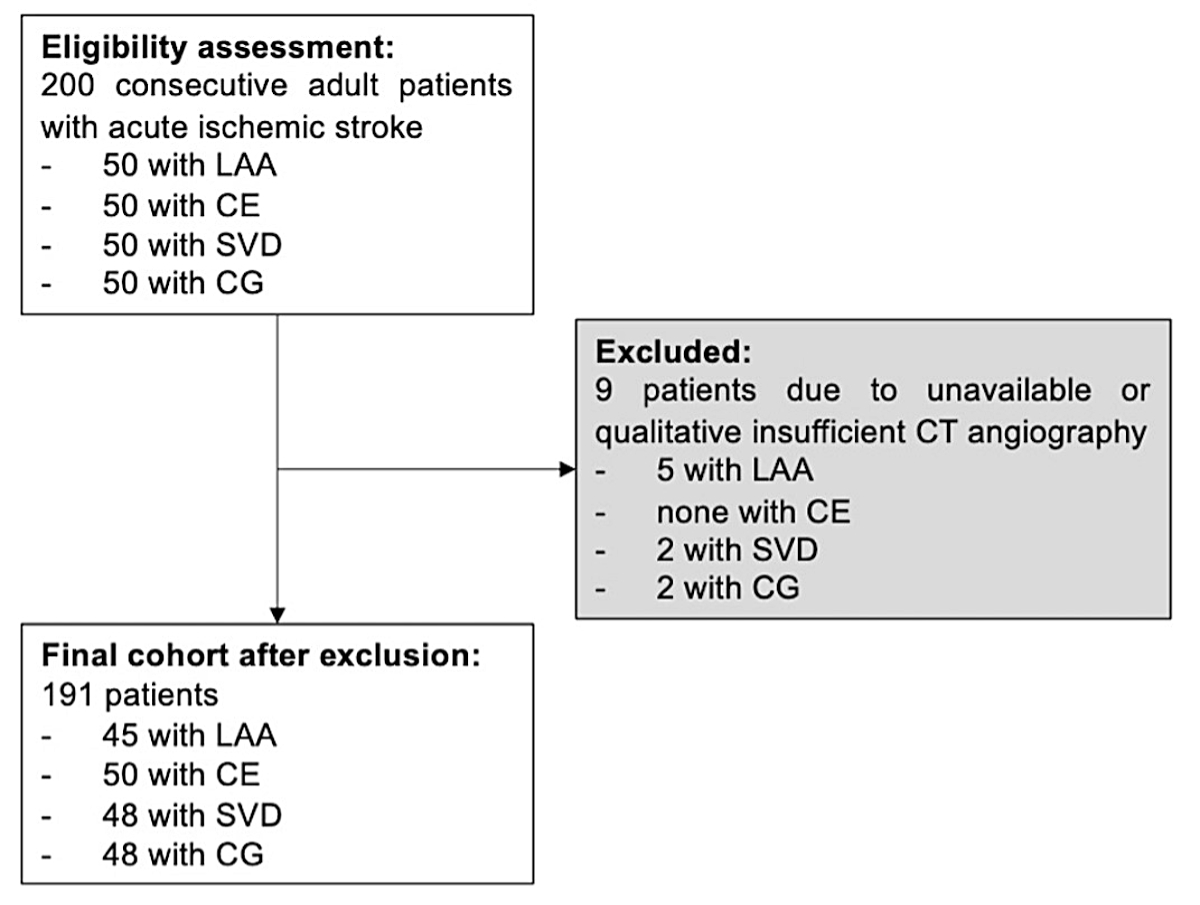
Figure 1Flowchart of the study. LAA: large-artery atherosclerosis; CE: cardioembolic; SVD: small-vessel disease; CG: cryptogenic.
DOI: https://doi.org/https://doi.org/10.57187/s.3584
Leptomeningeal collaterals are important for understanding the temporal evolution of ischaemic injury associated with acute stroke and the efficacy of recanalisation therapies. Leptomeningeal collaterals provide alternative blood flow pathways in the event of disrupted anterograde arterial flow [1].
Good collateralisation is associated with favourable functional outcomes in patients with acute large-vessel occlusions [2–5]. Possible protective mechanisms include a longer tolerance to ischaemia and a better endovascular device efficacy in terms of successful thrombus removal and avoidance of distal embolisation [1–6].
Several studies have investigated factors influencing the leptomeningeal collateral status assessed on admission with CT-angiography, such as age, sex and the presence of cardiovascular risk factors [7–9]. Emerging evidence indicates that collateral status differs based on the ischaemic stroke aetiology [10]. Chronic hypoperfusion was shown to be associated with better collateral recruitment whereas cardioembolic stroke aetiology was associated with worse collateralisation, potentially contributing to poorer functional outcomes in these patients [10–12].
However, these previous studies focused on the comparison between large-artery atherosclerosis and cardioembolic stroke aetiology. To our knowledge, less attention has been paid to leptomeningeal collateral status in relation to a wider set of aetiologies.
The primary aim of this retrospective study was to explore the association between ischaemic stroke aetiology, categorised according to the “Trial of Org 10172 in Acute Stroke Treatment” (TOAST) classification [13], and leptomeningeal collateral status, assessed by single-phase CT-angiography, in a Swiss cohort.
For this single-centre cohort study, we screened 200 consecutive adult patients with acute ischaemic stroke included in the PLEURA study [14]. PLEURA included all consecutive patients aged at least 18 years who were treated for an ischaemic or haemorrhagic cerebrovascular event at the University Hospital Basel between January 2014 and May 2021 and underwent CT-angiography during the index hospitalisation. We excluded patients if CT-angiography was not performed or unavailable. For this study, stratification by stroke aetiology was based on the TOAST classification with the aim of including 50 consecutive patients for every TOAST subgroup. Patients with cryptogenic aetiology were used as a proof-of-concept category due to the exclusion of tangible stroke aetiologies inherent to this category. In this stratum, we did not expect any influence on the degree of collateralisation given the lack of a pathophysiological stroke correlate. Individuals with a stroke of “Other determined aetiology” were excluded from our analyses due to the inhomogeneous pathomechanistic composition of this group. We assessed demographics (age, sex), medical history (premorbid modified Rankin Scale [mRS] score), known or newly diagnosed vascular risk factors (arterial hypertension [>140/90 mm Hg], diabetes [without glucose intolerance], dyslipidaemia [lipid-lowering treatment or LDL cholesterol >2.6 mmol/l], smoking [actively smoking or stopped <2 years ago]), coronary artery disease, myocardial infarction, peripheral artery disease) and prior medication (antiplatelets, anticoagulants, antihypertensives, lipid-lowering drugs), admission characteristics (body mass index, systolic blood pressure, National Institutes of Health Stroke Scale [NIHSS] score, total cholesterol, LDL cholesterol, blood glucose) and acute treatment with recanalisation therapies (intravenous thrombolysis and endovascular treatment).
The study was reviewed and approved by the Ethics Committee Northwest and Central Switzerland (EKNZ; 2021-01185 and 2023-00268). The study protocol, anonymised data and statistical code can be provided upon reasonable request.
The need for informed consent was waived by the EKNZ (article 34 of the Human Research Act).
We used the established TOAST criteria to classify the index stroke aetiology [13]. The TOAST classification distinguishes five aetiological subtypes: (1) large-artery atherosclerosis (LAA; ≥50% stenosis), (2) cardioembolic (CE; excluding patent foramen ovale and other rare cardiac causes), (3) small-vessel disease (SVD), (4) stroke of other determined aetiology, and (5) cryptogenic (CG; defined as unknown aetiology despite complete evaluation) [13]. Patients with more than one possible aetiology were excluded.
Collateral status was assessed visually using acquired baseline single-phase CT-angiography data with a previously validated 4-point scale [15] (0 points: no collaterals; 1 point: collaterals filling ≤50% of the occluded territory; 2 points: collaterals filling >50% but <100% of the occluded territory; 3 points: collaterals filling 100% of the occluded territory). For this study, each CT-angiography set was consistently evaluated by two independent reviewers (LS and either AMT or CG) who were blinded to the clinical characteristics and functional outcomes. In cases without consensus between the first two reviewers, an additional rating was performed by a third assessor (TDD), with the majority consensus determining the collateral status rating.
Before assessing leptomeningeal collateral status for this study, all reviewers were required to score a standardised training dataset consisting of 30 CT-angiography images with axial maximum intensity projection reconstructions. To qualify as an assessor, a minimum agreement of 80% with the previously defined collateral status consensus had to be achieved, without any time limit for the scoring.
Dichotomous variables are expressed as counts (with percentages), continuous variables as medians (with interquartile ranges [IQR]). For descriptive analyses, we considered all included individuals. We used Pearson’s chi-square test, the Wilcoxon rank-sum test and two-tailed t-tests, as appropriate. Bonferroni correction was performed to account for multiple comparisons.
We performed univariate and multivariate binary (poor [collaterals filling ≤50% of the occluded territory] vs good [collaterals filling >50% of the occluded territory] collateralisation; primary analysis) and ordinal (entire spectrum of collateral scores; secondary analysis) logistic regression to examine the effect of ischaemic stroke aetiology on collateral status. Univariate ordinal regression was used to identify covariables that were statistically significantly associated with the leptomeningeal collateral status. Subsequently, we fitted four separate multivariate ordinal regression models (one for each TOAST aetiology: LAA vs non-LAA, CE vs non-CE, SVD vs non-SVD, CG vs non-CG), adjusting for all covariables identified in univariate analyses and those previously described in the literature [7–9] as influencing leptomeningeal collateral recruitment. The included covariables were age, sex, arterial hypertension, dyslipidaemia and diabetes. We calculated adjusted predictions that reflect the probability of good collateralisation for each TOAST aetiology subgroup by holding the above-mentioned covariables at their average (for continuous variables) or reference category (for categorical variables). Only individuals with complete covariable sets were considered.
Weights (proportion of each TOAST aetiology of interest in the ischaemic stroke population divided by the respective proportions in the sample) were used for all analyses to address potential over- or under-representation due to the study’s sampling strategy. The proportion of each TOAST aetiology in the ischaemic stroke population was derived from data of the Swiss stroke registry (LAA 15%, CE 27%, SVD 11%, CG 31%) [16].
All analyses were performed with STATA version 17.0 (StataCorp LLC, College Station, Texas, USA). P values <0.05 were considered significant and 95% confidence intervals (CI) are reported.
We included 191 patients with acute ischaemic stroke (figure 1).

Figure 1Flowchart of the study. LAA: large-artery atherosclerosis; CE: cardioembolic; SVD: small-vessel disease; CG: cryptogenic.
There were several observed differences between TOAST aetiology subgroups. The detailed comparisons are presented in table 1.
Table 1Baseline characteristics of acute ischaemic stroke patients according to TOAST aetiology.
| Total (n = 191) | Stroke aetiology according to TOAST | ||||||||
| LAA (n = 45) | CE (n = 50) | SVD (n = 48) | CG (n = 48) | p value | Missing, n (%) | ||||
| Demographics | Age in years, median (IQR) | 78 (68–85) | 76 (68–82) | 82 (77–86) | 73 (64–82) | 77 (68–83) | 0.002a | 0 (0%) | |
| Female, n (%) | 72 (38%) | 12 (27%) | 21 (42%) | 17 (35%) | 22 (46%) | 0.24 | 0 (0%) | ||
| Patient characteristics, n (%) | Premorbid modified Rankin Scale, median (IQR) | 0 (0–1) | 0 (0–0) | 1 (0–2) | 0 (0–1) | 0 (0–1) | <0.001b | 21 (11%) | |
| Arterial hypertension, n (%) | 154 (81%) | 36 (80%) | 44 (88%) | 31 (65%) | 43 (90%) | 0.01c | 0 (0%) | ||
| Diabetes, n (%) | 41 (22%) | 9 (20%) | 10 (20%) | 13 (27%) | 9 (19%) | 0.75 | 0 (0%) | ||
| Dyslipidaemia, n (%) | 112 (59%) | 28 (62%) | 27 (54%) | 24 (50%) | 33 (69%) | 0.24 | 0 (0%) | ||
| Smoking, n (%) | 56 (29%) | 28 (62%) | 7 (14%) | 11 (23%) | 10 (21%) | <0.001d | 0 (0%) | ||
| Coronary artery disease, n (%) | 43 (23%) | 12 (27%) | 11 (22%) | 8 (17%) | 12 (28%) | 0.57 | 5 (3%) | ||
| Myocardial infarction, n (%) | 20 (11%) | 9 (20%) | 8 (16%) | 1 (2%) | 2 (4%) | 0.01e | 0 (0%) | ||
| Peripheral artery disease, n (%) | 7 (4%) | 3 (7%) | 2 (4%) | 1 (2%) | 1 (3%) | 0.66 | 8 (4%) | ||
| Prior medication, n (%) | Antiplatelets | 78 (41%) | 22 (49%) | 14 (28%) | 19 (40%) | 23 (48%) | 0.13 | 0 (0%) | |
| Anticoagulants | 23 (12%) | 6 (13%) | 14 (28%) | 0 (0%) | 3 (6%) | <0.001f | 1 (1%) | ||
| Antihypertensives | 126 (66%) | 32 (71%) | 38 (78%) | 21 (44%) | 35 (73%) | 0.002g | 1 (1%) | ||
| Lipid-lowering drugs | 57 (30%) | 18 (40%) | 9 (18%) | 13 (27%) | 17 (35%) | 0.10 | 1 (1%) | ||
| Admission characteristics | BMI in kg/m2, median (IQR) | 25 (23–28) | 25 (23–27) | 25 (23–28) | 26 (23–28) | 27 (24–29) | 0.69 | 11 (6%) | |
| Systolic blood pressure in mm Hg, median (IQR) | 153 (139–172) | 141 (125–150) | 155 (135–170) | 163 (150–184) | 153 (138–178) | <0.001h | 0 (0%) | ||
| NIHSS on admission, median (IQR) | 5 (2–9) | 1 (0–4) | 10 (6–16) | 5 (3–8) | 3 (0–7) | <0.001i | 2 (1%) | ||
| Collateral score, median (IQR) | 2 (2–3) | 2 (2–3) | 2 (1–2) | 2 (2–3) | 2 (2–3) | <0.001j | 0 (0%) | ||
| Collateral score, mean (standard deviation) | 2.1 (0.9) | 2.4 (0.7) | 1.6 (1.0) | 2.4 (0.7) | 2.2 (0.8) | ||||
| Laboratory parameters on admission, median (IQR) | Total cholesterol in mmol/l | 4.7 (4.0–5.4) | 4.4 (3.7–5.4) | 4.5 (3.7–5.1) | 4.8 (4.2–5.6) | 4.9 (4.2–5.6) | 0.09 | 5 (3%) | |
| LDL cholesterol in mmol/l | 2.5 (2.0–3.2) | 2.4 (1.8–3.4) | 2.4 (1.9–2.9) | 2.7 (2.1–3.2) | 2.8 (2.2–3.3) | 0.10 | 7 (4%) | ||
| Glucose in mmol/l | 6.4 (5.5–7.7) | 5.6 (5.0–6.6) | 7.1 (6.1–8.4) | 6.2 (5.4–8.5) | 6.5 (5.9–7.8) | <0.001k | 4 (2%) | ||
| Treatment characteristics, n (%) | Intravenous thrombolysis | 101 (53%) | 7 (16%) | 35 (70%) | 47 (98%) | 12 (25%) | <0.001l | 0 (0%) | |
| Endovascular treatment | 39 (20%) | 6 (13%) | 32 (64%) | 1 (2%) | 0 (0%) | <0.001m | 0 (0%) | ||
CE: cardioembolic; CG: cryptogenic; eGFR: estimated glomerular filtration rate; INR: International Normalised Ratio; LAA: large-artery atherosclerosis; LDL: low-density lipoprotein; NIHSS: National Institutes of Health Stroke Scale; SVD: small-vessel disease; TOAST: Trial of Org 10 172 in Acute Stroke Treatment
To identify which specific TOAST categories differ from each other, Bonferroni-corrected post-hoc tests were used (for continuous variables: ANOVA and Kruskal-Wallis test [for continuous variables with non-normal distribution]; for categorical variables: logistic regression with pairwise comparisons and Bonferroni adjustment). Statistically significant differences were found for:
a: CE vs SVD
b: LAA vs CE, CE vs CG
c: SVD vs CG
d: LAA vs CE, LAA vs SVD, LAA vs CG
e: Trend for differences for LAA vs SVD (p = 0.02), LAA vs CG (p = 0.03) and CE vs SVD (p = 0.04) which, however, did exceed the Bonferroni-corrected alpha level of ≈0.0083.
f: No statistically significant results after application of Bonferroni correction; probably model-related estimation problem due to zero cell count in the SVD group (no patients with previous anticoagulants).
g: CE vs SVD, SVD vs CG
h: LAA vs CE, LAA vs SVD, LAA vs CG
i: LAA vs CE, LAA vs SVD, CE vs SVD, CE vs CG
j: LAA vs CE, CE vs SVD, CE vs CG
k: LAA vs CE, LAA vs SVD, LAA vs CG
l: LAA vs CE, LAA vs SVD, CE vs SVD, CE vs CG, SVD vs CG
m: LAA vs CE, CE vs SVD, CE vs CG
In summary, CE patients were the oldest (on average 9 years older than SVD patients; poverall = 0.002), were more severely pre-morbidly functionally impaired (poverall <0.001), were most often pretreated with anticoagulants (poverall <0.001) and most affected by the index stroke (highest median NIHSS on admission, poverall <0.001). Regarding LAA aetiology, these patients had the highest proportion of smokers (poverall <0.001) and prior myocardial infarction (poverall = 0.01). Hypertension was generally very prevalent in the cohort with an overall proportion of 81%, but was highest in the SVD group at 65%, where it was also the least pretreated with antihypertensive medication. In line with these findings, SVD patients had the highest systolic blood pressure on admission (poverall <0.001) compared to the other TOAST subgroups.
Concerning collateral scores, CE patients had the lowest collateral scores, whereas LAA and SVD patients had the highest (poverall <0.001) (table 1). Almost all SVD patients received intravenous thrombolysis (98%), followed by the clinically most severely affected CE group with 70% (poverall <0.001). The latter group was also the most likely to receive endovascular treatment (64%; poverall <0.001).
In the unadjusted analyses, LAA and SVD had the strongest association with good collateralisation (LAA: odds ratio [OR] 3.73, 95% confidence interval [CI] 1.24–11.23; SVD: OR 3.84, 95% CI 1.28–11.50) (table 2), CE with poor collateralisation (OR 0.15, 95% CI 0.07–0.33). After adjustment for significant covariables from the univariate analyses (table 3) as well as prespecified predictors of collateralisation, the associations were shown to be consistent in the binary and ordinal logistic models (table 2). For CG aetiology, there was no significant effect on the collateral status in either the unadjusted or adjusted models. Figure 2 illustrates the predicted probabilities of good collateralisation for different TOAST aetiologies.
Table 2Impact of stroke aetiology on collateralisation status.
| OR | 95% CI | p value | |||
| Univariate logistic regression* | Binary (dichotomised collateral score) – good (vs bad) collateralisation** | LAA (vs non-LAA) | 3.73 | 1.24–11.23 | 0.02 |
| CE (vs non-CE) | 0.15 | 0.07–0.33 | <0.001 | ||
| SVD (vs non-SVD) | 3.84 | 1.28–11.50 | 0.02 | ||
| CG (vs non-CG) | 2.37 | 0.96–5.85 | 0.06 | ||
| Ordinal (collateral score) – shift towards higher collateral score category | LAA (vs non-LAA) | 2.15 | 1.19–3.91 | 0.01 | |
| CE (vs non-CE) | 0.22 | 0.11–0.45 | <0.001 | ||
| SVD (vs non-SVD) | 2.02 | 1.14–3.57 | 0.02 | ||
| CG (vs non-CG) | 1.57 | 0.88–2.78 | 0.13 | ||
| Multivariate logistic regression*** | Binary (dichotomised collateral score) – good (vs bad) collateralisation** | LAA (vs non-LAA) | 3.72 | 1.21–11.44 | 0.02 |
| CE (vs non-CE) | 0.17 | 0.07–0.41 | <0.001 | ||
| SVD (vs non-SVD) | 4.19 | 1.21–14.52 | 0.02 | ||
| CG (vs non-CG) | 1.99 | 0.76–5.20 | 0.16 | ||
| Ordinal (collateral score) – shift towards higher collateral score category | LAA (vs non-LAA) | 2.26 | 1.23–4.15 | 0.01 | |
| CE (vs non-CE) | 0.24 | 0.11–0.51 | <0.001 | ||
| SVD (vs non-SVD) | 1.94 | 1.03–3.66 | 0.04 | ||
| CG (vs non-CG) | 1.41 | 0.76–2.63 | 0.27 | ||
According to TOAST (Trial of Org 10 172 in Acute Stroke Treatment) classification: LAA: large-artery atherosclerosis; CE: cardioembolic; SVD: small-vessel disease; CG: cryptogenic. Collateral score: 0 = absent collaterals, 1 = collaterals filling ≤50% of the occluded territory, 2 = collaterals filling >50% but <100% of the occluded territory, 3 = collaterals filling 100% of the occluded territory.
* Unadjusted odds ratios (OR) (binary regression) and common odds ratios (cOR) (ordinal regression) are reported. We state 95% confidence intervals (CI).
** Good collateralisation: defined as collateral filling of >50% of the occluded territory.
*** Adjusted odds ratios (aOR) (binary regression) and adjusted common odds ratios (acOR) (ordinal regression) are reported. We state 95% confidence intervals (CI).
Table 3Univariate analysis of the association between baseline patient characteristics and shift towards higher collateral score.
| cOR | 95% CI | p value | |
| Age* | 0.97 | 0.95–1.00 | 0.04 |
| Female sex** | 1.44 | 0.79–2.63 | 0.23 |
| Premorbid mRS* | 0.75 | 0.52–1.06 | 0.10 |
| Arterial hypertension*** | 0.88 | 0.42–1.83 | 0.73 |
| Diabetes*** | 1.02 | 0.52–1.97 | 0.96 |
| Dyslipidaemia*** | 1.08 | 0.58–1.99 | 0.82 |
| Smoking*** | 1.30 | 0.74–2.32 | 0.36 |
| Coronary artery disease*** | 0.64 | 0.37–1.13 | 0.13 |
| Myocardial infarction*** | 0.42 | 0.17–1.05 | 0.06 |
| Peripheral artery disease*** | 0.58 | 0.26–1.30 | 0.19 |
| Prior antiplatelets*** | 0.81 | 0.46–1.42 | 0.46 |
| Prior anticoagulants*** | 0.93 | 0.31–2.81 | 0.89 |
| Prior antihypertensives*** | 0.76 | 0.41–1.41 | 0.39 |
| Prior lipid-lowering drugs*** | 0.97 | 0.52–1.78 | 0.91 |
cOR: common odds ratio; mRS: modified Rankin Scale.
* Interpretation of the coefficients: change in the log odds of a shift towards higher collateral score category for one unit increase in the predictor variable (e.g. age: per 1 year increase)
** Female vs male
*** Present vs not present
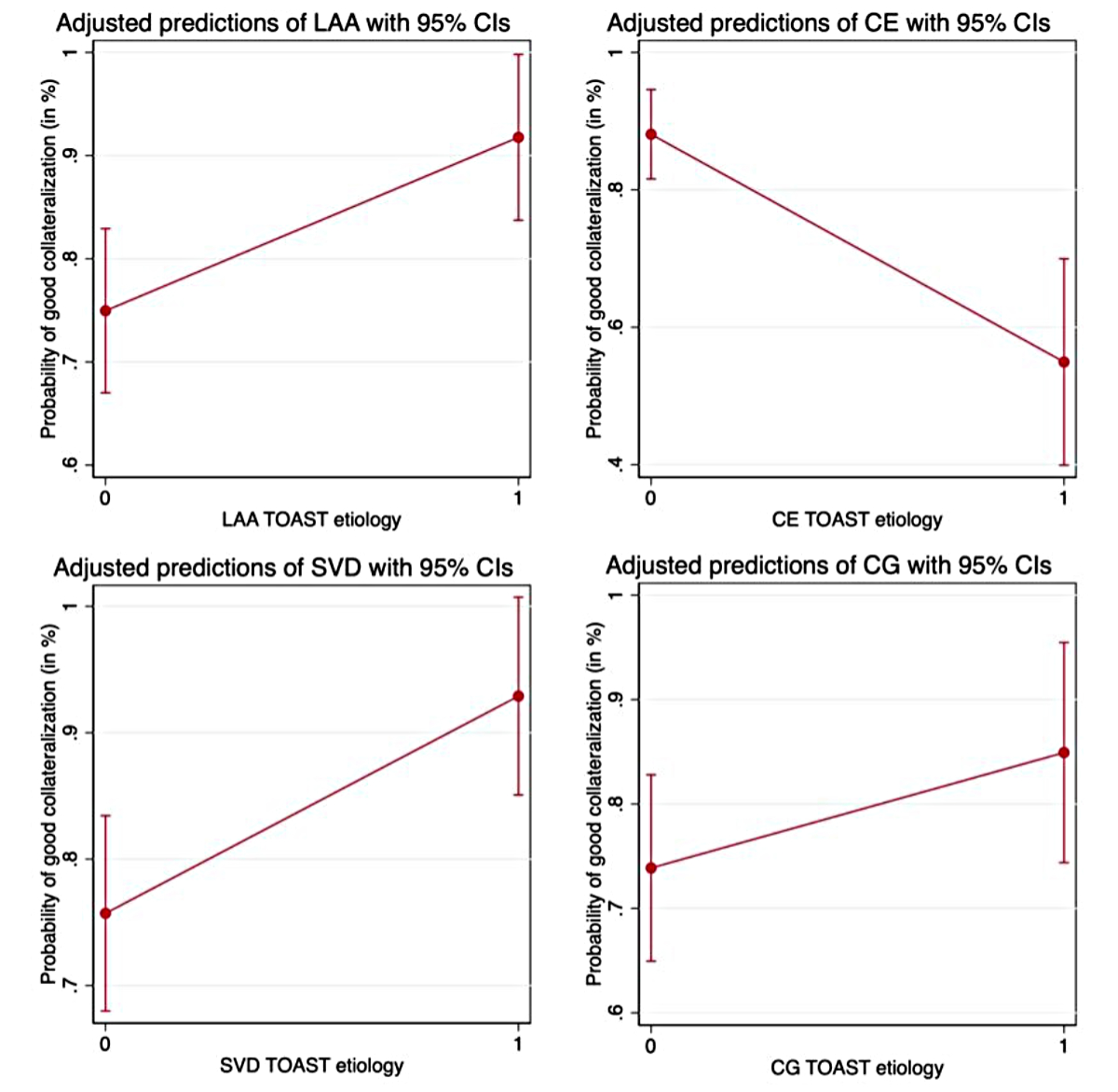
Figure 2Illustration of the predicted probabilities of good collateralisation (collaterals filling >50% of the occluded territory) for different TOAST aetiologies adjusted for covariables. Adjustment was performed for the following covariables: age, sex, arterial hypertension, dyslipidaemia and diabetes. LAA: large-artery atherosclerosis; CE: cardioembolic; SVD: small-vessel disease; CG: cryptogenic.
In a Swiss cohort of consecutive patients admitted for acute ischaemic stroke, we demonstrated that: (1) the TOAST subgroups differed considerably from one another with regard to several baseline characteristics, in particular traditional vascular risk factors; (2) collateral status varied across a wide range of TOAST aetiologies; and (3) LAA and SVD independently predicted favourable and CE unfavourable collateral status.
Our results are in line with those of a recent meta-analysis of seven studies that investigated the association of leptomeningeal collateral status with LAA and CE aetiology and found that LAA aetiology was associated with better collateralisation, and CE aetiology with worse collateralisation [17]. In contrast to the meta-analysis, we did not restrict to patients who received reperfusion therapies. It should also be noted that the type of collateral assessment varied among the studies included in the meta-analysis (i.e. multi-phase CT-angiography, cerebral angiography), which may have led to different assessments of the degree of collateralisation.
Leptomeningeal collateralisation has been intensively studied in its practical application for radiological patient selection for endovascular therapies as well as prognostication of reperfusion injury thereafter. Currently, there are no established pharmacological or interventional approaches to modulate leptomeningeal collateral flow in acute ischaemic stroke. This may partly be due to limited knowledge of the underlying mechanisms of collateral formation.
The relationship between the degree of leptomeningeal collateralisation and functional outcome in patients with ischaemic stroke due to large-vessel occlusions has been described in detail previously [2, 18, 3, 19, 20]. In these studies, better collateral status was shown to be an independent predictor of a favourable long-term functional outcome. In the ESCAPE trial, which compared endovascular therapy within 12 hours after symptom onset with standard medical therapy, patients with poor collateralisation – used as a surrogate of a large ischaemic core – were excluded a priori [18]. In the time window up to 24 hours after stroke onset, the recent MR CLEAN-LATE trial demonstrated that patient selection based on collateral status for endovascular treatment is feasible, including patients with poor collateralisation (grades 0 and 1) [21].
However, a substantial proportion of patients with successful mechanical recanalisation do not show significant clinical improvement [22, 23]. To date, little is known about the underlying pathophysiological mechanisms. Recent translational work involving 33 stroke patients with M1 or M2 segment occlusion of the middle cerebral artery suggests a pivotal role of the leptomeningeal collateral status [24]. Poor collateralisation in patients who received rapid successful mechanical reperfusion was associated with haemorrhagic transformation and higher disability [24].
Clinical determinants of collateral formation may provide indirect pathophysiological insights regarding collateral formation. Since ischaemic stroke represents a heterogeneous pathophysiological group, it is reasonable to investigate to what extent different underlying aetiologies influence collateral formation. We are not yet aware of any investigation focusing on the association of leptomeningeal collateralisation with different stroke aetiologies across a broad TOAST range. We can only speculate about the reasons. Probably the most relevant is that the single-phase CT-angiography collateral scores (such as the one proposed by Tan et al. that we used) [15] have only been validated for middle cerebral artery occlusions with or without concomitant internal carotid artery occlusion. Thus, the consideration of SVD and cryptogenic embolic strokes without the above-mentioned proximal vessel occlusion does not seem obvious. However, we believe that an exploratory study such as the one we conducted is justified. This is particularly due to its potential to generate pathophysiological hypotheses that need to be verified in future studies with larger case numbers. Chronic progressive hypoperfusion has been suggested as a possible driver of collateral formation, which could contribute to leptomeningeal collateralisation through activation of endothelial cells with consecutive activation of further downstream cascades [17]. This hypothesis would explain an association between the chronic impact of atherosclerotic plaques on haemodynamics in the context of LAA with better leptomeningeal collateral status via chronic stimulation of endothelial cells by shear forces [17]. In contrast, CE-induced ischaemia is not linked to chronic cerebral hypoperfusion that could promote collateral formation following this logic [17]. Data on leptomeningeal collateral status related to other TOAST subcategories are sparse. In our study, we saw that SVD aetiology independently predicted better leptomeningeal collateral status. It seems possible, considering the endothelial activation hypothesis, that progressive injury to the cerebral microperfusion with consecutive endothelial upregulation of several transcription factors and adhesion molecules might contribute to better collateral formation [25]. However, these results must be interpreted with caution given the limited number of cases and require further investigation in larger cohorts. In our study, we included patients without any detectable cardiac embolic source, relevant microangiopathy or macroangiopathy after aetiologic workup as a proof-of-concept subgroup. The fact that we did not see an association with leptomeningeal collateral status in this group reinforces a pathomechanistic link between stroke aetiology and collateral formation.
Our study has several strengths and limitations.
Strengths are that: (1) we included consecutive patients from clinical practice; (2) we studied the full spectrum of stroke aetiologies also including patients with cryptogenic aetiology; (3) leptomeningeal collateral status was assessed by at least two independent assessors; (4) collateral assessment was consistently performed using single-phase CT-angiography.
Limitations include: (1) the small sample size per aetiologic subgroup and the potential for TOAST misclassification; (2) missing information on occlusion localisation (which could have influenced the degree of collateralisation) for non-lacunar strokes. However, we did assess the NIHSS score, which can serve as a clinical surrogate marker for occlusion site; (3) well-known limitations of single-phase CT-angiography, whose correct interpretability depends on image acquisition during the arterial phase; (4) the limited generalisability of our findings due to the single-centre retrospective design. The applicability of our results to broader populations may therefore be limited given potential differences in, for example, patient demographics, healthcare systems and stroke management protocols.
The aetiology of ischaemic stroke is associated with leptomeningeal collateral status on baseline single-phase CT-angiography. Large-artery atherosclerosis and small-vessel disease are associated with better collateral status, while cardioembolic aetiology is linked to worse collateral status.
Data may be provided upon reasonable request to the corresponding authors.
Author contributions: LS, AMT, TDD and GMDM planned the work. LS, AMT and CG collected the data. LS, AMT, TDD and GMDM drafted the manuscript. TDD performed the statistical analyses. All authors interpreted the results and contributed significantly to the final manuscript.
This research received no funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012 Mar;33(3):576–82. 10.3174/ajnr.A2794
2. Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010 Oct;41(10):2316–22. 10.1161/STROKEAHA.110.592303
3. Berkhemer OA, Jansen IG, Beumer D, Fransen PS, van den Berg LA, Yoo AJ, et al.; MR CLEAN Investigators. Collateral Status on Baseline Computed Tomographic Angiography and Intra-Arterial Treatment Effect in Patients With Proximal Anterior Circulation Stroke. Stroke. 2016 Mar;47(3):768–76. 10.1161/STROKEAHA.115.011788
4. Park JS, Kwak HS, Chung GH, Hwang S. The Prognostic Value of CT-Angiographic Parameters After Reperfusion Therapy in Acute Ischemic Stroke Patients With Internal Carotid Artery Terminus Occlusion: Leptomeningeal Collateral Status and Clot Burden Score. J Stroke Cerebrovasc Dis. 2018 Oct;27(10):2797–803. 10.1016/j.jstrokecerebrovasdis.2018.06.010
5. Jansen IG, Mulder MJ, Goldhoorn RB, Boers AM, van Es AC, Yo LS, et al.; MR CLEAN Registry investigators. Impact of single phase CT angiography collateral status on functional outcome over time: results from the MR CLEAN Registry. J Neurointerv Surg. 2019 Sep;11(9):866–73. 10.1136/neurintsurg-2018-014619
6. García-Tornel Á, Ciolli L, Rubiera M, Requena M, Muchada M, Pagola J, et al. Leptomeningeal Collateral Flow Modifies Endovascular Treatment Efficacy on Large-Vessel Occlusion Strokes. Stroke. 2021 Jan;52(1):299–303. 10.1161/STROKEAHA.120.031338
7. Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Annals of Neurology. 2013 2013/08/01;74(2):241-48. 10.1002/ana.23906
8. Fujita K, Tanaka K, Yamagami H, Ide T, Ishiyama H, Sonoda K, et al. Detrimental Effect of Chronic Hypertension on Leptomeningeal Collateral Flow in Acute Ischemic Stroke. Stroke. 2019 2019/07/01;50(7):1751-57. 10.1161/STROKEAHA.119.025142
9. Hong Y, Fang J, Ma M, Su W, Zhou M, Tang L, et al. The Hyperdense middle cerebral artery sign is associated with poor leptomeningeal collaterals in acute ischemic stroke: a retrospective study. BMC Neurol. 2022 Feb;22(1):51. 10.1186/s12883-022-02566-9
10. Guglielmi V, LeCouffe NE, Zinkstok SM, Compagne KC, Eker R, Treurniet KM, et al.; MR-CLEAN Registry Investigators. Collateral Circulation and Outcome in Atherosclerotic Versus Cardioembolic Cerebral Large Vessel Occlusion. Stroke. 2019 Dec;50(12):3360–8. 10.1161/STROKEAHA.119.026299
11. Rebello LC, Bouslama M, Haussen DC, Grossberg JA, Dehkharghani S, Anderson A, et al. Stroke etiology and collaterals: atheroembolic strokes have greater collateral recruitment than cardioembolic strokes. Eur J Neurol. 2017 Jun;24(6):762–7. 10.1111/ene.13287
12. Hassler E, Kneihsl M, Deutschmann H, Hinteregger N, Magyar M, Wießpeiner U, et al. Relationship between stroke etiology and collateral status in anterior circulation large vessel occlusion. J Neurol. 2020 Nov;267(11):3362–70. 10.1007/s00415-020-10009-z
13. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan;24(1):35–41. 10.1161/01.STR.24.1.35
14. Dittrich TD, Aujesky M, Rudin S, Zietz A, Wagner B, Polymeris A, et al. Apical pulmonary lesions suspected of malignancy visible on neck CT angiography performed for acute stroke: Prevalence, treatment, and clinical implications – the PLEURA study. Eur Stroke J. 2023 Jun;8(2):549-56. 23969873231151488. 10.1177/23969873231151488
15. Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007 Jun;61(6):533–43. 10.1002/ana.21130
16. Silimon N, Drop B, Clénin L, Nedeltchev K, Kahles T, Tarnutzer AA, et al. Ischaemic stroke despite antiplatelet therapy: causes and outcomes. Eur Stroke J. 2023 Sep;8(3):692–702. 10.1177/23969873231174942
17. Sinha A, Stanwell P, Beran RG, Calic Z, Killingsworth MC, Bhaskar SM. Stroke Aetiology and Collateral Status in Acute Ischemic Stroke Patients Receiving Reperfusion Therapy-A Meta-Analysis. Neurol Int. 2021 Nov;13(4):608–21. 10.3390/neurolint13040060
18. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al.; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015 Mar;372(11):1019–30. 10.1056/NEJMoa1414905
19. Seyman E, Shaim H, Shenhar-Tsarfaty S, Jonash-Kimchi T, Bornstein NM, Hallevi H. The collateral circulation determines cortical infarct volume in anterior circulation ischemic stroke. BMC Neurol. 2016 Oct;16(1):206. 10.1186/s12883-016-0722-0
20. Al-Dasuqi K, Payabvash S, Torres-Flores GA, Strander SM, Nguyen CK, Peshwe KU, et al. Effects of Collateral Status on Infarct Distribution Following Endovascular Therapy in Large Vessel Occlusion Stroke. Stroke. 2020 Sep;51(9):e193–202. 10.1161/STROKEAHA.120.029892
21. Olthuis SG, Pirson FA, Pinckaers FM, Hinsenveld WH, Nieboer D, Ceulemans A, et al.; MR CLEAN-LATE investigators. Endovascular treatment versus no endovascular treatment after 6-24 h in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: a multicentre, open-label, blinded-endpoint, randomised, controlled, phase 3 trial. Lancet. 2023 Apr;401(10385):1371–80. 10.1016/S0140-6736(23)00575-5
22. Seker F, Qureshi MM, Möhlenbruch MA, Nogueira RG, Abdalkader M, Ribo M, et al. Reperfusion Without Functional Independence in Late Presentation of Stroke With Large Vessel Occlusion. Stroke. 2022 2022/12/01;53(12):3594-604. 10.1161/STROKEAHA.122.039476
23. Weyland CS, Vey JA, Mokli Y, Feisst M, Kieser M, Herweh C, et al. Full Reperfusion Without Functional Independence After Mechanical Thrombectomy in the Anterior Circulation. Clinical Neuroradiology. 2022 2022/12/01;32(4):987-95. 10.1007/s00062-022-01166-x
24. Nadine Felizitas B, Mohamad El A, Chaim G, William M, Anna Maria R, Adrien B, et al. Leptomeningeal collaterals regulate reperfusion in ischemic stroke. bioRxiv. 2023:2023.02.25.529915.
25. Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003 Jan;10(1):83–97. 10.1080/mic.10.1.83.97