Peripheral
lymphadenopathy of unknown origin in adults: a diagnostic approach emphasizing the
malignancy hypothesis
DOI: https://doi.org/https://doi.org/10.57187/s.3549
Ivana Hanzalová,
Maurice Matter
Department
of Surgery, University Hospital and Lausanne University, Lausanne, Switzerland
Summary
The term lymphadenopathy refers to an abnormality
in size, consistency or morphological aspect of one or several lymph nodes. Although
lymphadenopathies are commonly observed in everyday clinical practice, the difficulty
of differentiating benign and malignant disease may delay therapeutic approaches.
The present review aims to update diagnostic algorithms in different clinical
situations based on the currently available literature.
A literature review was performed to
assess current knowledge of and to update the diagnostic approach. A short
clinical vignette was used as an example of a typical clinical presentation.
This case of metastatic lymphadenopathy with incomplete patient history demonstrates
how misleading such lymphadenopathy may be, leading to a delayed diagnosis and even
a fatal outcome.
Any lymphadenopathy persisting
for more than 2 weeks should be considered suspicious and deserves further
investigation. Precise clinical examination, meticulous history-taking and a search
for associated symptomatology are still cornerstones for diagnosing the origin
of the condition. The next diagnostic step depends on the anatomical region and
the specific patient’s situation. Imaging starts with ultrasound, while computed
tomography (CT) and magnetic resonance imaging (MRI) allow assessment of the
surrounding structures. If the diagnosis remains uncertain, tissue sampling and
histological analyses should be performed.
Except for head and neck loco-regional
lymphadenopathy, there are no methodical guidelines for persistent lymphadenopathy.
The present review clarifies several confusing and complex situations. The
accuracy of fine needle aspiration cytology could be increased by using core needle
biopsy with immunocytologic and flow cytometric methods. Notably, except in the
head and neck area, open biopsy remains the best option when lymphoma is suspected
or when inconclusive results of previous fine needle aspiration cytology or
core needle biopsy are obtained. The incidence of malignant lymphadenopathy varies
with its location and the various diagnostic strategies. In metastatic lymphadenopathy
of unknown primary origin, European Society for Medical Oncology (ESMO)
guidelines and modern methods like next-generation sequencing (NGS) may help to
manage such complex cases.
Introduction
A clinical or radiological finding of
lymphadenopathy is defined as an anomaly in size, consistency or morphological
aspect of one or more lymph nodes. The definition of abnormal size depends on
the anatomical region and the patient’s age but starts at greater than 1 cm in
short-axis diameter [1]. While
jugulodigastric lymph nodes are considered normal up to 1.5 cm in size,
epitrochlear lymph nodes larger than 5 mm are already considered enlarged [1]. The
clinical context, for example, anal cancer, can decrease the lymph node size cut-off
value; they are considered abnormal at 5 mm and larger [2]. An
odd lymph node consistency, such as feeling rubbery or stiff upon palpation,
with fixation to the subcutaneous tissues and sometimes pain, contributes to
the definition of lymphadenopathy. The concept of lymphadenopathy includes both
localized and generalized lymphadenopathy, the latter of which is characterized
by an abnormal finding in at least two lymph node regions. The term
lymphadenitis refers to an enlarged lymph node with inflammation, generally due
to infection.
The diagnostic strategy includes considering
a detailed patient history, emphasizing exposures that could evoke an
infectious origin (e.g. travelling, risky behaviour, tick bite, specific
medication). The proportions of malignant and infectious origin of lymphadenopathy
vary according to the type of centre (primary care or specialized) and
geography [3, 4].
Constitutional symptoms are important,
as is the time elapsed since the first detection of enlarged lymph nodes. A complete
physical exam looks for possible signs associated with regional or generalized disease.
Then, laboratory tests are necessary, as well as various imaging methods
depending on the affected anatomical region. Biopsy is performed in cases of suspicion
of malignancy or inconclusive diagnosis despite all the methods applied.
This
review aims to assess
the main issues in the diagnostic and
therapeutic processes for unique or multiple enlarged peripheral lymph nodes in
adults. However,
the review of investigations of all lymphadenopathies is beyond the scope of
this article. The paediatric point of view on lymphadenopathy had been described
elsewhere [5].
Methodology
A literature review of lymphadenopathy
was performed using MEDLINE, PubMed, Web of Science and Google Scholar for sources
in English, French or German (IH, MM). The analysed period covered 1993–2023. In addition, algorithms
and guidelines of specialist associations were analysed (European Society for
Medical Oncology (ESMO), American College of Radiology and American Academy of
Otolaryngology–Head
and Neck Surgery Foundation). Large cohort studies were scarce, and some were
outdated. The majority of recent publications were case reports with specific
reviews and expert guidelines.
Epidemiology
Localized or generalized lymphadenopathy
is frequent among children and adults, with an estimated annual incidence of
0.5–0.6%/year in family doctor practices in 1981 and 1984 [6, 7]. The
distribution of localized lymphadenopathy, regardless of its aetiology, is
mainly the head and neck region (55%), followed by the supraclavicular (5%),
groin (14%) and
axillary (5%) regions [8, 9].
Among all patients with lymphadenopathy,
1.1–8.1% have
malignancy [6, 10]. Initial suspicion of malignancy has been confirmed in
14–17.3% of adult patients [4, 11]. However,
in the case of clear indication of biopsy in adults, the histologically proven malignancy
rate was much higher (47%) in a Malaysian study [3]. In
contrast, as inflammatory lymphadenopathy is a self-limited condition and does
not generally cause patients to be seen by a physician, data on its incidence
are lacking [12]. In
a British study with 342 patients (both adults and children) undergoing lymph
node biopsy, 45% had cytologically proven non-specific benign lymphadenopathy, most often reactive
lymphadenopathy [13]. Another British study showed a 40% incidence of reactive
lymphadenopathy among the 78%
non-malignant findings among 423 patients referred to a tertiary
cancer centre for suspicious lymphadenopathy [14]. As the prevalence of tuberculosis
varies geographically, tuberculosis lymphadenitis was found most frequently
(57%) in the non-malignant lymphadenopathy group in an Indian study [10]. However, in Switzerland, tuberculosis
is a rather rare cause of lymphadenopathy and also has shown a decreasing
incidence in the last 10 years (5/100,000) [15]. The majority of cases are in immunocompromised
patients or migrant populations, and they need investigation and care. This is
a reminder that this “old” disease must not be forgotten. In 2021, 357 cases of
tuberculosis were directly reported to the Swiss Federal Office of Public Health [16]. Tuberculosis was an extrapulmonary disease
in 27% of those patients. Despite a decreasing incidence in recent years, this
may change with population migration from high-risk areas.
Among confirmed adult
lymphoproliferative malignancies of any lymph node basin, the most frequent
diagnoses were Hodgkin lymphoma (31%), diffuse large B-cell lymphoma (29%) and
follicular lymphoma (16%). Among metastatic tumours, the most frequent primaries
were squamous head and neck carcinoma (35%) [14]. The
risk factors for
malignant lymphadenopathy were male
sex, Caucasian ethnicity and lymphadenopathy localized either in the
supraclavicular fossa or simultaneously in more than
one anatomic region [4, 17]. The
probability of malignancy in the case of unexplained cervical lymphadenopathy was associated with
increasing age. With increasing age, the probability of reactive lymphadenopathy decreased, while lymphoma or metastases were observed
in over 50% of patients [17].
Clinical presentation, patient history and related
symptomatology
Clinical presentation of both
localized and generalized lymphadenopathy
is variable; it can be combined with hepatomegaly,
splenomegaly, weight loss or fever [18].
There is a good correlation between palpable lymphadenopathy and final
pathological findings: soft, mobile and well-demarcated lymph nodes usually indicate
reactive lymphadenopathy or viral infections and rarely lymphoma, lymph node metastasis or tuberculosis [1]. In
cervical lymphadenopathy, malignancy may be suspected in the absence of
infection or given the persistence of enlarged lymph nodes for more than 2 weeks,
reduced lymph node mobility, firm structure or signs of cutaneous ulcerations.
Furthermore, malignancy is suspected patients older than 40 years with active
or past alcohol or tobacco consumption, sore throat, dysphagia, recent hearing
loss or voice changes, or any suspicious skin lesions found during clinical
examination [12] (figure 1).
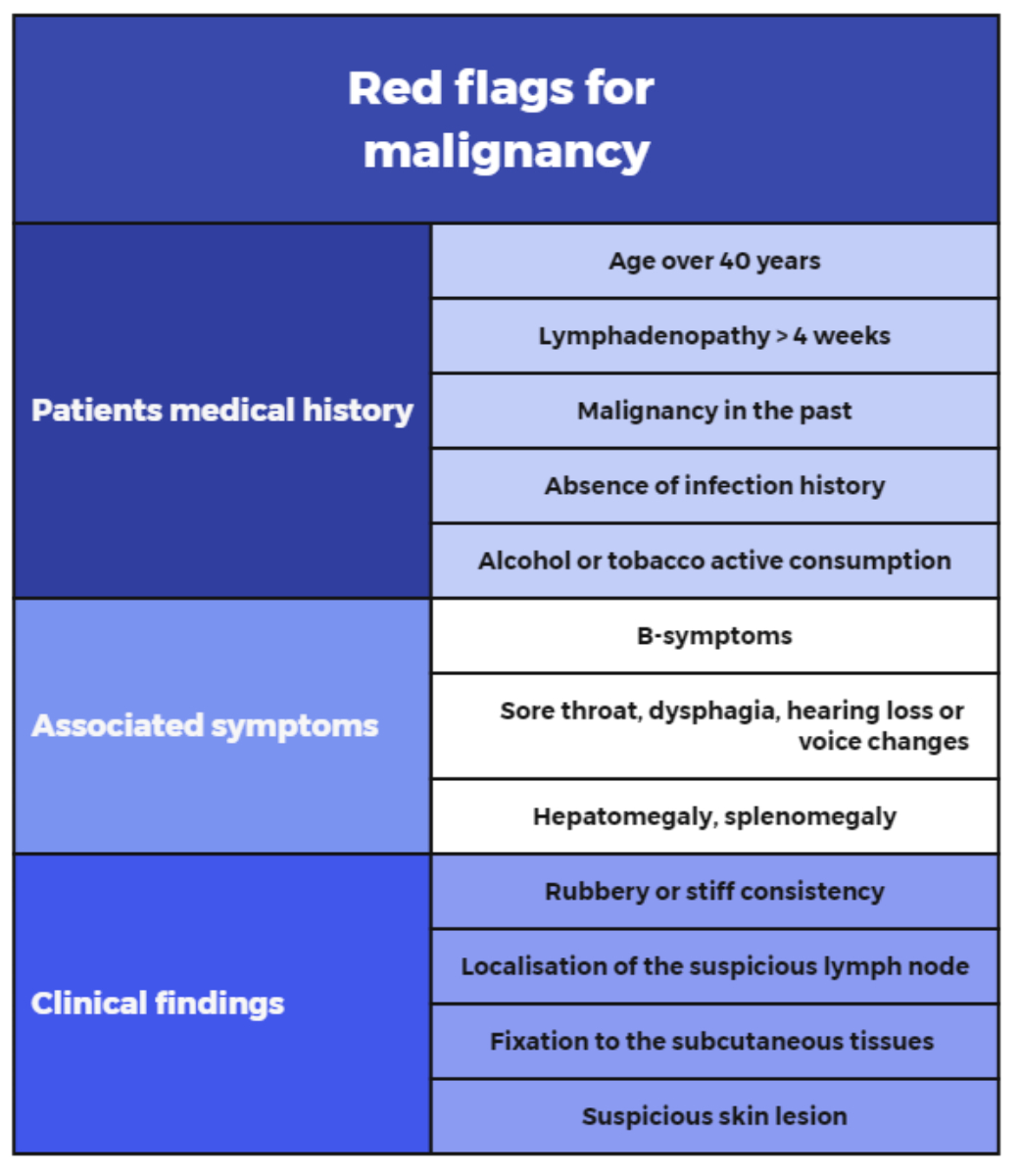
Figure 1Red flags for malignancy in lymphadenopathy. Based on: Gaddey HL, Riegel AM. Unexplained lymphadenopathy: evaluation and differential diagnosis. Am Fam Physician. 2016;94:896–903 [1] and Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723–32 [45].
Clinical vignette
A
70-year-old patient presented to an emergency department with a history of a painful
mass in the right groin. After 1 month, the pain increased, and the patient
experienced a local abscess and fistulization.
There were no previous problems with the ipsilateral lower limb or any
associated fever or rash symptoms. His past medical history included
anticoagulant therapy for atrial fibrillation, psoriasis (lower abdominal
region, both groins and scrotum) and a squamous cell carcinoma on the prepuce
with local surgery a year before (T1, N0, M0, R0). The 3 × 8 cm inguinal mass was drained
under local anaesthesia, followed by oral antibiotic therapy for 10 days. An
ultrasound-guided biopsy was performed without any clear histological findings.
Because of a worsening of the local status and a possible relationship with the
prepuce squamous cell carcinoma, a new examination of the glans revealed local
recurrence. A computed tomography (CT) scan showed multiple bilateral groin lymphadenopathies with an abcess on the right side (figure 2). Another biopsy
confirmed metastasis of the penile squamous cell carcinoma. After a multidisciplinary tumour board discussion, a radical
lymph node dissection was performed, which showed 13/15 positive superficial
(inguinal) nodes and 3/10 positive deep (iliac) nodes. The
postoperative course was complicated by lymphatic fistula and wound infection.
Immunotherapy (durvalumab and nivolumab) was later introduced, but the patient
died after 7 months of multisystemic complications.
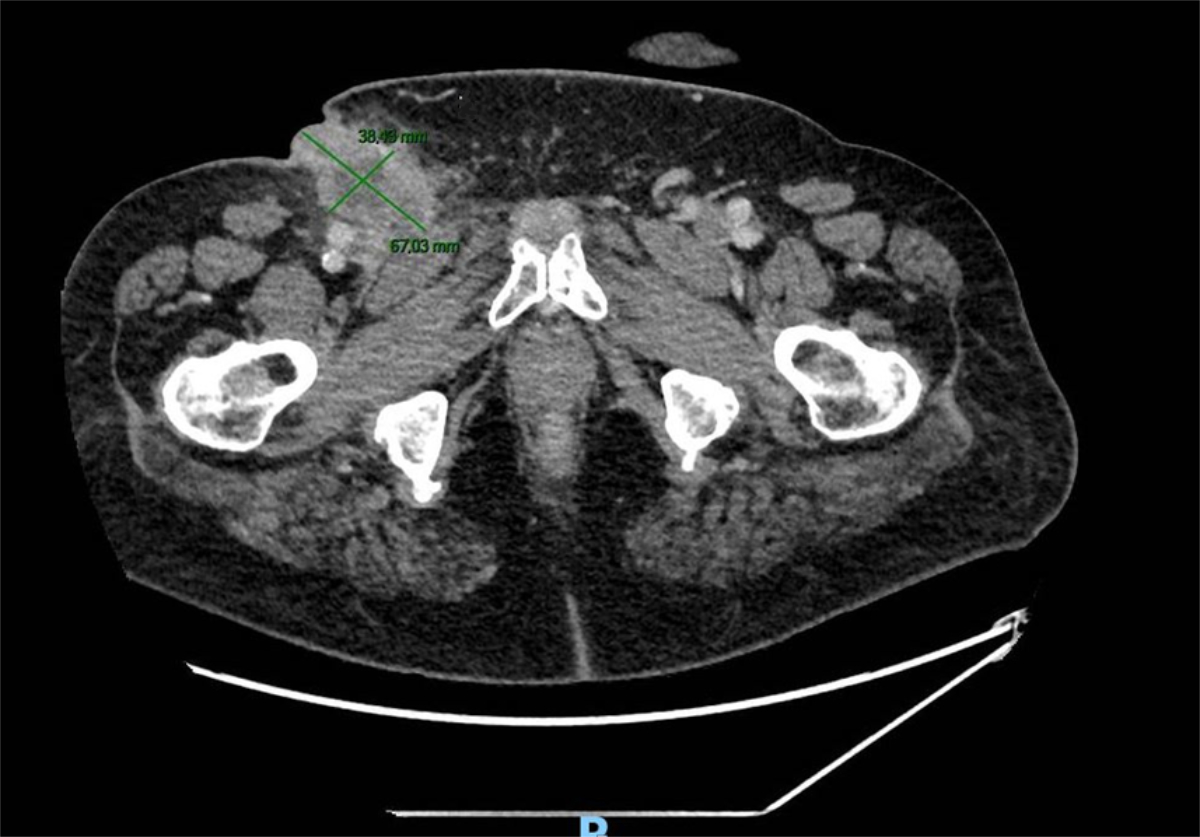
Figure 2CT imaging showing the ulcerated right-groin
lymphadenopathy.
Diagnostic approach
Any enlarged palpable lymph node
persisting for more than 2 weeks first requires a targeted physical
examination. Based on clinical history, the strategy should involve a search for infectious, malignant or other origins of the
lymph node enlargement. Critical aspects of the clinical history (apart from
any past malignancies) include exposure to recent insect or other animal bites,
recent or recurrent infections, travel-associated exposures, environmental or occupational
exposures and risky sexual behaviour. Cat-scratch
disease is a good example, with 90% of patients presenting with lymphadenopathy [19]. Some
medications can cause lymphadenopathy, including allopurinol, atenolol,
captopril, carbamazepine, some cephalosporins, gold, hydralazine, penicillin,
phenytoin, primidone, methylamine, quinidine, sulfonamides and sulindac
[20, 21]. Several attempts have been made to estimate which patients would or
would not benefit from biopsy based on epidemiologic records and clinical
findings, but the predictive value was poor [22]. Figure 3 illustrates an algorithm on how to approach a persisting peripheral lymphadenopathy in adults. Differential diagnosis of peripheral lymphadenopathy is summarized in figure 4.
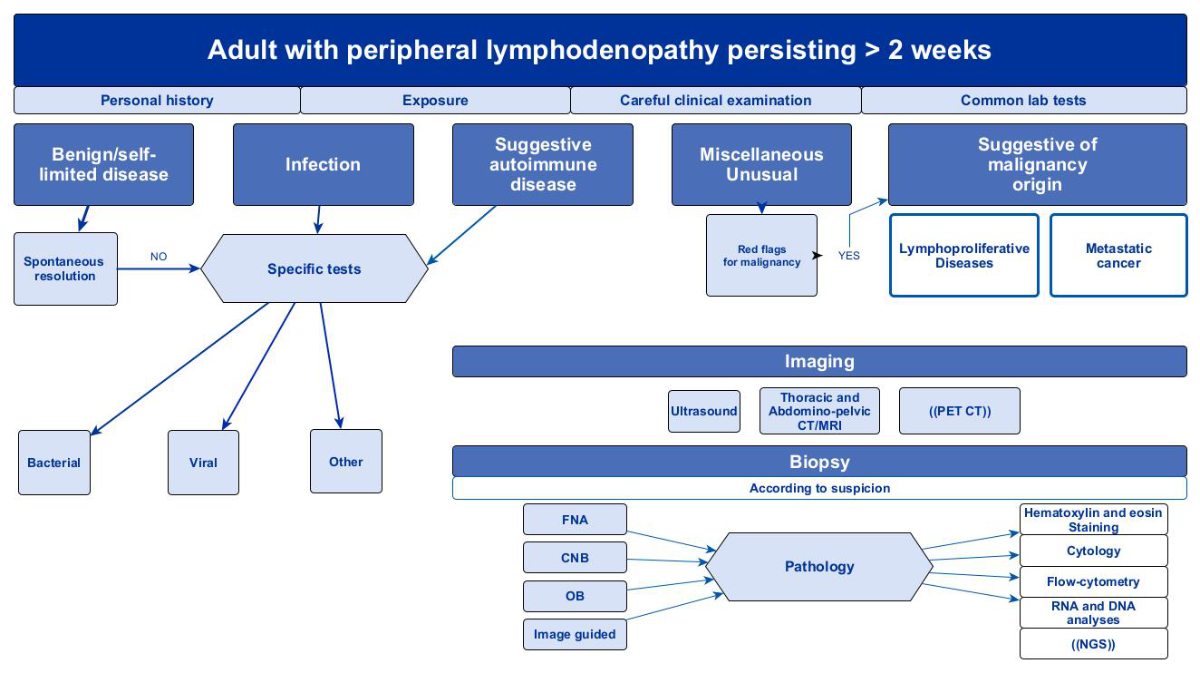
Figure 3Proposed diagnostic algorithm for
persistent lymphadenopathy of unknown origin. Adapted from [1, 18, 45, 87]. List of abbreviations used in the
diagram: CNB – core needle biopsy; CT – computed tomography; FNA – fine needle
aspiration; PET/CT – positron emission tomography–computed tomography; NGS – next-generation
sequencing; MRI – magnetic resonance imaging; (( ))– not recommended as a
first-line examination.
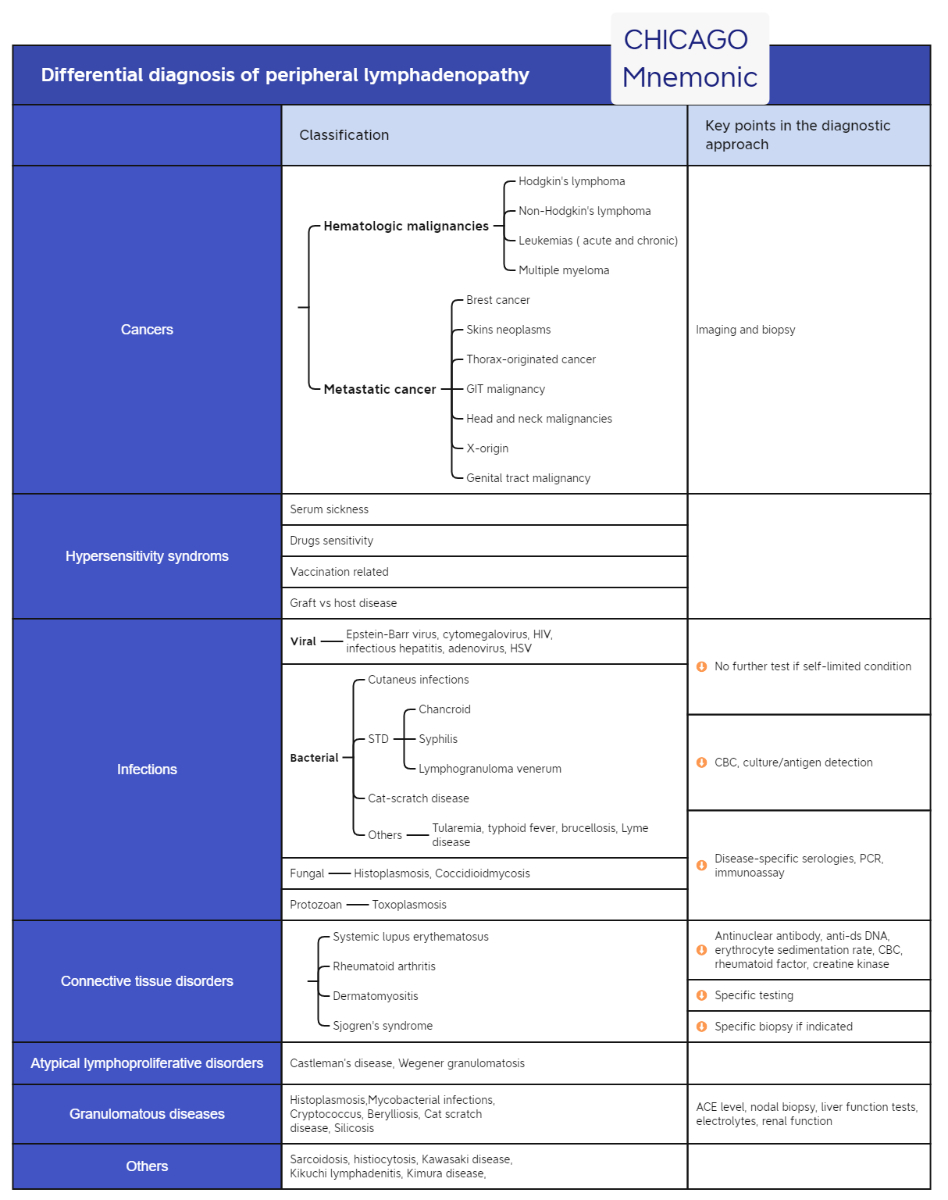
Figure 4Differential diagnosis of
peripheral lymphadenopathy. Based on: Gaddey HL, Riegel AM. Unexplained lymphadenopathy: evaluation and differential diagnosis. Am Fam Physician. 2016;94:896–903 [1] and Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723–32 [45].
The approach depends on the anatomical
region of the lymphadenopathy. In the head-neck region, a complete
examination of the scalp and skin and the oropharyngeal, nasopharyngeal and nasal
cavities, with larynx examination and otoscopy, is required. The palpation of
salivary glands, thyroid glands and cranial nerves is mandatory [12].
In the case of suspicion of malignancy
of an unknown primary site, the next step varies according to the region. Fine
needle aspiration cytology or core needle biopsy should be performed in the
head-neck
area before imaging. Endoscopic examination of the upper aerodigestive tract via
open biopsy is a second step only if the primary tumour remains unclear [12].
Laboratory tests do not help, due
to the low evidence of an association between complete blood count or lactate
dehydrogenase and malignancy. Some studies have suggested a predictive value of
leukopenia, thrombocytopenia or increased lactate
dehydrogenase levels
for malignancy, but this remains debated [23].
Specific markers may be used based on past medical history of malignancy. Moreover, if an invasive procedure
such as fine needle aspiration cytology is the next step in the diagnostic
process, coagulation testing and a blood count are reasonable.
Imaging
Chau et al. [14] reached
97% accuracy of malignancy detection in adults using ultrasound.
Several sonographic descriptors have attempted to define typical malignant characteristics
of lymph nodes. The probability of malignancy increases significantly with the following
criteria: increased long axis size; lower length-to-width ratio (Solbiati
index), with a higher probability of malignancy in “round lesions”;
inhomogeneity in inner structure; a hilum structure that is not clearly
detectable; adhesion to the surrounding structures; and excessive vascularization.
The accuracy of those criteria has been confirmed by biopsy in several studies [24].
The next lymphadenopathy diagnostic
imaging modality is contrast-enhanced CT or magnetic resonance imaging (MRI).
Both methods have been recognized as mandatory steps in cervical
lymphadenopathy diagnosis to define the extent and stage of the disease and
contact with surrounding structures. According to the American College of
Radiology, CT with contrast is recommended as the initial imaging method in
adults in the case of a non-pulsatile neck mass [25]. MRI,
however, is preferred in cases of suspicion of primary nasopharyngeal tumours,
skull base tumours or tumours at the base of the tongue [9]. The utility
of F-fluorodeoxyglucose (FDG)
positron
emission tomography-computed tomography (PET/CT) in the differential diagnosis of lymphadenopathy remains
debated due to its non-specific findings, but it may be helpful for the
diagnosis of cervical lymphadenopathy [26]. For investigating undiagnosed mediastinal and
upper abdominal lymphadenopathy, endoscopic ultrasound-guided fine needle
aspiration was found to be more effective than PET/CT [27]. When
investigating a patient with fever and lymphadenopathy of unknown origin and
suspected lymphoma, Chen et al. [28] showed in a prospective study that 18F-FDG
PET/CT had a sensitivity of 81% and a low specificity of 47.6% after lymphadenopathy
biopsy. However, combining this with clinical parameters may improve diagnostic
efficiency. As mentioned below, a PET scan is optional in the work-up of cancer
of unknown primary origin because of the low identification rate of the primary
tumour [29]. In the National Comprehensive Cancer
Network (NCCN) guidelines, PET is not a first-line radiological
investigation (except in the case of allergy to contrast media) [30].
Tissue biopsy
Despite
imaging and blood tests, the final diagnosis of the cause of lymphadenopathy may
still remain uncertain. The persistence of lymph node enlargement without a clear
origin requires histological diagnosis. There are three standard methods for
collecting tissue samples: fine needle aspiration cytology, core needle biopsy
and open lymph node biopsy.
Fine needle aspiration cytology
Fine needle
aspiration cytology is efficient because of its simplicity, cost-effectiveness
and high level of patient acceptance. However, it has limited value because of
the low quantity and quality of material obtained (cells without histology).
The main drawbacks of fine needle aspiration cytology are low sensitivity and a
low negative predictive value, which have been recorded as 56% (sensitivity)
and 72% (negative predictive value) in comparative studies. Fine needle aspiration
cytology is also significantly less sensitive than open biopsy in the case of suspicion
of tuberculosis [2, 3]. Besides sampling error and cytological similarity
between necrotized metastatic lymph nodes and the caseous necrosis of tuberculosis,
there is a lower efficacy in demonstrating acid-fast bacillus positivity by
Ziehl-Neelsen
staining of cyto-aspirated material than open biopsy material. This test is one
of the cornerstones of the cytological diagnosis of tuberculosis
lymphadenopathy [10]. However, diagnostic accuracy could be improved with
subsequent specific detection of mycobacterial DNA using a cartridge-based
nucleic acid amplification test or real-time PCR, which also permits an
analysis of drug resistance [25, 27].
Various studies
have shown the variability of the accuracy of fine needle aspiration cytology
depending on which tumours were included, with generally lower sensitivity for
lymphomas [4]. Moreover, ultrasound-guided core
needle biopsy is now recommended for suspected lymphoma in the cervical region
because of its higher sensitivity than fine needle aspiration cytology (92% vs
74%) [5]. The lack of accuracy of fine
needle aspiration cytology is due to insufficient sampling, possible fibrosis, the
collection of samples from reactive areas and deficiency of the cellular
structure in the aspirated samples [6]. Notably, the probability of obtaining
adequate results is significantly increased if the conventional cytology is
completed with immunocytology and/or flow cytometry, which allows lymphoma-type
differentiation in some cases. Nevertheless, fine needle aspiration cytology was
persistently limited in cases of T-cell and Hodgkin lymphomas [7].
In the case
of axillary lymph node biopsy in breast cancer, one meta-analysis demonstrated an
ultrasound-guided fine needle aspiration cytology sensitivity of 76%, compared
to definitive surgical axillary staging [8]. Another study involving fine
needle aspiration cytology and core needle biopsy in lymphadenopathy of unknown
origin in various anatomical regions recorded non-concluding histological
diagnoses in only 3% of patients, who then required open biopsy [7]. Fine needle
aspiration is a highly reliable tool for discriminating between COVID-19
vaccine-related and reactive lymphadenopathy and for excluding malignancy [31].
Core needle biopsy
Core needle biopsy obtains more tissues for tissue
architecture and adequate molecular testing, ideally with a 16- or 18-gauge
needle. Core needle biopsy is quick and less invasive and risky than open
biopsy.
Wilczynski et al. [32] showed in 7,093 core needle biopsies
for lymphadenopathy investigation the following diagnoses: non-Hodgkin lymphoma
in 245, Hodgkin lymphoma in 53, solid nonlymphocytic lymph node metastases in
359 and benign lymphadenopathy in 136. The overall accuracy was 95.0%.
Johl et al. [33] reviewed 1,510 lymph node specimens
in 2012 and found that core needle biopsy was less risky to perform than open
biopsy, but the diagnostic accuracy of core needle biopsy was lower. Furthermore,
non-diagnostic cases were nearly four times more frequent than with lymphadenopathy
open biopsy.
Open lymph node biopsy
Open biopsy
was considered the gold standard for a long time [7, 17]. Although the ESMO
guidelines recommend open biopsy for lymphoma diagnosis, a review by Seviar et
al. [34] showed a diagnostic efficacy of 79–97% (median 91%) for
ultrasound-guided core needle biopsy. He proposed core needle biopsy as the
first-line investigation in suspicious lymphoproliferative disease [35]. A meta-analysis
by Warshavsky et al. [36] reached the same conclusions for cervical lymphadenopathy
in which lymphoma was suspected.
For
lymphoproliferative disease, the comparison of paired samples by core needle
biopsy and open biopsy showed a 17.0% rate of incorrect diagnosis with the
former [37]. Shah et al. [38] recorded the
following primary diagnoses when an open biopsy was recommended: Hodgkin
lymphoma, various subtypes due to tissue heterogeneity and other subtypes (including
B-cell, follicular, composite lymphomas and EBV-associated lymphoproliferative
disorder).
The need for an operating room,
anaesthesia (local or general) and a possible hospital stay make open biopsies
more demanding. Clinicians must select the best option while considering the
diagnosis, convenience and patient compliance. Moreover, open biopsy has some
morbidity and complications, such as local haematoma or potential tumour
seeding, which has been observed in 7% of cervical lymph node open biopsies. However,
in a series conducted by Zenga et al. [12], as well as in a retrospective study by Akkina
et al. [17] in head and neck squamous cell carcinoma patients, the overall survival
rate was not worsened by open biopsy.
Tissue analysis
A complete discussion of the techniques involved in lymphadenopathy
analysis is beyond the scope of this review. Fine needle aspiration, cell
blocks and core needle biopsy provide small-volume biopsies. Such analyses are
improving due to the experience of the aspirator (ultrasound-guided), the size
of the biopsy needle, the number of aspirations, the preservation and
cellularity of the sample and the workflow of sample processing for flow
cytometry immunophenotyping [39]. The
standardization of the digital examination of lymphadenopathy cytopathology
using the Sydney system (evaluation of malignancy risk) was reviewed in an
international, multi-institutional study. This method showed excellent
interobserver reproducibility for benign and malignant lymphadenopathy [40]. The Sydney
system reviewed the clinical assessment and indications for fine needle
aspiration and the ultrasound-guided biopsy, including procedural and ancillary
techniques [41]. The material collected
by fine needle aspiration should include at least 10 × 106 cells for
the complete process, which involves cytology; immunohistochemistry; and RNA and
DNA analysis, including next-generation sequencing [42]. When suspecting
infectious lymphadenopathy, fresh tissues should be sent for molecular analysis
(PCR amplification) combined with standard cultures [43]. Next-generation
sequencing can detect nonspecific genomic alterations for malignancy and is not
recommended for first-line investigation in cases of suspected metastatic
malignancy [30]. The ESMO recommendations concerning
cancer of unknown
primary and some other series describe that further anatomopathological
investigations can specifically diagnose the origin of the lymph node
metastatic involvement. Metzgeroth et al. [7] have demonstrated that fine needle
aspiration cytology alone was able to determine the primary tumour site due to
cytokeratin pattern analysis and tumour markers in 75% of patients with
metastatic lymph nodes.
Generalized lymphadenopathy
The definition
of generalized lymphadenopathy requires at least two non-contiguous
lymph node groups to be affected and represents 25% of all lymphadenopathies
[1]. Benign origins involve numerous autoimmune
diseases like systemic lupus erythematosus and various
infections (e.g. AIDS, active tuberculosis, infectious mononucleosis, cytomegalovirus
infection) [44].
Generalized
lymphadenopathy can also be associated with
malignant diseases like leukaemias, lymphomas and advanced metastatic
carcinomas [1].
Which lymph node
should be biopsied in generalized lymphadenopathy?
The success rates of cytological/histological analyses
vary depending on the locations and characteristics of the lymph nodes. When
more than one region is affected, where to perform the needle aspiration/biopsy
should be decided. The recommendation
is to biopsy the largest lymph node outside the inguinal region [45]. According to another study (in an area
of high tuberculosis incidence), the inguinal and other lower limb lymph nodes have
a lower rate of successful diagnosis due to the frequent occurrence of nonspecific
reactive or chronic inflammatory changes and fibrotic changes [46]. There is limited evidence for how
to proceed in the case of multiple lymph nodes of a similar size, with only one
proposal of a decreasing diagnosis yield in the following order:
supraclavicular, cervical, epitrochlear and finally inguinal [1, 45]. It is also
possible to remove the largest lymphadenopathy [1].
Lymph node region-related aetiology and diagnostic
work-up
Unexplained head
and neck lymphadenopathy
A neck lymph node swelling may be caused by infectious, inflammatory,
congenital, traumatic, benign or malignant neoplastic diseases. An asymptomatic
neck lymphadenopathy is potentially the initial or only clinical manifestation
of a head and neck cancer, such as squamous cell carcinoma, lymphoma, thyroid cancer
or salivary gland cancer. When considering the initial lymph node involvement
in lymphomas, the neck is the most common peripheral lymphadenopathy location
(and overall second after the mediastinum), primarily in Hodgkin lymphomas [23,
47]. In squamous cell carcinoma lymphadenopathy, the primary tumour is
generally in the oral cavity, oropharynx, hypopharynx, nasopharynx or larynx.
Adults should be examined for any cervical lymphadenopathy lasting longer than 2
weeks to 1 month [9, 24]. Contrary to previous recommendations, empiric
antibiotics are unnecessary unless bacterial infection is suspected [9].
Based on the Robbins classification, the probability of malignancy in
the neck varies according to the level where regional lymphadenopathy is
located [24]. Guidelines for head and neck lymphadenopathy based on several
studies recommend contrast-enhanced CT as the first imaging modality in adults,
followed by ultrasound-guided fine needle aspiration cytology for tissue
analysis [9, 17].
Unexplained supraclavicular lymphadenopathy
Any detected
supraclavicular lymphadenopathy has a 34–86% risk of malignancy, especially in patients over
40 years of age [14, 48, 49]. The associated primary locations include the mediastinum,
lungs and oesophagus. In the case of the left supraclavicular lymph node (Troisier’s
sign or Virchow’s node), potential malignancy in the testes, ovaries, kidneys,
pancreas, prostate, stomach, or gallbladder must be investigated. Tuberculosis
is the most frequent non-malignant aetiology [49]. The work-up aims to identify
the origin of the primary tumour, including in the thoracic and abdominopelvic
regions (only 15% of tumours originate in the head and neck region), also using
fine needle aspiration cytology [49]. In male patients, prostate-specific
antigen level and digital rectal examination are recommended (to test for prostate
cancer) [50].
Unexplained
axillary and infraclavicular lymphadenopathies
Due to the lymphatic drainage of the upper limb, infectious and
post-trauma aetiologies are frequent, often due to bites from cats or other
animals [51]. Besides an infectious origin, accessory breast tissue can be
found in the axilla and may be confused with an enlarged lymph node [52].
Foreign body reaction due to silicone breast implants was also described as a
cause of reactive axillary lymphadenopathy [53]. In addition, malignant
aetiologies like Hodgkin lymphoma, non-Hodgkin lymphoma or breast cancer may be
suspected [54]. An isolated axillary metastasis can occur in occult breast
cancer, in which no breast tumour can be detected by physical or radiological
examination. This rare condition occurs in less than 1% of all newly diagnosed
breast cancers [55]. According to the American College of Radiology guidelines,
unilateral axillary lymphadenopathy without underlying abnormal breast findings
or known infection or inflammation is evaluated as problematic, and further
diagnostic approaches (first, imaging; second, fine needle aspiration cytology
or core needle biopsy) are required [56].
Other
malignancies can cause axillary lymphadenopathies, including lung, thyroid, stomach, colorectal, pancreatic, ovarian,
kidney and skin cancers (mainly melanoma).
Infraclavicular lymph node involvement has been observed in one-third of
locally advanced breast cancer patients [57].
Lymphadenopathy
following administration of mRNA vaccines for COVID-19
Recently, since the SARS-CoV-2
pandemic, infraclavicular lymphadenopathies post-mRNA vaccines have been described [31, 58]. The fact that these mRNA
vaccines were broadly administrated in a relatively short time and have a strong
immunogenic effect has resulted in more frequent reports of lymphadenopathy than
with other vaccines [31]. The COVID-19 vaccine can cause lymphadenopathy in
about 1% of people, depending on the type of vaccine. With the Moderna vaccine,
11% of lymphadenopathies appeared in the axilla, and 16% occurred after the
second dose [31]. Aside from peripheral lymphadenopathy, in up to 66% of cases
of COVID-19 infection, mediastinal lymphadenopathy was detected by CT [59]. COVID-19
vaccination history may also be relevant for interpreting FDG PET/CT results
because of the possible axillar or deltoid lymph node uptake positivity up to
circa 3 weeks after vaccination [60, 61, 62, 63]. There are some
recommendations for delaying a PET scan after COVID vaccination: for example, 2
weeks for oncology patients and 4–6 weeks for others
[60]. Unfortunately, other radiotracers also present false positive lymph node
uptakes, like 68Ga-DOTATATE, 18F-fluciclovine and
prostate-specific membrane antigen [63]. There are already guidelines from the
European Society of Breast Imaging about how to proceed with the diagnostic
approach. Within the first 12 weeks after a COVID-19 vaccine, radiologically non-suspicious
unilateral axillary lymphadenopathy is considered benign. However, if the
imaging evaluation reveals suspicious lymphadenopathy, or if the lymphadenopathy
appears contralateral or persists after 12 weeks, a standard work-up, including
tissue sampling, should be conducted [61].
Unexplained
inguinal lymphadenopathy
Inguinal lymph nodes are organized into superficial (groin) and deep (iliac) node groups. There is no consensus regarding the average size
of inguinal lymph nodes, but the mean size suspected to indicate malignancy in
a retrospective study was 5.4 ×11.7 mm. The number of lymph nodes in
both inguinal groups was 11, and their shape should have been oval [64]. In the differential diagnosis of
an unspecific groin lump, a hernia is the most common diagnosis [65]. Common benign lymphadenopathies
include reactive lymphadenopathy, sexually transmitted diseases and skin
infections. Inguinal lymphadenopathy can occur with a
periprosthetic joint infection (of the hip or knee), according to ultrasound
and the size of the lymphadenopathy: a size greater than a threshold of 19 mm signals
infection [66]. A recent systematic retrospective
review showed that 16% of lymphoma patients overall had an inguinal lymphadenopathy [23]. Metastatic inguinal lymphadenopathy
may originate from the male or female genital tract and anal malignancies, as
well as skin tumours (mainly melanoma). Chalif et al. [67] showed that, in a
series of 562 women with advanced epithelial ovarian cancer, 1.6% presented
with a palpable inguinal lymphadenopathy. Squamous cell carcinoma of the penis or vulva
may metastasize in the inguinal region without palpable lymphadenopathy [68].In addition, the reported rate of metastases
from ovarian cancer spreading along the round ligament to the inguinal lymph
nodes was found in 2% of all cases of confirmed ovarian cancer [69].
Mediastinal and
abdominal lymphadenopathy
The investigation of all lymphadenopathies is beyond
the scope of our review. For large (>10–15 mm) grouped mediastinal
lymphadenopathies, the best investigation is an endobronchial ultrasound-guided transbronchial needle
aspiration (EBUS-TBNA) [70]. This examination is valid for malignant and non-malignant diseases [71]. There are no guidelines for isolated mediastinal lymphadenopathy of
unclear aetiology below 10–15 mm (repeated CT scan to determine the evolution, PET/CT and EBUS-TBNA
to monitor progression) [72]. Abdominal (e.g. mesenteric, pelvic) lymphadenopathies are often
discovered incidentally or during investigation related to a condition or
disease. They entail an even larger differential diagnosis and are not
discussed in this article. As they relate to the investigation of cancers
of unknown primary origin presenting as lymphadenopathy, they will be briefly discussed
in that section.
Rare
lymphadenopathy localizations
Epitrochlear
lymphadenopathy
In the only published
series of 140 healthy adults with palpable epitrochlear (cubital or
supraepitrochlear) lymphadenopathy, the most frequent origins were lymphomas or
chronic lymphocytic leukaemia, followed by infectious mononucleosis and
rheumatoid arthritis [1, 73]. Cutaneous malignancies such as melanoma can show “interval”
lymphadenopathies and in-transit metastases between the primary site located
distally and the axillary lymph nodes [74, 75].
Popliteal lymphadenopathy
Popliteal
lymphadenopathy was shown in 36% of patients who underwent knee MRI for various
indications [76]. Aside from an infectious origin, malignant lymphadenopathies
are also seen in dermatologic malignancies or clear cell sarcoma of ankle
tendons. Popliteal lymph nodes belong to the group of interval nodes, meaning there
are additional sentinel lymph node locations apart from inguinal lymph nodes.
In any type of skin cancer assessed by lymphoscintigraphy, popliteal
lymphadenopathy was reported in 36% of patients, but it was reported in only 3%
of patients with infra-popliteal melanoma [77, 78]. The incidence of popliteal
melanoma metastasis was only 0.31% in a group of 4,262 patients with low limb
primary melanoma, despite a high frequency of popliteal sentinel nodes [79].
Delphian nodes
Prelaryngeal nodes (located between the thyroid and cricoid cartilage)
are eponymously called Delphian nodes. They are frequently seen in head and
neck malignancies, like thyroid cancer. Malignant Delphian lymphadenopathy is a
worsening prognostic factor and may be associated with primary invasion of
surrounding structures [80].
Other rare lymph
nodes with possible malignant lymphadenopathy
Lymphoscintigraphies
in sentinel lymph node procedures have shown many “interval nodes” in
lymphatics outside the usual basins, like the humeral, intercostal or scapular
lymph nodes, with the same metastatic risk as sentinel nodes in the usual basins [74].
Metastatic lymphadenopathy
of unknown primary origin
The definition of a metastatic lymph node of unknown primary origin is a
proven metastatic disease in a lymphadenopathy, without a known primary tumour,
even after appropriate investigation [29]. The ESMO recently
published updated guidelines [29]. In brief, like with
lymphadenopathy, they start with patient
history and physical examination, followed by blood tests and biochemical
analyses. Imaging includes either a CT scan with contrast media or an MRI of
the neck, thorax, abdomen and pelvis, with a mammogram in women.
Different endoscopies, additional biomarkers and
further radiological examination are indicated based on symptoms or the results
of previous analyses. Histology and immunohistochemistry markers are guided by
clinical information. Unlike the NCCN guidelines for cancers of unknown primary
origin, the ESMO guidelines suggest conducting next-generation sequencing routinely in cases
of cancers of unknown primary origin (a recommendation based on a case series).
However, there is no strong evidence that assessing gene expression may be helpful [29].
Whole body
PET/CT is recommended only for single-site lesions or oligometastatic patients or
patients with head and neck cancer of unknown primary origin (a recommendation
based on a case series). Nikolova et al. [81] showed that an FDG PET scan was
able to detect the primary tumour (head and neck and lung) in 36% of patients
with cervical lymphadenopathy and cancer of unknown primary origin. In a retrospective
study using PET/CT by Reinert et al. [82], 61.3% of cancers of unknown primary
origin were lymphadenopathies, half of them in the cervical area. PET/CT
findings were able to change the treatments in 45.8% of patients with cancer of
unknown primary origin.
The role
of PET/CT has been generally proven in cervical head-neck lymphadenopathy, axillary
adenopathy and single metastatic lesions [29]. However, with growing evidence that
PET/CT-based curative
therapy is associated with significantly longer patient survival,there is a tendency for
its general employment in work-ups of cancers of unknown primary origin [82]. There is already a first national
recommendation from the German Society for Haematology and Medical Oncology stating
that PET/CT plays an essential role in cases of any cancer of unknown primary
origin due to its high rate of identifying the primary tumour [83].
Based on
these analyses, and if the primary tumour remains unknown, patients can be divided
into two prognostic groups – favourable and poor prognosis – based on the
criteria defined by the Eastern Cooperative Oncology Group performance status [29]. All isolated lymph node metastases
(adenocarcinoma in the axilla, squamous cell carcinoma involving cervical lymph
and isolated squamous cell carcinoma-originated inguinal adenopathy) are
associated with the relatively favourable prognosis group. According to the
region affected, the therapy is then site-specific, for example, axillary nodal
dissection, mastectomy or breast irradiation and adjuvant chemotherapy [29]. In a retrospective
series of 365 patients with cervical lymphadenopathy and cancer of unknown
primary origin, the median survival was 45 months [84]. In the poor
prognosis group, which included 80% of patients with cancer of unknown primary
origin, several chemotherapies (mainly platinum-based doublet chemotherapy) did improve
survival significantly [29]. An approach involving only palliative
care is offered to patients with a life expectancy less than 4 months [85]. Wach et al. [86]
compared metastatic squamous cell carcinoma patients with inguinal (and
axillary) lymphadenopathy with cancer of unknown primary origin and those with an
identified primary tumour. They all had radical lymphadenectomies and showed
non-statistically different 5-year overall survival rates of 65% and 49%,
respectively.
Conclusion
The purpose
of this review was to summarize previous research conducted on this complex
topic and to discuss recent updates. The challenge of diagnosing unexplained lymphadenopathy
is the timing and a balance between over-diagnosing self-limiting benign lymphadenopathy
and underestimating life-threatening malignant lymphadenopathy. One of the
difficulties with diagnosing and treating lymphadenopathy is
the multidisciplinarity needed to achieve a correct diagnosis. This review and
the diagnostic algorithm could be used across medical specialities, primarily by
primary care specialists and emergency departments. The aim is to help with the
complex diagnostic process and to offer a clear diagnostic work-up for clinical
practice. The diagnostic strategy is a step-by-step procedure that can prevent unnecessary
surgery and favours ultrasound-guided fine needle aspiration and core needle
biopsy. New diseases like COVID-19 (its infection and vaccination), non-specific
imaging like PET/CT and access to molecular diagnosis techniques represent new
challenges and opportunities in managing lymphadenopathy and
cancers of unknown primary origin. The most important take-home messages have been summarized in figure 5.
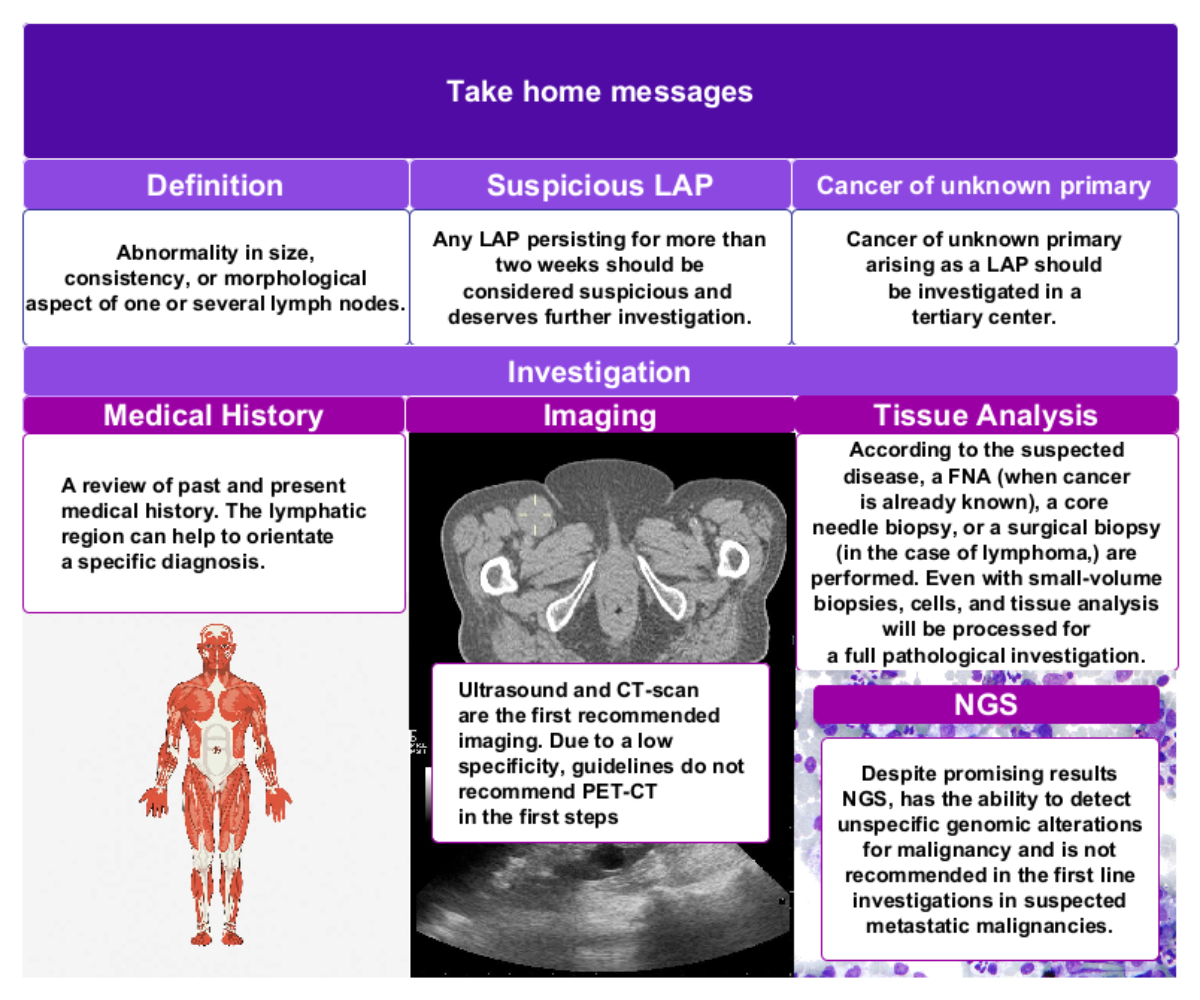
Figure 5Take-home messages. Images used in the figure: human body anatomy hand-drawn
illustration: free public domain CC0 image, no copyright, available from
rawpixel.com; CT imaging: our own archive; ultrasound images of renal cyst:
Creative Commons attribution, author Nevil Dilmen, available from Wikimedia Commons;
micrograph of Hodgkin lymphoma: Creative Commons attribution, author Nephron, available
from Wikimedia Commons.
Prof.
Maurice Matter
Centre
Hospitalier Universitaire Vaudois
Rue du
Bugnon 46
CH-1011
Lausanne
Maurice.Matter[at]chuv.ch
References
1. Gaddey HL, Riegel AM. Unexplained Lymphadenopathy: evaluation and differential diagnosis . Am Fam Physician. 2016 Dec;94(11):896–903. https://www.aafp.org/afp/2016/1201/p896.html
2. Newton MV, Ramesh RS, Manjunath S, ShivaKumar K, Nanjappa HG, Damuluri R, et al. Histological Surprises in Benign Cytologies after Lymph Node Biopsy-Surgeon’s Knife Improving Patient Care. Indian J Surg Oncol. 2017 Jun;8(2):113–8. 10.1007/s13193-016-0577-2
3. Farooq A, Ameen I. Comparison of FNAC vs Excision Biopsy for suspected Tuberculous Cervical Lymphadenopathy . Ann King Edw Med Univ. 2016 Jul;9(3): https://www.annalskemu.org/journal/index.php/annals/article/view/1343 10.21649/akemu.v9i3.1343
4. Health Quality Ontario. The Accuracy of Fine-Needle Aspiration Cytology in the Diagnosis of Lymphoma. Ontario; 2014 Oct. http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/rapid-reviews
5. Novoa E, Gürtler N, Arnoux A, Kraft M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: a meta-analysis and systematic review of the literature. Head Neck. 2012 Oct;34(10):1497–503. 10.1002/hed.21821
6. Morris-Stiff G, Cheang P, Key S, Verghese A, Havard TJ. Does the surgeon still have a role to play in the diagnosis and management of lymphomas? World J Surg Oncol. 2008 Feb;6(1):13. 10.1186/1477-7819-6-13
7. Metzgeroth G, Schneider S, Walz C, Reiter S, Hofmann WK, Marx A, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012 Sep;91(9):1477–84. 10.1007/s00277-012-1476-4
8. Pyo JS, Jung J, Lee SG, Kim NY, Kang DW. Diagnostic Accuracy of Fine-Needle Aspiration Cytology and Core-Needle Biopsy in the Assessment of the Axillary Lymph Nodes in Breast Cancer-A Meta-Analysis. Diagnostics (Basel). 2020 Sep;10(9):717. 10.3390/diagnostics10090717
9. Pynnonen MA, Gillespie MB, Roman B, Rosenfeld RM, Tunkel DE, Bontempo L, et al. Clinical Practice Guideline: Evaluation of the Neck Mass in Adults. Otolaryngol - Head Neck Surg (United States). 2017;157(2_suppl).
10. Khare P. Cytopathological Pattern of Tubercular Lymphadenopathy on FNAC: Analysis of 550 Consecutive Cases. J Clin DIAGNOSTIC Res; 2014.
11. Kühnl A, Cunningham D, Hutka M, Peckitt C, Rozati H, Morano F, et al. Rapid access clinic for unexplained lymphadenopathy and suspected malignancy: prospective analysis of 1000 patients. BMC Hematol. 2018 Aug;18(1):19. 10.1186/s12878-018-0109-0
12. Zenga J, Graboyes EM, Haughey BH, Paniello RC, Mehrad M, Lewis JS, et al. Definitive Surgical Therapy after Open Neck Biopsy for HPV-Related Oropharyngeal Cancer. United States: Otolaryngology - Head and Neck Surgery; 2016. 10.1177/0194599815627642
13. Moor JW, Murray P, Inwood J, Gouldesbrough D, Bem C. Diagnostic biopsy of lymph nodes of the neck, axilla and groin: rhyme, reason or chance? Ann R Coll Surg Engl. 2008 Apr;90(3):221–5. 10.1308/003588408X242105
14. Chau I, Kelleher MT, Cunningham D, Norman AR, Wotherspoon A, Trott P, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003 Feb;88(3):354–61. 10.1038/sj.bjc.6600738
15. World Health Organization GTR. Incidence of tuberculosis (per 100,000 people) - Switzerland. https://data.worldbank.org/indicator/SH.TBS.INCD?locations=CH. 2021.
16. Nathalie Gasser. Cantonal tuberculosis activities 2021. Bern; 2021 Oct.
17. Akkina SR, Kim RY, Stucken CL, Pynnonen MA, Bradford CR. The current practice of open neck mass biopsy in the diagnosis of head and neck cancer: A retrospective cohort study. Laryngoscope Investig Otolaryngol. 2019 Jan;4(1):57–61. 10.1002/lio2.240
18. Mohseni S, Shojaiefard A, Khorgami Z, Alinejad S, Ghorbani A, Ghafouri A. Peripheral lymphadenopathy: approach and diagnostic tools. Iran J Med Sci. 2014 Mar;39(2 Suppl):158–70.
19. Landes M, Maor Y, Mercer D, Habot-Wilner Z, Bilavsky E, Chazan B, et al. Cat Scratch Disease Presenting as Fever of Unknown Origin Is a Unique Clinical Syndrome. Clin Infect Dis. 2020 Dec;71(11):2818–24. 10.1093/cid/ciz1137
20. Freeman AM, Matto P. [] StatPearls []: ; 2024., Available from https://www.ncbi.nlm.nih.gov/books/NBK513250/
21. Miranda RN, Khoury JD, Medeiros LJ. Lymphadenopathy Secondary to Drug-Induced Hypersensitivity Syndrome. Atlas of Lymph Node Pathology. New York (NY): Springer New York; 2013. pp. 157–60. 10.1007/978-1-4614-7959-8_37
22. Mauch PM, Kalish LA, Kadin M, Coleman CN, Osteen R, Hellman S. Patterns of presentation of Hodgkin disease. Implications for etiology and pathogenesis. Cancer. 1993 Mar;71(6):2062–71. 10.1002/1097-0142(19930315)71:6<2062::AID-CNCR2820710622>3.0.CO;2-0
23. Laurent C, Do C, Gourraud PA, De Paiva GR, Valmary S, Brousset P. Prevalence of common non-hodgkin lymphomas and subtypes of hodgkin lymphoma by nodal site of involvement. Volume 94. United States: Medicine; 2015.
24. Al Kadah B, Popov HH, Schick B, Knöbber D. Cervical lymphadenopathy: study of 251 patients. Eur Arch Oto-Rhino-Laryngology. 2015;272(3). 10.1007/s00405-014-3315-9
25. Aulino JM, Kirsch CF, Burns J, Busse PM, Chakraborty S, Choudhri AF, et al.; Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® Neck Mass-Adenopathy. J Am Coll Radiol. 2019 May;16(5 5S):S150–60. 10.1016/j.jacr.2019.02.025
26. Ouyang L, Shi ZY, Lin ZG. 18F-FDG PET/CT makes a significant contribution to diagnosis of malignancy in patients with cervical lymphadenopathy: a study using optimal scale regression tests. Chin Med J (Engl). 2013 Feb;126(4):659–67. 10.3760/cma.j.issn.0366-6999.20121483
27. Redondo-Cerezo E, Martínez-Cara JG, Esquivias J, de la Torre-Rubio P, González-Artacho C, García-Marín MC, et al. Endoscopic ultrasonography-fine needle aspiration versus PET-CT in undiagnosed mediastinal and upper abdominal lymphadenopathy: a comparative clinical study. Eur J Gastroenterol Hepatol. 2015 Apr;27(4):455–9. 10.1097/MEG.0000000000000302
28. Chen J, Xu D, Sun WJ, Wang WX, Xie NN, Ruan QR, et al. Differential diagnosis of lymphoma with 18F-FDG PET/CT in patients with fever of unknown origin accompanied by lymphadenopathy. J Cancer Res Clin Oncol. 2023 Aug;149(10):7187–96. 10.1007/s00432-023-04665-7
29. Krämer A, Bochtler T, Pauli C, Baciarello G, Delorme S, Hemminki K, et al.; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Cancer of unknown primary: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023 Mar;34(3):228–46. 10.1016/j.annonc.2022.11.013
30. David S. Ettinger M, Marvaretta M. Stevenson MC. Occult Primary (Cancer of Unknown Primary [CUP]). 2022 Dec.
31. Caputo A, Caleo A, Cozzolino I, Zeppa P, Ciancia G, Ciliberti V. COVID-19 post-vaccination lymphadenopathy: A review of the use of fine needle aspiration cytology. Cytopathology. 2023 Sep;34(5):423–32. 10.1111/cyt.13221
32. Wilczynski A, Görg C, Timmesfeld N, Ramaswamy A, Neubauer A, Burchert A, et al. Value and Diagnostic Accuracy of Ultrasound-Guided Full Core Needle Biopsy in the Diagnosis of Lymphadenopathy: A Retrospective Evaluation of 793 Cases. J Ultrasound Med. 2020 Mar;39(3):559–67. 10.1002/jum.15134
33. Johl A, Lengfelder E, Hiddemann W, Klapper W; German Low-grade Lymphoma Study Group (GLSG). Core needle biopsies and surgical excision biopsies in the diagnosis of lymphoma-experience at the Lymph Node Registry Kiel. Ann Hematol. 2016 Aug;95(8):1281–6. 10.1007/s00277-016-2704-0
34. Eichenauer DA, Aleman BM, André M, Federico M, Hutchings M, Illidge T, et al.; ESMO Guidelines Committee. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018 Oct;29 Suppl 4:iv19–29. 10.1093/annonc/mdy080
35. Seviar D, Yousuff M, Chia Z, Ramesar K, Newman J, Howlett DC. Image-guided core needle biopsy as the first-line diagnostic approach in lymphoproliferative disorders-A review of the current literature. Eur J Haematol. 2021 Feb;106(2):139–47. 10.1111/ejh.13532
36. Warshavsky A, Rosen R, Perry C, Muhanna N, Ungar OJ, Carmel-Neiderman NN, et al. Core needle biopsy for diagnosing lymphoma in cervical lymphadenopathy: meta-analysis. Head Neck. 2020 Oct;42(10):3051–60. 10.1002/hed.26381
37. Pizzi M, Sbaraglia M, Dal Santo L, De Bartolo D, Santoro L, Scarmozzino F, et al. Higher accuracy of surgical over core needle biopsy for the diagnosis of lymphoproliferative disorders. Int J Lab Hematol. 2023 Aug;45(4):516–21. 10.1111/ijlh.14055
38. Shah A, Ross C, Sur M. An approach to small lymph node biopsies: pearls and pitfalls of reporting in the real world. J Am Soc Cytopathol. 2021;10(3):328–37. 10.1016/j.jasc.2020.12.006
39. Volaric AK, Lin O, Balassanian R, Cook S, Falchi L, Fitzpatrick MJ, et al.; Cyto-Heme Institutional Collaborative (CHIC) Consortium. Diagnostic Discrepancies in Small-volume Biopsy for the Initial Diagnosis, Recurrence, and Transformation of Follicular Lymphoma: A Multi-Institutional Collaborative Study. Am J Surg Pathol. 2023 Feb;47(2):212–7. 10.1097/PAS.0000000000001985
40. Caputo A, Fraggetta F, Cretella P, Cozzolino I, Eccher A, Girolami I, et al. Digital Examination of LYmph node CYtopathology Using the Sydney system (DELYCYUS): an international, multi-institutional study. Cancer Cytopathol. 2023 Nov;131(11):679–92. 10.1002/cncy.22741
41. Al-Abbadi MA, Barroca H, Bode-Lesniewska B, Calaminici M, Caraway NP, Chhieng DF, et al. A Proposal for the Performance, Classification, and Reporting of Lymph Node Fine-Needle Aspiration Cytopathology: the Sydney System. Acta Cytol. 2020;64(4):306–22. 10.1159/000506497
42. Peluso AL, Ieni A, Mignogna C, Zeppa P. Lymph Node Fine-Needle Cytology: Beyond Flow Cytometry. Acta Cytol. 2016;60(4):372–84. 10.1159/000447734
43. Prudent E, La Scola B, Drancourt M, Angelakis E, Raoult D. Molecular strategy for the diagnosis of infectious lymphadenitis. Eur J Clin Microbiol Infect Dis. 2018 Jun;37(6):1179–86. 10.1007/s10096-018-3238-2
44. Afzal W, Arab T, Ullah T, Teller K, Doshi KJ. Generalized Lymphadenopathy as Presenting Feature of Systemic Lupus Erythematosus: Case Report and Review of the Literature. J Clin Med Res. 2016 Nov;8(11):819–23. 10.14740/jocmr2717w
45. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000 Jul;75(7):723–32. 10.1016/S0025-6196(11)64620-X
46. Victor C, Kota AA, Colney L, Roopavathana B, Chase S, Nayak S. An audit to study the diagnostic yield of lymph node biopsies under local anaesthesia. Int Surg J. 2020;7(6):1804. 10.18203/2349-2902.isj20202385
47. Storck K, Brandstetter M, Keller U, Knopf A. Clinical presentation and characteristics of lymphoma in the head and neck region. Head Face Med. 2019 Jan;15(1):1. 10.1186/s13005-018-0186-0
48. Akinci S, Silay K, Hacibekiroglu T, Ulas A, Basturk A, Bakanay SM, et al. The predictive value of epidemiological characteristics, clinical and laboratory findings in adult lymphadenopathy etiology. Eur Rev Med Pharmacol Sci. 2015 Aug;19(16):2973–7.
49. Franzen A, Günzel T, Buchali A, Coordes A. Etiologic and differential diagnostic significance of tumor location in the supraclavicular fossa. Laryngoscope. 2018 Mar;128(3):646–50. 10.1002/lary.26775
50. Ahamed SH, Agarwal AK, Raju PP. Metastatic prostate carcinoma presenting as supraclavicular lymphadenopathy - is it unusual? Ann R Coll Surg Engl. 2006 Oct;88(6):W4-5. 10.1308/147870806X129278
51. Carrier C. Adénopathie biopsie ou observation? Méd Qué. 2012 Oct;47(10):39–44.
52. Husain M, Khan S, Bhat A, Hajini F. Accessory breast tissue mimicking pedunculated lipoma. Case Reports. 2014 Jul 8;2014(jul08 1):bcr2014204990–bcr2014204990. 10.1136/bcr-2014-204990
53. Shipchandler TZ, Lorenz RR, McMahon J, Tubbs R. Supraclavicular lymphadenopathy due to silicone breast implants. Arch Otolaryngol Head Neck Surg. 2007 Aug;133(8):830–2. 10.1001/archotol.133.8.830
54. Olivier JB, Verhaeghe JL, Butarelli M, Marchal F, Houvenaeghel G. Anatomie fonctionnelle du drainage lymphatique du sein : apport de la technique du lymphonoeud sentinelle. Ann Chir. 2006 Dec;131(10):608–15. 10.1016/j.anchir.2006.06.011
55. Lathrop KI, Kaklamani V. Unknown Primary Presenting With Axillary Lymphadenopathy. The Breast. Elsevier; 2018. pp. 1000–1003.e1. 10.1016/B978-0-323-35955-9.00079-9
56. D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS® -Atlas, breast imaging reporting and data system. 5th edn. Reston, VA, USA; 2013.
57. Newman LA, Kuerer HM, Fornage B, Mirza N, Hunt KK, Ross MI, et al. Adverse prognostic significance of infraclavicular lymph nodes detected by ultrasonography in patients with locally advanced breast cancer. Am J Surg. 2001 Apr;181(4):313–8. 10.1016/S0002-9610(01)00588-8
58. Hiller N, Goldberg SN, Cohen-Cymberknoh M, Vainstein V, Simanovsky N. Lymphadenopathy Associated With the COVID-19 Vaccine. Cureus. 2021 Feb;13(2):e13524.
59. Taweesedt PT, Surani S. Mediastinal lymphadenopathy in COVID-19: A review of literature. World J Clin Cases. 2021 Apr;9(12):2703–10. 10.12998/wjcc.v9.i12.2703
60. Schroeder DG, Jang S, Johnson DR, Takahashi H, Navin PJ, Broski SM, et al. Frequency and Characteristics of Nodal and Deltoid FDG and 11C-Choline Uptake on PET Performed After COVID-19 Vaccination. AJR Am J Roentgenol. 2021 Nov;217(5):1206–16. 10.2214/AJR.21.25928
61. Schiaffino S, Pinker K, Magni V, Cozzi A, Athanasiou A, Baltzer PA, et al. Axillary lymphadenopathy at the time of COVID-19 vaccination: ten recommendations from the European Society of Breast Imaging (EUSOBI). Insights Imaging. 2021 Aug;12(1):119. 10.1186/s13244-021-01062-x
62. Hagen C, Nowack M, Messerli M, Saro F, Mangold F, Bode PK. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med Wkly. 2021 Jul;151(2930):w20557. 10.4414/smw.2021.20557
63. Vaz N, Franquet E, Heidari P, Chow DZ, Jacene HA, Ng TS. COVID-19: findings in nuclear medicine from head to toe. Clin Imaging. 2023 Jul;99:10–8. 10.1016/j.clinimag.2023.04.003
64. Bontumasi N, Jacobson JA, Caoili E, Brandon C, Kim SM, Jamadar D. Inguinal lymph nodes: size, number, and other characteristics in asymptomatic patients by CT. Surg Radiol Anat. 2014 Dec;36(10):1051–5. 10.1007/s00276-014-1255-0
65. Hamilton W, Pascoe J, John J, Coats T, Davies S. Diagnosing groin lumps. Vol. 372. BMJ. 2021;n578. 10.1136/bmj.n578
66. Qin L, Zhao C, Wang H, Yang J, Chen L, Su X, et al. Detection of inguinal lymph nodes is promising for the diagnosis of periprosthetic joint infection. Front Cell Infect Microbiol. 2023 Apr;13:1129072. 10.3389/fcimb.2023.1129072
67. Chalif J, Yao M, Gruner M, Kuznicki M, Vargas R, Rose PG, et al. Incidence and prognostic significance of inguinal lymph node metastasis in women with newly diagnosed epithelial ovarian cancer. Gynecol Oncol. 2022 Apr;165(1):90–6. 10.1016/j.ygyno.2022.01.026
68. Swan MC, Furniss D, Cassell OC. Surgical management of metastatic inguinal lymphadenopathy. BMJ. 2004 Nov;329(7477):1272–6. 10.1136/bmj.329.7477.1272
69. Giri S, Shah SH, Batra K, Anu-Bajracharya, Jain V, Shukla H, et al. Presentation and Management of Inguinal Lymphadenopathy in Ovarian Cancer. Indian J Surg Oncol. 2016 Dec;7(4):436–40. 10.1007/s13193-016-0556-7
70. Sehgal IS, Dhooria S, Aggarwal AN, Agarwal R. Impact of Rapid On-Site Cytological Evaluation (ROSE) on the Diagnostic Yield of Transbronchial Needle Aspiration During Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Chest. 2018 Apr;153(4):929–38. 10.1016/j.chest.2017.11.004
71. Scano V, Fois AG, Manca A, Balata F, Zinellu A, Chessa C, et al. Role of EBUS-TBNA in Non-Neoplastic Mediastinal Lymphadenopathy: review of Literature. Diagnostics (Basel). 2022 Feb;12(2):512. 10.3390/diagnostics12020512
72. Pathak V, Adhikari N, Conklin C. Management of Isolated Thoracic Lymphadenopathy of Unclear Etiology: A Survey of Physicians and Literature Review. Cureus. 2023 Jul;15(7):e41867. 10.7759/cureus.41867
73. Yardimci VH, Yardimci AH. An Unusual First Manifestation of Hodgkin Lymphoma: Epitrochlear Lymph Node İnvolvement-A Case Report and Brief Review of Literature. J Investig Med High Impact Case Rep. 2017 Apr;5(2):2324709617706709. 10.1177/2324709617706709
74. Catalano O, Nunziata A, Saturnino PP, Siani A. Epitrochlear lymph nodes: Anatomy, clinical aspects, and sonography features. Pictorial essay(). J Ultrasound. 2010 Dec;13(4):168–74. 10.1016/j.jus.2010.10.010
75. Matter M, Nicod Lalonde M, Allaoua M, Boubaker A, Liénard D, Gugerli O, et al. The role of interval nodes in sentinel lymph node mapping and dissection for melanoma patients. J Nucl Med. 2007 Oct;48(10):1607–13. 10.2967/jnumed.107.041707
76. Musters GD, Kleipool RP, Bipat S, Maas M. Features of the popliteal lymph nodes seen on musculoskeletal MRI in a Western population. Skeletal Radiol. 2011 Aug;40(8):1041–5. 10.1007/s00256-010-1093-z
77. Hatta N, Morita R, Yamada M, Takehara K, Ichiyanagi K, Yokoyama K. Implications of popliteal lymph node detected by sentinel lymph node biopsy. Dermatol Surg. 2005 Mar;31(3):327–30. 10.1097/00042728-200503000-00014
78. Steen ST, Kargozaran H, Moran CJ, Shin-Sim M, Morton DL, Faries MB. Management of popliteal sentinel nodes in melanoma. J Am Coll Surg. 2011 Jul;213(1):180–6. 10.1016/j.jamcollsurg.2011.01.062
79. Thompson JF, Hunt JA, Culjak G, Uren RF, Howman-Giles R, Harman CR. Popliteal lymph node metastasis from primary cutaneous melanoma. Eur J Surg Oncol. 2000 Mar;26(2):172–6. 10.1053/ejso.1999.0765
80. Dy BM, Shaha AR, Tuttle RM. The Delphian node revisited: an uncommon site of recurrence. J Endocr Soc. 2017 Nov;1(12):1527–30. 10.1210/js.2017-00333
81. Nikolova PN, Hadzhiyska VH, Mladenov KB, Ilcheva MG, Veneva S, Grudeva VV, et al. The impact of 18F-FDG PET/CT in the clinical management of patients with lymph node metastasis of unknown primary origin. Neoplasma. 2021 Jan;68(1):180–9. 10.4149/neo_2020_200315N263
82. Reinert CP, Sekler J, la Fougère C, Pfannenberg C, Gatidis S. Impact of PET/CT on clinical management in patients with cancer of unknown primary-a PET/CT registry study. Eur Radiol. 2020 Mar;30(3):1325–33. 10.1007/s00330-019-06518-9
83. Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie e. V. Positronenemissionstomographie (PET) in der Onkologie. https://www.dgho.de/publikationen/stellungnahmen/g-ba/pet-pet-ct/positronenemissionstomographie-in-der-onkologie-2021.pdf. 2021.
84. Zhou M, Wu Y, Wu Y, Li H, Ye B, Yue K, et al. Clinical characteristics and outcomes of cervical lymph node metastasis from unknown primary sites: a single institution’s 14-year experience. Eur J Med Res. 2023 Jan;28(1):5. 10.1186/s40001-022-00957-9
85. Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G; ESMO Guidelines Committee. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up . Ann Oncol. 2015 Sep;26 Suppl 5:v133–8. https://linkinghub.elsevier.com/retrieve/pii/S0923753419471858 10.1093/annonc/mdv305
86. Wach MM, van Beek E, Ayabe R, Ruff S, Brown Z, Goldman DA, et al. Metastatic squamous cell carcinoma of known and unknown primary origin treated with axillary or inguinal lymphadenectomy. Am J Surg. 2018 Nov;216(5):963–8. 10.1016/j.amjsurg.2018.06.006
87. Richner S, Laifer G. Peripheral lymphadenopathy in immunocompetent adults. Swiss Med Wkly. 2010 Feb;140(7-8):98–104. 10.4414/smw.2010.12892




