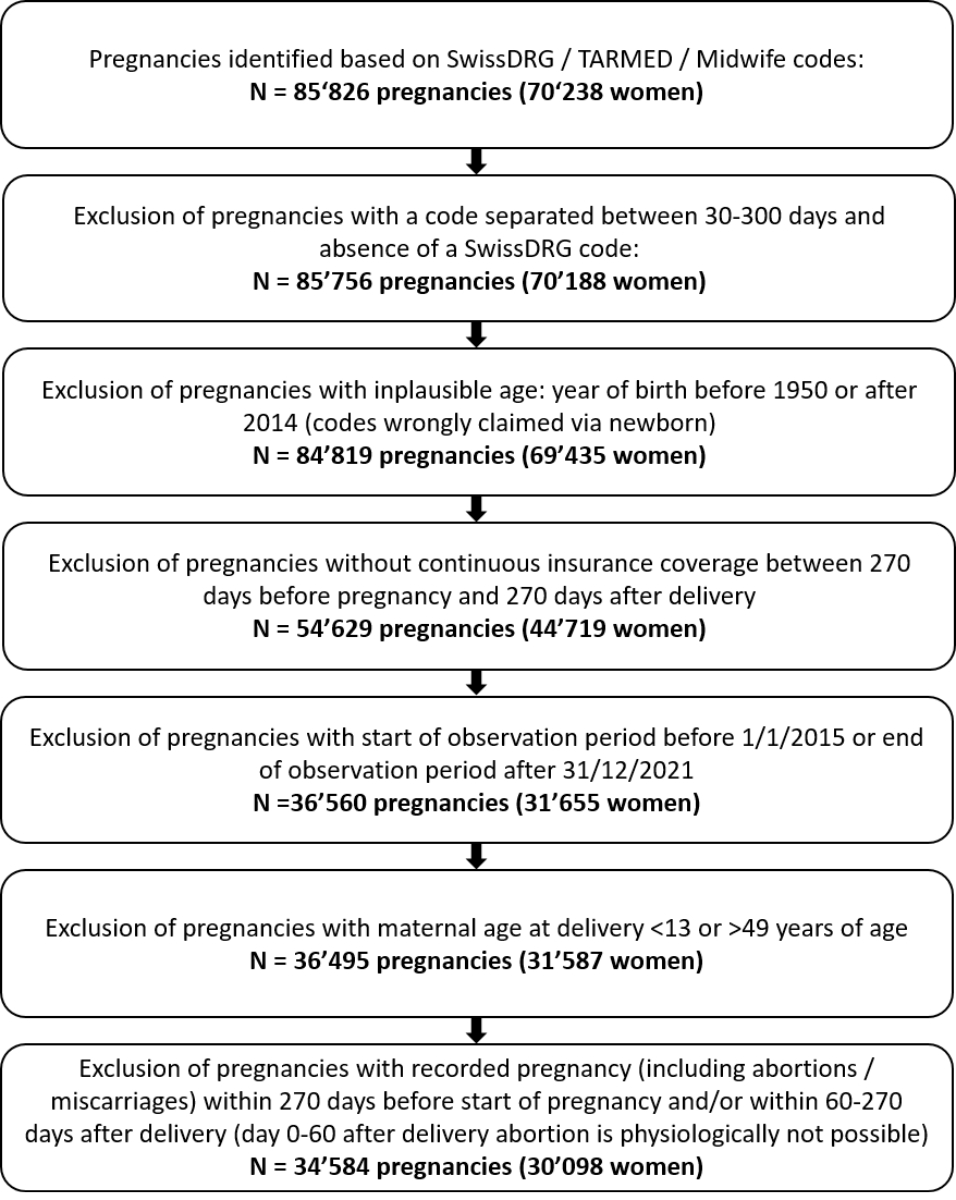
Figure 1Flow chart of the study population.
DOI: https://doi.org/https://doi.org/10.57187/s.3616
Many women require drug treatment during pregnancy to treat pre-existing or incident diseases, or obstetric complications. However, the safety of many drugs during pregnancy is not well understood [1–3] because pregnant women are excluded from most randomized controlled trials (RCTs). Despite this limitation, post-marketing studies allow possible teratogenicity to be evaluated for drugs that have been on the market for a long time. However, for new or rarely used drugs, evidence of in utero safety is insufficient or absent. In these situations, clinicians need to weigh benefits and risks to the pregnant woman and the unborn child based on insufficient evidence. In Switzerland, the use of prescribed drugs during pregnancy is not well understood, partly due to the fragmented health data landscape and the underdeveloped digitalization in the healthcare sector. It is important to understand which drugs are used during pregnancy in clinical practice to understand the medical needs and potential unknown risks to this vulnerable patient population. In a multinational web-based survey [4], 81% of pregnant women reported taking at least one drug during pregnancy, whether prescribed or over the counter (OTC), between 2011 and 2012. Of the 618 Swiss women who took part in the survey, 83% took at least one drug during pregnancy (excluding iron, mineral supplements, vitamins, herbal remedies, and any type of complementary medicine). However, this data is over a decade old and may be affected by volunteer bias. Three studies based on the Helsana claims database (2014–2018) quantified disease-specific utilisation of drugs to treat selected acute or chronic conditions during pregnancy [5–7], but did not evaluate the overall drug burden during pregnancy in Switzerland.
In this retrospective descriptive study based on the Swiss Helsana claims database, we evaluated the utilisation of all prescribed drugs dispensed during pregnancy in outpatient care in Switzerland between 2015 and 2021.
We conducted a retrospective study using the anonymized Helsana claims database for the years 2015–2021. The Helsana group is one of Switzerland’s leading health insurance companies, covering around 1.2 million individuals with mandatory health insurance from all 26 cantons (approximately 15% of the Swiss population). The database captures demographics, outpatient healthcare services, outpatient drug dispensations, as well as bundled diagnostic codes for hospitalizations (SwissDRG, Swiss Diagnoses Related Groups). For dispensation of prescription drugs, the corresponding codes of the anatomical therapeutic chemical (ATC) classification system are recorded.
This retrospective observational study using anonymous data did not require an ethics committee approval.
Patient consent was waived due to use of anonymous data.
Our study population included pregnant women between 13 and 49 years of age at delivery between 2015 and 2021. All women were (1) continuously insured with Helsana’s mandatory health insurance during the entire observation period (270 days before the start of pregnancy until 270 days after the delivery date), and (2) not pregnant during the pre-pregnancy or postpartum period (determined by recorded codes for delivery or abortion, reported in table S1 in the appendix). A woman may have contributed more than one pregnancy to the study population. A flow chart of cohort enrolment is shown in figure 1.

Figure 1Flow chart of the study population.
We identified inpatient and outpatient deliveries, and both live and stillbirths, covered by mandatory health insurance in the Helsana claims database between 01/01/2015 and 31/12/2021. Inpatient deliveries were captured by recorded SwissDRG codes, and outpatient deliveries by recorded TARMED codes (billing system for outpatient services in Switzerland) or billed deliveries by midwifes (table S1 in the appendix). In case there were multiple delivery codes recorded within a period of 30 days, these were regarded as pertaining to the same pregnancy [8], and the first recorded code was set as the date of delivery. Delivery codes separated by more than 300 days were considered as two separate pregnancies. When two successive codes were separated by between 30 and 300 days, the delivery date was set as the inpatient SwissDRG code, and were excluded if only outpatient codes were recorded [5–7]. Deliveries of twins were treated as one single pregnancy. A flow chart showing the number of excluded pregnancies is displayed in figure 1.
We estimated the date of the last menstrual period (i.e., start of pregnancy) because gestational length or start of pregnancy were not recorded in Swiss healthcare claims data during the study period. According to an algorithm validated in US claims data [9], the date of the last menstrual period was assigned to be 245 days before the date of delivery for pregnancies with a SwissDRG code indicating preterm delivery (<37 weeks, O01A, O01B, O01C, O01D, or O60A), and 270 days before the date of delivery for all other pregnancies. This algorithm has been used in previous studies evaluating pregnancies in the Helsana claims database [5–7].
We observed each pregnancy from 270 days before the last menstrual period (start of observation period) until 270 days after the delivery date (end of observation period). The observation period was divided into the following periods: a) pre-pregnancy period (270-day period before last menstrual period), b) pregnancy, and c) postpartum period (270-day period after the delivery date). Pregnancy was divided into trimesters of 90 days, and trimester 3 was shortened in case of a preterm delivery.
We captured maternal age at delivery. We identified reimbursed dispensing of any drugs based on recorded ATC codes [10]. Analysis omitted ATC codes not representing pharmacological treatment (e.g., prophylactic agents, surgical aids, or diagnostic agents; see full list with ATC codes in table S2 in the appendix). Due to the data structure of Swiss electronic claims data, we were not able to identify the indications for individual drugs.
We quantified the median (interquartile range, IQR) total number of drug dispensations (and the median (IQR) number of distinct drug dispensations separately) during the pre-pregnancy period, during pregnancy (overall and by trimester), and during the postpartum period. Analyses were conducted overall and within maternal age strata (<26, 26–35, and ≥36 years at delivery). We quantified the results with and without considering vitamins, mineral supplements, iron preparations, vitamin B12, folic acid, iodine therapy, and vaccines (ATC codes in appendix: table S2), as most women are exposed to those drugs during pregnancy.
We quantified (a) the proportion of pregnancies exposed to at least one dispensed drug (prevalence of exposure), and (b) the cumulative number of distinct dispensed drugs (0, 1, 2, 3, 4, and ≥5) per pregnancy during each period and within maternal age strata. Prevalence of exposure was quantified both with and without the dispensing of vitamins, mineral supplements, iron preparations, vitamin B12, folic acid, iodine therapy, and vaccines. For all other analyses, we did not consider those treatments.
We identified the 15 most frequently dispensed drugs (ATC 7 digits), quantified as the absolute number of exposed pregnancies divided by the total number of enrolled pregnancies, during the pre-pregnancy period, during pregnancy (overall, by trimester, and by maternal age strata), and during the postpartum period.
For all results, weighted numbers are shown in the appendix. Weighted results account for the demographic distribution of the population with mandatory insurance with Helsana relative to the Swiss population [5–7]. Weighting factors included calendar year, canton, age, and sex. All analyses were conducted using Python 3.11.0 [11].
The dataset included 34,584 pregnancies of 30,098 women (figure 1) with a median maternal age at delivery of 32 years (IQR = 29–36), 10.2% aged <26 years, 62.4% aged between 26–35 years, and 27.4% aged ≥36 years at delivery.
The median number of claimed drug dispensations during pregnancy was 8 (IQR = 5–13) and 6 (IQR = 3–8) in the case of distinct drugs. After excluding vitamins, supplements, and vaccines, these numbers decreased to 4 (IQR = 2–8) and 3 (IQR = 1–5). The median number of dispensed drugs (not including vitamins, supplements, and vaccines) during pregnancy (table 1) was highest among women aged <26 years at delivery (5, IQR = 2–9; distinct drug dispensations median = 4, IQR = 2–6) and similar for women aged 26–35 (median = 4, IQR = 1–8; distinct drug dispensations median = 3, IQR = 1–5) and for women aged ≥36 (median = 4, IQR = 2–9; distinct drug dispensations median = 3, IQR = 1–6).
Table 1Median (IQR) number of drug dispensations by period and within maternal age strata.
| Pre-pregnancy period | Pregnancy | Trimester 1 | Trimester 2 | Trimester 3 | Postpartum | ||||||||
| Age at delivery (years) | N pregnancies | Median (IQR) number of drug claims | Median (IQR) number of distinct drug claims* | Median (IQR) number of drug claims | Median (IQR) number of distinct drug claims* | Median (IQR) number of drug claims | Median (IQR) number of distinct drug claims* | Median (IQR) number of drug claims | Median (IQR) number of distinct drug claims* | Median (IQR) number of drug claims | Median (IQR) number of distinct drug claims* | Median (IQR) number of drug claims | Median (IQR) number of distinct drug claims* |
| All drug dispensations | |||||||||||||
| Total | 34584 | 3 (0–8) | 3 (0–6) | 8 (5–13) | 6 (3–8) | 2 (1–4) | 2 (1–3) | 2 (1–4) | 2 (1–4) | 3 (1–5) | 2 (1–4) | 5 (2–8) | 4 (2–7) |
| <26 | 3513 | 4 (1–9) | 3 (1–7) | 9 (5–13) | 6 (4–9) | 2 (1–4) | 2 (1–4) | 3 (1–5) | 2 (1–4) | 3 (1–5) | 2 (1–4) | 5 (2–9) | 4 (2–7) |
| 26–35 | 21592 | 3 (0–7) | 2 (0–6) | 8 (4–13) | 5 (3–8) | 2 (0–4) | 2 (0–3) | 2 (1–4) | 2 (1–4) | 3 (1–5) | 2 (1–4) | 4 (2–8) | 4 (2–6) |
| ≥36 | 9479 | 3 (1–8) | 3 (1–6) | 9 (5–14) | 6 (3–8) | 2 (0–4) | 2 (0–3) | 2 (1–5) | 2 (1–4) | 3 (2–6) | 3 (1–4) | 5 (2–9) | 4 (2–7) |
| Without vitamins, mineral supplements, iron preparations, vitamin B12, folic acid, iodine therapy, and vaccines | |||||||||||||
| Total | 34584 | 2 (0–7) | 2 (0–5) | 4 (2–8) | 3 (1–5) | 0 (1–3) | 1 (0–2) | 0 (1–2) | 1 (0–2) | 0 (1–3) | 1 (0–2) | 4 (2–7) | 3 (2–5) |
| <26 | 3513 | 3 (0–8) | 3 (0–6) | 5 (2–9) | 4 (2–6) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 4 (2–8) | 3 (2–6) |
| 26–35 | 21592 | 2 (0–6) | 2 (0–5) | 4 (1–8) | 3 (1–5) | 1 (0–3) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–3) | 1 (0–2) | 3 (2–7) | 3 (2–5) |
| ≥36 | 9479 | 3 (0–7) | 2 (0–5) | 4 (2–9) | 3 (1–6) | 1 (0–3) | 1 (0–2) | 1 (0–3) | 1 (0–2) | 1 (0–3) | 1 (0–3) | 4 (2–7) | 3 (2–5) |
IQR: interquartile range.
* Number of distinct drugs (distinct ATC-7 level) claimed during the indicated period, overall and within age strata.
Figure 2 shows the prevalence of exposure to at least one dispensed drug by period and within maternal age strata, with and without considering vitamins, supplements, and vaccines. During pregnancy, 87.5% of women claimed at least one drug (97.8% when including vitamins, supplements, and vaccines), with increasing proportions over the course of pregnancy (66.7% in trimester 3 vs. 59.2% and 58.6% in trimesters 1 and 2, respectively). Compared to older women, women aged <26 years more frequently claimed at least one drug during pregnancy (91.4% vs. 86.9% in 26–35 and 87.5% in ≥36 years).
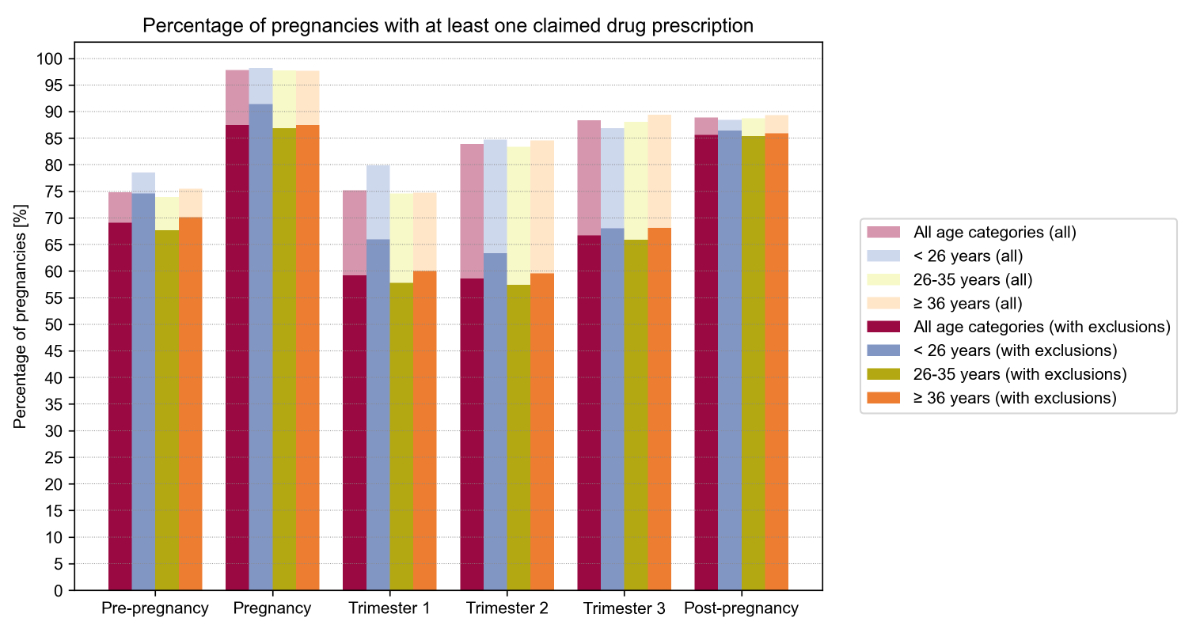
Figure 2Prevalence of exposure to at least one dispensed drug during the pre-pregnancy, pregnancy (overall and by trimester), and postpartum periods, overall and within maternal age strata, with and without considering vitamins, mineral supplements, iron preparations, vitamin B12, folic acid, iodine therapy, and vaccines. See table S3 in the appendix for exact values and for weighted results.
During the pre-pregnancy period, less women (69.1%) claimed at least one drug, whereas during the postpartum period, drug exposure was similar to that during pregnancy (85.6% and 87.5%, respectively). Further numerical data is given in figure 2 and table S3 in the appendix.
Overall, 71.9% of pregnant women claimed two or more distinct drugs during pregnancy (table 2). This is 15.8% more than during the pre-pregnancy period (56.1%), and 4.4% less than during the postpartum period (76.3%). In total, 33.3% of pregnant women claimed at least five distinct drugs during pregnancy (vs. 28.5% before pregnancy and 32.6% after pregnancy), of whom 8.2% claimed at least five distinct drugs during trimester 1 alone (vs. 6.8% and 7.8% in trimesters 2 and 3, respectively).
Table 2Prevalence of exposure to 0, 1, 2, 3, 4, and ≥5 drug dispensations during pre-pregnancy, pregnancy (overall and by trimester), and postpartum periods without considering vitamins, mineral supplements, iron preparations, vitamin B12, folic acid, iodine therapy, and vaccines. See table S4 in the appendix for weighted results.
| Pre-pregnancy period | Pregnancy | Trimester 1 | Trimester 2 | Trimester 3 | Postpartum | |
| Number of distinct drug claims* | N pregnancies (%) | N pregnancies (%) | N pregnancies (%) | N pregnancies (%) | N pregnancies (%) | N pregnancies (%) |
| 0 | 10695 (30.9) | 4324 (12.5) | 14101 (40.8) | 14306 (41.4) | 11509 (33.3) | 4968 (14.4) |
| 1 | 4501 (13.0) | 5395 (15.6) | 7738 (22.4) | 8362 (24.2) | 8910 (25.8) | 3224 (9.3) |
| 2 | 3707 (10.7) | 5127 (14.8) | 4912 (14.2) | 4898 (14.2) | 5765 (16.7) | 5943 (17.2) |
| 3 | 3187 (9.2) | 4535 (13.1) | 3157 (9.1) | 2918 (8.4) | 3666 (10.6) | 5134 (14.8) |
| 4 | 2632 (7.6) | 3702 (10.7) | 1828 (5.3) | 1739 (5.0) | 2034 (5.9) | 4032 (11.7) |
| ≥5 | 9862 (28.5) | 11501 (33.3) | 2848 (8.2) | 2361 (6.8) | 2700 (7.8) | 11283 (32.6) |
ATC: Anatomical Therapeutic Chemical.
* ATC-7 level.
Figure 3 shows the prevalence of exposure to 0, 1, 2, 3, 4, and ≥5 distinct drugs within maternal age strata. Pregnant women <26 years most frequently claimed at least two drugs (79.3%) followed by women ≥36 years (72.4%) and those between 26–35 years (70.5%). The same pattern was observed for exposure to at least five drugs (<26: 41.2%, 26–35: 31.6%, and ≥36: 34.2%).
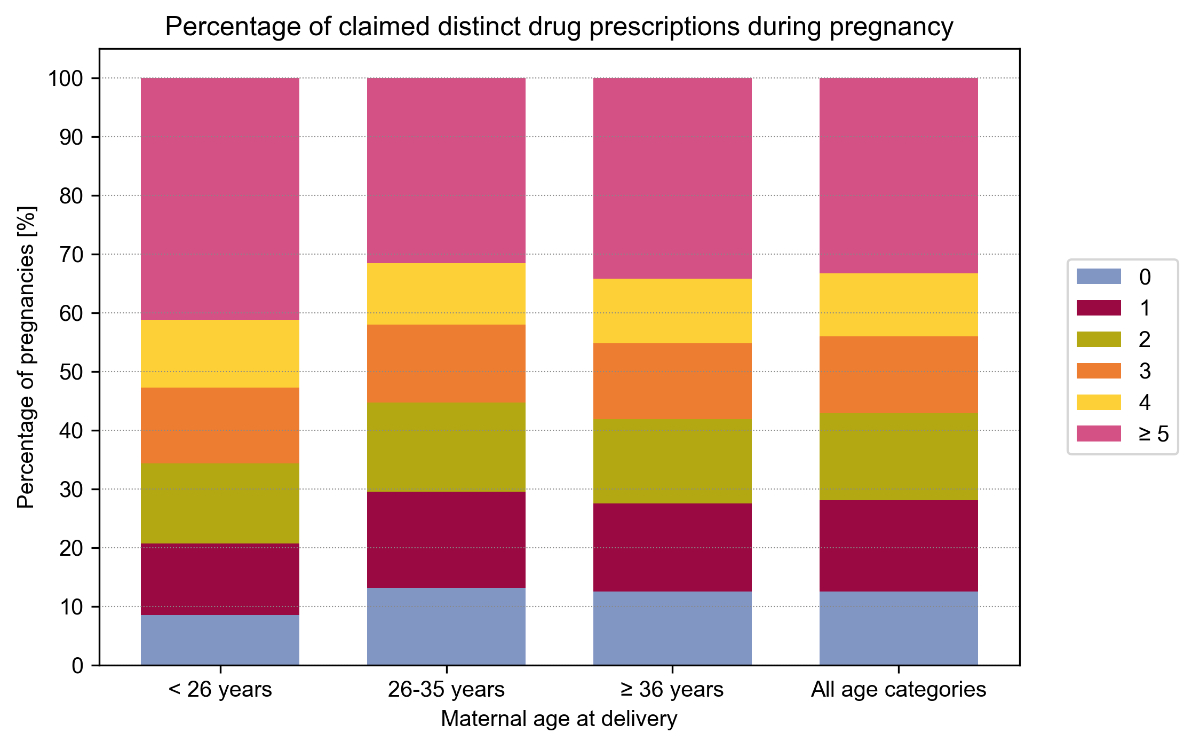
Figure 3Prevalence of exposure to 0, 1, 2, 3, 4, and ≥5 drug dispensations during pre-pregnancy, pregnancy (overall and by trimester), and postpartum periods, overall and within age strata, without considering vitamins, mineral supplements, iron preparations, vitamin B12, folic acid, iodine therapy, and vaccines. Exact values and weighted results are shown in table S5 in the appendix.
Paracetamol was dispensed to the largest proportion of women during pregnancy (29.8% vs. 22.0% before pregnancy and 63.3% after pregnancy, table 3).
Table 3Prevalence of exposure to the 15 most frequently dispensed drugs during pre-pregnancy, pregnancy (overall and by trimester), and postpartum periods, without considering vitamins, mineral supplements, iron preparations, vitamin B12, folic acid, iodine therapy, and vaccines. See table S6 in the appendix for weighted results.
| Pre-pregnancy period | Pregnancy | Trimester 1 | Trimester 2 | Trimester 3 | Postpartum | |||||||
| Rank | Drug substance (ATC code) | % of exposed pregnancies | Drug substance (ATC code) | % of exposed pregnancies | Drug substance (ATC code) | % of exposed pregnancies | Drug substance (ATC code) | % of exposed pregnancies | Drug substance (ATC code) | % of exposed pregnancies | Drug substance (ATC code) | % of exposed pregnancies |
| 1 | Paracetamol (N02BE01) | 22.0 | Paracetamol (N02BE01) | 29.8 | Paracetamol (N02BE01) | 13.1 | Paracetamol (N02BE01) | 14.1 | Paracetamol (N02BE01) | 12.0 | Paracetamol (N02BE01) | 63.3 |
| 2 | Ibuprofen (M01AE01) | 20.0 | Dequalinium (G01AC05) | 16.8 | Metoclopramide (A03FA01) | 12.7 | Dequalinium (G01AC05) | 6.6 | Anti-D (rh) immunoglobulin (J06BB01) | 8.2 | Ibuprofen (M01AE01) | 50.4 |
| 3 | Pantoprazole (A02BC02) | 9.0 | Metoclopramide (A03FA01) | 16.0 | Progesterone (G03DA04) | 10.9 | Levothyroxine sodium (H03AA01) | 6.1 | Dequalinium (G01AC05) | 7.3 | Mefenamic acid (M01AG01) | 16.3 |
| 4 | Amoxicillin and enzyme inhibitor (J01CR02) | 7.1 | Progesterone (G03DA04) | 13.4 | Dequalinium (G01AC05) | 6.6 | Amoxicillin and enzyme inhibitor (J01CR02) | 4.5 | Ordinary salt combinations (A02AD01) | 5.9 | Pantoprazole (A02BC02) | 12.2 |
| 5 | Metamizole sodium (N02BB02) | 6.7 | Amoxicillin and enzyme inhibitor (J01CR02) | 11.4 | Levothyroxine sodium (H03AA01) | 5.5 | Ordinary salt combinations (A02AD01) | 3.8 | Levothyroxine sodium (H03AA01) | 5.6 | Amoxicillin and enzyme inhibitor (J01CR02) | 10.7 |
| 6 | Diclofenac (M01AB05) | 5.4 | Anti-D (rh) immunoglobulin (J06BB01) | 11.1 | Ibuprofen (M01AE01) | 3.8 | Metoclopramide (A03FA01) | 3.5 | Pantoprazole (A02BC02) | 5.2 | Diclofenac (M01AB05) | 7.0 |
| 7 | Progesterone (G03DA04) | 5.3 | Ordinary salt combinations (A02AD01) | 10.3 | Amoxicillin and enzyme inhibitor (J01CR02) | 3.7 | Anti-D (rh) immunoglobulin (J06BB01) | 3.5 | Amoxicillin and enzyme inhibitor (J01CR02) | 4.4 | Heparin, combinations (C05BA53) | 5.6 |
| 8 | Mefenamic acid (M01AG01) | 5.2 | Pantoprazole (A02BC02) | 9.4 | Low-dose acetylsalicylic acid (B01AC06) | 3.3 | Low-dose acetylsalicylic acid (B01AC06) | 3.3 | Omeprazole (A02BC01) | 3.4 | Liquid paraffin (A06AA01) | 5.1 |
| 9 | Diclofenac (M02AA15) | 4.6 | Levothyroxine sodium (H03AA01) | 7.9 | Pantoprazole (A02BC02) | 3.1 | Pantoprazole (A02BC02) | 3.1 | Ranitidine (A02BA02) | 3.3 | Macrogol, combinations (A06AD65) | 4.7 |
| 10 | Levothyroxine sodium (H03AA01) | 4.5 | Fosfomycin (J01XX01) | 7.3 | Estriol (G03CC06) | 2.7 | Fosfomycin (J01XX01) | 2.9 | Nifedipine (C08CA05) | 3.2 | Levothyroxine sodium (H03AA01) | 4.7 |
| 11 | Dequalinium (G01AC05) | 4.5 | Estriol (G03CC06) | 7.0 | Fosfomycin (J01XX01) | 2.6 | Estriol (G03CC06) | 2.9 | Esomeprazole (A02BC05) | 3.0 | Dequalinium (G01AC05) | 4.5 |
| 12 | Fluconazole (J02AC01) | 4.4 | Ibuprofen (M01AE01) | 6.2 | Ordinary salt combinations (A02AD01) | 2.2 | Progesterone (G03DA04) | 2.5 | Fluconazole (J02AC01) | 2.8 | Diclofenac (M02AA15) | 4.4 |
| 13 | Fosfomycin (J01XX01) | 4.3 | Clotrimazole (D01AC01) | 5.9 | Clotrimazole (D01AC01) | 1.9 | Clotrimazole (D01AC01) | 2.4 | Fosfomycin (J01XX01) | 2.8 | Cabergoline (G02CB03) | 4.2 |
| 14 | Xylometazoline (R01AA07) | 4.3 | Fluconazole (J02AC01) | 5.6 | Clotrimazole (G01AF02) | 1.6 | Fluconazole (J02AC01) | 2.2 | Estriol (G03CC06) | 2.7 | Metamizole sodium (N02BB02) | 4.1 |
| 15 | Acetylcysteine (R05CB01) | 4.0 | Omeprazole (A02BC01) | 5.4 | Estradiol (G03CA03) | 1.6 | Ibuprofen (M01AE01) | 2.1 | Insulin detemir (A10AE05) | 2.5 | Fluconazole (J02AC01) | 4.1 |
ATC: anatomical therapeutic chemical.
In trimester 1, after paracetamol, metoclopramide (12.7%) was the second most prevalent prescription followed by progesterone (10.9%), topical dequalinium (vaginal antiseptic, 6.6%), levothyroxine (5.5%), ibuprofen (3.8%), amoxicillin (3.7%), low-dose acetylsalicylic acid (ASA, 3.3%), pantoprazole (3.1%), estriol (2.7%), fosfomycin (2.6%), ordinary salt combinations (antacids, 2.2%), clotrimazole (1.6%), and oestradiol (1.6%).
Dequalinium, levothyroxine, amoxicillin, pantoprazole, fosfomycin, and ordinary salt combinations (antacids) were also among the 15 most frequently claimed drugs during trimesters 2 and 3. In trimesters 2 and 3, anti-D (rh) immunoglobulin was also among the 15 most frequently dispensed drugs (3.5% and 8.2% of pregnancies, respectively). In trimester 3, proton pump inhibitors and H2 blockers (omeprazole 3.4%, ranitidine 3.3%, esomeprazole 3.0%), nifedipine (3.2%), and insulin detemir (2.5%) were also among the 15 most frequently dispensed drugs.
During the postpartum period, non-opioid analgesics were the predominantly claimed drugs, with paracetamol (63.3%) being the most prevalent followed by ibuprofen (50.4%), mefenamic acid (16.3%), diclofenac (7.0%), and metamizole (4.1%). Further commonly dispensed drugs after pregnancy were pantoprazole (12.2%), amoxicillin (10.7%), heparin (5.6%), agents used for treatment of constipation based on paraffin (5.1%), macrogol (4.7%), levothyroxine (4.7%), topical dequalinium (4.5%), cabergoline (4.2%), and fluconazole (4.1%).
During pre-pregnancy, commonly prescribed analgesics were also the most prevalent claims, although at a much lower proportion than in the postpartum period (paracetamol 22.0% vs. 63.3% of pregnancies, ibuprofen 20.0% vs. 50.4%, diclofenac 5.4% vs. 7.0%, mefenamic acid 5.2% vs. 16.3%, respectively). Additionally, progesterone was among the most frequently dispensed drugs before pregnancy (5.3%).
To further evaluate pregnant women <26 years of age, who had the highest exposure to at least one dispensed drug during pregnancy (see section “Mean (SD) number of drug dispensations”), we evaluated in a post-hoc analysis the prevalence of the 15 most prevalent drug dispensations within maternal age strata (table 4). Dispensation of most drugs was more prevalent to women <26 years of age than to those between 26–35 and ≥36 years of age, except for progesterone, anti-D (rh) immunoglobulin, levothyroxine sodium, omeprazole, and low dose ASA, which were more prevalent in the older age groups.
Table 4Prevalence of exposure to the 15 most prevalent drug dispensations during pregnancy within maternal age strata (without considering vitamins, mineral supplements, iron preparations, vitamin B12, folic acid, iodine therapy, and vaccines). See table S7 in the appendix for weighted results.
| <26 years (n = 3513) | 26–35 years (n = 21592) | ≥36 years (n = 9479) | ||||
| Rank | Drug substance (ATC code) | % of exposed pregnancies | Drug substance (ATC code) | % of exposed pregnancies | Drug substance (ATC code) | % of exposed pregnancies |
| 1 | Paracetamol (N02BE01) | 42.9 | Paracetamol (N02BE01) | 29.4 | Paracetamol (N02BE01) | 25.8 |
| 2 | Metoclopramide (A03FA01) | 24.5 | Dequalinium (G01AC05) | 17.0 | Progesterone (G03DA04) | 19.5 |
| 3 | Dequalinium (G01AC05) | 24.3 | Metoclopramide (A03FA01) | 16.2 | Dequalinium (G01AC05) | 13.7 |
| 4 | Amoxicillin and enzyme inhibitor (J01CR02) | 15.6 | Progesterone (G03DA04) | 11.4 | Metoclopramide (A03FA01) | 12.5 |
| 5 | Ordinary salt combinations (A02AD01) | 12.4 | Anti-D (rh) immunoglobulin (J06BB01) | 11.1 | Anti-D (rh) immunoglobulin (J06BB01) | 11.4 |
| 6 | Fosfomycin (J01XX01) | 12.3 | Amoxicillin and enzyme inhibitor (J01CR02) | 11.0 | Amoxicillin and enzyme inhibitor (J01CR02) | 10.7 |
| 7 | Pantoprazole (A02BC02) | 12.1 | Ordinary salt combinations (A02AD01) | 10.3 | Levothyroxine sodium (H03AA01) | 10.0 |
| 8 | Anti-D (rh) immunoglobulin (J06BB01) | 11.0 | Pantoprazole (A02BC02) | 9.3 | Ordinary salt combinations (A02AD01) | 9.4 |
| 9 | Eestriol (G03CC06) | 9.5 | Levothyroxine sodium (H03AA01) | 7.4 | Pantoprazole (A02BC02) | 8.6 |
| 10 | Progesterone (G03DA04) | 8.8 | Fosfomycin (J01XX01) | 7.3 | Low-dose acetylsalicylic acid (B01AC06) | 7.8 |
| 11 | Clotrimazole (D01AC01) | 8.3 | Estriol (G03CC06) | 7.0 | Estriol (G03CC06) | 6.2 |
| 12 | Ibuprofen (M01AE01) | 8.1 | Ibuprofen (M01AE01) | 6.0 | Ibuprofen (M01AE01) | 5.7 |
| 13 | Azithromycin (J01FA10) | 7.7 | Clotrimazole (D01AC01) | 5.7 | Clotrimazole (D01AC01) | 5.6 |
| 14 | Fluconazole (J02AC01) | 7.5 | Fluconazole (J02AC01) | 5.6 | Fosfomycin (J01XX01) | 5.6 |
| 15 | Clotrimazole (G01AF02) | 6.5 | Omeprazole (A02BC01) | 5.2 | Omeprazole (A02BC01) | 5.4 |
ATC: anatomical therapeutic chemical.
This study used Swiss health care claims data to evaluate the overall drug burden and pattern of utilisation of prescribed drugs before, during, and after pregnancy dispensed in outpatient care in Switzerland between 2015 and 2021. Our study population of 34,584 pregnancies (30,098 women) represents 5.6% of all pregnancies in Switzerland during this period [12–14].
Most women (87.5%) claimed at least one drug during pregnancy, not including vitamins, supplements, and vaccines. The median number of distinct drugs dispensed during pregnancy was 3 (IQR = 1–5), and one third of women claimed five or more distinct drugs. Even during the vulnerable trimester 1, during which organogenesis takes place, almost 1 of 10 women claimed five or more distinct drugs. Thus, our results show that drug use during pregnancy is common. This was expected, given that pregnant women are in close contact with the healthcare system and many pregnancy symptoms and complications may require medical treatment. These results highlight the need to understand which drugs are prescribed in routine care during pregnancy. Future studies should evaluate if the claimed drugs were medically indicated, which is not possible to determine from our data source due to the lack of diagnostic codes.
In a web-based survey [4], 83% of 618 pregnant women from Switzerland reported having used at least one drug (prescribed or OTC) during pregnancy, excluding iron, mineral supplements, vitamins, herbal remedies, and any type of complementary medicine. This survey population may have been affected by volunteer and recall bias, sample size was smaller than in our study, and results may be outdated (study period: 2011–2012). In our larger study, 88% of women claimed at least one prescribed drug during pregnancy, and the longitudinal nature of the data prevented recall bias. However, despite possible limitations, both studies suggest that more than 4 out of 5 women in Switzerland are exposed to drugs during pregnancy. Pregnant women aged <26 years most frequently claimed more than one drug during pregnancy (91.4% vs. 86.9–87.5% for ≥26 years). This may be because a pregnancy at a younger age is likely to be a first-time pregnancy and women may seek more medical advice than in subsequent pregnancies, resulting in more frequent prescriptions. However, channelling by socioeconomic status may also explain some of this difference, with younger women having undergone less education. In a large study among 19,874 Danish women, use of prescription drugs during pregnancy inversely correlated with the duration of education [15]. Younger women may also have lower incomes and may purchase less OTC drugs. However, the extent of OTC drug use cannot be evaluated in this data source.
Evidence on drug use in vulnerable populations such as pregnant women in Switzerland is of great relevance to public health but has only started to emerge in the past decade [16–19]. This is largely due to the fragmented health data landscape and underdeveloped digitalization in the healthcare sector. Other high-income countries, such as all Scandinavian countries, France, and the US, have used healthcare claims data for surveillance of drug use and safety, also during pregnancy, for decades [20–22]. However, inference on drug use in Switzerland from studies based on health care systems from other countries must be made very cautiously due to differences in reimbursement systems. For example, using comparable methodology, a study based on German claims data by the BARMER health insurer reported that 62.9% of 67,920 pregnant women in 2018 claimed at least one drug during pregnancy [23]. This almost 25% lower prevalence of pregnant women who had any drugs reimbursed is likely explained by the fact that, in Germany, health insurance almost exclusively reimburses prescription drugs. Thus, commonly used drugs such as paracetamol, which can be reimbursed in Switzerland, are not reimbursed in Germany, and thus went unrecorded. Thus, it is important to conduct studies evaluating the use of drugs during pregnancy using Swiss data.
Reassuringly, the 15 most frequently dispensed drugs recorded in our Helsana population have a relatively well-investigated safety profile [24]. The drugs most commonly taken during pregnancy are indicated to treat common acute and chronic conditions and are generally considered safe during pregnancy [5, 6]. These include paracetamol (29.8% of pregnancies), antibiotics (mainly amoxicillin [11.4%] and fosfomycin [7.3%]), vaginal disinfectants (dequalinium, 16.8%) [25], and levothyroxine (7.9%). Low-dose ASA was among the most prevalent drugs in trimesters 1 (3.3%) and 2 (3.3%). The Swiss Society for Gynaecology and Obstetrics (SGGG) recommends low-dose ASA between weeks 12 and 36 for the prevention of preeclampsia, in the presence of specific risk factors [26]. In trimester 3, ASA was not among the 15 most frequently dispensed drugs (1.8%), but true use in trimester 3 likely continued as ASA packages are large in Switzerland (90 pills/package [27]) and some women may not have required a prescription refill in trimester 3 [26].
As expected, antiemetics were among the most prevalent drugs in trimester 1, especially metoclopramide (12.7%). Nausea was also among the leading medication indications in the web-based survey of 628 women in Switzerland (2011–2012) [4]. The true use of antiemetics during trimester 1 is likely much higher than that captured in our study because the first-line antihistaminic antiemetic meclozine with pyridoxine, Itinerol®, is not reimbursed in Switzerland. In 2021, doxylamine (another antihistamine) with pyridoxine (Cariban®) was approved for the treatment of nausea and vomiting during pregnancy and is reimbursed [28]. Additionally in trimester 1, several hormones (progesterone, estriol, and oestradiol) were identified among the most prevalent drug exposures. These were likely prescribed in the context of assisted reproductive technology (ART) or in case of hormone insufficiencies. In contrast to the relatively well-investigated safety profile of the previously discussed drugs used during trimester 1, fluconazole (an antifungal) is discussed more critically in the literature suggesting an increased risk of congenital malformations [30]. A US study also reported that fluconazole was among the most frequently dispensed drugs among pregnant women. Our results reinforce the authors’ recommendation that future research should focus on commonly used anti-infectives not limited to antibiotics [31]. Among the 15 most frequently dispensed drugs were also anti-D (rh) immunoglobulins (3.5% in trimester 2 and 8.2% in trimester 3), which are indicated from gestational week 28–30 (earlier in case of an injury) to prevent rejection reactions in case of rhesus factor incompatibility between the mother and the unborn child [33].
In trimester 3, the use of drugs for acid related disorders increased (ordinary salt combinations, or antacids 5.9%, proton pump inhibitors 11.6%, and ranitidine 3.3%), which was expected given that gastro-oesophageal reflux affects between 30% and 50% of pregnant women and is an increasingly common problem as pregnancy progresses [34]. Insulin (2.5%) and the antihypertensive nifedipine (3.2%) were also among the most claimed drugs in trimester 3. Insulin is the treatment of choice for gestational diabetes, which usually manifests between weeks 24 and 28 [35], and is also the first-line treatment for pre-existing diabetes during pregnancy. Further valuable information on anti-diabetic drugs during pregnancy can be found in a Swiss study based on CSS health insurance claims data [36]. Nifedipine is considered safe during pregnancy and is used as an antihypertensive [37] and as a short-term tocolytic drug in the management of preterm labour (off-label) [38].
Drug use before and after pregnancy meaningfully differed from that during pregnancy. Before pregnancy, we mainly observed drugs which are commonly prescribed in routine care to treat frequent ailments such as pain and viral and bacterial infections. Additionally, progesterone was among the most frequently dispensed drugs, which was likely prescribed either in the context of ART or for a hormone deficiency in women who planned to get pregnant. After pregnancy, the vast majority of drug claims were oral analgesics (paracetamol 63.3%, ibuprofen 50.4%, mefenamic acid 16.3%, diclofenac 7.0%, and metamizole sodium 4.1%), which is expected postpartum. We further identified drugs to treat postpartum conditions such as cabergoline for ablactation, or laxatives to facilitate bowel movement. Recorded drug use was higher after pregnancy than before (median number of distinct dispensed drugs after pregnancy = 3 (IQR = 2–5) vs. 2 (IQR = 0–5) before pregnancy), and slightly higher than during pregnancy (median = 3, IQR = 1–5). This was mainly driven by increased postpartum use of analgesics, likely reflecting treatment of pain caused by the delivery. However, women are exempt from the deductible in the compulsory basic insurance between the 13th week of gestation and the 8th week after delivery in Switzerland. Thus, some drugs may not be billed to health insurance in the pre-pregnancy period if the deductible has not been reached [39].
The following limitations need to be considered. First, healthcare claims data do not provide information on whether any or all the tablets of a filled prescription were taken. Thus, our results draw the larger picture of overall drug prescribing during pregnancy in Switzerland, but they may not depict exact absolute levels of drug intake, especially because we also do not capture OTC medication intake. Second, results must be interpreted carefully, as they were estimated based on the 5.6% of all pregnant women in Switzerland who were insured with Helsana during this period. Furthermore, the study population was restricted to women with continuous insurance with Helsana between 270 days before until 270 days after pregnancy (810 days total). Thus, our study population was restricted to loyal customers of Helsana who did not change insurance during this time. Furthermore, the women in our study population may not be entirely representative of women in Switzerland in terms of socio-economic factors. However, maternal age is a known proxy for socio-economic status [40], and the age profile of our population was consistent with that provided by the Swiss Federal Statistical Office during our study period. Third, health care claims data do not capture OTC drug use. In a web-based survey study among 1293 pregnant women who were insured with the German health insurer BARMER in 2020, 75% of women who returned the questionnaire indicated having used at least one OTC drug during pregnancy. Swiss health insurance reimburses somewhat more drugs during pregnancy than German health insurance, but it can be assumed that unrecorded OTC drug use was also high in Switzerland. Fourth, as characteristics of Swiss billing codes did not allow inclusion of pregnancies which ended in termination or abortion into our study population, our prevalence of drug exposure is underestimated. The underestimation range is however difficult to predict, as the prevalence of spontaneous abortions in Switzerland is unknown and in case of terminations the last menstrual period cannot be accurately estimated in our data source. Finally, the start of pregnancy was estimated because it is not recorded in our data. This may have led to inaccurate start of pregnancy dates in some women, which could lead to some misclassification of the exposure time window.
In conclusion, this study suggests that 8 out of 10 women are exposed to prescribed drugs during pregnancy. Reassuringly, most drugs dispensed during pregnancy are comparatively well investigated and are generally considered safe. The frequent use of drugs during pregnancy highlights the importance of evidence on the benefit-risk profile of the individual drugs used. Healthcare claims databases are a valuable tool to study drug utilisation during pregnancy in Switzerland, but they have not been used to their full potential.
The datasets analysed during the current study are confidential and not publicly available. The analysis code is publicly available here: https://github.com/ebolac/dispensed-drugs-during-pregnancy-in-outpatient-care-between-2015-and-2021-in-Switzerland
This study was presented as a poster at the 39th International Conference on Pharmacoepidemiology & Therapeutic Risk Management (August 23–27, 2023; Halifax, Nova Scotia, Canada) and at the session “Wenn Schwangere und ihre Kinder Medikamente benötigen – Hotspots” (engl. “When pregnant women and their children need medication – hotspots”) organized by “Schweizerische Akademie für Perinatale Pharmakologie” (SAPP; November 2, 2023; Zurich, Switzerland).
We thank the Helsana Insurance Group for providing the data. We further thank the Freiwillige Akademische Gesellschaft (FAG) Basel for financially supporting the participation and poster presentation at the 39th International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE 2023, travel fund). We further thank Dr. Veronika Lappe (PMV Research Group, University of Cologne, Faculty of Medicine and University Hospital Cologne (AöR)) for providing the ATC codes of exclusion drugs (vitamins, mineral supplements, iron preparations, vitamin B12, folic acid, and iodine therapy) and for valuable exchange regarding comparability of our results with results presented in the 2021 Annual BARMER drug report [41] to which she contributed as an author).
Author contributions: CAM: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, software, visualisation, writing – original draft preparation, writing – review and editing. SG: conceptualisation, data curation, formal analysis, investigation, methodology, validation, writing – review and editing. DS: conceptualisation, investigation, methodology, validation, writing – review and editing. AP: conceptualisation, investigation, methodology, validation, writing – review and editing. CRM: conceptualisation, funding acquisition, project administration, resources, supervision, validation, writing – review and editing. JS: conceptualisation, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, software, supervision, validation, visualisation, writing – original draft preparation, writing – review and editing.
This research received no funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Frost Widnes SK, Schjøtt J. Advice on drug safety in pregnancy: are there differences between commonly used sources of information? Drug Saf. 2008;31(9):799–806.
2. Dashraath P, Nielsen-Saines K, Madhi SA, Baud D. COVID-19 vaccines and neglected pregnancy. Lancet. 2020 Sep;396(10252):e22.
3. Noh Y, Yoon D, Song I, Jeong HE, Bae JH, Shin JY. Discrepancies in the Evidence and Recommendation Levels of Pregnancy Information in Prescription Drug Labeling in the United States, United Kingdom, Japan, and Korea. J Womens Health (Larchmt). 2018 Sep;27(9):1086–92.
4. Lupattelli A, Spigset O, Twigg MJ, Zagorodnikova K, Mårdby AC, Moretti ME, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open. 2014 Feb;4(2):e004365.
5. Gerbier E, Graber SM, Rauch M, Marxer CA, Meier CR, Baud D, et al. Use of drugs to treat symptoms and acute conditions during pregnancy in outpatient care in Switzerland between 2014 and 2018: analysis of Swiss healthcare claims data. Swiss Med Wkly. 2021 Nov;151(4748):w30048.
6. Gerbier E, Graber SM, Rauch M, Marxer CA, Meier CR, Baud D, et al. Use of Prescribed Drugs to Treat Chronic Diseases during Pregnancy in Outpatient Care in Switzerland between 2014 and 2018: Descriptive Analysis of Swiss Health Care Claims Data. Int J Environ Res Public Health. 2022 Jan;19(3):1456.
7. Spoendlin J, Blozik E, Graber S, Rauch M, Marxer C, Rüegg S, et al. Use of valproate in pregnancy and in women of childbearing age between 2014 and 2018 in Switzerland: a retrospective analysis of Swiss healthcare claims data. Swiss Med Wkly. 2021 Jan;151(102):w20386.
8. MacDonald SC, Cohen JM, Panchaud A, McElrath TF, Huybrechts KF, Hernández-Díaz S. Identifying pregnancies in insurance claims data: methods and application to retinoid teratogenic surveillance. Pharmacoepidemiol Drug Saf. 2019 Sep;28(9):1211–21.
9. Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernández-Díaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013 Jan;22(1):16–24. 10.1002/pds.3284
10. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. https://www.whocc.no/atc_ddd_index/
11. Van Rossum G, Drake FL. Python 3 Reference Manual. Scotts Valley (CA): CreateSpace; 2009.ISBN: 978-1-4414-1269-0.
12. Federal Statistical Office. Births. https://www.bfs.admin.ch/bfs/en/home/statistics/population/births-deaths/births.html
13. Federal Statistical Office. Anzahl Todesfälle und Rate der perinatalen, Säuglings- und Kindersterblichkeit. https://www.bfs.admin.ch/bfs/en/home/statistics/health/state-health/mortality-causes-death/infant-stillbirths.assetdetail.23329947.html
14. Federal Statistical Office. Durchschnittsalter von Müttern und Vätern bei Geburt des Kindes nach Staatsangehörigkeitskategorie, 1971-2021. https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/geburten-todesfaelle/fruchtbarkeit.assetdetail.23328885.html
15. Olesen C, Thrane N, Henriksen TB, Ehrenstein V, Olsen J. Associations between socio-economic factors and the use of prescription medication during pregnancy: a population-based study among 19,874 Danish women. Eur J Clin Pharmacol. 2006 Jul;62(7):547–53. 10.1007/s00228-006-0119-x
16. Bornhauser C, Quack Lötscher K, Seifert B, Simões-Wüst AP. Diet, medication use and drug intake during pregnancy: data from the consecutive Swiss Health Surveys of 2007 and 2012. Swiss Med Wkly. 2017 Dec;147(51–52):w14572. 10.4414/smw.2017.14572
17. Gerbier E, Favre G, Maisonneuve E, Ceulemans M, Winterfeld U, Dao K, et al. Antidiabetic Medication Utilisation before and during Pregnancy in Switzerland between 2012 and 2019: An Administrative Claim Database from the MAMA Cohort. J Diabetes Res. 2023 May;2023:4105993.
18. Favre G, Gerbier E, Maisonneuve E, Pomar L, Winterfeld U, Lepigeon K, et al.; COVI-PREG and CONSIGN group. COVID-19-related medicine utilization study in pregnancy: the COVI-PREG cohort. Br J Clin Pharmacol. 2023 May;89(5):1560–74. 10.1111/bcp.15611
19. Schenkel L, Simões-Wüst AP, Hösli I, von Mandach U. Drugs in Pregnancy and Lactation - Medications Used in Swiss Obstetrics. Z Geburtshilfe Neonatol. 2018 Feb;222(4):152–64. 10.1055/s-0043-124975
20. Frank AS, Lupattelli A, Nordeng H. Risk factors for discontinuation of thyroid hormone replacement therapy in early pregnancy: a study from the Norwegian Mother and Child Cohort Study and the Medical Birth Registry of Norway. Acta Obstet Gynecol Scand. 2018 Jul;97(7):852–60.
21. Benevent J, Hurault-Delarue C, Araujo M, Montastruc JL, Lacroix I, Damase-Michel C. POMME: The New Cohort to Evaluate Long-Term Effects After Prenatal Medicine Exposure. Drug Saf. 2019 Jan;42(1):45–54.
22. Platt R, Brown JS, Robb M, McClellan M, Ball R, Nguyen MD, et al. The FDA Sentinel Initiative - An Evolving National Resource. N Engl J Med. 2018 Nov;379(22):2091–3. 10.1056/NEJMp1809643
23. Bérard A, Abbas-Chorfa F, Kassai B, Vial T, Nguyen KA, Sheehy O, et al. The French Pregnancy Cohort: medication use during pregnancy in the French population. PLoS One. 2019 Jul;14(7):e0219095.
24. Embryotox, Embryotox. www.embryotox.de
25. Mendling W, Weissenbacher ER, Gerber S, Prasauskas V, Grob P. Use of locally delivered dequalinium chloride in the treatment of vaginal infections: a review. Arch Gynecol Obstet. 2016 Mar;293(3):469–84.
26. Schweizerische Gesellschaft für Gynäkologie und Geburtshilfe (SGGG). Expertenbrief No. 57: Risikospezifizierung Präeklampsie im 1. Trimester; 2019.
27. Swissmedicinfo, Swissmedicinfo: Einzelabfrage. www.swissmedicinfo.ch
28. De Tejada BM, et al. Expertenbrief No 76, Nausea und Erbrechen in der Schwangerschaft, Hyperemesis gravidarum. Available: https://www.rosenfluh.ch/gynaekologie-2022-01/nausea-und-erbrechen-in-der-schwangerschaft-hyperemesis-gravidarum
29. Devall AJ, Papadopoulou A, Podesek M, Haas DM, Price MJ, Coomarasamy A, et al. Progestogens for preventing miscarriage: a network meta-analysis [Review]. Cochrane Database Syst Rev. 2021 Apr;4(4):CD013792.
30. Embryotox, Fluconazol. https://www.embryotox.de/arzneimittel/details/ansicht/medikament/fluconazol
31. Mansour O, Russo RG, Straub L, Bateman BT, Gray KJ, Huybrechts KF, et al. Prescription medication use during pregnancy in the United States from 2011 to 2020: trends and safety evidence. Am J Obstet Gynecol. 2023 Dec;(Dec):S0002-9378(23)02172-5. 10.1016/j.ajog.2023.12.020
32. Gerbier E, Graber SM, Rauch M, Marxer CA, Meier CR, Baud D, et al. Use of drugs to treat symptoms and acute conditions during pregnancy in outpatient care in Switzerland between 2014 and 2018: analysis of Swiss healthcare claims data [Internet]. Swiss Med Wkly. 2021 Nov;151(4748):w30048. [cited 2024 May 18] Available from: https://smw.ch/index.php/smw/article/view/3108 10.4414/SMW.2021.w30048
33. Schweizerische Gesellschaft für Gynäkologie und Geburtshilfe (SGGG). Empfehlungen zur Anti-D Immunglobulin Gabe in der Schwangerschaft (=Anti-D-Prophylaxe), Expertenbrief No. 68. https://www.sggg.ch/fileadmin/user_upload/PDF/68_Empfehlungen_zur_Anti-D_Immunglobulin_Gabe_in_der_Schwangerschaft__Anti-D-Prophylaxe_.pdf
34. Zielinski R, Searing K, Deibel M. Gastrointestinal distress in pregnancy: prevalence, assessment, and treatment of 5 common minor discomforts. J Perinat Neonatal Nurs. 2015;29(1):23–31.
35. Schweizerische Gesellschaft für Gynäkologie und Geburtshilfe (SGGG). Debistage du diabete gestationnel, Avis d’expert No 37. https://www.sggg.ch/fileadmin/user_upload/Dokumente/3_Fachinformationen/1_Expertenbriefe/Fr/37_Depistage_du_diabete_gestationnel_2011.pdf
36. Gerbier E, Favre G, Maisonneuve E, Ceulemans M, Winterfeld U, Dao K, et al. Antidiabetic Medication Utilisation before and during Pregnancy in Switzerland between 2012 and 2019: An Administrative Claim Database from the MAMA Cohort. J Diabetes Res. 2023 May;2023:4105993.
37. DGGG and OEGGG and SGGG, Hypertensive Schwangerschaftserkrankungen: Diagnostik und Therapie, 2019. https://register.awmf.org/de/leitlinien/detail/015-018
38. Schweizerische Gesellschaft für Gynäkologie und Geburtshilfe (SGGG). La tocolyse dans les menaces d’accouchement prématuré. https://www.sggg.ch/fileadmin/user_upload/PDF/41_Tocolyse_2013.pdf
39. Federal Office of Public Health (FOPH). Health insurance: Maternity services. https://www.bag.admin.ch/bag/en/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Leistungen-bei-Mutterschaft.html
40. De Wit ML, Rajulton F. Education and timing of parenthood among Canadian women: a cohort analysis. Soc Biol. 1992;39(1-2):109–22.
41. D. Grandt, V. Lappe, and I. Schubert, BARMER Arzneimittelreport 2021. 2021.
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3616.