Therapeutic management of fibrosis in systemic sclerosis patients – an analysis from
the Swiss EUSTAR cohort
DOI: https://doi.org/https://doi.org/10.57187/s.3630
Kevin Windirscha,
Suzana Jordana,
Mike Oliver Beckera,
Cosimo Brunia,
Rucsandra Dobrotaa,
Muriel Elhaia,
Ion-Alexandru Garaimana,
Carmen-Marina Mihaia,
Michele Iudicib,
Paul Haslerc,
Camillo Ribid,
Britta Maurere,
Armando Gabriellif,
Anna-Maria Hoffmann-Voldg,
Oliver Distlera
a Department of
Rheumatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland
b Division of Rheumatology, Geneva University Hospitals,
University of Geneva, Geneva, Switzerland
c Department of
Rheumatology,Kantonsspital Aarau, Aarau, Switzerland
d Department of Clinical Immunology and
Allergy, Centre hospitalier universitaire vaudois (CHUV), Lausanne, Switzerland
e Department of
Rheumatology, Inselspital, Bern University Hospital,
University of Bern, Bern, Switzerland
f Marche Polytechnic University, Institute of
Clinical Medicine, University of Ancona, Ancona, Italy
g Department of
Rheumatology, Rikshospitalet University Hospital, Oslo, Norway
Summary
OBJECTIVES: Systemic sclerosis is a chronic autoimmune
connective tissue disease leading to microvascular and fibrotic manifestations
in multiple organs. Several treatment options and recommendations from
different European countries are available. In this study, for which the ambit
is Switzerland specifically, we aim to describe the treatment patterns of systemic
sclerosis patients with fibrotic manifestations.
METHODS: Systemic sclerosis patients were selected from six Swiss tertiary centres
recorded in the multicentre, prospective European Scleroderma Trials and
Research (EUSTAR) registry. Patients fulfilling the 2013 ACR/EULAR systemic
sclerosis classification criteria at baseline were included. To determine the
differences in treatment of varying degrees of fibrosis, four groups were identified:
(1) patients with a modified Rodnan skin score (mRSS) >0; (2) those with
mRSS ≥7; (3) those with interstitial lung disease (SSc-ILD), diagnosed by
either chest X-Ray or high-resolution computed tomography; and (4) patients
fulfilling one of the additional criteria for extensive interstitial lung
disease, defined as interstitial lung disease involvement of >20% in high-resolution
computed tomography, dyspnea NYHA-stage 3/4, or a predicted forced vital
capacity (FVC) of <70%.
RESULTS: A total of 590 patients with systemic sclerosis fulfilled the inclusion
criteria. In this cohort, 421 (71.4%) had mRSS >0, of whom 195 (33.1%) had
mRSS ≥7; interstitial lung disease was diagnosed in 198 of 456 (43.4%), of whom
106 (18.0 %) showed extensive interstitial lung disease. Regarding non-biologic
disease-modifying medications (DMARDs), the most frequently prescribed was
methotrexate, followed by hydroxychloroquine and mycophenolate mofetil.
Rituximab and tocilizumab were most frequently used among the biologic DMARDs. Specifically,
148/372 (39.8%) of treated patients with skin fibrosis received methotrexate, mycophenolate
mofetil or rituximab, and 80/177 (45.2%) with interstitial lung disease
received cyclophosphamide, mycophenolate mofetil, tocilizumab or rituximab. Most
patients received a proton-pump inhibitor, and few patients underwent hematopoietic
stem cell transplantation.
CONCLUSION: Overall, in Switzerland, a wide range of medications is prescribed for
systemic
sclerosis patients. This includes modern, targeted treatments for which randomised
controlled clinical trial have been recently reported.
Introduction
Systemic
sclerosis is an autoimmune connective tissue disease characterised by increased
deposition of extracellular matrix, resulting from fibroblast dysfunction,
microvasculopathy, and autoimmunity [1–5]. The organ manifestations and
clinical course of systemic sclerosis vary greatly, and this complicates its
monitoring and treatment [4, 6].
Recommendations
regarding its treatment have been published by both the European League Against
Rheumatism (EULAR) and the European Scleroderma Trials and Research (EUSTAR)
groups in 2009 and were then updated in 2017 [6, 7]. Furthermore, other
national societies, such as the British Society of Rheumatology (BSR) and
British Health Professionals in Rheumatology (BHPR), have also published
recommendations for treating systemic sclerosis [8, 9]. Despite significant
agreement between both guidelines, especially regarding organ manifestations,
the BSR guidelines offer more suggestions regarding non-pharmacologic treatment;
they also cover topics like calcinosis, musculoskeletal, and cardiac symptoms [8].
In addition, consensus guidance for SSc-ILD management has been published by
European experts [10].
For systemic
sclerosis-related skin fibrosis, the recommendations suggest that methotrexate
may be considered for early diffuse systemic sclerosis, given its effect on
skin fibrosis [6, 8, 11]. However, the evidence behind these recommendations should
be interpreted with some caution. A randomised controlled trial of methotrexate
versus a placebo in early diffuse systemic sclerosis showed a trend in favour
of methotrexate, but it did not indicate statistical significance [12]. Another
randomised controlled trial with methotrexate versus a placebo was
statistically significant for methotrexate in treating early skin fibrosis, but
most patients in the methotrexate group had limited systemic sclerosis with
less severe organ involvement; as such, an mRSS reduction could hardly be
deemed as clinically relevant [11]. Furthermore, both trials had a relatively small
sample size.
The
EULAR recommends considering cyclophosphamide for treating progressive SSc-ILD,
despite the medication’s toxicity [6, 13, 14]. However, studies showed that
once cyclophosphamide is discontinued, its beneficial effects decline [15]. Mycophenolate
mofetil demonstrated improvement versus baseline similar to cyclophosphamide in
a large randomised controlled trial for the following: Forced Vital Capacity
(FVC), the transition dyspnea index (TDI), some but not all quantitative
measures of lung fibrosis on high-resolution computed tomography, and the
modified Rodnan skin score (mRSS) for skin fibrosis [16–19].
In the
two trials, it was observed that randomised placebo-controlled trials with
tocilizumab indicated a trend of improving skin fibrosis and had a strong
effect on stabilizing interstitial lung disease [20–22]. There was also a non-significant,
but consistent directionality of efficacy regarding skin and interstitial lung
disease in the EUSTAR real-life cohort treated with tocilizumab [23]. The
RECITAL trial revealed that rituximab was not superior to cyclophosphamide, but it
did
suggest comparable effects to cyclophosphamide when treating patients diagnosed
with connective tissue disease with associated interstitial lung disease, including
systemic
sclerosis [24, 25]. Similarly,
a recent single country, smaller double blind, placebo-controlled, randomised trial
with rituximab showed a significant improvement of skin sclerosis and lung
function in systemic sclerosis without major safety concerns [26].
In carefully
selected patients with rapidly progressive systemic sclerosis and a risk of
organ failure, hematopoietic stem cell transplantation should also be considered
[6]. Studies showed substantial improvement in skin fibrosis and a general
stability of internal organ involvement, which is estimated to extend at least
three years and was associated with significantly improved quality of life [7,
8, 27–30]. Nevertheless, haematopoietic stem cell transplantation is still associated
with high treatment-related
mortality of around 10% [29, 30].
This
study’s aim was to analyse the therapeutic management of systemic sclerosis
patients in the Swiss EUSTAR cohort in light of current recommendations, with a
focus on advanced skin fibrosis and systemic sclerosis-related-interstitial
lung disease.
Patients and methods
Study
population and criteria
Systemic
sclerosis patients from all six EUSTAR Swiss expert centres (Aarau, Basel,
Bern, Geneva, Lausanne, and Zurich) were extracted from the multicentre,
prospective EUSTAR database and included in this analysis (exported on
26.07.2019). Characteristics of the Swiss EUSTAR cohort have been reported
recently [31]. All Swiss centres obtained ethics approval and all patients
signed informed consent forms. The Cantonal Ethics Committee Zurich (BASEC
Nr.2017-02102) approved the data analysis.
In the
present study, only systemic sclerosis patients’ visits between 2013 and 2019
were analysed, namely because the extended data on patients’ treatments were
collected from 2013 onwards in the EUSTAR database.
The European Scleroderma Trials and Research
group (EUSTAR)is an
international research network, which was launched in 2004, that seeks to
raise the awareness, understanding, research, and management of systemic
sclerosis throughout Europe and worldwide. The main research tool is a
multicentre online registry with prospectively collected data. More than 100
clinical, laboratory, and demographic data are collected annually, with
patients having signed informed consent forms first. Over 200 international
centres have contributed since 2004. Patients had to fulfil the 2013 ACR/EULAR systemic
sclerosis classification criteria at baseline [32].
Furthermore,
eligible patients were sub-categorised according to the extent of their skin
and interstitial lung disease at baseline. Regarding skin fibrosis, two
different patient groups were formed. The first cohort was comprised of all
patients with a modified Rodnan skin score (mRSS) >0, identifying those with
skin fibrosis in general; the second was comprised of those with more advanced
skin fibrosis, indicated by a mRSS of ≥7. This threshold was chosen because it
reflects the lowest value classifiable as diffuse cutaneous systemic sclerosis [4,
33].
The
presence of interstitial lung disease was determined by either chest X-Ray or
high-resolution computed tomography, as listed in the EUSTAR database. The expert
radiologist from the local centres, following the method described by either Goh
et al or by local practice, assessed the extent of interstitial lung disease [34].
A patient was presumed to have more advanced interstitial lung disease if, in
addition to showing interstitial lung disease on chest X-Ray or high-resolution
computed tomography, one of the following criteria applied: interstitial lung
disease extent of >20% on high-resolution computed tomography, dyspnea by
New York Heart Association (NYHA) stage 3/4, or predicted FVC of <70%.
Treatment
analysis
Potentially
disease-modifying medications prescribed for each patient at the baseline visit
were recorded. These medications, DMARDs, included immunomodulatory medications
(hydroxychloroquine, intravenous immunoglobulins), conventional
immunosuppressives (azathioprine, cyclosporin A, cyclophosphamide,
D-penicillamine, leflunomide, methotrexate, mycophenolate mofetil, and glucocorticoids
[e.g., prednisone, sulfasalazine]), and biological DMARDs (abatacept,
rituximab, TNF-alpha antagonists, and tocilizumab). Prednisone was considered a
DMARD in doses >10 mg/d [35].
In
addition, haematopoietic stem cell transplantation lung transplantation, oxygen
supplementation and proton-pump inhibitor usage were recorded, the latter
because gastro-oesophageal reflux disease is hypothesised to initiate and
progress interstitial lung disease [6, 8, 36, 37]. The use frequency of each treatment
was compared with the above-mentioned sub-groups.
Statistical
analysis
For this
observational descriptive study, sample size calculation was not performed. All
available data from Swiss EUSTAR centres were used for this analysis. All
statistical analyses were performed using SPSS statistics version 25 software
(IBM). Data were expressed as frequencies and percentages for categorical
variables, or as a median and interquartile range (IQR) for continuous
variables according to their distribution. Continuous variables were compared
with the Mann–Whitney U test or t-test, and categorical variables with
Chi-square test or Fisher’s exact test, as applicable.
Results
Study
population
Patient
selection is summarised in figure 1.

Figure 1Patient selection overview. Of the 17,212 patients in the EUSTAR database at
the time of export, 812 were from Swiss EUSTAR centres (Aarau, Basel, Bern,
Geneva, Lausanne and Zurich). Because recording extended therapy data began in
2013, only data collected after 2013 were considered for analysis. Finally,
patients not meeting the 2013 ACR/EULAR classification criteria were excluded.
ACR:
American College of Rheumatology; EULAR: European league Against Rheumatism;
EUSTAR: European Scleroderma Trial and Research Group.
Among
812 Swiss patients in the EUSTAR database, 590 were eligible and their
demographic and clinical characteristics are listed in table 1. The population
was predominantly female (79.8%) with a median age of 68.0 (57–77) years. The median
disease duration was 6.0 (2–13) years, and most patients had been diagnosed
with limited cutaneous systemic sclerosis (74%).
Table 1Baseline demographic and clinical characteristics of the study cohort (n = 590).
Definitions of items and organ manifestation align with EUSTAR [7]. Data
of listed variables are presented as number (n)/total cases with available data
(N) (%). Disease duration was calculated as the difference between the dates of
the baseline visit and the first non-Raynaud’s symptom of the disease, as
reported by the patient. Pulmonary hypertension was judged on right heart
catheterisation (RHC). Active disease was defined as a score >3, determined
by calculating European Scleroderma Study Group disease activity indices for
systemic sclerosis, as proposed by Valentini [56].
| Demographics |
Median (IQR) |
Frequency (n/N)
(%) |
| Age, years (n = 590) |
68.0 (57–77) |
|
| Disease duration,
years (n = 538) |
6.0 (2–13) |
|
| Female |
|
471/590 (79.8%) |
| Male |
|
119/590 (20.2%) |
| Limited cutaneous systemic
sclerosis |
|
328/443 (74.0%) |
| Diffuse cutaneous systemic
sclerosis |
|
115/443 (26.0%) |
| Skin/vascular |
mRSS (n = 590) |
3 (0–9) |
|
| Raynaud’s Phenomenon |
|
563/586 (96.1%) |
| Digital ulcers |
|
189/516 (36.6%) |
| Active digital
ulcers |
|
66/516 (12.8%) |
| Pitting scars |
|
200/498 (40.1%) |
| Scleredema |
|
297/508 (58.5%) |
| Telangiectasia |
|
327/516 (63.4%) |
| Abnormal nailfold
capillaroscopy |
|
411/479 (85.8%) |
| Musculoskeletal |
Tendon friction rubs |
|
42/565 (7.4%) |
| Joint synovitis |
|
87/580 (15.0%) |
| Joint contractures |
|
170/573 (29.7%) |
| Muscle weakness |
|
71/578 (12.3%) |
| Gastrointestinal |
Esophageal symptoms |
|
315/582 (54.1%) |
| Stomach symptoms |
|
160/569 (28.1%) |
| Intestinal symptoms |
|
180/574 (31.4%) |
| Cardiopulmonary |
Dyspnea NYHA stage
1/2 |
|
486/543 (89.5%) |
| Dyspnea NYHA stage
3/4 |
|
57/543 (10.5%) |
| Diastolic
dysfunction |
|
150/481 (31.2%) |
| Pericardial effusion |
|
19/500 (3.8%) |
| Conduction blocks |
|
55/425 (12.9%) |
| LVEF <45% |
|
4/521 (0.8%) |
| PAH by RHC |
|
11/244 (4.5%) |
| Interstitial lung
disease on high-resolution computed tomography |
|
198/456 (43.4%) |
| Lung function |
| FVC, % predicted |
98 (83–111) |
|
| FEV1, % predicted |
94 (82–106) |
|
| TLC, % predicted |
100 (85–112) |
|
| DLCO, % predicted |
75 (61–88) |
|
| FVC <70%
predicted |
|
54/529 (10.2%) |
| DLCO <70%
predicted |
|
199/513 (38.8%) |
| Kidney |
Renal crisis |
|
12/584 (2.1%) |
| Laboratory parameters |
ANA positive |
|
525/537 (97.8%) |
| ACA positive |
|
230/490 (46.9%) |
| Anti-Scl-70 positive |
|
140/507 (27.6%) |
| Anti-RNA-polymerase
III positive |
|
51/433 (11.8%) |
| Creatinine kinase
elevation |
|
61/536 (11.4%) |
| Proteinuria |
|
55/542 (10.1%) |
| ESR >25 mm/h |
|
114/526 (21.7%) |
| CRP elevation |
|
113/558 (20.3%) |
| Active disease (VAI
>3) (56) |
|
166/363 (45.7%) |
Table 2 presents
the subclassification into more advanced skin fibrosis and interstitial lung disease.
Of the 590 patients, 421 (71.4%) had an mRSS >0, of whom 195
(33.1%) had an mRSS ≥7. Regarding interstitial lung disease, the database
included 198 (43.4%) patients with interstitial lung disease on either chest
X-Ray or high-resolution computed tomography, of whom 106 (18%) had more advanced
interstitial lung disease.
Table 2Fibrotic manifestations of systemic sclerosis
patients in Switzerland (total Swiss cohort n = 590). Classification
according to severity of skin and interstitial lung disease. Data of listed variables
are presented as number (n)/total cases with
available data (N) (%). Skin
fibrosis was defined as mRSS >0, and more advanced skin fibrosis as mRSS ≥7.
The presence of interstitial lung disease was determined by either chest X-Ray
or high-resolution computed tomography. Advanced interstitial lung disease was
defined by the parameters shown above.
| |
Frequency (n/N) |
(%) |
| Skin |
mRSS >0 |
421/590 |
(71.4) |
| mRSS ≥7 |
195/590 |
(33.1) |
| Lung |
Interstitial lung disease on CXR or high-resolution
computed tomography |
198/456 |
(43.4) |
| Advanced interstitial lung disease |
106/590 |
(18.0) |
| High-resolution computed tomography, fibrosis
>20% |
20/91 |
(21.9) |
| Dyspnea NYHA stage 3/4 |
57/543 |
(10.5) |
| FVC predicted <70% |
54/529 |
(10.2) |
Treatment
All
treatments with potentially disease modifying agents per patient are depicted
in figure 2. Regarding non-biologic DMARDs, the most frequently prescribed
medication was methotrexate, in 97 (16.4%) patients, followed by
hydroxy-/chloroquine, in 67 (11.4%), and mycophenolate mofetil in 55 (9.3%)
patients. Among biologic DMARDs, rituximab and tocilizumab were administered most
frequently, each in 23 (3.9%) patients. Additionally, 124 (21%) patients were
treated with low-dose prednisone (≤10 mg/d). Seven (1.2%) underwent haematopoietic
stem cell transplantation and two (0.3%) lung transplantation. Furthermore, 378
(64.1%) received proton-pump inhibitors and 20 (3.4%) required oxygen
supplementation.
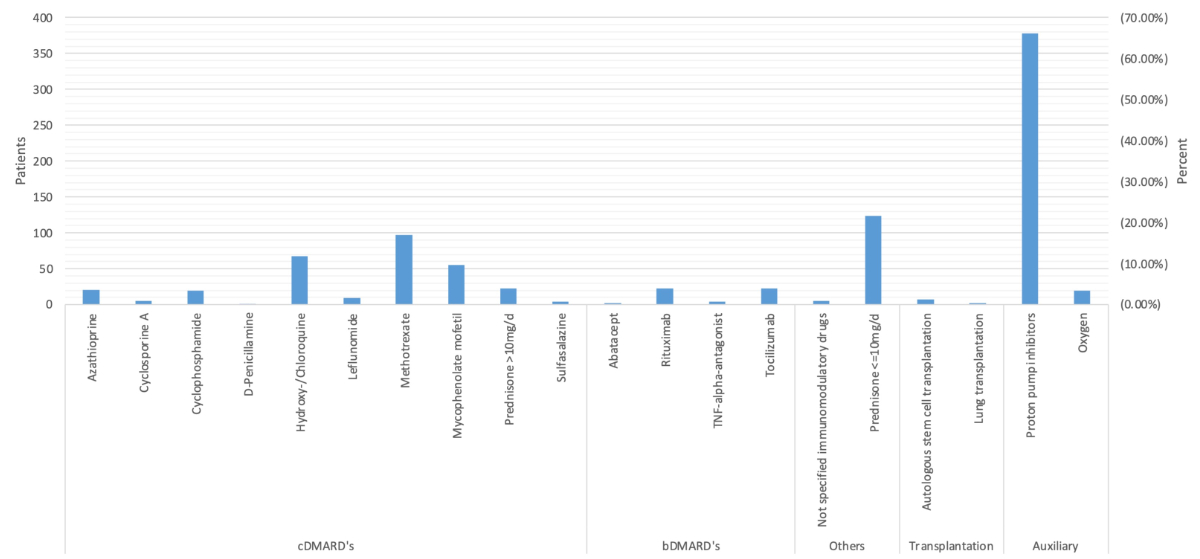
Figure 2Treatment
with potentially disease modifying agents in Swiss systemic sclerosis patients,
illustrated alphabetically as drugs per patient at baseline visit. Numbers and
percentage refer to Switzerland’s total patient population. Exact numbers are
listed in table S1 in the appendix.
Individual
treatments differ based on the extent of skin and interstitial lung disease, in
comparison to the entire cohort, are illustrated in figure 3. Cyclophosphamide
was used more frequently among patients with more advanced skin fibrosis and
patients with interstitial lung disease, including those with more advanced interstitial
lung disease. There was a higher prescription rate of methotrexate for patients
with more advanced skin fibrosis. Mycophenolate mofetil’s prescription rate was
higher among patients with interstitial lung disease and even greater for those
with more advanced interstitial lung disease. The data also indicate a higher
prescription rate for patients with more advanced skin fibrosis [38]. Rituximab
was more a widely used among patients with more advanced skin fibrosis or interstitial
lung disease. Furthermore, tocilizumab was used more frequently among patients
with more extended skin fibrosis and with advanced interstitial lung disease.

Figure 3Treatment
differences among SSc patients according to severity of skin fibrosis and interstitial
lung disease, compared
to the average baseline treatments of the study’s cohort.
CRX: Chest X-Ray;
HRCT: High-resolution computed tomography; mRSS: modified Rodnan skin score;
TNF: Tumor necrosis factor.
Of 421
patients with skin fibrosis (mRSS >0), 49 either had taken no medication at
all or had only proton-pump inhibitors, while 148/372 (39.8%) received
treatment withmethotrexate, mycophenolate
mofetil, or rituximab. For the 198 patients with interstitial lung disease, as
determined by X-ray or high-resolution computed tomography, 21 were without
treatment or had only proton-pump inhibitors; 80/177(45.2%) received therapy
with cyclophosphamide, mycophenolate mofetil, tocilizumab, or rituximab (figure
4).

Figure 4Percentages
of patients treated according to the EULAR/BSR Guidelines. For systemic sclerosis-related
skin fibrosis, the recommendation is
either methotrexate, mycophenolate mofetil, or rituximab; for systemic sclerosis-related
lung fibrosis, the recommendation
is cyclophosphamide, mycophenolate mofetil, tocilizumab, or rituximab. Patients
with gastrooesophageal reflux disease, for whom proton pump inhibitors have
been recommended for treatment, have also been listed.
CRX: Chest X-Ray;
HRCT: High-resolution computed tomography; mRSS: modified Rodnan skin score; BSR:
British Society of Rheumatology.
Low-dose
prednisone (≤10 mg/d) was used and often and, even more frequently so, among patients
with any type of interstitial lung disease. This was particularly the case for patients
with advanced interstitial lung disease patients. Finally, the prescription
rates for both proton-pump inhibitors and oxygen supplementation were higher in
the group of patients with advanced interstitial lung disease compared to the
other cohorts.
Only seven
systemic sclerosis patients were eligible for haematopoietic stem cell transplantation.
They were younger than the average population, with a median age of 57.0 (50.5–62.5),
yet the median disease duration had already been 5.0 (3–10) years. Most
patients (66.7%) were diagnosed with diffuse systemic sclerosis, had reported more
vascular and cardiopulmonary problems, and had worse lung function parameters;
40% had an active disease score (VAI >3). More detailed information is
listed in table S2 in the appendix.
A total
of 315 patients had recorded gastrooesophageal reflux disease and 251 (79.7 %)
were treated with proton-pump inhibitors (figure 4).
Discussion
Overall,
a wide range of medications is prescribed for systemic sclerosis patients in
Switzerland, nevertheless with consistent adherence to guidelines. Overall, 81 patients
(13.7 %) did not receive any medication and 39 (6.6 %) had only proton-pump. It
must be account for that treatment decisions are derived by means of a complex
process, with many influencing factors (e.g., contraindications, patient
preferences, financial considerations, etc.), which cannot be analysed from
registry data.
In
detail, cyclophosphamide was used more frequently among patients with advanced interstitial
lung disease or with interstitial lung disease in general than among the average
patient population; this aligns with EULAR recommendations and other guidelines
[6, 8]. In addition, 27.2% of this group fulfils the criteria for advanced interstitial
lung disease. Furthermore, skin fibrosis is associated with an increased risk
of internal organ manifestations [16].
We also ascertained
that methotrexate was used more commonly among patients with advanced skin
fibrosis [6, 8].
While
not yet recommended by EUSTAR, newer studies and BSR and BHPR guidelines recommend
mycophenloate mofetil to be used frequently among those in our study cohort for interstitial
lung disease, as well as for advanced skin fibrosis among
Swiss systemic sclerosis patients [8, 16, 17, 39].
In
Switzerland, there is a tendency to use rituximab for treating systemic
sclerosis patients with more advanced skin and lung fibrosis. Although it remains
a subject of current research, observational studies and smaller randomised
controlled clinical trials indicate that anti-CD20 mediated B cell depletion
positively effects both skin and lung involvement [35, 40–45]. Recent data from
the RECITAL trial showed similar effects of rituximab compared to
cyclophosphamide on FVC in patients with connective-tissue disease associated interstitial
lung disease, including systemic sclerosis [46]. This explains the wide use of
rituximab despite it not yet being recommended by EUSTAR and being only vaguely
mentioned in BSR and BHPR guidelines [6, 8]
Tocilizumab use among patients with interstitial lung diseace can be generally explained
by the findings of the faSScinate trial; the outcomes indicated a beneficial
effect on stabilizing lung function regarding FVC [20, 47]. This is consistent
with the more recent randomised placebo-controlled phase III focuSSced trial of
tocilizumab in systemic sclerosis, which led to the Federal Drug Administration
(FDA) of the United States approving tocilizumab for treating SSc-ILD [48].
These studies also showed a numeric, but not statistically significant,
reduction in mRSS changes at week 24. In the phase III trial, in which systemic
sclerosis patients received subcutaneous tocilizumab for 48 weeks, there was
again a numeric, but not significant, difference between tocilizumab and a placebo
in the primary endpoint mRSS at week 48 [21, 47, 48].
Glucocorticoids,
such as prednisone, are frequently used for treating systemic sclerosis despite
their efficacy being supported by limited evidence [49, 50]. Often, there is no
correlation between the prescription pattern and the clinical signs of
inflammation [50]. In Switzerland, this pattern is also reflected by prescriptions
given to systemic sclerosis patients. As seen in figure 2, especially daily,
low-prednisone doses (≤10 mg/d) are frequently prescribed for patients with systemic
sclerosis-related interstitial lung disease and advanced skin fibrosis. There
is also a tendency to prescribe doses higher than 10 mg per day among the advanced
interstitial lung disease cohort. Higher prednisone doses are usually avoided
due to a risk of scleroderma renal crisis.
Regarding
haematopoietic stem cell transplantation, there was a slight tendency to offer
this treatment for patients with more advanced interstitial lung disease. Among
the entire Swiss patient population, there were only seven who underwent haematopoietic
stem cell transplantation at the baseline visit, or short afterwards. EUSTAR
recommends haematopoietic stem cell transplantation only in carefully selected
patients with rapidly progressive systemic sclerosis; this accounts for the
high risk of treatment-related morbidity and mortality [6]. Following EUSTAR
recommendations, in Switzerland, systemic sclerosis patients were younger, the
majority of whom had diffuse systemic sclerosis with worse lung parameters; 40%
had active disease.
Finally,
proton-pump inhibitors were prescribed most commonly of the analysed medications
among our study population. Accordingly, both EUSTAR and BSR/BHPR guidelines
recommend using proton-pump inhibitors in cases of systemic sclerosis-related
gastro-oesophageal reflux disease (GERD), despite there being a dearth of
specific randomised controlled trials [6, 8]. Most of our study population
(66.4%) had recorded oesophageal symptoms. Another reason, especially for the
additional use of proton-pump inhibitors among patients with advanced interstitial
lung disease, why proton-pump inhibitors are frequently used among patients
with interstitial lung disease could be because of a suspected causal
correlation between interstitial lung disease and GERD [36, 37, 51].
Regarding
the limitations of our study, these are inevitable due to missing values based
on the observational, multicentre nature of the registry. This study captured
neither non-pharmacologic treatments, nor alternative medicines. At the time of
exporting data from the EUSTAR database, nintedanib (a drug more recently
approved for SSc-ILD treatment, both in Switzerland and worldwide) was not
recorded in the EUSTAR database [52]. Thus, such treatments could not be analysed
using the current dataset. Data were drawn in 2019, and the treatment landscape
may have changed since then. Notably, we have no indications that general
adherence to guidelines and recommendations has changed since then; as such, we
have strong reason to believe that this study’s general conclusions remain
valid. Finally, we did not have longitudinal data available to assess treatment
duration.
In
conclusion, in Switzerland, systemic sclerosis patients are being prescribed a
wide range of medications. This includes modern, targeted treatments for which
randomised controlled clinical trial have been recently reported. Future
research could therefore focus on treatment for other manifestations of systemic
sclerosis, such digital vasculopathy, pulmonary arterial hypertension, or systemic
sclerosis-related gastrointestinal disease. New guidelines and recommendations,
which are expected to be published soon, will have increasing complexity due to
the high number of new medications citing strong evidence for efficacy. This
will challenge their implementation in clinical practice. It is to be noted
that the Swiss EUSTAR database has proven useful in monitoring this process.
Acknowledgments
We thank
all patients for allowing us to collect their data and for providing informed
consent. Primary data are available upon reasonable request, submitted to the
corresponding author, following EUSTAR rules.We thank all patients for allowing us
to collect their data and for providing informed consent. Primary data are available
upon reasonable request, submitted to the corresponding author, following EUSTAR rules.
Prof. Dr. med. Oliver Distler
Department of
Rheumatology
University Hospital
Zurich
University of Zurich
Rämistrasse 100
CH-8091 Zurich
Oliver.Distler[at]usz.ch
References
1. Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017 Oct;390(10103):1685–99. 10.1016/S0140-6736(17)30933-9
2. Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009 May;360(19):1989–2003.
10.1056/NEJMra0806188
3. Wollheim FA. Classification of systemic sclerosis. Visions and reality. Rheumatology
(Oxford). 2005 Oct;44(10):1212–6. 10.1093/rheumatology/keh671
4. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013
classification criteria for systemic sclerosis: an American College of Rheumatology/European
League against Rheumatism collaborative initiative. Arthritis Rheum. 2013 Nov;65(11):2737–47.
10.1002/art.38098
5. Katsumoto TR, Whitfield ML, Connolly MK. The pathogenesis of systemic sclerosis. Annu
Rev Pathol. 2011;6(1):509–37. 10.1146/annurev-pathol-011110-130312
6. Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al.; EUSTAR
Coauthors. Update of EULAR recommendations for the treatment of systemic sclerosis.
Ann Rheum Dis. 2017 Aug;76(8):1327–39. 10.1136/annrheumdis-2016-209909
7. Kowal-Bielecka O, Landewé R, Avouac J, Chwiesko S, Miniati I, Czirjak L, et al.; EUSTAR
Co-Authors. EULAR recommendations for the treatment of systemic sclerosis: a report
from the EULAR Scleroderma Trials and Research group (EUSTAR). Ann Rheum Dis. 2009 May;68(5):620–8.
10.1136/ard.2008.096677
8. Denton CP, Hughes M, Gak N, Vila J, Buch MH, Chakravarty K, et al.; BSR and BHPR Standards,
Guidelines and Audit Working Group. BSR and BHPR guideline for the treatment of systemic
sclerosis. Rheumatology (Oxford). 2016 Oct;55(10):1906–10. 10.1093/rheumatology/kew224
9. Hachulla E, Agard C, Allanore Y, Avouac J, Bader-Meunier B, Belot A, et al.; Collaborators.
French recommendations for the management of systemic sclerosis. Orphanet J Rare Dis.
2021 Jul;16(S2 Suppl 2):322. 10.1186/s13023-021-01844-y
10. Hoffmann-Vold AM (Rheumatology L, editor). Distler O et al. The identification and
management of interstitial lung disease in systemic sclerosis: evidence-based European
consensus statements. 2020. pp. E71–83. 10.1016/S2665-9913(19)30144-4
11. van den Hoogen FHJ, Boerbooms AMT, Swaak AJG, Rasker JJ, van Lier HJJ, van de Putte LBA.
Comparison of Methotrexate with placebo in the treatment of systemic sclerosis: a
24 Week randomized double-blind trial, followed by a 24 week observational trial.
1996;35:364–72.
12. Pope JE, Bellamy N, Seibold JR, Baron M, Ellman M, Carette S, et al. A randomized,
controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis
Rheum. 2001 Jun;44(6):1351–8. 10.1002/1529-0131(200106)44:6<1351::AID-ART227>3.0.CO;2-I
13. Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter,
prospective, randomized, double-blind, placebo-controlled trial of corticosteroids
and intravenous cyclophosphamide followed by oral azathioprine for the treatment of
pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006 Dec;54(12):3962–70. 10.1002/art.22204
14. Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al.; Scleroderma
Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease.
N Engl J Med. 2006 Jun;354(25):2655–66. 10.1056/NEJMoa055120
15. Volkmann ER, Tashkin DP, Sim M, Li N, Khanna D, Roth MD, et al. Cyclophosphamide for
Systemic Sclerosis-related Interstitial Lung Disease: A Comparison of Scleroderma
Lung Study I and II. J Rheumatol. 2019 Oct;46(10):1316–25. 10.3899/jrheum.180441
16. Namas R, Tashkin DP, Furst DE, Wilhalme H, Tseng CH, Roth MD, et al.; Participants
in the Scleroderma Lung Study I and members of the Scleroderma Lung Study II Research
Group. Efficacy of Mycophenolate Mofetil and Oral Cyclophosphamide on Skin Thickness:
Post Hoc Analyses From Two Randomized Placebo-Controlled Trials. Arthritis Care Res
(Hoboken). 2018 Mar;70(3):439–44. 10.1002/acr.23282
17. Volkmann ER, Tashkin DP, Li N, Roth MD, Khanna D, Hoffmann-Vold AM, et al. Mycophenolate
Mofetil Versus Placebo for Systemic Sclerosis-Related Interstitial Lung Disease: An
Analysis of Scleroderma Lung Studies I and II. Arthritis Rheumatol. 2017 Jul;69(7):1451–60.
10.1002/art.40114
18. Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al.; Scleroderma
Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease.
N Engl J Med. 2006 Jun;354(25):2655–66. 10.1056/NEJMoa055120
19. Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al.; Sclerodema
Lung Study II Investigators. Mycophenolate mofetil versus oral cyclophosphamide in
scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind,
parallel group trial. Lancet Respir Med. 2016 Sep;4(9):708–19. 10.1016/S2213-2600(16)30152-7
20. Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety
and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate):
a phase 2, randomised, controlled trial. Lancet. 2016 Jun;387(10038):2630–40. 10.1016/S0140-6736(16)00232-4
21. Khanna D, Lin CJ, Furst DE, Goldin J, Kim G, Kuwana M, et al.; focuSSced investigators.
Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled,
phase 3 trial. Lancet Respir Med. 2020 Oct;8(10):963–74. 10.1016/S2213-2600(20)30318-0
22. Distler O, Distler JH. Tocilizumab for systemic sclerosis: implications for future
trials. Lancet. 2016 Jun;387(10038):2580–1. 10.1016/S0140-6736(16)00622-X
23. Kuster S, Jordan S, Elhai M, Held U, Steigmiller K, Bruni C, et al.; EUSTAR collaborators.
Effectiveness and safety of tocilizumab in patients with systemic sclerosis: a propensity
score matched controlled observational study of the EUSTAR cohort. RMD Open. 2022 Nov;8(2):e002477.
10.1136/rmdopen-2022-002477
24. Maher TM, Tudor VA, Saunders P, Gibbons MA, Fletcher SV, Denton CP, et al.; RECITAL
Investigators. Rituximab versus intravenous cyclophosphamide in patients with connective
tissue disease-associated interstitial lung disease in the UK (RECITAL): a double-blind,
double-dummy, randomised, controlled, phase 2b trial. Lancet Respir Med. 2023 Jan;11(1):45–54.
10.1016/S2213-2600(22)00359-9
25. Saunders P, Tsipouri V, Keir GJ, Ashby D, Flather MD, Parfrey H, et al. Rituximab
versus cyclophosphamide for the treatment of connective tissue disease-associated
interstitial lung disease (RECITAL): study protocol for a randomised controlled trial.
Trials. 2017 Jun;18(1):275. 10.1186/s13063-017-2016-2
26. al SEe. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): open-label
extension of a double blind, investigators-initiated, randomised, placebo-controlled
trial. Lancet Rheumatology. 2022;Volume 4, Issue 8, E546-E555, August 2022.
27. Tsukamoto H, Nagafuji K, Horiuchi T, Mitoma H, Niiro H, Arinobu Y, et al. Analysis
of immune reconstitution after autologous CD34+ stem/progenitor cell transplantation
for systemic sclerosis: predominant reconstitution of Th1 CD4+ T cells. Rheumatology
(Oxford). 2011 May;50(5):944–52. 10.1093/rheumatology/keq414
28. Vonk MC, Marjanovic Z, van den Hoogen FH, Zohar S, Schattenberg AV, Fibbe WE, et al. Long-term
follow-up results after autologous haematopoietic stem cell transplantation for severe
systemic sclerosis. Ann Rheum Dis. 2008 Jan;67(1):98–104. 10.1136/ard.2007.071464
29. Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative
haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per
month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet.
2011 Aug;378(9790):498–506. 10.1016/S0140-6736(11)60982-3
30. van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al.; EBMT/EULAR
Scleroderma Study Group. Autologous hematopoietic stem cell transplantation vs intravenous
pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical
trial. JAMA. 2014 Jun;311(24):2490–8. 10.1001/jama.2014.6368
31. Hernández J, Jordan S, Dobrota R, Iudici M, Hasler P, Ribi C, et al.; The Eustar Collaborators.
The burden of systemic sclerosis in Switzerland - the Swiss systemic sclerosis EUSTAR
cohort. Swiss Med Wkly. 2021 Jul;151(2728):w20528. 10.4414/smw.2021.20528
32. Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, et al. Continuous
intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum
of disease. A randomized, controlled trial. Ann Intern Med. 2000 Mar;132(6):425–34.
10.7326/0003-4819-132-6-200003210-00002
33. Dobrota R, Maurer B, Graf N, Jordan S, Mihai C, Kowal-Bielecka O, et al.; EUSTAR coauthors.
Prediction of improvement in skin fibrosis in diffuse cutaneous systemic sclerosis:
a EUSTAR analysis. Ann Rheum Dis. 2016 Oct;75(10):1743–8. 10.1136/annrheumdis-2015-208024
34. Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial
lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care
Med. 2008 Jun;177(11):1248–54. 10.1164/rccm.200706-877OC
35. Daoussis D, Melissaropoulos K, Sakellaropoulos G, Antonopoulos I, Markatseli TE, Simopoulou T,
et al. A multicenter, open-label, comparative study of B-cell depletion therapy with
Rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthritis
Rheum. 2017 Apr;46(5):625–31. 10.1016/j.semarthrit.2016.10.003
36. Bandeira CD, Rubin AS, Cardoso PF, Moreira JS, Machado MM. Prevalence of gastroesophageal
reflux disease in patients with idiopathic pulmonary fibrosis. J Bras Pneumol. 2009 Dec;35(12):1182–9.
10.1590/S1806-37132009001200004
37. Qi J, Shang S, Li Z, Kang J, Kong L. [The relationship between idiopathic pulmonary
fibrosis and gastroesophageal reflux disease]. Zhonghua Nei Ke Za Zhi. 2015 Aug;54(8):695–8.
38. Wu W, Jordan S, Becker MO, Dobrota R, Maurer B, Fretheim H, et al. Prediction of progression
of interstitial lung disease in patients with systemic sclerosis: the SPAR model.
Ann Rheum Dis. 2018 Sep;77(9):1326–32. 10.1136/annrheumdis-2018-213201
39. Shenoy PD, Bavaliya M, Sashidharan S, Nalianda K, Sreenath S. Cyclophosphamide versus
mycophenolate mofetil in scleroderma interstitial lung disease (SSc-ILD) as induction
therapy: a single-centre, retrospective analysis. Arthritis Res Ther. 2016 Jun;18(1):123.
10.1186/s13075-016-1015-0
40. Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Paliogianni F, Sirinian C,
et al. Effect of long-term treatment with rituximab on pulmonary function and skin
fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol. 2012;30(2 Suppl
71):S17–22.
41. Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y, et al.; EUSTAR
Rituximab study group. Effects and safety of rituximab in systemic sclerosis: an analysis
from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2015 Jun;74(6):1188–94.
10.1136/annrheumdis-2013-204522
42. Elhai M, Boubaya M, Distler O, Smith V, Matucci-Cerinic M, Alegre Sancho JJ, et al.;
for EUSTAR network. Outcomes of patients with systemic sclerosis treated with rituximab
in contemporary practice: a prospective cohort study. Ann Rheum Dis. 2019 Jul;78(7):979–87.
10.1136/annrheumdis-2018-214816
43. Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C, et
al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle
study. Rheumatology (Oxford). 2010 Feb;49(2):271–80. 10.1093/rheumatology/kep093
44. Ebata S, Yoshizaki-Ogawa A, Sato S, Yoshizaki A. New Era in Systemic Sclerosis Treatment:
Recently Approved Therapeutics. J Clin Med. 2022 Aug;11(15):4631. 10.3390/jcm11154631
45. Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs
rituximab for the treatment of early diffuse scleroderma lung disease: open label,
randomized, controlled trial. Rheumatology (Oxford). 2018 Dec;57(12):2106–13. 10.1093/rheumatology/key213
46. Maher TM, Tudor VA, Saunders P, Gibbons MA, Fletcher SV, Denton CP, et al. Rituximab
versus intravenous cyclophosphamide in patients with connective tissue disease-associated
interstitial lung disease in the UK (RECITAL): a double-blind, double-dummy, randomised,
controlled, phase 2b trial. Lancet Respir Med. 2022.
47. Khanna D, Denton CP, Lin CJ, van Laar JM, Frech TM, Anderson ME, et al. Safety and
efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label
period of a phase II randomised controlled trial (faSScinate). Ann Rheum Dis. 2018 Feb;77(2):212–20.
10.1136/annrheumdis-2017-211682
48. Khanna D, Lin CJ, Furst DE, Goldin J, Kim G, Kuwana M, et al. A randomised placebo-controlled
phase 3 trial of tocilizumab in systemic sclerosis. Lancet Respir Med. 2020. 10.1016/S2213-2600(20)30318-0
49. Hunzelmann N, Moinzadeh P, Genth E, Krieg T, Lehmacher W, Melchers I, et al.; German
Network for Systemic Scleroderma Centers. High frequency of corticosteroid and immunosuppressive
therapy in patients with systemic sclerosis despite limited evidence for efficacy.
Arthritis Res Ther. 2009;11(2):R30. 10.1186/ar2634
50. Iudici M, Fasano S, Iacono D, Russo B, Cuomo G, Valentini G. Prevalence and factors
associated with glucocorticoids (GC) use in systemic sclerosis (SSc): a systematic
review and meta-analysis of cohort studies and registries. Clin Rheumatol. 2014 Feb;33(2):153–64.
10.1007/s10067-013-2422-0
51. Salaffi F, Di Carlo M, Carotti M, Fraticelli P, Gabrielli A, Giovagnoni A. Relationship
between interstitial lung disease and oesophageal dilatation on chest high-resolution
computed tomography in patients with systemic sclerosis: a cross-sectional study.
Radiol Med. 2018 Sep;123(9):655–63. 10.1007/s11547-018-0894-3
52. Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al.; SENSCIS
Trial Investigators. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung
Disease. N Engl J Med. 2019 Jun;380(26):2518–28. 10.1056/NEJMoa1903076
53. Valentini G, D’Angelo S, Della Rossa A, Bencivelli W, Bombardieri S. European Scleroderma
Study Group to define disease activity criteria for systemic sclerosis. IV. Assessment
of skin thickening by modified Rodnan skin score. Ann Rheum Dis. 2003 Sep;62(9):904–5.
10.1136/ard.62.9.904
Appendix
Table S1Numbers of systemic sclerosis patients treated with potentially disease
modifying agents in the Swiss EUSTAR cohort (total Swiss cohort n = 590). The exact
numbers and percentage
values referred to in figure 2 are listed below.
| |
|
Number
(n/N; [%]) |
| Disease
modifying anti-rheumatic drugs |
Azathioprine |
21/590
(3.6%) |
| Cyclosporine
A |
5/590
(0.9%) |
| Cyclophosphamide |
20/590
/590 (3.4%) |
| D-Penicillamine |
1/590
(0.2%) |
| Hydroxy-/Chloroquine |
67/590
(11.4%) |
| Leflunomide |
9/590
(1.5%) |
| Methotrexate |
97/590
(16.4%) |
| Mycophenolate
mofetil |
55/590
(9.3%) |
| Prednisone
(>10 mg/d) |
23/590
(3.9%) |
| Sulfasalazine |
4/590
(0.7%) |
| B
Disease modifying anti-rheumatic drugs |
Abatacept |
2/590
(0.3%) |
| Rituximab |
23/590
(3.9%) |
| TNF-alpha-antagonists |
4/590
(0.7%) |
| Tocilizumab |
23/590
(3.9%) |
| Other
Immunosuppressants |
Prednisone
(≤10 mg/d) |
124/590
(21.1%) |
| Not specified
immunomodulatory drugs |
5/590
(0.9%) |
| Transplantations |
Autologous
stem cell transplantation |
7/590
(1.2%) |
| Lung
transplantation |
2/590
(0.3%) |
| Auxiliary
drugs |
Oxygen
supplementation |
20/590
(3.4%) |
| Proton
pump inhibitor |
378/590
(64.1%) |
Table S2A comparison
of demographic and clinical characteristics between the total number of
patients in the Swiss systemic sclerosis cohort and Swiss patients with
recorded haematopoietic stem cell transplantation at baseline visit (seven in
total). Definitions of items and organ manifestation align
with EUSTAR. Data are presented as number (n)/total valid cases (N) (%).
Disease duration was calculated as the difference between the dates of the
baseline visit and the first non-Raynaud’s symptom of the disease, per patient
reports. Pulmonary hypertension was judged based on RHC. Active disease was
defined as a score >3, which was derived by calculating European Scleroderma
Study Group disease activity indices for systemic sclerosis, as proposed by
Valentini [48].
| |
Total Swiss
cohort (n = 590%) |
Haematopoietic
stem cell transplantation (n = 7%) |
| |
|
Median (IQR%) |
Frequency (n/N;
%) |
Median (IQR%) |
Frequency (n/N;
%) |
| Demographics |
Age, years |
68.0 (57–77) |
|
57.0 (50.5–62.5) |
|
| Disease duration,
years |
6.0 (2–13) |
|
5.0 (3–10) |
|
| Female |
|
471/590 (79.8%) |
|
5/7 (71.4%) |
| Male |
|
119/590 (20.2%) |
|
2/7 (28.6%) |
| Limited cutaneous systemic
sclerosis |
|
328/443 (74.0%) |
|
2/6 (33.3%) |
| Diffuse cutaneous systemic
sclerosis |
|
115/443 (26.0%) |
|
4/6 (66.7%) |
| Skin/Vascular |
mRSS |
3 (0–9) |
|
2 (1–8) |
|
| Raynaud’s Phenomenon |
|
563/586 (96.1%) |
|
6/6 (100.0%) |
| Digital ulcers |
|
189/516 (36.6%) |
|
5/6 (83.3%) |
| Active digital
ulcers |
|
66/516 (12.8%) |
|
2/6 (33.3%) |
| Pitting scars |
|
200/498 (40.1%) |
|
5/5 (100.0%) |
| Scleredema |
|
297/508 (58.5%) |
|
1/5 (20.0%) |
| Telangiectasia |
|
327/516 (63.4%) |
|
4/5 (80.0%) |
| Abnormal nailfold
capillaroscopy |
|
411/479 (85.8%) |
|
2/2 (100.0%) |
| Musculoskeletal |
Tendon friction rubs |
|
42/565 (7.4%) |
|
None (0.0%) |
| Joint synovitis |
|
87/580 (15.0%) |
|
1/6 (16.7%) |
| Joint contractures |
|
170/573 (29.7%) |
|
None (0.0%) |
| Muscle weakness |
|
71/578 (12.3%) |
|
None (0.0%) |
| Gastrointestinal |
Esophageal symptoms |
|
315/582 (54.1%) |
|
5/7 (71.4%) |
| Stomach symptoms |
|
160/569 (28.1%) |
|
2/6 (33.0%) |
| Intestinal symptoms |
|
180/574 (31.4%) |
|
1/6 (16.7%) |
| Cardiopulmonary |
Dyspnea NYHA stage
1/2 |
|
486/543 (89.5%) |
|
4/6 (66.6%) |
| Dyspnea NYHA stage
3/4 |
|
57/543 (10.5%) |
|
2/6 (33.4%) |
| Diastolic
dysfunction |
|
150/481 (31.2%) |
|
3/4 (75.0%) |
| Pericardial effusion |
|
19/500 (3.8%) |
|
1/4 (25.0%) |
| Conduction blocks |
|
55/425 (12.9%) |
|
None (0.0%) |
| LVEF<45% |
|
4/521 (0.8%) |
|
None (0.0%) |
| PAH by RHC |
|
11/244 (4.5%) |
|
2/5 (40.0%) |
| Interstitial lung
disease on high-resolution computed tomography |
|
198/456 (43.4%) |
|
2/6 (66.7%) |
| Lung function |
| FVC, % predicted |
98 (83–111%) |
|
73.5 (53.3–93.5) |
|
| FEV1, % predicted |
94 (82–106) |
|
77 (60–96.5) |
|
| TLC, % predicted |
100 (85–112) |
|
81 (63–93.5) |
|
| DLCO, % predicted |
75 (61–88) |
|
55.5 (50.8–61) |
|
| FVC<70% predicted |
|
54/529 (10.2%) |
|
3/7 (42.9%) |
| DLCO<70%
predicted |
|
199/513 (38.8%) |
|
4/5 (80.0%) |
| Kidney |
Renal crisis |
|
12/584 (2.1%) |
|
None (0.0%) |
| Laboratory
parameters |
ANA positive |
|
525/537 (97.8%) |
|
5/6 (83.3%) |
| ACA positive |
|
230/490 (46.9%) |
|
1/6 (16.7%) |
| Anti–Scl–70 positive |
|
140/507 (27.6%) |
|
3/6 (50.0%) |
| Anti–RNA–polymerase
III positive |
|
51/433 (11.8%) |
|
None (0.0%) |
| Creatinine kinase
elevation |
|
61/536 (11.4%) |
|
1/6 (16.7%) |
| Proteinuria |
|
55/542 (10.1%) |
|
4/6 (66.7%) |
| ESR >25 mm/h |
|
114/526 (21.7%) |
|
3/6 (50.0%) |
| CRP elevation |
|
113/558 (20.3%) |
|
2/7 (28.6%) |
| Active disease (VAI
>3%)[48] |
|
166/363 (45.7%) |
|
2/5 (40.0%) |



