Successful treatment of immune checkpoint inhibitor-related periaortitis
DOI: https://doi.org/https://doi.org/10.57187/s.3631
Elias D. Bührera,
Ian L. Albertsb,
Lisa Christa,
Berna C. Özdemirc
a Department of Rheumatology and Immunology, Inselspital, Bern University Hospital,
University of Bern, Bern, Switzerland
b Department of Nuclear Medicine, Inselspital, Bern University Hospital, University
of Bern, Bern, Switzerland
c Department of Oncology, Inselspital, Bern University Hospital, University of Bern,
Bern,
Switzerland
Summary
We report a
64-year-old patient with melanoma receiving ipilimumab and nivolumab therapy
who presented with a periaortic soft tissue mass around the abdominal aorta on
restaging fluorodeoxyglucose positron emission tomography/computed tomography
imaging. Clinical, laboratory, and radiologic findings resulted in a diagnosis
of immune checkpoint inhibitor-related periaortitis. Periaortitis is a rare
disease presenting with fibro-inflammatory tissue around the aorta and may lead
to serious complications. Immune checkpoint inhibitors were discontinued, and
the patient was treated with glucocorticoids, leading to a complete resolution
of the periaortitis. To our knowledge, this is only the third reported case of immune
checkpoint inhibitor-related periaortitis.
Case report
Chronic periaortitis is a rare disease characterised by fibro-inflammatory tissue
around the aorta that is more common in males and those aged 40–60 years. It commonly
involves the abdominal aorta. Because of its unspecific symptoms, such as lower back
or abdominal pain, and rarity, its diagnosis is frequently delayed, and patients might
present with complications such as urethral obstruction, vascular stenosis or aneurysm.
Periaortitis may be idiopathic or secondary to infections, drugs, or malignancies
[1]. Furthermore, immunoglobulin G4 (IgG4)-related disease can present as periaortitis
[2].
Immune
checkpoint inhibitors have revolutionised cancer treatment and can lead to
long-lasting remission, even in patients with metastatic disease [3]. However,
immune-related adverse events might limit their use. The most common immune-related
adverse events include dermatitis, colitis, hepatitis and thyroiditis. Cancer
patients routinely receive follow-up imaging to determine treatment response. Consequently,
treatment-related side effects, such as periaortitis, can be detected early. To
our knowledge, only two cases of immune checkpoint inhibitor-related periaortitis
have been previously reported [4, 5]. Herein, we report a case of immune
checkpoint inhibitor-related periaortitis.
A 64-year-old
Caucasian male was diagnosed with local melanoma on the left flank (Breslow
thickness = 2.5 mm) in 2015. Five years later, he was diagnosed with metastatic
disease (B-Raf proto-oncogene serine/threonine kinase [BRAF] V600E-mutated, CD274
molecule [CD274/PD-L1] combined positive score = 80) and began combination
immunotherapy with ipilimumab and nivolumab. After the onset of immune
checkpoint inhibitor-associated colitis and hepatitis (no histological analysis
was performed), which resolved with glucocorticoid treatment, maintenance
therapy with nivolumab was continued during a partial remission with two small
brain metastases, one lung, two intramuscular, and one bone lesion. After 32
cycles of nivolumab (i.e. 16 months of treatment), restaging 2-deoxy-2-[fluorine-18]fluoro-D-glucose
(2-[18F]FDG) positron emission tomography (PET) / computed tomography
(CT) imaging revealed a new periaortic soft tissue mass (10 × 5 cm) with intensive
FDG uptake (max
standardised uptake value [SUV] = 8.3) along the distal abdominal aorta (figure
1A, B).
Clinically,
the patient was asymptomatic except for an unspecific dermatitis treated with
topical glucocorticoids. Histology of the exanthema revealed infiltration by
plasma cells (no increased IgG4 positivity) and eosinophils without evidence of
a sarcoid-like reaction. His current medications were levothyroxine, amlodipine,
and valsartan, which are not known to induce periaortitis [6]. Laboratory
work-up revealed an elevated C-reactive protein (CRP) level of 40 mg/l (normal:
<5 mg/l), an elevated erythrocyte sedimentation rate (ESR) of 81 mm/hour (normal:
0–20 mm/hour),
increased serum IgG4 concentration of 3.32 g/l (normal: 0.08–1.4 g/l), and
mildly reduced kidney function (creatinine = 110 µmol/l [normal: <104 µmol/l]).
The antinuclear antibody (ANA) titer was 1:80 (nuclear speckled) without
specific autoantibodies corresponding to the staining pattern (e.g. anti-Sjögren’s
syndrome [SS]-A, SS-B, myositis-associated antibodies, or Smith antigen [Sm])
in additional enzyme-linked immunosorbent assays. The antineutrophil cytoplasmic
antibodies (ANCA) test, infectious serologies (hepatitis B and C, HIV, and syphilis),
and QuantiFERON test were negative.
Radiological,
molecular imaging, and biological findings led us to suspect nivolumab-induced
periaortitis. A sarcoid-like reaction was unlikely given the atypical
periaortic location [7]. A biopsy was not obtained from this patient due to
potential complication risks. After a multidisciplinary discussion, nivolumab
was discontinued, and systemic glucocorticoid treatment (prednisolone
equivalent: 1 mg/kg body weight) resulted in complete imaging remission of the
soft tissue density within three months (figure 1C). After glucocorticoid
treatment, the patient remained in partial remission from melanoma without new lesions.
B-cell targeting approaches (e.g. rituximab) have shown efficacy in
IgG4-related disease [8] and may be used as a treatment option for periaortitis
in cases with relapsing disease after glucocorticoid taper.
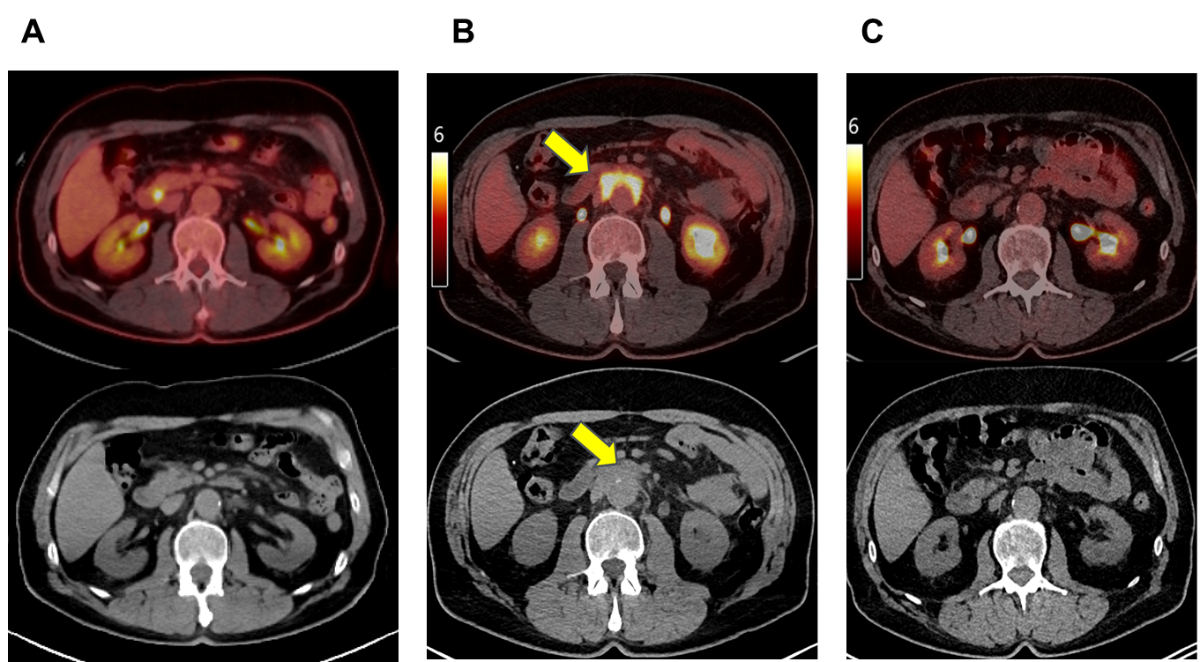
Figure 12-[18F]FDG PET/CT imaging findings. (A) A co-registered PET/CT and
non-contrast-enhanced CT show the aorta without periaortic soft tissue
abnormality before immune checkpoint inhibitor (ICI) therapy. (B) A
co-registered PET/CT and non-contrast-enhanced CT show an intensively FDG-enhanced
(max SUV = 8.3) periaortic soft tissue mass (10 × 5 cm) after ICI therapy. (C) Post-glucocorticoid
treatment PET/CT and non-contrast-enhancedCTshowtotal
regression of the periaortic soft tissue mass and FDG avidity.
The two previously reported cases of immune checkpoint inhibitor-induced periaortitis
occurred in male patients with lung cancer (aged 57 and 66 years) and a history of
aortic aneurysm and were related to nivolumab [4, 5]. In both patients, nivolumab
was discontinued, and the periaortitis regressed within eight weeks, suggesting a
drug-related process. Glucocorticoid treatment was added for one case. IgG4 levels
were reported for one case and appeared within the normal range. Our case’s IgG4 levels
were elevated, possibly implicating an IgG4-related process [2]. However, the temporal
relationship with immune checkpoint inhibitor treatment and previous immune-related
adverse events (colitis and hepatitis) led us to suspect a diagnosis of immune checkpoint
inhibitor-associated periaortitis. A biopsy would be needed to definitively rule out
IgG4-related disease. Immune checkpoint inhibitor-induced IgG4-related disease has
been previously reported, but none of the cases were associated with periaortitis
[9, 10].
Our patient
had a known history of atherosclerotic disease of the aorta with infrarenal
ectasia. Aortic aneurysms and atherosclerosis are associated with chronic
inflammation and might trigger chronic periaortitis [11, 12]. The immune
responses induced by immune checkpoint inhibitors can facilitate periaortitis
development.
In conclusion, periaortitis can be a late immune-related adverse event. Patients with
a history of aortic ectasia or aneurysm might be more susceptible to this immune-related
adverse event. Treatment options vary depending on the clinical presentation and include
discontinuation of immune checkpoint inhibitor treatment and additional corticosteroid
treatment.
Acknowledgments
An Informed consent has been obtained from the patient.
Berna C. Özdemir
Freiburgstrasse 18
CH-3011 Bern
berna.oezdemir[at]insel.ch
References
1. Marvisi C, Accorsi Buttini E, Vaglio A. Aortitis and periaortitis: the puzzling spectrum
of inflammatory aortic diseases. Presse Med. 2020 Apr;49(1):104018. 10.1016/j.lpm.2020.104018
2. Mizushima I, Kasashima S, Fujinaga Y, Kawano M, Ishizaka N. IgG4-related periaortitis/periarteritis:
an under-recognized condition that is potentially life-threatening. Mod Rheumatol.
2019 Mar;29(2):240–50. 10.1080/14397595.2018.1546367
3. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun.
2020 Jul;11(1):3801. 10.1038/s41467-020-17670-y
4. Roy AK, Tathireddy HR, Roy M. Aftermath of induced inflammation: acute periaortitis
due to nivolumab therapy. BMJ Case Rep 2017/bcr-2017-221852. 10.1136/bcr-2017-221852
5. Hotta M, Naka G, Minamimoto R, Takeda Y, Hojo M. Nivolumab-induced periaortitis demonstrated
by FDG PET/CT. Clin Nucl Med. 2020 Nov;45(11):910–2. 10.1097/RLU.0000000000003215
6. Gianfreda D, Superchi E, Peyronel F, Mazzariol M, Vaglio A. Chronic periaortitis:
A clinical approach. Rev Med Interne. 2023 Feb;44(2):79–84. 10.1016/j.revmed.2022.11.009
7. Gkiozos I, Kopitopoulou A, Kalkanis A, Vamvakaris IN, Judson MA, Syrigos KN. Sar-
coidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018 Aug;13(8):1076–82.
10.1016/j.jtho.2018.04.031
8. Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, et al. Rituximab
for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015 Jun;74(6):1171–7.
10.1136/annrheumdis-2014-206605
9. Joob B, Wiwanitkit V. Immunoglobulin G4 associated autoimmune cholangitis and pancreatitis
and nivolumab. World J Clin Cases. 2022 Dec;10(35):13146–7. 10.12998/wjcc.v10.i35.13146
10. Terashima T, Iwami E, Shimada T, Kuroda A, Matsuzaki T, Nakajima T, et al. IgG4-related
pleural disease in a patient with pulmonary adenocarcinoma under durvalumab treatment:
a case report. BMC Pulm Med. 2020 Apr;20(1):104. 10.1186/s12890-020-1150-x
11. Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2045–51.
10.1161/ATVBAHA.108.179705
12. Jois RN, Gaffney K, Marshall T, Scott DG. Chronic periaortitis. Rheumatology (Oxford).
2004 Nov;43(11):1441–6. 10.1093/rheumatology/keh326
