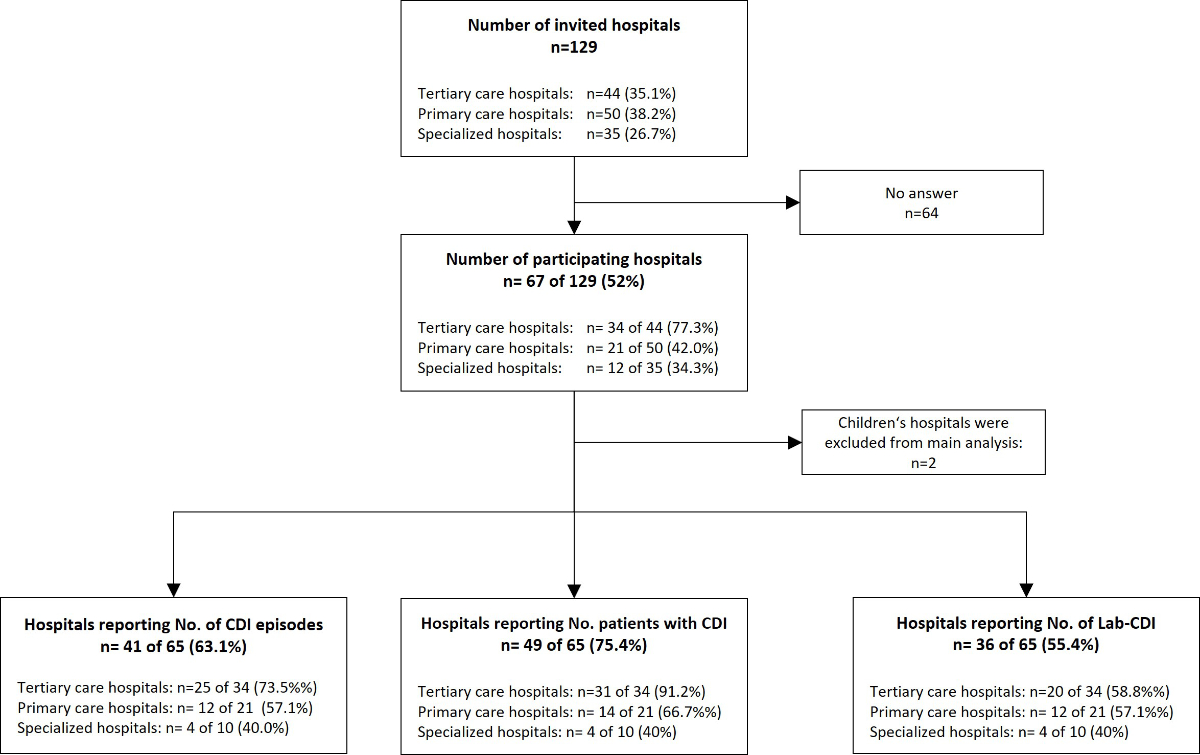
Figure 1Flowchart showing the number of participating hospitals in the Swiss C. difficile survey in 2022 and the response rates for key measurements.
DOI: https://doi.org/https://doi.org/10.57187/s.3571
Clostridioides difficile is the most common gastrointestinal healthcare-associated infection worldwide [1]. The spectrum of C. difficile infection ranges from diarrhoea to life-threatening toxic megacolon [2, 3]. This infection significantly contributes to morbidity and mortality and associated costs, with approximately 500,000 cases annually in the United States and 30-day mortality ranging from 6–11% [1,4] and even higher in ICU patients [5]. C. difficile infection was previously thought to be mainly transmitted in hospitals but is increasingly recognized in ambulatory settings as well [6]. Three components are key to the development of infection: acquisition of C. difficile,e.g. from the environment; exposure to antibiotic therapy; and host susceptibility to infection. Risk factors include age >65 years, multiple comorbidities, chemotherapy or immunosuppressive treatments, recent surgery of any type and proton pump inhibitor therapy. Despite treatment, recurrence affects up to 30% of C. difficile infection patients [7, 8]. Surveillance is crucial for comprehending the interplay of these factors and their effects on infection rates and for developing intervention strategies. Prevention strategies focus on two modifiable components: antibiotic exposure and acquisition of C. difficile. Antibiotic stewardship programs target both factors and can result in a 30% reduction in infection rates. More importantly, up to 80% of cases are preventable if antibiotic stewardship programs are paired with effective infection control measures [9, 10].
A systematic review and meta-analysis of 13 US studies found that the incidence of hospital-onset C. difficile infection was 8.3 cases per 10,000 patient-days [11]. The European C. difficile infection study (ECDIS) – under the guidance of the European Centre for Disease Prevention and Control (ECDC) – has published several estimates of C. difficile infection incidence in Europe over the last decade. In 2008, the weighted mean incidence rate per 10,000 patient-days was 4.1 C. difficile infection cases per hospital (range 0.0–36.3). In 2013, the rate increased to 5.78, and the most recent ECDC report for 2016–17 indicated a crude incidence density of 3.48 C. difficile infections per 10,000 patient-days [12–14]. Many European countries have nationwide surveillance programs, and several, such as Belgium, France, Ireland, Malta and UK (Scotland), are performing comprehensive C. difficile infection surveillance. Switzerland currently has no nationwide surveillance system, despite the active contributions of Swiss experts to C. difficile research and guideline development, such as the guidelines for prevention of C. difficile infection for the European Society for Clinical Microbiology and Infection (ESCMID). These guidelines, like other international recommendations, advise surveillance for C. difficile infection [15, 16]. Swissnoso – the Swiss National Centre for Infection Prevention – is an association of infection control experts that formulates national infection control guidelines for acute care hospitals within the federally regulated Swiss healthcare system. The system serves 9 million inhabitants, with average life expectancies of 81.6 years for men and 85.7 years for women, through 278 hospitals [17]. In 2017, with the Swiss Centre for Antibiotic Resistance (ANRESIS), Swissnoso launched a laboratory-based surveillance system that allows automated import of C. difficile infection laboratory results into a database. However, currently too few laboratories participate to allow an estimate of the burden of C. difficile infection in Switzerland. As part of this project, a questionnaire was administered to Swiss acute care hospitals in 2023 to estimate the incidence of C. difficile infection and to assess current clinical practices and diagnostic approaches for C. difficile infection. Here we report the most up-to-date estimate of the national burden of C. difficile infection in acute care hospitals in Switzerland.
A C. difficile infection surveillance reporting form was crafted by Swissnoso and underwent pretesting in several hospitals. The reporting form encompassed C. difficile infection diagnoses, management measures, hospital parameters and the C. difficile testing algorithm and frequency. Inclusion criteria involved inpatients with C. difficile infection in acute care hospitals. Long-term care, psychiatric and maternity hospitals were excluded, aligning with international surveillance protocols. Diagnostic criteria for C. difficile infection adhered to definitions by the ECDC and the Centers for Disease Control and Prevention (CDC; Atlanta, USA) [18]. They were adapted to the Swiss hospital setting while still allowing for comparison with the minimal surveillance protocol of the ECDC [19]. C. difficile infection was defined as a positive test result for toxigenic C. difficile from clinical faecal samples. “Lab-based C. difficile infection” referred to diagnoses based solely on laboratory results, while “C. difficile infection episode” was used for symptomatic patients with C. difficile infection. Recurrences were recorded, and duplicates, defined as repeated tests within 14 days of C. difficile infection diagnosis, were excluded. Our methodology deviated from the reference protocols [19] mainly in the following characteristics: we omitted the a priori distinction between community- and healthcare-associated infections, as the routine collection of information regarding the timing of previous hospitalisations is not a standard practice in Switzerland. Only adult patients were included in our analysis, and our survey captured both clinical C. difficile infection and lab-based C. difficile infection cases. A web-based survey created using SurveyMonkey was sent to all Swiss acute care hospitals participating in the Swissnoso surveillance system. Trained infection control practitioners or infectious disease physicians submitted information for their institutions for the entire year from 1 January to 31 December 2022. The completion period for the electronic questionnaire spanned 10 weeks, with data submitted to Swissnoso in aggregate form, devoid of patient-specific details. The survey was available in German or French. Data entry was possible for individual institutions or for a whole hospital group, which could submit cumulative data. All participants were provided with instructions and explanations of key parameters and were given opportunities to seek professional support directly from the investigators and to provide written feedback throughout the survey period.
Survey data were collected on SurveyMonkey [20] and underwent processing, including plausibility checks and data cleaning. Subsequent statistical analyses were conducted using Stata version 16.1 (College Station, TX: StataCorp LLC) by a professional biostatistician (AS).
Variation in the completeness of participant responses necessitated separate analyses for each question, resulting in nine different incidences to accommodate response heterogeneity. For the incidence rates, 95% Poisson confidence intervals (CIs) were calculated. Incidence rates, expressed as the number of C. difficile episodes per 10,000 patient-days, were used as the main rates for comparison and mean incidence rate determination, as recommended by the CDC and ECDC surveillance protocols [18, 19]. Stratification aligned with hospital categories – tertiary care hospitals, primary care hospitals and specialized surgery hospitals – according to Federal Statistical Office (BFS) classification [17]. In summary, the 278 hospital facilities were categorized into three main groups: tertiary hospitals, which provide advanced medical services; primary care hospitals, which serve as essential community healthcare providers; and a diverse group of specialized clinics (63.7% of the institutions), mainly non-acute care facilities focusing on specific medical areas, such as rehabilitation or psychiatry. Our survey included acute care hospitals covering tertiary care, primary care hospitals and specialized surgery hospitals that exclusively provide specialized surgeries. Clustering within hospital types was not explicitly considered in this analysis.
In March 2023, the survey was sent to 129 facilities (acute care hospitals or hospital networks), from which 67 responses were received, representing a response rate of 52% (figure 1). Ten respondents provided cumulative data for a hospital group; therefore, the actual number of participating institutions may be higher than indicated. Two children’s hospitals were excluded from subsequent analyses. Response rates varied by hospital type: the participation rates were 77% (34/44 invited hospitals) for tertiary care hospitals, 42% (21/50 invited) for primary care hospitals and 34% (12/35 invited) for specialized hospitals. Our sample covered 51% of acute care hospitals, as defined by the Federal Office of Public Health (FOPH), across all regions of Switzerland.

Figure 1Flowchart showing the number of participating hospitals in the Swiss C. difficile survey in 2022 and the response rates for key measurements.
Due to diverse monitoring practices, not all participants could provide all parameters based on our case definitions. Asking for the number of patients yielded the most answers: in total, 1,593 patients with C. difficile infection were reported from 49 participating facilities. The infection rates were analysed for each incidence calculation separately, including only hospitals that provided the variables needed for each calculation. To account for the heterogeneity in the completeness of responses, this resulted in nine different C. difficile infection incidences. The mean incidence rate was 3.8 (Poisson 95% CI: 3.2–4.5) C. difficile infection episodes per 10,000 patient-days for 2022. We observed significant variation in C. difficile infection incidence rates among hospital types (figure 2). When calculating incidence from lab-based C. difficile infection diagnoses, the rate increased to 4.2 (Poisson 95% CI: 3.3–5.1) lab-based C. difficile infections per 10,000 patient-days per hospital, indicating a more than 10% increase solely by altering the case definition in the numerator. A recurrence rate of 10.7% (153/1,425 patients) was calculated from the participants who provided data. Twenty-nine hospitals were able to stratify by community-associated C. difficile infection and healthcare-associated C. difficile infection. The majority used a simplified definition for healthcare-associated infections, with a hospitalization duration of >48 hours as a cut-off, resulting in 50.4% of C. difficile infections being classified as community-associated infections (442 of 860 C. difficile infections).
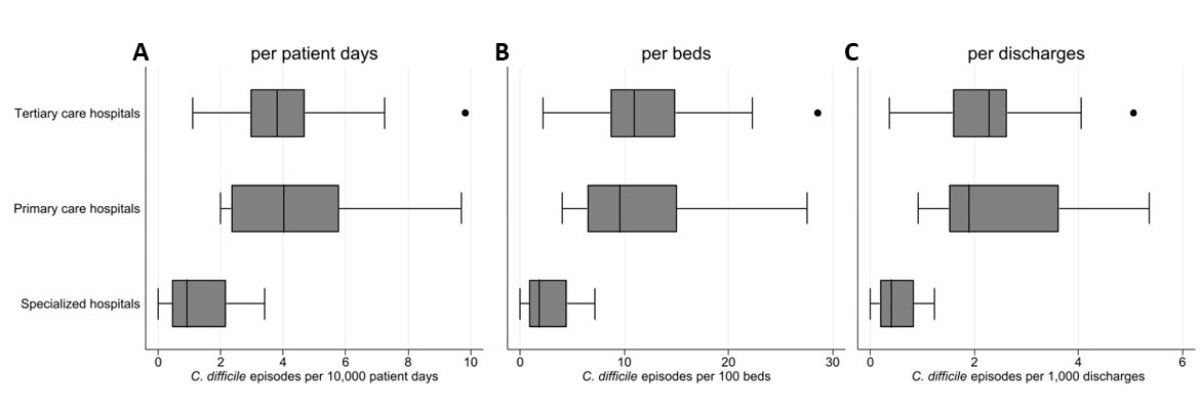
Figure 2The incidence of C. difficile infections stratified by different hospital types. A: C. difficile episodes per 10,000 patient-days; B: C. difficile episodes per 100 beds; C: C. difficile episodes per 1,000 discharges. Boxes represent the interquartile range (IQR), with the median denoted by the central line. Whiskers: Extend to the minimum and maximum values within 1.5 times the IQR. Outliers: individually marked points beyond this range. Each boxplot is derived from independent incidence calculators.
In our sample, surveillance and infection control measures were often institution specific and lacked nationwide consistency. Notably, isolation practices differed from international guidelines that generally recommend single-room isolation. Instead, Switzerland widely employs a risk-adapted approach, with half of the respondents indicating the use of additional individual criteria for isolation, primarily for infection with hypervirulent ribotypes or for uncooperative or incontinent patients.
Forty-three participants provided the numbers of laboratory tests for C. difficile conducted in 2022. The mean number of tests performed was 76.5 per 10,000 patient-days per hospital, with a mean positivity rate of 9.2% (table 1). Different diagnostic algorithms were reported. Overall, 58% of the laboratories followed internationally recommended diagnostic algorithms. The most common screening test was a glutamate dehydrogenase (GDH)–based enzyme immunoassay (EIA), either alone or in combination with toxin EIA, followed by a nucleic acid amplification test (NAAT) for discordant results. Initial screening with a NAAT was reported by 19.2% of respondents. Toxigenic culture and susceptibility testing were available in only a minority of hospitals (11.1%).
Table 1Mean C. difficile incidence rate in Swiss acute care hospitals in 2022.
| Measured indicator | Incidence per 10,000 patient-days (95% CI) | Incidence per 1,000 discharges (95% CI) |
| C. difficile episodes | 3.8 (3.2–4.5) | 2.1 (1.7–2.6) |
| Patients with C. difficile | 3.4 (2.9–4.0) | 1.9 (1.6–2.4) |
| Positive test results for C. difficile | 4.2 (3.3–5.1) | 2.4 (1.9–3.0) |
CI: confidence interval
This analysis provides the first nationwide estimate of C. difficile infection incidence in Switzerland, utilizing the most current data available. Our survey’s participation rate, which exceeded 50%, is considerably higher than the average national representation in the European ECDC survey, especially for countries without ongoing surveillance. Our results align with earlier annual reports of ICD codes for C. difficile in hospitalized patients that have been provided routinely by the Federal Statistical Office. Although these numbers cannot be directly compared to our results, they indicate that our sample adequately represented the burden of C. difficile infection infections in the Swiss healthcare system.
These results can serve as a baseline for estimating the burden of disease in Switzerland and for comparing infection rates in the European context. In the latest ECDC publication, a crude incidence density of 3.48 C. difficile infection cases per 10,000 patient-days was reported [14]; this closely resembles our survey’s rate of 3.83 (95% CI: 3.2–4.5) C. difficile infection episodes per 10,000 patient-days. Like in the European results, tertiary care hospitals exhibited the highest rates, at 3.87 C. difficile infection cases per 10,000 patient-days, while the group of specialized hospitals reported the lowest infection rates (figure 2). The recurrence rates observed, both in Switzerland (10.7%) and in the broader European context (12%), are lower than the 20–30% recurrence rates reported in earlier literature [7, 21].
Community-associated infections accounted for >50% of C. difficile infections (442 cases of community-associated C. difficile infection and 418 cases of healthcare-associated C. difficile infection). This finding is surprising, as C. difficile infection is traditionally considered a healthcare-associated pathogen, and there are few data available on C. difficile infection in ambulatory patients in Switzerland. However, most hospitals used a simplified definition of healthcare-associated C. difficile infection. International recommendations suggest that the infections of patients whose symptom onset occurred in the community but who had recently been hospitalized should be classified as healthcare-associated infections [22]. Swiss hospitals do not routinely monitor for previous hospitalizations, so this information was not readily available. The use of a simplified definition for healthcare-associated C. difficile infection therefore may have led to misclassification of cases. Nevertheless, such a large proportion of community-associated infections indicates that surveillance solely focused on hospitalized patients might lead to substantial underdiagnosis of C. difficile infection in the community by overlooking a substantial portion of infections.
Different tests are available for the detection of C. difficile,and the approach to testing varied across participants: only 58% adhered to internationally recommended multistep algorithms [23]. This figure is lower than the ECDC’s reported value of 76.8% [14]. Tertiary care hospitals exhibited the highest compliance, at 73.3%, contrasting with primary care hospitals, at 25%. The most common screening test was an EIA, often used with other testing methods if needed. EIAs are rapid, simple and inexpensive and, as part of a multistep algorithm, can accurately diagnose C. difficile infection. Participants employing testing strategies not recommended by guidelines were more likely to rely on NAAT alone for the diagnosis of C. difficile infection. NAATs are accurate tests for C. difficile infection, but, with their potential for overdiagnosis and higher costs, they should be interpreted only in conjunction with other parameters for C. difficile infection diagnosis. Notably, expertise in toxigenic culture, the reference test for C. difficile infection, was confined to a few laboratories in our survey. Cultures are a prerequisite for susceptibility testing and molecular typing of C. difficile strains, which is uniformly recommended by international surveillance programs [18, 19].
With the use of suboptimal testing, both under- and overreporting are concerns, as demonstrated in previous studies and ESCMID guidelines[23, 24]. Standardization of diagnostic approaches is needed to enhance the comparability and accuracy of results in future C. difficile infection surveillance efforts.
The lack of uniform national recommendations explains the heterogeneity in the management of C. difficile infection. Even larger hospitals used individual case definitions. While some institutions tracked symptomatic patients, others focused solely on patients with a first C. difficile infection, and a distinct subset employed automatic surveillance with positive test results (lab-based C. difficile infection). While most hospitals received notifications of positive C. difficile test results, only a modest 15.8% (9/57 responses) routinely investigated healthcare-associated transmission of C. difficile infections within their facilities. Notably, two tertiary hospitals reported episodes of increased infection rates, suggestive of potential transmission events, during the survey period.
Important limitations must be considered when interpreting our results. First, our sample did not include all hospitals; however, we had a high participation rate among larger hospitals. As these larger facilities care for most patients with C. difficile infections, we believe that our analysis represents the care of C. difficile infection patients very well. Comparing our sample with the numbers of ICD codes for C. difficile infection published by the Federal Statistical Office for previous years, our results seem plausible. Additional comparison and validation would be valuable once those statistics become publicly available for 2022. Second, the test algorithm was not reported by all participants, which could have altered our results. Third, the absence of clinical or outcome data precluded the assessment of mortality, risk factors and the proportion of preventable infections. We estimate that up to half the 1,593 C. difficile infection cases in 2022 could have been prevented through rigorous adherence to antibiotic stewardship and concomitant infection control measures, assuming a preventable percentage of 30% by antibiotic stewardship coupled with at least a 20% reduction through adequate infection control [9, 10].
Our evaluation of C. difficile infection in Swiss healthcare institutions underscores the relevance of C. difficile infection as a healthcare-associated infection, as well as the necessity for standardized surveillance methodologies. We propose the establishment of a continuous nationwide surveillance system, as outlined by the ECDC, to provide a basis for benchmarking and tailored interventions to reduce C. difficile infection rates. While the patient-related approach remains the benchmark, its reliance on substantial resources and annual reporting intervals poses inherent limitations. In contrast, laboratory-based surveillance allows for more frequent, potentially daily, reporting, thereby enabling hospitals and authorities to promptly address escalating C. difficile infection rates. As the prerequisites for an automated laboratory-based surveillance system already exist in Switzerland, now a larger database is required, and participation needs to be promoted among diagnostic laboratories. Continuous monitoring will empower authorities in assessing the burden of infections and evaluating whether additional strategies are needed. Laboratory-based surveillance has additional benefits for hospitals, including promptly detecting outbreaks and serving as a tool in antibiotic stewardship programs by correlating infection rates with antibiotic consumption.
A timely and concerted effort towards the establishment of a standardized surveillance program for Switzerland is essential to provide insights into the current C. difficile infection epidemiology and effective prevention strategies.
We thank the participating hospitals for data sharing.
This project is financially supported by the Federal Office of Public Health, Switzerland as part of the national initiative against antibiotic resistance (StAR-2). The contents are those of the authors and do not necessarily represent the official views of the FOPH and Swissnoso. The scientific work regarding this publication did not receive funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015 Feb;372(9):825–34. 10.1056/NEJMoa1408913
2. Burden of Clostridium difficile Infection in the United States. N Engl J Med. 2015;372(24):2368–70. 10.1056/NEJMc1505190
3. Durovic A, Widmer AF, Tschudin-Sutter S. New insights into transmission of Clostridium difficile infection-narrative review. Clin Microbiol Infect. 2018 May;24(5):483–92. 10.1016/j.cmi.2018.01.027
4. Feuerstadt P, Theriault N, Tillotson G. The burden of CDI in the United States: a multifactorial challenge. BMC Infect Dis. 2023 Mar;23(1):132. 10.1186/s12879-023-08096-0
5. Kenneally C, Rosini JM, Skrupky LP, Doherty JA, Hollands JM, Martinez E, et al. Analysis of 30-day mortality for clostridium difficile-associated disease in the ICU setting. Chest. 2007 Aug;132(2):418–24. 10.1378/chest.07-0202
6. Lim SC, Knight DR, Riley TV. Clostridium difficile and One Health. Clin Microbiol Infect. 2020 Jul;26(7):857–63. 10.1016/j.cmi.2019.10.023
7. McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999 Jan;20(1):43–50. 10.1086/501553
8. Johnson TM, Molina KC, Howard AH, Schwarz K, Allen L, Huang M, et al. Real-World Comparison of Bezlotoxumab to Standard of Care Therapy for Prevention of Recurrent Clostridioides difficile Infection in Patients at High Risk for Recurrence. Clin Infect Dis. 2022 May;74(9):1572–8. 10.1093/cid/ciab674
9. Dingle KE, Didelot X, Quan TP, Eyre DW, Stoesser N, Golubchik T, et al.; Modernising Medical Microbiology Informatics Group. Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis. 2017 Apr;17(4):411–21. 10.1016/S1473-3099(16)30514-X
10. Baur D, Gladstone BP, Burkert F, Carrara E, Foschi F, Döbele S, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017 Sep;17(9):990–1001. 10.1016/S1473-3099(17)30325-0
11. Marra AR, Perencevich EN, Nelson RE, Samore M, Khader K, Chiang HY, et al. Incidence and Outcomes Associated With Clostridium difficile Infections: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020 Jan;3(1):e1917597. 10.1001/jamanetworkopen.2019.17597
12. Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, et al.; ECDIS Study Group. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011 Jan;377(9759):63–73. 10.1016/S0140-6736(10)61266-4
13. van Dorp SM, Kinross P, Gastmeier P, Behnke M, Kola A, Delmée M, et al.; European Clostridium difficile Infection Surveillance Network (ECDIS-Net) on behalf of all participants. Standardised surveillance of Clostridium difficile infection in European acute care hospitals: a pilot study, 2013. Euro Surveill. 2016 Jul;21(29):
14. Dey A. ECDC - Annual epidemiological report for 2016-2017. Clostridioides (Clostridium) difficile infections. Annual epidemiological report for 2016–2017.
15. Tschudin-Sutter S, Kuijper EJ, Durovic A, Vehreschild MJ, Barbut F, Eckert C, et al.; Committee. Guidance document for prevention of Clostridium difficile infection in acute healthcare settings. Clin Microbiol Infect. 2018 Oct;24(10):1051–4. 10.1016/j.cmi.2018.02.020
16. McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar;66(7):e1–48. 10.1093/cid/cix1085
17. BFS. BFS - Bundesamt für Statistik Katalogdatenbanken: Federal office of Statistics Internet cited 2023 Oct 8. Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/katalogedatenbanken.assetdetail.23568235.html
18. CDC. https://www.cdc.gov/hai/eip/cdiff-tracking.html cited 2023 Oct 8.
19. ECDC. https://www.ecdc.europa.eu/en/clostridioides-difficile-infections Internet cited 2023 Oct 8. Available from: https://www.ecdc.europa.eu/en/clostridioides-difficile-infections
20. SurveyMonkey. San Mateo, California, USA: SurveyMonkey Inc.
21. Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, et al.; MODIFY I and MODIFY II Investigators. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med. 2017 Jan;376(4):305–17. 10.1056/NEJMoa1602615
22. Krutova M, Kinross P, Barbut F, Hajdu A, Wilcox MH, Kuijper EJ, et al.; survey contributors. How to: surveillance of Clostridium difficile infections. Clin Microbiol Infect. 2018 May;24(5):469–75. 10.1016/j.cmi.2017.12.008
23. Crobach MJ, Planche T, Eckert C, Barbut F, Terveer EM, Dekkers OM, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2016 Aug;22 Suppl 4:S63–81. 10.1016/j.cmi.2016.03.010
24. Gupta A, Cifu AS, Khanna S. Diagnosis and Treatment of Clostridium difficile Infection. JAMA. 2018 Sep;320(10):1031–2. 10.1001/jama.2018.12194