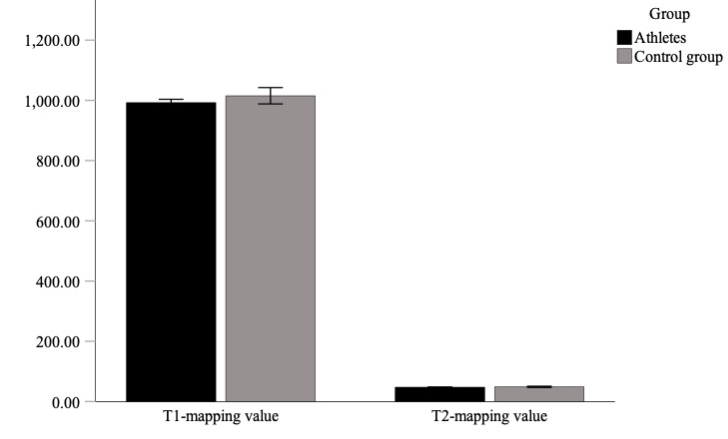
Figure 1Bar graph illustrating the comparison of T1 and T2 mapping values in athletes and controls, with p = 0.530 for T1 mapping and p = 0.313 for T2 mapping.
DOI: https://doi.org/https://doi.org/10.57187/s.3534
The global pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), is still ongoing despite the rapid progress of vaccination and other containment efforts. Since the early report [1] of myocardial involvement in COVID-19 detected by cardiovascular magnetic resonance (CMR), extensive research has raised concerns about myocardial damage due to COVID-19 infection. A study by Puntmann et al. [2] reported cardiac involvement in an alarming 78% of patients, with signs of ongoing myocarditis in 60%. These data were collected from random middle-aged patients in need of hospital care. A multicentre study revealed myocarditis-like injury patterns in 28% of troponin-positive hospitalised patients with COVID-19 infection [3], prompting many follow-up studies. Myocarditis and myocarditis-like patterns are serious myocardial injuries and can cause cardiac complications, such as heart failure, arrhythmia, and cardiogenic shock [4]. Cardiac magnetic resonance offers a unique combination of high-resolution functional imaging, parametric mapping, and late gadolinium enhancement (LGE) imaging and thereby provides detailed insight into myocardial texture. Thus, cardiac magnetic resonance is the imaging modality of choice and serves as the gold standard for detecting myocardial injury in suspected myocarditis [5, 6]. Because myocarditis is one of the leading causes of sudden cardiac death in athletes [7], early detection of myocardial damage is essential. The current Graduated Return to Play Protocol highlights the crucial role of cardiac magnetic resonance in athletes [8], especially in athletes with prolonged symptoms [9].
Studies on athletes with COVID-19 infection have presented inconsistent results. The COMPETE-CMR study observed myocarditis-like injury in 3% of the athletes [10], and a study by Rajpal et al. [11] found myocarditis-like injury in 15%; by contrast, Malek et al. [12] found no signs of acute myocarditis but observed other cardiac magnetic resonance abnormalities in 19% of athletes. These heterogeneous trials are limited in several regards: some did not include control patients, some were not focused on elite athletes, and some were not cardiac magnetic resonance-only studies. So far, only two cardiac magnetic resonance-only studies involving elite athletes have been conducted; these studies included 12 and 26 athletes [12, 13] and investigated myocardial damage shortly after infection. Furthermore, no data are available on mid- to longer-term myocardial damage due to COVID-19. At the time our study was initiated, an expert analysis by Sharma et al. [14] provided a thorough overview of the existing data and studies. Thus, our study investigated the potential mid- to long-term myocardial effects of prior COVID-19 infection in elite athletes.
The project was approved by the ethics committee of the German Sport University, Cologne, on June 22, 2020 (no. 087/2020) and was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all athletes and controls.
This study utilised data from an observational COVID-19 study titled “COVID-19 in High-Performance Sports” involving 65 elite athletes from the Olympic Centre of North Rhine-Westphalia (NRW)/Rhineland participating in different sports. The study was conducted from January 2020 to October 2021 at the Institute of Cardiology and Sports Medicine, which is licensed by the German Olympic Sports Confederation (DOSB) and located at the German Sport University. Among the study participants were two Olympic gold medalists, four world championship gold medalists, two world championship silver medalists, three world championship bronze medalists, three European champions, and seven national champions.
Inclusion criteria were (1.) a proven infection with SARS-CoV-2 assessed by polymerase chain reaction (PCR) or positive serum SARS-CoV-2 IgG, (2.) registration as a member of the German Federal Squad or Paralympic Federal Squad and regular annual routine sports medical examination at our DOSB-licensed institute, (3.) an age of 14 years or older, and (4.) no acute illness (e.g., febrile disease). Recruitment was conducted either through an antibody screening during the sports medical examination or a request for athletes with positive PCR test results. Written informed consent was required for inclusion and was obtained from all athletes. Prior to enrollment, all participants were informed about the objectives, procedure, and design of the study; subsequent data saving; and anonymity regarding the data. If study participants were under 18 years old, their written consent also had to be signed by a parent or guardian. Participants could withdraw from the study at any time without providing a reason.
Because of the initial reports of a high risk of myocardial injury from COVID-19 infection, 27 of the 65 athletes were randomly selected for cardiac magnetic resonance, with special regard to upcoming competitions or high-stress events (e.g., training camps). A negative PCR result and a symptom-free period of at least 10 days were required before enrollment. As part of the larger study, additional tests (electrocardiogram [ECG] and laboratory tests, including high-sensitivity [hs] troponin I) were performed on all athletes, and a standardised questionnaire was distributed to record the clinical manifestations of COVID-19 and the infection mechanisms and to assess the subjective performance at the clinical visits. The symptoms assessed included fever, cough, loss of taste or smell, runny nose, sore throat, shortness of breath at rest and during exertion, diarrhoea, headache, palpitations, dizziness, chest pain, syncope, muscle pain, joint pain, performance reduction, sleep disturbance, mood swings, concentrations disorders, and skin changes. The normal range of hs troponin-I was below the 99th percentile upper cutoff value (<80 ng/l).
The control group comprised nine healthy volunteers (five female and four male) with no relevant medical history or regular medication and no prior COVID-19 infection or clinical signs of infection within the past 6 months. The volunteers did not have any cardiac conditions, and the cardiac magnetic resonance examinations were assessed to establish new cardiac magnetic resonance protocols and scanner-specific T1 and T2 mapping values. All participants provided written informed consent for the use of their data.
Data were collected by administering COVID-19-specific paper-based questionnaires, implementing SARS-CoV-2 antibody testing, and conducting study-specific diagnostics (e.g., venous blood sampling and resting ECG) along with functional analyses (e.g., spiroergometric and echocardiography including strain analysis). Study-specific diagnostics and data collection were performed at two time points: t0 and t1. Baseline diagnostics at t0 were scheduled after complying with the quarantine guidelines recommended by the Robert Koch Institute (quarantine period of seven days, symptom-free period of 48 hours, and a negative PCR test), and a follow-up visit at t1 was conducted 16 weeks after t0. Data were documented in a study-specific patient chart for which each participant was assigned a unique eight-digit code. To ensure the accuracy and validity of the data, well-established tools, internal monitoring, and quality checks were implemented in addition to external random visits to our study centre. If any data were not available, they were marked as N/A, and a comment was included if needed.
Cardiac magnetic resonance was performed in the follow-up after recovery from COVID-19 on a Siemens Aera 1.5 Tesla scanner (Erlangen, Germany). The study followed a standard protocol including scout images and cine-balanced steady-state free precession (bSSFP) breath-hold sequences in the long axis (two-, three-, and four-chamber) and short axis (SAX) orientations. SAX images were acquired as a stack of images including the ventricles from their valvular plane to their apex. Additionally, parametric mapping was performed in three SAX slices (basal, medial, and apical). T1-mapping utilised a conventional modified look-locker inversion recovery (MOLLI) sequence, and T2-mapping was performed with a T2-prepared SSFP sequence. Dark-blood T2-weighted images with fat suppression were performed in the SAX stack orientation. For late gadolinium enhancement (LGE) imaging, a gadolinium contrast agent (gadoteric acid, DotaVision©, b-e-imaging GmbH) was administered at 0.15 mmol/kg followed by a flush of 30 ml of isotonic saline. Late gadolinium enhancement images were obtained with a breath-hold technique using a phase-sensitive inversion recovering sequence (PSIR) in combination with a conventional bSSFP LGE sequence. Late gadolinium enhancement images were acquired 10 minutes after contrast agent administration. The correct inversion time was determined by a time of inversion finder (TI finder) sequence. Late gadolinium enhancement images were acquired in all three long-axis orientations and as a stack of SAX ccine images covering the full ventricle length.
Image analysis was performed with cardiac magnetic resonance-dedicated software (Circle cvi42 version 5.13.5). Both end-diastolic and end-systolic endocardial and epicardial contours were drawn automatically for the left ventricle (LV) and right ventricle (RV) in the SAX stack of bSSFP cine images. Manual correction was conducted when needed. For the quantification of left ventricular end-diastolic volume (LVEDV), right ventricular end-diastolic volume (RVEDV), left ventricular systolic volume (LVSV), right ventricular systolic volume (RVSV), left and right stroke volumes (LSV and RSV), LV and RV ejection fractions (LVEF/RVEF), and LV mass (LVM), the delineated contours were used, and the assessed volumes were indexed to the body surface area (LVEDVi; RVEDVi, LVSVi, RVSVi, LSVi, and RSVi). T1 and T2 relaxation times were calculated with a T1 and T2 map with motion correction (MOCO) in the midventricular SAX orientation. For this purpose, the slice was divided into six segments following the American Heart Association (AHA) segmentation. Epicardial and endocardial contours were drawn by hand. An offset of 10% was used to avoid including blood pools or extracardiac tissue. The presence of late gadolinium enhancement was assessed visually. Strain analysis was performed with the Circle Cardiovascular Imaging (CVI)-dedicated plug-in for strain analysis. The global longitudinal strain (GLS) was evaluated by using the two-, three-, and four-chamber cine images and the global circumferential strain (GCS) of the SAX cine stack. Images were analysed by two cardiologists (CS and RM) with more than 12 years of experience in cardiac magnetic resonance and a cardiac magnetic resonance level III certification from the European Society of Cardiology (ESC) and the Society of Cardiac Magnetic Resonance (SCMR).
The Shapiro-Wilk test was used to assess the normality of the data distribution. All variables were normally distributed except for LVEDVi and RVEF. The inspection of boxplots revealed no extreme outliers in the data, except for one athlete with a value of +3 SD in LVM and one control with a value of −3 SD in LVEDVi. Levene’s test for equality of variance revealed homogeneity in the variances for the T1 mapping values but not for the T2 mapping values or LVEF. Accordingly, depending on the normal distribution and variance homogeneity, t-tests, Welch’s t-tests, or Mann-Whitney tests for independent samples were used to assess the significance of between-group differences (athletes vs control group). A p-value of less than 0.05 was considered indicative of a statistically significant difference. Given the 16 multiple comparisons conducted in the analysis, a Bonferroni correction was applied, resulting in significance thresholds of p <0.003 for two-sided tests and p <0.006 for one-sided tests. All values are presented with the standard deviation (SD). Statistical analysis was performed with SPSS V.28 (IBM).
The study group consisted of 16 male (59%) and 11 female (41%) elite athletes as well as 5 female (55%) and 4 male (45%) controls. Further details are presented in table 1. The median (interquartile range [IQR]) time interval between COVID-19 diagnosis and the first clinical examination at the Olympic medical centre was 34 (23–54 days) days. Most athletes (92%) experienced a symptomatic course, and 14 athletes (54%) reported having symptoms for more than 4 weeks. Only three athletes (11%) mentioned chest pain at the initial presentation. Of the 14 athletes with symptoms persisting for >4 weeks, none had cardiac symptoms, and fatigue was the predominant complaint amongst this subgroup. Since none of the athletes experienced major complications, no specific treatment was required beyond symptomatic therapy with anti-inflammatory and anti-rheumatic drugs, such as ibuprofen. To ensure the safe resumption of sports, we followed the return-to-play guidelines outlined by Elliott et al. for SARS-CoV-2-positive athletes. At the time of the study, this included a minimum training break of 10 days after testing positive for COVID-19 and a symptom-free period of at least 7 days before returning to activity.
Table 1Characteristics of athletes and controls.
| Athletes (n = 27) | Control group (n = 9) | ||
| Age (years) | Mean = 23.69 (SD = 5.05) | Mean = 41.11 (SD = 6.27) | |
| Body mass index (kg/m2) | Mean = 23.30 (SD = 2.82) | Mean = 23.18 (SD = 3.59) | |
| Gender | 11 female, 16 male | 5 female, 4 male | |
| Sporting disciplines, n (%) | Fencing | 9 (34.62%) | |
| Judo | 4 (15.38%) | ||
| Boxing | 3 (11.54%) | ||
| Athletics | 2 (7.69%) | ||
| Swimming | 2 (7.69%; 1 excluded because of ARVC) | ||
| Taekwondo | 2 (7.69%) | ||
| Wrestling | 2 (7.69%) | ||
| Basketball | 1 (3.85%) | ||
| Hockey | 1 (3.85%) | ||
| Rowing | 1 (3.85%) | ||
| Symptoms, n (%) | Initially | 24 (92.31%) | |
| After 4 weeks | 14 (53.80%) | ||
| Elevated high-sensitivity troponin I, n (%) | 2 (7.69%) | ||
ARVC: arrhythmogenic right ventricular cardiomyopathy
In two athletes, elevated troponin values were observed at the initial clinical visit, one of whom was the excluded athlete (see “Cardiac magnetic resonance findings”). The other athlete experienced no cardiac symptoms at any time and was asymptomatic at the time of cardiac magnetic resonance; troponin was 108 ng/l and had normalised to <80 ng/l 3 days later. The athlete had trained intensely prior to the initial troponin elevation. The median time interval between the positive PCR test and the cardiac magnetic resonance study was 182 days (SD 99).
The ECG was normal in all athletes (bradycardia is a physiological feature in trained athletes). None of the athletes were treated with any specific pharmacological agent for COVID-19.
One male athlete (a swimmer) was excluded from the study because of cardiac magnetic resonance findings indicating arrhythmogenic right ventricular cardiomyopathy (ARVC). In another male athlete, an incidental mass of the right ventricle at the free lateral wall was found and diagnosed as a benign fibroma. Athletes had significantly enlarged left and right ventricle volumes and an increased left ventricular myocardial mass in comparison with the healthy control group (LVEDVi: 103.4 vs 91.1 ml/m2, p = 0.031; RVEDVi: 104.1 vs 86.6 ml/m2, p = 0.007; LVMi: 59.0 vs 46.2 g/m2, p = 0.002). Global longitudinal strain values did not differ between the athlete and the control cohort (global longitudinal strain –16.6 vs –17.8 % with p = 0.066). Global circumferential strain values were significantly lower in athletes compared with controls (global circumferential strain −16.7 vs −18.7 %, p = 0.029), but this difference was reduced to a tendency after applying the Bonferroni correction for multiple comparisons. No differences were observed in left ventricular ejection fraction (LVEF: 56.5 vs 58.9%, p = 0.072), whereas the right ventricular ejection fraction of athletes was reduced in comparison with the control group (RVEF: 51 vs 54.8%, p = 0.008). The T1 and T2 mapping values of the athletes did not differ from those of the control group (T1 values: 992.5 vs 1015.4 ms; T2 values: 47.8 vs 48.7 ms; p = 0.530 and p = 0.313); see figure 1.

Figure 1Bar graph illustrating the comparison of T1 and T2 mapping values in athletes and controls, with p = 0.530 for T1 mapping and p = 0.313 for T2 mapping.
LGE imaging did not reveal any abnormalities in myocardial tissue characterisation indicating myocardial damage. None of the athletes met the current Lake Louise criteria for acute myocarditis [15]. To account for the potential confounding effects of age, weight, height, and BMI, we also performed analysis of covariance (ANCOVA). After this adjustment, no differences retained statistical significance. However, the inclusion of control variables in the ANCOVA model was constrained by the sample size; our small sample size may have limited statistical power, and the introduction of multiple control variables warrants careful consideration given the potential complications in interpretation. The full details of the cardiac magnetic resonance findings are presented in table 2; figure 2 shows exemplary cardiac magnetic resonance images and strain analyses of a control (A–D) and an athlete (E–I).
Table 2Details of cardiac magnetic resonance values and comparison between athletes and control subjects. The between-group comparisons show p-values, mean differences (non-standardised) and 95% CI. All comparisons between athletes and controls were made using independent t-tests. Levene’s test for quality of variance showed no homogeneity of variances. Accordingly, Welch’s t-test was used to assess the between-group significance. Because the data were not normally distributed, comparisons for LVEDVi (ml/m2) and RVEF were conducted with Mann-Whitney tests; distributions did not differ between groups, Kolmogorov-Smirnov p = 0.118 and p = 0.059.
| Athletes (n = 26) | Control group (n = 9) | Group difference | ||||||||
| Mean | SD | Median | IQR | Mean | SD | Median | IQR | p-value (two-sided) | Mean difference (95% CI) | |
| LVEDD basal (mm) | 52.47 | 4.62 | 51.70 | 4.62 | 50.81 | 5.17 | 51.50 | 7.30 | 0.374 | 1.7 (−2.1 to 5.4) |
| Left ventricle mass (g) | 112.77 | 30.45 | 103.50 | 30.45 | 87.44 | 32.55 | 69.00 | 61.50 | 0.099 | 21.1 (−4.7 to 47.0) |
| Left ventricle mass / body surface area (g/m2) | 59.00 | 9.83 | 56.90 | 9.83 | 46.16 | 10.41 | 41.10 | 19.10 | 0.002 | 12.8 (5.0 to 20.7) |
| LVEDV (ml) | 196.54 | 42.87 | 182.50 | 42.87 | 162.78 | 41.61 | 155.00 | 65.00 | 0.048 | 33.8 (0.3 to 67.3) |
| LVEDVi (ml/m2) | 103.39 | 15.62 | 99.70 | 99.70 | 84.77 | 20.96 | 91.00 | 17.00 | 0.031 | 12.3 (0.35 to 24.2) |
| LVESV (ml) | 89.65 | 21.27 | 84.50 | 21.27 | 67.89 | 19.21 | 66.00 | 30.00 | 0.011 | 21.8 (5.4 to 38.1) |
| LVEF (%) | 56.50 | 2.00 | 56.00 | 2.00 | 58.89 | 3.37 | 59.00 | 6.50 | 0.072 | −2.4 (−5.0 to 0.3) |
| RVEDV (ml) | 197.96 | 44.64 | 183.50 | 44.64 | 158.22 | 42.22 | 148.00 | 70.50 | 0.026 | 39.7 (5.1 to 74.4) |
| RVEDVi (ml/m2) | 104.06 | 16.52 | 101.15 | 16.52 | 86.64 | 12.69 | 84.90 | 15.15 | 0.007 | 17.4 (5.1 to 29.8) |
| RVESV (ml) | 98.08 | 25.99 | 88.50 | 25.99 | 73.89 | 20.20 | 66.00 | 31.00 | 0.016 | 24.2 (4.7 to 43.6) |
| RVESVi (ml/m2) | 51.43 | 10.33 | 50.10 | 10.33 | 39.69 | 6.13 | 40.30 | 3.40 | 0.003 | 11.7 (4.2 to 19.2) |
| RVEF (%) | 51.04 | 2.86 | 51.00 | 2.86 | 54.78 | 3.80 | 56.00 | 30.00 | 0.008 | −3.7 (−6.2 to −1.3) |
| Native T1 (ms) | 992.54 | 27.27 | 991.43 | 41.61 | 1015.36 | 35.31 | 1009.72 | 62.27 | 0.530 | −22.8 (−46.0 to 0.3) |
| Native T2 (ms) | 47.75 | 1.45 | 47.77 | 2.03 | 48.74 | 2.64 | 49.57 | 4.08 | 0.313 | −1.0 (−3.1 to 1.1) |
| Global longitudinal strain (%) | 16.59 | 1.45 | 16.50 | 1.45 | 17.84 | 2.32 | 18.20 | 4.45 | 0.066 | −1.3 (−2.6 to 0.1) |
| Global circumferential strain (%) | 16.73 | 1.07 | 16.85 | 1.07 | 18.69 | 2.20 | 17.80 | 4.30 | 0.029 | −1.9 (−3.1 to 0.8) |
| Late gadolinium enhancement | 0 | n.a. | ||||||||
LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; LVEDV: left ventricular end-diastolic volume; RVEDV: right ventricular end-diastolic volume; RVESVi: right ventricle end-systolic volume indexed to the body surface area.
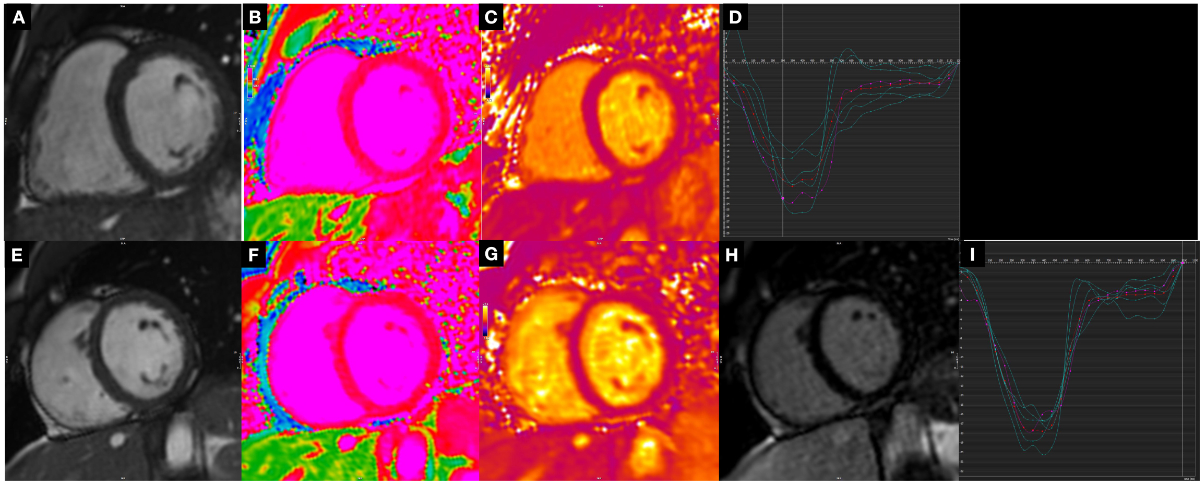
Figure 2Panels A–D show example images from a participant in the control group: (A) still frame of a medial short-axis cine image, (B) the corresponding T1 map, (C) the related T2 map, and (D) the associated strain analysis with image A. Panels E–I show examples from an athlete: (E) still frame of a medial short axis cine image, (F) the corresponding T1 map, (G) the related T2 map, (H) the late gadolinium enhancement image, and (I) the circumferential strain curve associated with image E.
It is well-documented that COVID-19 infection can cause myocardial involvement with a myocarditis-like pattern in the cardiac magnetic resonance diagnostic assessment [1, 3]. The present study examined the mid- to long-term effects of COVID-19 infection on the hearts of elite athletes as assessed by cardiac magnetic resonance. The main findings of the study were as follows: (a) no signs of myocarditis or myocardial damage were documented in 26 elite athletes with previous COVID-19 infection; (b) the hearts of the athletes demonstrated elevated left and right ventricular volumes and left ventricular mass as well as slightly lower RVEF values in comparison with the control group; and (c) the ejection fractions and strain values were within the normal range for athletes.
Athletes are considered a low-risk population for severe COVID-19 because of their young age and good general health. However, because of the persistence of the pandemic and the rising number of athletes with acute and prior infections (some repeatedly), research into the potential cardiac effects is highly relevant, especially considering that myocarditis is one of the leading causes of sudden cardiac death in athletes [16]. As return-to-play guidelines continue to evolve, [17] the data of the present study support the key insight that mid- to long-term myocardial damage seems to be very rare in athletes. As recently demonstrated by Wroblewsi et al. [18], a differentiation between acute and sub-acute to mid- and long-term effects must be differentiated; using cardiac magnetic resonance, the authors found that 38 out of 117 (32%) college athletes had signs of cardiac inflammation, but all of these signs had resolved by the follow-up cardiac magnetic resonance 6–8 weeks later [19].
Interestingly, this study did not reveal any signs of myocardial inflammation even though 54% of athletes still reported symptoms at the time of data collection. However, these were unspecific symptoms, with most athletes reporting lingering fatigue (no chest pain or dyspnea).
Generally, the results of the present study are in line with a growing body of evidence indicating a very low incidence of myocarditis in athletes [14]. So far, the largest COVID-19 study in professional athletes by Martinez et al. [19] showed a low incidence of abnormal cardiac screening results (3.8%) during standard return-to-play examinations. Additional indication-based cardiac magnetic resonance in 30 of >800 college athletes found signs of myocarditis in only 0.6% of athletes. Another cardiac magnetic resonance-only study in 12 professional athletes showed no signs of cardiac involvement [13]. In almost all studies to date, cardiac magnetic resonance examinations have been performed in the acute or early phase after infection. Insights from a longitudinal study involving patients without COVID-19 with cardiac magnetic resonance-established myocarditis showed that the amount of LGE increased in up to 20% of the patients in the 3 months following initial diagnosis, despite the normalisation of cardiac enzymes [20]. In the present study, cardiac magnetic resonance was performed 182 days after the positive COVID-19 PCR result; therefore, this study elucidates the cardiac effects during long follow-up periods after infection. Our data suggest that the risk for mid to long-term myocardial damage is low to negligible in elite athletes after COVID-19 infection. These findings are in line with the results of a recently published study reporting that initial cardiac magnetic resonance signs of myocardial damage (elevated T1-mapping values and signs of LGE) were not observed in non-athletic patients after 6 months, despite symptom persistence in 52% of cases [18]. Although cardiac magnetic resonance is the most sensitive imaging test and the gold standard for diagnosis of myocardial inflammation, cardiac magnetic resonance should not be used as direct screening, as summarised in the current guidelines [21]. According to our data and the results of recent trials, cardiac magnetic resonance should primarily be conducted in athletes if it is indicated by symptoms or pathological findings via ECG, echocardiogram [19], or elevated relevant cardiac enzymes (e.g., hs troponin).
The current recommendations [21] are based on published data and the experience of the past three years. In summary, our results indicate that despite the initial awareness and results of the signs of myocardial inflammation at the beginning of the pandemic, the true incidence of myocardial damage in elite athletes is very low.
It should be noted that the observed cardiac magnetic resonance values for ventricle volumes and ejection fractions are typical physiological adaptations of elite athletes commonly described as “athlete’s heart” [22, 23].
Hence, future research should focus on when a safe return to sports is possible when cardiac magnetic resonance indicates signs of myocardial damage without active oedema. Is there a safe way – especially for elite athletes – to return to full sports activity despite cardiac magnetic resonance signs of previous myocarditis? So far there are no existing data on that. The current guidelines recommend at least 3 to 6 months of rest after a diagnosis of myocarditis in athletes. Future studies should focus on whether cardiac magnetic resonance is a valuable tool to guide a safe early return to play.
The main strength of this study is the elite status of the included athletes. All were part of the German Olympic team at the time of inclusion, and many were international and national medalists. The cardiologists in charge of magnetic resonance imaging (MRI) had extensive experience in the field of cardiac MRI and sports medicine, reducing the margin for error in the assessment.
The sample was limited by its size (26 athletes) and the non-athletic random control group. Normally, athletes are only referred to cardiac magnetic resonance because of specific findings in baseline tests or cardiac-related symptoms. During the first pandemic wave, healthy athletes were advised to strictly avoid unnecessary contact. Therefore, no cardiac magnetic resonance data are available for healthy elite athletes during this period. Because COVID-19 has now affected such a large portion of the population, it seems impossible to find a control group that has not been infected since this study was conducted. The time points of data collection do not allow for acute and long-term conclusions, but the explicit aim of this study was to assess the midrange period after infection. Since the study population consisted of elite athletes only, the results cannot be generalised to the general population.
The findings of this study add to the growing body of evidence that the risk for mid- to long-term myocardial damage following COVID-19- is very low in elite athletes.
Author contributions: JZ, TS and H-GP conceived the study design (‘Covid-19 in high-performance sport’), recruited funding and obtained ethics approval. KD, JZ and CS participated in coordination and data collection. KD, JZ, TS and H-GP examined the athletes as part of study and the return to play protocol. CS performed and evaluated, together with RM, the CMR exams and collected the CMR-specific data. CSy was responsible for the statistical analysis. CS and KD contributed equally to the manuscript and drafted the manuscript; therefore, both will serve as joint first authors. As JZ and RM contributed equally with their expertise and mentorship in their fields, there was the consensus of all authors that both should be stated as joint last authors. HGP, TS, JZ and RM revised and edited the manuscript and participated in data interpretation. All authors have read and approved the final manuscript and take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
This study was funded by the Bundesinstitut für Sportwissenschaft.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Manka R, Karolyi M, Polacin M, Holy EW, Nemeth J, Steiger P, et al. Myocardial edema in COVID-19 on cardiac MRI. J Heart Lung Transplant. 2020 Jul;39(7):730–2. 10.1016/j.healun.2020.04.025
2. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020 Nov;5(11):1265–73. 10.1001/jamacardio.2020.3557
3. Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021 May;42(19):1866–78. 10.1093/eurheartj/ehab075
4. Phelan D, Kim JH, Elliott MD, Wasfy MM, Cremer P, Johri AM, et al. Screening of Potential Cardiac Involvement in Competitive Athletes Recovering From COVID-19: An Expert Consensus Statement. JACC Cardiovasc Imaging. 2020 Dec;13(12):2635–52. 10.1016/j.jcmg.2020.10.005
5. Captur G, Manisty C, Moon JC. Cardiac MRI evaluation of myocardial disease. Heart. 2016 Sep;102(18):1429–35. 10.1136/heartjnl-2015-309077
6. Patriki D, Gresser E, Manka R, Emmert MY, Lüscher TF, Heidecker B. Approximation of the Incidence of Myocarditis by Systematic Screening With Cardiac Magnetic Resonance Imaging. JACC Heart Fail. 2018 Jul;6(7):573–9. 10.1016/j.jchf.2018.03.002
7. Peterson DF, Kucera K, Thomas LC, Maleszewski J, Siebert D, Lopez-Anderson M, et al. Aetiology and incidence of sudden cardiac arrest and death in young competitive athletes in the USA: a 4-year prospective study. Br J Sports Med. 2021 Nov;55(21):1196–203. 10.1136/bjsports-2020-102666
8. Nieß A, Bloch W, Friedmann-Bette B, Grim C, Halle M, Hirschmüller A, et al. Position stand: return to sport in the current Coronavirus pandemic (SARS-CoV-2 / COVID-19). Dtsch Z für SportmedGer J Sports Med. 2020;71(5):E1–4.
9. Elliott N, Martin R, Heron N, Elliott J, Grimstead D, Biswas A. Infographic. Graduated return to play guidance following COVID-19 infection. Br J Sports Med. 2020 Oct;54(19):1174–5. 10.1136/bjsports-2020-102637
10. Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, et al. COVID-19 Myocardial Pathology Evaluation in Athletes With Cardiac Magnetic Resonance (COMPETE CMR). Circulation. 2021 Feb;143(6):609–12. 10.1161/CIRCULATIONAHA.120.052573
11. Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, et al. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2021 Jan;6(1):116–8.
12. Małek ŁA, Marczak M, Miłosz-Wieczorek B, Konopka M, Braksator W, Drygas W, et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: A magnetic resonance study. J Magn Reson Imaging. 2021 Jun;53(6):1723–9. 10.1002/jmri.27513
13. Vago H, Szabo L, Dohy Z, Merkely B. Cardiac Magnetic Resonance Findings in Patients Recovered From COVID-19: Initial Experiences in Elite Athletes. JACC Cardiovasc Imaging. 2021 Jun;14(6):1279–81. 10.1016/j.jcmg.2020.11.014
14. Sharma G, Blumenthal RS, Martinez MW. COVID-19, Myocarditis, and Cardiac MRI in Athletes: Distinguishing Signal from Noise.pdf. American College in Cardioilogy.
15. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: expert Recommendations. J Am Coll Cardiol. 2018 Dec;72(24):3158–76. 10.1016/j.jacc.2018.09.072
16. Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009 Mar;119(8):1085–92. 10.1161/CIRCULATIONAHA.108.804617
17. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Russ J Cardiol. 2021;26(5):4488. 10.15829/1560-4071-2021-4488
18. Wroblewski J, Mahajan P, Li RL, Busch J, Rhee B. Long term characterization of athletes recovering from COVID-19 infection and return to play. J Am Coll Cardiol. 2022;79(9):2061. 10.1016/S0735-1097(22)03052-2
19. Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, et al. Prevalence of Inflammatory Heart Disease Among Professional Athletes With Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol. 2021 Jul;6(7):745–52. 10.1001/jamacardio.2021.0565
20. Berg J, Kottwitz J, Baltensperger N, Kissel CK, Lovrinovic M, Mehra T, et al. Cardiac Magnetic Resonance Imaging in Myocarditis Reveals Persistent Disease Activity Despite Normalization of Cardiac Enzymes and Inflammatory Parameters at 3-Month Follow-Up. Circ Heart Fail. 2017 Nov;10(11):e004262. 10.1161/CIRCHEARTFAILURE.117.004262
21. Gluckman TJ, Bhave NM, Allen LA, Chung EH, Spatz ES, Ammirati E, et al.; Writing Committee. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022 May;79(17):1717–56. 10.1016/j.jacc.2022.02.003
22. Małek ŁA, Mazurkiewicz Ł, Marszałek M, Barczuk-Falęcka M, Simon JE, Grzybowski J, et al. Deformation Parameters of the Heart in Endurance Athletes and in Patients with Dilated Cardiomyopathy-A Cardiac Magnetic Resonance Study. Diagnostics (Basel). 2021 Feb;11(2):374. 10.3390/diagnostics11020374
23. D’Ascenzi F, Anselmi F, Piu P, Fiorentini C, Carbone SF, Volterrani L, et al. Cardiac Magnetic Resonance Normal Reference Values of Biventricular Size and Function in Male Athlete’s Heart. JACC Cardiovasc Imaging. 2019 Sep;12(9):1755–65. 10.1016/j.jcmg.2018.09.021