
Figure 1Number of claims per 100,000 adults between 2014 and 2019.
DOI: https://doi.org/https://doi.org/10.57187/s.3535
Metamizole is a controversial non-opioid analgesic drug due to its potential toxicity. In various countries, including France, the US, England and Sweden, metamizole has not been approved or has been withdrawn from the market due to the risk of drug-induced agranulocytosis. However, the reported absolute risk of metamizole-induced agranulocytosis varies between 1 per 1439 and 1.1 per million metamizole prescriptions across different studies [1–13]. Agranulocytosis is defined as a rapid decrease of peripheral neutrophil granulocytes, leading to an increased susceptibility to serious infections with a mortality of approximately 5% [13, 14]. Additionally, European drug authorities have recently warned about a potentially increased risk of drug-induced liver injury (DILI) associated with metamizole [15–17].
In Switzerland, metamizole is approved for the treatment of severe pain or fever if other treatments have failed [18]. Despite these potential adverse drug events and the restricted label, the number of claims for metamizole in Switzerland has increased from 4018 per 100,000 people in 2006 to 13,729 per 100,000 people in 2013 (+ 242%) [19]. Moreover, metamizole has been listed by a large Swiss health insurance company (Helsana) as one of the 10 most frequently claimed medications since 2014 [20]. Possible reasons may be that many physicians presume that metamizole has a more favourable safety profile and fewer drug-drug interactions than non-steroidal anti-inflammatory drugs (NSAIDs), especially in older adults and in those with advanced, progressive diseases [21, 22]. These patients are more susceptible to the adverse drug reactions of NSAIDs, such as gastroduodenal bleeding, cardiovascular events or renal toxicity [23–32]. However, it remains unknown which non-opioid analgesic drugs are preferably claimed by older adults in Switzerland.
This study aimed to investigate the use of metamizole and the three other most frequently claimed non-opioid analgesic drugs in Switzerland (ibuprofen, diclofenac and paracetamol) between 2014 and 2019 in subgroups comparing sex, age and region (canton) of its users.
We conducted a retrospective descriptive study using outpatient administrative claims data from the Swiss health insurance company Helsana for the period from January 2014 to December 2019. In Switzerland, basic health insurance is mandatory and insurance companies must accept all applicants for basic insurance coverage. Patients can choose between various private insurance companies, but all of them have to cover the same catalogue of health services. The Helsana claims database provides anonymised basic health insurance data of approximately 1.2 million individuals across all Swiss cantons (approximately 15% of the overall Swiss population for the year 2019) and thus provides information on a representative sample of the Swiss population [33]. The Helsana claims database captures longitudinal records of patients, comprising demographics and all reimbursed dispensations of outpatient prescription drugs, including information on the Anatomical Therapeutic Chemical (ATC) Classification System, dose, route of administration and pack size.
In the first part of this study we evaluated the number of claims, defined daily doses and geographical regions (cantons) of the claims of the four non-opioid analgesic drugs of interest in Switzerland [34]. We included all claims (based on recorded ATC codes) between 1 January 2014 and 31 December 2019 of metamizole, ibuprofen, diclofenac and paracetamol by adults aged 18 years or older (ATC codes in table S1 in the appendix). In the second part of the study, we characterised new users of these non-opioid analgesic drugs over a 1-year period. Therefore, we categorised users who had at least one claim of a non-opioid analgesic drug of interest in 2019 into four groups (i.e. metamizole, ibuprofen, diclofenac or paracetamol). New users had to have been continuously insured for at least 180 days before the first claim of interest, during which they must not have had any recorded claims for the respective non-opioid analgesic drug. If new users had claims of different non-opioid analgesic drugs of interest, they were included in each respective group.
According to the Swiss Law of Human Research, this study did not require ethical approval since data were anonymised. We conducted the study following the principles of Good Clinical Practice and in accordance with the Declaration of Helsinki.
We identified all drugs of interest based on their respective ATC codes. The non-opioid analgesic drugs of interest were metamizole, ibuprofen, diclofenac and paracetamol (without combination products). We captured the canton in which these non-opioid analgesic drugs were claimed. Additionally, we used comedications as proxies for underlying comorbidities since outpatient diagnoses were not systematically recorded in a standardised manner in the Swiss outpatient setting. Pre-existing cardiovascular, gastrointestinal and renal comorbidities might influence the choice of non-opioid analgesic drug given the known cardiovascular risk associated with NSAIDs, which might result in channelling of non-opioid analgesic drug users. We identified claims of anticoagulants, platelet aggregation inhibitors, lipid-modifying drugs, antihypertensive drugs and antidiabetics within 180 days before the first non-opioid analgesic drug claim of interest (ATC codes in table S1 in the appendix).
We applied descriptive statistics and reported results as counts and proportions. In the first analysis, we assessed the number of claims per year and the number of dispensed defined daily doses per year per non-opioid analgesic drug of interest per 100,000 adults during the study period. To calculate the number of claimed defined daily doses per non-opioid analgesic drug of interest per year, we summed the dispensed cumulative dose of each claimed non-opioid analgesic drug of interest per year and divided it by the defined daily dose of the respective drug. The World Health Organization defines a defined daily dose as the average maintenance dose per day for a drug used for its main indication in adults [35]. We analysed the number of adults with at least one metamizole claim per calendar year between 2014 and 2019 overall and stratified by age. Additionally, we calculated the number of claims per 100,000 adults of each non-opioid analgesic drug of interest per canton and assessed its percentage difference compared to the Swiss average number of claims per 100,000 adults of the respective non-opioid analgesic drug of interest in the year 2019.
In the second analysis, we characterised new users of each non-opioid analgesic drug of interest in the year 2019 regarding age (median and interquartile range [IQR] as well as age groups 18–45, 46–65, 66–75, 76–85 and ≥85 years), sex and comedications. We calculated the median and IQR of the number of claims of metamizole per metamizole user per calendar year overall and within each age group during the study period. We performed all analyses using SAS 9.4 software (SAS Institute, Cary, NC, USA).
We identified an average annual total of 955,638 adults in the Helsana claims database between 2014 and 2019 (total number of adults per year are displayed in table S2 in the appendix). Paracetamol was the most frequently claimed non-opioid analgesic drug of interest in 2014 (50,596 claims/100,000 adults), followed by ibuprofen (22,533/100,000 adults), metamizole (19,297/100,000 adults) and diclofenac (18,128/100,000 adults). However, we observed the largest increase in claims between 2014 and 2019 for metamizole (+ 50%) followed by ibuprofen (+ 30%). Claims for diclofenac decreased by 30% during the same period, whereas claims for paracetamol slightly increased (+ 7%, figure 1). Absolute numbers of claims (per 100,000 adults) per non-opioid analgesic drug of interest and year are displayed in table S2.

Figure 1Number of claims per 100,000 adults between 2014 and 2019.
Paracetamol had the highest number of claimed defined daily doses with 827,684 claimed defined daily doses per 100,000 adults in 2014, followed by diclofenac (456,199), ibuprofen (366,166) and metamizole (103,630). However, between 2014 and 2019, we observed the largest increase in claimed defined daily doses per 100,000 adults for metamizole (+84%), followed by ibuprofen (+22%) and paracetamol (+6%). The number of claimed defined daily doses per 100,000 adults of diclofenac decreased during this period (–22%) (figure 2). Absolute values of defined daily doses (per 100,000 adults) per non-opioid analgesic drug of interest and year are displayed in table S2. Our post hoc analysis showed that the number of adults with at least one claim of metamizole increased by 33% overall and by 47% in adults aged over 85 years between 2014 and 2019 (table S3 in the appendix).
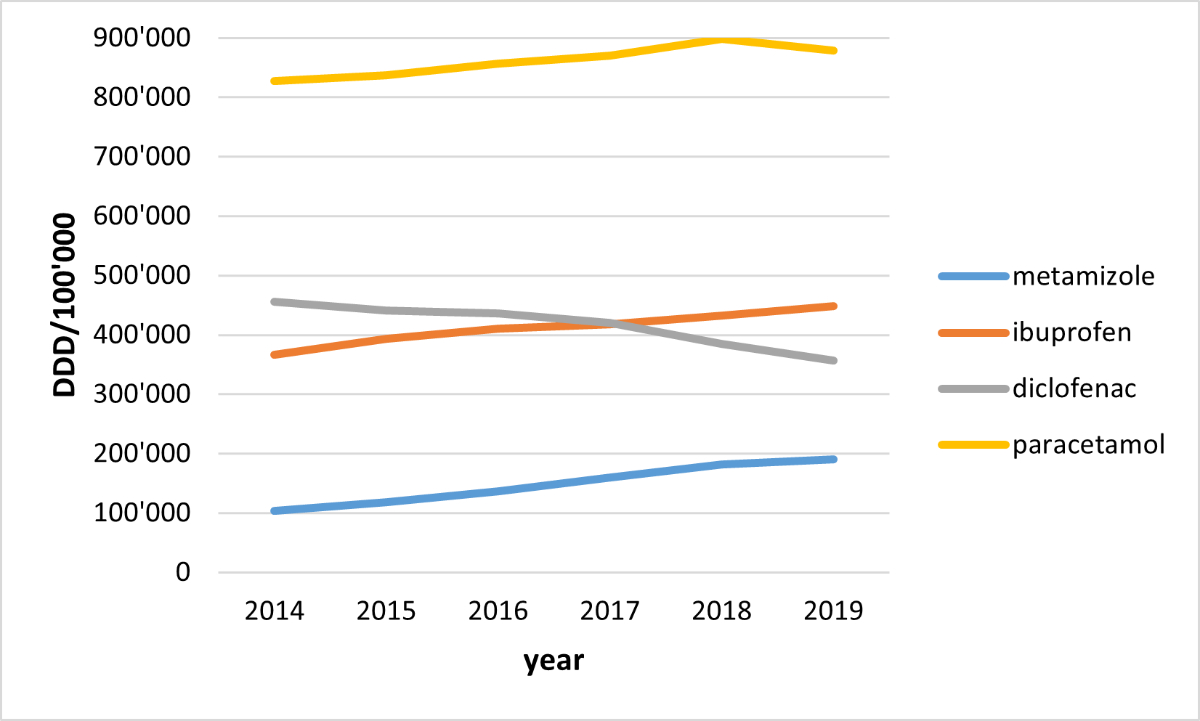
Figure 2Number of claimed defined daily doses (DDD) per 100,000 adults between 2014 and 2019.
Figure 3 displays the relative difference between the number of metamizole claims per 100,000 adults per canton and the Swiss average in 2019 (28,843 metamizole claims per 100,000 adults). Compared to the Swiss average, the French- and Italian-speaking cantons of Switzerland showed much lower use of metamizole (e.g. Geneva: –94%, Ticino: –37%, Fribourg: –49%). On the other hand, in most German-speaking cantons, more metamizole was claimed compared to the Swiss average, with the highest use of metamizole in Basel-Land, Zürich, St Gallen and Glarus (all at least +25% compared with the Swiss average) In the French-speaking part of Switzerland, ibuprofen and paracetamol were claimed more frequently, whereas diclofenac was more often claimed in the German- and Italian-speaking parts of Switzerland, compared with the Swiss average (table S4 and figure S1 in the appendix).
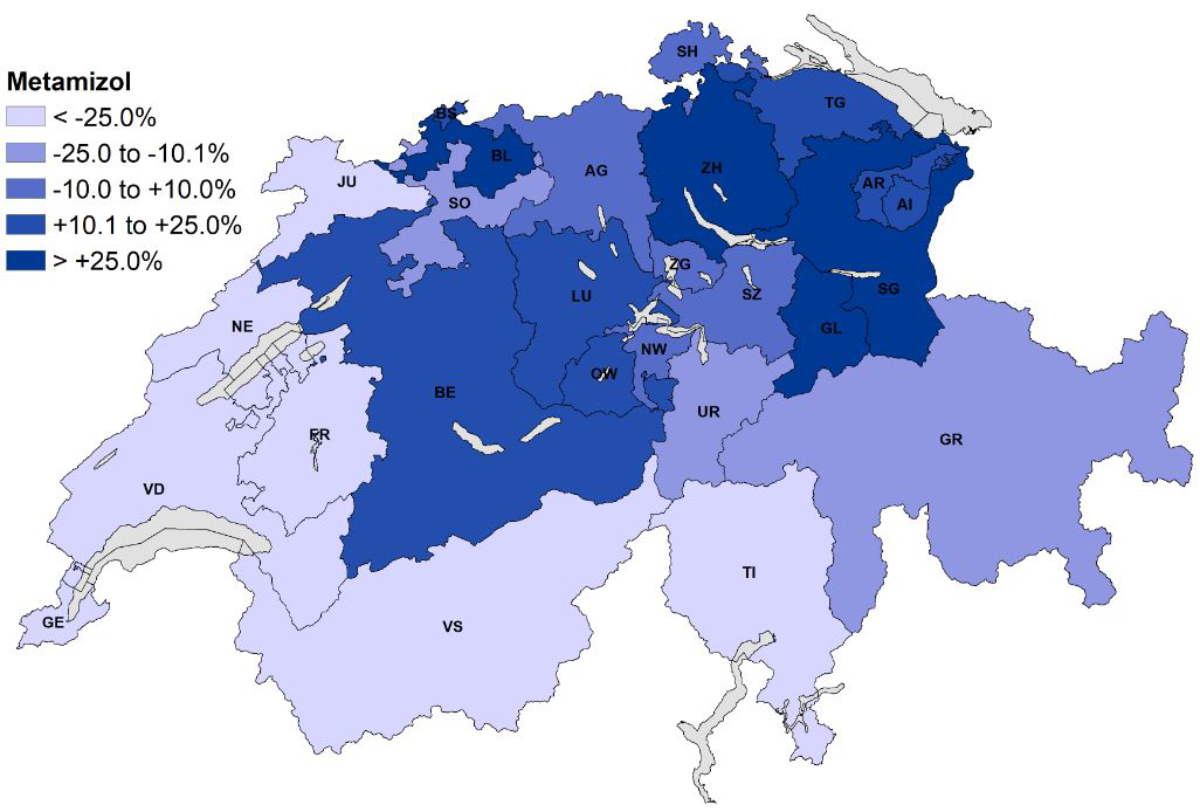
Figure 3Relative differences between the number of claims of metamizole per 100,000 adults per canton and the Swiss average. Abbreviations: AG: Aargau, AI: Appenzell Innerrhoden, AR: Appenzell Ausserrhoden, BE: Bern, BL: Basel-Land, BS: Basel-Stadt, FR: Fribourg, GE: Geneva, GL: Glarus, GR: Graubünden, JU: Jura, LU: Luzern, NE: Neuchâtel, NW: Nidwalden, OW: Obwalden, SG: St Gallen, SH: Schaffhausen, SO: Solothurn, SZ: Schwyz, TG: Thurgau, TI: Ticino, UR: Uri, VD: Vaud, VS: Valais, ZG: Zug, ZH: Zürich.
In 2019, new users of metamizole had the highest median age (62 years) and the highest percentage of elderly users aged 65 years or over (46%) followed by paracetamol (58 years; ≥65 years: 40%), diclofenac (57 years; ≥65 years: 36%) and ibuprofen users (47 years; ≥65 years: 22%, table 1).
Overall, new users of metamizole claimed more comedications of interest in the preceding 180 days, with paracetamol users showing the most similar pattern of comedications. In total, 40% of new metamizole users previously claimed at least one antihypertensive drug (vs 36% paracetamol, 32% diclofenac, 22% ibuprofen users), 13% claimed at least one anticoagulant (vs 10% paracetamol, 6% diclofenac, 5% ibuprofen users) and 11% claimed at least one antidiabetic drug (vs 10% paracetamol, 8% diclofenac, 6% ibuprofen users). The median number of claims of metamizole per metamizole user per calendar year did not increase in our study period, independent of age (table S5 in the appendix).
Table 1Characteristics of non-opioid analgesic drug users in 2019 regarding sex, age and number of claimed oral comedications.
| Metamizole | Ibuprofen | Diclofenac | Paracetamol | ||
| N | 98,910 | 159,705 | 74,902 | 215,770 | |
| Female sex (%) | 59,086 (60%) | 98,430 (62%) | 40,563 (54%) | 132,644 (61%) | |
| Age, median (IQR) | 62 (44–77) | 47 (33–62) | 57 (43–71) | 58 (39–75) | |
| Age groups (%) | 18–45 years | 25,078 (25%) | 71,940 (45%) | 20,292 (27%) | 67,667 (31%) |
| 46–65 years | 28,261 (29%) | 52,492 (33%) | 27,328 (36%) | 60,612 (28%) | |
| 66–75 years | 15,985 (16%) | 18,742 (12%) | 13,791 (18%) | 32,268 (15%) | |
| 76–85 years | 17,114 (17%) | 12,286 (7%) | 10,316 (14%) | 32,713 (15%) | |
| >85 years | 12,472 (13%) | 4245 (3%) | 3175 (4%) | 22,510 (10%) | |
| Claimed oral comedications (%) | PPIs | 47,592 (48%) | 58,704 (37%) | 33,744 (45%) | 82,818 (38%) |
| Anticoagulants | 13,100 (13%) | 7984 (5%) | 4764 (6%) | 21,896 (10%) | |
| Platelet aggregation inhibitors | 17,815 (18%) | 13,118 (8%) | 9511 (13%) | 34,759 (16%) | |
| Lipid-modifying drugs | 19,573 (20%) | 17,633 (11%) | 12,921 (17%) | 40,374 (19%) | |
| Antihypertensive drugs | 40,036 (40%) | 34,972 (22%) | 24,255 (32%) | 78,266 (36%) | |
| RAAS inhibitors | 30,451 (31%) | 26,587 (17%) | 19,065 (25%) | 59,846 (28%) | |
| Calcium-channel blockers | 14,849 (15%) | 11,516 (7%) | 8125 (11%) | 27,812 (13%) | |
| Diuretics | 22,726 (23%) | 16,171 (10%) | 11,827 (16%) | 42,304 (20%) | |
| Beta blockers | 19,226 (19%) | 14,218 (9%) | 9786 (13%) | 36,369 (17%) | |
| Antidiabetics | 10,401 (11%) | 9505 (6%) | 6007 (8%) | 21,699 (10%) | |
| Metformin | 7259 (7%) | 7315 (5%) | 4785 (6%) | 15,846 (7%) | |
| SGLT-2 inhibitors | 1459 (1%) | 1557 (1%) | 999 (1%) | 3099 (1%) | |
| GLP-1 receptor agonists (subcutaneous application) | 1175 (0%) | 1091 (1%) | 648 (1%) | 2211 (1%) | |
| Other antidiabetics | 3070 (3%) | 2244 (1%) | 1552 (2%) | 6168 (3%) | |
The present study investigated the claims pattern of metamizole, ibuprofen, diclofenac and paracetamol in Switzerland between 2014 and 2019. Paracetamol was the most frequently claimed non-opioid analgesic drug of interest during this 6-year period, but metamizole showed the largest increase in claims and defined daily doses per 100,000 adults (+50%; +84%), followed by ibuprofen (+30%; +22%) and paracetamol (+7%; +6%), whereas diclofenac claims and defined daily doses declined (–30%; –22%). The median number of claims of metamizole per metamizole user per calendar year did not change, either overall or stratified by age group, between 2014 and 2019. On the other hand, the number of adults with at least one metamizole claim increased by 33% overall and by 47% in adults older than 85 years of age during this time period. This suggests that the observed increase in metamizole claims is mainly driven by an increase in the number of adults using metamizole and not by individual adults using more metamizole. We found that metamizole users were older than NSAID (ibuprofen, diclofenac) or paracetamol users (median ages in 2019: 62 vs 47, 57, 58 years) and more often had claims of PPIs, anticoagulants and platelet aggregation inhibitors than NSAID and paracetamol users. Metamizole was less often claimed in French- and Italian-speaking Switzerland than in German-speaking regions of Switzerland, whereas the number of claims of ibuprofen and paracetamol was highest in the French-speaking regions and that of diclofenac in Italian- and German-speaking regions.
A previous study conducted with Helsana claims data found increasing popularity of metamizole and showed a similar pattern of non-opioid analgesic drug claims although with an even steeper increase in claims per 100,000 adults of metamizole (+242%) between 2006 and 2013 (vs ibuprofen [+68%], paracetamol [+32%], diclofenac [–2.7%]) [19]. Given its similar methodology, the increase of claims of metamizole might have slowed down since 2013. Also, in Germany, an increase in metamizole prescriptions was reported between 2009 and 2018 (2009: 115 million defined daily doses; 2018: 225 million defined daily doses) [36]. We observed that metamizole had the largest increase in claims of all non-opioid analgesic drugs, whereas ibuprofen increased less steeply and use of diclofenac dropped. In addition to its rather potent antipyretic and analgesic effects, the increasing use of metamizole may be due to increasing awareness of potential risks associated with NSAID use. NSAIDs are associated with gastrointestinal (e.g. dyspepsia, gastroduodenal bleeding), cardiovascular (e.g. myocardial infarction, worsening of heart failure, hypertension) and renal (e.g. worsening of renal function) adverse drug events [23–32]. In particular, diclofenac (more than ibuprofen) has been associated with cardiovascular events over the past years, which might explain a certain shift from diclofenac towards metamizole and ibuprofen over time [26]. These adverse drug events are especially problematic in older adults, who have more comorbidities such as hypertension, heart failure, atherosclerosis and chronic renal failure [37]. Consequently, older adults are often exposed to polypharmacy, which can further increase the risk of developing adverse drug reactions when taking NSAIDs (e.g. increased risk of gastroduodenal bleeding in combination with anticoagulants, antiplatelet drugs, serotonergic antidepressants) [38, 39]. Therefore, the American Geriatrics Society recommends avoiding chronic NSAID use in older adults (aged > 65 years) and many classifications of drug appropriateness for older people recommend that NSAIDs should be avoided in older adults [40–43]. Paracetamol is not associated with gastrointestinal, cardiovascular or renal toxicity, but is a less effective analgesic especially for certain indications such as lower back pain or arthritis, and therefore is not always a viable treatment option [44, 45].
Metamizole users were older than ibuprofen, diclofenac or paracetamol users (median age in 2019: 62 vs 47, 57, 58 years), which is also supported by the “Fit for The Aged” (FORTA) expert consensus list [41]. Metamizole users also claimed more comedications, such as PPIs, indicating possible underlying comorbidities. PPIs are often prescribed as prophylaxis to prevent gastroduodenal bleeding in adults with risk factors thereof, such as advanced age and/or comedication with anticoagulants or platelet aggregation inhibitors [39, 46]. Metamizole users also had more claims of antihypertensive and lipid-modifying drugs than ibuprofen and diclofenac users, which are commonly prescribed in adults with cardiovascular diseases such as hypertension, heart failure and myocardial infarction, conditions which can be worsened or caused by NSAIDs [30, 47, 48]. Metamizole users had slightly more claims for antidiabetics than NSAID users. Adults with diabetes often develop renal and heart failure among other cardiovascular diseases [49]. Since NSAIDs can worsen existing renal or heart failure, they may not be a viable treatment option in these patients either [31, 48, 50]. Interestingly, metamizole seems to be used for shorter treatment periods than ibuprofen or diclofenac. Metamizole claims per 100,000 adults were higher than diclofenac claims and almost equalled ibuprofen claims in 2019 (metamizole: 28,843 vs ibuprofen: 29,354 per 100,000 adults), but the number of claimed defined daily doses of metamizole per 100,000 adults was markedly lower than that of ibuprofen and diclofenac during the whole study period. This suggests that metamizole is claimed in smaller pack sizes or dosages than ibuprofen or diclofenac.
Additionally, we observed regional differences in the claiming patterns of metamizole and the other non-opioid analgesic drugs of interest. These regional differences might be present because French- and Italian-speaking cantons might more often use pharmaceutical and medical information from France and Italy, both countries in which metamizole is not approved.
Some limitations of this study need to be considered. Firstly, in Switzerland, ibuprofen, diclofenac and paracetamol can also be purchased over the counter, which is not captured in administrative Helsana claims data. Moreover, use of non-opioid analgesic drugs in the inpatient setting is also not captured as they are reimbursed as part of bundled Diagnosis-Related Groups. Therefore, we likely underestimated the real extent of use of ibuprofen, diclofenac and paracetamol during the study period. Secondly, the data is based on 1.2 million Swiss insured by the Helsana basic health insurance scheme and is approximately representative for the general Swiss population. The insured population may have a slightly higher proportion of women and people aged 65 years or older than the Swiss population [34]. Thirdly, we had no information on diagnoses of non-opioid analgesic drug users, which is why we used the main indication of claimed medications as proxies for potential underlying chronic diseases. Since medications are also used for conditions other than their main indications, we cannot be certain that the claimed medications were always a good proxy for the underlying diseases. Fourthly, we had no information about the socioeconomic status of the insured people, a factor that could influence the amount of claimed health services and medications.
The presented results strengthen the assumption that metamizole is preferably used in older, frailer adults in whom comorbidities and comedications may prevent the use of NSAIDs owing to their safety profile, and paracetamol may not be effective enough. In the future, metamizole claims may further increase due to ageing of the population. In Switzerland, 19% of the population were 65 years or older in 2020, and it is assumed that by 2050 this percentage will increase to 25.6% [51, 52]. Despite the increasing use of metamizole, which may even be more pronounced in the future, little is known about its safety profile apart from the rare risk of blood disorders [53]. Recently, warnings have been issued by European drug authorities, associating metamizole use with drug-induced liver injuries [16, 17, 54]. To date, no increased cardiovascular risk associated with metamizole use has been reported, but studies on this safety outcome are scarce [55]. Although one study reported that short-term use of metamizole did not affect renal function in healthy adults [56], little is known about the nephrotoxic potential of metamizole. In view of its growing popularity, it is important to further investigate the safety profile of metamizole in future studies, especially in older adults with comorbidities and comedications.
We observed increasing use of metamizole between 2014 and 2019, mainly in the German-speaking parts of Switzerland. Metamizole users were older and claimed more comedications, suggesting that metamizole is preferably prescribed to patients with contraindications to NSAIDs. Given that the safety profile of metamizole remains incompletely understood, studies to evaluate its effectiveness and safety in this patient population are needed.
Study data cannot be shared due to strict laws of privacy protection, which requires presence of legal agreements and contracts for data provision.
This research received no specific grant from any funding agency.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Lutz M. Metamizole (Dipyrone) and the Liver: A Review of the Literature. J Clin Pharmacol. 2019 Nov;59(11):1433–42.
2. The International Agranulocytosis and Aplastic Anemia Study, Risks of Agranulocytosis and Aplastic Anemia. JAMA. 1986 Oct;256(13):1749.
3. Ibáñez L, Vidal X, Ballarín E, Laporte JR. Agranulocytosis associated with dipyrone (metamizol). Eur J Clin Pharmacol. 2005 Jan;60(11):821–9.
4. Shapiro S, Issaragrisil S, Kaufman DW, Anderson T, Chansung K, Thamprasit T, et al.; Aplastic Anemia Study Group. Agranulocytosis in Bangkok, Thailand: a predominantly drug-induced disease with an unusually low incidence. Am J Trop Med Hyg. 1999 Apr;60(4):573–7.
5. van der Klauw MM, Goudsmit R, Halie MR, van’t Veer MB, Herings RM, Wilson JH, et al. A population-based case-cohort study of drug-associated agranulocytosis. Arch Intern Med. 1999 Feb;159(4):369–74.
6. Hamerschlak N, Maluf E, Biasi Cavalcanti A, Avezum Júnior A, Eluf-Neto J, Passeto Falcão R, et al. Incidence and risk factors for agranulocytosis in Latin American countries—the Latin Study: a multicenter study. Eur J Clin Pharmacol. 2008 Sep;64(9):921–9.
7. Huber M, Andersohn F, Sarganas G, Bronder E, Klimpel A, Thomae M, et al. Metamizole-induced agranulocytosis revisited: results from the prospective Berlin Case-Control Surveillance Study. Eur J Clin Pharmacol. 2015 Feb;71(2):219–27.
8. Maj S, Lis Y. The incidence of metamizole sodium-induced agranulocytosis in Poland. J Int Med Res. 2002;30(5):488–95.
9. Basak GW, Drozd-Sokołowska J, Wiktor-Jedrzejczak W. Update on the incidence of metamizole sodium-induced blood dyscrasias in Poland. J Int Med Res. 2010;38(4):1374–80.
10. Lampl C, Likar R. Metamizol: Wirkmechanismen, Interaktionen und Agranulozytoserisiko. Schmerz. 2014 Dec;28(6):584–90.
11. Hedenmalm K, Spigset O. Agranulocytosis and other blood dyscrasias associated with dipyrone (metamizole). Eur J Clin Pharmacol. 2002 Jul;58(4):265–74.
12. Blaser LS, Tramonti A, Egger P, Haschke M, Krähenbühl S, Rätz Bravo AE. Hematological safety of metamizole: retrospective analysis of WHO and Swiss spontaneous safety reports. Eur J Clin Pharmacol. 2015 Feb;71(2):209–17.
13. Huber M, Andersohn F, Bronder E, Klimpel A, Thomae M, Konzen C, et al. Drug-induced agranulocytosis in the Berlin case-control surveillance study. Eur J Clin Pharmacol. 2014 Mar;70(3):339–45.
14. Andrès E, Maloisel F. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol. 2008 Jan;15(1):15–21.
15. European Medicines Agency. Metamizole: Risk of drug-induced liver injury, 2020. https://www.ema.europa.eu/en/medicines/dhpc/metamizole-risk-drug-induced-liver-injury
16. Federal Institute for Drugs and Medical Devices. Direct Healthcare Professional Communication (DHPC) on metamizole: risk of drug-induced liver injury, 2020. https://www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/EN/RHB/2020/rhb-metamizol.pdf?__blob=publicationFile/
17. swissmedic, DHPC – Metamizol, 2021. https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/marktueberwachung/health-professional-communication--hpc-/dhpc-metamizol.html (accessed Aug. 08, 2022).
18. S.-A. (Suisse). Fachinformation Novalgin. https://www.swissmedicinfo.ch/
19. Wertli MM, Reich O, Signorell A, Burgstaller JM, Steurer J, Held U. Changes over time in prescription practices of pain medications in Switzerland between 2006 and 2013: an analysis of insurance claims. BMC Health Serv Res. 2017 Feb;17(1):167.
20. Helsana, Arzneimittelreport. https://www.helsana.ch/de/helsana-gruppe/medien-publikationen/helsana-reports/arzneimittelreport.html
21. Frechen S, Zoeller A, Ruberg K, Voltz R, Gaertner J. Drug interactions in dying patients: a retrospective analysis of hospice inpatients in Germany. Drug Saf. 2012 Sep;35(9):745–58. 10.1007/BF03261971
22. Gaertner J, Ruberg K, Schlesiger G, Frechen S, Voltz R. Drug interactions in palliative care—it’s more than cytochrome P450. Palliat Med. 2012 Sep;26(6):813–25.
23. García Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994 Mar;343(8900):769–72.
24. Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ. 2000 Nov;321(7270):1183–7.
25. Rostom A, Muir K, Dubé C, Jolicoeur E, Boucher M, Joyce J, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a Cochrane Collaboration systematic review. Clin Gastroenterol Hepatol. 2007 Jul;5(7):818–28.
26. Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al.; Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013 Aug;382(9894):769–79.
27. Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006 Jun;332(7553):1302–8.
28. McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006 Oct;296(13):1633–44.
29. Helin-Salmivaara A, Virtanen A, Vesalainen R, Grönroos JM, Klaukka T, Idänpään-Heikkilä JE, et al. NSAID use and the risk of hospitalization for first myocardial infarction in the general population: a nationwide case-control study from Finland. Eur Heart J. 2006 Jul;27(14):1657–63.
30. Schmidt M, Sørensen HT, Pedersen L. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ. 2018 Sep;362:k3426.
31. Huerta C, Castellsague J, Varas-Lorenzo C, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005 Mar;45(3):531–9.
32. Harirforoosh S, Jamali F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2009 Nov;8(6):669–81.
33. R. Schneider, N. Schur, R. Daphne, S. Matthias, and C. R. Meier, Helsana Arzneimittelreport, 2017.
34. Twerenbold S, et al. Helsana-Arzneimittelreport für die Schweiz 2021, 2021. Online. Available: https://reports.helsana.ch/arzneimittel2021/
35. World Health Organization. Defined Daily Dose (DDD). https://www.who.int/tools/atc-ddd-toolkit/about-ddd
36. Knecht B, Lohmüller J, Telschow C. Arzneiverordnungs-Report 2019. Berlin, Heidelberg: Springer Berlin Heidelberg; 2019.
37. Fabbri LM, Ferrari R. Chronic disease in the elderly: back to the future of internal medicine. Breathe (Sheff). 2006;3(1):40–9.
38. Linjakumpu T, Hartikainen S, Klaukka T, Veijola J, Kivelä SL, Isoaho R. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol. 2002 Aug;55(8):809–17.
39. Tielleman T, Bujanda D, Cryer B. Epidemiology and Risk Factors for Upper Gastrointestinal Bleeding. Gastrointest Endosc Clin N Am. 2015 Jul;25(3):415–28.
40. Fick DM, et al.; By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019 Apr;67(4):674–94.
41. Pazan F, Weiß C, Wehling M. The FORTA List Fit forThe Aged, 2021. https://forta.umm.uni-heidelberg.de/
42. Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010 Aug;107(31-32):543–51.
43. Lavan AH, Gallagher P, Parsons C, O’Mahony D. STOPPFrail (Screening Tool of Older Persons Prescriptions in Frail adults with limited life expectancy): consensus validation. Age Ageing. 2017 Jul;46(4):600–7.
44. Saragiotto B, Machado G, Ferreira M, Pinheiro M, Abdel Shaheed C, Maher C. Paracetamol for low back pain (Review) SUMMARY OF FINDINGS FOR THE MAIN COMPARISON. Cochrane. 2016;(6):10–2.
45. Leopoldino AO, Machado GC, Ferreira PH, Pinheiro MB, Day R, McLachlan AJ, et al. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database Syst Rev. 2019 Feb;2(2):CD013273.
46. Kamboj AK, Hoversten P, Leggett CL. Upper Gastrointestinal Bleeding: etiologies and Management. Mayo Clin Proc. 2019 Apr;94(4):697–703.
47. Sowers JR, White WB, Pitt B, Whelton A, Simon LS, Winer N, et al.; Celecoxib Rofecoxib Efficacy and Safety in Comorbidities Evaluation Trial (CRESCENT) Investigators. The Effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-hour blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med. 2005 Jan;165(2):161–8.
48. Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Fosbøl EL, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti-inflammatory drugs in chronic heart failure. Arch Intern Med. 2009 Jan;169(2):141–9.
49. Long AN, Dagogo-Jack S. Comorbidities of diabetes and hypertension: mechanisms and approach to target organ protection. J Clin Hypertens (Greenwich). 2011 Apr;13(4):244–51.
50. Brater DC. Renal effects of cyclooxygyenase-2-selective inhibitors. J Pain Symptom Manage. 2002 Apr;23(4 Suppl):S15–20.
51. Bundesamt für Statistik. Ständige Wohnbevölkerung nach Geschlecht und Altersklasse, definitive Jahresergebnisse, 2015-2020. https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/stand-entwicklung/alter-zivilstand-staatsangehoerigkeit.assetdetail.18344300.html
52. Bundesamt für Statistik, Szenarien zur Bevölkerungsentwicklung der Schweiz und der Kantone 2020-2050. https://www.viz.bfs.admin.ch/assets/01/ga-01.03.01/de/index.html
53. Hoffmann F, Schmiemann G. Pain medication in German nursing homes: a whole lot of metamizole. Pharmacoepidemiol Drug Saf. 2016 Jun;25(6):646–51.
54. EMA. Metamizole: Risk of drug-induced liver injury, 2020. https://www.ema.europa.eu/en/medicines/dhpc/metamizole-risk-drug-induced-liver-injury
55. de Abajo FJ, Gil MJ, García Poza P, Bryant V, Oliva B, Timoner J, et al. Risk of nonfatal acute myocardial infarction associated with non-steroidal antiinflammatory drugs, non-narcotic analgesics and other drugs used in osteoarthritis: a nested case-control study. Pharmacoepidemiol Drug Saf. 2014 Nov;23(11):1128–38.
56. Blaser LS, Duthaler U, Bouitbir J, Leuppi-Taegtmeyer AB, Liakoni E, Dolf R, et al. Comparative Effects of Metamizole (Dipyrone) and Naproxen on Renal Function and Prostacyclin Synthesis in Salt-Depleted Healthy Subjects – A Randomized Controlled Parallel Group Study. Front Pharmacol. 2021 Sep;12(September):620635.
The appendix is available in the pdf version of the article.