Transcatheter aortic valve implantation with SAPIEN 3 versus surgical aortic valve
replacement in patients with symptomatic severe aortic stenosis at low risk of surgical
mortality: a cost-utility analysis for Switzerland
DOI: https://doi.org/https://doi.org/10.57187/s.3558
Christophe Alain Wyssa,
Roberto Cortia,
Thomas Nestelbergerb,
Pascal Candolfic,
Alexis Delbaerec*,
Barbara Fischerd,
Matthias Schwenkglenkse,
Harry Telserdf
a University of Zurich, HerzKlinik Hirslanden, Zurich, Switzerland
b University of Basel, Basel,
Switzerland
c Edwards
Lifesciences, Nyon, Switzerland
d Polynomics
AG, Olten, Switzerland
e Health Economics Facility,
Department of Public Health, University of Basel,
Basel, Switzerland
f Center
for Health, Policy and Economics, University of Lucerne, Lucerne, Switzerland
* Alexis Delbaere was an employee of
Edwards Lifesciences when this work was performed but now works for Cell-Easy,
Toulouse, France
Summary
AIMS OF THE STUDY: The 2021 European Society
of Cardiology Guidelines on valvular heart disease recommend transcatheter aortic
valve implantation (TAVI) for patients with symptomatic severe aortic stenosis at
low surgical risk and age ≥75 years who are suitable for a transfemoral approach
(recommendation class IA) based on two large randomised controlled trials (PARTNER
3 and Evolut Low Risk) comparing transcatheter aortic valve implantation with surgical
aortic valve replacement (SAVR). Whether such an approach is cost-effective in Switzerland
remains unclear. The aim of this cost-utility analysis was to compare transcatheter
aortic valve implantation with SAPIEN 3 versus surgical aortic valve
replacement in symptomatic severe aortic stenosis patients at low risk of surgical
mortality from the perspective of Swiss compulsory health insurance using data from
the PARTNER 3 trial (reflecting specifically the safety and efficacy of the SAPIEN
3 TAVI device).
METHODS: A previously published two-stage Markov-based
model that captured clinical outcomes from the PARTNER 3 trial was adapted from the
perspective of the Swiss compulsory health insurance system, using local or geographically
close general population mortality and utility data, unit costs and medical resource
use from publicly available sources and based on expert opinion. The model had a
lifetime horizon with a 3% yearly discounting factor. The cost–utility analysis
estimated changes in both direct healthcare costs and health-related quality-adjusted
life years for transcatheter aortic valve implantation compared with surgical
aortic valve replacement in patients with symptomatic severe aortic stenosis at
low risk of surgical mortality.
RESULTS: Overall, transcatheter aortic
valve implantation with SAPIEN 3 resulted in lifetime costs per patient of CHF 79,534
and quality-adjusted life years per patient of 9.64, compared with surgical
aortic valve replacement lifetime costs and quality-adjusted life years per patient
of CHF 76,891 and 8.96, respectively. Compared with surgical aortic valve
replacement, transcatheter aortic valve implantation was estimated to offer an incremental
improvement of +0.68 quality-adjusted life years per patient at an increased cost
of +CHF 2643 per patient over a lifetime horizon. The incremental cost-effectiveness
ratio was CHF 3866 per quality-adjusted life year gained and remained below CHF
50,000 per quality-adjusted life year gained across several sensitivity analyses.
CONCLUSIONS: This analysis suggests that transcatheter
aortic valve implantation using the SAPIEN 3 device is likely to be a highly cost-effective
alternative for symptomatic severe aortic stenosis patients at a low risk of surgical
mortality, treated in the contemporary Swiss setting. These findings may help to
inform a holistic approach when making policy decisions for the management of this
patient group.
Abbreviations
- CHF:
-
Swiss franc
- EQ-5D(-5L):
-
European Quality of Life 5
Dimensions (5 Level Version)
- ESC:
-
European Society of Cardiology
- PARTNER 3 trial:
-
Placement of Aortic
Transcatheter Valves 3 trial
- SAVR:
-
surgical
aortic valve replacement
- TAVI:
-
transcatheter
aortic valve implantation
Introduction
Severe aortic
stenosis is a common valvular disease [1]
with survival probabilities as low as 50% at two years and 20% at five years [2] without
valve replacement. Since its introduction in 2002, transcatheter
aortic valve implantation (TAVI) has become the treatment of choice for the treatment
of symptomatic severe aortic stenosis in elderly and high-risk surgical patients
[3, 4]. Continuous development of the technology
improved patients’ outcomes, patients’ quality of life and reduced complication
rates, leading to an unprecedented expansion towards lower-risk patient populations,
namely intermediate-risk and low-risk patients [5–8].
The Placement of Aortic Transcatheter Valve
Study (PARTNER) 3 trial was a multicentre randomised controlled study in patients
with symptomatic severe aortic stenosis considered at low risk of surgical mortality
[9–11]. In this study, transfemoral TAVI using the SAPIEN
3 transcatheter heart valve (Edwards Lifesciences) was compared to surgical aortic
valve replacement
(SAVR) [9–11]. TAVI reduced
the composite outcome of death, stroke or rehospitalisation compared with SAVR after
2 years (11.5% vs 17.4%; hazard ratio [HR]: 0.63; 95% confidence interval: 0.45–0.88;
p = 0.007) [9, 10] and after 5 years (22.8%
vs 27.2%; HR: 0.79; 95% confidence interval: 0.61–1.02; p = 0.07) [11] but slightly
short of statistical significance. TAVI also resulted in significantly lower rates
of stroke and new-onset atrial fibrillation (AF), shorter index hospitalisation,
higher functional status and improved quality of life, at 30 days. Last, there were
no significant between-group differences in major vascular complications, new permanent
pacemaker insertions, or moderate or severe paravalvular regurgitation [9–11].
Based on the clinical benefits of TAVI versus
SAVR in patients with symptomatic severe aortic stenosis across all risk groups,
the latest European Society of Cardiology (ESC)/European Association for Cardio-Thoracic
Surgery (EACTS) guidelines on valvular heart disease recommend TAVI in all patients
aged 75 years or older who are suitable for a transfemoral approach, regardless
of the degree of surgical risk (recommendation class IA) [5, 12].
ESC guidelines were endorsed by the Swiss Society
of Cardiology, and the Swiss compulsory health
insurance scheme (on the legal basis of the Federal Health Insurance Act [13]) recently
added coverage for the TAVI procedures
in patients with symptomatic severe aortic stenosis at low surgical risk in their
latest policy – effective from 1 July 2023. The regulations state that there should
be mandatory coverage for inoperable, high- and intermediate- surgical risk patients,
and provisional coverage regarding evidence development for those at low risk. Criteria
for reimbursement in Switzerland are
based on efficacy, effectiveness, expediency and economic efficiency. While both
efficacy and expediency were convincingly shown in previous randomised controlled
trials, cost-effectiveness largely depends on national tariffs and prosthesis prices,
and thus requires a dedicated cost-utility analysis.
Such evaluations have already been performed
in various countries, with publications in France [14], Italy [15], Spain [16], Germany
[17],
Belgium [18] and the Netherlands [19] all showing the cost-effectiveness of TAVI
with SAPIEN 3 compared with SAVR; however data for Switzerland is lacking.
We thus aimed to conduct a cost-utility analysis
comparing TAVI with SAPIEN 3 with SAVR in symptomatic severe aortic stenosis patients
at low risk of surgical mortality from the perspective of Swiss compulsory health
insurance, using data from the PARTNER 3 trial and other relevant sources.
Methods
A cost-utility analysis was built using methodology
validated for the French [14], Italian [15], Spanish [16],
German [17], Belgian [18] and Dutch [19] populations to estimate changes in both direct
healthcare costs and health-related quality
of life with the use of TAVI with SAPIEN 3 versus SAVR in symptomatic severe
aortic stenosis patients at low risk of surgical mortality (<4% as defined by
the Society of Thoracic Surgeons [STS]) from the perspective of the Swiss compulsory
health insurance system. Ethical approval of research was not required as this cost-utility
analysis was based on data from previously conducted studies and did not include
any new studies with human participants.
Model structure
Details of the two-stage model structure and the rationale of the four distinct
health states have been described previously [14]. In brief, survival, quality of
life, costs
and early adverse events (AEs) linked to the TAVI procedure were captured using
the 30-day AEs dataset from the PARTNER 3 study [10] in a decision tree (figure 1A).
This data was then fed into a Markov model that
included four distinct health states (“alive and well”, “treated
AF”, “disabling stroke” and “dead”) to capture
longer-term outcomes of patients, post-TAVI or post-SAVR intervention (figure 1B).
The model was considered appropriate for the Swiss context by the authors, based
on their clinical and health economics expertise.
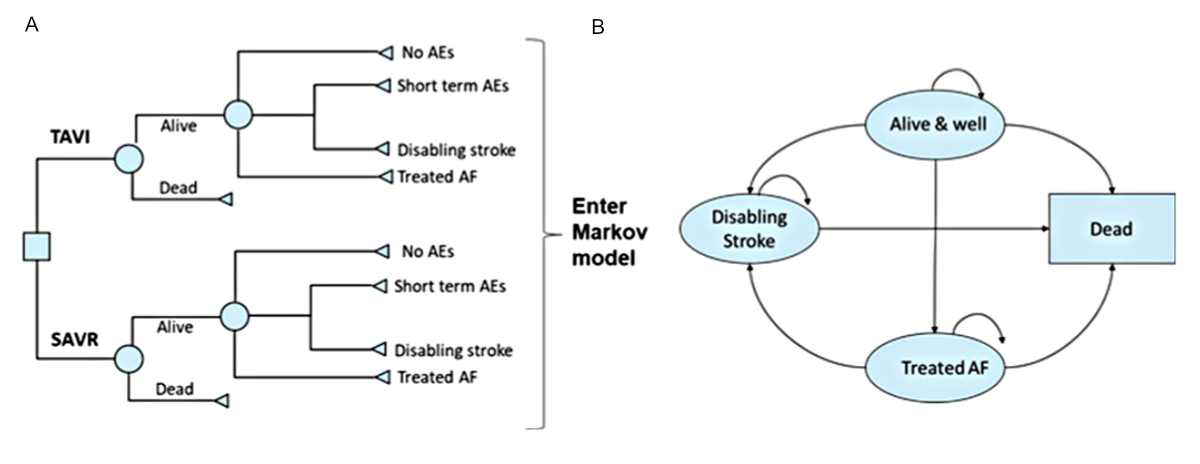
Figure 1The
cost-effectiveness model had two stages: (A) early adverse events (AE)
from the PARTNER 3 trial were captured in a decision tree, which fed into (B)
a Markov model that captured longer-term outcomes of patients, with four
distinct health states: “Alive and well” = patients have
undergone the procedure and survived with only short-term or no AEs; patients
in this health state can transition to “disabling stroke”, “treated
AF” or “dead” at any point during the model time horizon. “Treated
AF” = patients have undergone the procedure and survived but developed atrial
fibrillation (AF) requiring specific treatment; this can either occur within
the first 30 days or during the rest of the time horizon of the model, and
patients in this health state can transition to “disabling stroke” or “dead” at
any point during the model time horizon. “Disabling stroke” =
patients have undergone the procedure and survived but had a disabling stroke;
this can either occur within the first 30 days or during the rest of the time
horizon of the model, and patients in this health state can only transition to
the “dead” state at any point during the model time horizon. “Dead” is
the absorbing state in the model: all patients in the model are at risk of
dying due to general all-cause mortality; patients with “treated
AF” and “disabling stroke” are at an increased risk of dying. SAVR:
surgical aortic valve replacement; TAVI: transcatheter aortic valve
implantation. Reproduced from Gilard M, et al. [14]. Value Health 2021doi: https://doi.org/10.1016/j.jval.2021.10.003, under the terms of
the Creative Commons licence “Attribution 4.0 International”.
Considering that the initial treatment decision
has long-term consequences and that symptomatic severe aortic stenosis requires
life-long valve replacement, a lifetime horizon (50 years) was selected for the
cost-utility analysis. This time horizon was chosen to reflect all possible consequences
in individuals with symptomatic severe aortic stenosis over their lifetime.
A discounting factor per year of 3% was applied
for both future costs and benefits. Such a discount rate is frequently used in Health
Technology Assessment (HTA) reports for the Federal Office of Public Health (FOPH)
and is thus accepted by the FOPH [20]. Details
for input variable definitions have been published previously [10, 14] and are summarised
in the sections below.
The cost-utility model generated total per-patient
costs and quality-adjusted
life years for each intervention and the incremental cost-effectiveness
ratio for TAVI compared with SAVR. Output definitions can be found at www.yhec.co.uk/glossary/.
For non-experts of
economic evaluation, a reader’s guide to facilitate reading and interpretation is
recommended [21].
Study overview
The model was informed by the study
population of PARTNER 3 (ClinicalTrials.gov number: NCT02675114), a multicentre
randomised clinical trial that compared TAVI with transfemoral placement of a third-generation
balloon-expandable
valve with standard SAVR in patients with symptomatic
severe aortic stenosis who were considered at low risk of mortality from surgery
(STS-Predicted Risk of Mortality [STS-PROM] score <4%). The trial protocol was designed
by the
trial sponsor (Edwards Lifesciences) and the steering committee, with guidance from
the Food and Drug Administration (FDA). The sponsor funded all trial-related activities
and participated in site selection, data collection and monitoring, and statistical
analyses. Patients with clinical frailty, bicuspid aortic
valves or other anatomical features that increased the risk of complications associated
with either surgery or transcatheter aortic valve implantation were excluded. In
PARTNER 3, 1000 patients were enrolled, of whom 503 were randomised to TAVI and
497 to SAVR, with “as treated” groups of 496 and 454 patients, respectively [10].
The trial comprised patients with an average
age of 73 years and 69% of patients were male.
All-cause mortality was determined from general
population normal mortality risk, with relative risks applied from published literature
corresponding to each health state. Costs and resources used were based on costing
information from Swiss Diagnosis-Related Groups (DRGs), regional tariffs, literature
and expert interviews. Utility values used age-adjusted population norms from Germany
in the absence of robust Swiss population norms [22],
with decrements (disutilities) applied from published literature corresponding to
each health state.
Clinical events
Probabilities of clinical events, such as health
state transition probabilities, rehospitalisation rates, aortic reintervention rates
and intercurrent events (such as myocardial infarction, bleeding and transient ischaemic
attack), used in the model were sourced from the PARTNER 3 trial and from Swiss-specific
literature sources when available and relevant (table S1 in the appendix). Monthly
transition probabilities between health states for the Markov model were estimated
based on data from PARTNER 3 (up to 5-year outcomes) or other literature sources
where there were too few events in PARTNER 3 for reliable estimates (table S1).
Input data for permanent pacemaker insertion at 30 days was based on PARTNER 3 data
for SAVR [10] and estimates from the Swiss
TAVI Registry [23] to reflect more recently
available SAPIEN 3 TAVI data specific to the Swiss population. Rehospitalisation
rates were based on data from the PARTNER 3 study up to 5 years [9–11] and assumed
to remain constant over the time
horizon of the model thereafter. Reintervention rates were also based on data from
the PARTNER 3 study up to 5 years [9–11] and
by competing risk estimates for the 73-year-old cohort from a study by Bourguignon
et al. from Year 6 onwards [24]. The same
reintervention rate was used for both TAVI with SAPIEN 3 and SAVR in the base case;
this simplifying assumption allowed best use of the available data.
Survival extrapolation
All-cause mortality was determined from general
population normal mortality risk, with relative risks applied from published literature
corresponding to each health state. In the base case, transition probabilities were
taken from the literature (compared to the general population, relative risks of
death with “treated AF” and “disabling stroke” are 1.46 and 2.30, respectively) due
to immaturity
of survival data from the PARTNER 3 trial producing clinically implausible estimates
(because of the very low rate of death in the study [9–11]). Annual mortality risk
for “alive and well” and other relative risks for other health states
are shown in table S2 in the appendix.
Health utilities
The PARTNER 3 trial collected EQ-5D-based utilities;
however, given that few clinical events were observed, we decided it was more appropriate
to consider estimates from the literature. We used age-specific utility values representing
population norms from Germany in the absence of Swiss population norms covering
all language regions [22]. Disutilities by
health state were calculated as weighted averages of disutilities in neighbouring
countries, namely Germany [17], France [14] and Italy [15],
with weights based on the distribution of main languages in Switzerland, as reported
in the Structural Survey of the Federal Statistical Office (FSO). The resulting
disutilities were 0.14 for “treated AF” and 0.38 for “disabling
stroke” (table S3 in the appendix).
Cost inputs
Costs were based on costing information from
Swiss Diagnosis Related Groups (DRGs), regional tariffs and literature. Costs associated
with TAVI and SAVR (procedure, complications and long-term) are shown in table 1.
Base case procedure cost information was drawn from a composite of SwissDRG version
13.0 AG 2024 [25]: F98B and F98C (TAVI); F03C
and F03E (SAVR). The breakdown of TAVI and SAVR procedure costs is shown in table
S4 in the appendix. For pacemaker complication costs, in the absence of Swiss-specific
data we used data from a German study [31].
To adjust the costs to the Swiss price level, we used purchasing power parity corrections
(Germany: 1.544 for 2020 and 1.490 for 2021 [32]).
All costs were adjusted to 2022 Swiss franc (CHF) using the Consumer Price Index.
Table 1Costs associated with transcatheter aortic valve implantation and surgical
aortic valve replacement (procedure, complications, long-term).
| Unit cost
components |
Transcatheter
aortic valve implantation with SAPIEN 3 |
Surgical aortic
valve replacement |
Source |
| Procedure |
Intervention |
CHF 45,211 |
CHF 36,099 |
Composite of
SwissDRG Version 13 [25]: F98B and F98C
(TAVI); F03C and F03E (SAVR). |
| Rehabilitation |
CHF 4934 |
CHF 9422 |
SwissDRG ST
Reha Version 1.0 / 2022 [26]. |
| Associated with
health state |
Treated AF – month 1 |
CHF 6649 |
SwissDRG AG
2021 [25]: F50B and F50C; + cost of
anticoagulation drug and beta-blocker; + outpatient costs calculated as per
TARMED [27], expert interview. |
| Treated AF ≥ month 2 |
CHF 93 |
Cost of
anticoagulation drug (20 mg Xarelto) and beta-blocker (5 mg Bilol),
assumption of one tablet each per day [28]. |
| Disabling
stroke – month 1 |
CHF 20,662 |
Pletscher et
al. 2013 [29]. |
| Disabling
stroke ≥ month 2 |
CHF 3648 |
| Alive and well
– Year 1 (per month) |
CHF 103 |
CHF 34 |
TARMED [27], expert interview (CHF 413 per check-up
for echocardiography, consultation and report. Assumption of one check-up per
year; with TAVI, three check-ups in the first year). |
| Alive and well
– year 2+ (per month) |
CHF 34 |
CHF 34 |
| Other costs considered |
Myocardial
infarction |
CHF 8924 |
CHF 8924 |
Reinhold et al.
2011. Value adjusted to inflation rate (Dec 2020) using the following
converter: [30]. |
| Pacemaker
procedure |
CHF 13,176 |
CHF 13,176 |
SwissDRG AG
2021 [25]: F17A. |
| Pacemaker complications
(monthly) |
CHF 329 |
CHF 329 |
SwissDRG AG
2021 [25]: F17A + TARMED [27], expert interview + Ludwig et al. 2019 [31]. |
| Rehospitalisation |
CHF 9259 |
CHF 9259 |
SwissDRG AG
2021 [25]: F62A, F62B, F62C, F62D. |
| Reintervention |
CHF 50,145 |
CHF 50,145 |
Assumed equal
to cost of initial procedure plus rehabilitation associated with procedure. |
Cost-effectiveness threshold per quality-adjusted
life year
In the absence of an official willingness-to-pay
threshold for Switzerland, we assumed the cost-effectiveness threshold to be CHF
50,000 per quality-adjusted life year gained.
Sensitivity and scenario analyses
To evaluate uncertainty, 1-way deterministic
sensitivity analyses were performed by varying inputs using confidence intervals
and ranges from the literature when available, and plausible ranges when data was
unavailable (appendix table S5). All parameters were changed and the impact on the
results explored. Overall parameter uncertainty was addressed using a probabilistic
sensitivity analysis (PSA). Probability distributions for all input parameters were
specified and 1000 Monte Carlo simulations were run using random draws of all parameters
from within their assigned distributions (appendix table S6).
Finally, several scenario analyses were performed
to account for uncertainties not captured by the standard sensitivity analyses.
The impact of increased risk of reintervention was explored in Scenario
1, based on data at 5 years from the PARTNER 2 trial [7]. Scenarios 2 and 3 considered
parametric survival fitting based on Kaplan-Meier data from the PARTNER
3 trial, utilising various HRs. Among the three parametric distributions considered
(Weibull, Exponential, Gompertz), the Weibull was best in terms of goodness-of-fit
statistics, minimising the Akaike information criterion (AIC) and the Bayesian information
criterion (BIC), and was adjusted to the survival of the overall Swiss population.
In Scenario 2, the HR from the PARTNER 3 trial at two years (HR = 0.75) was used
and adjusted to the Swiss population overall mortality. Scenario 3 removed any survival
benefit with the SAPIEN 3 valve (HR = 1). Scenario 4 considered utility decrements
for each treatment arm from the PARTNER 3 trial – individually extracted at baseline,
after 30 days, 6 months and 1 year [33].
Scenarios 5 and 6 considered various costing estimates (SAVR based on minimal
invasive tariff and assuming inpatient rehabilitation only). Lastly, Scenarios
7 to 11 looked at various model time horizons (from 5 to 30 years). All analyses
were performed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA).
Results
Base case
Compared with SAVR, TAVI is estimated to offer
significant benefits by increasing quality-adjusted life years (incremental improvement
of +0.68 per patient) at a slightly increased cost (+CHF 2643 per patient) over
a lifetime horizon. This represents an incremental cost-effectiveness ratio of CHF
3866 per quality-adjusted life year gained. Overall, TAVI with SAPIEN 3 resulted
in lifetime costs per patient of CHF 79,534 and lifetime quality-adjusted life
year per patient of 9.64; SAVR in CHF 76,891 and 8.96 quality-adjusted life
years respectively.
The estimated incremental
cost-effectiveness ratio is much lower than the considered highly-cost-effectiveness
threshold of CHF 50,000 per quality-adjusted life year gained (table 2). Further
examination of the breakdown of costs for TAVI with SAPIEN 3 versus SAVR revealed
that, despite initial higher procedural costs in the model with TAVI, costs with
respect to “disabling stroke”, “treated AF” and “rehospitalisation” were lower
(figure 2).
Table 2Base case results with acute and lifetime costs.
| Summary results
|
Transcatheter aortic
valve implantation with SAPIEN 3 |
Surgical aortic
valve replacement |
Incremental |
| Cost per
patient |
CHF 79,534 |
CHF 76,891 |
CHF 2643 |
| Life years
gained (undiscounted) |
14.99 |
14.44 |
0.55 |
| Life years
gained (discounted) |
11.67 |
11.29 |
0.38 |
| Median survival
(years) |
17.83 |
15.92 |
1.92 |
| Quality-adjusted
life years per patient |
9.64 |
8.96 |
0.68 |
| Incremental
cost-effectiveness ratio (ICER)* |
CHF 3866 |
| Acute phase
cost (first hospitalisation and rehabilitation) |
Index
hospitalisation |
CHF 45,211 |
CHF 36,099 |
CHF 9112 |
| Rehabilitation
(inpatient and outpatient) |
CHF 4934 |
CHF 9422 |
–CHF 4488 |
| Acute phase costs |
CHF 50,145 |
CHF 45,521 |
CHF 4624 |
| Additional
costs at 1 year |
Myocardial
infarction |
CHF 135 |
CHF 117 |
CHF 18 |
| Costs of
pacemaker complications |
CHF 457 |
CHF 139 |
CHF 318 |
| Costs of
rehospitalisations |
CHF 645 |
CHF 947 |
–CHF 313 |
| Reintervention
costs |
CHF 224 |
CHF 250 |
CHF 2 |
|
| “Alive and well”
health state costs |
CHF 1153 |
CHF 255 |
CHF 898 |
| “Treated atrial
fibrillation” health state costs |
CHF 389 |
CHF 2785 |
–CHF 2397 |
| “Disabling
stroke” health state costs |
CHF 21 |
CHF 303 |
–CHF 283 |
| Total costs at 1 year |
CHF 53,168 |
CHF 50,314 |
CHF 2854 |
| Additional
lifetime costs |
Costs of
pacemaker complications |
CHF 5474 |
CHF 1617 |
CHF 3857 |
| Costs of
rehospitalisations |
CHF 1626 |
CHF 1476 |
CHF 150 |
| Reintervention
costs |
CHF 10,774 |
CHF 10,149 |
CHF 624 |
|
| “Alive and well”
health state costs |
CHF 3694 |
CHF 2674 |
CHF 1020 |
| “Treated atrial
fibrillation” health state costs |
CHF 2223 |
CHF 4611 |
–CHF 2388 |
| “Disabling
stroke” health state costs |
CHF 2574 |
CHF 6050 |
–CHF 3475 |
| Additional lifetime costs |
CHF 26,365 |
CHF 26,577 |
–CHF 212 |
| Total lifetime costs |
CHF 79,534 |
CHF 76,891 |
CHF 2643 |
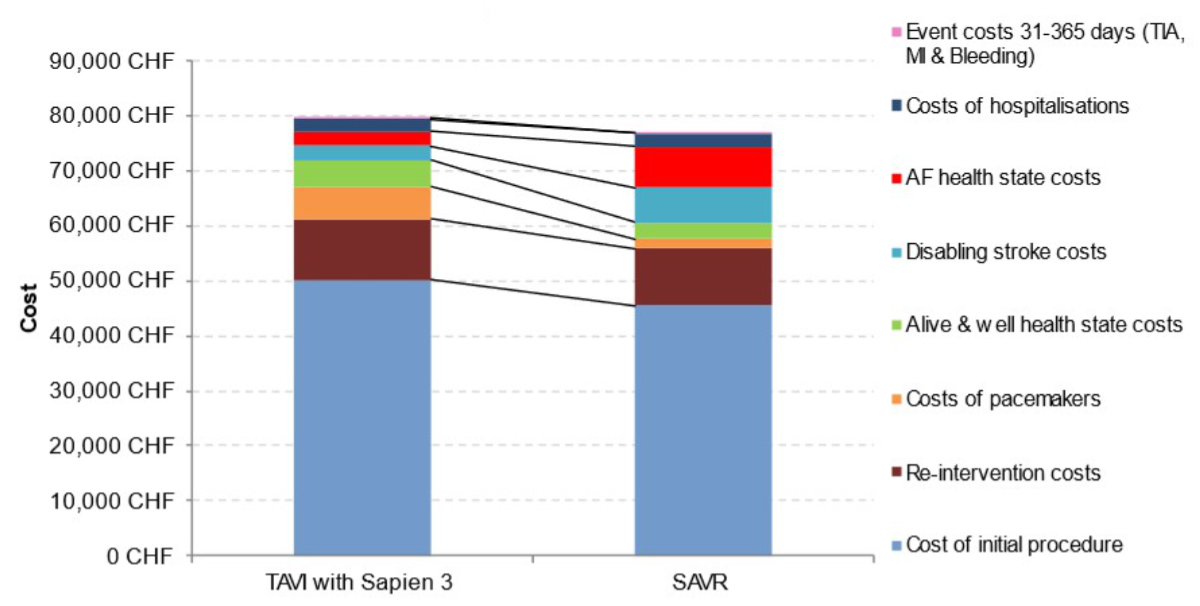
Figure 2Cost
breakdown for transcatheter aortic valve implantation (TAVI) with SAPIEN 3 and
for surgical aortic valve replacement (SAVR). AF: atrial
fibrillation; CHF: Swiss franc; MI: myocardial infarction; TIA: transient ischaemic
attack.
Probabilistic sensitivity analyses
The findings of the PSA corroborate those of
the base case analysis. At the considered highly-cost-effectiveness threshold of
CHF 50,000/quality-adjusted life year, TAVI with SAPIEN 3 remained cost-effective
compared with SAVR in 99.9% of simulations (figure 3). Even at a lower threshold
of CHF 30,000/quality-adjusted life year, TAVI with SAPIEN 3 still had a high probability
(97.7%) of being cost-effective (figure 4).
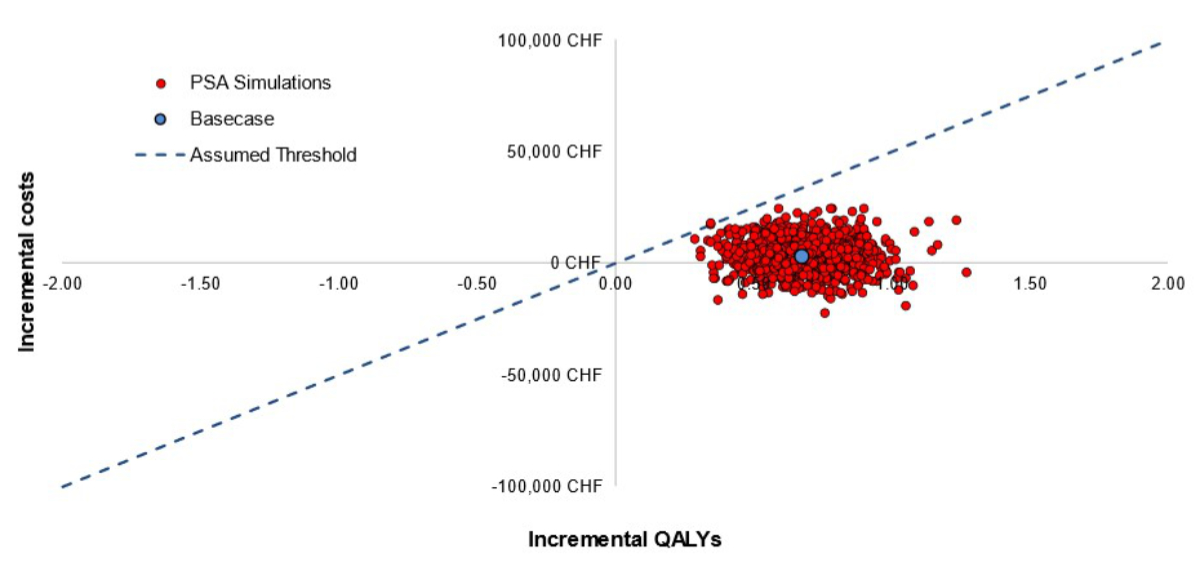
Figure 3Probabilistic sensitivity analysis (PSA): Cost-effectiveness scatter plot. “Assumed
threshold” is the willingness-to-pay threshold that corresponds to Swiss francs (CHF)
50,000
per quality-adjusted life year (QALY) gained. The scatter plot is shown on a
cost-effectiveness plane. The cost-effectiveness plane plots incremental QALYs
against incremental costs for each probabilistic simulation. As an example,
simulations in the top-right quadrant represent simulations in which transcatheter
aortic valve implantation (TAVI) is more costly and more effective than
surgical aortic valve replacement (SAVR).
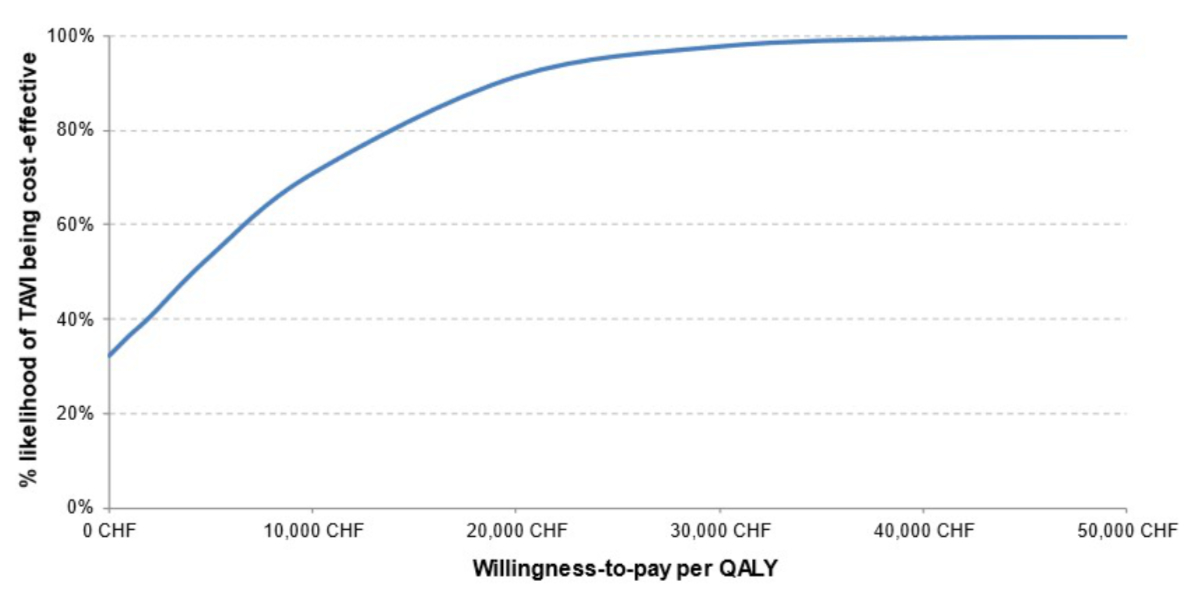
Figure 4Probabilistic sensitivity analysis: Cost-effectiveness acceptability curve. CHF: Swiss
franc; QALYs: quality-adjusted life years; TAVI: transcatheter aortic valve
implantation.
Deterministic sensitivity analyses
Univariate sensitivity analyses showed that
TAVI remained cost-effective irrespective of plausible changes in individual model
parameters (figure 5). The model was most sensitive to the procedure and the reintervention
costs of both strategies, the risk of new onset of AF at 30 days for SAVR, and the
starting age of patients entering the model (only those 10 parameters with the greatest
influence on the model’s results are displayed).
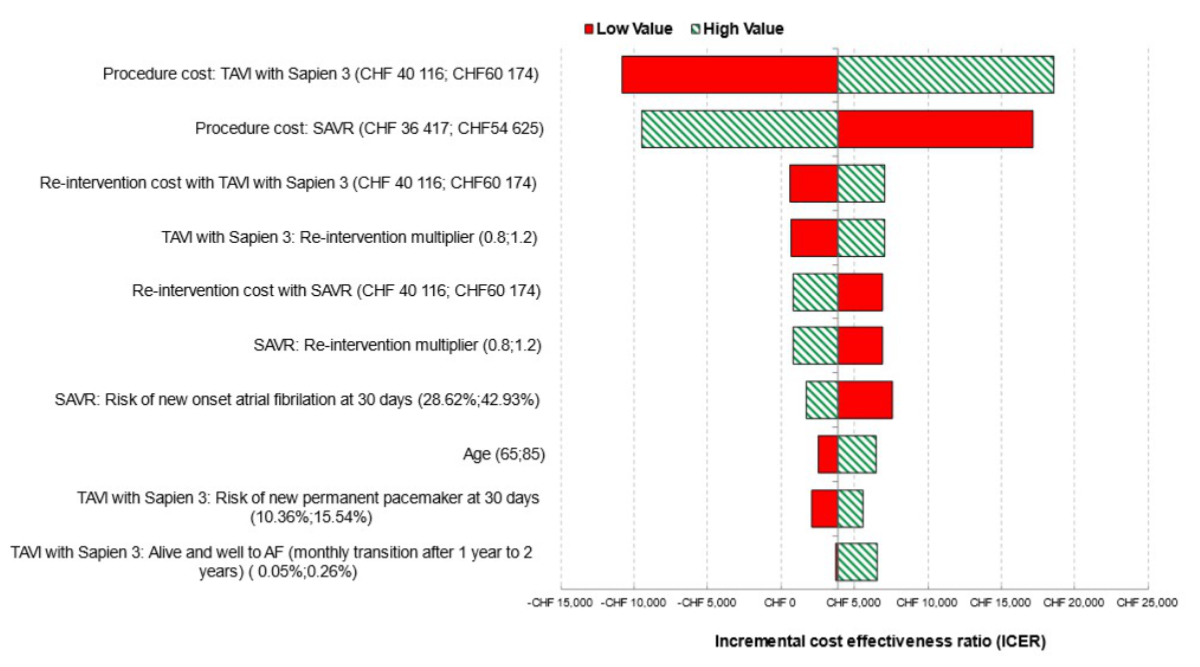
Figure 5Deterministic sensitivity analysis: Tornado diagram showing the 10 parameters
with greatest influence on the model. CHF: Swiss franc; ICER: incremental cost-effectiveness
ratio; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic
valve implantation. Interpretation note: This chart presents the results of the
10 univariate sensitivity analyses that had the greatest influence on the model
ICER. Each analysis is summarised using a horizontal bar which represents the
variation in the ICER around a central value corresponding to the base case
analysis as the relevant parameter is varied between two plausible but extreme
values. The horizontal bars are ordered so that those with the greatest spread
(i.e. parameters to which the model output is most sensitive) are at the top of
the diagram, and those with the lowest spread at the bottom.
Scenario analyses
The results from the various scenario analyses
demonstrated the comparative robustness of the model reported (table 3).
Table 3Scenario analyses.
| No. |
Description |
Incremental costs (TAVI vs SAVR), in CHF |
Incremental quality-adjusted life years (TAVI vs SAVR), in quality-adjusted
life years |
Incremental cost-effectiveness ratio: CHF / quality-adjusted life
year |
|
Base case |
2643 |
0.68 |
3866 |
| 1 |
More aggressive reintervention rate for transcatheter aortic valve
implantation (PARTNER 2A 5 years) |
26,117 |
0.67 |
39,267 |
| 2 |
Survival data from PARTNER 3, as reported in the study (HR = 0.75) |
5517 |
1.37 |
4033 |
| 3 |
Survival data from PARTNER 3, estimating there is no survival
benefit (HR = 1) |
187 |
0.48 |
390 |
| 4 |
Utility from PARTNER 3 EQ-5D-5L (disutility by treatment) |
2643 |
0.34 |
7866 |
| 5 |
Procedure cost with all SAVRs based on minimal-invasive tariff
(F03C) |
–9317 |
0.68 |
Dominant |
| 6 |
Only inpatient rehabilitation (no outpatient rehabilitation) |
880 |
0.68 |
1287 |
| 7 |
Time horizon = 5 years |
2407 |
0.24 |
9890 |
| 8 |
Time horizon = 10 years |
1940 |
0.43 |
4468 |
| 9 |
Time horizon = 15 years |
1939 |
0.57 |
3385 |
| 10 |
Time horizon = 20 years |
2329 |
0.65 |
3566 |
| 11 |
Time horizon = 30 years |
2640 |
0.68 |
3862 |
Discussion
This analysis indicates that TAVI with SAPIEN
3 is expected to be a cost-effective valve replacement choice for patients with
symptomatic severe aortic stenosis at low risk of surgical mortality in Switzerland.
Incremental cost-effectiveness ratio benefits shown in this analysis suggest a cost-effective
intervention in the Swiss system, even with a considered cost-effectiveness threshold
of CHF 50,000 per quality-adjusted life year. Sensitivity analyses were used to
assess uncertainty and the results appeared robust.
The findings of the current analyses are reinforced
by other cost-effectiveness studies which show that TAVI with SAPIEN 3 is either
dominant or cost-effective in patients at low risk of surgical mortality [34–38].
The Swiss findings are also consistent
with cost-effectiveness analyses of TAVI with SAPIEN 3 versus SAVR in France [14],
Italy [15], Spain [16], Germany [17], Belgium [18] and the Netherlands [19] using
the same model structure.
The cost-effectiveness of TAVI in low-risk patients
in Switzerland appears to be driven by lower long-term management costs, particularly
those costs related to “treated AF” and “disabling
stroke”; cost savings in these areas were also seen in France, Italy, Spain,
Germany, Belgium and the Netherlands [13–19].
Our analysis showed that initial procedure costs for TAVI with SAPIEN 3 were higher
than for SAVR in Switzerland; this was also the case in Italy and Spain, whereas
the initial cost for performing TAVI was lower than for SAVR in France, mainly driven
by the higher rehabilitation costs that SAVR patients experience.
The results of this cost-effectiveness study
in Switzerland are valuable for supporting the use of TAVI as a minimally invasive
treatment option in patients with symptomatic severe aortic stenosis at low risk
of surgical mortality. Data suggests that, with TAVI, rehospitalisation risk is
reduced, there is a lower risk of procedural complications and recovery rates improve,
resulting in overall quality of life gains. There are also many societal benefits
associated with the use of TAVI. Reducing hospital stays as well as resource use (e.g.
lower general anaesthesia, less intensive care/ICU stays, improvement in efficiencies
during the index hospitalisation) allows for more patients to be treated in the
same hospital. The former is an important element as long waiting lists after the
COVID-19 pandemic occurred in some countries and an expected increase in number
of TAVI procedures due to demographic changes have put health systems, already in
high demand, under even further stress [39].
Following the update to the European guidelines
[5, 12] and the potential update to the Swiss
guidelines, it would be expected that the number of TAVIs will increase in the coming
months and years, as large numbers of symptomatic severe aortic stenosis patients
at low surgical risk become eligible to benefit from this treatment. It is likely
that the TAVI procedure will be further simplified, with shorter admission times
and lengths of stay post-procedure, leading to decreasing costs. In this regard,
the results of this analysis could inform policymakers on the management of patients
with symptomatic severe aortic stenosis in Switzerland and improve access to TAVI
for these patients.
Limitations
Some limitations relate to those of any cost-effectiveness
analysis and include assumptions made where there is “best fit” data or
paucity of data, extrapolations modelled for time horizons beyond the scope of existing
input data, and potential for under- and over-estimations due to differences in
healthcare systems or by the intervention/treatment selection criteria within a
specific system. First, neither utilities nor estimates for annual mortality rates
were derived from aortic stenosis patients. Both are likely different in an aortic
stenosis population than the average normal population.
Second, the reintervention rate was assumed
to stay constant after 22 years; the effect of this assumption on modelled outcomes
was thought to be minimal based on an expectation that around 15% of patients would
still be alive in the model after this time point, with limited need for reintervention.
Nevertheless, uncertainty about the longer-term durability of the TAVI device and
consequent reintervention rates in younger patients cannot be disregarded. Third,
disutilities were not included for intercurrent events because it would risk them
being counted twice with the health state utilities being applied to patients in
the “treated AF” and “disabling stroke” states. This was a conservative assumption
because,
apart from pacemaker complications, rates of intercurrent events were generally
lower for TAVI with SAPIEN 3 compared with SAVR [10].
Fourth, the literature data used to calculate the utility decrements for “treated
AF” and “disabling stroke” could imply a limitation. The disutilities were
calculated through an average weighting of disutilities in neighbouring countries,
namely Germany, France and Italy. Although the best available option and methodologically
sound, further investigation into disutilities specific to the Swiss population
on these conditions may be valuable. Moreover, utilities were taken from population
norms in Germany that were recorded 20 years ago; hence, they may not be applicable
to current times. Fifth, additional charges (Zusatzentgelte), which would
have a greater impact on SAVR costs (transfusion, haemofiltration in acute kidney
injury) were not taken into consideration. Sixth, to calculate some costs, such
as the rehabilitation costs following a TAVI and SAVR procedure, the “treated
AF” cost, the “alive and well” cost and the pacemaker cost, expert interviews
were partially relied upon. This was seen as the best available option to localise
the cost. The generalisability of the PARTNER 3 results was a limitation. Patients
with unfavourable coronary anatomy were excluded from PARTNER 3, so any conclusions
cannot be generalised to the overall population with aortic stenosis. In addition,
findings from this model cannot be generalised to populations outside of Switzerland.
Seventh, variations may occur across regions of Switzerland. Finally, the employment
of some of the authors by Edwards Lifesciences could be considered a limitation.
Conclusions
This analysis suggests that TAVI using the SAPIEN
3 device is likely to be a cost-effective alternative for symptomatic severe
aortic stenosis patients at a low risk of surgical mortality, treated in the contemporary
Swiss setting. The findings are consistent with cost-effectiveness analyses of TAVI
with SAPIEN 3 versus SAVR in other European countries using the same model structure.
While the initial procedure costs for TAVI with SAPIEN 3 are higher than those of
SAVR in Switzerland, the overall cost-effectiveness of TAVI is driven by lower long-term
management costs. TAVI with SAPIEN 3 offers efficiency gains by limiting healthcare
resource use, reducing postoperative complications and shortening hospital length
of stay compared with SAVR, while also meeting patients’ preference for a minimally
invasive option and improving patients’ quality of life. We propose that this analysis
is valuable for clinical decision-making and for policymakers specifically considering
the 2021 European Society of Cardiology / European Association for
Cardio-Thoracic Surgery guidelines that recommend TAVI in all patients ≥75 years
who are suitable for a transfemoral approach regardless of the degree of surgical
risk.
Data availability statement
Input parameters values used and data generated
during this cost-utility study are wholly included within this published article
and the associated supplementary material in the appendix.
Acknowledgments
This work was funded by Edwards Lifesciences (Nyon,
Switzerland). We would like to thank Prof. Lorenz Räber and Christophe Alain Wyss
who were part of the scientific committee that provided guidance regarding the clinical
outcomes and costs specific to Switzerland. The original model was developed by
Michelle Green and Judith Short from York Health Economics Consortium (YHEC) (University
of York, United Kingdom) [14]. Tom Bromilow,
Daniela Afonso and Karin Butler from YHEC also contributed to this adaptation for
Switzerland. Polynomics AG (Olten, Switzerland) provided support to structure the
local data in the report that can be found in the appendix. Writing support was provided
by Zenith Healthcare Communications Ltd (Chester,
United Kingdom) and was funded by Edwards Lifesciences.
Pascal Candolfi, PhD
Edwards
Lifesciences SA
CH-1260
Nyon
pascal_candolfi[at]edwards.com
References
1. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, et al. A prospective
survey of patients with valvular heart disease in Europe: The Euro Heart Survey on
Valvular Heart Disease. Eur Heart J. 2003 Jul;24(13):1231–43. doi: https://doi.org/10.1016/S0195-668X(03)00201-X
2. Otto CM. Timing of aortic valve surgery. Heart. 2000 Aug;84(2):211–8. doi: https://doi.org/10.1136/heart.84.2.211
3. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous
transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis:
first human case description. Circulation. 2002 Dec;106(24):3006–8. doi: https://doi.org/10.1161/01.CIR.0000047200.36165.B8
4. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al.; PARTNER Trial
Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients
who cannot undergo surgery. N Engl J Med. 2010 Oct;363(17):1597–607. doi: https://doi.org/10.1056/NEJMoa1008232
5. Beyersdorf F, Vahanian A, Milojevic M, Praz F, Baldus S, Bauersachs J, et al.; ESC/EACTS
Scientific Document Group. Erratum to: 2021 ESC/EACTS Guidelines for the management
of valvular heart disease. Eur J Cardiothorac Surg. 2022 Mar;61(4):964. doi: https://doi.org/10.1093/ejcts/ezab557
6. Falk V, Baumgartner H, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al.; ESC Scientific
Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease.
Eur J Cardiothorac Surg. 2017 Oct;52(4):616–64. doi: https://doi.org/10.1093/ejcts/ezx324
7. Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, Webb JG, et al.; PARTNER 2
Investigators. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement.
N Engl J Med. 2020 Jan;382(9):799–809. doi: https://doi.org/10.1056/NEJMoa1910555
8. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, et al.;
SURTAVI Investigators. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk
Patients. N Engl J Med. 2017 Apr;376(14):1321–31. doi: https://doi.org/10.1056/NEJMoa1700456
9. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, et al.; PARTNER 3 Investigators.
Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical
Risk. J Am Coll Cardiol. 2021 Mar;77(9):1149–61. doi: https://doi.org/10.1016/j.jacc.2020.12.052
10. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al.; PARTNER 3 Investigators.
Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk
Patients. N Engl J Med. 2019 May;380(18):1695–705. doi: https://doi.org/10.1056/NEJMoa1814052
11. Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, et al.; PARTNER 3 Investigators.
Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N Engl
J Med. 2023 Nov;389(21):1949–60. doi: https://doi.org/10.1056/NEJMoa2307447
12. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al.; ESC/EACTS
Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular
heart disease. Eur Heart J. 2022 Feb;43(7):561–632. doi: https://doi.org/10.1093/eurheartj/ehab395
13. 832.112.31. KS. Anhang 1 der Krankenpflege-Leistungsverordnung (KLV). Vergütungspflicht
der obligatorischen Krankenpflegeversicherung für bestimmte ärztliche Leistungen 2023.
Available from: https://www.bag.admin.ch/dam/bag/de/dokumente/kuv-leistungen/leistungen-und-tarife/aerztliche-leistungen/Anhang1-KLV/aenderungen_klv_anh1_010723.pdf.download.pdf/aenderungen_klv_anh1_010723.pdf
14. Gilard M, Eltchaninoff H, Iung B, Lefèvre T, Spaulding C, Dumonteil N, et al. Cost-Effectiveness
Analysis of SAPIEN 3 Transcatheter Aortic Valve Implantation Procedure Compared With
Surgery in Patients With Severe Aortic Stenosis at Low Risk of Surgical Mortality
in France. Value Health. 2022 Apr;25(4):605–13. doi: https://doi.org/10.1016/j.jval.2021.10.003
15. Mennini FS, Meucci F, Pesarini G, Vandoni P, Lettino M, Sarmah A, et al. Cost-effectiveness
of transcatheter aortic valve implantation versus surgical aortic valve replacement
in low surgical risk aortic stenosis patients. Int J Cardiol. 2022 Jun;357:26–32.
doi: https://doi.org/10.1016/j.ijcard.2022.03.034
16. Vázquez Rodríguez J, Pinar Bermúdez E, Zamorano Gómez J, Burgos J, Diaz-Fernandez J,
Garcia del Blanco B, et al. Cost-effectiveness of SAPIEN 3 transcatheter aortic valve
implantation in low surgical mortality risk patients in Spain. REC Interv Cardiol.
2023;5:38–45. doi: https://doi.org/10.24875/RECICE.M22000340
17. Kuck KH, Leidl R, Frankenstein L, Wahlers T, Sarmah A, Candolfi P, et al. Cost-Effectiveness
of SAPIEN 3 Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement
in German Severe Aortic Stenosis Patients at Low Surgical Mortality Risk. Adv Ther.
2023 Mar;40(3):1031–46. doi: https://doi.org/10.1007/s12325-022-02392-y
18. Dubois C, Adriaenssens T, Annemans L, Bosmans J, Callebaut B, Candolfi P, et al. Transcatheter
aortic valve implantation versus surgical aortic valve replacement in severe aortic
stenosis patients at low surgical mortality risk: a cost-effectiveness analysis in
Belgium. Acta Cardiol. 2024 Feb;79(1):46–57. doi: https://doi.org/10.1080/00015385.2023.2282283
19. Eerdekens R, Kats S, Grutters JP, Green M, Shore J, Candolfi P, et al. Cost-utility
analysis of TAVI compared with surgery in patients with severe aortic stenosis at
low risk of surgical mortality in the Netherlands. Cost Eff Resour Alloc. 2024 Mar;22(1):24.
doi: https://doi.org/10.1186/s12962-024-00531-6
20. FOPH. Federal Office of Public Health. Health technology assessment (HTA). Available
from: https://www.bag.admin.ch/bag/en/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/hta.html
21. Abbott JH, Wilson R, Pryymachenko Y, Sharma S, Pathak A, Chua JY. Economic evaluation:
a reader’s guide to studies of cost-effectiveness. Arch Physiother. 2022 Dec;12(1):28.
doi: https://doi.org/10.1186/s40945-022-00154-1
22. Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International
Perspective based on EQ-5D. Dordrecht (NL): Springer open. Copyright 2014.
23. SGK. Swiss TAVI Registry. Data provided by SGK 2021 2021. Available from: https://www.swisstavi.ch/
24. Bourguignon T, El Khoury R, Candolfi P, Loardi C, Mirza A, Boulanger-Lothion J, et
al. Very Long-Term Outcomes of the Carpentier-Edwards Perimount Aortic Valve in Patients
Aged 60 or Younger. Ann Thorac Surg. 2015 Sep;100(3):853–9. doi: https://doi.org/10.1016/j.athoracsur.2015.03.105
25. Swiss DR. Datenspiegel SwissDRG 13.0 2024. Available from: https://www.swissdrg.org/de/akutsomatik/swissdrg-system-1302024/fallpauschalenkatalog
26. Swiss DR. RCG Katalog ST Reha 1.0 / 2022 2022. Available from: https://www.swissdrg.org/application/files/4216/1598/6342/ST_Reha_1.0_RCG_Katalog.pdf
27. Swiss Medical Association. Ambulante Tarife Tarmed. Version 01.09.00_BR_KVG. Available
from: https://browser.tartools.ch/de/tarmed_kvg
28. FOPH. Federal Office of Public Health. Spezialitätenliste (SL). Available from: https://www.spezialitätenliste.ch/Default.aspx
29. Pletscher M, Plessow R, Eichler K, Wieser S. Cost-effectiveness of dabigatran for
stroke prevention in atrial fibrillation in Switzerland. Swiss Med Wkly. 2013 Jan;143:w13732.
doi: https://doi.org/10.4414/smw.2013.13732
30. Statbureau. Germany Inflation Calculators 2020. Available from: https://www.statbureau.org/en/germany/inflation-calculators?dateBack=2005-1-1&dateTo=2020-12-1&amount=7266.42
31. Ludwig S, Theis C, Wolff C, Nicolle E, Witthohn A, Götte A. Complications and associated
healthcare costs of transvenous cardiac pacemakers in Germany. J Comp Eff Res. 2019 Jun;8(8):589–97.
doi: https://doi.org/10.2217/cer-2018-0114
32. OECD. OECD Data. Purchasing power parities (PPP) 2021. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm
33. Baron SJ, Wang K, House JA, Magnuson EA, Reynolds MR, Makkar R, et al. Cost-Effectiveness
of Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe
Aortic Stenosis at Intermediate Risk. Circulation. 2019 Feb;139(7):877–88. doi: https://doi.org/10.1161/CIRCULATIONAHA.118.035236
34. HAS-Sante. SAPIEN 3 treatment of severe symptomatic aortic stenosis in low-risk surgical
patients in France 2021. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2021-04/sapien3_9022021_avis_economique_vf2.pdf
35. HIQA (Health Information and Quality Authority). Health Technology Assessment of transcatheter
aortic valve implantation (TAVI) in patients with severe symptomatic aortic stenosis
at low and intermediate risk of surgical complications 2019. Available from: https://www.hiqa.ie/sites/default/files/2019-12/TAVI_HTA.pdf
36. NIPH (Norwegian Institute of Public Health). Transcatheter aortic valve implantation
(TAVI) versus surgical aortic valve replacement (SAVR) for patients with severe aortic
stenosis and low surgical risk and across surgical risk groups: a health technology
assessment 2021. Available from: https://www.fhi.no/en/publ/2021/TAVI-vs-SAVR-for-patients-with-severe-aortic-stenosis-and-low-surgical-risk-and-across-surgical-risk-groups/
37. Tam DY, Azizi PM, Fremes SE, Chikwe J, Gaudino M, Wijeysundera HC. The cost-effectiveness
of transcatheter aortic valve replacement in low surgical risk patients with severe
aortic stenosis. Eur Heart J Qual Care Clin Outcomes. 2021 Oct;7(6):556–63. doi: https://doi.org/10.1093/ehjqcco/qcaa058
38. Zhou JY, Liew D, Duffy SJ, Walton A, Htun N, Stub D. Cost-Effectiveness of Transcatheter
Versus Surgical Aortic Valve Replacement in Low-Risk Patients With Severe Aortic Stenosis.
Heart Lung Circ. 2021 Apr;30(4):547–54. doi: https://doi.org/10.1016/j.hlc.2020.09.934
39. Huygens SA, van der Kley F, Bekkers JA, Bogers AJ, Takkenberg JJ, Rutten-van Mölken MP.
Beyond the clinical impact of aortic and pulmonary valve implantation: health-related
quality of life, informal care and productivity. Eur J Cardiothorac Surg. 2019 Apr;55(4):751–9.
doi: https://doi.org/10.1093/ejcts/ezy382
40. SAFE (Stroke Alliance For Europe). The burden of stroke in Germany 2017. Available
from: https://www.safestroke.eu/wp-content/uploads/2017/12/SAFE_STROKE_GERMANY.pdf
41. Blum S, Aeschbacher S, Coslovsky M, Meyre PB, Reddiess P, Ammann P, et al.; BEAT-AF
and Swiss-AF investigators. Long-term risk of adverse outcomes according to atrial
fibrillation type. Sci Rep. 2022 Feb;12(1):2208. doi: https://doi.org/10.1038/s41598-022-05688-9
42. FSO. Federal Statistical Office. Kohortensterbetafeln für die Schweiz (1876-2030)
nach Geburtsjahrgang, Geschlecht und Alter 2019. Available from: https://www.pxweb.bfs.admin.ch/pxweb/de/px-x-0102020300_101/px-x-0102020300_101/px-x-0102020300_101.px/
43. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation
and risks of cardiovascular disease, renal disease, and death: systematic review and
meta-analysis. BMJ. 2016 Sep;354:i4482. doi: https://doi.org/10.1136/bmj.i4482
44. Gandjour A, Stock S. A national hypertension treatment program in Germany and its
estimated impact on costs, life expectancy, and cost-effectiveness. Health Policy.
2007 Oct;83(2-3):257–67. doi: https://doi.org/10.1016/j.healthpol.2007.01.003
45. Ali M, MacIsaac R, Quinn TJ, Bath PM, Veenstra DL, Xu Y, et al. Dependency and health
utilities in stroke: data to inform cost-effectiveness analyses. Eur Stroke J. 2017 Mar;2(1):70–6.
doi: https://doi.org/10.1177/2396987316683780
46. Walter E, Voit M, Eichhober G. Cost-effectiveness analysis of apixaban compared to
other direct oral anticoagulants for prevention of stroke in Austrian atrial fibrillation
patients. Expert Rev Pharmacoecon Outcomes Res. 2021 Apr;21(2):265–75. doi: https://doi.org/10.1080/14737167.2020.1798233
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3558.




