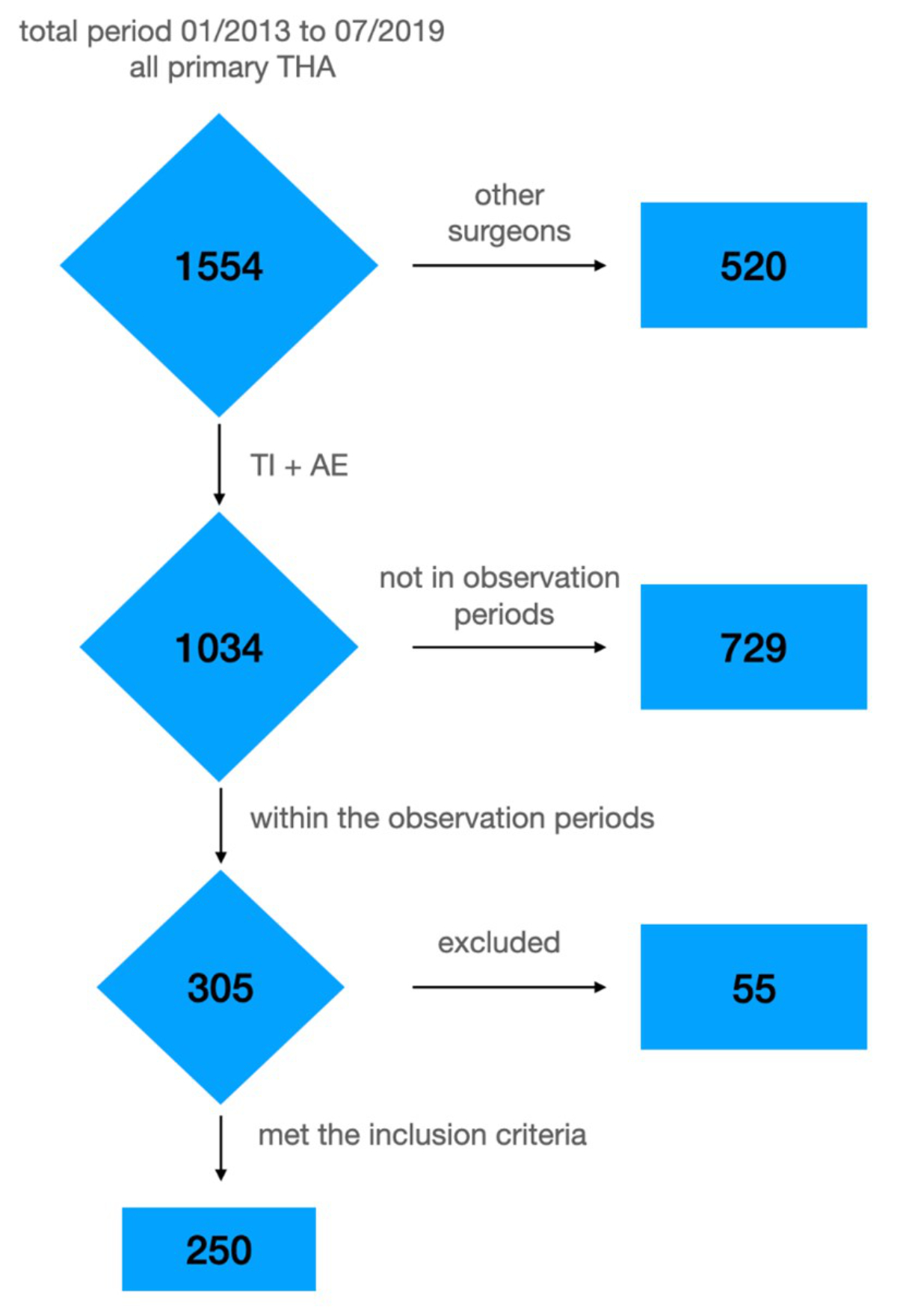
Figure 1Flowchart of all hip replacements performed at the hospital and the excluded hips.THA: total hip arthroplasty; AE: Anke Eckardt; TI: Thomas Ilchmann.
DOI: https://doi.org/https://doi.org/10.57187/s.3537
The number of total hip arthroplasties is growing each year in almost every Organisation for Economic Cooperation and Development (OECD) country [1], leading to a high economic burden on the healthcare systems. Furthermore, in ageing societies, the increased number of polymorbid patients necessitates more complex treatment. Therefore, it is mandatory to use available medical resources more efficiently by implementing evidence-based, safe, and cost-effective treatment pathways.
Several enhanced recovery programs for total hip arthroplasty have been introduced during the past decade [2–6]. The purpose of these programs is to optimise the whole treatment process, reduce the length of hospital stay, and decrease overall costs without compromising treatment quality [6]. The effects of implementing such programs depend on the healthcare systems and can differ considerably between countries. So far, to the best of our knowledge, no such data available for the Swiss population.
In 2014, a surgeon at our hospital introduced a standardised treatment protocol for his patients. In 2018, this protocol was developed further as an enhanced recovery program by adding a more extensive preoperative workup and incorporating all care providers.
This retrospective cohort study investigated the effects of these changes to clinical routines on peri- and postoperative outcome parameters.
We hypothesised that with the implementation process of the standardised treatment protocol and the enhanced recovery program, the primary outcomes (length of stay and discharge destination) and other clinical parameters would improve without affecting the rate of adverse events.
The study was performed at Hirslanden Klinik Birshof, (Münchenstein, Switzerland), a privately owned hospital at which orthopaedic surgeons work as independent consultants. The hospital has a public order to provide care for all patients with health insurance in Switzerland and is thus open to the whole local population.
The study was approved by the local ethics committee (Project-ID: 2019-01798). Patients provided informed global consent that their clinical data could be used for scientific studies.
This study included patients scheduled for elective, primary, unilateral total hip arthroplasty without previous hip surgery, disregarding their other musculoskeletal health or insurance status. Patients with bilateral simultaneous hip replacement, urgent total hip replacement due to trauma or acute hip disease, or previous surgery on the same hip were excluded (figure 1).

Figure 1Flowchart of all hip replacements performed at the hospital and the excluded hips.THA: total hip arthroplasty; AE: Anke Eckardt; TI: Thomas Ilchmann.
A baseline group (series 0) of 50 consecutive patients who underwent operations by a single experienced surgeon (AE) and met the inclusion criteria were assessed retrospectively (January to September 2013). This group reflects the routine protocol at the hospital before the implementation of the standardised treatment protocol. The implant was either a cemented MS-30® stem or an uncemented Alloclassic® stem combined with an uncemented Allofit® cup (all implants were from Zimmer/Biomet®, Winterthur, Switzerland). Patients in this group were admitted to the hospital the day before surgery and received benzodiazepine in the morning (midazolam p.o., Dormicum® 7.5 mg) and right before surgery (i.v., e.g. 0.5 mg midazolam), which was typically repeated during the surgery, combined with an opioid (i.v., 50 μg fentanyl). All patients had a urinary catheter for 24 hours. Methadone or morphine (i.v.) was routinely administered in the recovery room, combined with non-steroidal anti-inflammatory drugs (NSAIDs), metamizole, or paracetamol (i.v.). Standardised peroral pain medication was administered postoperatively (NSAIDs = 2 × 300 mg etodolac (Lodine®), 4 × 1 g paracetamol, 1 g metamizole up to four times in 24 hours as backup medication, and morphine i.v., if needed). Low-molecular-weight heparin (dalteparin, Fragmin® 5000 IU s.c. daily) was used as prophylaxis for thromboembolism starting on the day of operation, and rivaroxaban (Xarelto®) was administered after discharge for 4 weeks. No drainages were used. Tranexamic acid was not administered. First-time mobilisation was expected on the day of surgery or the first postoperative day. No standardised protocol was followed for postoperative erythrocyte transfusions.
In April 2014, a new surgeon (TI) started his practice at our hospital and introduced a standardised treatment protocol for his patients. From that date, data were collected prospectively. The operations on these patients were performed with a direct anterior approach (Smith–Peterson). All implants were uncemented cups (RM Pressfit vitamys, Mathys, Bettlach, Switzerland) combined with cemented or uncemented femoral stems (both twinSys, Mathys, Bettlach, Switzerland). No drainages were applied; occlusive dressings were used and changed on the day of discharge. With the introduction of the standardised treatment protocol, the following measures were implemented: admission on the day of surgery, no preoperative sedative, dexamethasone 4–8 mg i.v., tranexamic acid 1 g i.v. 30 min before surgery and 1 g into the joint at the end of the operation, strict transfusion criteria depending on clinical symptoms, and no urinary catheters. In the recovery room, ketorolac (Tora-dol®) 30 mg i.v. and metamizol (Novalgin®) 1 g p.o. were administered postoperatively. The later postoperative analgesia was standardised (ibuprofen 600 mg p.o. 3×/d and metamizole 1 g p.o. 3×/d, if appropriate for the patient). Additionally, oxycodone p.o. was administered on demand. Low-molecular-weight heparin (dalteparin, Fragmin® 5000 IU s.c. daily) was used as prophylaxis for thromboembolism from the day of operation, and rivaroxaban (Xarelto®) was prescribed after discharge for 4 weeks.
Patients were mobilised on the day of surgery, and full weight bearing without further restrictions was encouraged. Discharge was suggested when the patient fulfilled the defined criteria (independent mobilisation and stairclimbing) and felt prepared enough to leave the hospital. No maximum length of stay was defined preoperatively, and no pressure was placed on patients regarding discharge even if all criteria were fulfilled. The patients were discharged to a rehabilitation hospital if they were assumed to be unable to manage their daily life at home or if it was the patient’s wish and it was covered by their insurance.
Data from two series of 50 consecutive patients who underwent operations and met the inclusion criteria were analysed, being treated according to standardised treatment protocol. The first series (series 1, April 2014 to January 2015) included the first 50 patients after the protocol was introduced; these patients were part of a pilot phase to allow adjustment to any possible learning curve. The second series (series 2, November 2016 to June 2017) included patients who underwent operations after the protocol had been well-established.
In January 2018, an enhanced recovery program was initiated, and further measures were added: An information booklet was developed by all care providers (surgeons, anaesthesiologists, nursing staff, and physiotherapists) and handed out after the operation was scheduled at an interdisciplinary information event (involving all care providers) that gathered patients and their relatives to outline the upcoming hospital stay. A standardised preoperative workup was organised, including the consultation of a nurse who reported illnesses and actual medications and a consultation with a specialist in internal medicine if needed (e.g. in cases of anaemia, immunomodulating medications, or medications affecting haemostasis). It was communicated that the planned length of stay would be 5 days maximum but that no pressure would be applied to the patients regarding the actual discharge date. The criteria for inpatient rehabilitation remained the same.
The third series (series 3, January 2018 to June 2018) included the first 50 patients treated under the enhanced recovery program. Like series 1, this series was meant to allow for adjustment to a potential learning curve. The fourth series (series 4, January 2019 to July 2019) included patients who underwent surgery 1 year after the introduction of the enhanced recovery program when the protocol was well-established.
The time periods of operation for the groups analysed are illustrated in figure 2. The number of 50 patients per group was chosen to ensure that a reasonable number of patients was included in each group and that sufficient time had passed between the observation periods to assess changes in the outcomes.
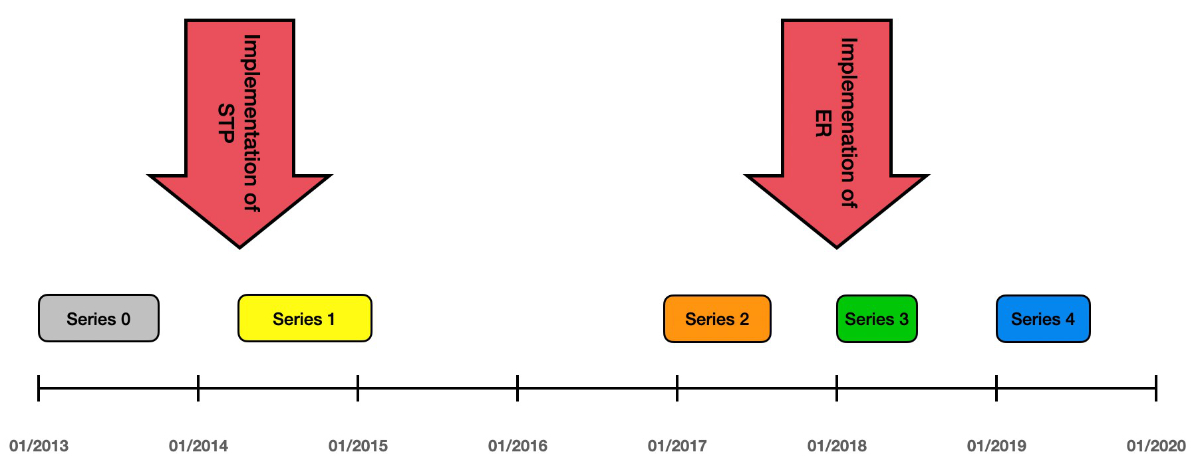
Figure 2Treatment periods of series 0 to 4 on a time axis. ER: enhanced recovery program; STP: standardised treatment protocol.
The patient and perioperative data were routinely documented for any operation performed at the hospital and were taken from the charts without following a specific protocol. Postoperative consultations with the surgeon were scheduled for all patients at 6 and 12 weeks and after 1 year.
On the day of admission, length of stay (nights postoperatively) and discharge destination (home or rehabilitation) were assessed. The following data were recorded for all patients: age, sex, living alone, body mass index, American Society of Anaesthesiologists (ASA) score, medication affecting haemostasis (Aspirin®, vitamin K antagonists, and factor Xa inhibitors), preoperative anaemia (World Health Organization 2011, haemoglobin level <120 g/l (women), <130 g/l (men) [7]), and cemented or uncemented stems (table 1). In addition, the following outcomes were analysed: the use of urinary catheters, blood transfusions, difference in pre- and postoperative haemoglobin, administration of opioid drugs at any time, and adverse events within 3 months of surgery.
Table 1Baseline characteristics, series 0–4 (50 patients each).
| Series 0 | Series 1 | Series 2 | Series 3 | Series 4 | p-value | |
| Age, years: median (IQR) | 65 (52.5 to 73) | 72 (64 to 77.8) | 71.5 (64 to 75) | 68.5 (59.2 to 74.8) | 71 (64 to 78.8) | 0.029* |
| Sex – female: n (%) | 30 (60%) | 28 (56%) | 29 (58%) | 29 (58%) | 24 (48%) | 0.799** |
| Living alone (yes): n (%) | 6 (12%) | 15 (30%) | 17 (34%) | 13 (26%) | 15 (30%) | 0.086** |
| Body mass index: median (IQR) | 26.2 (22.9 to 29.9) | 26.4 (23.5 to 28.4) | 24.7 (22.7 to 28.8) | 27 (24.4 to 30.1) | 25.1 (23.6 to 28.3) | 0.455* |
| ASA score – 1: n (%) | 9 (18%) | 10 (20%) | 11 (22%) | 11 (22%) | 10 (20%) | 0.989** |
| ASA score – 2: n (%) | 26 (52%) | 26 (52%) | 26 (52%) | 29 (58%) | 26 (52%) | |
| ASA score – 3: n (%) | 15 (30%) | 14 (28%) | 13 (26%) | 10 (20%) | 14 (28%) | |
| Medication affecting haemostasis: n (%) | 11 (22%) | 18 (36%) | 17 (34%) | 8 (16%) | 13 (26%) | 0.132** |
| Preoperative anaemia: n (%) | 2 (4%) | 5 (10%) | 5 (10%) | 3 (6%) | 0 | 0.132** |
| Implants – cemented stem: n (%) | 30 (60%) | 15 (30%) | 21 (42%) | 19 (38%) | 29 (58%) | 0.009** |
| Admission at the day of surgery: n (%) | 1 (2%) | 14 (28%) | 33 (66%) | 50 (100%) | 49 (98%) | <0.001** |
* Kruskal–Wallis rank sum test
** Fisher’s exact test
Clinical and radiological follow-ups were routinely performed in all patients 6 and 12 weeks after surgery. All patients had further routine follow-ups 1 year after surgery that were unrelated to this study.
Descriptive data analyses were performed to summarise baseline and procedure characteristics. Qualitative data are summarised as the number of observations and percentages. Quantitative data are reported with appropriate descriptive statistics; normally distributed data are reported as the mean and standard deviation, and non-normally distributed data are reported as the median and interquartile range (IQR).
First, an omnibus test was employed to identify differences in outcome measures between the five series (0 to 4). Second, a post hoc analysis was used to assess differences between series 4 and the other series (0 to 3). For continuous data, QQ plots and Shapiro-Wilk normality tests were used to check the normality of the distribution. Since none of the continuous variables tested were normally distributed, Kruskal-Wallis tests were used to check for differences between the series. To compare series 0–3 with series 4, a nonparametric Dunn comparison test was used for Kruskal-type ranked data. For categorical data, Fisher’s exact test was used as an omnibus test to check for differences between series. For post hoc comparison of series 0–3 with series 4, pairwise Fisher’s exact tests with the Benjamini-Hochberg method were used to control for the false discovery rate due to multiple testing.
Furthermore, the outcomes of series 0 patients were compared with those of patients in series 1–4 combined with the Wilcoxon rank sum test for continuous data and Fisher’s exact test for categorical data. Fisher’s exact test was used to investigate whether the proportion of blood transfusions differed between patients with and without anaemia. The Wilcoxon rank sum test was used to test whether the length of stay differed between patients discharged directly home and patients discharged to a rehabilitation clinic.
In the subgroup of patients living alone, the proportion of patients discharged to a rehabilitation clinic was assessed using Fisher’s exact test.
All statistical analyses were performed in R (R Core Team, http://www.R-project.org/).
All patients were seen 6 and 12 weeks after surgery at the corresponding surgeon’s practice. Patients in series 0 were younger than those in series 1 to 4 (p = 0.006) and a lower percentage of patients were living alone in series 0 than in the other series (12% in series 0 vs. 26–34% in series 1–4, p = 0.011). More uncemented stems were used in series 1, 2, and 3 than in series 4 (70%, 58%, and 62% vs 42%, p = 0.034, p = 0.215, and p = 0.142, respectively, table 1). No statistically significant differences were observed in sex, body mass index, ASA score, or medications affecting haemostasis between the five groups (%) 1). Moreover, the incidence of anaemia did not differ significantly between series 0 and series 1 to 3, but no patients in series 4 had anaemia (p = 0.132, table 1)
In series 0, the median length of stay was 10 days, whereas the median length of stay was 7 days in series 1 and only 5 days in series 4 (table 2; series 1 vs. series 4: p <0.001). Only one patient had a length of stay of ≤5 days in series 0, and this increased to 10 patients (20%) in series 1 and 36 patients (72%) in series 4 (table 2; series 1 vs. series 4: p <0.001). No patients were discharged early (length of stay ≤3 days) in series 0, whereas 7 patients could be discharged early in each series from series 2 to 4 (table 2). The median length of stay of all patients was longer in patients who were discharged to a rehabilitation hospital compared with those who were discharged home (7 vs. 5 days, p <0.001). This difference decreased with the implementation of the enhanced recovery program (table 2).
Table 2Study outcomes by series.
| Series 0 | Series 1 | Series 2 | Series 3 | Series 4 | p-value | |
| Delta haemoglobin: median (IQR) | 29 (25–36) | 25.5 (21–33) | 24 (22–31.5) | 24 (18.8–34) | 25 (21.2–31) | 0.172* |
| Blood loss (ml): median (IQR) | 500 (338–600) | 350 (200–475) | 300 (250–400) | 300 (200–400) | 300 (200–400) | <0.001* |
| Blood transfusion: n (%) | 5 (10%) | 2 (4%) | 3 (6%) | 0 | 2 (4%) | 0.245** |
| Urinary catheter: n (%) | 50 (100%) | 36 (72%) | 3 (6%) | 0 | 0 | <0.001** |
| No opioids: n (%) | 5 (10%) | 3 (6%) | 12 (24%) | 17 (34%) | 18 (36%) | <0.001** |
| Discharge home: n (%) | 33 (66%) | 29 (58%) | 29 (58%) | 39 (78%) | 42 (84%) | 0.013** |
| Length of stay: median (IQR) | 10 (9–11) | 7 (6–8) | 5 (4–6) | 5 (4–5) | 5 (4–6) | <0.001* |
| Length of stay ≤5 days: n (%) | 1 (2%) | 10 (20%) | 31 (62%) | 41 (82%) | 36 (72%) | <0.001** |
| Length of stay ≤3 days: n (%) | 0 | 2 (4%) | 7 (14%) | 7 (14%) | 7 (14%) | 0.01** |
| Length of stay when discharged to a rehab clinic: median (IQR) | 10 (9–11) | 7 (7–8) | 6 (5–7) | 5 (5–5.5) | 5 (4.8–5.2) | <0.001* |
| Length of stay when discharged to home: median (IQR) | 9 (8–10) | 7 (5–8) | 5 (4–5) | 5 (4–5) | 5 (4–6) | <0.001* |
* Kruskal-Wallis rank sum test
** Fisher’s exact test
In series 0, 33 patients (66%) were discharged home. The number of patients who were discharged home increased steadily from 29 patients (58%) in series 1 to 42 patients (84%) in series 4 (p = 0.015; table 3). Living alone was positively associated with discharge to a rehabilitation hospital (p <0.001). In series 0, five of six patients who lived alone were discharged to a rehabilitation centre. In series 1–4, fewer patients who lived alone were discharged to an institution (11 of 15 in series 1; 5 of 15 in series 4) (p = 0.104, table 3).
Table 3Patients living alone discharged to rehab centres vs. those discharged to their homes.
| Series 0 | Series 1 | Series 2 | Series 3 | Series 4 | p-value | ||||||
| Rehab | Home | Rehab | Home | Rehab | Home | Rehab | Home | Rehab | Home | ||
| Living alone: n (%) | 5 (83.3%) | 1 (16.7%) | 11 (73.3%) | 4 (26.7%) | 12 (70.6%) | 5 (29.4%) | 7 (53.8%) | 6 (46.2%) | 5 (33.3%) | 10 (66.7%) | <0.001* |
| Living with a partner: n (%) | 12 (27.3%) | 32 (72.7%) | 10 (28.6%) | 25 (71.4%) | 9 (27.3%) | 24 (72.7%) | 4 (10.8%) | 33 (89.2%) | 3 (8.6%) | 32 (91.4%) | |
*Cochran-Mantel-Haenszel Chi-Squared Test
One patient in series 0 was admitted on the day of surgery. This number increased from 14 patients (28%) in series 1 to all patients in series 3 and all but one in series 4 (series 1 vs series 4: p <0.001).
The median blood loss (500 ml vs. 300 ml, p <0.001), median difference in pre- and postoperative haemoglobin (29 g/dl vs. 25 g/dl, p = 0.145), and the number of blood transfusions (5 versus 2, p = 0.99) were higher in the series 0 than in series 4.
There was no difference in blood loss, pre- and postoperative haemoglobin and blood transfusions between series 1 to 4. Blood transfusions were performed in 4 of 15 patients with preoperative anaemia (27%) and 8 of the 235 patients without anaemia (3%).
Five patients in series 0 were treated without the use of opioids. The number of patients treated without opioids increased in a stepwise pattern from 3 (6%) in series 1 to 18 (36%) in series 4 (series 1 vs. series 4: p = 0.002, table 2). Furthermore, in series 3 and 4, most of the patients received just one peroral opioid administration on the first postoperative night.
All patients in series 0 received a urinary catheter. The use of urinary catheters decreased from 36 in series 1 to no catheters in series 3 and 4 (series 1 vs. series 4: p <0.001, table 2).
No reoperations were performed in series 0; one superficial wound infection occurred (treated with antibiotics during the same hospital stay), and no further adverse events occurred. Six patients (3%) required reoperation in series 1–4. One reoperation was necessary because of early subsidence of the stem 5 days after surgery (technical error, series 1), which was treated with a change of the stem; one was performed to evacuate a postoperative hematoma (during the same hospital stay, series 2), one was performed for deep infection (series 2, treated with debridement and antibiotics, overall incidence of deep infection: 0.4%), two were necessary because of periprosthetic fractures (series 3 and 4, overall incidence of periprosthetic fracture: 0.8%), and one was performed for superficial wound infection (during the same hospital stay, revision 1 week after surgery, series 3, overall incidence of superficial infection: 0.8%).
One hip dislocation occurred (readmission, closed reduction) after six weeks (series 3, overall incidence of dislocation: 0.4%). One patient had a thrombophlebitis (series 2) and one had a deep venous thrombosis (series 4), both of whom were treated uneventfully. No pulmonary embolisms were observed, and the overall rate of thromboembolic events in series 0–4 was 0.4%.
Fewer adverse events occurred in series 0 than in series 1–4, but this difference was not significant (p = 0.699).
Several studies on enhanced recovery after total hip arthroplasty have been published [2, 3, 5, 6, 8]. These must be interpreted considering the socio-economic background, differences in healthcare systems, and the social structure and acceptance in the society [9, 10]. Treatment principles might be generalised, but the implementation of these protocols can be quite different because they are determined by the political and economic context in each country. Switzerland has one of the most expensive healthcare systems in the world [11], with a large number of hospital beds and many inpatient rehabilitation facilities. In the past, there was not much economic pressure concerning cost reduction, but now the situation is changing. To detect potentially unnecessary treatments, improve outcomes, and increase efficiency while maintaining a high quality of patient care, population-specific analyses are of utmost importance [12]. However, so far, such data are lacking for the Swiss healthcare system. Total hip arthroplasty is suitable to account for the effects of changes in treatment protocols, as it is a standardised and common procedure. Furthermore, comparisons with other treatment pathways are possible.
Tranexamic acid was not used at our hospital before the introduction of a standardised treatment protocol, but the anaesthetic department was somewhat concerned about bleeding, so it was administered to almost all patients after introducing the standardised treatment (series 1). Because the surgeon was well familiar with the procedure, there was no operative learning curve; thus, no decreases in blood loss or difference in pre- and postoperative haemoglobin were expected in series 1–4. The lower blood loss and difference in pre- and postoperative haemoglobin in series 1–4 compared with series 0 might be explained by the use of tranexamic acid, as this effect is well described in the literature [14]. The anterior approach might be another explanation for these changes, but no data support this approach causing less blood loss than the lateral approach used in series 0 [15].
Apart from higher blood loss and difference in pre- and postoperative haemoglobin, the transfusion rate was already low (10%) in series 0 compared with the rate found in the literature (Yoshihara et al.: 11.2–19.1%, Bedard et al.: 12.7%) [16, 17]. With the introduction of tranexamic acid and transfusion criteria, the transfusion rate decreased even more in series 1–4 (overall 3.5%). We found an association between preoperative anaemia and the need for transfusions, which is well-described [18]. With the introduction of the enhanced recovery program (series 3 and 4), anaemia was controlled preoperatively, and the transfusion rate decreased further, matching the published values for fast-track programs [19, 20].
Opioids were seen as mandatory in joint replacement surgery for decades and were standard at our hospital (series 0). Because of their well-documented side effects, a multimodal pain management strategy that limits opioid use is a key part of any fast-track treatment protocol [21, 22]. Such a protocol should have been introduced from the beginning of series 1, but it took time to incorporate it into the perioperative pain medication plan because of the concerns of the anaesthetic department. Most patients were treated with opioids, and many still received intravenous opioids in series 1, but this rate decreased steadily. It is debatable whether opioid-free treatment is an achievable goal for all patients; however, with restricted and peroral application, the side effects of opioids can be effectively reduced [22].
For many years, urinary catheterisation was seen as mandatory in patients with joint replacement to monitor urinary output and guide fluid management [23]; all patients had a urinary catheter in series 0. However, the routine use of perioperative urinary catheterisation may increase the risk of urinary tract infection and does not eliminate the need for subsequent re-catheterisation [24]. In fast-track total hip arthroplasty and total knee arthroplasty (TKA), standardised urinary catheterisation is not recommended. However, urinary retention is seen in up to 40% of patients with fast-track total hip arthroplasty and TKA [25, 26]. In our study, the use of urinary catheters was meant to be avoided from series 1 on, but the vast majority of the patients still received a catheter because of anaesthesiological habits. Two years later (series 2) the standard placement of urinary catheters had almost disappeared.
All patients were seen at 6 and 12 weeks after surgery by the treating surgeons, and no patients missed their follow-ups; thus, all reoperations and adverse events were recorded. The rate of reoperations and adverse events was low (series 1–4 average: 4.5%), in line with the published data on fast-track programs [8, 27] and the Swiss implant registry SIRIS [13]. The reoperation rate was evenly distributed across the whole observation period without an increase after the introduction of the standardised treatment protocol and enhanced recovery program. Fewer adverse events occurred in the baseline group (series 0) than in series 1–4 (p = 0.699). However, the number of patients and the incidence of adverse events were too low to distinguish whether this was due to mere coincidence, differences in demographics, more restrictive rehabilitation, or the use of the lateral approach.
Discharge to a rehabilitation centre is a well-established practice in Switzerland and is seen as necessary by healthcare providers and patients, especially for patients living alone. This is a known predictor of discharge to an institution [28–30], which our findings confirmed. As a part of the standardised treatment protocol and even more so in the enhanced recovery program, patients had more preparation for the time after hospitalisation. This might have been why more patients could be discharged home, even if they lived alone.
Admission on the day of surgery was not an established practice at the hospital; thus, it took some time for this practice to be managed and introduced. Several anaesthesiological, social, and financial concerns had to be overcome, and these concerns may differ considerably between healthcare systems [31–35]. The preoperative assessment has to be anticipated in the outpatient setting, which requires time and organisational effort. With the introduction of the enhanced recovery program (series 3 and 4) the hospital committed to this strategy and facilitated structural changes, and thus almost no patients were admitted the day before surgery.
The median length of stay was 3 days longer in series 0 than in the first series treated under the standardised treatment protocol. In series 0, only one patient had a length of stay of less than 5 days, whereas shorter lengths of stay became frequent in series 1–4. This difference is even more remarkable considering that patients in series 0 were admitted the day before surgery (the day of admission did not count toward the length of stay), had a lower age, and were less likely to be living alone. The length of stay decreased with the introduction of the standardised treatment protocol and further decreased with the enhanced recovery program (series 3). It remained unchanged in series 4, and a further decrease is unlikely because of the lack of pressure regarding discharge. Very short hospital stays (≤2 days) still are uncommon in the regional health system; patients do not expect this, and financial consequences can occur in cases of very short hospital stays, as determined by the Swiss Diagnosis-Related Group (DRG). Shorter hospital stays are common in other countries [27, 36|, with some performing total hip arthroplasty as an outpatient procedure, but more structural changes are needed for this, along with financial stimulus and a broader acceptance in society.
In cases of discharge to a rehabilitation institution, the length of stay depends on many organisational aspects. This was not anticipated in series 0–2, and thus patients admitted to an institution had a longer length of stay. In series 4, patients with different discharge destinations showed almost no differences in length of stay, and the enhanced recovery program also affected the administrative processes.
The study has several limitations. The hospital had no ICU, and patients with an ASA score of 4 were not admitted. However, these patients are rarely scheduled for elective total hip arthroplasty, and most of them would not have met the inclusion criteria. All other patients with health insurance in Switzerland could have been treated. This study had no major selection bias, and the demographic data of the studied patients were comparable to the data available in the national Swiss Implant Registry SIRIS [13].
The use of cemented stems was higher than the mean use in Switzerland [13]. Cemented stems were used for older adults with poor bone quality according to the judgement of the surgeons, and the indication and technique did not change with time. Cementing requires a longer operating time for curing of the cement but otherwise does not affect peri- and postoperative treatment. This should not have affected the results.
The sample size was limited, and an adjustment for baseline characteristics was not possible. The control group (series 0) differed considerably in age and the percentage of patients living alone. However, these differences in the patient demographics would have favoured the outcomes in the control group (series 0) [40, 28].
Operations in the baseline group were performed via the lateral approach by one surgeon (AE), and the operations in series 1–4 were performed by another surgeon via the anterior approach. The anterior approach is assumed to facilitate recovery [37]. No data have been published regarding differences in recovery between the anterior approach and the direct lateral approach; the use of different approaches might be a limitation of the study, favouring outcomes for the standardised treatment protocol and the enhanced recovery program [38]. However, no operative learning curve was present for either approach, as both surgeons were familiar with the approaches, and a significant difference in outcomes between approaches has not been shown [39]. Thus, the differences in approach do not explain the described differences in outcomes.
Furthermore, the study did not include a control group, but this would not have been feasible because changes in clinical processes could affect other processes at the same institution. Despite these limitations, it can be assumed that series 0 represents the standard of care in many Swiss hospitals at that time.
Two parameters showed a lack of significant improvement due to the limited sample size, but all other parameters improved; this aligns with the published data on enhanced recovery programs [21, 27, 41]. Many treatment details were changed, and the studied parameters were not independent. A multifactorial analysis would have been necessary to study the effects of single measures, but the number of patients was too small for such an analysis.
Another limitation is the lack of patient-reported outcomes, which would have been needed to assess the effect of the standardised treatment protocol and enhanced recovery program on clinical outcomes and patient satisfaction. However, all patients were seen regularly by the responsible surgeons even 1 year after surgery, and the patients were operated on in a competitive environment. None of the healthcare providers at the hospital would have suggested stopping the enhanced recovery program. Thus, we strongly believe that patient satisfaction at least did not decrease with the introduction of the standardised treatment protocol and enhanced recovery program.
The strengths of the study are as follows: the operations in the study group (series 1–4) were performed by a single surgeon using the same implants and surgical technique; there was no learning curve concerning the operative technique; and a complete dataset of all patients was available.
In a Swiss population, we showed for the first time that the implementation of an enhanced treatment protocol for total hip arthroplasty resulted in a shorter length of stay and a lower rate of discharge to a rehabilitation institution. There was an improvement in almost all examined parameters, and the rate of adverse events was not affected and remained low. These data can be used as a benchmark, but the details of these findings need to be confirmed in larger cohorts.
This research received no specific grant from any funding agency.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Health at a Glance 2017 | READ online. OECD iLibrary https://read.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2017_health_glance-2017-en
2. Antrobus JD, Bryson GL. Enhanced recovery for arthroplasty: good for the patient or good for the hospital? Can J Anaesth. 2011 Oct;58(10):891–4. 10.1007/s12630-011-9564-9
3. Raphael M, Jaeger M, van Vlymen J. Easily adoptable total joint arthroplasty program allows discharge home in two days. Can J Anaesth. 2011 Oct;58(10):902–10. 10.1007/s12630-011-9565-8
4. Fawcett WJ, Mythen MG, Scott MJ. Enhanced recovery: more than just reducing length of stay? Br J Anaesth. 2012 Nov;109(5):671–4. 10.1093/bja/aes358
5. Okamoto T, Ridley RJ, Edmondston SJ, Visser M, Headford J, Yates PJ. Day-of-Surgery Mobilization Reduces the Length of Stay After Elective Hip Arthroplasty. J Arthroplasty. 2016 Oct;31(10):2227–30. 10.1016/j.arth.2016.03.066
6. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012 Oct;83(346):1–39. 10.3109/17453674.2012.700593
7. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1
8. Zhu S, Qian W, Jiang C, Ye C, Chen X. Enhanced recovery after surgery for hip and knee arthroplasty: a systematic review and meta-analysis. Postgrad Med J. 2017 Dec;93(1106):736–42. 10.1136/postgradmedj-2017-134991
9. Schneider M, Kawahara I, Ballantyne G, McAuley C, Macgregor K, Garvie R, et al. Predictive factors influencing fast track rehabilitation following primary total hip and knee arthroplasty. Arch Orthop Trauma Surg. 2009 Dec;129(12):1585–91. 10.1007/s00402-009-0825-9
10. Napier RJ, Spence D, Diamond O, O’Brien S, Walsh T, Beverland DE. Modifiable factors delaying early discharge following primary joint arthroplasty. Eur J Orthop Surg Traumatol. 2013 Aug;23(6):665–9. 10.1007/s00590-012-1053-5
11. OECD. Health at a Glance 2019: OECD Indicators. OECD; 2019.
12. Husted H, Gromov K, Malchau H, Freiberg A, Gebuhr P, Troelsen A. Traditions and myths in hip and knee arthroplasty. Acta Orthop. 2014 Dec;85(6):548–55. 10.3109/17453674.2014.971661
13. Swiss National Hip and Knee Joint Registry. Report 2020. Internet: https://www.anq.ch/wp-content/uploads/2021/01/ANQakut_SIRIS_Hips-Knee_Annual-Report_2020.pdf
14. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009 Jun;91(6):776–83. 10.1302/0301-620X.91B6.22393
15. Alecci V, Valente M, Crucil M, Minerva M, Pellegrino CM, Sabbadini DD. Comparison of primary total hip replacements performed with a direct anterior approach versus the standard lateral approach: perioperative findings. J Orthop Traumatol. 2011 Sep;12(3):123–9. 10.1007/s10195-011-0144-0
16. Yoshihara H, Yoneoka D. National trends in the utilization of blood transfusions in total hip and knee arthroplasty. J Arthroplasty. 2014 Oct;29(10):1932–7. 10.1016/j.arth.2014.04.029
17. Bedard NA, Pugely AJ, Lux NR, Liu SS, Gao Y, Callaghan JJ. Recent Trends in Blood Utilization After Primary Hip and Knee Arthroplasty. J Arthroplasty. 2017 Mar;32(3):724–7. 10.1016/j.arth.2016.09.026
18. Saleh E, McClelland DB, Hay A, Semple D, Walsh TS. Prevalence of anaemia before major joint arthroplasty and the potential impact of preoperative investigation and correction on perioperative blood transfusions. Br J Anaesth. 2007 Dec;99(6):801–8. 10.1093/bja/aem299
19. Loftus TJ, Spratling L, Stone BA, Xiao L, Jacofsky DJ. A Patient Blood Management Program in Prosthetic Joint Arthroplasty Decreases Blood Use and Improves Outcomes. J Arthroplasty. 2016 Jan;31(1):11–4. 10.1016/j.arth.2015.07.040
20. Madsen RV, Nielsen CS, Kallemose T, Husted H, Troelsen A. Low Risk of Thromboembolic Events After Routine Administration of Tranexamic Acid in Hip and Knee Arthroplasty. J Arthroplasty. 2017 Apr;32(4):1298–303. 10.1016/j.arth.2016.10.015
21. Husted H, Solgaard S, Hansen TB, Søballe K, Kehlet H. Care principles at four fast-track arthroplasty departments in Denmark. Dan Med Bull. 2010 Jul;57(7):A4166.
22. Schlosser MJ, Korwek KM, Dunn R, Poland RE. Reduced post-operative opioid use decreases length of stay and readmission rates in patients undergoing hip and knee joint arthroplasty. J Orthop. 2020 Mar;21:88–93. 10.1016/j.jor.2020.03.003
23. Huang Z, Ma J, Shen B, Pei F. General anesthesia: to catheterize or not? A prospective randomized controlled study of patients undergoing total knee arthroplasty. J Arthroplasty. 2015 Mar;30(3):502–6. 10.1016/j.arth.2014.09.028
24. Bjerregaard LS, Homilius M, Bagi P, Hansen TB, Kehlet H. Indwelling urinary catheterisation may increase risk of complications in hip and knee arthroplasty. Dan Med J. 2019 Apr;66(4).
25. Bjerregaard LS, Bogø S, Raaschou S, Troldborg C, Hornum U, Poulsen AM, et al. Incidence of and risk factors for postoperative urinary retention in fast-track hip and knee arthroplasty. Acta Orthop. 2015 Apr;86(2):183–8. 10.3109/17453674.2014.972262
26. Bjerregaard LS, Jorgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-Track Hip and Knee Replacement Collaborative Group. Serious renal and urological complications in fast-track primary total hip and knee arthroplasty; a detailed observational cohort study. Minerva Anestesiol. 2016 Mar.
27. Berg U, BüLow E, Sundberg M, Rolfson O. No increase in readmissions or adverse events after implementation of fast-track program in total hip and knee replacement at 8 Swedish hospitals: an observational before-and-after study of 14,148 total joint replacements 2011-2015. Acta Orthop. 2018 Oct;89(5):522–7. 10.1080/17453674.2018.1492507
28. de Pablo P, Losina E, Phillips CB, Fossel AH, Mahomed N, Lingard EA, et al. Determinants of discharge destination following elective total hip replacement. Arthritis Rheum. 2004 Dec;51(6):1009–17. 10.1002/art.20818
29. MacDonald V, Ottem P, Wasdell M, Spiwak R. Predictors of prolonged hospital stays following hip and knee arthroplasty. Int J Orthop Trauma Nurs. 2010;14(4):198–205. 10.1016/j.ijotn.2010.06.001
30. Jørgensen CC, Kehlet H; Lundbeck Foundation Centre for Fast-track Hip and Knee Replacement Collaborative Group. Role of patient characteristics for fast-track hip and knee arthroplasty. Br J Anaesth. 2013 Jun;110(6):972–80. 10.1093/bja/aes505
31. Husted H. Fast-track hip and knee arthroplasty: clinical and organizational aspects. Acta Orthop Suppl. 2012 Oct;83(346):1–39. 10.3109/17453674.2012.700593
32. Füssenich W, Gerhardt DM, Pauly T, Lorenz F, Olieslagers M, Braun C, et al. A comparative health care inventory for primary hip arthroplasty between Germany versus the Netherlands. Is there a downside effect to fast-track surgery with regard to patient satisfaction and functional outcome? Hip Int. 2020 Jul;30(4):423–30. 10.1177/1120700019876881
33. Büttner M, Mayer AM, Büchler B, Betz U, Drees P, Susanne S. Economic analyses of fast-track total hip and knee arthroplasty: a systematic review. Eur J Orthop Surg Traumatol. 2020 Jan;30(1):67–74. 10.1007/s00590-019-02540-1
34. Nöth U, Rackwitz L, Clarius M. [Challenges of fast-track arthroplasty in Germany]. Orthopade. 2020 Apr;49(4):334–7.
35. Nöth U, Geiser T, Kranich T, von Rottkay E, Reichert JC, Reyle-Hahn M, et al. [Fast track strategies in hip arthroplasty]. Orthopade. 2019 Apr;48(4):330–6.
36. Petersen PB, Jørgensen CC, Kehlet H; Lundbeck Foundation Center for Fast-track Hip and Knee Replacement collaborative group. Temporal trends in length of stay and readmissions after fast-track hip and knee arthroplasty. Dan Med J. 2019 Jul;66(7).
37. Galakatos GR. Direct Anterior Total Hip Arthroplasty. Mo Med. 2018;115(6):537–41.
38. Ilchmann T. Approaches for primary total hip replacement. Hip Int. 2014 Oct;24(10_suppl Suppl 10):S2–6. 10.5301/hipint.5000163
39. Lloyd JM, Wainwright T, Middleton RG. What is the role of minimally invasive surgery in a fast track hip and knee replacement pathway? Ann R Coll Surg Engl. 2012 Apr;94(3):148–51. 10.1308/003588412X13171221590214
40. Dall GF, Ohly NE, Ballantyne JA, Brenkel IJ. The influence of pre-operative factors on the length of in-patient stay following primary total hip replacement for osteoarthritis: a multivariate analysis of 2302 patients. J Bone Joint Surg Br. 2009 Apr;91(4):434–40. 10.1302/0301-620X.91B4.21505
41. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012 Jan;132(1):101–4. 10.1007/s00402-011-1396-0