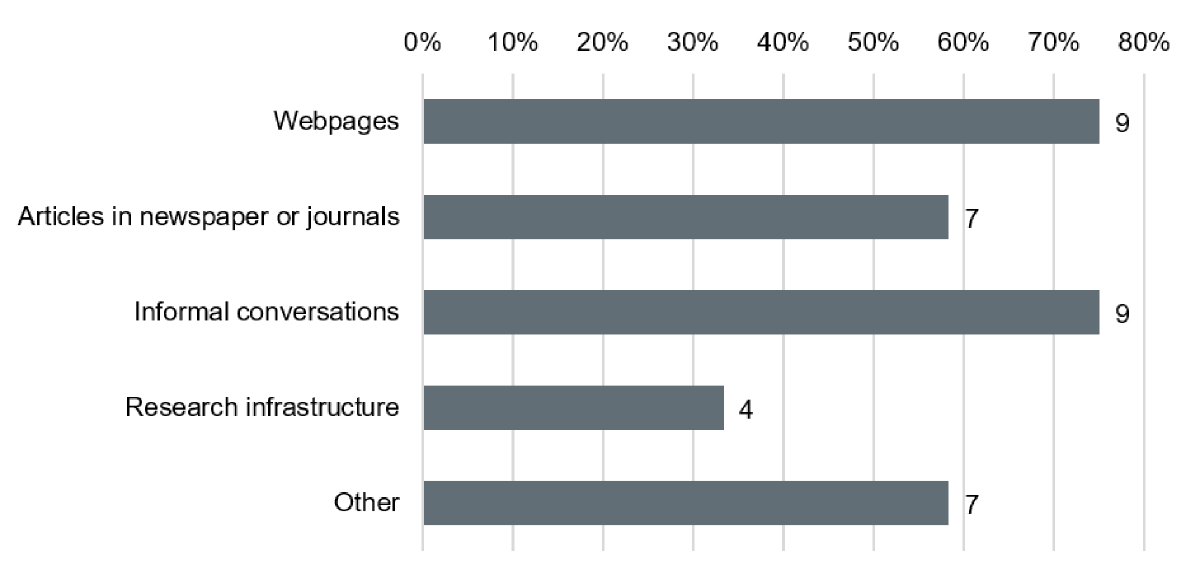
Figure 1Research sources for patient and public involvement (PPI) according to researchers. Resources used by Researchers (n = 12) to obtain information on PPI.
DOI: https://doi.org/https://doi.org/10.57187/s.3563
Academic Research Infrastructure
Clinical Trials Center
European Patients’ Academy on Therapeutic Innovation
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
National Institute for Health Research
Patient and Public Involvement
Swiss Clinical Trial Organisation
Swiss National Science Foundation
Strengths, Weaknesses, Opportunities, Threats
United Kingdom
Historically, clinical research has been characterised by a clear distinction between researchers as experts and the role of patients as passive trial participants [1]. However, in recent years this paradigm has shifted and patients are increasingly recognised as experts who can provide unique insights and inputs based on personal experience [1].
Patient and public involvement (PPI), also known as patient engagement [2], describes the active involvement of patients and the public in roles that go beyond their role as trial participants [1]. Patients participating in such active roles during a research project are often designated as patient representatives [3].
There are strong ethical and moral reasons for the active involvement of patients and the public in clinical research [1]. Further, PPI has been suggested to increase the quality of research [4] and may address several shortcomings related to the lack of a patient-centred approach in clinical research:
Using PPI approaches in the study design has been found to improve enrolment and decrease premature termination of trials [2], and may help to ensure that the kind and extent of diagnostic procedures reflect patient needs, that all study procedures are clearly related to the research question, that the setting is as close as possible to everyday clinical practice, and that study endpoints and outcomes are relevant to patients [4].
PPI can be integrated at various levels and stages of a research project, depending on the project’s objective, nature and the expertise of patient representatives [1]. In a consultative role, patient representatives are asked for their opinions but are not directly involved in decision-making. In collaborative partnerships, the researcher and the patient representatives work together in an ongoing process, engaging in shared decision-making. At the leadership level, patients themselves or patient organisations take an active role in directing research.
While PPI has gained significant momentum globally, particularly in countries like the UK, it remains a relatively new and evolving concept in other regions, including Switzerland. Therefore, the aim of this study was to evaluate the current state, opportunities and challenges of PPI in academic clinical research in Switzerland.
To assess the status quo and to identify opportunities and challenges of PPI in academic clinical research in Switzerland, we designed a sequential explanatory mixed-methods design [9]. As a first step, we conducted a stakeholder analysis followed by a survey among the different groups of interest [10] (see appendix). The results were then qualitatively explored and discussed in semi-structured interviews and depicted as a SWOT analysis to draft recommendations for the future of PPI in Swiss academic clinical research.
Our study was reported according to the STROBE guidelines (STrengthening the Reporting of OBservational studies in Epidemiology) [11].
This study did not use personal data from survey respondents. Therefore, it falls outside the scope of the Swiss Human Research Act and thus did not require ethics approval or informed consent.
The literature research for this study was conducted systematically using a combination of academic databases and supplementary sources. We used Swisscovery along with major databases such as MEDLINE, EMBASE, Web of Science Core Collection and Scopus to ensure comprehensive coverage of relevant literature. Additionally, open web searches were performed to capture more general information, and we also consulted the subject-specific journal Research Involvement and Engagement for targeted insights. This multi-source approach provided a robust foundation for the study, ensuring that the research was well-informed and aligned with current knowledge and practices in the field.
We conducted a stakeholder analysis to understand the interests, influence and needs of those involved in or affected by PPI in academic clinical research. This process involved several key steps: identifying relevant stakeholders, such as patients, healthcare professionals, researchers, policymakers and academic research infrastructures; grouping and categorising them by their level of involvement, power and influence and interest in PPI; and assessing their needs, expectations and potential impact. This analysis enabled us to develop targeted strategies for effectively engaging each group. The detailed stakeholder analysis is provided in the appendix.
The survey population consisted of different groups of interest, which were previously identified by stakeholder analysis: “Patients and Public”, “Researchers”, “Staff Members of Academic Research Infrastructure (ARI)” and “Regulatory and Funding Bodies” [10] (see appendix). For simplicity and because of their similar backgrounds and findings from the stakeholder analysis, “Staff Members of ARIs” and “Regulatory and Funding Bodies” were combined into a single group. Thus, there were three distinct groups in total.
We developed three German-language questionnaires based on the literature search to gather relevant data by aligning questions with research objectives and targeting specific stakeholder groups.
Although the surveys followed a similar structure, they were customised for each group: “Patients and Public” (14 questions), “Researchers” (15 questions) and “Staff Members of ARI, Regulatory and Funding Bodies” (10 questions). Each included a mix of closed-ended (e.g. multiple-choice, Likert scales) and open-ended questions. The questionnaires were organised into sections on general awareness, risks and benefits, challenges, PPI activities and implementation. A pilot test and clear instructions ensured user-friendliness and effective completion. Full surveys are available in the appendix. The patient survey was published on 22 March 2021 on the publicly accessible Clinical Trials Center (CTC) Zurich website (15), where it was available for one month to recruit patients. The second survey was promoted on the University Hospital Zurich (USZ) intranet, which is restricted to USZ staff and aimed at recruiting researchers.
For the stakeholder groups Researchers and ARI, Regulatory and Funding Bodies, prospective participants were also personally invited by email.
Microsoft Excel version 16.48 (Microsoft Corporation, Redmond, WA, USA) was used for descriptive analysis of the survey data. This involved importing and cleaning the data (missing data was handled by pairwise deletion), calculating summary statistics (frequencies, percentages, means and/or standard deviations) and categorising responses. We created visualisations, such as bar charts and pie charts, to illustrate patterns and trends. Excel’s pivot tables and conditional formatting helped identify key findings and anomalies, facilitating clear presentation and interpretation of the data.
To ensure a diverse and representative sample of interviewees, we used a multi-stage recruitment process. We first established criteria based on survey findings and study objectives, focusing on relevant expertise and diverse roles. Potential candidates were contacted via email with details about the study. After confirming eligibility and interest through preliminary screenings, we recruited and scheduled interviews with those who met the criteria. This process ensured a relevant and varied sample aligned with our research goals.
To develop the interview guidelines, we followed a systematic and iterative approach based on the survey results. We first analysed the survey data to identify key patterns, themes and insights, examining both quantitative trends and qualitative responses. Next, we pinpointed recurring issues and concerns highlighted by the survey respondents.
We then formulated open-ended questions designed to explore these themes in greater depth. To ensure clarity and relevance, we pilot-tested the questions with a small group of participants and refined them based on the feedback received. This approach allowed us to develop interview questions that effectively built on the survey findings and provided a comprehensive understanding of the research topic.
Interview partners received the questions three weeks in advance. The interviews, guided by these questions but not limited to them, were scheduled for one hour. With participants’ consent, interviews were audio-recorded and the recordings were deleted after the transcripts were completed. We conducted a qualitative content analysis of interviews by first transcribing and thoroughly reviewing the data. We then coded the text to identify key concepts and grouped these codes into broader themes. The themes were analysed in relation to our research objectives. To ensure consistency, we used peer review. Finally, we reported our findings with illustrative quotes from participants, providing a detailed and nuanced understanding of their experiences and perspectives.
Using a SWOT analysis [12], we classified the collected information into the four areas strengths, weaknesses, opportunities and threats. Findings from literature research were integrated into the internal analysis of inherent strengths and weaknesses of the concept of PPI in clinical research. Findings from the online survey and semi-structured interviews were integrated into the external analysis of opportunities and threats of PPI in Swiss academic clinical research.
The surveys yielded 123 responses including 42 (completion rate: 85%) from ARI, Regulatory and Funding Bodies, 39 (completion rate: 74%) from Researchers and 42 (completion rate: 52%) from Patients and Public.
Three semi-structured interviews were held with representatives from the SCTO, the SNSF as well as the Zurich cantonal ethics committee.
A vast majority (97.3%) of ARI, Regulatory and Funding Bodies endorsed the concept of PPI, but only half are familiar with the possibilities of PPI and only one third offered PPI services or launched initiatives to promote PPI. The main reasons mentioned for advocating PPI were the promotion of patient-relevant research and ethical reasons. Funding Bodies further confirmed their intentions that patients should be the focus of the funded research. During the semi-structured interview, the SNSF representative stated that the SNFS set PPI as a funding criterion and included patient representatives in the evaluation panel for investigator-initiated clinical trials (IICTs) in order to cope with that issue. The interviewee also confirmed that this step increased the evaluation panel’s sensitivity for patient-relevant research.
Almost two thirds (61.5%) of all Researchers were familiar with the term PPI. Also, half of Researchers (51%) were familiar with possibilities to include patient representatives in different activities along the research cycle and a majority of Researchers (76%) affirmed to be willing to educate themselves about PPI. Resources used for information were mainly webpages, informal conversations and articles (figure 1); however most Researchers required more easily accessible information about the practical implementation of PPI and support offers as well as contact opportunities to patient representatives. In addition, the need for exchange within and between stakeholder groups was expressed.

Figure 1Research sources for patient and public involvement (PPI) according to researchers. Resources used by Researchers (n = 12) to obtain information on PPI.
Only a minority of Patients and Public (31.0%) was familiar with the concept of PPI. However, the readiness to actively contribute was very high (95.7%) and a majority of Patients and Public (74%) affirmed to be willing to educate themselves about PPI.
The main goal of implementing PPI for Patients and Public as well as for Researchers was to foster the conduct of patient-relevant research (figure 2). Only a minority of Researchers stated to implement PPI as a requirement of funding bodies and none of them conducted PPI for the sole purpose of additional funding. Reasons for PPI selected by a majority of Patients and Public were finding new or improved therapies for own illness or illness of others and learning about the latest research activities and findings.
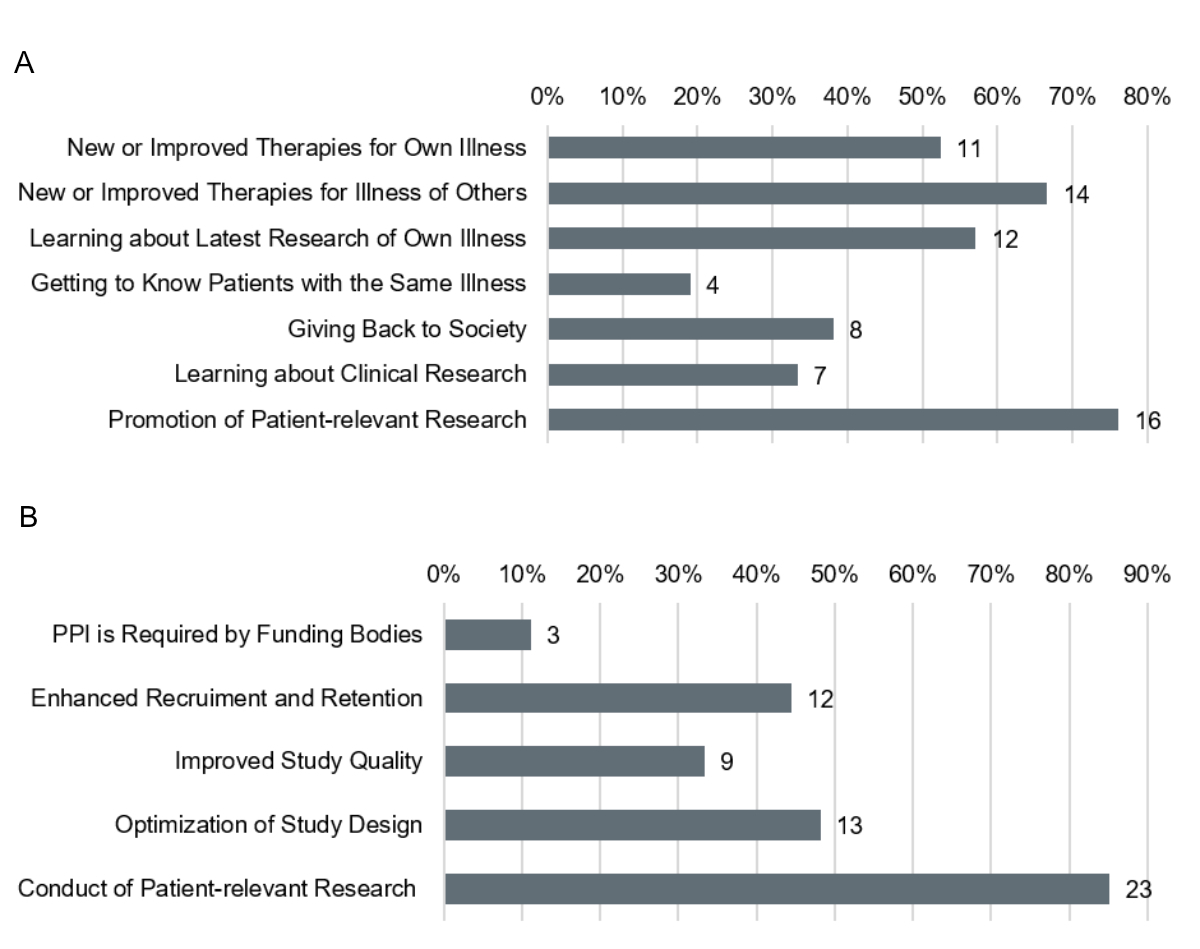
Figure 2Reasons for Patient and Public Involvement in Clinical Research. (A) Reasons of Patients and Public to engage as patient representatives (n = 21). (B) Reasons of Researchers for engaging patient representatives (n = 27).
During the interview with the SCTO representative, it was confirmed that there is still a lack of awareness about the concept of PPI. However, they also observed a growing interest in PPI among both researchers and the public. In response, the SCTO has made introductory information about PPI available on its website.
ARI, Regulatory and Funding Bodies identified the greatest benefits of PPI as the promotion of patient-relevant research and the strengthening of patient autonomy (figure 3a). No direct risks were attributed to improvements in study quality by involving patient representatives and to the democratisation of knowledge, but many participants took up a neutral stance. Similarly, many participants rated the optimisation of study resources, improvement of risk-benefit ratio of a research project as well as the protection of the rights and safety of study participants neutral. There existed some disagreement about the risk of jeopardising data protection of patient representatives’ personal and health data generated during PPI activities. Further risks identified during a semi-structured interview with a representative of an ethics committee were tokenistic approaches to PPI as well as the instrumentalisation of patient representatives.
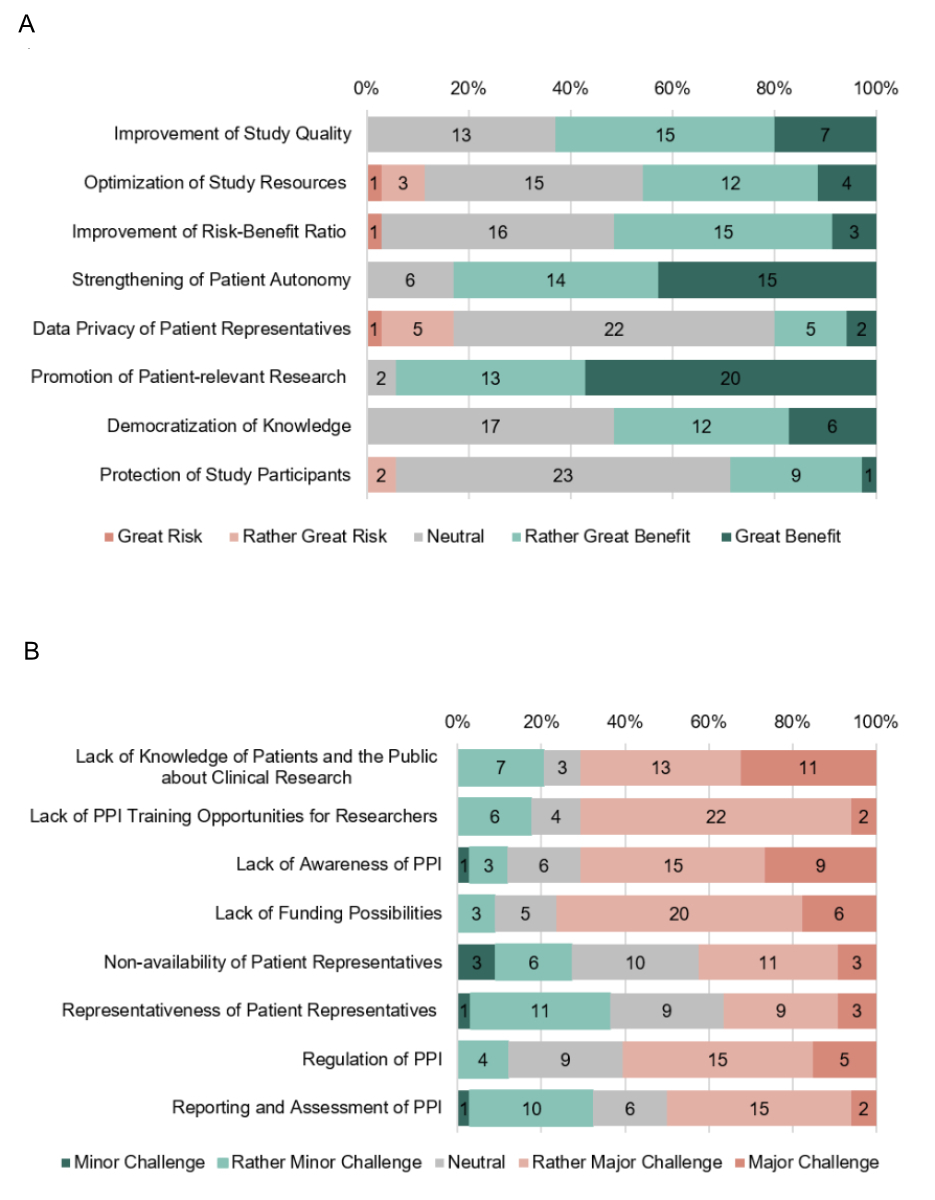
Figure 3Benefits, risks and challenges of Patient and Public Involvement (PPI) in clinical research. (A) Benefits and risks of PPI in clinical research, rated as great risk (red), rather great risk (light red), neutral (grey), rather great benefit (light green) and great benefit (green), judged by ARI, Regulatory and Funding Bodies in Switzerland (n = 35). (B) Challenges of PPI in clinical research, rated as minor challenge (green), rather minor challenge (light green), neutral (grey), rather major challenge (light red) and major challenge (red), judged by ARI, Regulatory and Funding Bodies in Switzerland.
ARI, Regulatory and Funding Bodies disagreed on the significance of the challenges associated with PPI (figure 3b). They considered the greatest challenge to be the lack of funding possibilities for PPI. Further identified as challenges by more than half of all participants were a general lack of awareness of the concept of PPI, unavailable education of patients and the public about clinical research and researchers about PPI, as well as missing PPI regulations. The SCTO representative explained in the semi-structured interview that the lack of education possibilities for patient representatives was currently being tackled by the SCTO in collaboration with the Swiss European Patients’ Academy on Therapeutic Innovation (EUPATI CH) to develop a training module for patient representatives. The representative of an ethics committee also deemed regulations to be crucial in order to avoid tokenistic approaches and to have a validated impact. He also considered the lack of definitions of the role and responsibilities of a patient representative a fundamental gap. A further challenge mentioned was the declaration and regulation of conflicts of interest of patient representatives.
Survey participants selected activities considered implementable in PPI (figure 4).
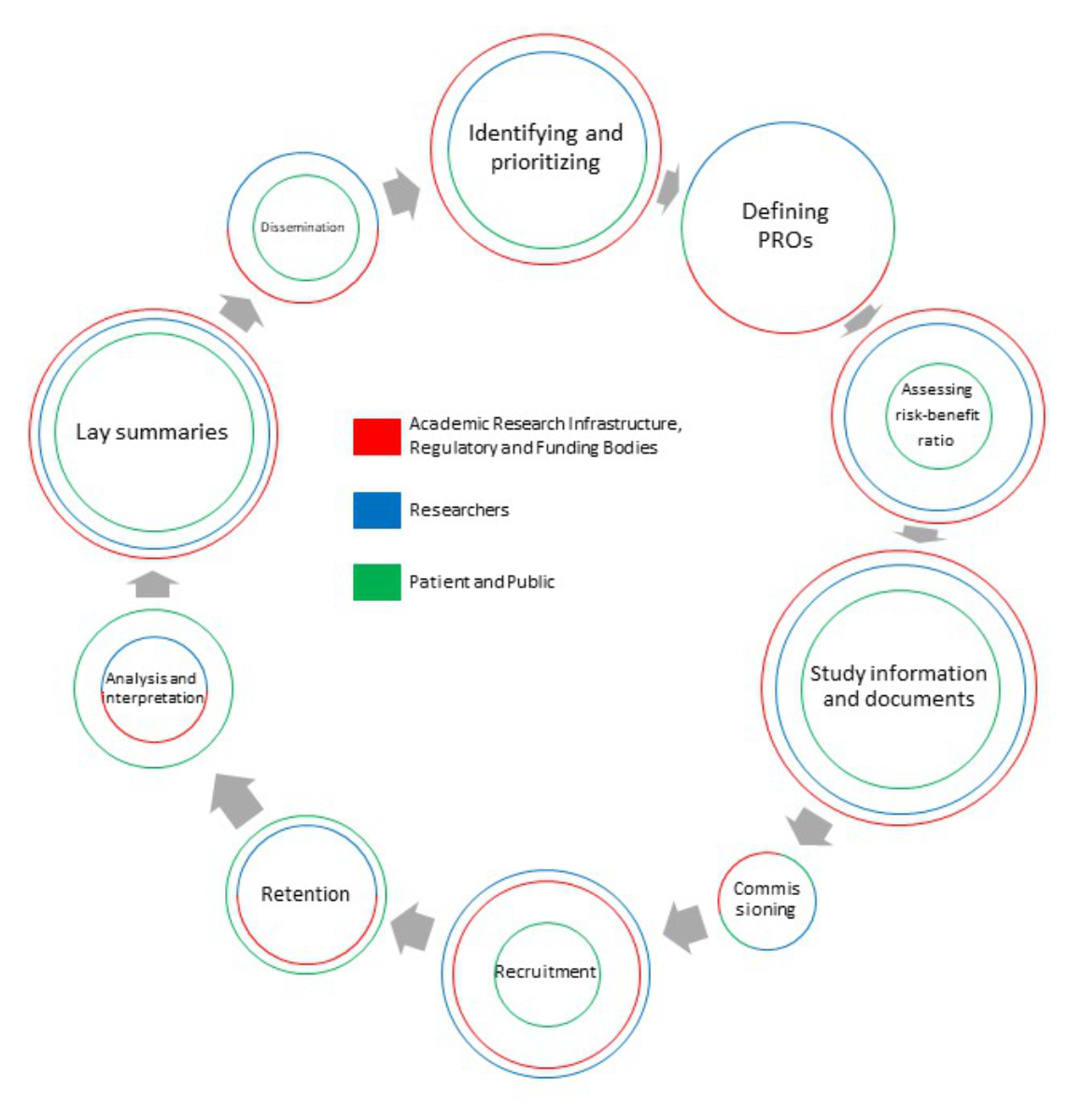
Figure 4PPI activities. Results of ARI, Regulatory and Funding Bodies (red) / Researchers (blue) / Patients and Public (green): Actively participating in the development of research questions (76.5% / 65.5% / 65.5%). Defining patient-reported outcomes (PROs ) (70.6% / 69.0% / 65.5%). Assessing the risk-benefit ratio of a study (70.6% / 62.2% / 34.8%). Compiling study information and documents in lay language (91.2% / 82.8% / 56.5%). Commissioning studies (32.4% / 34.5% / 30.4%). Recruiting study participants (61.8% / 69.0% / 34.8%). Retaining study participants (47.0% / 44.8% / 52.2%). Analysing and interpreting data from a patient perspective (35.3% / 34.8% / 52.2%). Summarising study results as lay summaries (82.4% / 75.9% / 65.5%). Disseminating study results (44.1% / 48.3% / 34.8%).
ARI, Regulatory and Funding Bodies largely supported the following activities: compiling study information and documents in lay language, summarising study results as lay summaries, actively participating in the development of research questions, defining patient-reported outcomes and assessing the risk-benefit ratio of a study. ARI, Regulatory and Funding Bodies did not support the following activities: commissioning studies, analysing and interpreting data from a patient perspective, disseminating study results and retaining study participants. This opinion was shared by most Researchers. Only a minority of Researchers stated that involvement of patient representatives in their own research projects was not conceivable at all.
In the Patients and Public group, more than half endorsed active participation in developing research questions, defining patient-reported outcomes, developing lay summaries, compiling study information and documents in lay language, retaining patients, and analysing and interpreting data from the patient perspective. Patients and Public did not consider it to be their role to support commissioning of studies, assessment of risk-benefit ratio, recruitment of patients and dissemination of study results. Only one survey participant was not ready to participate in any PPI activity. Reasons were lack of time and knowledge about clinical research as well as direct and indirect costs linked with the engagement.
Half (52.6%) of all Researchers have already, to some extent, involved patient representatives in their research. Overall, the experience of involving patient representatives was mixed. Positive feedback included that research projects were more meaningful, feasible and accepted by patients. Critical voices stated that PPI complicated the planning and conduct of the research project and that PPI was a lengthy and resource-intensive process. The latter was confirmed in semi-structured interviews and all interview partners agreed that more-efficient PPI processes are needed in Switzerland.
Out of 42 Patients and Public survey participants, only one had already engaged as a patient representative in clinical research. The participant had a mixed experience. The main point of criticism was the unstructured approach to involving patient representatives, but overall the representative’s input was appreciated by the research team.
Most Researchers could imagine consulting patient representatives on selected topics and matters and only a minority could imagine involving patient representatives at a collaboration or leadership level. Patients and Public however were equally willing to engage at a consultation, collaboration or leadership level (figure 5a). Most Researchers and Patients and Public would also devote at least several days or evenings a year to PPI activities (figure 5b).
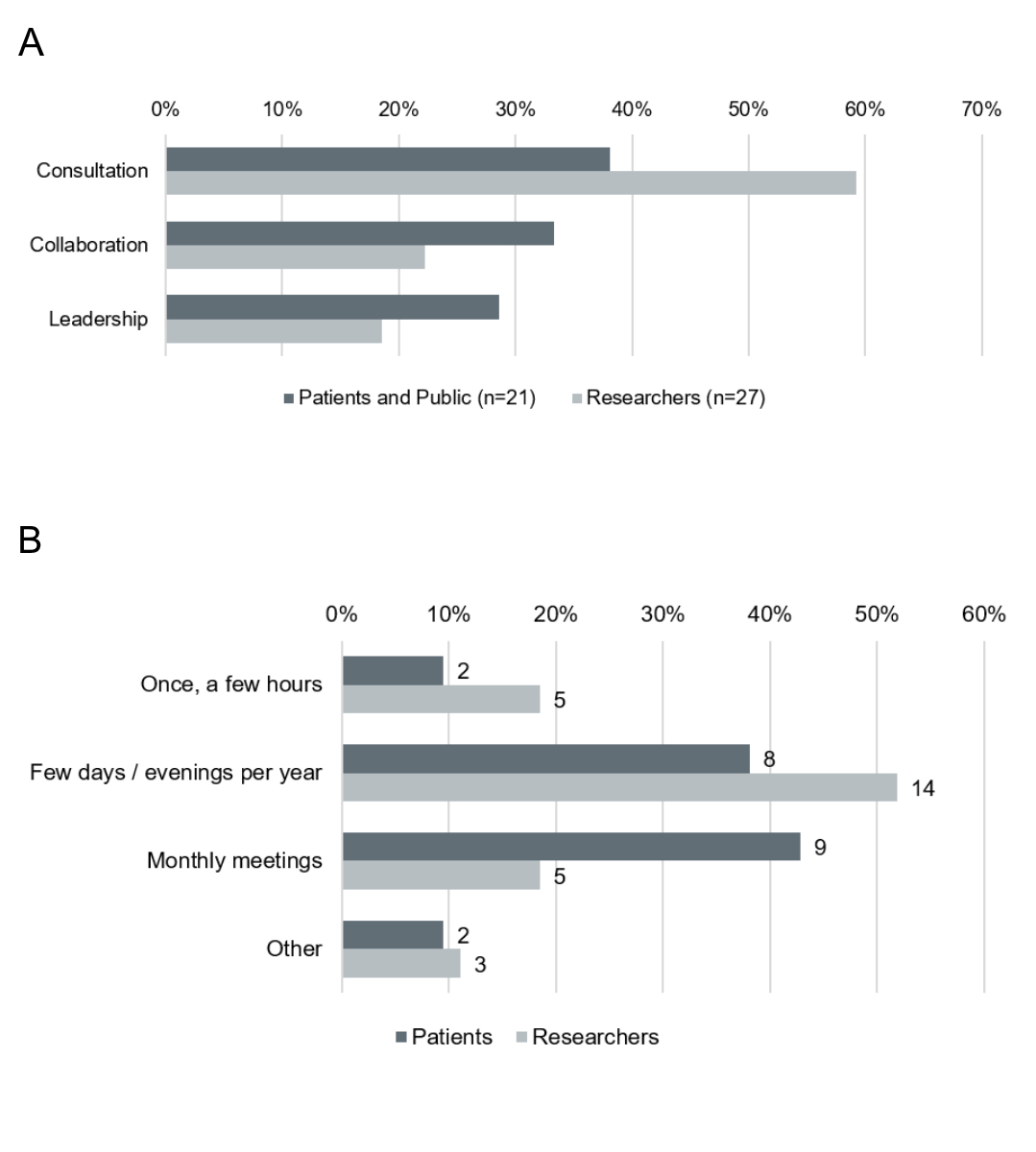
Figure 5Level of involvement for PPI. (A) Desired level of involvement of Patients and Public (n = 21) (grey) and Researchers (n = 27) (light grey). (B) Possible expenditure of time for PPI activities of Patients and Public (n = 21) (grey) and Researchers (n = 27) (light grey).
Survey results are summarised and depicted as a SWOT analysis in figure 6. The internal analysis summarises strengths and weaknesses of the concept of PPI. A literature search has shown that strengths of PPI include a strong ethical rationale and the adoption of a new paradigm of seeing patients as experts [1]. Thereby, patient-centredness and relevancy in clinical research can be increased as well as public understanding of clinical research [1]. Overall, PPI can increase health literacy and thus also informed decision-making [1]. Further, PPI presents an approach to solve present issues in clinical research and can thereby increase research quality [4–8]. Weaknesses include the lack of clear PPI methodology [13], and that PPI is time- and resource-intensive for both financial and personnel resources [1].
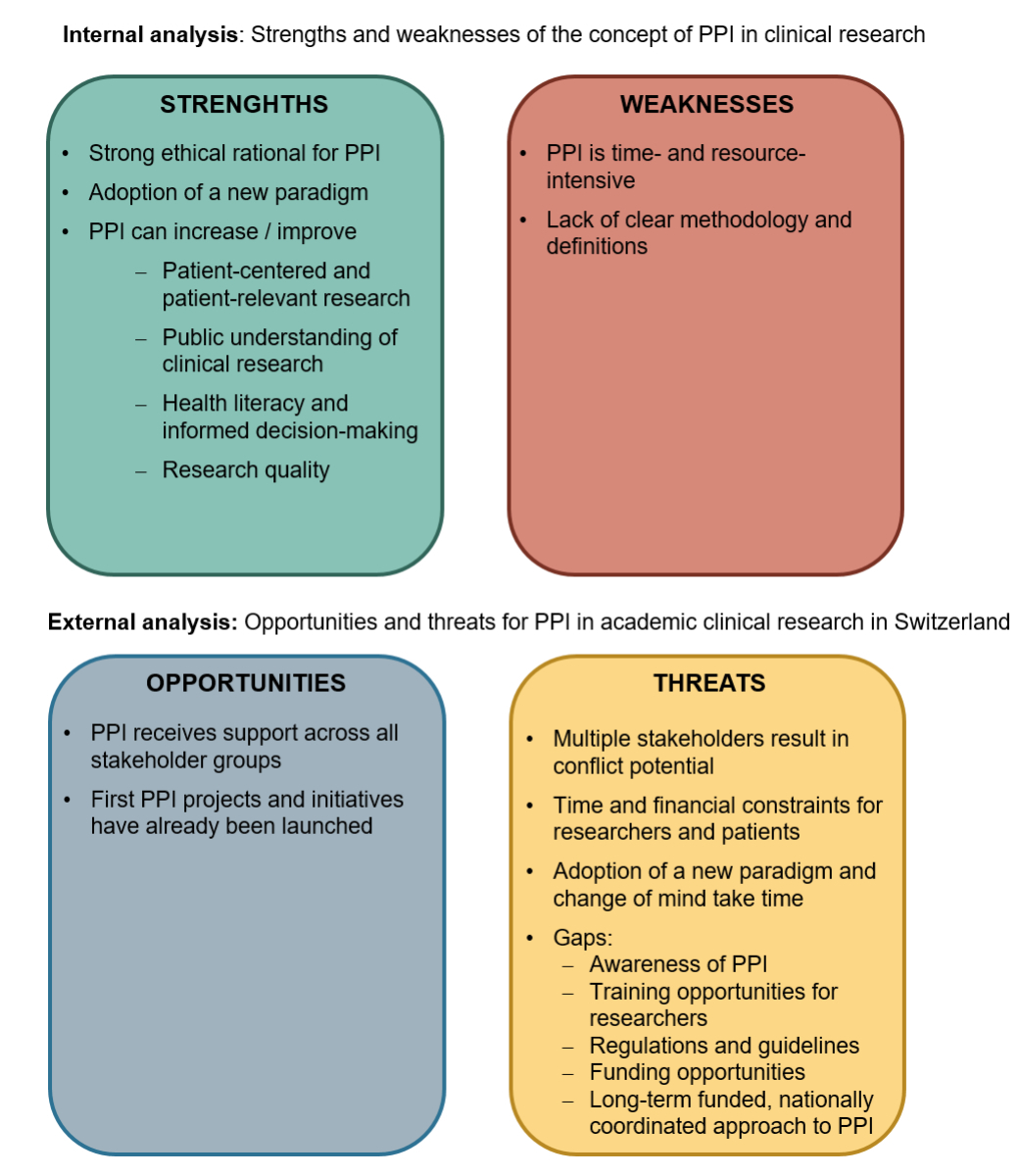
Figure 6Results of the SWOT analysis, split into internal (strengths and weaknesses) and external (opportunities and threats) analyses.
The external analysis summarises opportunities and threats of PPI in Swiss academic clinical research. Opportunities to promote PPI show in the survey results, as PPI is supported across all stakeholder groups and its importance is being recognised. Further, the first PPI projects and initiatives have already been launched and allow Swiss stakeholders to collaborate and to define common understandings, which strengthens the relevance of Swiss academic clinical research. Threats include a general lack of awareness of the concept of PPI as well as multiple stakeholders involved in the processes, which results in conflict potential. This conflict potential is enhanced by the fact that PPI imposes additional time and financial expenses for researchers and patients, and the lack of appropriate funding opportunities to cover such expenses. Training opportunities are not only essential for educating about PPI methodology to enable meaningful cooperation, but also for bringing about the change in mindset required to see patients as experts. This mindset shift could also be supported by providing a regulatory basis including guidelines as well as a long-term funded, nationally coordinated approach to PPI.
The surveys showed that research organisations, researchers and the public in Switzerland generally highly supported the concept of PPI and that they were willing to actively contribute. Also, survey participants agreed that PPI can bring great benefits to various aspects of clinical research. In addition, some organisations and researchers were already implementing PPI or establishing PPI initiatives. However, there was no established nationwide initiative to support and promote PPI on a large scale.
The divergent opinions on the significance of PPI might not only be based on different perspectives and priorities, but also on the lack of coordination and overview of PPI initiatives and support offers. A coordinated approach is also suggested by interview partners as well as the Swiss Academy of Medical Sciences [14]. The SCTO has plans to streamline PPI processes by creating a Swiss PPI Hub, which would act as a contact point for all stakeholders.
The survey also revealed that Patients and Public are insufficiently informed about the general concept of PPI. In comparison, Researchers were better informed, but more than one third of all Researchers still had not yet heard of PPI. This was confirmed by ARI, Regulatory and Funding Bodies, which rated the lack of awareness of PPI as one of the major challenges. These findings further support the need for a central point of contact as well as widespread, easily accessible and understandable information for all stakeholder groups.
The lack of funding for PPI was rated the most challenging issue. After this survey was completed, the SNSF announced the newly created opportunity for researchers to apply for a preparatory PPI grant to cover reimbursement and compensation of patient representatives before receiving an SNSF investigator-initiated clinical trial grant [15]. Further initiatives are needed to close this gap for other grant applications.
Trial discontinuation due to poor recruitment was found to be rather common for SNSF-founded randomised controlled trials [16]. The funding criterion PPI as well as the involvement of patient representatives in the commissioning of research grants could lead to better success rates of SNSF-supported investigator-initiated clinical trials.
Reasons for the involvement of or engagement as patient representative were diverse and differed between Researchers and Patients and Public. The overarching motivation for both groups however was the promotion of patient-relevant research, which was also confirmed by the interviewees.
Also the feasibility of PPI activities in clinical research was rated differently by the stakeholders. Overall, involving patient representatives to support the compilation of documents in a lay-friendly way was rated to be the most valuable activity. This is a promising basis for the mandatory publication of lay summaries of study results of clinical trials with medical devices in Switzerland [17]. With the upcoming revision of the ordinances of the Swiss Human Research Act, all studies subject to the Clinical Trials Ordinance (ClinO) will be required to publish their results in the form of a lay summary [18].
Lack of regulations and guidelines for PPI was identified as a major challenge. Available documents do not sufficiently regulate PPI activities and according to the Federal Council there are no intentions to legally anchor involvement processes [19].
Even though there is no legal framework for PPI itself in Switzerland, there are other regulations to consider, e.g. federal and cantonal data protection acts as well as general data protection principles [20]. To avoid tokenistic approaches to PPI, it should be clearly defined what role and responsibility a patient representative is expected to cover. It is thus crucial that the SCTO and its CTU network is focusing on establishing guidelines, best practices, recommendations as well as training and consulting opportunities.
All interview partners urged that a cultural change in Swiss academic clinical research is needed to sustainably implement PPI. Also, survey results suggest Researchers might not yet be ready to accept patients at higher engagement levels. Patients and Public were evenly ready to engage at a consultation, collaboration and leadership level, whereas the majority of Researchers preferred only to consult with patient representatives. Also a lack of time and insufficient knowledge on the Researchers’ side about how to involve patient representatives at a higher engagement level and the possible benefits thereof might be a reason. To sustainably promote this mindset shift, widespread training, including PPI methodology for researchers as well as long-term funding for communication and coordination, is needed.
The SWOT analysis shows great inherent strengths of the concept of PPI that outweigh the weaknesses. In contrast, threats outweigh opportunities for the situation of PPI in Switzerland. This confirms insufficient frame conditions present in Switzerland. Other countries in comparable early PPI stages as Switzerland, e.g. Australia or Japan, report similar struggles such as lack of financial and staff resources and a gap in training opportunities [21, 22]. In contrast, other countries have established national PPI initiatives to support and promote PPI in clinical research. In the UK, the NIHR has launched the Centre for Engagement and Dissemination, which brought together INVOLVE, its former national advisory group, and the Dissemination Centre [23]. The Canadian Institute of Health Research launched Canada’s Strategy for Patient-Oriented Research that aims to fund research that is important to patients and to create hubs to bring stakeholders together [24]. The Patient-Centered Outcome Research Institute is a non-governmental organisation in the USA that was established to fund clinical effectiveness research with patient-relevant outcomes [25]. Besides national initiatives, the ICH has addressed PPI in a reflection paper and announced compilation of two guidelines focusing on patient-centred outcomes as well as PPI methodology [26].
In this study, we applied a thorough methodology that combined a literature search with surveys and semi-structured interviews. This approach allowed for direct effects at the University Hospital Zurich by initiating a PPI project and facilitated an in-depth exploration of stakeholder experiences. These efforts led to the identification of specific milestones and the creation of a roadmap towards the improved establishment of PPI in Switzerland.
Despite these strengths, several limitations were noted. The surveys were only available in German, which excluded a portion of Switzerland’s population. Additionally, there was a low completion rate for the Patients and Public surveys, likely due to the lengthy and detailed section on PPI activities, where most participants discontinued. This highlighted the importance of layperson-adjusted language in terms of length and complexity.
Another potential bias was introduced by placing the survey banner on the CTC Zurich webpage. However, this also proved to be a strength, as it allowed for participation beyond personally invited respondents, potentially resulting in a more diverse sample.
Finally, while the number of semi-structured interviews was not representative, we carefully selected interview partners due to the limited number of experts available in Switzerland. These interviews provided valuable insights and a detailed understanding of the key issues PPI faces, contributing significantly to the overall objectives of this study.
In general, this study showed great support for PPI across all stakeholder groups, but the Swiss framework for PPI is in need of improvement. Basic standards for PPI in Swiss clinical research should be implemented, including regulations and guidelines for PPI as well as widespread information for patients, the public and researchers. Further, training opportunities in PPI concepts for clinical research as well as a sustainable source of funding for PPI are required. A joint effort by all stakeholders is needed to keep the momentum going and to catch up with international PPI developments to match high-level ethical and quality standards.
Surveys and survey results can be provided upon reasonable request.
Authors’ contributions: DE, AB, RG were involved in the conception of the survey. DE analysed the data and drafted the tables and graphics. DE and AB wrote the manuscript. RG supervised the project. RG, JR, CL reviewed the manuscript.
We thank Francisca Jörger, Karin Huber, Anja Eskat and Gabriela Senti for reviewing the survey. Additionally, we would like to thank Peter Kleist and Frederique Lachmann for reviewing the manuscript and sharing their valuable thoughts and input.
No funding was received for this study.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1.Schilling I, Herbon C, Jilani H, Rathjen KI, Gerhardus A. [Patient and public involvement in clinical research: an introduction] [ZEFQ]. Z Evid Fortbild Qual Gesundhwes. 2020 Sep;155:56–63. doi: https://doi.org/10.1016/j.zefq.2020.06.007
2.Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014 Feb;14(1):89. doi: https://doi.org/10.1186/1472-6963-14-89
3.Klingmann I, Heckenberg A, Warner K, Haerry D, Hunter A, May M, et al. EUPATI and patients in medicines research and development: guidance for patient involvement in ethical review of clinical trials. Front Med (Lausanne). 2018 Sep;5:251. doi: https://doi.org/10.3389/fmed.2018.00251
4.Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014 Oct;17(5):637–50. doi: https://doi.org/10.1111/j.1369-7625.2012.00795.x
5.Crowe S, Fenton M, Hall M, Cowan K, Chalmers I. Patients’, clinicians’ and the research communities’ priorities for treatment research: there is an important mismatch. Res Involv Engagem. 2015;1:1–10.
6.Sacristán JA, Aguarón A, Avendaño-Solá C, Garrido P, Carrión J, Gutiérrez A, et al. Patient involvement in clinical research: why, when, and how. Patient Prefer Adherence. 2016 Apr;10:631–40. doi: https://doi.org/10.2147/PPA.S104259
7.Kasenda B, von Elm E, You J, Blümle A, Tomonaga Y, Saccilotto R, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014 Mar;311(10):1045–51. doi: https://doi.org/10.1001/jama.2014.1361
8.Briel M, Elger B, von Elm E, Satalkar P. Insufficient recruitment and premature discontinuation of clinical trials in Switzerland: qualitative study with trialists and other stakeholders. Swiss Med Wkly. 2017 Nov;147:w14556. doi: https://doi.org/10.4414/smw.2017.14556
9.Nicolau B, Castonguay G, Levine A, Hong QN, Pluye P, Afrashtehfar KI, et al. Applied mixed methods in oral health research: importance and example of a training program. JDR Clin Trans Res. 2017;2(3):206–10. doi: https://doi.org/10.1177/2380084417705823
10.Varvasovszky Z, Brugha R. A stakeholder analysis. Health Policy Plan. 2000 Sep;15(3):338–45. doi: https://doi.org/10.1093/heapol/15.3.338
11.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007 Oct;4(10):e296. doi: https://doi.org/10.1371/journal.pmed.0040296
12. Kotler P, Berger R, Bickhoff N. The Quintessence of Strategic Management - What You Really Need to Know to Survive in Business. The Quintessence of Strategic Management. Berlin, Heidelberg: Springer-Verlag; 2010.
13.Fergusson D, Monfaredi Z, Pussegoda K, Garritty C, Lyddiatt A, Shea B, et al. The prevalence of patient engagement in published trials: a systematic review. Res Involv Engagem. 2018 May;4(1):17. doi: https://doi.org/10.1186/s40900-018-0099-x
14.Swiss Academy of Medical Sciences (SAMS). White Paper: Clinical Research. Swiss Academies Communications 16, (2021).
15.Investigator initiated clinical trials (IICT). https://www.snf.ch/en/hyaJhBlfsmd618fO/funding/programmes/iict (2022).
16.Amstutz A, Schandelmaier S, Frei R, Surina J, Agarwal A, Olu KK, et al. Discontinuation and non-publication of randomised clinical trials supported by the main public funding body in Switzerland: a retrospective cohort study. BMJ Open. 2017 Aug;7(7):e016216. doi: https://doi.org/10.1136/bmjopen-2017-016216
17. Swiss Federal Council. Ordinance on Clinical Trials with Medical Devices of 1 July 2020 (Status as of 26 May 2021). (2020).
18. Federal Office of Public Health. Teilrevision Des Ausführungsrechts Zum Bundesgesetz Über Die Forschung Am Menschen (HFG) - Erläuternder Bericht. (2023).
19. Schweizerische Eidgenossenschaft. Patientenrechte und Patientenpartizipation in der Schweiz. Bericht in Erfüllung der Postulate 12.3100 Kessler, 12.3124 Gilli und 12.3207 Steiert. (2015).
20. Eberle, D. & Hansen, M. M. B. PPI in Switzerland: A regulatory perspective. Regulatory Affairs Watch 4–7 (2021).
21.McKenzie A, Bowden J, Zalcberg JR, Conroy K, Fallon-Ferguson J, Jesudason S, et al. A snapshot of consumer engagement in clinical trials in Australia: results of a national survey of clinical trial networks and research organisations. Res Involv Engagem. 2022 Feb;8(1):3. doi: https://doi.org/10.1186/s40900-022-00338-w
22. Tanemura, N., Sasaki, T., Sato, J. & Urushihara, H. Real World Survey of Patient Engagement Status in Clinical Research: The First Input from Japan. The Patient - Patient-Centered Outcomes Research 13, 623–632 (2020).
23.NIHR - National Institute for Health Research. NIHR launches new Centre for Engagement and Dissemination. https://www.nihr.ac.uk/news/nihr-launches-new-centre-for-engagement-and-dissemination/24576 (2020).
24.Canadian Institutes of Health Research. Strategy for Patient-Oriented Research. https://cihr-irsc.gc.ca/e/41204.html (2021).
25.Patient-Centered Outcomes Research Institute. PCORI - Patient-Centered Outcomes Research Institute. https://www.pcori.org (2021).
26. ICH Reflection paper. Proposed ICH Guideline Work to Advance Patient Focused Drug Development This. (2021).
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3563.