–c, see appendix) and table 2 (d–e). Briefly, predicted proportions from
sensitivity analyses a, b, and c were similar to those of the primary analysis.
In sensitivity analysis d (therapeutic hypothermia), all proportions of target
achievement were lower, with only the subpopulation of late preterm or term neonates
outside the newborn period (post-menstrual age 35–44 weeks, post-natal age ≥14
days) achieving Ctrough <5 mg/l in at least 80% of neonates
(89.9% versus 15.6–59.8% in the other subpopulations under the initially proposed
dosing regimen). In sensitivity analysis e (ibuprofen treatment), all
proportions of target achievement were lower, but most subpopulations still
achieved Ctrough <5 mg/l in at least 80% of neonates.
Discussion
We applied pharmacometric in silico approaches in the context of a
national dose standardisation process in neonatology to illustrate their
utility and prerequisites. For our motivational example of amikacin, all three
prerequisites for performing a pharmacometric simulation study were met (figure
1). First, a suitable, externally evaluated population pharmacokinetic model
was available for the drug and population of interest [16]. Second, representative
demographic patient population data
could be taken from a large real-world data set for neonates treated with antibiotics
[27]. Third, the clinical target exposure
of interest was quantitatively defined (Ctrough <5 mg/l), as was
the outcome of interest (percentage of neonatal patients achieving the target: ≥80%).
In our case, this approach confirmed the clinically
observed risk of amikacin accumulation with elevated Ctrough (>5 mg/l
prior to the third dose) in a neonatal subpopulation of clinical concern (preterm
neonates with post-menstrual age 30–35 weeks, post-natal age 0–14 days) following
the introduction of a new nationally standardised dose recommendation (i.e., a 24
h-interval with a dosage of 15 mg/kg). Simulations provided a scientific,
quantitative rationale indicating that extension of the dosing interval to 36 h
would be sufficient for similar target achievement as in the other neonatal subpopulations
for whom no safety concerns were raised. An extended dosing interval had
already been discussed for a subgroup of preterm neonates during the
SwissPedDose standardisation process but was finally set to 24 hours for
practical reasons. To simplify dosing across different subpopulations and to guarantee
sufficient peak plasma concentration (Cmax), which is considered
relevant for aminoglycoside efficacy [16],
a reduction of the dose was not discussed. Figure 3 demonstrates that simulated
Cmax remained nearly constant despite the prolongation of the dosing
interval. The results presented herein show that performing such pharmacometric
simulations can facilitate scientific discussions by formulating and providing
model-based pharmacological predictions. This can help to better define optimal
dosing recommendations among clinical experts, in particular where study data
are sparse or missing or when discussions are controversial or have not reached
a consensus. Our simulations suggest that elevated Ctrough values were
indeed highly associated with post-natal age <7 days (<50% achieving Ctrough
<5 mg/l versus >80% for 7–14 days, figure 5) facilitating
dose standardisation towards this cutoff, in line with the approach chosen for several
other renally eliminated anti-infectives such as gentamicin and beta-lactam
antibiotics [6].
As a result of developmental and maturational
processes, neonatal dosing is a complex process. On one hand, optimised dosing
approaches in neonatology should consider not only safety and efficacy aspects
but also quickly developing physiology and pharmacokinetics. On the other hand,
it is crucial that dosing be practical and simple to mitigate the risk of
medication errors in this vulnerable patient population [35–37]. In the development
of new medicines, similar pharmacometric
simulation approaches are regularly applied to translate initial complex
approaches to dosing (e.g., based on body surface area) into simpler and more practical
(e.g., stratified fixed dose) recommendations [7,
38, 39]. As our understanding of neonatal pharmacology is constantly
increasing for many anti-infective drugs [40–43],
it is time to bring such quantitative pharmacological knowledge into clinical
practice, particularly in the context of national dose standardisation initiatives
such as SwissPedDose.
Neonatal real-world dataset
Especially in the neonatal population,
generating a virtual patient population with a representative multidimensional
covariate distribution may be a challenge. Whereas the general neonatal
population would show a uniform post-natal age distribution that can easily be
simulated, our real-live demographic data show that post-natal age distribution
in neonates treated with antibiotics is skewed to the left (i.e., an over-representation
of neonates with post-natal age <7 days). Given the rapid improvement in kidney
function and renal drug clearance with post-natal age, the predicted percentage
of patients with amikacin Ctrough <5 mg/l (the defined safety
target) would increase if more neonates with older post-natal age were included.
Utilizing a large real-world demographic dataset of a representative population
has the advantage of fewer assumptions concerning a complex covariate distribution
and correlations but may require the handling of missing demographic data [44]. In
our case, neonates with missing
information on birth weight and gestational age most likely represented
neonates that were treated on a general paediatric ward (older gestational age
and post-natal age, higher body weight as compared to “complete-information”
neonates). Optimal dosing approaches may hence differ between neonates treated
on general paediatric versus neonatal wards as well as in patients receiving
additional potentially nephrotoxic drugs or therapeutic hypothermia
(sensitivity analyses d–e).
In our simulations, we assumed that the covariates
did not change over time. Our simulations may hence be considered conservative,
as renal clearance improves with each day of life and increasing weight in
neonates. A combination with neonatal weight-prediction models [45] could be of interest
in other studies
regarding questions as to when to adapt the dosing strategy in an individual
patient.
Pharmacometric model
A suitable pharmacometric model may not be
available for all dosing questions, or several candidate models may be
available and compared regarding their predictions [46]. In cases where no suitable
model is available for the drug
and population of interest, physiology-based pharmacokinetic models may be considered
to formulate semi-quantitative exposure comparisons for different neonatal or paediatric
subgroups, which then may be updated quantitatively upon the availability of
actual pharmacokinetic data by population pharmacokinetic modelling [8]. Ideally,
not only dose-exposure but also
exposure-outcome simulations can be performed, such as for clinical cure, bacterial
killing, and resistance development [47].
Pharmacodynamic studies in neonates, however, remain scarce due to practical
and ethical difficulties [8].
In our case, we did not compare simulations
from other models [18, 19], but the model
we used represents by far the most extensively evaluated neonatal model, having
additionally demonstrated favourable predictive performance regarding
post-natal renal function maturation [20].
A large number of trough and peak amikacin concentration measurements were used
to develop the model, suggesting its suitability for Ctrough prediction.
Pharmacometric in
silico studies
In general, many different exposure
questions can be addressed, but clinical limitations with respect to formulated
targets need to be kept in mind for final dosing decisions, as illustrated in figure
1 by expert opinion relevance (e.g., in our case uncertainty concerning the actual
risk of oto-/nephrotoxicity associated with short-term high Ctrough,
an optimal Ctrough cutoff to define “elevated” Ctrough values
under varying dosing intervals, and duration of post-antibiotic effect [48–50]). Simulations
may be easily adjusted
and updated if new evidence emerges regarding exposure targets, such as
increasing minimal inhibitory concentration (MIC), as has been observed for
gentamicin [12, 51]. Improved
infrastructures allowing the collection and use of real-world data for
scientific purposes [52] will further
facilitate the development and evaluation of such model-informed dosing
approaches and their implementation in a learn-and-confirm cycle (figure 1).
In our case, simulations may also be used to
evaluate amikacin Cmax, a key parameter related to aminoglycoside
efficacy [16]; Cmax >20 mg/l
was achieved in the majority of patients (minimum efficacy target used in other
studies [40]). As no random inter-individual variability was incorporated in the model
applied, a factor that might be
expected, this outcome may require a more cautious interpretation. Interestingly,
however, the proportion of patients achieving Cmax >20 mg/l was least
in the “oldest” subpopulation (i.e., post-menstrual age 35–44 weeks, post-natal
age 14–28 days) treated with single doses of 15 mg/mg, supporting the use of
single doses up to 20 mg/kg in this subpopulation (table 1). Antibiotic
coverage for efficacy (normally with a target ratio of Cmax / minimal inhibitory concentration >8–10)
may be more important in the first days of treatment compared to the risk of
short-term drug accumulation [53].
Conclusion
In conclusion, pharmacometric in silico studies based on high-quality real-world
demographic datasets can provide a quantitative scientific rationale and rating
for dose optimisation and facilitate national dose standardisation, particularly
in neonatology. In the present motivational example, this approach allowed us to
translate pharmacological expectations for amikacin based on birth weight and post-natal
age to exposure predictions stratified by post-natal age and post-menstrual age
for various dosing intervals. Further, computer simulations indicated that a post-natal
age stratification ≤7 or >7 days may be considered in the future, in line with dosing
recommendations for other renally eliminated anti-infective drugs employed in
neonatology. Implementation of pharmacometrics into the decision-making process
of neonatal dose standardisation should not be unidirectional, but rather part
of a continuous learn-and-confirm process combining scientific evidence, clinical
experience, and expert opinion (figure 1). In addition to facilitating the
standardisation of existing neonatal dose recommendations, such an iterative
approach will increase clinical acceptance of fine-tuned dose recommendations
and support their implementation.
Data availability statement
All demographic and simulated data are presented
in the manuscript/supplemental material. Further inquiries can be directed to
the corresponding authors.
Acknowledgments
This project was realised thanks to close
collaboration between the team establishing the Swiss database for dosing
medicinal products in paediatrics (SwissPedDose; www.swisspeddose.ch) and the Swiss
Research Network of Clinical Pediatric Hubs (SwissPedNet). SwissPedDose is
supported by the Swiss Federal Office of Public Health (FOPH). We thank Herman
Goossens and Ann Versporten, University of Antwerp and ARPEC project group, for
providing the ARPEC data set. We thank clinical SwissPedDose and SwissPedNet experts,
in particular Matteo Fontana (harmonisation expert neonatology) for initiating
discussions on the presented case example.
Author contributions: Conception and Design: Clinical question of
case example formulated by SwissPedDose experts: EG (SwissPedDose harmonisation
specialist, neonatology), MB and CB (SwissPedDose harmonisation experts
paediatric infectious diseases). Design of scientific approach: VG, PP, CC, MP (SwissPedDose/SwissPedNet
collaboration expert team). Data acquisition: JB. Analysis: VG, DB.
Interpretation of the data and approach: all. Drafting the article: VG with JB,
PP, CC, MP. Revising the manuscript: all.
Verena Gotta
Pediatric Pharmacology and Pharmacometrics
University Children’s Hospital Basel
CH-4000 Basel
verena.gotta[at]ukbb.ch
References
1 Leroux S, Zhao W, Bétrémieux P, Pladys P, Saliba E, Jacqz-Aigrain E; French Society
of Neonatology. Therapeutic guidelines for prescribing antibiotics in neonates should
be evidence-based: a French national survey. Arch Dis Child. 2015 Apr;100(4):394–8.
10.1136/ARCHDISCHILD-2014-306873 10.1136/archdischild-2014-306873
2 Kadambari S, Heath PT, Sharland M, Lewis S, Nichols A, Turner MA. Variation in gentamicin
and vancomycin dosage and monitoring in UK neonatal units. J Antimicrob Chemother.
2011 Nov;66(11):2647–50. 10.1093/jac/dkr351
3 Metsvaht T, Nellis G, Varendi H, Nunn AJ, Graham S, Rieutord A, et al. High variability
in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence
study: implications for research and dissemination. BMC Pediatr. 2015 Apr;15(1):41.
10.1186/s12887-015-0359-y
4 Liem TB, Slob EM, Termote JU, Wolfs TF, Egberts AC, Rademaker CM. Comparison of antibiotic
dosing recommendations for neonatal sepsis from established reference sources. Int
J Clin Pharm. 2018 Apr;40(2):436–43. 10.1007/s11096-018-0589-9
5 Tilen R, Panis D, Aeschbacher S, Sabine T, Meyer Zu Schwabedissen HE, Berger C. Development
of the Swiss Database for dosing medicinal products in pediatrics. Eur J Pediatr.
2022 Mar;181(3):1221–31. 10.1007/S00431-021-04304-8 10.1007/s00431-021-04304-8
6 SwissPedDose Association SwissPedDose. National pediatric drug doses. https://swisspeddose.ch/
7 Mehrotra N, Bhattaram A, Earp JC, Florian J, Krudys K, Lee JE, et al. Role of Quantitative
Clinical Pharmacology in Pediatric Approval and Labeling. Drug Metab Dispos. 2016 Jul;44(7):924–33.
10.1124/DMD.116.069559 10.1124/dmd.116.069559
8 Vinks AA, Emoto C, Fukuda T. Modeling and simulation in pediatric drug therapy: application
of pharmacometrics to define the right dose for children. Clin Pharmacol Ther. 2015 Sep;98(3):298–308.
10.1002/cpt.169
9 Pfiffner M, Berger-Olah E, Vonbach P, Pfister M, Gotta V. Pharmacometric Analysis
of Intranasal and Intravenous Nalbuphine to Optimize Pain Management in Infants. Front
Pediatr. 2022 Mar;10:837492. 10.3389/FPED.2022.837492 10.3389/fped.2022.837492
10 Pfiffner M, Gotta V, Pfister M, et al (2022) Pharmacokinetics and tolerability of
intranasal or intravenous administration of nalbuphine in infants. Arch Dis Child
archdischild-2022-323807. https://doi.org/10.1136/archdischild-2022-323807
11 van Donge T, Samiee-Zafarghandy S, Pfister M, Koch G, Kalani M, Bordbar A, et al. Methadone
dosing strategies in preterm neonates can be simplified. Br J Clin Pharmacol. 2019 Jun;85(6):1348–56.
10.1111/BCP.13906 10.1111/bcp.13906
12 van Donge T, Pfister M, Bielicki J, Csajka C, Rodieux F, van den Anker J, et al. Quantitative
analysis of gentamicin exposure in neonates and infants calls into question its current
dosing recommendations. Antimicrob Agents Chemother. 2018 Mar;62(4):e02004-17. 10.1128/AAC.02004-17
13 Darlow CA, da Costa RM, Ellis S, Franceschi F, Sharland M, Piddock L, et al. Potential
Antibiotics for the Treatment of Neonatal Sepsis Caused by Multidrug-Resistant Bacteria.
Paediatr Drugs. 2021 Sep;23(5):465–84. 10.1007/S40272-021-00465-Z 10.1007/s40272-021-00465-z
14 Ramirez MS, Tolmasky ME. Amikacin: Uses, Resistance, and Prospects for Inhibition.
Molecules. 2017 Dec;22(12):2267. 10.3390/MOLECULES22122267 10.3390/molecules22122267
15 Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017 Oct;390(10104):1770–80.
10.1016/S0140-6736(17)31002-4
16 Cristea S, Smits A, Kulo A, Knibbe CA, van Weissenbruch M, Krekels EH, et al. Amikacin
Pharmacokinetics To Optimize Dosing in Neonates with Perinatal Asphyxia Treated with
Hypothermia. Antimicrob Agents Chemother. 2017 Nov;61(12):e01282–17. 10.1128/AAC.01282-17
17 Jenkins A, Thomson AH, Brown NM, Semple Y, Sluman C, MacGowan A, et al.; BSAC Working
Party on Therapeutic Drug Monitoring. Amikacin use and therapeutic drug monitoring
in adults: do dose regimens and drug exposures affect either outcome or adverse events?
A systematic review. J Antimicrob Chemother. 2016 Oct;71(10):2754–9. 10.1093/JAC/DKW250 10.1093/jac/dkw250
18 Wilbaux M, Fuchs A, Samardzic J, Rodieux F, Csajka C, Allegaert K, et al. Pharmacometric
Approaches to Personalize Use of Primarily Renally Eliminated Antibiotics in Preterm
and Term Neonates. J Clin Pharmacol. 2016 Aug;56(8):909–35. 10.1002/jcph.705
19 Illamola SM, Sherwin CM, van Hasselt JG. Clinical Pharmacokinetics of Amikacin in
Pediatric Patients: A Comprehensive Review of Population Pharmacokinetic Analyses.
Clin Pharmacokinet. 2018 Oct;57(10):1217–28. 10.1007/s40262-018-0641-x
20 Wu Y, Allegaert K, Flint RB, Simons SH, Krekels EH, Knibbe CA, et al. Prediction of
glomerular filtration rate maturation across preterm and term neonates and young infants
using inulin as marker. AAPS J. 2022 Feb;24(2):38. 10.1208/S12248-022-00688-Z/FIGURES/4 10.1208/s12248-022-00688-z
21 Salem F, Johnson TN, Hodgkinson AB, Ogungbenro K, Rostami-Hodjegan A. Does “Birth”
as an Event impact maturation trajectory of renal clearance via glomerular filtration?
Reexamining data in preterm and full-term neonates by avoiding the creatinine bias.
J Clin Pharmacol. 2021 Feb;61(2):159–71. 10.1002/jcph.1725
22 Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal
function maturation: a quantitative description using weight and postmenstrual age.
Pediatr Nephrol. 2009 Jan;24(1):67–76. 10.1007/s00467-008-0997-5
23 van Donge T, Allegaert K, Gotta V, Smits A, Levtchenko E, Mekahli D, et al. Characterizing
dynamics of serum creatinine and creatinine clearance in extremely low birth weight
neonates during the first 6 weeks of life. Pediatr Nephrol. 2021 Mar;36(3):649–59.
10.1007/S00467-020-04749-3 10.1007/s00467-020-04749-3
24 Bi Y, Liu J, Li L, Yu J, Bhattaram A, Bewernitz M, et al. Role of Model-Informed Drug
Development in Pediatric Drug Development, Regulatory Evaluation, and Labeling. J
Clin Pharmacol. 2019 Sep;59(S1 Suppl 1):S104–11. 10.1002/jcph.1478
25 Madabushi R, Seo P, Zhao L, Tegenge M, Zhu H. Review: Role of Model-Informed Drug
Development Approaches in the Lifecycle of Drug Development and Regulatory Decision-Making.
Pharm Res. 2022 Aug;39(8):1669–80. 10.1007/s11095-022-03288-w
26 Hartman SJ, Swaving JG, van Beek SW, van Groen BD, de Hoop M, van der Zanden TM, et
al. A New Framework to Implement Model-Informed Dosing in Clinical Guidelines: Piperacillin
and Amikacin as Proof of Concept. Front Pharmacol. 2020 Dec;11:592204. 10.3389/FPHAR.2020.592204 10.3389/fphar.2020.592204
27 Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H; ARPEC project group.
The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point
prevalence survey: developing hospital-quality indicators of antibiotic prescribing
for children. J Antimicrob Chemother. 2016 Apr;71(4):1106–17. 10.1093/jac/dkv418
28 Versporten A, Sharland M, Bielicki J, Drapier N, Vankerckhoven V, Goossens H; ARPEC
Project Group Members. The antibiotic resistance and prescribing in European Children
project: a neonatal and pediatric antimicrobial web-based point prevalence survey
in 73 hospitals worldwide. Pediatr Infect Dis J. 2013 Jun;32(6):e242–53. 10.1097/INF.0b013e318286c612
29 De Cock RF, Allegaert K, Schreuder MF, Sherwin CM, de Hoog M, van den Anker JN, et
al. Maturation of the glomerular filtration rate in neonates, as reflected by amikacin
clearance. Clin Pharmacokinet. 2012 Feb;51(2):105–17. 10.2165/11595640-000000000-00000
30 Allegaert K, Scheers I, Cossey V, Anderson BJ. Covariates of amikacin clearance in
neonates: the impact of postnatal age on predictability. Drug Metab Lett. 2008 Dec;2(4):286–9.
10.2174/187231208786734157
31 Allegaert K, Anderson BJ, Cossey V, Holford NH. Limited predictability of amikacin
clearance in extreme premature neonates at birth. Br J Clin Pharmacol. 2006 Jan;61(1):39–48.
10.1111/j.1365-2125.2005.02530.x
32 Sherwin CM, Svahn S, Van der Linden A, Broadbent RS, Medlicott NJ, Reith DM. Individualised
dosing of amikacin in neonates: a pharmacokinetic/pharmacodynamic analysis. Eur J
Clin Pharmacol. 2009 Jul;65(7):705–13. 10.1007/S00228-009-0637-4 10.1007/s00228-009-0637-4
33 Schreuder MF, Wilhelm AJ, Bökenkamp A, Timmermans SM, Delemarre-van de Waal HA, van
Wijk JA. Impact of gestational age and birth weight on amikacin clearance on day 1
of life. Clin J Am Soc Nephrol. 2009 Nov;4(11):1774–8. 10.2215/CJN.02230409
34 Smits A, De Cock RF, Allegaert K, Vanhaesebrouck S, Danhof M, Knibbe CA. Prospective
Evaluation of a Model-Based Dosing Regimen for Amikacin in Preterm and Term Neonates
in Clinical Practice. Antimicrob Agents Chemother. 2015 Oct;59(10):6344–51. 10.1128/AAC.01157-15
35 Gotta V, van den Anker J, Pfister M. [Understanding and reducing the risk of adverse
drug reactions in pediatric patients]. Ther Umsch. 2015 Dec;72(11-12):679–86. 10.1024/0040-5930/a000737
36 Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al. Medication
errors and adverse drug events in pediatric inpatients. JAMA. 2001 Apr;285(16):2114–20.
10.1001/jama.285.16.2114
37 Kaushal R, Goldmann DA, Keohane CA, Abramson EL, Woolf S, Yoon C, et al. Medication
errors in paediatric outpatients. Qual Saf Health Care. 2010 Dec;19(6):e30. 10.1136/qshc.2008.031179
38 Vinks AA, Emoto C, Fukuda T. Modeling and simulation in pediatric drug therapy: application
of pharmacometrics to define the right dose for children. Clin Pharmacol Ther. 2015 Sep;98(3):298–308.
10.1002/cpt.169
39 Hong Y, Kowalski KG, Zhang J, Zhu L, Horga M, Bertz R, et al. Model-based approach
for optimization of atazanavir dose recommendations for HIV-infected pediatric patients.
Antimicrob Agents Chemother. 2011 Dec;55(12):5746–52. 10.1128/AAC.00554-11
40 Wilbaux M, Fuchs A, Samardzic J, Rodieux F, Csajka C, Allegaert K, et al. Pharmacometric
Approaches to Personalize Use of Primarily Renally Eliminated Antibiotics in Preterm
and Term Neonates. J Clin Pharmacol. 2016 Aug;56(8):909–35. 10.1002/JCPH.705 10.1002/jcph.705
41 Dao K, Fuchs A, André P, Giannoni E, Decosterd LA, Marchetti O, et al. Dosing strategies
of imipenem in neonates based on pharmacometric modelling and simulation. J Antimicrob
Chemother. 2022 Feb;77(2):457–65. 10.1093/JAC/DKAB394 10.1093/jac/dkab394
42 Dao K, Guidi M, André P, Giannoni E, Basterrechea S, Zhao W, et al. Optimisation of
vancomycin exposure in neonates based on the best level of evidence. Pharmacol Res.
2020 Apr;154:104278. 10.1016/J.PHRS.2019.104278 10.1016/j.phrs.2019.104278
43 van Donge T, Fuchs A, Leroux S, Pfister M, Rodieux F, Atkinson A, et al. Amoxicillin
Dosing Regimens for the Treatment of Neonatal Sepsis: Balancing Efficacy and Neurotoxicity.
Neonatology. 2020;117(5):619–27. 10.1159/000509751
44 Bräm DS, Nahum U, Atkinson A, Koch G, Pfister M. Evaluation of machine learning methods
for covariate data imputation in pharmacometrics. CPT Pharmacometrics Syst Pharmacol.
2022 Dec;11(12):1638–48. 10.1002/PSP4.12874 10.1002/psp4.12874
45 Wilbaux M, Kasser S, Wellmann S, Lapaire O, van den Anker JN, Pfister M. Characterizing
and Forecasting Individual Weight Changes in Term Neonates. J Pediatr. 2016 Jun;173:101–107.e10.
10.1016/J.JPEDS.2016.02.044 10.1016/j.jpeds.2016.02.044
46 Gotta V, Buclin T, Csajka C, Widmer N. Systematic review of population pharmacokinetic
analyses of imatinib and relationships with treatment outcomes. Ther Drug Monit. 2013 Apr;35(2):150–67.
10.1097/FTD.0b013e318284ef11
47 Alhadab AA, Ahmed MA, Brundage RC. Amikacin Pharmacokinetic-Pharmacodynamic Analysis
in Pediatric Cancer Patients. Antimicrob Agents Chemother. 2018 Mar;62(4):e01781-17.
10.1128/AAC.01781-17
48 Rao SC, Srinivasjois R, Moon K. One dose per day compared to multiple doses per day
of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database
Syst Rev. 2016 Dec;12(12):CD005091. 10.1002/14651858.CD005091.PUB4 10.1002/14651858.CD005091.pub4
49 Hanberger H, Edlund C, Furebring M, G Giske C, Melhus A, Nilsson LE, et al.; Swedish
Reference Group for Antibiotics. Rational use of aminoglycosides—review and recommendations
by the Swedish Reference Group for Antibiotics (SRGA). Scand J Infect Dis. 2013 Mar;45(3):161–75.
10.3109/00365548.2012.747694
50 Craig WA. Optimizing aminoglycoside use. Crit Care Clin. 2011 Jan;27(1):107–21. 10.1016/J.CCC.2010.11.006 10.1016/j.ccc.2010.11.006
51 Paioni P, Jäggi VF, Tilen R, Seiler M, Baumann P, Bräm DS, et al. Gentamicin population
pharmacokinetics in pediatric patients—a prospective study with data analysis using
the saemix package in r. Pharmaceutics. 2021 Oct;13(10):1596. 10.3390/PHARMACEUTICS13101596/S1 10.3390/pharmaceutics13101596
52 Goers R, Coman Schmid D, Jäggi VF, Paioni P, Okoniewski MJ, Parker A, et al. SwissPKcdw
- A clinical data warehouse for the optimization of pediatric dosing regimens. CPT
Pharmacometrics Syst Pharmacol. 2021 Dec;10(12):1578–87. 10.1002/PSP4.12723 10.1002/psp4.12723
53 van Donge T, Bielicki JA, van den Anker J, Pfister M. Key Components for Antibiotic
Dose Optimization of Sepsis in Neonates and Infants. Front Pediatr. 2018 Oct;6:325.
10.3389/FPED.2018.00325 10.3389/fped.2018.00325
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3632.




