Impact of respiratory tract infections on spinal muscular atrophy with focus on respiratory
syncytial virus infections: a single-centre cohort study
DOI: https://doi.org/https://doi.org/10.57187/s.3573
Christina
T. Rüschabc,
Miriam
Sturza,
Elea
Galiarta,
Patrick M. Meyer Sauteurd,
Maarja Soomanne,
Johannes Trücke,
Georg M. Stettnera
a Neuromuscular Centre Zurich and
Department of Pediatric Neurology, University Children’s Hospital Zurich,
University of Zurich, Zurich, Switzerland
b Swiss Epilepsy Centre, Klinik Lengg, Zurich,
Switzerland
c Department of Pediatrics, Stadtspital Triemli, Zurich, Switzerland
d Division of Infectious Diseases and
Hospital Epidemiology, University Children’s Hospital Zurich, University of
Zurich, Zurich, Switzerland
e Division of Immunology, University
Children’s Hospital Zurich, University of Zurich, Zurich, Switzerland
Summary
AIMS OF THE STUDY: Spinal muscular atrophy (SMA) is a degenerative neuromuscular disorder
leading to muscle hypotonia,
weakness, and respiratory and bulbar impairment. Infants with SMA have an increased
risk of respiratory tract infections (RTI) including
severe respiratory syncytial virus (RSV) infections. Therefore, guidelines for
the treatment of SMA recommend RSV prophylaxis with
palivizumab for patients aged below two years who have compromised motor
functions (“non-sitters”). Since palivizumab is not approved for RSV
prophylaxis in SMA patients in Switzerland, payers usually
do not grant cost approvals for this indication. Therefore, this study aimed to
investigate the frequency of severe RTI among SMA patients focusing on RSV infections
requiring hospital
treatment and to determine the long-term impact of RSV infections on the natural
history of SMA.
METHODS: A single-centre cohort study at the
tertiary paediatric Neuromuscular Centre Zurich, Switzerland, including data of
SMA patients with a genetic-based therapy initiated below
two years of age between May 2019 and December 2022. All hospitalisations were
analysed with a focus on severe RTI and especially RSV
infections, and their impact on nutritional and respiratory function. The costs
of inpatient treatment of RSV infections were determined and compared with
estimated expenses for RSV prophylaxis with palivizumab.
RESULTS: 12 SMA patients (median age at treatment initiation: 3.5 months, range: 0–17
months)
were followed for a cumulative period of 25.75 years (7 SMA type 1; 5 SMA type
2 including one presymptomatic individual). With an incidence rate of 2.34 per
patient-year, the risk of severe RTI was especially
high in SMA type 1 (versus 0.1 in SMA type 2, p = 0.044). A total of 37 hospitalisations
(279 hospital days) was necessary for the treatment of RTI in general; 9 of them were
attributed to RSV infections (in 5 SMA type 1
patients; 84 hospital days). Only 3/12 SMA patients had received
seasonal RSV prophylaxis with palivizumab. No RSV infections requiring hospital
treatment occurred in patients while receiving seasonal RSV prophylaxis. During
RTI, nutritional support had to be commonly initiated
and continued after discharge. In 3/7 SMA type 1 patients, non-invasive
ventilation was started during acute treatment for RTI and continued to the end of
follow-up.
CONCLUSION: We observed a high risk
of RTI, especially RSV infections, among young SMA patients. Failure to adhere to
established care protocols, for
example by omitting RSV prophylaxis, may be linked to a heightened risk of
morbidity in these children.
Introduction
Chromosome 5q-associated SMA is an autosomal recessive, neurodegenerative motor neuron
disorder characterised by loss of motor neurons in the brainstem and spinal
cord. SMA leads to progressive muscle hypotonia, weakness
and atrophy, reduced or absent deep tendon reflexes, tongue fasciculation and
respiratory and bulbar impairment [1]. SMA is caused by biallelic mutations of the
survival motor
neuron 1 gene (SMN1), mapped on chromosome 5q11.1-13.3 and coding for the
survival motor neuron protein (SMN) [2]. Owing
to its broad phenotypic spectrum, SMA has been classified
into subtypes 0 to 4 according to disease severity, itself based on age at
manifestation and maximum motor functions achieved [3,
4]. The majority of SMA patients have a severe,
infantile-onset phenotype (SMA type 1) with a mean life expectancy of 6–10
months without disease-modifying treatment. With an overall incidence of
approximately 1 in 6000 to 10,000 live births [5],
SMA was therefore a leading genetic cause of infant
mortality prior to the introduction of disease-modifying therapies [6–9].
Recently, different disease-modifying
treatment approaches were developed and introduced for the treatment of SMA. These
new treatments include nusinersen and risdiplam, two
splicing modifiers of the SMN2 gene, a low-functioning paralogue of the SMN1
gene. Nusinersen and risdiplam were approved for the treatment of SMA in Switzerland
in 2017 and 2021, respectively. In 2021, the gene-addition
therapy onasemnogene abeparvovec was approved for the treatment of SMA in Switzerland.
Real-world outcome data of SMA
patients treated in Switzerland with nusinersen and onasemnogene abeparvovec
was published recently using data that was prospectively collected within the
Swiss Registry for Neuromuscular Disorders (Swiss-Reg-NMD) [10, 11]. These disease-modifying
treatments have significantly
changed the natural history of SMA. Therefore, recent
standards of care have recommended using new phenotypic disease categories
based on motor function to guide therapeutic and prophylactic interventions [12, 13].
Trajectories of respiratory function for SMA of various severities have been described
[14, 15]. Progressive lung function decline increases
susceptibility to RTI [16]
and is considered to be the most important cause of morbidity and
mortality in SMA [17, 18].
This applies in particular to the most severely affected patients with
infantile-onset SMA. Even with disease-modifying treatment,
lung function of patients with infantile-onset SMA might
remain compromised or decline [19, 20]. This
patient group, similar to patients with other severe neuromuscular diseases
with impaired motor and respiratory function, has an increased risk of RTI in general
and of severe RSV
infections in particular [21, 22].
RSV is a common cause of lower RTI in infants and young children. The burden of RSV
infections is
high, since virtually all children become infected with RSV in their first two
years of life [23]. In addition, 50–90% of
hospitalisations due to bronchiolitis are caused by RSV [24]. RSV prophylaxis is possible
using the anti-RSV monoclonal
antibody palivizumab. Palivizumab is a humanised monoclonal antibody against
the F-protein of RSV and should be given monthly during the RSV season. It has
been shown that RSV prophylaxis with palivizumab reduces RSV-related hospitalisations
and morbidity especially in vulnerable patient populations [25, 26].
In severe neuromuscular disorders,
including SMA, acute RTI often
lead to deterioration of the underlying condition with motor regression and
need for prolonged nutritional and/or respiratory support, e.g. tube feeding
and nocturnal non-invasive ventilation, respectively. Therefore, it is often
accompanied by increased care requirements and need for specialised nursing
care [27]. Acute RTI
may also delay therapeutic measures, including implementation or continuation
of disease-modifying therapies [21, 28].
Internationally recognised guidelines for
the treatment of SMA recommend prophylaxis with palivizumab
for children with SMA and compromised motor function
(“non-sitters”) aged below two years [12, 13, 29].
However, not all treatment centres adhere to these
guidelines, for different reasons. For example, in Switzerland payers commonly
refuse to grant cost approvals for RSV prophylaxis with palivizumab, because
palivizumab is not approved in Switzerland for this condition nor generally
recommended by the Swiss guidelines for the usage of palivizumab in 2016 [30].
This study aims to investigate (1) the
frequency of severe RTI requiring hospital treatment,
focusing on RSV infections in SMA patients aged below two
years, (2) the long-term impact of RTI and RSV
infections, in particular, on the need for nutritional and/or respiratory
support in patients with SMA, and (3) whether non-adherence
to standards of care (by omitting RSV prophylaxis) is associated with increased
morbidity due to RSV infections in a population of young SMA patients followed in
a Swiss tertiary neuromuscular centre.
Materials and methods
This study was conducted in the tertiary paediatric
Neuromuscular Centre Zurich (Department of Paediatric Neurology, University
Children’s Hospital Zurich, University of Zurich, Switzerland). The
Neuromuscular Centre Zurich is a national reference centre for rare
neuromuscular diseases, accredited by kosek (Nationale Koordination Seltene
Krankheiten / National Coordination of Rare Diseases). Treatment of SMA with genetically based,
disease-modifying therapies and
follow-up consultations in Switzerland are only carried out in accredited
national reference centres due to corresponding regulations. Frequency of
follow-up consultations immediately after initiation of SMA
treatment depends on the therapy, with multiple consultations during the first
four months, and are then scheduled approximately every four months in the
outpatient clinic of the Neuromuscular Centre Zurich.
All patients treated in the Neuromuscular
Centre Zurich with a genetically confirmed diagnosis of 5q-associated SMA and below
two years of age at diagnosis and initiation of a
genetic-based, targeted disease-modifying therapy during the period between 1 May
2019 and 31 December 2022 were included in the study.
The source for data extractions was the
clinical information system of the University Children’s Hospital Zurich. The
following data of all included patients was extracted from the clinical
information system: SMA type (historical types [SMA types 1
and 2] and types defined by maximal motor function [non-sitter, sitter, walker]);
age at treatment onset; type of disease-modifying therapy; motor function
assessment scores (depending on age and individual motor function, either the Children’s
Hospital of Philadelphia Infant Test of Neuromuscular Disorders [CHOP Intend,
total score 0–64] or the Hammersmith Functional Motor Scale Expanded [HFMSE,
total score 0–66]) at treatment onset and end of follow-up; palivizumab vaccination
status. In addition, any documented hospitalisation of the included patients
was pulled from the clinical information system. The following data was
extracted from the clinical information system for all hospitalisations: number
of, the reason for, and the total duration of the hospitalisation; the duration
of intensive care treatment; nutritional support before, during and after
hospitalisation for RTI; respiratory support before,
during and after hospitalisation for RTI; the duration
of intensive care treatment with ventilation; the delay of planned
interventions and the need for additional specialised support following acute
hospitalisations. All this data was verified against the information obtained
during follow-up visits to the outpatient clinic of the Neuromuscular Centre
and documented in the consultation reports. In addition, the hospitalisation
costs charged to the payers for the treatment of RSV infections were calculated
via the hospital’s accounting system, and the cost of RSV prophylaxis with
palivizumab was estimated on the basis of the published price. Data was
transferred into a predefined Excel spreadsheet. The cut-off date for follow-up
data was 30 June 2023.
The study was approved by the local ethics
committee on 27 June 2023 and registered with the Swiss project database (BASEC
2023-01003). Written informed consent
(general consent) was obtained from the caregivers prior to inclusion of
participants in the study.
This work is mainly descriptive. If not
stated otherwise, the median and range are reported. Incidence rates were
calculated per patient-year. For comparison of groups, the unpaired t-test was
applied for normally distributed data and the Mann-Whitney U test for
non-normally distributed data. A p-value <0.05 was considered statistically
significant. All analyses were performed using the statistical software
GraphPad Prism version 10.2.1 for Windows (GraphPad Software; Boston, Massachusetts,
USA; www.graphpad.com).
A specific study protocol has not been
published.
Results
Demographics and study population
From May 2019 to December 2022, a total of
12 patients with SMA with a median age of 3.5 months (range:
0–17 months) at treatment initiation with one of the new genetic-based, disease-modifying
therapies were included in this study. The cut-off date for follow-up data was 30
June 2023. Prior to this date, the study cohort was followed for a cumulative
period of 25.75 years (mean follow-up duration per patient: 25.75 months [SD: 11.51]).
Mean follow-up duration per patient with SMA type 1 and SMA type 2 (including
the patient with presymptomatic treatment) was 26.43 (SD: 12.44) and 24.8 (SD: 11.43)
months (p = 0.82). For a summary of patient demographics, treatment data and
number of RTI-associated hospitalisations, please refer
to table 1.
Table 1Summary of patient
demographics, motor function, treatment data and RTI.
| Pt# |
At treatment onset |
During 1st & 2nd RSV seasons |
≥3 RSV season |
Severe non-RSV RTI
[n] |
At end of
follow-up |
| SMA type |
Age [m] |
DMT |
CHOP Intend |
HFMSE |
RSV prophylaxis |
Severe RSV infections [n] |
Severe RSV infections [n] |
Age [m] |
Respiratory support |
Nutritional support |
CHOP Intend |
HFMSE |
SMA type |
| 1 |
1 |
Non-sitter |
4 |
N |
25 |
– |
Palivizumab |
0 |
1 |
1 |
34 |
– |
– |
44 |
12 |
Sitter |
| 2 |
1 |
Non-sitter |
2 |
OA |
24 |
– |
Palivizumab |
0 |
na |
2 |
28 |
NIV |
gs |
48 |
14 |
Sitter |
| 3 |
1 |
Non-sitter |
2 |
OA |
13 |
– |
Palivizumab |
0 |
na |
2 |
8 |
NIV |
– |
35 |
– |
Non-sitter |
| 4 |
1 |
Non-sitter |
3 |
N* |
7 |
– |
– |
0 |
1 |
4 |
49 |
– |
– |
54 |
27 |
Sitter |
| 5 |
1 |
Non-sitter |
6 |
OA |
35 |
– |
– |
1 |
na |
0 |
24 |
NIV |
– |
50 |
– |
Sitter |
| 6 |
1 |
Non-sitter |
0 |
N |
23 |
– |
– |
3 |
0 |
8 |
33 |
NIV |
gs |
50 |
13 |
Sitter |
| 7 |
1 |
Non-sitter |
3 |
OA |
12 |
– |
– |
3 |
na |
9 |
29 |
NIV |
gs |
44 |
– |
Non-sitter |
| |
| 8 |
2 |
Sitter |
13 |
N |
31 |
– |
– |
0 |
0 |
0 |
39 |
– |
– |
48 |
14 |
Sitter |
| 9 |
2 |
Sitter |
14 |
N |
59 |
25 |
– |
0 |
0 |
0 |
56 |
– |
– |
- |
48 |
Walker |
| 10 |
2 |
Sitter |
17 |
OA |
46 |
14 |
– |
0 |
0 |
1 |
40 |
– |
– |
64 |
45 |
Sitter |
| 11 |
2 |
Sitter |
10 |
OA |
59 |
– |
– |
0 |
na |
0 |
20 |
– |
– |
60 |
32 |
Sitter |
| |
| 12 |
ps |
Non-sitter |
1 |
OA |
52 |
– |
– |
0 |
na |
0 |
24 |
– |
– |
64 |
36 |
Walker |
Hospitalisations
The incidence rate of all hospitalisations
after diagnosis and initiation of genetic treatment was 2.14 per patient-year
in the entire cohort (3.5 and 0.1 hospitalisations per patient-year in SMA type
1 and SMA type 2, respectively; p = 0.0037).
This incidence corresponds to a total of 55
hospitalisations with a total number of 347 hospital days (median 4 days, range
1–25) in 8 patients (67% of all SMA patients; 7 SMA type 1
and 1 SMA type 2). Four SMA patients did not require
inpatient care during the observation period (33%; 3 SMA type 2 patients, 1
presymptomatic patient). The number of hospitalisations per patient varied
between 0 and 17.
Elective hospitalisations
The incidence rate of elective hospitalisations
in patients with SMA type 1 and SMA type 2 was 0.91 and 0 per patient-year,
respectively; p = 0.015. In total, 14 elective hospitalisations were recorded
(26% of all hospitalisations). These elective hospitalisations accounted for a
total of 60 inpatient hospital days. Reasons for elective inpatient treatment (figure
1) were percutaneous endoscopic gastrostomy (PEG) feeding tube placement or
change from PEG to a gastro button (n = 6), orchidopexy (n = 2), respiratory
polygraphy (n = 1) and initiation/adjustment of non-invasive ventilation (n = 4).
Two elective PEG placements resulted in an extended hospital stay because of a hospital-acquired
RTI (non-RSV). These hospitalisations therefore were
also added to the number of acute hospitalisations due to RTI.
The median duration of elective inpatient treatment was 3 days (range 1–20).
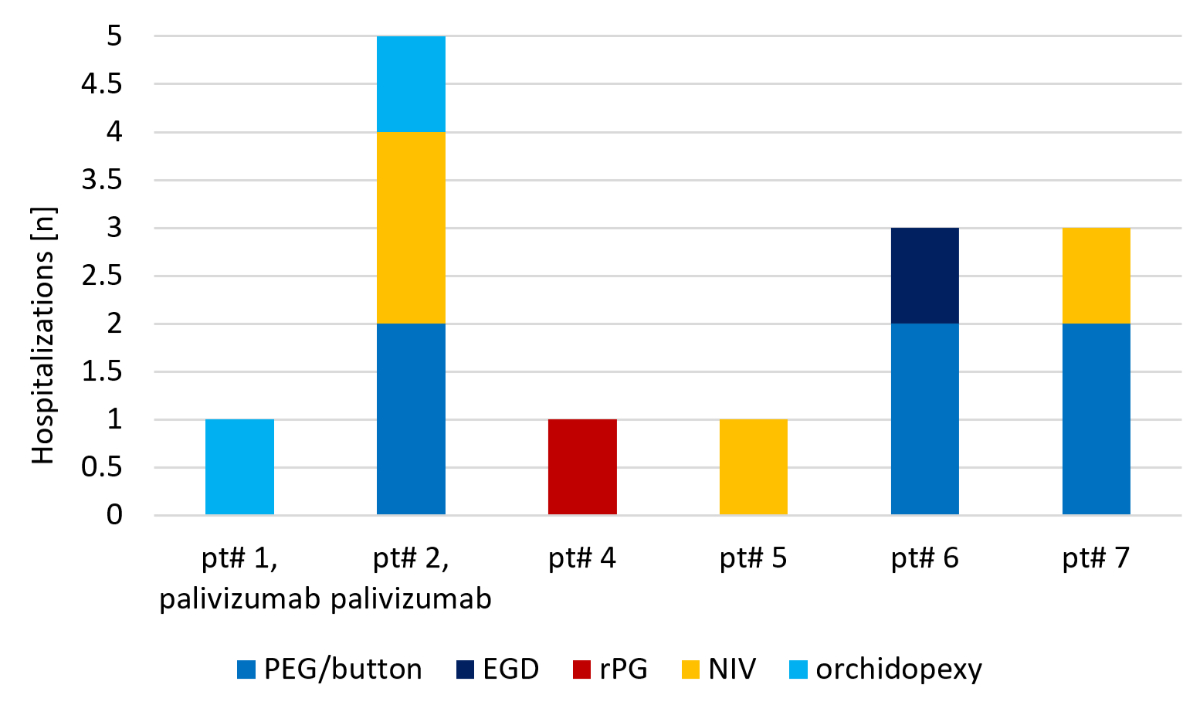
Figure 1Individual indications for elective
hospitalisations. EGD: oesophagoduodenoscopy; NIV: initiation/adjustment of
nighttime non-invasive ventilation; PEG/button: percutaneous endoscopic
gastrostomy (PEG) feeding tube placement or change from PEG to a gastro button;
rPG: respiratory polygraphy.
Acute hospitalisations
The incidence rate of acute hospitalisations
in patients with SMA type 1 and SMA type 2 was 2.60 and 0.1 per patient-year,
respectively (p = 0.022). In total, 41 hospitalisations were for acute reasons
(74% of all hospitalisations). These acute hospitalisations totalled 287
hospital days. A total of 37 hospitalisations (279 hospital days including 104
days in the intensive care unit) were due to RTI
(including RSV and non-RSV infections) and accounted for 90% of all acute
hospitalisations. Other reasons for acute hospitalisations were
gastrointestinal infections (n = 2), pyelonephritis (n = 1) and fever of
unknown origin (n = 1) (figure 2).
The incidence rate of RTI-related hospitalisations in patients with SMA type 1 and
SMA type 2
was 2.34 and 0.1 per patient-year, respectively (p = 0.044). Of the RTI-related hospitalisations,
28 were due to non-RSV infections (195
hospital days including 73 days in the intensive care unit) and 9 were due to
RSV infections (84 hospital days including 31 days in the intensive care unit).
Thus, 24% of all RTI-related hospitalisations were due
to RSV infections.
The 9 RSV infections occurred in 5 patients
(all SMA type 1), 2 of whom required multiple hospitalisations for RSV
infections (three admissions each). In both patients, the first two hospitalisations
occurred during their first RSV season which fell into the atypical summer RSV
surge during the COVID-19 pandemic in 2021. These two RSV infections were one
month and three months apart from each other. The third hospitalisation of
these two patients occurred in their second RSV season in autumn 2022.
Interestingly, two hospitalisations due to RSV infection occurred in patients
during their 3rd or later RSV season. These two hospitalisations were short (2
and 3 days, respectively) and did not require intensive care treatment. No RSV
infection requiring hospital treatment occurred in patients while receiving
seasonal RSV prophylaxis with palivizumab.
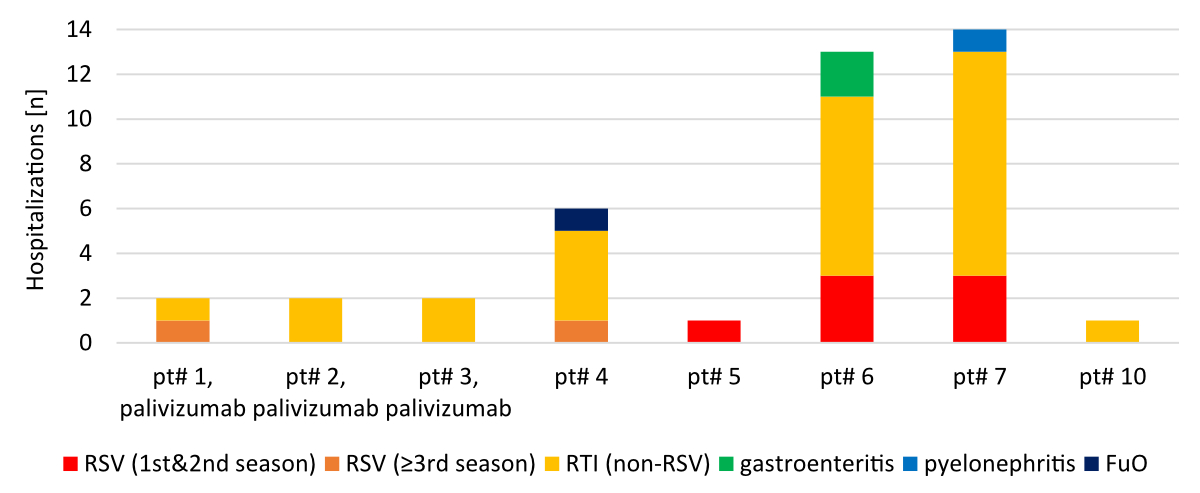
Figure 2Individual
indications for acute hospitalisations. FuO: fever of unknown origin; RSV: respiratory
syncytial virus infection; RTI (non-RSV): RTI other
than RSV.
Palivizumab vaccination status
Seven patients of our cohort fulfilled the
criteria of RSV prophylaxis due to their motor status (non-sitters) and age (2 years
or below) according to current care recommendations [12,
29]. Of these seven patients, only three received RSV prophylaxis cost
approvals from the payers for palivizumab during their first two RSV seasons.
Neither of them required hospitalisation due to RSV infection while receiving
seasonal RSV prophylaxis. An eighth, presymptomatically treated individual was
a non-sitter by motor function when receiving a disease-modifying treatment but
acquired the ability to sit before entering the first RSV season.
Long-term impact of respiratory tract infections
Nutritional support
Six of eight SMA
patients who needed hospital treatment for RTI required
nasogastric tube feeding during the acute phase of the hospital treatment. Only
two of these patients required tube feeding during acute RTI treatment and could be
discharged from inpatient treatment with oral
nutrition solely. However, four of these patients remained dependent on
nutritional support beyond discharge from the hospital. RSV infection was the
reason for three out of four of these hospitalisations. While one patient (pt#5
in table 1) was able to switch back to oral nutrition two weeks after discharge
from the hospital for RSV RTI treatment, the remaining
three remained dependent on tube feeding in the long term and were still almost
exclusively tube fed at the end of follow-up, after 20, 23 and 25 months. Pt#2
became permanently dependent on nutritional support during the second severe
non-RSV RTI. Pt#6 and pt#7 (see table 1) were
temporarily dependent on nutritional support during a first non-RSV RTI, but were
initially discharged without tube feeding. During a
subsequent RSV RTI, both then became permanently
dependent on nutritional support. Consecutively, a PEG tube was placed in all
three of these patients.
Respiratory support
Respiratory support (including high-flow
therapy and BiPAP non-invasive ventilation) had to be newly introduced during
seven acute hospitalisations in four patients, and pre-existing respiratory
support had to be continued in four hospitalisations in one patient. In 3 of
the 9 RSV-associated hospitalisations (33%), respiratory support was necessary:
high-flow respiratory support during the acute phase (n = 2, for a total of 14
days); intensification of previously started non-invasive nighttime ventilation
to full-time non-invasive ventilation (n = 1, for 11 days). Altogether, 25 ICU
days with ventilation were attributable to RSV infections.
At the end of data collection, 5 of 12
patients (42%, all SMA type 1) were dependent on non-invasive nocturnal
ventilation. In three patients (pt#3, pt#6, pt#7 – see table 1), non-invasive
ventilation was started during acute treatment for RTI
and was continued until the end of follow-up in all three patients. In two
patients (pt#2 and pt#5 – see table 1), non-invasive nocturnal ventilation was
started electively.
Delay of interventions, need for additional care
In three patients, a planned surgical
intervention (PEG placement, change of PEG to button, achillotomy) had to be
postponed due to an acute hospitalisation, all of them due to non-RSV RTI. The achillotomy
mentioned was done later in an outpatient
setting and did not require inpatient treatment.
Regular motor function assessments are
mandatory following / during treatment of SMA with genetic
therapies in Switzerland, in particular for extensions of cost approvals for
their continuation. In two patients (one with RTI due
to RSV), these assessments had to be postponed due to RTI.
Nutritional and respiratory support usually
require specialised outpatient nursing services, at least in the initial phase
after discharge from the hospital. For example, all four SMA patients dependent on
nutritional support beyond inpatient treatment
for RTI received such a home care service. In addition,
these patients had a need for new or intensified existing therapies and support
(e.g. orofacial therapy by a speech therapist, physiotherapy, nutritional
counselling by a dietitian).
Costs due to RSV infections
RSV infections resulted in 84 days of
hospitalisation, with 31 days occurring in the intensive care unit primarily
because of the need for advanced respiratory support (25 ICU days with
ventilation) and nutritional support.
The direct costs of RSV-associated hospitalisation
amounted to more than CHF 200,000 (approximately USD 230,000), including costs
for 31 days of intensive care. However, the total economic burden of these
severe RSV infections on the healthcare system was much greater, since
subsequent costs must be added to inpatient costs resulting, for example, from
the ongoing need for respiratory and nutritional support, specialised home care
services and other supportive symptomatic therapies. However, within this
study, it was only possible to estimate the direct costs of inpatient
treatment.
The direct costs of inpatient treatment of SMA patients with RSV infections contrast
with the costs of RSV
prophylaxis. In the observation period, the costs of RSV prophylaxis with
palivizumab would have amounted to approximately CHF 80,000 (approximately USD 90,000)
(7 patients with SMA type 1 / non-sitter below age 2 years, treatment for two
seasons each).
Discussion
5q-associated SMA is a neurodegenerative
neuromuscular disorder leading to compromised motor functions including
respiratory and bulbar function in more severely affected individuals.
Respiratory muscle weakness, impaired mucus mobilisation, high prevalence of
gastro-oesophageal reflux and bulbar dysfunction are well known risk factors
for RTI in neuromuscular disorders in general [21, 22]. They have also been recognised
as risk
factors, in particular, for more severe RSV
infections in neuromuscular diseases including SMA [31].
New genetic therapies have established a
new era in the treatment of SMA. They significantly prolong
survival and enable acquisition of motor milestones in patients with infantile-onset
SMA (type 1). In more mildly affected patients (later-onset
SMA, types 2 and 3), they prevent progression of
neuromuscular symptoms and lead to long-term preservation of already acquired
motor skills [32]. Prior to the availability
of these disease-modifying treatments, most children with SMA type 1 died in
the first two years of life or required permanent ventilation. Today, under
treatment with these new therapies, these children with SMA type 1 survive but
develop varying degrees of muscle weakness, which limits motor development and
function, and potentially compromises bulbar and respiratory function. To
protect this fragile SMA population, well-defined
recommendations for preventive and symptomatic therapeutic measures were
developed, including care recommendations for pulmonary symptoms and
prophylaxis for RSV infections with palivizumab in severely affected infants
with SMA (“non-sitters”) below 2 years of age [12, 29].
RSV prophylaxis with palivizumab has not
yet been approved for the treatment of patients with SMA in
Switzerland. Payers therefore generally refuse to cover the costs of RSV
prophylaxis. Therefore, the aim of this study was to investigate the role of
RSV infections among severe RTI with inpatient treatment in
a population for which adherence to otherwise internationally recognised
standards was not possible.
In summary, our data shows that the risk of
severe RSV infections requiring hospitalisation is high in SMA patients. This especially
concerns patients with severely compromised
motor function (SMA type 1 and/or non-sitters) during early infancy. In our
cohort of 7 SMA type 1 patients / non-sitters, a total of 84 hospitalisation
days, including 31 days in ICU, were necessary for the treatment of RSV
infection. In our small cohort, severe RSV infection only occurred in patients
without seasonal RSV prophylaxis with palivizumab. The cost of these
RSV-associated hospitalisations far exceeds the estimated cost of RSV
prophylaxis for the subgroup of our study cohort defined in the care standards
(patients with SMA type 1 / “non-sitters” and age below two years) – even without
taking into account indirect follow-up costs arising from prolonged nutritional
and/or respiratory support following RSV infections. Our study shows that such
support is often required in SMA patients beyond hospital
treatment for severe RTI in general, especially if the
reason was an RSV infection. In addition, our data shows a clear long-term
impact of RSV infections for the patients. Three patients remained in need of
nutritional support beyond hospitalisation. Ventilatory support was needed in 3
of 8 hospitalisations due to RSV infection and one child needed intensified
ventilatory support after the hospitalisation. In addition, severe RSV
infections led to a delay of planned surgical interventions and therapies.
Thus, our study may indicate that non-adherence to care standards (by omitting
RSV prophylaxis) might be associated with increased morbidity in the studied
patient population. In addition, there is a cost to society that is potentially
significantly higher than the cost of providing RSV prophylaxis according to
care recommendations.
At the time of our study, RSV prophylaxis
with palivizumab was broadly available in many regions worldwide. Palivizumab
can prevent severe RSV infections and their long-term sequelae. In Switzerland,
a consensus statement for the prevention of RSV infections with palivizumab was
published by the Paediatric Infectious Disease Group of Switzerland (PIGS) in
2016 [30]. According to this statement, RSV
prophylaxis is recommended for children with severe bronchopulmonary dysplasia
and uncorrected haemodynamically significant congenital heart defects. Only for
these indications was RSV prophylaxis reimbursed by the Swiss invalid insurance
after an individual cost approval. Since July 2022, cost coverage has
been provided by the mandatory healthcare insurance in Switzerland. The
PIGS consensus statement specified that a general indication for RSV
prophylaxis is not given for a broad range of other diseases including SMA because
of the wide clinical variability of these conditions
and a lack of efficacy data regarding RSV prophylaxis for these disease
populations. In fact, the present report is to our knowledge the first cohort
study that investigates the occurrence and consequences of RSV infections in SMA patients.
The 2018 guideline of the Working Group of the
Scientific Medical Societies (Arbeitsgemeinschaft der Wissenschaftlichen
Medizinischen Fachgesellschaften, AWMF) on prophylaxis of severe RSV disease
in high-risk children [33] states that
patients with neuromuscular disorders and RSV infection more often require
intensive care and invasive ventilation and have higher lethality. These
guidelines list SMA among other high-risk diseases, such as
patients with immunodeficiencies and cystic fibrosis.
Therefore, internationally recognised
guidelines for the treatment of SMA and standards of care
recommend prophylaxis with palivizumab for children with SMA and significantly impaired
motor function (“non-sitters”) aged below 2
years [12, 29].
In recent years, great progress has been
made in the development of new measures for RSV prophylaxis. A new antiviral
monoclonal antibody, nirsevimab, was approved for RSV prophylaxis by the European
Medicines Agency (EMA) and the FDA in October 2022 and July 2023, respectively.
According to the EMA prescribing information, nirsevimab is indicated in all
neonates and infants during their first RSV season regardless of whether there
is a predisposing underlying disease [34]. The
FDA prescribing information extends the indication to children up to 24 months
of age who remain vulnerable to severe RSV disease through their second RSV
season [35]. In addition, both the EMA and the
FDA approved Abrysvo in August 2023, the first active immunisation of pregnant women
for the prevention of severe RSV infections in infants from birth to 6 months
of age [36, 37]. During the revision of the
present report, nirsevimab was also approved by Swissmedic in late December
2023. The approval label largely follows the FDA’s decision [38]. Recently, an expert
working group consisting of
representatives from various Swiss medical societies, the Federal Commission
for Vaccination Issues (EKIF / CFV) and the Federal Office of Public Health
(BAG / FOPH) issued a new consensus statement / recommendation on the
prevention of respiratory syncytial virus infections with the monoclonal
antibody nirsevimab [39]. The implementation
of this consensus recommendation still requires further regulatory processes,
such as the inclusion of nirsevimab prophylaxis in the national vaccination
recommendations and the specialty list for reimbursement by payers. This
development provides improved RSV prophylaxis in many regions worldwide and may
be to the benefit of patients with SMA.
Parallel to this development, SMA has been included in numerous national newborn screening
programmes worldwide. In the US and Europe up to 100% and >65% of all newborns, respectively,
are screened for the presence of SMA. The rationale for the inclusion
of SMA in newborn screening programmes is that a
significantly better prognosis is seen when therapy is initiated at a
presymptomatic disease stage vs at a symptomatic stage. Presymptomatic
treatment results in normal or near-normal motor development for the majority
of individuals with a genetic diagnosis of SMA, at least in
the first years of life [40–43]. These
coincidental developments result in a rapidly decreasing SMA population at risk (“non-sitters”)
and at the same time improved and
extended measures for RSV prophylaxis, at least in regions with appropriate
approvals.
This study has several potential limitations.
First, it is a single-centre cohort study relying on existing medical records.
Since SMA is a rare disorder, only a comparatively small
patient population could be included. Further investigations are needed to
investigate the impact on severe RTI and RSV infections
on patients with SMA. However, this study is one of the
first to investigate the frequency of severe RSV infections in SMA and its impact
on the more long-term wellbeing of affected individuals
of this fragile patient group. Second, the study focused on severe RTI and RSV infections
and thus might have missed milder cases not
requiring hospital care and their impact on the outcome. Third, the study
period coincided with the COVID-19 pandemic. The aim of this study was not to
investigate the incidence of severe COVID-19 infections. However, during and
following the COVID-19 pandemic, the epidemiology of RSV changed. Atypical
surges in RSV cases were observed during the summer months, i.e. between
typical RSV seasons [44].
Whether and to what extent the results of this study may have been influenced
by a temporal association with the COVID-19 pandemic and the change of RSV
epidemiology can only be speculated upon. Fourth, this study might be
representative only for Switzerland and other regions where RSV prophylaxis is
not routinely available.
However, this specific regional situation
depicts well the consequences of non-adherence to respected care
recommendations. International care recommendations for rare disorders should
be respected by healthcare professionals, payers and other stakeholders
involved in the care of patients with a rare disorder, especially when national
or local regulations and recommendations do not include a particular rare
disorder and/or do not reflect current knowledge or new developments. The study
results should provide a loud voice for reinforcing current treatment
recommendations for rare disorders.
Data sharing statement
The data that supports the findings of this
study is available in anonymised and aggregated form from the corresponding
author upon reasonable request. The data is not publicly available as it contains
information that could compromise the privacy of research participants in this
small cohort study.
Acknowledgments
We thank the patients and their caregivers
for contributing their data. We would like to thank K. Benincasa Herr for
language editing.
Authorsʼ contributions: CTR and GMS initiated and designed the study,
extracted data from the hospital’s clinical information system, analysed the
data and wrote the manuscript. MSt and EG extracted data from the clinical
information system, analysed the data and revised the manuscript. CTR, EG and
GMS were directly involved in patient care and collected clinical data during
follow-up consultations. MSo analysed the data and revised the manuscript. PMMS
and JT co-designed the study and revised the manuscript. All authors read and
approved the final manuscript.
PD Dr. med.
Georg M. Stettner
Neuromuscular
Centre Zurich and Department of Pediatric Neurology
University
Children’s Hospital Zurich
University
of Zurich
Steinwiesstrasse 75
CH-8032 Zurich
georg.stettner[at]kispi.uzh.ch
References
1. Prior TW, Leach ME, Finanger E. Spinal Muscular Atrophy. GeneReviews®. Seattle (WA).
Seattle: University of Washington; 1993.
2. Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification
and characterization of a spinal muscular atrophy-determining gene. Cell. 1995 Jan;80(1):155–65.
doi: https://doi.org/10.1016/0092-8674(95)90460-3
3. Kolb SJ, Kissel JT. Spinal Muscular Atrophy. Neurol Clin. 2015 Nov;33(4):831–46. doi: https://doi.org/10.1016/j.ncl.2015.07.004
4. Farrar MA, Park SB, Vucic S, Carey KA, Turner BJ, Gillingwater TH, et al. Emerging
therapies and challenges in spinal muscular atrophy. Ann Neurol. 2017 Mar;81(3):355–68.
doi: https://doi.org/10.1002/ana.24864
5. Verhaart IE, Robertson A, Wilson IJ, Aartsma-Rus A, Cameron S, Jones CC, et al. Prevalence,
incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature
review. Orphanet J Rare Dis. 2017 Jul;12(1):124. doi: https://doi.org/10.1186/s13023-017-0671-8
6. Ou SF, Ho CS, Lee WT, Lin KL, Jones CC, Jong YJ; SMA Study Group. Natural history
in spinal muscular atrophy Type I in Taiwanese population: A longitudinal study. Brain
Dev. 2021 Jan;43(1):127–34. doi: https://doi.org/10.1016/j.braindev.2020.07.012
7. Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB, et al.; NeuroNEXT
Clinical Trial Network on behalf of the NN101 SMA Biomarker Investigators. Natural
history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017 Dec;82(6):883–91.
doi: https://doi.org/10.1002/ana.25101
8. Finkel RS, McDermott MP, Kaufmann P, Darras BT, Chung WK, Sproule DM, et al. Observational
study of spinal muscular atrophy type I and implications for clinical trials. Neurology.
2014 Aug;83(9):810–7. doi: https://doi.org/10.1212/WNL.0000000000000741
9. Cances C, Vlodavets D, Comi GP, Masson R, Mazurkiewicz-Bełdzińska M, Saito K, et al.;
ANCHOVY Working Group. Natural history of Type 1 spinal muscular atrophy: a retrospective,
global, multicenter study. Orphanet J Rare Dis. 2022 Jul;17(1):300. doi: https://doi.org/10.1186/s13023-022-02455-x
10. Tscherter A, Rüsch CT, Baumann D, Enzmann C, Hasselmann O, Jacquier D, et al.; Swiss-Reg-NMD
group. Evaluation of real-life outcome data of patients with spinal muscular atrophy
treated with nusinersen in Switzerland. Neuromuscul Disord. 2022 May;32(5):399–409.
doi: https://doi.org/10.1016/j.nmd.2022.02.001
11. Stettner GM, Hasselmann O, Tscherter A, Galiart E, Jacquier D, Klein A. Treatment
of spinal muscular atrophy with Onasemnogene Abeparvovec in Switzerland: a prospective
observational case series study. BMC Neurol. 2023 Feb;23(1):88. doi: https://doi.org/10.1186/s12883-023-03133-6
12. Finkel RS, Mercuri E, Meyer OH, Simonds AK, Schroth MK, Graham RJ, et al.; SMA Care
group. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and
acute care; medications, supplements and immunizations; other organ systems; and ethics.
Neuromuscul Disord. 2018 Mar;28(3):197–207. doi: https://doi.org/10.1016/j.nmd.2017.11.004
13. Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al.; SMA Care Group.
Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis,
rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018 Feb;28(2):103–15.
doi: https://doi.org/10.1016/j.nmd.2017.11.005
14. Trucco F, Ridout D, Scoto M, Coratti G, Main ML, Muni Lofra R, et al.; International
SMA Consortium (iSMAc). Respiratory Trajectories in Type 2 and 3 Spinal Muscular Atrophy
in the iSMAC Cohort Study. Neurology. 2021 Jan;96(4):e587–99. doi: https://doi.org/10.1212/WNL.0000000000011051
15. Vicino A, Bello L, Bonanno S, Govoni A, Cerri F, Ferraro M, et al. Respiratory function
in a large cohort of treatment-naïve adult spinal muscular atrophy patients: a cross-sectional
study. Neuromuscul Disord. 2023 Dec;33(12):911–6. doi: https://doi.org/10.1016/j.nmd.2023.10.002
16. Guo W, Meng L, Cao L. Risk factors for recurrent respiratory tract infections and
acute respiratory failure in children with spinal muscular atrophy. Pediatr Pulmonol.
2023 Feb;58(2):507–15. doi: https://doi.org/10.1002/ppul.26218
17. Wijngaarde CA, Veldhoen ES, van Eijk RP, Stam M, Otto LA, Asselman FL, et al. Natural
history of lung function in spinal muscular atrophy. Orphanet J Rare Dis. 2020 Apr;15(1):88.
doi: https://doi.org/10.1186/s13023-020-01367-y
18. Veldhoen ES, Wijngaarde CA, Hulzebos EH, Wösten-van Asperen RM, Wadman RI, van Eijk RP,
et al. Natural history of respiratory muscle strength in spinal muscular atrophy:
a prospective national cohort study. Orphanet J Rare Dis. 2022 Feb;17(1):70. doi: https://doi.org/10.1186/s13023-022-02227-7
19. Menard J, Seferian AM, Fleurence E, Barzic A, Binoche A, Labouret G, et al. Respiratory
management of spinal muscular atrophy type 1 patients treated with Nusinersen. Pediatr
Pulmonol. 2022 Jun;57(6):1505–12. doi: https://doi.org/10.1002/ppul.25899
20. Panagiotou P, Kanaka-Gantenbein C, Kaditis AG. Changes in Ventilatory Support Requirements
of Spinal Muscular Atrophy (SMA) Patients Post Gene-Based Therapies. Children (Basel).
2022 Aug;9(8):1207. doi: https://doi.org/10.3390/children9081207
21. Wilkesmann A, Ammann RA, Schildgen O, Eis-Hübinger AM, Müller A, Seidenberg J, et
al.; DSM RSV Ped Study Group. Hospitalized children with respiratory syncytial virus
infection and neuromuscular impairment face an increased risk of a complicated course.
Pediatr Infect Dis J. 2007 Jun;26(6):485–91. doi: https://doi.org/10.1097/INF.0b013e31805d01e3
22. Panitch HB. Viral respiratory infections in children with technology dependence and
neuromuscular disorders. Pediatr Infect Dis J. 2004 Nov;23(11 Suppl):S222–7. doi: https://doi.org/10.1097/01.inf.0000144670.78558.c7
23. Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection
with respiratory syncytial virus. Am J Dis Child. 1986 Jun;140(6):543–6.
24. Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001 Jun;344(25):1917–28.
doi: https://doi.org/10.1056/NEJM200106213442507
25. Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH Jr, et al.; Cardiac
Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory
syncytial virus in young children with hemodynamically significant congenital heart
disease. J Pediatr. 2003 Oct;143(4):532–40. doi: https://doi.org/10.1067/S0022-3476(03)00454-2
26. Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al.; Respiratory Virus
Global Epidemiology Network; RESCEU investigators. Global, regional, and national
disease burden estimates of acute lower respiratory infections due to respiratory
syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet.
2022 May;399(10340):2047–64. doi: https://doi.org/10.1016/S0140-6736(22)00478-0
27. Chen KA, Widger J, Teng A, Fitzgerald DA, D’Silva A, Farrar M. Real-world respiratory
and bulbar comorbidities of SMA type 1 children treated with nusinersen: 2-Year single
centre Australian experience. Paediatr Respir Rev. 2021 Sep;39:54–60.
28. Simon A, Prusseit J, Müller A. Respiratory syncytial virus infection in children with
neuromuscular impairment. Open Microbiol J. 2011;5(1):155–8. doi: https://doi.org/10.2174/1874285801105010155
29. Kölbel H, Müller-Felber W. Spinale Muskelatrophie (SMA), Diagnostik und Therapie.
AWMF Leitlinie. 2020 Dec 2; Available from: https://register.awmf.org/de/leitlinien/detail/022-030
30. Agyeman P, Barazzone C, Hammer J, Heininger U, Nadal D, Pfammatter JP, et al. Konsensus
Statement zur Prävention von Respiratory Syncytial Virus (RSV)-Infektionen mit dem
humanisierten monoklonalen Antikörper Palivizumab (Synagis®). Paediatrica. 2017;28(2):13–5.
31. Resch B, Manzoni P, Lanari M. Severe respiratory syncytial virus (RSV) infection in
infants with neuromuscular diseases and immune deficiency syndromes. Paediatr Respir
Rev. 2009 Sep;10(3):148–53. doi: https://doi.org/10.1016/j.prrv.2009.06.003
32. Antonaci L, Pera MC, Mercuri E. New therapies for spinal muscular atrophy: where we
stand and what is next. Eur J Pediatr. 2023 Jul;182(7):2935–42. doi: https://doi.org/10.1007/s00431-023-04883-8
33. Liese J, Forster J, Herting E. Leitlinie zur Prophylaxe von schweren Erkrankungen
durch Respiratory Syncytial Virus (RSV) bei Risikokindern. AWMF Leitlinie. 2023 Sep 25;
Available from: https://www.awmf.org/service/awmf-aktuell/prophylaxe-von-schweren-rsv-erkrankungen-bei-risikokindern-mit-monoklonalen-antikoerpern
34. Beyfortus: EPAR - Product Information. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/beyfortus#product-info
35. Beyfortus Prescribing Information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761328s000lbl.pdf
36. Abrysvo: EPAR - Product Information. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/abrysvo#product-info
37. Abrysvo Prescribing Information. Available from: https://www.fda.gov/vaccines-blood-biologics/abrysvo
38. Beyfortus, Injektionslösung in einer Fertigspritze (Nirsevimab). Available from: https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/authorisations/new-medicines/beyfortus-injlsg-nirsevimabum.html
39. Consensus statement / recommendation on the prevention of respiratory syncytial virus
(RSV) infections with the monoclonal antibody Nirsevimab (Beyfortus®). Available from:
https://www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/rsv.html
40. De Vivo DC, Bertini E, Swoboda KJ, Hwu WL, Crawford TO, Finkel RS, et al.; NURTURE
Study Group. Nusinersen initiated in infants during the presymptomatic stage of spinal
muscular atrophy: interim efficacy and safety results from the Phase 2 NURTURE study.
Neuromuscul Disord. 2019 Nov;29(11):842–56. doi: https://doi.org/10.1016/j.nmd.2019.09.007
41. Crawford TO, Swoboda KJ, De Vivo DC, Bertini E, Hwu WL, Finkel RS, et al.; NURTURE
Study Group. Continued benefit of nusinersen initiated in the presymptomatic stage
of spinal muscular atrophy: 5-year update of the NURTURE study. Muscle Nerve. 2023 Aug;68(2):157–70.
doi: https://doi.org/10.1002/mus.27853
42. Strauss KA, Farrar MA, Muntoni F, Saito K, Mendell JR, Servais L, et al. Onasemnogene
abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal
muscular atrophy type 1: the Phase III SPR1NT trial. Nat Med. 2022 Jul;28(7):1381–9.
doi: https://doi.org/10.1038/s41591-022-01866-4
43. Strauss KA, Farrar MA, Muntoni F, Saito K, Mendell JR, Servais L, et al. Onasemnogene
abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal
muscular atrophy: the Phase III SPR1NT trial. Nat Med. 2022 Jul;28(7):1390–7. doi: https://doi.org/10.1038/s41591-022-01867-3
44.Abu-Raya B, Viñeta Paramo M, Reicherz F, Lavoie PM. Why has the epidemiology of RSV
changed during the COVID-19 pandemic? EClinicalMedicine. 2023 Jul;61:102089. doi: https://doi.org/10.1016/j.eclinm.2023.102089

