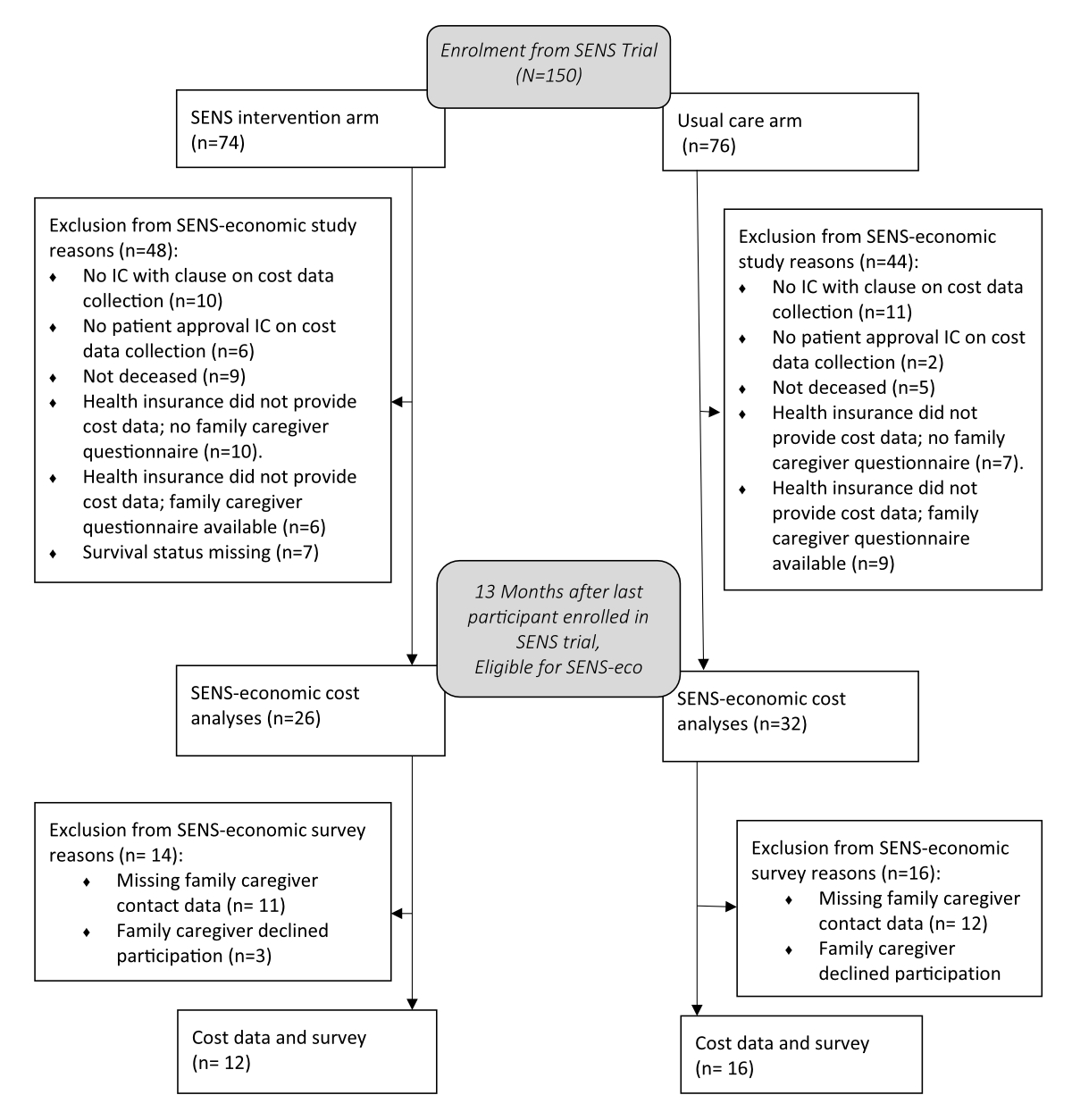
Figure 1Flow diagram of participant inclusion in the SENS-economic study. IC = Informed consent.
DOI: https://doi.org/https://doi.org/10.57187/s.3591
Health care costs often increase at the end of life [1], especially those related to emergency admissions and hospital care [2]. To empower patients to decide autonomously about their life and care, their needs and support options should be identified early [3]. Early integration of palliative care into pathways of patients with severe, progressive, life-limiting illnesses, such as cancer, can support their autonomy and aims to maximise quality of life and family support. Randomised controlled trials (RCTs) and meta-analyses have confirmed the positive effects of early palliative care on symptoms, quality of life and care satisfaction among patients with advanced cancer [4, 5]. In addition, the American Society of Clinical Oncology and the European Society for Medical Oncology recommend early integration of palliative care services alongside usual oncology treatment [6, 7].
Studies on early integration of palliative care show decreased health care utilisation and health care costs [8–10]. However, details about types of health care utilisation and cost reductions vary between studies.
To understand how cost reductions can be reproduced in daily practice, it is therefore important to know what specific elements or parts of an early palliative care intervention programme cause reductions. Although reducing costs is not a goal of palliative care, palliative care interventions that reduce health costs are important for efforts to keep health care affordable and to allocate reimbursement to measures corresponding to patient goals. Palliative care interventions should be characterised as successful if healthcare costs match patient needs, expectations and preferences, not only if they reduce costs. To build excellent early palliative care services that also reduce unnecessary health care costs, health care managers require information about the kind and intensity level of every early palliative care service element and their effects on health care costs and on care utilisation.
The SENS trial was a multicentre RCT on SENS, an early palliative care intervention. SENS is a practice-orientated, thematic intervention structure for assessing, planning and evaluating the treatment of chronic progressive or potentially life-limiting diseases used at any stage requiring palliative support. The SENS trial examined the effects of a single, in-hospital conversational palliative care intervention among patients with advanced cancer. SENS topics include Symptoms, End-of-life decisions, Network building and Support for carers. Details of the SENS trial are published elsewhere [11, 12] (NCT01983956 on www.clinicaltrials.gov). Figure S1 in the appendix shows the SENS intervention structure.
The SENS intervention was performed within 16 weeks of diagnosis of a tumour stage not amenable or responsive to curative treatment. The SENS intervention is therefore defined as early palliative care. SENS helps uncover patients’ needs and concerns from their perspective; set priorities; and organise support needed for patients and their families, while accommodating patients’ wishes and values. The goal of SENS is to empower participants to find individual and optimal solutions for their specific needs and goals. Following the SENS-structured conversational intervention, we provided participants with specific questions to enhance further discussions with professionals [13, 14]. The intervention consisted of one single consultation with a senior palliative care physician and advanced practice nurse (APN); however, we allowed follow-up visits upon participant request (average 1.45 early specialist palliative care consultations). As a result of the SENS intervention, patients and their families understand and prepare for controlling symptoms and make relevant treatment and care decisions for redirecting care towards quality of life and function, which may eventually reduce emergency hospitalisations.
An additional study, SENS-economic, which placed no additional burden on SENS trial participants, collected and analysed information about costs and care utilisation within the SENS study. Potential cost-reducing effects of the SENS intervention are most likely highest at the end of life. For instance, when advance directives or anticipatory care planning for emergency situations lower the number of expensive health procedures, such as hospitalisations or diagnosis-specific treatment, no longer aligned with patient life goals. Therefore, we focused our cost analyses on the last month of life. The SENS-economic research question was: “Among participants with advanced cancer, is early palliative care associated with reduced costs and utilisation of care when compared with usual oncology care at the end of life?”. We hypothesised that the SENS intervention reduces costs.
However, if healthcare costs align with patient needs, expectations and preferences, palliative care interventions should be considered successful.
SENS trial participants were inpatients and outpatients with advanced cancer from the departments of medical and radiation oncology or internal medicine at University Hospital Bern, Inselspital, Switzerland. Participants were older than 18 years and had histologically confirmed advanced cancer no longer amenable to curative treatment. Cancer progression had been diagnosed at most 16 weeks before inclusion in the SENS trial. We included participants with good functional status [15]. Details of the study population are described elsewhere [11].
For the SENS-economic study, additional inclusion criteria required participants to have approved the use of their health insurance data in a separate clause in the informed consent and to have died by the end of the study. We considered eligible patients to be completely or partially lost to follow-up if we could not contact family caregivers, family caregivers did not reply or health insurance schemes refused to provide patient cost data. For pragmatic reasons, we began the SENS-economic study after the first 21 participants enrolled in the SENS trial.
Participants in the control arm received usual oncology care, i.e. without SENS-structured conversations, from oncologists with training in communication and tumour-centred care. Unlike patients in the intervention arm, they were treated primarily by oncologists without training in specialised palliative care. Outpatient consultations involved a systematic, structured oncology survey sheet providing information only about (a) drug therapy; (b) severity of major symptoms and physical examination; (c) imaging; and (d) laboratory diagnostics.
On request, a trained palliative care physician and APN performed specialist palliative care consultations. In addition, psycho-oncologists, social workers, pastoral workers and dietitians were available on demand, rather than actively offered to participants [11].
We adopted the health insurance perspective in the SENS-economic study and focused on health care service use and costs [16]. We followed our protocol to collect health care data about participants during the last month of life from two sources: (1) questionnaires about health utilisation filled out by family caregivers after participants had died and (2) medical cost data derived from patient compulsory basic health insurance administrative information. Details of our data collection process are given in table S1 in the appendix.
Three months after each participant’s death, we invited family caregivers by mail or phone to complete questionnaires about health care utilisation during the participant’s last month of life. The study nurse mailed family caregivers study information forms and questionnaires about health care utilisation. Family caregiver questionnaires included questions about caregiver characteristics, place where the participant died, specialisations of treating physicians and health care utilisation during the last month of life, including number of general practitioner (GP) and specialist visits, hospital admissions, emergency consultations and calls to emergency numbers, nursing home care and home care services by district nurses/mobile palliative care teams.
Our health insurance data only includes costs eligible for reimbursement as per Swiss basic health insurance guidelines. Health insurance is compulsory for all permanent inhabitants of Switzerland. It includes health care costs, such as hospitalisation, medications and ambulatory care. Only costs reported to health insurance schemes could be analysed.
We included only costs accumulated in the last month of life. Some health care treatments started more than one month before the participant died and continued until the participant’s death. In these situations, we were unable to extract health care costs generated only in the last month of life from our health insurance data because only the total cost of these services was provided. Therefore, in our analyses we included only health care costs in proportion to the time these services were performed in the last month before death. We based cost types on health insurance data labeling and information. We categorised costs reported as GP services to health insurance schemes in communities and hospitals as outpatient physician care – a category also including outpatient care from other specialist physicians.
We extracted data from SENS trial questionnaires, such as health service use, Palliative Outcome Scale [17], Functional Assessment of Cancer Therapy – General [18] and Lubben Social Network Scale [19] from the last follow-up time point before participant death.
We calculated intervention treatment costs by using the total time needed for each intervention and its resources. We only included costs directly related to the intervention, such as information leaflets, since those costs would also apply when integrating the intervention into usual care. We excluded variable study-related costs, such as screening eligible patients or helping participants complete study questionnaires, but included variable intervention-related costs for renting the hospital consultation room. Average total costs included variable personnel costs for a senior palliative care physician and APN and costs for materials and hospital consultation room rent. Material costs only included leaflets given to participants.
According to the null hypothesis, change in total health care costs is the same among participants who received the SENS intervention alongside usual oncology care and those who received usual oncology care alone. We tested our null hypothesis against a two-tailed alternative, providing 95% confidence intervals (CI) for all reported effect measures and using two-tailed p values.
We represented continuous data as mean ± standard deviation (SD) and median (interquartile range [IQR]) because some variables are zero-inflated, so the median (IQR) does not provide a meaningful overview. Mean and SD, although certainly not perfect, at least allow rudimentary comparison. We compared groups using the Wilcoxon-Mann-Whitney test. We presented categorical variables as number (%) and compared them using Fisher’s exact test. To increase comparability between the studies, we based our analysis on the intention-to-treat principle, as previously done in another published SENS trial study [11].
We analysed all randomised participants and their clusters according to randomised assignment regardless of treatment. As a sensitivity analysis to better understand the cost, we also performed per-protocol analyses.
We performed all analyses using Stata 16 (Stata Corp, College Station, Texas, USA).
The cantonal ethics committee of Bern, Switzerland approved the study (KEK number: 102/13, 29-05-2017). The study protocol is registered with the Swiss ethical committee (102/2013).
According to inclusion and exclusion criteria, 58 of 150 participants with advanced cancer included in the SENS trial were eligible for the SENS-economic study. Of these 58 participants eligible for the SENS-economic study, matching family caregivers provided surveys. Figure 1 shows the CONSORT inclusion flow diagram.

Figure 1Flow diagram of participant inclusion in the SENS-economic study. IC = Informed consent.
Participants in the intervention and control arms included in the SENS-economic study showed similar characteristics at baseline and at the last follow-up measurement of the SENS trial (table 1).
Table 1Baseline and follow-up characteristics of 58 participants with advanced cancer. Outcomes are expressed as median (interquartile range), mean ± standard deviation or n (%).
| Total (n = 58) | SENS (n = 26) | Control (n = 32) | p value | ||
| Baseline measurements | |||||
| Age (years) | 67 (60–75) | 66 (59–74) | 68 (62–75) | 0.24 | |
| Female sex | 18 (31%) | 8 (31%) | 10 (31%) | 1.00 | |
| Marital status | 0.07 | ||||
| Single | 5 (8.6%) | 2 (7.7%) | 3 (9.4%) | ||
| Married | 37 (64%) | 14 (54%) | 23 (72%) | ||
| Widowed | 5 (8.6%) | 5 (19%) | 0 (0.00%) | ||
| Divorced | 11 (19%) | 5 (19%) | 6 (19%) | ||
| Religion | 0.53 | ||||
| Catholic | 15 (26%) | 6 (23%) | 9 (28%) | ||
| Protestant | 33 (57%) | 15 (58%) | 18 (56%) | ||
| None | 8 (14%) | 5 (19%) | 3 (9.4%) | ||
| Other | 2 (3.4%) | 0 (0%) | 2 (6.3%) | ||
| Availability of advance directive | 0.72 | ||||
| No | 20 (34%) | 8 (31%) | 12 (38%) | ||
| Yes | 5 (8.6%) | 3 (12%) | 2 (6.3%) | ||
| Unknown | 33 (57%) | 15 (58%) | 18 (56%) | ||
| Cancer entity | 0.94 | ||||
| Lung cancer | 20 (34%) | 8 (31%) | 12 (38%) | ||
| Colorectal cancer | 4 (6.9%) | 2 (7.7%) | 2 (6.3%) | ||
| Prostate cancer | 5 (8.6%) | 3 (12%) | 2 (6.3%) | ||
| Breast cancer | 4 (6.9%) | 2 (7.7%) | 2 (6.3%) | ||
| Urothelial cancer | 3 (5.2%) | 2 (7.7%) | 1 (3.1%) | ||
| Pancreatic cancer | 22 (38%) | 9 (35%) | 13 (41%) | ||
| ECOG PS | 0.25 | ||||
| 0 | 18 (31%) | 11 (42%) | 7 (22%) | ||
| 1 | 28 (48%) | 10 (38%) | 18 (56%) | ||
| 2 | 12 (21%) | 5 (19%) | 7 (22%) | ||
| Comorbidities | 52 (90%) | 23 (88%) | 29 (91%) | 1.00 | |
| Last questionnaire in SENS trial patient follow-up | |||||
| Physician visit | 36 (62%) | 18 (69%) | 18 (56%) | 0.42 | |
| Hospital emergency room visit | 17 (29%) | 6 (23%) | 11 (34%) | 0.40 | |
| Hospital overnight stay | 16 (28%) | 6 (23%) | 10 (31%) | 0.56 | |
| FACT-G | 54 (50–60) | 53 (51–55) | 56 (50–62) | 0.56 | |
| POS | 9.0 (6.0–13) | 8.0 (6.0–14) | 9.0 (7.0–12) | 0.69 | |
| LSNS-6 | 19 (15–23) | 17 (14–23) | 20 (17–23) | 0.36 | |
| NCCN distress thermometer | 4.0 (2.0–5.5) | 4.0 (2.0–5.0) | 4.0 (2.0–6.0) | 0.52 | |
ECOG PS: Eastern Cooperative Oncology Group Performance Status; NCCN: National Comprehensive Cancer Network; FACT-G: Functional Assessment of Cancer Therapy–General scale; POS: Palliative care Outcome Scale; LSNS-6: abbreviated Lubben Social Network Score.
The average time between the last follow-up measurement in the SENS trial and death was 168 days in the intervention arm and 144 days in the control arm (p = 0.93). There were no significant differences in this follow-up time point for any reported outcome between study arms. We show characteristics between study arms, which were similar, in our display of characteristics of family caregivers and patients who answered the family caregiver questionnaire (tables 2 and 3 in the appendix).
On average, participants in the intervention arm had CHF 600 lower absolute overall health care costs in the last month of life when compared with participants in the usual care arm (p = 0.98) (table 2). Costs for inpatient care were the highest cost subcategory. In both study arms, inpatient costs were higher than all outpatient cost types together. No cost type showed a significant difference between the two study arms.
Table 2Compulsory Swiss basic health insurance eligible care costs of 58 participants in the last month of life in Swiss Francs (CHF). Outcomes are expressed as median (interquartile range).
| Cost type | Total(n = 58) | SENS(n = 26) | Control(n = 32) | pValue |
| Total health care costs | 7985 (5637–12450) | 7892 (5637–13489) | 8492 (5411–12012) | 0.98 |
| Inpatient care | 5974 (0–10970) | 6214 (3257–10970) | 3848 (0–10614) | 0.50 |
| Outpatient physician care | 556 (46–1386) | 498 (16–1342) | 691 (220–1706) | 0.36 |
| Outpatient nursing care | 243 (0–950) | 3.3 (0–808) | 336 (0–1323) | 0.17 |
| Outpatient laboratory | 0 (0–79) | 0 (0–92) | 0 (0–62) | 0.12 |
| Outpatient medications | 316 (50–607) | 125 (0–670) | 389 (119–584) | 0.28 |
| Outpatient care products | 0 (0–232) | 0 (0–124) | 1.4 (0–369) | 0.18 |
| Transportation | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–19) | 0.27 |
| Outpatient health professionals* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.23 |
| Other care | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.78 |
* Excluding physician and nurses.
Total health care costs decreased with increasing age in both arms (figure 2).
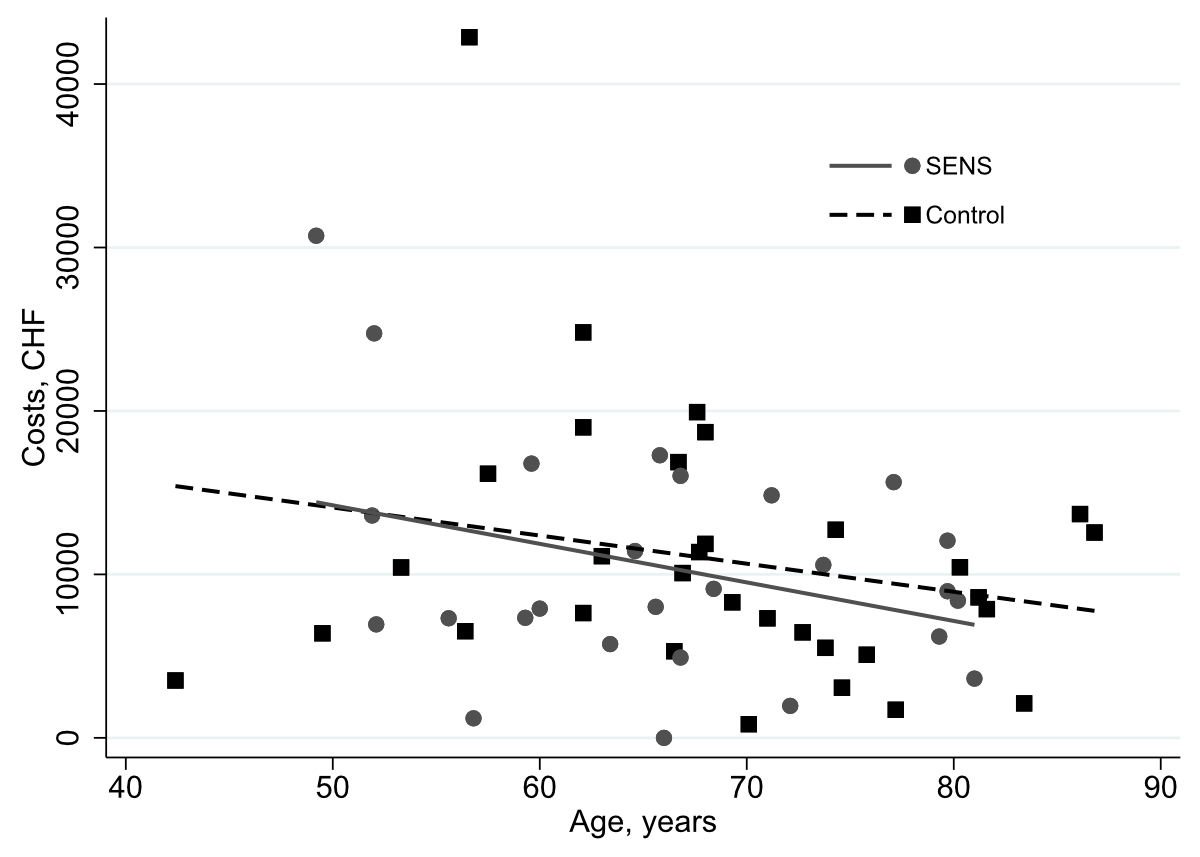
Figure 2Total compulsory Swiss basic health insurance eligible care costs of 58 participants for intervention and control arms during the last month of life in Swiss Francs (CHF) by participant age at study enrollment.
In the appendix (table S4), we show similar results from the per-protocol analyses of the costs, excluding three participants who did not receive the SENS intervention according to study protocol. In per-protocol analyses, the intervention arm showed CHF 1204 lower absolute overall health care costs in the last month of life when compared with the control arm (p = 0.66).
The total average cost of the SENS intervention was CHF 380 per participant. The average time of intervention consultations (one or more consultations) for participants was 52 min (range: 5–170). In table S5 in the appendix, we provide cost details.
Health care utilisation analyses showed no significant differences between the intervention and control arm at the last SENS trial follow-up measurement (table 1) and in the last month of life (table 3). Although not significant, descriptive data showed family caregivers of participants in the intervention arm reported fewer deaths in hospital (42% vs 56%), lower use of hospital emergency departments (25% vs 44%), lower non-use of nursing home care (42% vs 56%), higher rate of outpatient visits by specialists (50% vs 31%) and lower rate of outpatient visits by GPs (42% vs 50%) when compared with participants in the control arm (table 3).
In the last month of life, most participants in both arms renounced medical care and support (table 3). Participants most frequently declined “Resuscitation” (n = 10) followed by “Continuation with radiation therapy, chemotherapy or hormone therapies” (n = 8), “Starting new radiation therapy, chemotherapy or hormone therapies” (n = 7) or “Receiving artificial nutrition” (n = 7) (table S6 in the appendix).
Table 3Health care utilisation reported in family caregiver questionnaire of participants included in cost analyses.
| SENS(n =12) | Control(n = 16) | pvalue | ||
| Place of participant’s death | 0.56 | |||
| Home | 5 (42%) | 3 (19%) | ||
| Nursing home | 0 (0.0%) | 2 (13%) | ||
| Hospital | 5 (42%) | 9 (56%) | ||
| Other | 2 (17%) | 2 (13%) | ||
| Specialisation of treating physician | 1.00 | |||
| General Practitioner | 3 (25%) | 4 (25%) | ||
| Oncologist | 6 (50%) | 9 (56%) | ||
| Palliative care specialist | 1 (8.3%) | 1 (6.3%) | ||
| Unknown | 1 (8.3%) | 1 (6.3%) | ||
| Other | 1 (8.3%) | 1 (6.3%) | ||
| Missing data | 3 (25%) | 4 (25%) | ||
| Visited hospital emergency room? | 0.42 | |||
| No | 8 (67%) | 8 (50%) | ||
| Yes | 3 (25%) | 7 (44%) | ||
| Missing data | 1 (8.3%) | 0 (0.0%) | ||
| Unknown | 0 (0.0%) | 1 (6.3%) | ||
| Stayed at least one night in hospital? | 1.00 | |||
| No | 3 (25%) | 4 (25%) | ||
| Yes | 9 (75%) | 12(75%) | ||
| Used nursing home care? | 0.75 | |||
| No | 5 (42%) | 9 (56%) | ||
| Yes, fewer than once per day | 0 (0.0%) | 1 (6.3%) | ||
| Yes, once per day | 4 (33%) | 3 (19%) | ||
| Yes, twice per day | 2 (17%) | 3 (19%) | ||
| Yes, three times per day | 1 (8.3%) | 0 (0.0%) | ||
| Stayed in retirement home or nursing home? | 0.49 | |||
| No | 12 (100%) | 14 (88%) | ||
| Yes | 0 (0.0%) | 2 (13%) | ||
| Renounced medical care or support? | 0.90 | |||
| No | 4 (33%) | 5 (31%) | ||
| Yes | 7 (58%) | 10 (63%) | ||
| Missing data | 0 (0.0%) | 1 (6.3%) | ||
| Unknown | 1 (8.3%) | 0 (0.0%) | ||
| Outpatient medical specialist visits with medical practice outside or inside hospital | 6 (50%) | 5 (31%) | 0.44 | |
| Outpatient general practitioner visits with medical practice inside or outside hospital | 5 (42%) | 8 (50%) | 0.72 | |
Our results showed no significant differences in health care utilisation or overall costs for participants with cancer who received the SENS intervention alongside usual oncology care and those who received usual oncology care alone. In both study arms, inpatient costs generated most health care costs in the last month of life. In concordance with these results, descriptive analyses showed that participants in the intervention arm used less hospital care and died more often at home. When compared with the control arm, the average reduction of overall health care costs in the intervention arm outweighed the costs of the SENS intervention.
The literature on effects of early palliative care on health care costs has contradictory results. Comparing study results is challenging since study methods, timing, health care systems and content of early palliative care interventions are heterogeneous. A Swiss study using administrative hospital data of deceased patients instead of health insurance data showed similar results [20]. Their overall hospital cost analyses of the final hospitalisation before death showed no significant cost reduction for patients receiving specialist palliative care compared with those receiving usual care. The study was not specifically about early palliative care, which complicates comparison of cost results because timing influences care costs [21]. However, detailed analyses showed that among palliative care populations, average daily hospital costs significantly decreased CHF –3224 (95% CI: –3811 – –2631). The data showed a shift in type of costs after specialist palliative care. Deceased patients who received in-hospital specialist palliative care showed fewer costs for diagnostic interventions and medications when compared with participants receiving no specialist palliative care; however, participants receiving specialist palliative care demonstrated higher costs for catering, rooms, nursing care, social counselling and nonmedical therapists [20]. Our study method disallowed analysis of potential shifts between medical care and comfort care costs. However, our study demonstrates that our inpatient SENS intervention showed no negative effect on increasing costs in the outpatient setting.
Heterogeneity was also displayed in a study of 12 Dutch hospitals, which reported no significant differences in total mean inpatient costs [22]. The intervention was similar to our SENS structure, assessing patient symptoms and physical, emotional, social and spiritual problems and coordination of care. In addition, the health care systems of both counties are similar. However, the Dutch study defined early palliative care as a palliative care consultation within three days of hospital admission, which could be at a later time point than our early palliative care consultation, which was within 16 weeks of the diagnosis of advanced cancer no longer amenable to curative treatment. The Dutch study also used 3-month follow-up data, including participants who survived during this period, which indicates a higher variety of disease stages compared with our study, focusing on the last month of life only. These differences possibly influence health care costs [23, 24].
Our study confirmed a trend in the literature showing older participants with lower health care costs than younger participants at end of life [25, 26]. Our study results contradict studies showing significant reductions in health care costs related to early palliative care among patients with cancer [23, 27, 28]; yet again, these are studies with heterogeneous study methods and interventions. A meta-analysis showed total direct hospital costs decreased with hospital palliative care consultation within 3 days of hospital admission among patients with cancer (–4251 USD; 95% CI –4664 – –3837 USD; p <0.001) [23]. A cohort study reported a cost reduction derived mainly from shorter hospital stays [28]. Since available literature focused on inpatient health care costs mostly for single hospital admissions, it is unclear whether patients generated more health care costs in outpatient sectors, during later hospital admissions or from out-of-pocket payments for patients/families. In these studies, early palliative care was mostly defined by the number of days after hospital admission, such as fewer than three days, when palliative care consultations occurred, not by disease stage. Such differences make it difficult to compare results. Our study defined early palliative care based on time since diagnosis of a life-limiting cancer disease and included outpatient costs – a setting where cost savings are more difficult to measure [29].
Even though the expected effect on inpatient costs is higher than outpatient costs and inpatient cost data are often better available, future economic analyses of palliative care should not focus on inpatient settings only.
Outpatient analyses are important for future palliative care. If patients have fewer (expensive) inpatient days, the need for information on excellent, affordable alternatives in outpatient care is of high relevance. Finally, use of hospital care during the last weeks of life – including length of stay and costs – remains a complex outcome and possibly reflects reimbursement incentives within the Swiss health care system. With outpatient and long-term care demands, a high proportion of out-of-pocket expenses and affordable care solutions might not always be immediately available; whereas hospital costs are mostly covered by health insurance in Switzerland. Thus, patients and family carers in Switzerland and elsewhere possibly prefer (expensive) hospital care because of such regulations.
Since RCT design decreases bias between two study arms, our study design is a strength. The clarity of our intervention is also a strength because treatment costs are more transparent when compared with integrated, comprehensive palliative care intervention programmes. As another strength, the single structured conversational intervention allowed us to study a systematic, step-wise increase in intensity of specialist palliative care and to determine the effects of each added intervention element. We conducted our study directly alongside the RCT before results of the studied intervention were available; therefore, the added analysis maximises use of data. Furthermore, results from economic analyses simplify decisions when planning next research steps for an intervention and possible implementation into usual (oncology) care [30].
A small sample size is the most important limitation; it decreases the robustness of our results. Since cost data provision required approval by 16 different health insurance schemes and each provided their own database characteristics, challenges led to missing or loss of data details. A complete, accessible national database on health care costs for research decreases missing cost data, yet does not exist. Other countries possibly desire a national health care database including costs; our study illustrates that missing health care cost data limit analyses.
Given that we excluded participants after randomisation for not meeting additional inclusion criteria and encountered missing data, (unobserved) bias was possibly introduced. Another limitation involves unrepresented national languages and geographical regions, decreasing result generalisability. Studies have shown that health care utilisation and costs vary between geographic, language and hospital regions [31–33]. Knowledge and attitudes about palliative care also vary between Swiss language regions. A survey of the Swiss government showed that the general population in German-speaking regions of Switzerland more often reported having advance directives (19%) when compared with French-speaking (9%) and Italian-speaking (7%) regions [34]. However, in German-speaking regions, 55% reported knowing the term “palliative care” compared with 73% in French-speaking and 64% in Italian-speaking regions [34]. Such findings possibly indicate that our German-language region study population is more ideal since they seemed willing to define advance directives early – a part of the SENS intervention.
Also, they were less aware of palliative care services, with participants in the control arm less likely to request it. Another limitation involves allowing control arm participants to request palliative care services. The SENS trial showed this to be a relevant yet small effect [11].
Focusing our analyses on the last month of life makes the study population more heterogeneous and clear about short life expectancy and disease stage, which increases the probability that patients decide to prioritise their personal end-of-life goals over another hospitalisation or intensive treatment. However, the short time period also prevented us from providing any information about an earlier effect from the intervention; an earlier effect is especially important since studies have clearly shown reduced hospital costs immediately following palliative care interventions [23].
The health service payer perspective is another limitation. As a result, our data cannot determine whether health care costs shifted from health service payers to participants and families as out-of-pocket expenses. Revealing a fuller picture of interventions causing cost reductions and potential shifts in health care costs requires more detailed health care data about inpatient and outpatient care and studies with higher statistical power.
The SENS trial included three studies. The quantitative study showed that the single SENS intervention did not decrease distress nor improve quality of life among participants with cancer [11, 12]. Yet, the nested interview study (12) showed that participants experienced the SENS intervention as beneficial and felt it should be incorporated into routine oncology care. Lastly, the present study showed the relatively low costs for the intervention; however, we did not identify any significant difference in health care costs between the intervention and control arms. Following the hierarchy of evidence, health care managers should cautiously evaluate implementing our intervention into usual care because primary outcomes – costs and participant distress – did not statistically improve. Nevertheless, instead of providing no early palliative care at all, we advise the implementation of an early palliative care intervention based on SENS and in a repeated format, thus giving more weight to individual patient voices. A permanent evaluation determining whether the allocation of costs at end of life corresponds to individual patient goals is necessary. Since participants valued the intervention (12), treatment costs were low with no negative effects, such as increased distress. As a health care cost-reducing measure, care managers should evaluate integrated and more intensive early palliative care interventions.
Although integrating an early palliative care intervention into usual oncology care showed no significant statistical differences in health care utilisation or overall health care costs between the intervention and control arm among participants with cancer, integrating such an intervention is beneficial to individuals. However, a small sample size reduces the robustness of this conclusion. We recommend further economic research on early palliative care focusing on extracting large, detailed cost databases showing potential shifts in cost and cost effectiveness.
We thank all participants for their willingness to share their experiences and data. We thank Kristin Marie Bivens, the scientific editor for the Institute of Social and Preventive Medicine at the University of Bern, for her editorial contributions to this article. We also thank the participating health insurance employees who performed data extractions.
Author contributions: S. Zwahlen, S. Eychmüller, M. Maessen and D. M. Aebersold designed the study. M. Maessen, M. Maier and M. C. Fliedner collected the data. B. Gahl and M. Maessen performed the analysis and designed the figures. M. Maessen drafted the manuscript. S. Exchmüller, M. Maessen, M. Maier, B. Gahl, M. C. Fliedner, S. Zwahlen and D. M. Aebersold critically reviewed the manuscript. All authors discussed the results and commented on the manuscript. M. Maessen is responsible for the overall content of the manuscript.
The study received financial support from the Swiss Cancer Research Foundation; they played no role in identifying, designing, conducting, or reporting our analysis.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Reeve R, Srasuebkul P, Langton JM, Haas M, Viney R, Pearson SA; EOL-CC study authors. Health care use and costs at the end of life: a comparison of elderly Australian decedents with and without a cancer history. BMC Palliat Care. 2017 Jun;17(1):1. 10.1186/s12904-017-0213-0
2. von Wyl V, Telser H, Weber A, Fischer B, Beck K. Cost trajectories from the final life year reveal intensity of end-of-life care and can help to guide palliative care interventions. BMJ Support Palliat Care. 2018 Sep;8(3):325–34. 10.1136/bmjspcare-2014-000784
3. Sobanski P, Krajnik M, Beattie JM. Integrating the complementary skills of palliative care and cardiology to develop care models supporting the needs of those with advanced heart failure. Curr Opin Support Palliat Care. 2016 Mar;10(1):8–10. 10.1097/SPC.0000000000000197
4. Mathews J, Hannon B, Zimmermann C. Models of Integration of Specialized Palliative Care with Oncology. Curr Treat Options Oncol. 2021 Apr;22(5):44. 10.1007/s11864-021-00836-1
5. Huo B, Song Y, Chang L, Tan B. Effects of early palliative care on patients with incurable cancer: A meta-analysis and systematic review. Eur J Cancer Care (Engl). 2022 Nov;31(6):e13620. 10.1111/ecc.13620
6. Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017 Jan;35(1):96–112. 10.1200/JCO.2016.70.1474
7. Jordan K, Aapro M, Kaasa S, Ripamonti CI, Scotté F, Strasser F, et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol. 2018 Jan;29(1):36–43. 10.1093/annonc/mdx757
8. Ma J, Chi S, Buettner B, Pollard K, Muir M, Kolekar C, et al. Early Palliative Care Consultation in the Medical ICU: A Cluster Randomized Crossover Trial. Crit Care Med. 2019 Dec;47(12):1707–15. 10.1097/CCM.0000000000004016
9. Morrison RS, Dietrich J, Ladwig S, Quill T, Sacco J, Tangeman J, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff (Millwood). 2011 Mar;30(3):454–63. 10.1377/hlthaff.2010.0929
10. Greer JA, Tramontano AC, McMahon PM, Pirl WF, Jackson VA, El-Jawahri A, et al. Cost Analysis of a Randomized Trial of Early Palliative Care in Patients with Metastatic Nonsmall-Cell Lung Cancer. J Palliat Med. 2016 Aug;19(8):842–8. 10.1089/jpm.2015.0476
11. Eychmüller S, Zwahlen S, Fliedner MC, Jüni P, Aebersold DM, Aujesky D, et al. Single early palliative care intervention added to usual oncology care for patients with advanced cancer: A randomized controlled trial (SENS Trial). Palliat Med. 2021 Jun;35(6):1108–17. 10.1177/02692163211005340
12. Fliedner M, Zambrano S, Schols JM, Bakitas M, Lohrmann C, Halfens RJ, et al. An early palliative care intervention can be confronting but reassuring: A qualitative study on the experiences of patients with advanced cancer. Palliat Med. 2019 Jul;33(7):783–92. 10.1177/0269216319847884
13. Eychmüller S. [SENS is making sense - on the way to an innovative approach to structure Palliative Care problems]. Ther Umsch. 2012 Feb;69(2):87–90.
14. Fliedner MC, Mitchell G, Bueche D, Mettler M, Schols JM, Eychmueller S. Development and Use of the ‘SENS’-Structure to Proactively Identify Care Needs in Early Palliative Care-An Innovative Approach. Healthcare (Basel). 2019 Feb;7(1):32. 10.3390/healthcare7010032
15. de Kock I, Mirhosseini M, Lau F, Thai V, Downing M, Quan H, et al. Conversion of Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group Performance Status (ECOG) to Palliative Performance Scale (PPS), and the interchangeability of PPS and KPS in prognostic tools. J Palliat Care. 2013;29(3):163–9. 10.1177/082585971302900305
16. Garrison LP, Jr., Pauly MV, Willke RJ, Neumann PJ. An Overview of Value, Perspective, and Decision Context-A Health Economics Approach: An ISPOR Special Task Force Report [2]. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2018;21(2):124-30.
17. Hearn J, Higginson IJ; Palliative Care Core Audit Project Advisory Group. Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Qual Health Care. 1999 Dec;8(4):219–27. 10.1136/qshc.8.4.219
18. Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res. 2002 May;11(3):207–21. 10.1023/A:1015276414526
19. Lubben J, Blozik E, Gillmann G, Iliffe S, von Renteln Kruse W, Beck JC, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist. 2006 Aug;46(4):503–13. 10.1093/geront/46.4.503
20. Hagemann M, Zambrano SC, Bütikofer L, Bergmann A, Voigt K, Eychmüller S. Which Cost Components Influence the Cost of Palliative Care in the Last Hospitalization? A Retrospective Analysis of Palliative Care Versus Usual Care at a Swiss University Hospital. J Pain Symptom Manage. 2020 Jan;59(1):20–29.e9. 10.1016/j.jpainsymman.2019.08.026
21. May P, Normand C. Analyzing the Impact of Palliative Care Interventions on Cost of Hospitalization: Practical Guidance for Choice of Dependent Variable. J Pain Symptom Manage. 2016 Jul;52(1):100–6. 10.1016/j.jpainsymman.2016.01.009
22. Brinkman-Stoppelenburg A, Polinder S, Olij BF, van den Berg B, Gunnink N, Hendriks MP, et al. The association between palliative care team consultation and hospital costs for patients with advanced cancer: an observational study in 12 Dutch hospitals. Eur J Cancer Care (Engl). 2020 May;29(3):e13198. 10.1111/ecc.13198
23. May P, Normand C, Cassel JB, Del Fabbro E, Fine RL, Menz R, et al. Economics of Palliative Care for Hospitalized Adults With Serious Illness: A Meta-analysis. JAMA Intern Med. 2018 Jun;178(6):820–9. 10.1001/jamainternmed.2018.0750
24. Scibetta C, Kerr K, Mcguire J, Rabow MW. The Costs of Waiting: Implications of the Timing of Palliative Care Consultation among a Cohort of Decedents at a Comprehensive Cancer Center. J Palliat Med. 2016 Jan;19(1):69–75. 10.1089/jpm.2015.0119
25. Panczak R, Luta X, Maessen M, Stuck AE, Berlin C, Schmidlin K, et al. Regional Variation of Cost of Care in the Last 12 Months of Life in Switzerland: Small-Area Analysis Using Insurance Claims Data. Med Care. 2016.
26. Kalseth J, Halvorsen T. Health and care service utilisation and cost over the life-span: a descriptive analysis of population data. BMC Health Serv Res. 2020 May;20(1):435. 10.1186/s12913-020-05295-2
27. May P, Garrido MM, Cassel JB, Kelley AS, Meier DE, Normand C, et al. Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients With Advanced Cancer: Earlier Consultation Is Associated With Larger Cost-Saving Effect. J Clin Oncol. 2015 Sep;33(25):2745–52. 10.1200/JCO.2014.60.2334
28. May P, Garrido MM, Cassel JB, Kelley AS, Meier DE, Normand C, et al. Cost analysis of a prospective multi-site cohort study of palliative care consultation teams for adults with advanced cancer: where do cost-savings come from? Palliat Med. 2017 Apr;31(4):378–86. 10.1177/0269216317690098
29. Davis MP, Temel JS, Balboni T, Glare P. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med. 2015 Jul;4(3):99–121.
30. Petrou S, Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ. 2011 Apr;342 apr07 2:d1548. 10.1136/bmj.d1548
31. Panczak R, Luta X, Maessen M. Regional Variation of Cost of Care in the Last 12 Months of Life in Switzerland: Small-area Analysis Using Insurance Claims Data. Volume 55. LIPPINCOTT WILLIAMS & WILKINS; 2017.
32. Matter-Walstra KW, Achermann R, Rapold R, Klingbiel D, Bordoni A, Dehler S, et al. Days spent in acute care hospitals at the end of life of cancer patients in four Swiss cantons: a retrospective database study (SAKK 89/09). Eur J Cancer Care (Engl). 2016.
33. Bähler C, Rapold R, Signorell A, Reich O, Panczak R, Blozik E. Regional differences in healthcare costs at the end of life: an observational study using Swiss insurance claims data. Int J Public Health. 2020 Jul;65(6):969–79. 10.1007/s00038-020-01428-w
34. Bevölkerungsbefragung Palliative Care 2017. Bern: Bundesamt für Gesundheit BAG 2018.
Table S1Overview of the data collection process showing time points of data collection, differentiating between questionnaires of SENS and SENS-economic studies and intervention start point.
| Study | SENS | SENS-economic | |||
| Time points of data collation | Month 0 (Baseline) | Month 2 | Month 4 | Month 6 | After death |
| SENS intervention | no | yes* | yes* | yes* | yes* |
| SENS questionnaire for patients | yes | yes | yes | yes | no |
| SENS-economic: health insurance data from last month of life | no | no | no | no | yes |
| SENS-economic questionnaire for family caregivers | no | no | no | no | yes |
*The SENS intervention is integrated into usual oncology care and consists of a single specialist palliative care consultation. Further specialist palliative care consultations were provided if needed.
Table S2Characteristics of 28 family caregivers who answered the family caregivers’ questionnaire.
| Data from family caregiver survey | SENS (n = 12) | Control (n = 16) | p value |
| Relationship to patient | 1.0 | ||
| Partner | 9 (75%) | 11 (69%) | |
| Child | 1 (8.3%) | 2 (13%) | |
| Brother / sister | 0 (0.0%) | 1 (6.3%) | |
| Other | 2 (17%) | 2 (13%) | |
| Age | 62 (11) | 64 (9.0) | 0.75 |
| Female sex | 11 (92%) | 9 (56%) | 0.09 |
Table S3Baseline and follow-up characteristics of a subgroup of 28 patients with advanced cancer with family caregivers who answered family caregivers’ questionnaire. Outcomes are expressed as median (interquartile range), mean ± standard deviation or n (%).
| Total (n = 28) | SENS (n = 12) | Standard (n = 16) | P value | |
| Baseline measurements | ||||
| Age (years) | 67 (62–73) | 67 (58–73) | 67 (62–73) | 0.75 |
| Female sex | 9 (32%) | 3 (25%) | 6 (38%) | 0.69 |
| Marital Status | 0.63 | |||
| Single | 4 (14%) | 1 (8.3%) | 3 (19%) | |
| Married | 17 (61%) | 8 (67%) | 9 (56%) | |
| Widowed | 1 (3.6%) | 1 (8.3%) | 0 (0.00%) | |
| Divorced | 6 (21%) | 2 (17%) | 4 (25%) | |
| Religion | 0.84 | |||
| Catholic | 6 (21%) | 2 (17%) | 4 (25%) | |
| Protestant | 18 (64%) | 8 (67%) | 10 (63%) | |
| None | 3 (11%) | 2 (17%) | 1 (6.3%) | |
| Other | 1 (3.6%) | 0 (0.00%) | 1 (6.3%) | |
| Availability of an advance directive | 0.81 | |||
| No | 7 (25%) | 3 (25%) | 4 (25%) | |
| Yes | 1 (3.6%) | 1 (8.3%) | 0 (0.00%) | |
| Unknown | 20 (71%) | 8 (67%) | 12 (75%) | |
| Cancer diagnosis | 0.60 | |||
| Lung cancer | 11 (39%) | 5 (42%) | 6 (38%) | |
| Colorectal cancer | 2 (7.1%) | 1 (8.3%) | 1 (6.3%) | |
| Prostate cancer | 2 (7.1%) | 1 (8.3%) | 1 (6.3%) | |
| Breast cancer | 1 (3.6%) | 1 (8.3%) | 0 (0.00%) | |
| Urothelial cancer | 3 (11%) | 2 (17%) | 1 (6.3%) | |
| Pancreatic cancer | 9 (32%) | 2 (17%) | 7 (44%) | |
| ECOG PS | 1.00 | |||
| 0 | 6 (21%) | 3 (25%) | 3 (19%) | |
| 1 | 17 (61%) | 7 (58%) | 10 (63%) | |
| 2 | 5 (18%) | 2 (17%) | 3 (19%) | |
| Comorbidities | 25 (89%) | 11 (92%) | 14 (88%) | 1.00 |
| Last questionnaire in patient follow-up | ||||
| Physician visit | 20 (71%) | 8 (67%) | 12 (75%) | 0.69 |
| Hospital emergency room visit | 8 (29%) | 1 (8.3%) | 7 (44%) | 0.09 |
| Hospital overnight stay | 8 (29%) | 2 (17%) | 6 (38%) | 0.40 |
| FACT-G | 56 (52– 62) | 53 (52–56) | 61 (51–63) | 0.44 |
| POS | 10 (7.0–14) | 8.5 (6.0–14) | 10 (7.0–14) | 0.43 |
| LSNS-6 | 21 (16–24) | 17 (14–24) | 22 (19–25) | 0.12 |
| NCCN distress thermometer | 4.0 (2.0–5.0) | 4.0 (2.5–5.5) | 4.0 (2.0–5.0) | 1.00 |
ECOG PS: Eastern Cooperative Oncology Group Performance Status; NCCN: National Comprehensive Cancer Network; FACT-G: Functional Assessment of Cancer Therapy – General scale; POS: Palliative care Outcome Scale; LSNS-6: abbreviated Lubben Social Network Score.
Table S4Per-protocol analysis of costs in Swiss Francs (CHF); three participants in the study population did not receive the SENS intervention according to protocol.
| Total (n = 55) | SENS (n = 23) | Control (n = 32) | p | |
| Total health care costs | 7632 (5214–12367) | 7288 (5214–12367) | 8492 (5411–12012) | 0.66 |
| Outpatient physician care | 580 (59–1386) | 512 (27–1342) | 691 (220–1706) | 0.48 |
| Outpatient nursing care | 270 (0.00–1037) | 0.00 (0.00–808) | 336 (0.00–1323) | 0.19 |
| Outpatient laboratory | 0.00 (0.00–79) | 0.00 (0.00–92) | 0.00 (0.00–62) | 0.17 |
| Outpatient medication | 386 (62–620) | 275 (18–843) | 389 (119–584) | 0.64 |
| Outpatient care products | 0.00 (0.00–232) | 0.00 (0.00–124) | 1.4 (0.00–369) | 0.54 |
| Transport | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–19) | 0.20 |
| Outpatient health professionals (excluding physicians and nurses) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.29 |
| Other care | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.96 |
| Inpatient | 4010 (0.00–9602) | 5833 (0.00–9123) | 3848 (0.00–10614) | 0.79 |
Table S5Overview of intervention treatment costs in Swiss Francs (CHF).
| Cost type | Average consultation timein minutes | Administrative time in minutes | Costs* (CHF) | Total costs (CHF) |
| Specialist palliative care physician | 52 | 20 | 192 | 230.4 |
| Advanced practice nurse | 52 | 20 | 96 | 115.2 |
| 3 leaflets (design and print) | - | - | 10.5 | 10.5 |
| Consultation room rent (1 hour) | - | - | 24.3 | 24.3 |
| Total | 380.4 | |||
* Provided by hospital administration in matching year.
Table S6Overview of health care that participants declined according to family caregivers.
| Care type declined by patient* | SENS (n = 12) | Control (n = 16) |
| Resuscitation in the event of cardiovascular arrest | 6 (50%) | 4 (25%) |
| Continuation of radiation therapy, chemotherapy or hormone therapy | 3 (25%) | 5 (31%) |
| Starting new radiation therapy, chemotherapy or hormone therapy | 3 (25%) | 4 (25%) |
| Enteral nutrition | 3 (25%) | 4 (25%) |
| Surgery | 2 (17%) | 3 (19%) |
| Continuation of medication | 2 (17%) | 3 (19%) |
| Starting new medication | 2 (17%) | 2 (13%) |
| Special examinations (such as PET or blood tests) | 2 (17%) | 2 (13%) |
| Parenteral nutrition | 1 (8.3%) | 1 (6.3%) |
| (More) specialist palliative nursing home care | 1 (8.3%) | 1 (6.3%) |
| (More) nursing home care | 0 (0.0%) | 1 (6.3%) |
| Planned hospitalisation | 0 (0.0%) | 1 (6.3%) |
| Emergency hospitalisation | 0 (0.0%) | 1 (6.3%) |
| Blood transfusions | 1 (8.3%) | 0 (0.0%) |
| Other | 1 (8.3%) | 2 (13%) |
| Total | 27 | 32 |
PET: Positron Emission Tomography.
*Multiple answers per family caregiver are possible.

Figure S1SENS structure as provided on a summary pocket card.