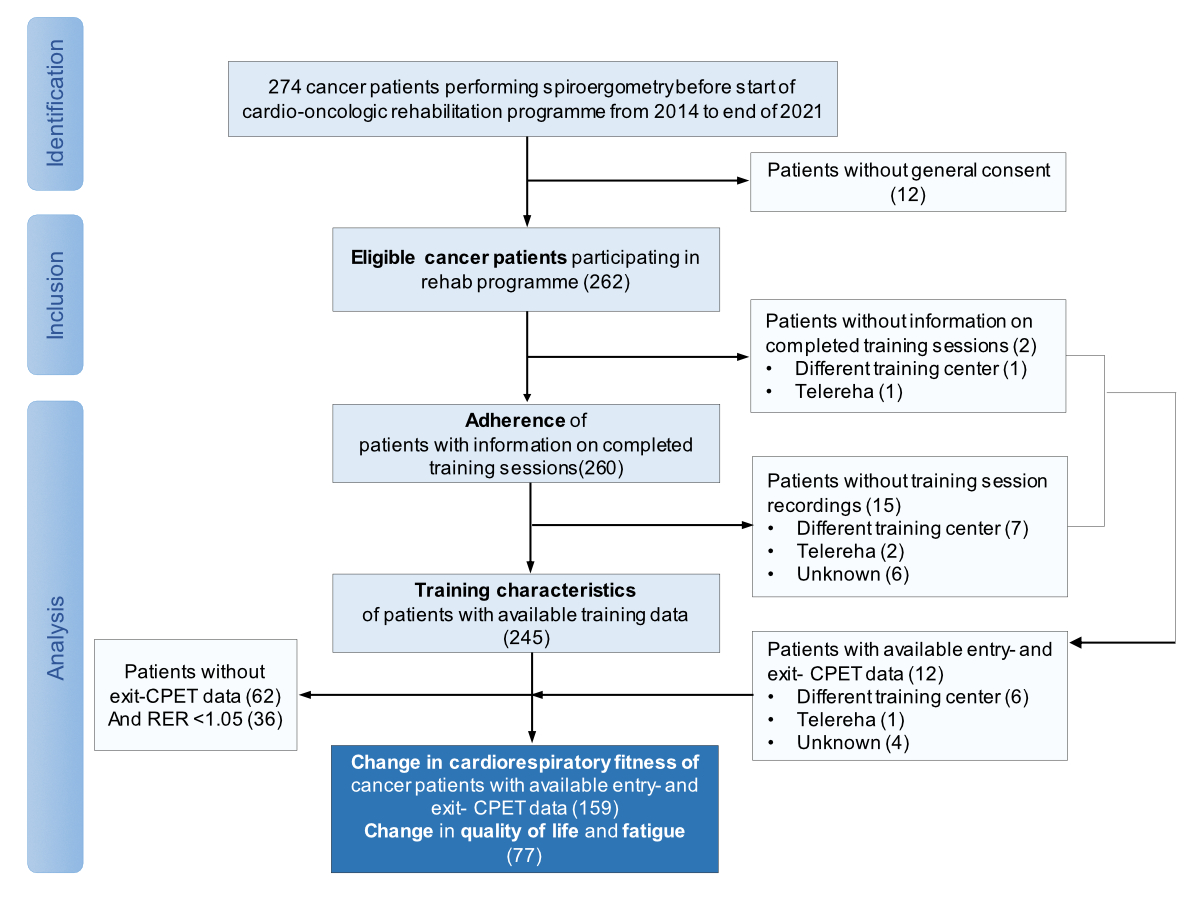
Figure 1Study flow. CORE: Cardio-oncology rehabilitation programme; ET: Exercise training; CPET: Cardiopulmonary exercise test; RER: Respiratory exchange ratio.
DOI: https://doi.org/https://doi.org/10.57187/s.3588
Lower cardiorespiratory fitness has been observed in patients with cancer compared to healthy age- and sex-matched sedentary individuals [1–3]. While lower levels of physical activity due to diagnosis and treatment may be a driving factor in the decline of cardiorespiratory fitness, measured as peak oxygen consumption (peak VO2), there is increasing interest in understanding other physiological mechanisms that may be driving this observation. Reduced cardiorespiratory fitness may compromise health-related quality of life and is a strong independent predictor of cancer- and cardiac-specific mortality as well as overall mortality in cancer survivors [4, 5]. A better understanding of these mechanisms will allow for the development of interventions to improve cardiorespiratory fitness during and following cancer diagnosis and treatment.
Lower cardiorespiratory fitness has been found in the absence of cancer treatment (surgery and/or chemotherapy and/or radiotherapy). This finding may be explained by tumour-induced damage and resulting systemic inflammation, affecting multiple organs [6]. Moreover, cancer therapies may cause skeletal muscle myopathy [6, 7] and can have a direct negative effect on the heart and vascular endothelial function [8], further reducing peak VO2 [9]. A proposed mechanism for cancer therapy-associated toxicity is the generation of reactive oxygen species (ROS), induced by anthracycline-based chemotherapy [10, 11], that may negatively affect both skeletal and cardiac muscle tissue [6]. In the muscle, ROS may induce mitochondrial dysfunction, contribute to the inflammation process and can affect muscle repair by reducing the replenishment of the satellite pool [12]. These combined mechanisms amplify the myopathy process and may ultimately result in skeletal muscle wasting and dysfunction, leading to reduced peak oxygen uptake [12]. In the heart, increased ROS production may cause cardiac myocyte apoptosis and necrosis (i.e. anthracycline-induced cardiac toxicity) [13], thereby negatively affecting oxygen transport. Deconditioning and weight gain, occurring secondary to therapies, may result in suboptimal cardiovascular disease prevention. Moreover, the presence of shared risk factors occurring in combination (i.e. inactive lifestyle, obesity and smoking) may adversely affect cardiorespiratory fitness and predispose patients with cancer to the development of cardiovascular disease [14].
Structured exercise training as part of cardio-oncology rehabilitation (CORE) programmes has the potential to prevent and/or restore loss in cardiorespiratory fitness and to alleviate side effects of cancer therapies (i.e. reduce fatigue and improve quality of life) [15]. While the increase in peak VO2 with exercise training was generally found to be greater when it was done after rather than during cancer treatment [16, 17], no study has compared the timing effect in patients receiving cardiotoxic vs those receiving non-cardiotoxic treatments.
We therefore aimed to evaluate the association of timing of exercise-based CORE with change in cardiorespiratory fitness (primary outcome), quality of life and cancer-related fatigue (secondary outcomes) in patients with anthracyclines, other cardiotoxic therapies (non-anthracycline) and non-cardiotoxic therapies.
We included patients with cancer who participated in a 3-month exercise-based CORE programme at the University Hospital of Bern, Switzerland, between January 2014 and the end of January 2022 and who underwent a cardiopulmonary exercise test (CPET) at the beginning and end of the programme. The indication for cardio-oncological rehabilitation in Switzerland depends on referral by oncologists and by different reimbursement strategies of different health insurance schemes. Upon referral, patients underwent a cardiorespiratory fitness assessment at the Centre of Rehabilitation & Sports Medicine and completed the CORE programme at the Department of Physiotherapy.
Patients with a missing follow-up CPET or invalid peak VO2 at one or both visits, but available data on training characteristics and participation were included in the analysis of adherence and training characteristics but excluded from the primary analysis on predictors for change in peak VO2.
We categorised disease as early stage or advanced stage using the UICC (Union for International Cancer Control) classification and the International Staging System (ISS), which is used for classifying multiple myeloma. “Early stage” was defined as all cancers classified as UICC 0 to III and ISS I, whereas “advanced stage” included cancers classified as UICC IV and ISS II to III [18].
Cardiovascular risk factors were recorded and a sum score was calculated. The presence of established atherosclerotic cardiovascular disease (ASCVD, i.e. prior myocardial infarction, stroke or presence of atherosclerosis) or other cardiovascular disease (i.e. thrombosis, hypertensive cardiomyopathy, pulmonary embolism) at the beginning of the programme was documented.
For patients who had provided signed general consent, non-genetic, health-related data such as age, sex and medical history were derived from the electronic health record. Every training session was recorded and stored in the training monitoring system until the time of data extraction in January 2022. Likewise, CPET data was stored in the databank of the cardiopulmonary exercise test software and extracted at the same time point. CPET data was merged with clinical data and data of monitored training sessions if available. The study was approved by the local ethics committee of the canton of Bern as part of the CAPRICE study (NCT03850171).
The primary outcome of the study was change in cardiorespiratory fitness (peak VO2) from before to after CORE according to type of therapy, namely anthracycline-based chemotherapy, other cardiotoxic therapies (non-anthracycline) and non-cardiotoxic therapies and timing of exercise training. As secondary outcomes, the study evaluated the association of timing of exercise training with changes in quality of life and cancer-related fatigue in patients with anthracyclines, non-anthracyclines or non-cardiotoxic therapies.
Cardiopulmonary exercise tests were performed on a cycle ergometer with an individualised ramp protocol aiming to achieve exhaustion within 8 to 12 minutes of ramp duration. The protocol consisted of a 3-minute warm-up at a workload of 5–50 watts followed by an increase of 10, 15 or 20 watts every minute until voluntary exhaustion then a 2-minute active cool-down period. Throughout the cardiopulmonary exercise test, patients were monitored by a cardiologist with continuous assessment of a 12-lead ECG. Gas exchange was measured using the spirometry system Jaeger Oxycon Pro (Masterscreen CPX, PanGas Healthcare GmbH) up to the end of February 2020, then using the Quark spirometry system (Cosmed, Rome, Italy). Peak values from the cardiopulmonary exercise tests were determined and included VO2, power, heart rate as well as ventilatory and gas exchange parameters. Ventilatory thresholds (VT1 and VT2) were determined using established criteria [19]. To ensure that only valid peak VO2 values were used in our analysis, we excluded cardiopulmonary exercise tests with a respiratory exchange ratio below 1.05, since below this value the identified peak VO2 is from a submaximal test and does not indicate a true peak value.
Quality of life was assessed with the validated Functional Assessment of Cancer Therapy–General (FACT-G) scale, which contains subscales for physical, functional, emotional and social/family wellbeing. These questionnaires were routinely administered from the beginning of 2019. The subscales were summed to obtain a FACT-G score, which ranged from 0 to 108, with higher values indicating better quality of life. Fatigue was assessed using the 13-item Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), the fatigue scale of the FACT measurement system which gives a score ranging from 0 to 52, with lower values indicating a higher level of fatigue [20]. Patients completed the FACT-G and FACIT-F questionnaires on the days of their cardiopulmonary exercise test visits either electronically or on a print-out.
Methods for training monitoring and characteristics can be found in the data supplement in the appendix.
The following clinical factors were assessed for their predictive value for change in peak VO2 with exercise training: type of chemotherapy (categorical variable), timing of exercise training (categorical variable) in relation to cancer treatment, exercise training compliance and number of cardiovascular risk factors. Type of cancer and disease classification were not used in the model because they were collinear with type of chemotherapy. Age, sex, change in body fat percentage and baseline peak VO2 were included as confounding variables. To avoid entering too many training-related variables into the models, we calculated a composite training impulse variable (including endurance sessions only) according to the following formula: Total number of training sessions [n] × average duration of training sessions [min] × average training load as percentage of peak power at baseline [%]. Training impulse was included in the model for patients completing centre-based exercise training only and tested interchangeably with exercise training compliance to assess whether one factor was a better predictor. Since the model did not change substantially when compliance was included, these results are not shown. The type of chemotherapy was categorised into the following groups: anthracycline-based chemotherapy with or without other cardiotoxic treatment; cardiotoxic treatment other than anthracycline-based chemotherapy, e.g. trastuzumab, left-sided radiotherapy, fluorouracil or cyclophosphamide; and non-cardiotoxic treatment. Timing of exercise training was categorised as “during cancer treatment” or “following cancer treatment”. For patients who did not receive cardiotoxic therapies, exercise training participation during cancer treatment refers to any other therapy that was prescribed. For patients receiving anthracyclines, irrespective of whether other cardiotoxic therapies were administered, timing of exercise training participation refers to anthracyclines. For patients who participated in the exercise programme during other cardiotoxic treatment but did not receive anthracycline-based chemotherapy, timing of exercise training refers to other cardiotoxic therapies. Since the group of patients receiving no treatment was small (five patients), they were included in the non-cardiotoxic therapy “during” cancer treatment group.
All statistics were performed with R Studio (version 2022.02.0 +443) and SPSS (version 25). Baseline data was described as frequencies or means with standard deviations (or medians with interquartile ranges [IQRs]) as appropriate. Differences at baseline between patients receiving anthracycline-based chemotherapy vs those not receiving anthracycline-based chemotherapy were assessed with the Wilcoxon rank-sum test, the chi-squared test or Fisher’s exact test as appropriate.
Robust linear models were performed for change in peak VO2 [ml/kg/min] between baseline and conclusion cardiopulmonary exercise tests (lmrob function from the robustbase package). Robust regression was used since it is less sensitive to outliers than standard linear regression. The following factors were included as predictor variables: type and timing of exercise training; and the interaction effect of type of therapy and timing of exercise training. Age and peak exercise capacity at baseline were included as confounding variables. Cardiovascular risk factors (sum score), sex and change in body fat were included as additional predictor variables in the main model; however since results did not change substantially, we removed these factors from the analysis. We could not enter change in presence of anaemia at baseline as a confounding factor into the model because this data was only available in 62.2% of our patients. Therefore we only performed Pearson correlation between changes in haemoglobin with changes in VO2 to assess the influence of this variable on our primary outcome. Given that patients with no pharmaceutical or other cancer therapies and those receiving other cardiotoxic treatment showed comparable improvements in peak VO2 in the linear model, results of these patients were summarised in one group for presentation in tables (the without-anthracyclines group) and compared to the anthracycline therapy group (tables 1, 2 and 3). Furthermore, a corresponding model was performed for the subpopulation of patients attending the centre-based programme only (excluding patients enrolled in hybrid or pure tele-rehab programmes during the COVID-19 pandemic). In the subpopulation model, exercise training compliance or training impulse (in % to peak power at baseline) were included as additional predictor variables. Additionally, cumulative linked models (clm function from the ordinal package) were performed for change in quality of life and fatigue including type and timing of exercise training and the interaction effect of type of therapy and timing of exercise training as predictor variables, adjusted for FACT-G and FACIT-F scores at baseline. Cumulative linked models are used for regression analysis of dependent variables with ordinal data (rather than continuous numerical data that can be analysed with linear regression models). Missing data was not imputed. The alpha level was set at 0.05 for all analyses (two-tailed for Wilcoxon rank-sum test).
We collected the data of 262 patients at the beginning of their CORE (figure 1).

Figure 1Study flow. CORE: Cardio-oncology rehabilitation programme; ET: Exercise training; CPET: Cardiopulmonary exercise test; RER: Respiratory exchange ratio.
Adherence to exercise training during CORE was assessed in 260 patients whereas analysis on training characteristics was performed in 245 patients. A total of 205 patients (78.2%) completed the CORE with centre-based exercise training sessions only, whereas 54 patients (20.6%) attended a hybrid programme with both centre-based and home-based exercise training. Three additional patients completed the CORE programme as tele-rehabilitation with no centre-based exercise training. Hybrid and tele-rehabilitation was mainly offered to patients due to COVID-19-related closure of centre-based training facilities. Baseline characteristics of all included patients are shown in table 1.
Table 1Baseline characteristics of the study population, expressed as n (%) or median (interquartile range).
| Characteristics | All cancer patients | AC group | Without-AC group* | p value | |
| n = 262 | n = 141 | n = 121 | |||
| Female | 180 (68.7%) | 103 (73.0%) | 77 (63.6%) | 0.132 | |
| Age (years) | 52 (40–61) | 47 (37–57) | 56 (47–64.5) | <0.0001 | |
| Body mass index (kg/m2) | 24.7 (22.1–28) | 24.5 (21.9–27.8) | 25.1 (22.7–28.5) | 0.447 | |
| Systolic blood pressure (mm Hg) | 117 (109–130) | 115 (107–127) | 120 (110–130) | 0.013 | |
| Diastolic blood pressure (mm Hg) | 70 (62–80) | 70 (62–80) | 72 (63–80) | 0.063 | |
| Haemoglobin (g/l) ** | 123 (110–135) | 122 (111–133.5) | 123 (108.3–137.5) | 0.801 | |
| Time from haemoglobin assessment to spiroergometry (weeks) | 0 (-2.0–2.0) | 0 (-1.8–0.0) | -0.29 (-2.1–0) | 0.473 | |
| Cardiorespiratory fitness (ml/min/kg) | 21.0 (17.2–25.7) | 21.7 (18.2–26.6) | 20.2 (16.2–24.8) | 0.029 | |
| Cardiorespiratory fitness (% of predicted) | 87% (71.0–103%) | 87.1% (70–103%) | 86.3% (71.2–102.9%) | 0.943 | |
| Tumour site | Breast | 107 (40.8%) | 80 (56.7%) | 27 (22.3%) | <0.0001 |
| Lymphoma | 52 (19.8%) | 44 (31.2%) | 8 (6.6%) | ||
| Blood cancer | 32 (12.2%) | 11 (7.8%) | 21 (17.4%) | ||
| Other | 71 (27.1%) | 6 (4.3%) | 65 (53.7%) | ||
| Disease stage (I, II, III, IV) | Stage 0 | 2 (0.8%) | 1 (0.7%) | 1 (0.8%) | 0.937 |
| Stage I | 48 (18.3%) | 27 (19.1%) | 23 (19.0%) | ||
| Stage II | 39 (14.9%) | 24 (17.0%) | 15 (12.4%) | ||
| Stage III | 85 (32.4%) | 46 (32.6%) | 39 (32.2%) | ||
| Stage IV | 37 (14.1%) | 22 (15.6%) | 16 (13.2%) | ||
| Disease stage (early, advanced, other) | Early stage (UICC 0-III and ISS I) | 182 (69.5%) | 99 (70.2%) | 86 (71.1%) | 0.184 |
| Advanced stage (UICC IV and ISS II-III) | 48 (18.3%) | 22 (15.6%) | 26 (21.5%) | ||
| Leukaemia, other classification or unknown | 27 (10.3%) | 19 (13.5%) | 9 (7.4%) | ||
| Cancer therapy | Anthracycline-containing | 141 (53.8%) | |||
| Herceptin (trastuzumab) | 20 (7.6%) | 14 (9.9%) | 5 (4.1%) | 0.118 | |
| Radiotherapy | 118 (45.0%) | 65 (46.1%) | 53 (43.8%) | 0.804 | |
| Left-sided | 55 (21.0%) | 37 (26.2%) | 18 (23.1%) | 0.036 | |
| Other cardiotoxic treatment (cyclophosphamide, 5-fluorouracil) | 28 (10.7%) | 0 (0%) | 28 (23.1%) | <0.0001 | |
| Smoking | Never | 153 (58.4%) | 80 (56.7%) | 73 (60.3%) | 0.843 |
| Current | 30 (11.5%) | 17 (12.1%) | 13 (10.7%) | ||
| Former (more than 3 months ago) | 79 (30.2%) | 44 (31.2%) | 35 (28.9%) | ||
| Pre-existing cardiovascular conditions / comorbidities | Diabetes mellitus | 29 (11.1%) | 12 (8.5%) | 17 (14.0%) | 0.220 |
| Hyperlipidaemia | 47 (17.9%) | 18 (12.8%) | 29 (24.0%) | 0.028 | |
| Obesity | 49 (18.7%) | 25 (17.7%) | 24 (19.8%) | 0.782 | |
| Hypertension | 57 (21.8%) | 23 (16.3%) | 35 (28.9%) | 0.021 | |
| Anaemia** | 78 (48.4%) | 45 (31.9%) | 33 (27.3%) | 0.494 | |
| Sum score for cardiovascular risk factors | 1 (0–2) | 1 (0–1) | 1 (0–2) | ||
| Existing atherosclerotic cardiovascular disease (e.g. myocardial infarction, coronary vascular disease, stroke, peripheral artery disease) | 16 (6.1%) | 5 (3.5%) | 11 (9.1%) | 0.107 | |
| Other cardiovascular disease (e.g. thrombosis, pulmonary embolism, hypertensive cardiopathy) | 61 (23.3%) | 24 (17.0%) | 37 (30.6%) | 0.015 | |
AC: anthracycline therapy; ISS: International Staging System; UICC: Union for International Cancer Control.
* The without-anthracyclines group consists of patients who received cardiotoxic therapy other than anthracyclines (non-anthracyclines group) and patients who received non-cardiotoxic therapy (non-CTOX group).
** 163 patients had haemoglobin data at baseline, of whom 97 were in the anthracycline group and 66 in the non-anthracycline group. Anaemia was defined as haemoglobin level <120 g/l for women and <130 g/l for men.
Of patients with available data on haemoglobin levels, 31.9% and 27.3% of patients with and without anthracyclines, respectively, had anaemia (table S1 in the appendix). Patients with anthracyclines were 9 years younger than patients without anthracyclines (p <0.001). Cardiorespiratory fitness at baseline (when related to age- and sex-matched predicted values) did not differ between the two groups. Patients were comparable with regard to the sum of cardiovascular risk factors. A total of 141 patients received anthracyclines, of whom 94 patients received anthracyclines only (35.9%) and 47 patients (17.9%) additionally received another cardiotoxic therapy (i.e. trastuzumab, left-sided radiotherapy). Forty-nine patients (18.7%) received other cardiotoxic treatments without anthracyclines and 72 patients (27.5%) had surgery only or were treated with a non-cardiotoxic therapy. Some patients received both, chemotherapy and radiotherapy.
Table 2Baseline values and changes in cardiorespiratory fitness from baseline to conclusion cardiopulmonary exercise test in patients with respiratory exchange ratio (RER) ≥1.05. Values are expressed as median (interquartile range). n refers to the number of patients with available data for calculation of the change from the baseline to the conclusion cardiopulmonary exercise test.
| AC group (n = 97) | Without-AC group* (n = 67) | |||||||||||
| ET during therapy (n = 21) | ET after therapy (n = 76) | ET during therapy (n = 25) | ET after therapy (n = 42) | |||||||||
| Resting parameters | Baseline | Change | n | Baseline | Change | n | Baseline | Change | n | Baseline | Change | n |
| Heart rate (/min) | 77 (67–82) | –4.0 (–7.5–10.0) | 19 | 74 (69–86) | –2.0 (–7.5–3.5) | 75 | 77 (72–93) | –8.0 (–13–3) | 25 | 74 (65–85) | –6.0 (–13.3–5.0) | 40 |
| VO2 (ml/min/kg) | 5.1 (4.8–6.8) | –0.6 (–1.2–0.3) | 21 | 5.1 (4.2–5.9) | 0.0 (–1.0–3.8) | 75 | 5.3 (4.7–6.3) | –0.5 (–1.5–0.9) | 25 | 5.3 (3.9–6.1) | 0.1 (–1.1–0.9) | 41 |
| Ventilation (l/min) | 12.3 (11.2–14.0) | –0.8 (–2.3–1.0) | 20 | 12.0 (10.6–15.0) | 0.0 (–2.0–2.0) | 75 | 13 (11.0–15.5) | –1.0 (–3.0–0.8) | 25 | 12.4 (10.2–15.0) | 0.0 (–2.0–1.1) | 41 |
| Respiratory exchange ratio | 0.83 (0.80–0.88) | –0.01 (–0.04–0.06) | 20 | 0.83 (0.78–0.87) | –0.01 (–0.05–0.04) | 75 | 0.82 (0.80–0.86) | 0.00 (–0.06–0.05) | 25 | 0.79 (0.76–0.85) | 0.02 (–0.04–0.1) | 41 |
| Parameters at VT1 | ||||||||||||
| Power output (W/kg) | 0.9 (0.6–1.1) | –0.1 (–0.2–0.2) | 19 | 0.8 (0.6–1.0) | 0.2 (0.0–0.4) | 71 | 0.6 (0.4–0.8) | 0.1 (–0.02–0.3) | 23 | 0.7 (0.5–1.01) | 0.2 (0.0–0.3) | 41 |
| Heart rate (/min) | 121 (111–126) | 2.0 (–7–12) | 21 | 118 (110–132) | –2.0 (–11.8–9.8) | 74 | 119 (114–131) | –11.0 (–15.3–0.5) | 24 | 113 (102–125) | –0.5 (–9.3–6.0) | 40 |
| VO2 (ml/min/kg) | 14.1 (13.2–15.9) | –0.2 (–1.5–2.5) | 21 | 13.5 (11.1–16.1) | 1.8 (0.5–4.0) | 74 | 12.8 (11.2–14.2) | 0.8 (–0.8–2.4) | 25 | 13.7 (11.4–16.2) | 1.2 (–0.5–2.5) | 42 |
| VO2 (% of peak) | 58.6 (52.4– 62.9) | 2.2 (–3.1–11.0) | 21 | 60.7 (53.6–66.7) | 1.5 (–6.7–6.5) | 74 | 59.7 (53.6–68.7) | –2.9 (–13.1–4.0) | 25 | 61.9 (56.9–69.2) | –0.6 (–8.8–4.3) | 41 |
| Parameters at peak | ||||||||||||
| Power output (W/kg) | 2.4 (2.1–2.7) | 0.1 (–0.3–0.1) | 15 | 1.9 (1.5–2.4) | 0.4 (0.2–0.5) | 73 | 1.8 (1.3–2.1) | 0.4 (0.1–0.5) | 23 | 1.8 (1.3–2.1) | 0.3 (0.2–0.5) | 40 |
| Heart rate (/min) | 168 (157–180) | 0.0 (–5.0–6.0) | 21 | 167 (155–185) | –1.0 (–7.0–7.5) | 75 | 171 (153–179) | –3.0 (–9.0–4.0) | 25 | 156 (137–168) | 4.0 (–1.5–12.0) | 39 |
| VO2 (ml/kg/min) | 24.7 (21.7–29.2) | –2.1 (–4.7–2.0) | 21 | 23.0 (18.7–28.2) | 2.8 (1.2–5.3) | 76 | 21.6 (17.3–25.8) | 4.1 (0.7–7.7) | 25 | 21.5 (18.4–25.4) | 2.3 (0.1–4.6) | 42 |
| VO2 (% of predicted) | 97.0 (84.0–113.0) | –5.2 (–13.0–6.0) | 21 | 90.9 (75.7–106.3) | 11.2 (5.4–23.0) | 76 | 92.1 (77.0–102.6) | 16.0 (1.4–32.0) | 25 | 93.0 (77.6–103.8) | 7.6 (1.4–19.7) | 42 |
| Ventilation (l/min) | 72 (63–85) | –1.0 (–7.0–10.6) | 21 | 69 (51–84) | 8.0 (–2.0–15.2) | 75 | 77 (62–88.0) | 4.0 (–3.3–16.0) | 25 | 69 (56.0–80.0) | 6.0 (–1.0–13.0) | 41 |
| Respiratory exchange ratio | 1.22 (1.16–1.29) | 0.01 (–0.08–0.07) | 21 | 1.22 (1.16–1.26) | –0.02 (–0.08–0.03) | 76 | 1.21 (1.16–1.32) | –0.07 (–0.09–0.01) | 25 | 1.16 (1.11–1.24) | 0.02 (–0.04–0.08) | 42 |
AC: anthracycline therapy; ET: exercise training; VO2: Oxygen uptake; VT1: Ventilatory threshold 1.
*The without-AC group consists of patients who received cardiotoxic therapy other than anthracyclines (non-AC group) and patients who received non-cardiotoxic therapy (non-CTOX group).
Of 262 available patients, 62 were excluded from the analysis of peak VO2 due to a missing conclusion visit, leaving 200 patients with complete CPET data from the beginning to the end of the CORE programme. Of these patients, 36 did not reach sufficient metabolic exhaustion (respiratory exchange ratio ≥1.05) in either the baseline or conclusion CPET or both and were also removed from the dataset, leaving 164 patients for the final analysis. Missing data was not imputed. In these 164 patients, median (IQR) peak VO2 was 23.1 (18.9–26.9) ml/kg/min, corresponding to 92% (IQR: 77–106%) of sex- and age-predicted value before exercise training and increased to 25.6 (20.7–30.3) ml/kg/min, corresponding to 103% (IQR: 85–118%) after completion of exercise training. Median changes in peak VO2 from before to after exercise training were 2.3 ml/kg/min (range: –10.1–15.9), with 71.3% of patients showing an improvement in peak VO2 and 28.7% showing no changes or a decrease in oxygen uptake (see figure S1 in the appendix). Thirteen patients (6.5%) did not perform both CPETs on the same spirometry system; their median change in peak VO2 from before to after exercise training was 2.4 ml/kg/min (IQR: 1.2–3.7).
The final model included 156 of 164 patients and is shown in table 3. The centre-based model only included 108 of 125 patients, with missing data related to unavailable training data.
We found a significant interaction effect for cardiotoxic therapy and timing of CORE with regard to these therapies on change in peak VO2 (figure 2, table 3). Patients performing exercise training during anthracycline therapy had a mean adjusted decrease of –1.0 ml/kg/min, while those performing exercise training after anthracycline therapy had a mean adjusted increase of 3 ml/kg/min. In patients performing CORE during or after cardiotoxic treatment without anthracyclines (non-anthracycline), the adjusted increases were 4 ml/kg/min and 3 ml/kg/min, respectively. Changes in peak VO2 of 4 ml/kg/min and 2.0 ml/kg/min were found in patients without other cardiotoxic therapy who completed the exercise training during and after treatment, respectively. Older age (per 1 year) and higher baseline VO2 (per 1 ml/kg/min lesser increase in peak VO2) were negatively associated with changes in peak VO2 with a 0.07 ml/kg/min lesser increase in peak VO2 per year of increasing ageand a 0.11 ml/kg/min lesser increase per additional 1 ml/kg/min of higher baseline peak VO2. On the other hand, training impulse or compliance were positively associated with changes in peak VO2. However even in the centre-based model including training impulse or compliance, the large independent interaction effect of timing and type of chemotherapy persisted. Changes in haemoglobin were available in 105 patients and declines were greatest with anthracyclines (see table S1 in the appendix).
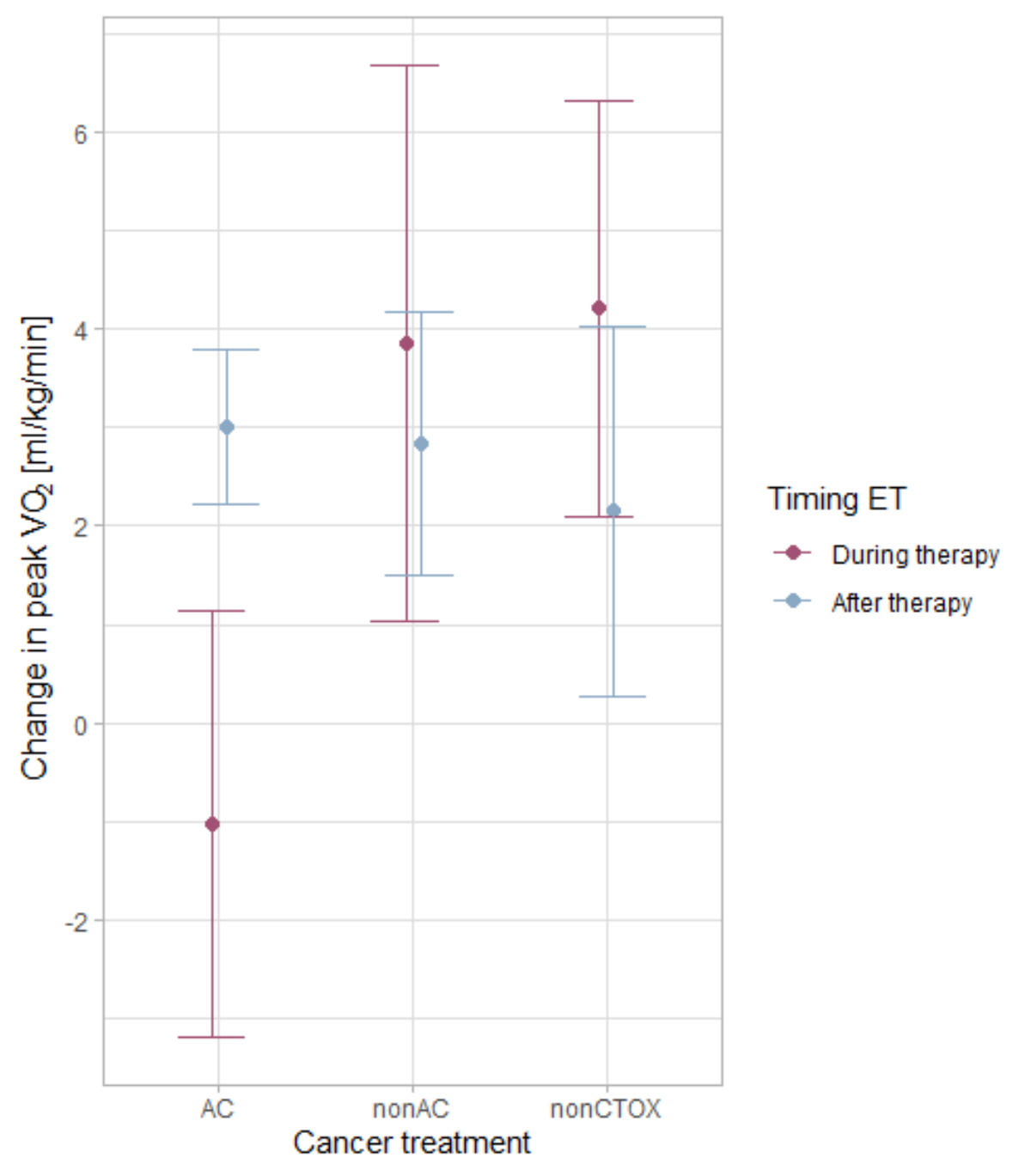
Figure 2Interaction plot showing changes in peak oxygen uptake (VO2) depending on timing of exercise training (ET) and cancer treatment adjusted for age, change in body fat percentage, baseline peak VO2 and training impulse. AC: anthracycline treatment; Non-AC: cardiotoxic treatment other than AC; Non-CTOX: non-cardiotoxic cancer treatment.
Table 3Robust linear models for change in peak VO2 from before to after exercise training. Independent parameters were age, peak VO2 at baseline, therapy (non-cardiotoxic therapy, anthracycline, non-anthracycline), timing of exercise training participation (during or after cancer therapy). The reference category for chemotherapy (anthracycline and non-anthracycline) was non-cardiotoxic therapy; for exercise training after cancer therapy, it was exercise training during cancer therapy. The model for change in peak VO2 explained 14.1% of total variance. In the subgroup of patients participating in the centre-based programme only, training impulse was included as an additional independent parameter. The model for change in peak VO2 explained 23.8 % of total variance.
| Change in peak VO [ml/kg/min] | Estimate (95% confidence interval) | t value | p value | |
| Model 1: all patients (n = 156) | ||||
| Intercept | 10.29 | (5.81–14.76) | 4.51 | <0.0001 |
| Age (years) | –0.07 | (–0.12––0.02) | –2.87 | 0.0046 |
| Peak VO2 at baseline (ml/kg/min) | –0.11 | (–0.22––0.01) | –2.07 | 0.0398 |
| Anthracycline treatment | –5.24 | (–8.23––2.25) | –3.43 | 0.0008 |
| Non-anthracycline treatment | –0.36 | (–3.84–3.12) | –0.20 | 0.8407 |
| Exercise training after cancer therapy | –2.05 | (–4.84–0.73) | –1.45 | 0.1503 |
| Anthracycline treatment X exercise training after cancer therapy* | 6.10 | (2.54–9.65) | 3.36 | 0.0010 |
| Non-AC X exercise training after cancer therapy* | 1.04 | (–3.05–5.12) | 0.50 | 0.6193 |
| Model 2: centre-based only (n = 108) | ||||
| Intercept | 8.51 | (2.25–14.77) | 2.66 | 0.0089 |
| Age (years) | –0.09 | (–0.15––0.03) | –2.98 | 0.0035 |
| Peak VO2 at baseline (ml/kg/min) | –0.11 | (–0.24–0.02) | –1.66 | 0.0992 |
| Training impulse (% power baseline) | 0.01 | (0.00–0.01) | 3.09 | 0.0025 |
| Anthracycline treatment | –5.41 | (–8.54––2.28) | –3.39 | 0.0010 |
| Non-anthracycline treatment | –0.46 | (–4.58–0.33) | –0.19 | 0.8537 |
| Exercise training after cancer therapy | –2.12 | (–4.58–0.14) | –1.69 | 0.0934 |
| AC X exercise training after cancer therapy* | 6.08 | (–2.53–9.63) | 3.36 | 0.0011 |
| Non-AC X exercise training after cancer therapy* | 0.85 | (–4.58–6.27) | 0.31 | 0.7608 |
VO2: oxygen uptake; Non-AC: cardiotoxic treatment other than anthracyclines.
* X indicates the interaction between cancer treatment (anthracycline therapy and cardiotoxic treatment other than anthracycline therapy) and exercise training timing.
Baseline data and changes in the FACT-G and FACIT-F scores after exercise training participation were available in 77 patients only, given that routine assessment and storage in the clinical database started in January 2021 only (table 4). Missing data was not imputed. Both quality of life and fatigue were lowest in the groups who attended the exercise training programme after chemotherapy completion. Exercise training participation during chemotherapy did not prevent a reduction in general wellbeing in either the anthracycline group or the without-anthracycline group whereas clinically meaningful increases occurred in the groups attending the exercise training programme after chemotherapy completion. Fatigue decreased with exercise training participation after cancer treatment, while an increase occurred in the anthracycline group with exercise training participation during anthracycline therapy (figure 3), with both changes being clinically meaningful [21]. In the linear model (table 5), neither timing nor cancer therapies had an effect on prediction of changes in general wellbeing. We found a trend for a greater decrease in the FACIT-F score (indicating an improvement in fatigue) when exercise training was completed after cancer treatment; however anthracycline-based chemotherapy or other cardiotoxic therapies did not affect changes in fatigue. For both quality of life and fatigue, higher FACT-G and FACIT-F scores at baseline were negatively associated with changes in quality of life and fatigue.
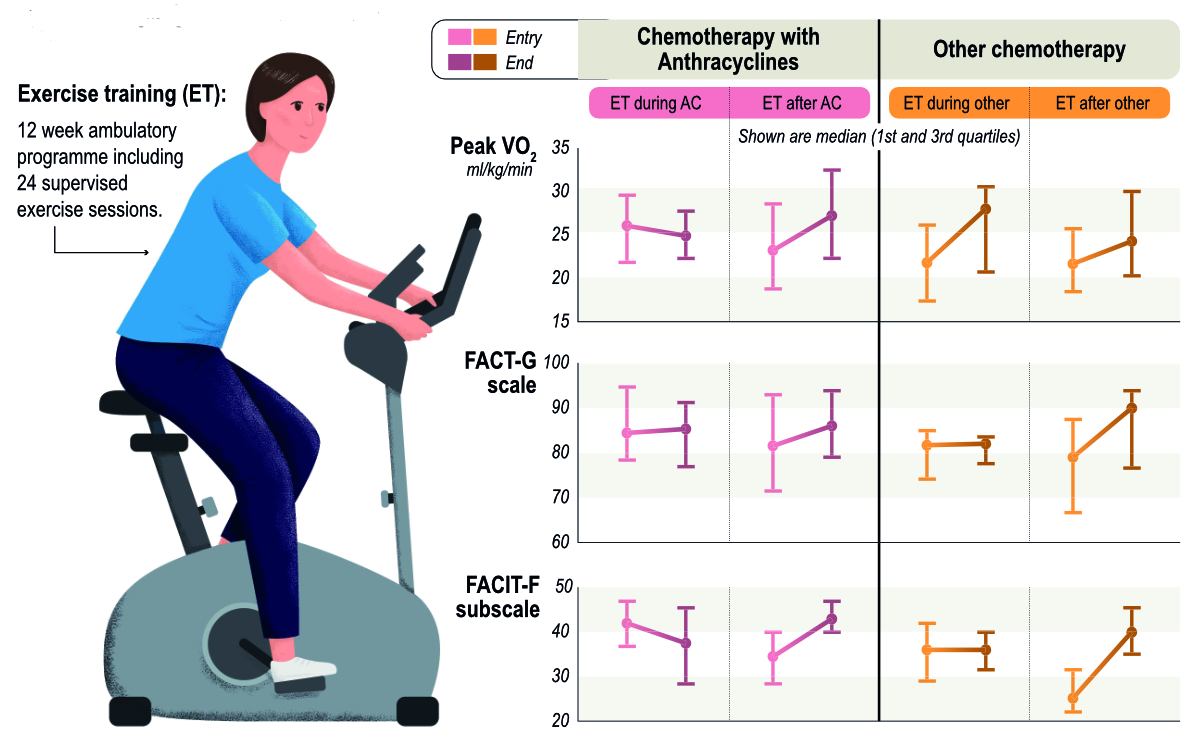
Figure 3Timing of exercise-based cardio-oncological rehabilitation in patients with cardiotoxic chemotherapies and changes in cardiorespiratory fitness and quality of life.Peak VO2: Maximal oxygen consumption; FACT-G: Functional Assessment of Cancer Therapy-General; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue.
Table 4Quality of life (FACT-G) and fatigue (FACIT-F) scores at baseline and changes with exercise training programmes. Values expressed as median (interquartile range).
| AC group | Without-AC group* | ||||||||
| Exercise training during therapy | Exercise training after therapy | Exercise training during therapy | Exercise training after therapy | ||||||
| Baseline | Change | Baseline | Change | Baseline | Change | Baseline | Change | ||
| n = 18 | n = 18 | n = 34 | n = 33 | n = 7 | n = 7 | n = 19 | n = 19 | ||
| FACT-G | 84.8 (78.3–94.8) | 0.8 (–5.8–6.5) | 81.5 (71.3–93.1) | 5.7 (0.0–10.0) | 81.6 (74–85) | –1.0 (–7.5–9.5) | 79.0 (66.5–87.5) | 10.0 (5.0–14.0) | |
| FACIT-F | 42 (36.8–47.0) | –1.5 (–14.5–6.5) | 34.5 (28.3–40.0) | 8.0 (2.0–12.0) | 36.0 (29.0–42.0) | 0.0 (–5.5–5.5) | 25.0 (22.0–31.5) | 13.0 (5.5–20.5) | |
AC: anthracyclines.
* The without-AC group consists of patients who received cardiotoxic therapy other than anthracyclines (non-AC group) and patients who received non-cardiotoxic therapy (non-CTOX group).
Table 5Cumulative linked models for change in quality of life and fatigue from before to after exercise training. Independent parameters were type of therapy (non-cardiotoxic treatment, anthracycline, other cardiotoxic treatment), timing of exercise training participation (during or after cancer therapy) and baseline quality of life and fatigue levels. Other predictor variables (e.g. age or training impulse) were not assessed due to the small number of patients with available data. The reference category for anthracycline therapy and non-anthracycline therapy was non-cardiotoxic treatment; for exercise training after cancer therapy, it was exercise training during cancer therapy.
| Estimate (95% confidence interval) | t value | p value | ||
| Change in quality of life: Subset of patients with available data on quality of life (n = 77) | ||||
| Anthracycline therapy | 0.69 | (–1.64–3.02) | 0.58 | 0.5650 |
| Non-anthracycline therapy | 1.24 | (–1.66–4.14) | 0.84 | 0.4030 |
| Exercise training after cancer therapy | 1.91 | (–0.54–4.36) | 1.52 | 0.1280 |
| FACT-G score at baseline | –0.08 | (–0.12–0.04) | –4.14 | 0.0000 |
| Anthracycline therapy x exercise training after cancer therapy | –1.20 | (–3.85–1.45) | –0.89 | 0.3740 |
| Cardiotoxic treatment other than anthracycline therapy x exercise training after cancer therapy | –1.05 | (–4.38–2.28) | –0.62 | 0.5350 |
| Change in fatigue: Subset of patients with available data on fatigue (n = 77) | ||||
| Anthracycline therapy | 0.98 | (–0.80–2.76) | 1.08 | 0.2811 |
| Non-anthracycline therapy | 2.26 | (–0.50–5.02) | 1.61 | 0.1085 |
| Exercise training after cancer therapy | 2.02 | (–0.02–4.06) | 1.94 | 0.0524 |
| Fatigue subscale at baseline | –0.19 | (–0.25–0.13) | –6.87 | 0.0000 |
| Anthracycline therapy x exercise training after cancer therapy | –0.54 | (–2.77–1.69) | –0.47 | 0.6378 |
| Cardiotoxic treatment other than anthracycline x exercise training after cancer therapy | –1.03 | (–4.24–2.18) | –0.63 | 0.5290 |
FACT-G: Functional Assessment of Cancer Therapy–General.
Training characteristics according to type and timing of anthracycline treatment are summarised in table S2 in the appendix. Median compliance for all patients (centre-based and hybrid exercise training) was 91.6% (IQR: 62.5–100%; range: 4–100%), with higher compliances after compared to during cancer therapy, particularly when exercise training during anthracycline treatment was compared to exercise training after anthracycline treatment (75% vs 96%, table S2).
The key finding of our study was that the increase in cardiorespiratory fitness with exercise training was diminished by concurrent anthracycline treatment in our cohort. For patients with cardiotoxic treatments other than anthracycline-based chemotherapy, cardiorespiratory fitness and fatigue were not associated with timing of exercise training (figure 3). Overall, greater positive changes in peak VO2 were seen in individuals who achieved a greater training impulse, whereas higher age and higher baseline VO2 resulted in smaller gains in peak VO2. This is to our knowledge the first study to evaluate changes in cardiorespiratory fitness, quality of life and fatigue with regard to timing of exercise training in patients with anthracycline treatment and other cardiotoxic chemotherapies.
Our findings of a median increase in peak VO2 of 2.3 ml/kg/min are in line with two meta-analyses in patients with cancer, reporting a weighted mean difference of 2.1 ml/kg/min [16] and 2.9 ml/kg/min [17] from before to after exercise training in exercising patients compared to the usual care group. Interestingly, in both studies changes in peak VO2 in exercising patients were greater following the completion of adjuvant therapy, compared to exercise training during therapies. Scott et al. describe a weighted mean difference of -1.1 ml/kg/min between groups participating in exercise training during vs after therapy and Jones et al. report an increase of 3.4 ml/kg/min in the three included studies conducting exercise training after chemotherapy [22–24] compared to 1.2 ml/kg/min for the two studies conducting exercise training during adjuvant treatment [25, 26]. In both meta-analyses, a great proportion of the included studies were conducted in middle-aged women with breast cancer; thus it can be expected that most patients were treated with anthracycline treatment. The meta-analysis by Scott et al. further investigated the effect of intervention timing (during vs before surgery and during vs after primary adjuvant therapy); however, they could not identify any moderating effect on the response in exercise capacity, contradicting our findings.Neither of the two meta-analyses differentiated anticancer treatment into anthracycline-based chemotherapy, cardiotoxic and non-cardiotoxic therapies. Our study extends the current literature by suggesting that for anthracycline treatment, changes in peak VO2 are time-dependent, but that for other cardiotoxic or non-cardiotoxic therapies, timing is not associated with reduced benefits on cardiorespiratory fitness, with the greatest changes seen in patients completing exercise training during these therapies.
The finding that quality of life decreased (anthracycline and non-anthracycline groups) and fatigue increased (anthracycline group only) in the groups performing exercise training during cancer treatment is in contrast to the results from a recent meta-analysis by Buffart et al., which reported a beneficial effect of exercise training on quality of life and did not find differences in the groups who exercised during compared to after cancer treatment [27]. However, cancer treatment was not categorised into anthracyclines and other non-cardiotoxic therapies. Another meta-analysis by McNeely et al. found a greater increase (by 4.6 points) in quality of life analysed by the FACT-G scale with exercise training compared to usual care in female breast cancer patients during and after cancer treatment (including anthracyclines) [28]. They also found a reduction in fatigue with exercise training. However, of the individual studies included, the two with exercise training performed following cancer treatment showed significant fatigue reductions, whereas the other four studies with exercise training performed during adjuvant cancer treatment showed non-significant improvements in fatigue, underpinning our findings. It should be noted that exercise training is only one component of a comprehensive CORE programme, which further includes counselling on psychosocial issues, nutrition, cardiovascular risk factors and pain management. Thus, changes in quality of life could have resulted from participation in other components of CORE and/or social interactions during exercise training. However, participation in other modules of CORE was very heterogeneous between patients and evaluation of comprehensive CORE was not the aim of the study.
The overall median increase of 2.3 ml/kg/min in peak VO2 in our cohort is likely to be of clinical importance, given the inverse association of cardiorespiratory fitness with all-cause and cardiovascular mortality in the general population and in patients with cancer [4, 5]. In an observational cohort study including 1691 male cancer survivors, high cardiorespiratory fitness (defined as those above the 60th percentile, achieving mean metabolic equivalent of task [MET] of 13.0 ± 1.8 in a treadmill test) was associated with a 32% risk reduction of cancer mortality and a 68% reduction of cardiovascular disease mortality, compared with patients with low cardiorespiratory fitness (defined as those below the 20th percentile, achieving 8.4 ± 1.2 MET) [29]. In the same study, every 1 MET increase in cardiorespiratory fitness corresponded to 17% and 9% relative risk reductions in lung and colorectal cancer risk, respectively. A population-based follow-up study in 579 apparently healthy men observed changes in peak VO2 over 11 years and found that after adjusting for various risk factors, baseline VO2 and physical activity, a 1 ml/kg/min lesser decrease in peak VO2 was associated with a 9% relative risk reduction of all-cause mortality after approximately 13 years of follow-up [30].
The increase in peak VO2 was highest when patients completed the exercise training during non-anthracycline therapies (4.1 ml/kg/min) and after receiving anthracyclines (2.8 ml/kg/min), even if they received other cardiotoxic therapies during exercise training. It is difficult to judge whether changes in exercise capacity occurred due to exercise training participation and factors related to exercise intensity and dose, spontaneous recovery of peak VO2 or due to unmeasured confounders. However, since training impulse was highest in the exercise training after anthracycline group, followed by the exercise training after non-anthracycline group, exercise training during non-anthracycline and exercise training during anthracycline group (table S2 in the appendix), it does not sufficiently explain the highest changes seen in patients completing exercise training during non-anthracycline therapies. In addition, cardiorespiratory fitness can be reduced due to prevailing anaemia, which often develops over the course of anticancer therapy with haemoglobin levels lowest immediately post-therapy, but generally recovering within 12 weeks of treatment cessation [31]. Since patients in the exercise training during non-anthracycline therapies and exercise training after anthracycline groups showed the lowest haemoglobin values at baseline and the greatest increase over the course of exercise training (table S1 in the appendix), the larger improvement in peak VO2 may be partly explained by spontaneous recovery of haemoglobin values. In fact, haemoglobin only declined in the group performing exercise training during anthracycline therapy (table S1). However, when changes in haemoglobin levels from before to after exercise training were included in the model for change in peak VO2, this factor was not significant, possibly due to the reduced sample size of patients with haemoglobin data. There was a significant albeit weak correlation between changes in peak VO2 and changes in haemoglobin level (0.260, p <0.001), suggesting at best a minor role of anaemia on changes in peak VO2.
Our finding that training impulse (and compliance) was positively and independently associated with changes in peak VO2 is in line with results by Bjørke et al. who evaluated the effects of training modes and intervention duration on peak VO2 in patients exercising during (neo-) adjuvant treatment [32]. Indeed, other studies in patients with cancer and sedentary older adults have shown that higher weekly exercise durations and intensities are associated with greater improvements in peak VO2 [33–35]. It should be noted that part of the poor improvement in peak VO2 in the exercise training during anthracycline therapy group may have resulted from lower compliance with the exercise programme, resulting in a lower training impulse (table S3 in the appendix). However, a recent study by Foulkes et al. observed similar changes in peak VO2 from before to after a 12-week supervised exercise programme (–1.5 ml/kg/min, 6% reduction compared to baseline) with higher exercise compliance (median 83%) and greater training impulse (3 exercise sessions per week, including 1 interval session) [36]. This suggests that the systemic adverse effect of anthracycline therapy may counteract exercise training-induced beneficial adaptations.
This is the first study to suggest that timing of exercise training participation with regard to cancer treatment should be considered when analysing changes in peak VO2, quality of life and fatigue.
Exercise training during anthracycline-based chemotherapy was not associated with improvements in cardiorespiratory fitness, quality of life or fatigue.
Inherent to the study’s observational design, no conclusions can be made about the causal association between anthropometric, clinical and exercise training-related moderators (i.e. training characteristics) and changes in peak VO2 and quality of life. The observational design of the study further prevents the appraisal of the potential of exercise training to mitigate a larger decline of cardiorespiratory fitness and quality of life during anthracycline therapy. In addition, our sample size allowed for a somewhat crude analysis only and did not permit differentiation into further subgroups based on timing of exercise training with regard to primary cancer diagnosis, type of cardiotoxic treatments other than anthracycline therapy or cancer treatments additional to anthracyclines. Furthermore, the timing with regard to anthracyclines always overrode other categories (even if they were cardiotoxic, such as trastuzumab) and the exercise training after anthracycline therapy group included 12 patients on trastuzumab, which may have led to an underestimation of recovery of peak VO2 with exercise training after anthracycline therapy. Unfortunately, 62 patients had to be excluded from our primary outcome analysis of peak VO2 due to a missing conclusion visit, mainly related to the COVID-19 pandemic. Based on the baseline characteristics of these patients, it was not possible to estimate how the missing data would have affected our results; therefore data was not imputed.
Another limitation linked to the clinical setting of this study was that patients did not complete a familiarisation CPET. In a study by Jones et al., an increase in peak VO2 of 0.9 ml/kg/min was found from the first to the second CPET. Nevertheless, when subtracting 0.9 ml/kg/min from the median improvement in peak VO2 observed in our study, our patients still show a clinically meaningful improvement of 1.4 ml/kg/min. 6.5% of our patients had follow-up CPETs on a different spirometry system; however changes in peak VO2 were comparable to those observed in the other patients.
Despite the limitations of observational design, our real-life setting of patients with cancer participating in an exercise training programme make our study more representative than randomised trials with strict selection criteria. Indeed, with 59% of the study cohort consisting of patients with various cancer diagnoses, our findings expand on data that has typically been obtained from studies consisting of exclusively or primarily breast cancer patients.
The present study evaluated the predictive value of clinical and training-related factors on short-term changes in cardiorespiratory fitness, quality of life and fatigue with exercise training participation. This is the first study to suggest that the change in peak VO2, quality of lifeand fatigue may be dependent on type and timing of exercise training with regard to cardiotoxic cancer treatment. The modest (negative) changes in peak VO2 observed in our study may be explained by simultaneous adverse effects of anthracycline therapy on skeletal and cardiac muscle tissue, impairing oxygen transport and utilisation. For patients with cardiotoxic treatments other than anthracycline therapy, timing had a minimal effect and was not related to reduced benefits on cardiorespiratory fitness and fatigue with exercise training. Higher age and higher baseline VO2 were negatively associated with changes in peak VO2 while greater training impulse was positively associated with them. The observational study design precludes the assessment of the potential of exercise training to mitigate a larger decline of cardiorespiratory fitness, quality of life and fatigue during anthracycline therapy.Nevertheless, whether changes in exercise capacity occurred secondary to changes in haemoglobin values, to factors related to exercise intensity and dose or to unmeasured confounders remains to be determined in future studies.
Data sharing may be available upon request to the corresponding author.
We want to acknowledge Susana Perez Alves for her contribution in the design of the graphical abstract (figure 3).
The study was partly funded by a Swiss Cancer Research Grant to the CAPRICE study (Clinical Trials.gov: NCT03850171) under grant number HSR-4360-11-2017.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, et al. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol. 2014 Sep;64(13):1310–9. 10.1016/j.jacc.2014.07.948
2. Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012 Jul;30(20):2530–7. 10.1200/JCO.2011.39.9014
3. Lipshultz SE, Landy DC, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012 Apr;30(10):1050–7. 10.1200/JCO.2010.33.7907
4. Groarke JD, Payne DL, Claggett B, Mehra MR, Gong J, Caron J, et al. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes. 2020 Oct;6(4):315–22. 10.1093/ehjqcco/qcaa015
5. Schmid D, Leitzmann MF. Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol. 2015 Feb;26(2):272–8. 10.1093/annonc/mdu250
6. Rausch V, Sala V, Penna F, Porporato PE, Ghigo A. Understanding the common mechanisms of heart and skeletal muscle wasting in cancer cachexia. Oncogenesis. 2021 Jan;10(1):1. 10.1038/s41389-020-00288-6
7. Campelj DG, Goodman CA, Rybalka E. Chemotherapy-Induced Myopathy: The Dark Side of the Cachexia Sphere. Cancers (Basel). 2021 Jul;13(14):3615. 10.3390/cancers13143615
8. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al.; ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016 Sep;37(36):2768–801. 10.1093/eurheartj/ehw211
9. Klassen O, Schmidt ME, Scharhag-Rosenberger F, Sorkin M, Ulrich CM, Schneeweiss A, et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol. 2014 Oct;53(10):1356–65. 10.3109/0284186X.2014.899435
10. Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014 Sep;64(9):938–45. 10.1016/j.jacc.2014.06.1167
11. Angsutararux P, Luanpitpong S, Issaragrisil S. Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxid Med Cell Longev. 2015;2015:795602. 10.1155/2015/795602
12. Campelj DG, Goodman CA, Rybalka E. Chemotherapy-Induced Myopathy: The Dark Side of the Cachexia Sphere. Cancers (Basel). 2021 Jul;13(14):3615. 10.3390/cancers13143615
13. Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49(5):330–52. 10.1016/j.pcad.2006.10.002
14. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J. 2022;43(41):4229-4361.
15. Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, et al.; American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Peripheral Vascular Disease. Cardio-Oncology Rehabilitation to Manage Cardiovascular Outcomes in Cancer Patients and Survivors: A Scientific Statement From the American Heart Association. Circulation. 2019 May;139(21):e997–1012. 10.1161/CIR.0000000000000679
16. Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018 Aug;36(22):2297–305. 10.1200/JCO.2017.77.5809
17. Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–20. 10.1634/theoncologist.2010-0197
18. Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al.; ESMO Guidelines Committee. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015 Sep;26 Suppl 5:v8–30. 10.1093/annonc/mdv298
19. Corrà U, Piepoli MF, Adamopoulos S, Agostoni P, Coats AJ, Conraads V, et al. Cardiopulmonary exercise testing in systolic heart failure in 2014: the evolving prognostic role: a position paper from the committee on exercise physiology and training of the heart failure association of the ESC. Eur J Heart Fail. 2014 Sep;16(9):929–41. 10.1002/ejhf.156
20. Cella D. Assessment methods for quality of life in cancer patients: the FACIT measurement system. Int J Pharm Med. 2000;14(2):78–81. 10.2165/00124363-200004000-00007
21. Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002 Dec;24(6):547–61. 10.1016/S0885-3924(02)00529-8
22. Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc. 2002 Dec;34(12):1863–7. 10.1097/00005768-200212000-00001
23. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003 May;21(9):1660–8. 10.1200/JCO.2003.04.093
24. Herrero F, San Juan AF, Fleck SJ, Balmer J, Pérez M, Cañete S, et al. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med. 2006 Jul;27(7):573–80. 10.1055/s-2005-865848
25. Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007 Oct;25(28):4396–404. 10.1200/JCO.2006.08.2024
26. Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009 Jan;27(3):344–51. 10.1200/JCO.2007.15.4963
27. Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017 Jan;52:91–104. 10.1016/j.ctrv.2016.11.010
28. McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006 Jul;175(1):34–41. 10.1503/cmaj.051073
29. Lakoski SG, Willis BL, Barlow CE, Leonard D, Gao A, Radford NB, et al. Midlife Cardiorespiratory Fitness, Incident Cancer, and Survival After Cancer in Men: The Cooper Center Longitudinal Study. JAMA Oncol. 2015 May;1(2):231–7. 10.1001/jamaoncol.2015.0226
30. Laukkanen JA, Zaccardi F, Khan H, Kurl S, Jae SY, Rauramaa R. Long-term Change in Cardiorespiratory Fitness and All-Cause Mortality: A Population-Based Follow-up Study. Mayo Clin Proc. 2016 Sep;91(9):1183–8. 10.1016/j.mayocp.2016.05.014
31. Kirkham AA, Lloyd MG, Claydon VE, Gelmon KA, McKenzie DC, Campbell KL. A Longitudinal Study of the Association of Clinical Indices of Cardiovascular Autonomic Function with Breast Cancer Treatment and Exercise Training. Oncologist. 2019 Feb;24(2):273–84. 10.1634/theoncologist.2018-0049
32. Bjørke AC, Sweegers MG, Buffart LM, Raastad T, Nygren P, Berntsen S. Which exercise prescriptions optimize V̇O2 max during cancer treatment?-A systematic review and meta-analysis. Scand J Med Sci Sports. 2019 Sep;29(9):1274–87. 10.1111/sms.13442
33. Kampshoff CS, Chinapaw MJ, Brug J, Twisk JW, Schep G, Nijziel MR, et al. Randomized controlled trial of the effects of high intensity and low-to-moderate intensity exercise on physical fitness and fatigue in cancer survivors: results of the Resistance and Endurance exercise After ChemoTherapy (REACT) study. BMC Med. 2015 Oct;13(1):275. 10.1186/s12916-015-0513-2
34. van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol. 2015 Jun;33(17):1918–27. 10.1200/JCO.2014.59.1081
35. Huang G, Wang R, Chen P, Huang SC, Donnelly JE, Mehlferber JP. Dose-response relationship of cardiorespiratory fitness adaptation to controlled endurance training in sedentary older adults. Eur J Prev Cardiol. 2016 Mar;23(5):518–29. 10.1177/2047487315582322
36. Foulkes SJ, Howden EJ, Haykowsky MJ, Antill Y, Salim A, Nightingale SS, et al. Exercise for the Prevention of Anthracycline-induced Functional Disability and Cardiac Dysfunction: The BReast Cancer Randomized EXercise InTervention (BREXIT) Study. Circulation. 2023;147(7): 532–45. 10.1161/CIRCULATIONAHA.122.062814
37. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020 Dec;54(24):1451–62. 10.1136/bjsports-2020-102955
38. Medicine ACoS. ACSM’s guidelines for exercise testing and prescription testing and prescription. 6th ed. Baltimore: ACSM2000.
39. Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993 Jan;25(1):71–80. 10.1249/00005768-199301000-00011
Data supplement: Training monitoring
The exercise-based CORE programme is a 12-week ambulatory multidisciplinary programme, including 24 supervised exercise sessions, counselling on physical activity, psychosocial aspects, nutrition, cardiovascular risk factors and pain. The exercise training is offered twice per week to groups of up to 10 cancer patients with exercise sessions lasting 90 minutes and supervised by experienced exercise therapists. Sessions start with approximately 30–40 minutes of cycling on an ergometer at moderate intensity, increased on a weekly basis if possible. After the cycling training, patients continue the exercise session with 45 minutes of strength, coordination and/or balance training. As a consequence of COVID-19, in June 2020, CORE was changed to a hybrid model comprising one supervised individual ET session and one non-supervised ET session at home. During COVID-19, some patients completed a CORE without centre-based sessions. All patients were encouraged to perform at least 150 minutes of moderate physical activity per week, and two weekly strength sessions for major muscle groups [37].
Training workload and duration of endurance training sessions on cycle ergometers were monitored using the Ers2 system, version 1.01 (ergoline GmbH, Bitz, Germany). In addition, heart rate and rhythm were continuously recorded with 3-lead ECG. During every training session, patients were asked about their perceived exertion using the established Borg scale (scale of perceived exertion from 6 to 20). Resistance, coordination or other endurance sessions performed outside the centre (outdoors, at home or at a different training centre) were not recorded.
For the descriptive analysis of training characteristics, we calculated means from all sessions of each patient for the following parameters: training load, duration and heart rate (HR). Training load was expressed in percent of peak power achieved at baseline CPET and also in percent of the mean from peak power at baseline and conclusion CPET. HR was also expressed as HR relative to peak HR achieved at baseline CPET and also relative to the mean from peak HR at baseline and conclusion CPET. The same was done for expressing HR relative to HR reserve (difference between resting HR and peak HR).
For patients completing all exercise training sessions at the centre (i.e. the centre-based cohort), weekly exercise volume and MET-minutes per week including endurance and strength training were calculated. For endurance sessions on stationary bikes, the following ACSM formula was used to estimate oxygen consumption based on the individual training load: 10.8 × average training load/weight + 7 [38]. Subsequently, VO2 values were divided by 3.5 (1 MET = 3.5 ml/kg/min) to obtain MET values for each endurance session and then multiplied by the average duration of the endurance session. Each centre-based training session typically included 40 minutes of strength training, performed either on weight machines or as circuit training in the gym. Average intensity during strength training was estimated based on the Compendium for Physical Activity table [39] with 3.5 MET (low to moderate intensity). Therefore, 140 MET-minutes were added for each strength session to the endurance-based exercise volume to approximate overall MET-minutes per week.
Compliance with exercise training was determined by the number of planned training sessions divided by the number of completed training sessions. In order to achieve 100% compliance, two supervised centre-based training sessions per week had to be completed over the duration of the exercise training programme (12 weeks). For patients enrolled in the hybrid rehabilitation model, full compliance was achieved when patients attended one centre-based exercise session per week, assuming an additional non-monitored training session was completed at home. For patients without centre-based sessions, compliance was not documented, which is why these patients were excluded from the analysis of compliance.
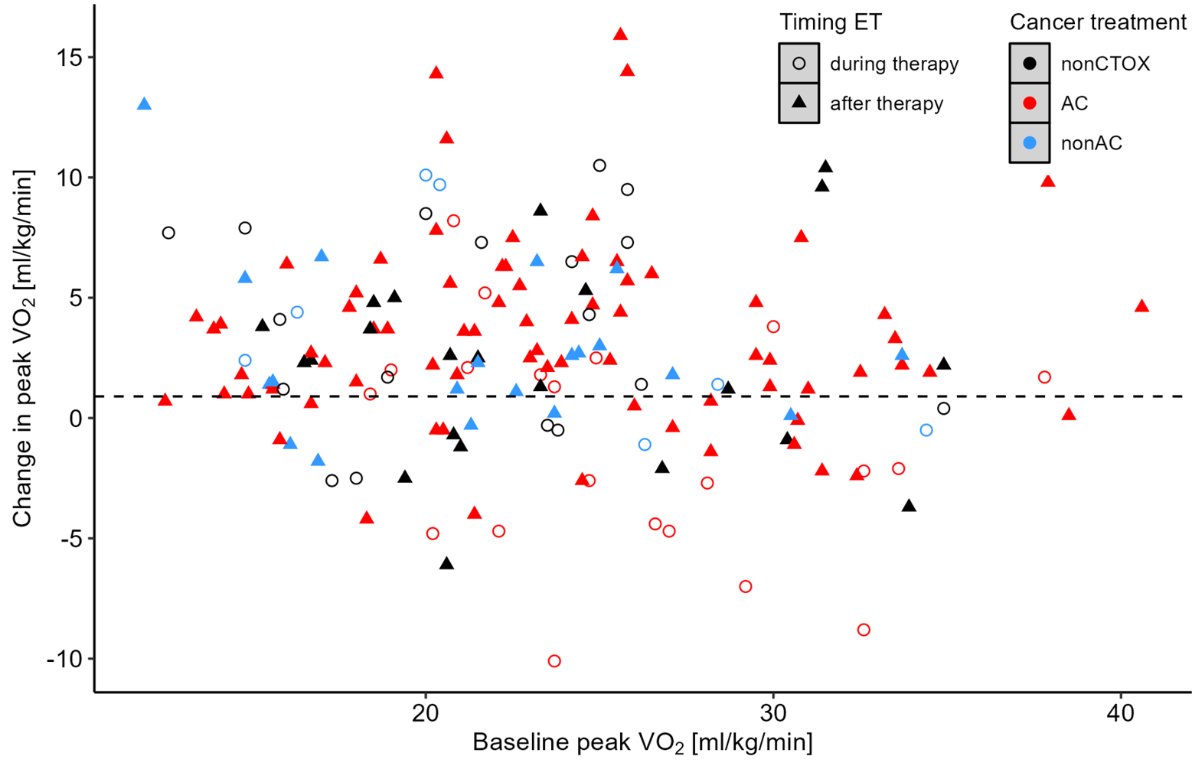
Figure S1Changes in peak VO2 from before to after exercise training (ET) relative to peak oxygen uptake (VO2) at baseline, depending on timing of exercise training and cancer treatment. AC: anthracycline treatment; Non-AC: Cardiotoxic treatment other than AC; Non-CTOX: Non-cardiotoxic cancer treatment.
Table S1Anaemia status at baseline and changes after exercise training programme. Values expressed as median (interquartile range).
| AC group | Without-AC group* | |||||||
| ET during therapy | ET after therapy | ET during therapy | ET after therapy | |||||
| Baseline | Change | Baseline | Change | Baseline | Change | Baseline | Change | |
| n = 22 | n = 19 | n = 75 | n = 51 | n = 28 | n = 12 | n = 40 | n = 23 | |
| Haemoglobin (g/l) | 126 (113–134) | -12 (-26–0.5) | 120 (111–132.5) | 7 (-1–18.5) | 117 (102–129) | 6 (4.3–7.5) | 126 (113–138) | 0 (-6–5) |
ET: exercise training.
* The without-AC group consists of patients who received cardiotoxic therapy other than anthracyclines (non-AC group) and patients who received non-cardiotoxic therapy (non-CTOX group).
Table S2Training data from patients with available baseline and conclusion cardiopulmonary exercise tests performing exercise training during or after cancer therapy. Values expressed as median (interquartile range).
| Training characteristics | AC group (n = 141) | Without-AC group* (n = 121) | ||
| Timing of exercise training | During cancer therapy | After cancer therapy | During cancer therapy | After cancer therapy |
| Exercise training sessions per week | 1.1 (0.6–1.7) | 1.3 (0.9–1.6) | 1.3 (0.8–1.6) | 1.3 (0.8–1.7) |
| Compliance with exercise training (%) | 75% (45.8–95.8%) | 95.8% (70.8–100%) | 83.3% (63.5–100%) | 91.7% (50–100%) |
| Centre-based (n = 19): 70.8% (43.8–87.5%) | Centre-based (n = 85): 87.5% (66.7–100%) | Centre-based (n = 40): 83.3% (65.6–100%) | Centre-based (n = 61): 91.7% (50–100%) | |
| Hybrid (n = 6): 100% (81.3–100%) | Hybrid (n = 28): 100% (100–100%) | Hybrid (n = 6): 100% (62.5–100%) | Hybrid (n = 13): 100% (50–100%) | |
| Time from cancer diagnosis to start of exercise training (weeks) | 8.7 (5.9–12.3) | 40.0 (29.9–66.1) | 41.9 (30.2–119.1) | 41.5 (30.7–89.3) |
| Training intensity relative to mean peak power (%)* | 46.7% (44.4–50.8%) | 46.7% (41.8–50.7%) | 48.8% (41.6–51.7%) | 47.3% (42.0–51.3%) |
| Training intensity relative to peak power at baseline (%) | 46.2% (42.3–49.4%) | 50.5% (45.7–56.7%) | 50.1% (42.3–56.7%) | 50.7% (43.6–56.6%) |
| Training intensity relative to mean power at VT1 (%)* | 114.9% (104.4–128.4%) | 118.5% (93.9–164.5%) | 130.9% (106.2–160.3%) | 115.3% (90.3–140.2%) |
| Training intensity relative to mean HR peak (%)* | 81.5% (78.9–83.6%) | 79.9% (75.4–85.4%) | 77.5% (74.3–84.8%) | 77.0% (74.2–83.9%) |
| Training intensity relative to heart rate reserve (%)** | 65% (60.8–69.7%) | 62.4% (54.6–69.0%) | 59.7% (55.6–66.1%) | 57.2% (47–67.3%) |
| Mean MET | 5.3 (5.1–5.8) | 4.6 (3.9–5.5) | 4.0 (3.5–4.8) | 4.4 (3.8–5.2) |
| Weekly endurance volume (MET-minutes/week) | 228 (149–276) | 192 (121–257) | 160.7 (98.2–219) | 188 (128–245) |
| Weekly exercise volume including strength and endurance training (MET-minutes/week) | 427 (297–519) | 405 (294–487) | 370 (286–433) | 386 (272–475) |
| Training impulse based on peak power at baseline (min × %) | 241 (193–335) | 351 (259–451) | 314 (201–430) | 330 (192–447) |
| Training impulse based on mean peak power (min × %)* | 248 (189–338) | 341(268–407) | 324 (249–394) | 348 (276–404) |
MET: metabolic equivalent of task.
Training impulse: number of training sessions × session duration [min] × load [% of peak power at baseline].
Missing cases in each group were 13 for anthracycline-based chemotherapy (AC) during therapy, 48 for AC after therapy, 20 for non-AC during therapy and 29 for non-AC after therapy.
* The without-AC group consists of patients who received cardiotoxic therapy other than anthracyclines (non-AC group) and patients who received non-cardiotoxic therapy) non-CTOX group).
** calculated as mean from cardiopulmonary exercise tests at exercise trainings baseline and conclusion.
*** two patients were removed for calculation of intensities based on erroneous values.