Prostate cancer screening in Switzerland: a literature review and
consensus statement from the Swiss Society of Urology
DOI: https://doi.org/https://doi.org/10.57187/s.3626
Christoph Würnschimmelab,
Dominik Mengesc,
Maciej Kwiatkowskide,
Silvan Sigga,
Lukas Praused,
Agostino Matteiab,
Daniel Engelerf,
Daniel Eberlig,
Helge Seiferth,
Massimo Valerioi,
Cyrill A. Rentschh,
Ashkan Mortezavih
a Department of Urology, Luzerner Kantonsspital, Lucerne, Switzerland
b Faculty
of Health Sciences and Medicine, University of Lucerne, Lucerne, Switzerland
c Epidemiology, Biostatistics and Prevention Institute, University of Zurich,
Zurich, Switzerland
d Department of Urology, Kantonsspital Aarau, Aarau, Switzerland
e Faculty
Member, University Hospital Basel, Basel, Switzerland
f Department
of Urology, Kantonsspital St. Gallen, St. Gallen, Switzerland
g Department of Urology, University Hospital Zurich, Zurich, Switzerland
h Department of Urology, University Hospital Basel, Basel, Switzerland
i Department of Urology, University Hospital Geneva, Geneva, Switzerland
Summary
Over a decade ago, the United States Preventive Services
Taskforce (USPSTF) recommended against prostate-specific antigen (PSA)-based
screening for prostate cancer in all men, which considerably influenced
prostate cancer screening policies worldwide after that. Consequently, the
world has seen increasing numbers of advanced stages and prostate cancer
deaths, which later led the USPSTF to withdraw its initial statement.
Meanwhile, the European Union has elaborated a directive to address the problem
of implementing prostate cancer screening in “Europe’s Beating Cancer
Plan”. In Switzerland, concerned urologists formed an open Swiss Prostate
Cancer Screening Group to improve the early detection of prostate cancer. On
the 20th of September 2023, during the annual general assembly of
the Swiss Society of Urology (SGU/SSU) in Lausanne, members positively voted
for a stepwise approach to evaluate the feasibility of implementing organised prostate
cancer screening programs in Switzerland. The following article will summarise
the events and scientific advances in the last decade during which evidence and
promising additional modalities to complement PSA-based prostate cancer screening
have emerged. It also aims to provide an overview of contemporary
strategies and their potential harms and benefits.
Introduction
Over a decade ago, the United States Preventive Services
Taskforce (USPSTF) recommended against prostate-specific antigen (PSA)-based
screening for prostate cancer in all men due to concerns about a high rate of over
diagnosis and overtreatment. This considerably influenced prostate cancer
screening policies worldwide [1]. Emerging evidence suggests
that the incidence of advanced-stage and metastatic prostate cancers increased
in the United States after that [2–5]. As the evidence from sizeable
international screening trials matured, the USPSTF amended its statement
against PSA-based prostate cancer screening in 2018, recommending PSA-based
screening in men aged 55–69 years based on an individual evaluation and shared
decision-making [6, 7]. Furthermore, various studies
have demonstrated the potential value of diagnostic tests, such as magnetic
resonance imaging (MRI), and biomarkers and PSA testing for prostate cancer
screening. As a result, the European Union recently issued a directive
supporting its member states in evaluating the feasibility of organised prostate
cancer screening through their “Europe’s Beating Cancer Plan” [8].
In Switzerland, concerned urologists formed an open Swiss
Prostate Cancer Screening Group to improve prostate cancer screening. On the 20th
of September 2023, during the annual general assembly of the Swiss Society of
Urology (SGU/SSU) in Lausanne, members positively voted for a stepwise approach
to evaluate the feasibility of implementing organised prostate cancer screening
programs in Switzerland. The following article summarises the discussions and
results of this voting, which should play a pivotal role in the future of
prostate cancer screening in Switzerland (figure 1). The board of the Swiss Society
of Urology (SGU/SSU) has
approved the wording of this manuscript. Finally, the article summarises the
events and scientific advances in the last decade during which evidence and
promising additional modalities to complement PSA-based prostate cancer screening
have emerged. It also aims to provide an overview of contemporary strategies
and their potential harms and benefits.
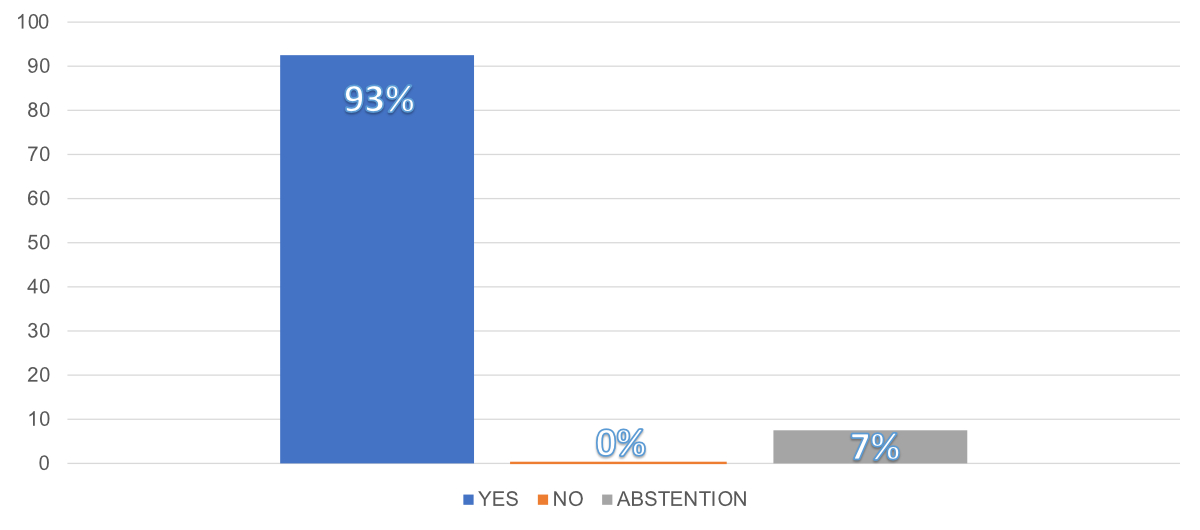
Figure 1Results of the Swiss Society of Urology
voting on 20th September 2023 concerning the potential future of
prostate cancer screening in Switzerland: “Considering the evidence and the
significant amount of ongoing opportunistic screening, Switzerland should take
a stepwise approach, including piloting and further supporting research to
evaluate the feasibility of implementation of organised programs aimed at
assuring appropriate management and quality of prostate cancer screening”.
How it all began
The story of prostate cancer screening mirrors the story of PSA
testing. This serum tumour marker was first identified in the 1960s and was
used until the late 1980s exclusively to monitor disease progression in men who
had been diagnosed with prostate cancer [9]. In the early 1990s, the PSA
test gained popularity as a tool for prostate cancer screening because it was
believed that it may lead to better patient outcomes [10]. To support this hypothesis,
two large-scale clinical trials named European Randomized Study of Screening
for Prostate Cancer (ERSPC) and Prostate, Lung, Colorectal and Ovarian Cancer
Screening (PLCO) were initiated to assess the effectiveness of prostate cancer
screening using PSA testing in Europe and in the USA, respectively [11, 12]. These
studies aimed to
determine whether PSA-based screening could reduce prostate cancer mortality
rates.
Prostate cancer screening trials
European Randomized Study of Screening for Prostate Cancer (ERSPC)
The multinational ERSPC trial was initiated in 1993. The
protocols differed slightly between the eight participating countries. Men aged
50 to 74 were randomly assigned to the screening or control group. The
screening group received regular PSA tests (every two to four years) and
follow-up if PSA levels exceeded an established threshold. Men with a PSA ≥3.0
ng/mL were referred for a systematic prostate biopsy. The screening interval
was four years in most centres. The control group was not offered PSA testing. The
first results were reported in 2009 after a median of nine years of follow-up
of over 160,000 men [11]. The rate ratio for prostate
cancer death in the screening versus the control group was 0.80 (95% CI 0.65 to
0.98). The absolute prostate cancer mortality risk difference was 0.71 prostate
cancer deaths per 1000 men. This, together with an excess incidence of 34
prostate cancer patients per 1000 men, translated into 1410 invited men (number
needed to invite) and 48 additional prostate cancer patients (number needed to diagnose)
to avoid one death from prostate cancer. Of note, of the men who underwent
biopsy for an elevated PSA value, 13,308 (75.9%) had a negative biopsy result.
Of those diagnosed with prostate cancer, 72.2% had a Gleason score of 6 or
less, which means that the majority of patients harboured low-grade disease.
Prostate, Lung, Colorectal and Ovarian Cancer Screening (PLCO)
In the same year (2009), the first results of the prostate
arm of the PCLO trial were reported [12]. This trial enrolled 76,693
men in the United States between the ages of 55 and 74 from 1993 to 2001. Those
in the screening group received annual PSA testing for six years and digital
rectal examinations annually for four years. After follow-ups of seven years [12]
and 13 years [13], the trial failed to
demonstrate an effect of PSA screening on prostate cancer mortality. The
relative incidence of prostate cancer in the screening group was 1.12 (95% CI
1.07 to 1.17), and the relative risk of prostate cancer-related death was 1.09
(95% CI 0.87 to 1.36] compared to the control group. In this trial, 67.7% of
the biopsied men had no prostate cancer, and from those with malignant
histology, 65.7% had a Gleason score of 6 or less [14].
The “United States Preventive Service Task Force (USPSTF)” statements
and statements by medical societies
In subsequent years, the USPSTF weighed mortality benefits
against the harms associated with PSA testing, detection, and treatment based
on the reported data from the ERSPC and PLCO studies. In 2012, the USPSTF panel
stated that there was convincing evidence that the number of prostate cancer
deaths prevented by PSA testing was minimal and harms associated with the
diagnosis and treatment of prostate cancer were common. The panel concluded
that the benefits of PSA-based screening for prostate cancer do not outweigh
the harms and recommended against testing for all men [1]. A health technology
assessment by the Swiss Medical Board published in 2011 came to similar conclusions
and recommended against routine PSA testing in Switzerland [15]. Readers may remember
these
announcements, which also affected how men and primary care physicians in
Switzerland dealt with PSA testing. In 2012, the Swiss Society of Urology
(SGU/SSU) articulated a statement from a Swiss perspective. Diverging from the
USPSTF's stringent recommendation, the SGU/SSU still suggested that PSA testing
could be extended to "well-informed men" from the age of 50 or 45 in
the presence of risk factors and a life expectancy >10 years. However, it
explicitly withheld support for PSA testing within a population-wide screening
program [16]. The same recommendation is
still endorsed by the current European Association of Urology Guidelines (EAU) and
by the American Association of Urology Guidelines (AUA), which emphasise shared
decision-making and patient education before PSA testing [17, 18].
It has taken several years to evaluate objectively the potential
effects of the recommendations against PSA testing for prostate cancer
screening. Following the initial USPSTF recommendation, recent study results
from the United States indicated a significant reduction in the detection rate
of localised prostate cancer and an increase in the incidence of advanced and
metastatic prostate cancer (figure 2). Furthermore, the annual continuous mortality
reduction since the introduction of widespread PSA testing in the early 1990s
until 2012 was no longer observed but reached a plateau [3].
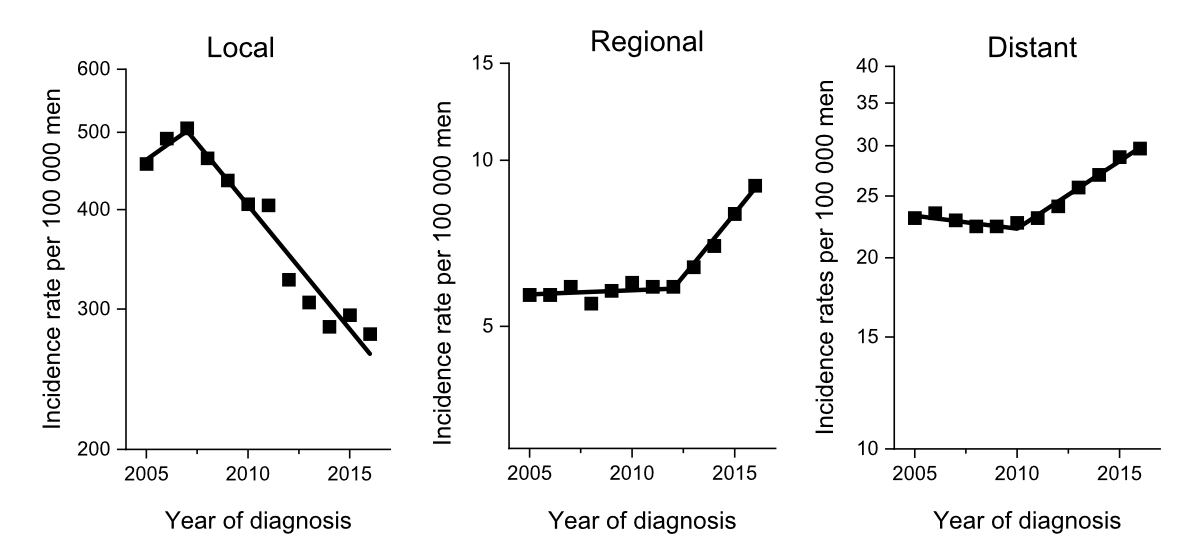
Figure 2Incidence rates of prostate cancer between
2005 and 2015 in the United States, stratified by localised disease, regional
lymph node metastasis, and distant metastasis, demonstrating the effects of the
United States Preventive Service Task Force recommendations against PSA testing
of men above 75 years of age in 2008 and in all men in 2012. From: Jemal A, Culp MB,
Ma J, Islami F, Fedewa SA. Prostate Cancer Incidence 5 Years After US Preventive Services
Task Force Recommendations Against Screening. J Natl Cancer Inst. 2021 Jan 4;113(1):64-71.
doi: 10.1093/jnci/djaa068, by permission of Oxford University Press.
The contentious interpretation of the data is believed to
result from either overlooking or misinterpreting the significant
methodological flaws in the PLCO trial [19, 20] and the preliminary nature of
the ERSPC data. In the PLCO trial, the contamination rate was too elevated to
draw conclusions from PSA screening because nearly half of the men had
undergone previous PSA testing before entering the trial, and 90% of the
control group had received PSA testing [19]. Furthermore, less than half
of the men with elevated PSA levels underwent prostate biopsies [20–22]. With
longer follow-up of the ERSPC study, the absolute prostate cancer mortality
risk difference increased and the numbers needed to invite and diagnose
decreased [23, 24]. After 16 years, the number needed to invite decreased to 570 and
the number needed to diagnose decreased to 18. The absolute
risk reduction, although low, doubled from 0.07 to 0.18%. Twelve-year follow-up
data from four centres showed a 50% reduction in metastatic disease at the time
of diagnosis and a 30% reduction overall, including metastasis detected during
follow-up [23, 25]. As a comparison, in the Nordic-European
Initiative on Colorectal Cancer trial (NordICC), 455 were invited to avoid one
case of colon cancer in a screening setting [26]. Furthermore, colorectal
cancer mortality could not be significantly reduced by screening (relative
risk: 0.90; 95% CI: 0.64 to 1.16), and 3,333 invitations would have been
necessary to prevent one death from colon cancer (NNI). Furthermore, in breast
cancer screening, the effectiveness depends heavily on the age group examined.
Accordingly, the spread of the numbers given in the literature is vast (377 to 2000
invitations to prevent one breast cancer death). In the more “optimistic”
scenarios, at best, it overlaps with those for ESPRC but certainly not in the
less effective screening scenarios (table 1, [27]). In 2018, the USPSTF guidelines
changed prior guidance against routine screening for prostate cancer, issuing
new recommendations similar to the SGU/SSU endorsing individual decision-making
for men aged 55 to 69 years based on a discussion of the potential benefits and
harms [2, 6]. Since then, no further
recommendations have been made, but we await future amendments [28].
Table 1The number needed to invite (NNI),
number needed to diagnose (NND), and number needed to treat (NNT) to avoid
one prostate cancer-specific death.
|
NNI |
NND/NNT |
| Prostate cancer [24] |
570 |
18 |
| Colon cancer [26] |
3333 (PPA: 667) |
– |
| Breast cancer [27] |
337–2000 |
– |
| Beta blocker after myocardial infarction
[66] |
– |
42 |
| Antiplatelet medication after myocardial
infarction [66] |
– |
153 |
| Statins after myocardial infarction [66] |
– |
94 |
| ICD implantation [67] |
– |
6–22 |
Prostate cancer screening in Europe
In Switzerland, organised programs for screening breast and
colorectal cancer are active. Additionally, efforts are underway to establish a
similar program for detecting lung cancer [29]. However, regarding prostate
cancer, no comparable initiative has been considered thus far.
Across other European countries, the topic of screening for
prostate cancer has been the subject of intense discussion in recent years,
driven in part by the efforts of the European Association of Urology (EAU). In
December 2022, the Council of the European Union updated its recommendations on
cancer prevention, responding to a proposal from the European Commission. This
update included a decision to expand targeted cancer screening to include
prostate cancer based on emerging scientific evidence [8, 30]. The recommendation
urges
member states to explore the feasibility and effectiveness of organised screening
programs for prostate cancer. Sweden has already taken a proactive stance in
this regard. In 2018, the Confederation of Regional Cancer Centres in Sweden
was tasked with assisting all regions in establishing organised prostate cancer
testing programs [31]. This initiative was spurred
by various factors, including prostate cancer being the leading cause of cancer
death in Sweden, unlike the rest of Europe, where lung cancer holds that
position, owing to Sweden's low smoking rate. As a result, such programs have
been implemented or are in the process of initiation across almost all regions
in Sweden [32].
Reducing harms of prostate cancer screening
The policy change on prostate cancer screening in
Switzerland, the European Union and North America is only partially motivated by (1)
the reported methodological flaws of the PLCO trial, (2) the maturing
results of the ERSPC and (3) the increase in advanced and metastatic prostate
cancer patients through reduced PSA testing. Even so, the policy
change is strongly supported by emerging evidence indicating that innovative novel
diagnostic strategies have the potential to decrease the risk of over diagnosis
and overtreatment resulting from organised screening programs for prostate
cancer. Simultaneously, these strategies aim to improve the detection of
aggressive forms of the disease.
Studies have indicated that in the context of traditional
diagnostic algorithms [33], unorganised, opportunistic
PSA testing is routinely practised in Switzerland with a rate of up to 50%
[34–36] and is associated with a negative cost-benefit ratio [37]. In the current
standard
practice of opportunistic PSA testing, men who receive an abnormal PSA result
would most likely undergo a diagnostic process, including imaging and prostate
biopsy, even though only a small proportion of them are likely to have
aggressive prostate cancer [3, 38]. Moreover, relying on shared decision-making
to guide opportunistic PSA testing has led to an uneven distribution of
prostate cancer screening rates, with higher rates of PSA testing among those
who are wealthier and more educated [34, 35]. Finally, many men diagnosed
with cancer through biopsies opt for either surgery or radiotherapy, sometimes
along with androgen deprivation therapy, even when they have low-risk tumours
that are unlikely to result in cancer-related health problems or death [39].
Recognising the limitations of traditional prostate cancer
diagnosis, intensive research efforts have been made in recent decades to
develop new diagnostic methods and screening algorithms. These are specifically
designed to optimise the identification of men with clinically relevant
prostate cancer, defined as a Gleason Score ≥7 (or ISUP Grade Group ≥2), while minimising
the detection of clinically irrelevant cancers. While the PSA value exhibits
good sensitivity for detecting early-stage prostate cancer, it is characterised
by low specificity. In the moderately elevated range (3–10 ng/ml), the cause is
often non-specific inflammation or hyperplasia rather than carcinoma. While MRI
of the prostate is minimally invasive and radiation-free, a prostate biopsy,
even when performed transperineally according to current standards, is
associated with potential risks [40]. Both MRI and biopsy expose
healthy and asymptomatic men to physical and psychological stress. With improved
diagnostics, rates of false-negative biopsies and the detection of clinically
irrelevant cancers have been significantly reduced. For example, the use of
multiparametric prostate MRI to detect prostate lesions in combination with
targeted prostate biopsies has resulted in a significant reduction in the
detection rate of clinically non-relevant tumours [41]. Moreover, the detection rate
of clinically significant diseases, which were previously often missed by systematic
biopsies (e.g. in the ERSPC study), has been increased through image-guided
fusion biopsies. Indeed, two new prospective randomised studies from Sweden
have demonstrated the benefits of MRI in a screening setting and suggest that
men with a normal MRI may no longer need to undergo a biopsy [42, 43]. Even though
both studies
exclusively used a biparametric MRI without contrast agents (as opposed to
multiparametric MRI), the necessary resources and associated costs on a
population level for such a program, where imaging would only be performed
based on PSA values, cannot be underestimated. Therefore, future screening
programs should evaluate additional triage tests that relieve valuable and time-consuming
MRI resources while still being effective and cost-efficient in screening [44].
Biomarkers as a triage test
PSA is a well-established triage test for screening. Below a
value of 1.5 μg/l, the risk of clinically relevant prostate cancer is extremely
low. Fortunately, 65% of men (across all age groups) exhibit a value lower than
this threshold [45].
However, over one-third of men above 40 exhibit a PSA level
above this threshold. A higher cut-off in the range of 3.0 to 4.0 μg/l has been
used in most screening trials to reduce the number of false positive tests [46], but
a relevant number of prostate
cancers in the range of 1.5 to 3.0 μg/l have been missed with this approach [47].
A suggested concept to
increase the screening program’s efficiency to reduce costs has been the
development of biomarkers as reflex tests in men with elevated PSA as a triage before
imaging. Several tests have become commercially available in recent years, such
as the Prostate Health Index (PHI), isoPSA, Proclarix, PCA3, ExoDx, MyProstateScore
(MPS), Select MDx, 4Kscore and Stockholm3. All tests except the latter two have
been developed and validated exclusively in high-risk populations planned for
prostate biopsy, which questions the calibration in a screening setting. Furthermore,
urine tests require a prior digital rectal examination, making them less
suitable for organised screening. While consistent data on the performance of
the Stockholm3 test in population-based screening programs in Sweden have been
reported, the 4Kscore is currently being investigated in Finland in a
comparable setting. To our knowledge, only Proclarix, SelectMDX and Stockholm3
are commercially available in Switzerland.
The Stockholm-3 test
The Stockholm-3 blood test combines five protein biomarkers
(total PSA, free PSA, human kallikrein 2, beta-microseminoprotein and macrophage-inhibitor
cytokine) and 232 genetic single nucleotide polymorphisms (SNPs). SNPs are
variations in DNA sequences that occur at specific positions within a genome
and are the most common type of genetic variation in humans, which are associated
with certain diseases. The Stockholm-3 test can provide an estimate of the risk
of having clinically relevant prostate cancer with clinical parameters that can
be assessed without the involvement of a specialist (age, family history of
prostate cancer, previous negative prostate biopsy, use of a 5-alpha-reductase
inhibitor) and may thus be used within a population-based prostate cancer screening
program. The test was compared to PSA in two extensive population-based studies
in Sweden [47, 48]. Remarkably, clinically
significant cancers were found in the PSA range of 1.5 to 3.0 μg/l. Depending
on the selected Stockholm-3 threshold (“11%” or “15%” risk of prostate cancer),
the use of the Stockholm3 test in men with a PSA level of >1.5 μg/l may increase
the detection of significant carcinomas by 20% or reduce the number of needed MRIs
by almost half, all without any urological assessment or investigation before
imaging. The validity of the test for Swiss men has been recently shown in a
prospective multicentre trial [49].
The 4Kscore
The 4Kscore test measures free, intact, and total PSA levels
and human kallikrein 2 in the blood and is combined with age, digital rectal
examination findings, and prior biopsy history [50]. This application of the
4Kscore test results in a notable reduction in unnecessary biopsies, ranging
from 30 to 50%, while maintaining >90% detection of Gleason scores ≥7 and
>97% of Gleason scores ≥4+3 = 7 cancers [51, 52]. Although performed prospectively
with a robust methodology, all three trials were performed in men with elevated
PSA, and its performance in a screening setting is eagerly awaited. The ongoing
Finnish ProScreen trial will evaluate a screening algorithm, including PSA, the
4Kscore test, and MRI, with targeted biopsies for a population-based screening
program [53]. Although prostate cancer-specific
mortality after 15 years will be the primary endpoint, data on the performance
and cost-effectiveness of the 4Kscore will soon be available.
Developing a roadmap for prostate cancer screening in Switzerland
Given the described developments, the USPSTF recommendation
against general PSA testing has been withdrawn [11]. In response to the ongoing
developments in the European Union, the Swiss Prostate Cancer Screening Group
took proactive measures. On the 20th of September 2023, they
facilitated an official vote to determine the overall stance of the members of
the SGU/SSU regarding screening for prostate cancer in Switzerland. Closely
following the official statement from the European Union, the following wording
was voted on and agreed upon (Figure 1; 80 yes, 6 abstentions, 0 no):
“Considering the evidence and the significant amount of
ongoing opportunistic screening, Switzerland should take a stepwise approach,
including piloting and further supporting research to evaluate the feasibility
of implementation of organised programs aimed at assuring appropriate
management and quality of prostate cancer screening.”
Starting from this full endorsement, the Swiss Prostate
Cancer Screening Group aims to prepare and execute pilot studies to develop a
roadmap for the potential introduction of prostate cancer screening in Switzerland.
After extensive literature research and including expert opinions, the group
has identified several areas to be considered in future research.
Identification of ideal age groups for prostate cancer screening
Recent study results from a randomised Swedish screening
cohort of the ERSPC Trials ("Göteborg-1") indicated that systematic
early PSA testing provides a significant survival advantage [54]. If the first PSA
screening
is conducted at age 55, the risk of death from prostate cancer is halved compared
to men who receive their first PSA test at age 60. Others have proposed to
begin screening at an earlier age, as the PSA value in younger men is less
influenced by confounding factors, such as benign prostatic hyperplasia [55]. These
considerations should
be weighed against the potential to detect insignificant prostate cancer at
young ages.
Standardisation and optimisation of active surveillance
One of the fundaments of reducing the risk for overtreatment
includes the concept of active surveillance for prostate cancer. Long-term
studies have recently demonstrated that active surveillance is a safe approach
for low-risk prostate cancer [56, 57] and should, therefore, be
investigated and implemented further to establish standardised inclusion
criteria and follow-up routines in Switzerland. In this context, it should be
evaluated if the inclusion criteria for active surveillance might include
patients with favourable ISUP Grade 2 and otherwise low-risk features [58].
Cost-effectiveness analyses
First, the willingness of the Swiss male population to
undergo different screening scenarios should be evaluated to receive a rough
estimate of the potential costs associated with it. Furthermore, the
cost-effectiveness and cost impact of an organised prostate cancer-screening
program depends on several factors, including the strategies used for
screening. In this context, the performance and the cost-effectiveness of MRI-
and potential biomarker-based screening algorithms (based on local
availability) need to be determined in the context of Switzerland. Hence,
different promising screening algorithms should be evaluated and compared to
the cost-effectiveness of the current situation with ongoing opportunistic PSA testing
in approximately 50% of all Swiss men [34, 35] versus a situation without
screening. This should consider the potential increased uptake of opportunistic
PSA testing, MRI testing and/or biomarker testing in the Swiss male population in
the following years, based on the increased visibility and discussion of
prostate cancer screening in the Swiss and European health politics.
MRI studies
After triage, some men will proceed to receive an MRI of the
prostate. The "Göteborg-2" trial suggested that prostate cancer diagnostics
should include PSA testing and, to prevent over diagnosis, a biopsy may only be
performed in the case of abnormal prostate MRI [42]. This novel approach should also
be evaluated in a Swiss population and compared to screening algorithms that include
“non-targeted” randomised biopsies [41]. The MRI capacity and
associated healthcare resources in Switzerland should also be evaluated, and
the role of biparametric MRI should be investigated further because the latter
could be performed quicker, potentially saving healthcare resources and
allowing a higher capacity with better cost-efficiency [59]. The role of bpMRI as
a
primary screening tool is being evaluated further, and we await the results
(VISIONING, NCT03749993). Finally, our ability to implement artificial
intelligence (AI) to interpret and deliver high-quality imaging beyond expert
centres should be investigated [60], such as within the current
ongoing research of AI in the Prostate Imaging-Cancer AI (PI-CAI) challenge
(https://pi-cai.grand-challenge.org/). Finally, the quality of MRI protocols
and readings is known to differ between institutions, and therefore, protocol
differences and inter-reader variability should be assessed. Moreover, programs
to increase standardisation and comparability throughout Switzerland should be
enforced [61, 62].
Risk calculators and risk factors
Prostate cancer risk calculators can be sophisticated tools
for prostate cancer risk evaluation [63] and have been suggested as a
measure for risk stratification for prostate cancer screening. Such risk
calculators have been derived from specific populations, such as large
prospective trials like the ERSPC [64]. However, they may provide different
discriminative properties when evaluating Swiss men, and their generalizability
and calibration need to be carefully evaluated [65]. Data availability must be
considered when discussing the use of risk calculators for population-based
prostate cancer screening. While some risk calculators rely on anamnestic,
clinical information (e.g., age, family cancer history, personal cancer
history, and use of 5-alpha reductase inhibitors), others require additional
data, such as results from digital rectal examinations, prostate volume, PSA
dynamics, or MRI. In a population-based screening program, such additional
information from testing or imaging may not be available. Therefore, risk
calculators, including readily available anamnestic information, may be
considered for population-based prostate cancer screening, while a more
specific risk prediction could be provided for men for which additional data is
available (e.g., after MRI examination in case of an elevated PSA). Several European-organised
testing programs in the framework of PRAISE-U (https://uroweb.org/praise-u)
will evaluate the feasibility of a risk-adapted approach using risk calculators
in the future.
Conclusion
Recent studies have shown that a contemporary risk-based approach
to prostate cancer screening, which combines PSA testing with MRI and/or modern
biomarkers as well as targeted biopsies, reduces the over diagnosis of
non-life-threatening prostate cancer and improves the identification of men
with clinically relevant prostate cancer. Further research is needed to
determine how these promising novel diagnostic tools and risk stratifications
can be best utilised to optimise individual and population outcomes.
Considering the significant scope of ongoing opportunistic PSA testing in
Switzerland, the introduction of a Swiss population-based organised prostate
cancer screening program should be evaluated following a stepwise approach,
including pilot projects and targeted studies to assess the acceptance, feasibility,
effectiveness and cost-effectiveness of its implementation. These programs
should be supported by health policymakers and coordinated by the Federal
Office of Public Health by leveraging the individual benefits of PSA,
biomarkers and MRI in prostate cancer screening in Switzerland.
Christoph
Würnschimmel
Department
of Urology
Luzerner Kantonsspital
Spitalstrasse
CH-6000 Luzern
christoph.wuernschimmel[at]luks.ch
References
1. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S.
Preventive Services Task Force recommendation statement. Ann Intern Med. 2012 Jul;157(2):120–34.
10.7326/0003-4819-157-2-201207170-00459
2. Jemal A, Culp MB, Ma J, Islami F, Fedewa SA. Prostate Cancer Incidence 5 Years After
US Preventive Services Task Force Recommendations Against Screening. J Natl Cancer
Inst. 2021 Jan;113(1):64–71. 10.1093/jnci/djaa068
3.Welch HG, Albertsen PC. Reconsidering Prostate Cancer Mortality — The Future of PSA
Screening. Malina D, editor. N Engl J Med. 2020 Apr 16;382(16):1557–63.
4. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin.
2023 Jan;73(1):17–48. 10.3322/caac.21763
5. Desai MM, Cacciamani GE, Gill K, Zhang J, Liu L, Abreu A, et al. Trends in Incidence
of Metastatic Prostate Cancer in the US. JAMA Netw Open. 2022 Mar;5(3):e222246. 10.1001/jamanetworkopen.2022.2246
6. Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, et al.;
US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services
Task Force Recommendation Statement. JAMA. 2018 May;319(18):1901–13. 10.1001/jama.2018.3710
7. Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic
prostate cancer in the United States (2004-2013). Prostate Cancer Prostatic Dis. 2016 Dec;19(4):395–7.
10.1038/pcan.2016.30
8. Council Recommendation on strengthening prevention through early detection: A new
EU approach on cancer screening replacing Council Recommendation 2003/878/EC. Available
from: https://ec.europa.eu/commission/presscorner/detail/en/ip_22_7548
9. Rao AR, Motiwala HG, Karim OM. The discovery of prostate-specific antigen. BJU Int.
2007 Aug.
10. Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, et al. Measurement
of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl
J Med. 1991 Apr;324(17):1156–61. 10.1056/NEJM199104253241702
11. Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al.; ERSPC Investigators.
Screening and prostate-cancer mortality in a randomized European study. N Engl J Med.
2009 Mar;360(13):1320–8. 10.1056/NEJMoa0810084
12. Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al.; PLCO Project
Team. Mortality results from a randomized prostate-cancer screening trial. N Engl
J Med. 2009 Mar;360(13):1310–9. 10.1056/NEJMoa0810696
13. Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al.; PLCO Project
Team. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and
Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl
Cancer Inst. 2012 Jan;104(2):125–32. 10.1093/jnci/djr500
14. Pinsky PF, Parnes HL, Andriole G. Mortality and complications after prostate biopsy in the Prostate, Lung, Colorectal and Ovarian Cancer Screening (PLCO) trial. BJU Int.
2014 Feb;113(2):254–9. 10.1111/bju.12368
15. Swiss Medical Board (SMB). Stellenwert des PSA-Wertes bei der Früherkennung des Prostatakarzinoms.
Health Technology Assessment Report. Available from: https://www.swissmedicalboard.ch
16. Gasser T, Iselin C, Jichlinksi P, Kreienbühl B, Merz V, Recker F, et al. PSA-Bestimmung ‒ Empfehlungen der Schweizerischen Gesellschaft für Urologie (SGU). Swiss Med
Forum ‒ Schweizerisches Medizin-Forum. 2012 Feb 8;12(06).
17. Mottet N, Cornford P, Van den Bergh R, et al. EAU - EANM - ESTRO - ESUR - ISUP - SIOG
Guidelines on Prostate Cancer 2023. European Association of Urology Guidelines. 2023
Edition. Arnhem, The Netherlands: European Association of Urology Guidelines Office;
2023., Available from https://uroweb.org/guidelines/prostate-cancer
18. Wei JT, Barocas D, Carlsson S, Coakley F, Eggener S, Etzioni R, et al. Early Detection
of Prostate Cancer: AUA/SUO Guideline Part I: Prostate Cancer Screening. J Urol. 2023 Jul;210(1):46–53.
10.1097/JU.0000000000003491
19. Cooperberg MR. Prostate cancer: why the prostate arm of the PLCO trial failed and
what it has taught us. Nat Rev Urol. 2016 Aug;13(8):439–40. 10.1038/nrurol.2016.116
20. Shoag JE, Mittal S, Hu JC. Reevaluating PSA Testing Rates in the PLCO Trial. N Engl
J Med. 2016 May;374(18):1795–6. 10.1056/NEJMc1515131
21. Pinsky PF, Andriole GL, Kramer BS, Hayes RB, Prorok PC, Gohagan JK; Prostate, Lung,
Colorectal and Ovarian Project Team. Prostate biopsy following a positive screen in
the prostate, lung, colorectal and ovarian cancer screening trial. J Urol. 2005 Mar;173(3):746–50.
10.1097/01.ju.0000152697.25708.71
22. Pinsky PF, Prorok PC, Yu K, Kramer BS, Black A, Gohagan JK, et al. Extended mortality
results for prostate cancer screening in the PLCO trial with median follow-up of 15
years. Cancer. 2017 Feb;123(4):592–9. 10.1002/cncr.30474
23. Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al.; ERSPC Investigators.
Screening and prostate cancer mortality: results of the European Randomised Study
of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014 Dec;384(9959):2027–35.
10.1016/S0140-6736(14)60525-0
24. Hugosson J, Roobol MJ, Månsson M, Tammela TL, Zappa M, Nelen V, et al.; ERSPC investigators.
A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer.
Eur Urol. 2019 Jul;76(1):43–51. 10.1016/j.eururo.2019.02.009
25. Schröder FH, Hugosson J, Carlsson S, Tammela T, Määttänen L, Auvinen A, et al. Screening
for prostate cancer decreases the risk of developing metastatic disease: findings
from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur Urol.
2012 Nov;62(5):745–52. 10.1016/j.eururo.2012.05.068
26. Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al.; NordICC
Study Group. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related
Death. N Engl J Med. 2022 Oct;387(17):1547–56. 10.1056/NEJMoa2208375
27. Gøtzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database
Syst Rev. 2006 Oct 18;(4):CD001877. doi: 10.1002/14651858.CD001877.pub2. Update in: Cochrane Database Syst Rev. 2009;(4):CD001877. PMID: 17054145. 10.1002/14651858.CD001877.pub3
28. Draft for Update of USPSTF Statement on Prostate Cancer Screening. Available from:
https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/prostate-cancer-screening-adults
29. Jungblut L, von Garnier C, Puhan M, Tomonaga Y, Kaufmann C, Azzola A, et al. The Swiss
Approach - feasibility of a national low-dose CT lung cancer screening program. Swiss
Med Wkly. 2022 Apr;152(15-16):w30154. 10.4414/SMW.2022.w30154
30. Proposal for a Council Recommendation on strengthening prevention through early detection:
A new EU approach on cancer screening replacing Council Recommendation 2003/878/EC.
Available from: https://health.ec.europa.eu/system/files/2022-09/com_2022-474_annex_en.pdf
31. Bratt O, Godtman RA, Jiborn T, Wallström J, Akre O, Carlsson S, et al. Population-based
Organised Prostate Cancer Testing: Results from the First Invitation of 50-year-old
Men. Eur Urol. 2024 Mar;85(3):207–14. 10.1016/j.eururo.2023.11.013
32. Swedish Organised Prostate Cancer Testing (OPT). Available from: https://cancercentrum.se/samverkan/vara-uppdrag/prevention-och-tidig-upptackt/prostatacancertestning/organised-prostate-cancer-testing/
33. Wurnschimmel C, Grande P, Moschini M, Ferrari M, Mordasini L, Mattei A. Accuracy of
standardized 12-core template biopsies versus non-standardized biopsies for detection
of Epstein Grade 5 prostate cancer regarding the histology of the prostatectomy specimen.
Prostate . 2018/01/26. 2018;78(5):365–9.
34. Ulyte A, Wei W, Dressel H, Gruebner O, von Wyl V, Bähler C, et al. Variation of colorectal,
breast and prostate cancer screening activity in Switzerland: Influence of insurance,
policy and guidelines. Van Hemelrijck M, editor. PLoS One. 2020 Apr 16;15(4):e0231409.
35. Guessous I, Cullati S, Fedewa SA, Burton-Jeangros C, Courvoisier DS, Manor O, et al. Prostate
cancer screening in Switzerland: 20-year trends and socioeconomic disparities. Prev
Med. 2016 Jan;82:83–91. 10.1016/j.ypmed.2015.11.009
36. Scherer TP, Saba K, Wettstein MS, Lucca I, Mortezavi A, Waisbrod S, et al. Do Swiss
urologists and Swiss internists screen themselves and their relatives for prostate
cancer? A questionnaire study. Swiss Med Wkly. 2023 Sep;153(9):40115. 10.57187/smw.2023.40115
37. Vickers A, O’Brien F, Montorsi F, Galvin D, Bratt O, Carlsson S, et al. Current policies
on early detection of prostate cancer create overdiagnosis and inequity with minimal
benefit. BMJ. 2023 May;381:e071082. 10.1136/bmj-2022-071082
38. Shoag JE, Nyame YA, Gulati R, Etzioni R, Hu JC. Reconsidering the Trade-offs of Prostate
Cancer Screening. N Engl J Med. 2020 Jun;382(25):2465–8. 10.1056/NEJMsb2000250
39. Washington SL 3rd, Jeong CW, Lonergan PE, Herlemann A, Gomez SL, Carroll PR, et al. Regional
Variation in Active Surveillance for Low-Risk Prostate Cancer in the US. JAMA Netw
Open. 2020 Dec;3(12):e2031349. 10.1001/jamanetworkopen.2020.31349
40. Hogenhout R, Remmers S, van Leenders GJ, Roobol MJ. The transition from transrectal
to transperineal prostate biopsy without antibiotic prophylaxis: cancer detection
rates and complication rates. Prostate Cancer Prostatic Dis. 2023 Sep;26(3):581–7.
10.1038/s41391-022-00641-3
41. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et
al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med .
2018/03/20. 2018;378(19):1767–77.
42. Hugosson J, Månsson M, Wallström J, Axcrona U, Carlsson SV, Egevad L, et al.; GÖTEBORG-2
Trial Investigators. Prostate Cancer Screening with PSA and MRI Followed by Targeted
Biopsy Only. N Engl J Med. 2022 Dec;387(23):2126–37. 10.1056/NEJMoa2209454
43. Nordström T, Discacciati A, Bergman M, Clements M, Aly M, Annerstedt M, et al.; STHLM3
study group. Prostate cancer screening using a combination of risk-prediction, MRI,
and targeted prostate biopsies (STHLM3-MRI): a prospective, population-based, randomised,
open-label, non-inferiority trial. Lancet Oncol. 2021 Sep;22(9):1240–9. 10.1016/S1470-2045(21)00348-X
44. Würnschimmel C, Chandrasekar T, Hahn L, Esen T, Shariat SF, Tilki D. MRI as a screening
tool for prostate cancer: current evidence and future challenges. World J Urol. 2023 Apr;41(4):921–8.
10.1007/s00345-022-03947-y
45. Crawford ED, Rosenberg MT, Partin AW, Cooperberg MR, Maccini M, Loeb S, et al. An
Approach Using PSA Levels of 1.5 ng/mL as the Cutoff for Prostate Cancer Screening
in Primary Care. Urology. 2016 Oct;96:116–20. 10.1016/j.urology.2016.07.001
46. Lin K, Croswell J, Koenig H. Prostate-Specific Antigen-Based Screening for Prostate
Cancer: An Evidence Update for the U.S. Preventive Services Task Force. Rockv Agency
Healthc Res Qual (US); Evidence S.
47. Grönberg H, Adolfsson J, Aly M, Nordström T, Wiklund P, Brandberg Y, et al. Prostate
cancer screening in men aged 50-69 years (STHLM3): a prospective population-based
diagnostic study. Lancet Oncol. 2015 Dec;16(16):1667–76. 10.1016/S1470-2045(15)00361-7
48. Grönberg H, Eklund M, Picker W, Aly M, Jäderling F, Adolfsson J, et al. Prostate Cancer
Diagnostics Using a Combination of the Stockholm3 Blood Test and Multiparametric Magnetic
Resonance Imaging. Eur Urol. 2018 Dec;74(6):722–8. 10.1016/j.eururo.2018.06.022
49. Elyan A, Saba K, Sigle A, Wetterauer C, Engesser C, Püschel H, et al. Prospective
Multicenter Validation of the Stockholm3 Test in a Central European Cohort. Eur Urol
Focus. 2023 Oct;S2405-4569(23)00216-X. 10.1016/j.euf.2023.09.016
50. Parekh DJ, Punnen S, Sjoberg DD, Asroff SW, Bailen JL, Cochran JS, et al. A multi-institutional
prospective trial in the USA confirms that the 4Kscore accurately identifies men with
high-grade prostate cancer. Eur Urol. 2015 Sep;68(3):464–70. 10.1016/j.eururo.2014.10.021
51. Bryant RJ, Sjoberg DD, Vickers AJ, Robinson MC, Kumar R, Marsden L, et al. Predicting
high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured
in blood in the ProtecT study. J Natl Cancer Inst. 2015 Apr;107(7):djv095. 10.1093/jnci/djv095
52. Bhattu AS, Zappala SM, Parekh DJ, Punnen S. A 4Kscore Cut-off of 7.5% for Prostate
Biopsy Decisions Provides High Sensitivity and Negative Predictive Value for Significant
Prostate Cancer. Urology. 2021 Feb;148:53–8. 10.1016/j.urology.2020.11.008
53. Auvinen A, Rannikko A, Taari K, Kujala P, Mirtti T, Kenttämies A, et al. A randomized
trial of early detection of clinically significant prostate cancer (ProScreen): study
design and rationale. Eur J Epidemiol. 2017 Jun;32(6):521–7. 10.1007/s10654-017-0292-5
54. Carlsson SV, Arnsrud Godtman R, Pihl CG, Vickers A, Lilja H, Hugosson J, et al. Young
Age on Starting Prostate-specific Antigen Testing Is Associated with a Greater Reduction
in Prostate Cancer Mortality: 24-Year Follow-up of the Göteborg Randomized Population-based
Prostate Cancer Screening Trial [Erratum in: Eur Urol. 2022 Dec 8; PMID: 36334968;
PMCID: PMC10481420]. Eur Urol. 2023 Feb;83(2):103–9. 10.1016/j.eururo.2022.10.006
55. Vickers AJ, Ulmert D, Sjoberg DD, Bennette CJ, Bjork T, Gerdtsson A, et al. Strategy
for detection of prostate cancer based on relation between prostate specific antigen
at age 40-55 and long term risk of metastasis: case-control study. BMJ . 2013 Apr 16;346(apr15
5):f2023–f2023.
56. Ventimiglia E, Bill-Axelson A, Bratt O, Montorsi F, Stattin P, Garmo H. Long-term
Outcomes Among Men Undergoing Active Surveillance for Prostate Cancer in Sweden. JAMA
Netw Open. 2022 Sep;5(9):e2231015. 10.1001/jamanetworkopen.2022.31015
57. Hamdy FC, Donovan JL, Lane JA, Metcalfe C, Davis M, Turner EL, et al.; ProtecT Study
Group. Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate
Cancer. N Engl J Med. 2023 Apr;388(17):1547–58. 10.1056/NEJMoa2214122
58. Willemse PM, Davis NF, Grivas N, Zattoni F, Lardas M, Briers E, et al. Systematic
Review of Active Surveillance for Clinically Localised Prostate Cancer to Develop
Recommendations Regarding Inclusion of Intermediate-risk Disease, Biopsy Characteristics
at Inclusion and Monitoring, and Surveillance Repeat Biopsy Strategy. Eur Urol. 2022 Apr;81(4):337–46.
10.1016/j.eururo.2021.12.007
59. Porter KK, King A, Galgano SJ, Sherrer RL, Gordetsky JB, Rais-Bahrami S. Financial
implications of biparametric prostate MRI. Prostate Cancer Prostatic Dis. 2020 Mar;23(1):88–93.
10.1038/s41391-019-0158-x
60. Winkel DJ, Wetterauer C, Matthias MO, Lou B, Shi B, Kamen A, et al. Autonomous Detection
and Classification of PI-RADS Lesions in an MRI Screening Population Incorporating
Multicenter-Labeled Deep Learning and Biparametric Imaging: Proof of Concept. Diagnostics
(Basel, Switzerland) . 2020 Nov 14;10(11).
61. Giganti F, Ng A, Asif A, Chan VW, Rossiter M, Nathan A, et al.; PRIME Quality Improvement
Group. Global Variation in Magnetic Resonance Imaging Quality of the Prostate. Radiology.
2023 Oct;309(1):e231130. 10.1148/radiol.231130
62. Giganti F, Allen C, Emberton M, Moore CM, Kasivisvanathan V; PRECISION study group.
Prostate Imaging Quality (PI-QUAL): A New Quality Control Scoring System for Multiparametric
Magnetic Resonance Imaging of the Prostate from the PRECISION trial. Eur Urol Oncol.
2020 Oct;3(5):615–9. 10.1016/j.euo.2020.06.007
63. Saba K, Wettstein MS, Lieger L, Hötker AM, Donati OF, Moch H, et al. External Validation
and Comparison of Prostate Cancer Risk Calculators Incorporating Multiparametric Magnetic
Resonance Imaging for Prediction of Clinically Significant Prostate Cancer. J Urol.
2020 Apr;203(4):719–26. 10.1097/JU.0000000000000622
64. SWOP Prostate Cancer Research Foundation Risk Calculator. https://www.prostatecancer-riskcalculator.com/
65. Poyet C, Wettstein MS, Lundon DJ, Bhindi B, Kulkarni GS, Saba K, et al. External Evaluation
of a Novel Prostate Cancer Risk Calculator (ProstateCheck) Based on Data from the
Swiss Arm of the ERSPC. J Urol. 2016 Nov;196(5):1402–7. 10.1016/j.juro.2016.05.081
66. Ong HT. β blockers in hypertension and cardiovascular disease. BMJ. 2007 May;334(7600):946–9.
10.1136/bmj.39185.440382.47
67. Uhlig K, Balk EM, Earley A, Persson R, Garlitski AC, Chen M, Lamont JL, Miligkos M,
Avendano EE. Assessment on Implantable Defibrillators and the Evidence for Primary
Prevention of Sudden Cardiac Death [Internet]. Rockville (MD): Agency for Healthcare
Research and Quality (US); 2013 Jun 26. PMID: 25356453.

