Clinical outcomes of HeartMate 3 left ventricular assist device support with a Bridge
to Transplant vs a Destination Therapy strategy: a single-centre retrospective cohort
DOI: https://doi.org/https://doi.org/10.57187/s.3529
John Kikoïnea,
Anna Nowackab,
Sara Schukrafta,
Tamila Abdurashidovaa,
Patrick Yerlya,
Piergiorgio
Tozzib,
Zied Ltaiefc,
Lorenzo
Rosnerd,
Roger
Hullina,
Matthias
Kirschb
a Department of Cardiology,
University Hospital, Lausanne, Switzerland.
b Department of Cardiac Surgery,
University Hospital, Lausanne, Switzerland
c Department of Intensive Care
Medicine, University Hospital, Lausanne, Switzerland
d Department of Anaesthesiology,
University Hospital, Lausanne, Switzerland
Summary
INTRODUCTION: Real-world outcomes with the HeartMate 3
left ventricular assist device (LVAD) depending on whether it’s a bridge to
transplantation (BTT) or destination therapy (DT) are poorly studied. We aimed
to compare the profile and clinical outcomes of patients supported with HeartMate 3
according to a BTT or a DT pre-implantation strategy.
METHODS: All patients consecutively
implanted with HeartMate 3 at our centre (University Hospital of
Lausanne, Switzerland) in 2015–2022 were analysed in a retrospective
observational study. Indications for HeartMate 3 implantation were
advanced heart failure despite optimal medical treatment. Patients were treated
with a vitamin K antagonist anticoagulant combined with antiplatelet therapy
after HeartMate 3 implantation and were followed up monthly at our institution.
RESULTS: Among 71 patients implanted with HeartMate 3
between 2015 and 2022, 51 (71.8%) were implanted as a BTT and 20 (28.2%) as DT.
Their median age was 58 (IQR: 52–69)
years and 84% of patients were classified as INTERMACS profiles 2–4. The
median follow-up duration was 18.3 (IQR: 7.5–33.9) months. Patients in the DT
group were older than those in the BTT group (p <0.001) and had more chronic renal
failure (p <0.001). They also had
a lower 5-year survival rate (mean ± standard error: 87.3 ± 5.6% vs 49.4 ± 15.1%) and more adverse
events such as renal dysfunction
requiring temporary perioperative dialysis (p = 0.08) or bleeding (p = 0.06).
CONCLUSION: Although patients supported
with HeartMate 3 have favourable survival, those with LVAD-DT have poorer
outcomes. There is a need to better select patients eligible for LVAD-DT in
order to limit the burden of adverse events and improve their prognosis.
Abbreviations
- BTT
-
bridge to transplantation
- DT
-
destination therapy
- ECLS
-
extracorporeal life support
- LVAD
-
left ventricular assist device
- RV
-
right ventricle
Introduction
Heart failure (HF) is a common disease in
developed countries with a prevalence of around 1–2% in adults [1–3]. Most HF patients
progress to advanced HF and face a significant risk of mortality if treatment is
only pharmacological. The use of left ventricular assist devices (LVADs) has
transformed the management of advanced HF by offering two primary indications:
bridge to transplantation (BTT) and destination therapy (DT) [4]. As a BTT, an LVAD
provides temporary mechanical circulatory support to patients enabling them to
maintain haemodynamic stability, improve peripheral organ function and enhance
functional capacity while awaiting the availability of a suitable donor organ.
As part of DT, the device is a long-term treatment option for patients who are
not eligible for heart transplantation.
The HeartMate 3 LVAD (Abbott, Abbott
Park, IL, USA) has significantly advanced the field of left ventricular assist device
therapy using a continuous-flow centrifugal pump with a fully magnetically
levitated rotor, wide blood flow passages and an intrinsic pseudopulse (which
reduces blood stasis in the pump without generating noticeable pulsed pressure
in the arterial circulation) [5]. This resulted in a significantly improved haemocompatibility
profile, reducing complications such as pump thrombosis, stroke and bleeding
compared to previous LVAD generations [6]. In the French-speaking part of
Switzerland, an algorithm has been established for the care of advanced HF patients
in need of long-term mechanical support [7].
Understanding the real-world results and implications of using the HeartMate 3
device as a BTT or DT is essential to optimise patient selection and improve
clinical decision-making.
We aimed to compare the profile and
clinical outcomes of patients supported with a HeartMate 3 device in our
institution according to a BTT or DT pre-implantation strategy.
Methods
Population
We evaluated, through an exploratory and retrospective
observational study, all consecutive patients implanted with a HeartMate 3
LVAD at our centre (University Hospital of Lausanne, Switzerland) between
November 2015 and October 2022. Patients supported during the same period with
other implantable VADs such as Abbott HeartMate 2 (n = 2, implanted as isolated
right ventricular [RV] assist device) were not included. No patients were
excluded from the analysis. Indications for HeartMate 3 implantation have been
described previously [7]. Briefly, patients with New York Heart Association
(NYHA) class IIIB or IV symptoms with an ejection fraction ≤25% and a cardiac
index ≤2.2 l/min/m2
without inotropic support despite optimal medical management, or
inotrope-dependent patients, or listed for heart transplant according to the
recommendations of the International Society for Heart Lung Transplantation [8]
were eligible. The DT programme at our institution was started in June 2017. Depending
on transplant eligibility at the time of LVAD support, each patient was
assigned to a pre-implantation strategy: BTT or DT. Patients with possible eligibility
for transplantation (bridge to candidacy, n = 2) were analysed in the BTT
group.
Surgical technique
Immediately prior to HeartMate 3
implantation, transoesophageal echocardiography was always performed to exclude
the presence of coexisting conditions requiring additional surgical procedure:
moderate to severe aortic regurgitation, severe tricuspid regurgitation, patent
foramen ovale, atrial septal defect or thrombus in the left ventricle after
myocardial infarction. Valvular replacement with a bioprosthesis was
performed in patients with a mechanical aortic prosthesis, whereas mechanical
prostheses in mitral position were retained.
Surgical techniques have already been
described [9]. Briefly, three different surgical approaches were used: “median
sternotomy”, “double mini-thoracotomy” and “left thoracotomy”. In “median
sternotomy” (the default approach) or “double mini-thoracotomy” (accessed
through a left anterior mini-thoracotomy and an upper mini-sternotomy) [10],
the left ventricular assist device was implanted between the left ventricular
apex and the ascending aorta. In “left thoracotomy”, the outflow graft was
implanted in the descending thoracic aorta via a left anterolateral thoracotomy
[11]. The latter approach was
preferred in patients with a history of cardiac surgery (especially coronary
artery bypass grafting [CABG]) to avoid a high-risk resternotomy. All
implantations were performed with central or peripheral cardiopulmonary bypass.
No aortic cross-clamping was used, except in the case of concomitant left-sided
cardiac procedures. The apical sewing ring was sutured to the apex of the left
ventricle using the “core
and sew with back stitch” technique [12]. The driveline was placed
using the double tunnelling technique [13] and was stabilised
immediately after surgery using the Hollister’s horizontal tube attachment
device (Hollister Inc., Libertyville, IL, USA).
In cases of severe postoperative right
ventricular dysfunction, a temporary right ventricle support device was
installed through a venoarterial extracorporeal life support (ECLS) (venous
inflow cannula in the right atrium through a femoral vein and outflow cannula
in the main pulmonary artery) [14]. Severe right ventricular dysfunction was
defined as a right ventricular failure visually assessed by transoesophageal
echocardiography associated with the inability to achieve a stable LVAD output
≥2.5 l/min despite adequate LVAD placement, sufficient volume loading and
maximal inotropic and pulmonary vasodilator support.
Antithrombotic treatment
Anticoagulation with intravenous heparin
was started 6–12 h after surgery, with target anti-factor Xa activity of 0.3 to
0.45 IU/ml. Vitamin K antagonist (VKA) therapy was initiated after extubation
and removal of chest drains with a target international normalised ratio (INR) of
2–3. In addition, in the absence of bleeding or thrombocytopenia, antiplatelet
therapy as aspirin 100 mg/day was systematically added to the anticoagulation
when patients were discharged from the intensive care unit. During follow-up,
we bridged to low-molecular-weight heparin or unfractionated heparin (if
estimated glomerular filtration rate was less than 30 ml/min/1.73 m2)
only when patients had an INR <1.8. The combination of vitamin K antagonist therapy
and antiplatelet therapy was indicated lifelong in the absence of prohibitive
bleeding risk such as the occurrence of a bleeding event leading to hospitalisation,
in which case aspirin was definitely discontinued.
Follow-up
After hospital discharge, patients were
followed up every month at our outpatient Heart Failure Clinic by an experienced
HF specialist to assess their clinical and biological status. A skin culture was
also routinely taken from the driveline exit site at each visit. Driveline dressings
were
renewed three times per week in accordance with our local protocol.
Data collection and outcomes
Baseline characteristics, intra- and peri-operative
data, and clinical outcomes including follow-up data were retrospectively
collected in a local database by reviewing patients’ electronic medical files.
Data integrity was verified secondarily by one of the study investigators.
Preoperative clinical profiles were established for each patient according to
INTERMACS definitions [15] and assessed in the 24 hours prior to HeartMate 3
implantation. Patients with acute cardiogenic shock stabilised by venoarterial ECLS
with recovery of peripheral organ
function were classified as INTERMACS class 2. Outpatients treated with
repeated (monthly) elective levosimendan infusions (administered at a dose of
0.1 μg/kg/min over 24 hours) were classified as INTERMACS class 4. Adverse
events were reported as per the definitions of the Mechanical Circulatory Support
Academic
Research Consortium [16]. Postoperative infections were defined as
VAD-specific or VAD-related in accordance with previous definitions [17]. Driveline
infection was defined by the
presence of drainage or inflammation around the driveline exit site associated
with a positive culture. This study was
conducted with the approval of the local ethics committee (CER Number
2019-00697).
Statistical analysis
All patients were analysed within their
initially assigned groups (BTT or DT) although three patients shifted from BTT to
DT during follow-up because of contraindications to heart transplantation that
emerged after LVAD implantation, namely refusal of heart transplantation, diagnosis
of lung cancer and onset of a depressive syndrome. No crossovers were observed
from DT to BTT. Categorical variables are presented as counts (percentages) and
continuous variables as medians (interquartile ranges [IQR]). Comparisons
between qualitative variables were made using the chi-square or Fisher’s exact
test, as appropriate. Continuous variables were compared using a Wilcoxon rank-sum
test. Patient survival rates were estimated by the Kaplan-Meier method with its
95% confidence interval (CI). For patient survival estimates on HeartMate 3
support, patients were censored at the time of LVAD explantation (heart
transplantation [n = 29] or device weaning [n = 1]) or at the date of last
follow-up. Statistical comparisons of survival rates between the DT and BTT groups
were not carried out due to the wide disparity between the two groups. All tests
were 2-sided and conducted at a 0.05 level of significance. Statistical
analyses were performed using SPSS BASE 17.0 statistical software (SPSS
Inc., Chicago, IL, USA).
Results
Pre-implantation population characteristics
We included a total of 71 HeartMate 3 patients:
51 (71.8%) were implanted as BTT and 20 (28.2%) as DT. The main reasons for DT were
age ≥70 years in 17/20 (85%) patients, and chronic obstructive pulmonary
disease, polyvascular disease and neurological conditions in the three others. Primary
causes of heart failure by study group are shown in figure 1. The most common
cause of heart failure was dilated ischaemic cardiomyopathy in 48% of patients,
followed by primary dilated cardiomyopathy in 30% and recent acute myocardial
infarction in 20%. There was a trend towards a higher frequency of recent acute
myocardial infarction in the BTT patients than in DT patients (26% vs 5%, p = 0.09).
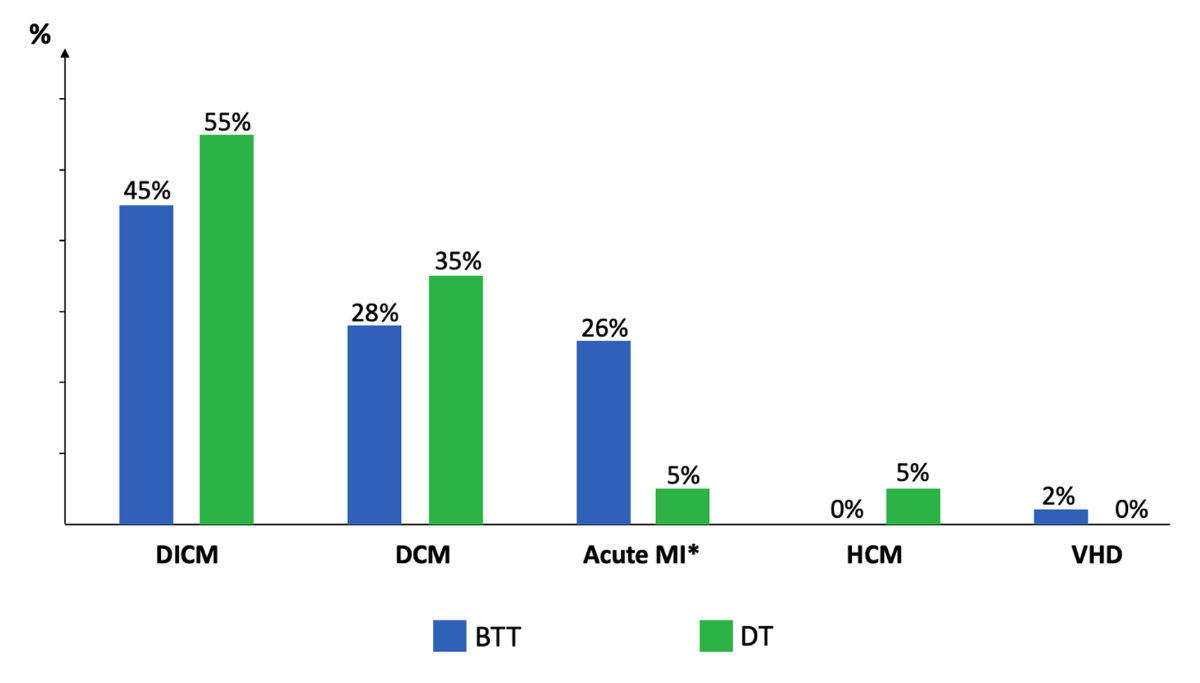
Figure 1Primary causes
of heart failure by study group. Proportions of
patients are expressed as percentages for each study group (blue bar for bridge
to transplantation [BTT] and green bar for destination therapy [DT]).
Comparisons between groups: Acute MI* (p = 0.09); DICM (p = 0.60); DCM (p = 0.57).
DCM: dilated cardiomyopathy; DICM: dilated ischaemic cardiomyopathy; HCM: hypertrophic
cardiomyopathy; MI: myocardial infarction; VHD: valvular heart disease. *
myocardial infarction <3 months.
Baseline clinical characteristics of the
study population are displayed in table 1. The median age was 58 (52–69) yearsand
63 (89%) patients were men. Patients
in the BTT group were younger than those in the DT group (53 [47–60] years vs 71
[69–74] years, p <0.001). A total of 10 (14%)
patients had a history of cardiac surgery, including coronary artery bypass
grafting (n = 7, one with associated mitral annuloplasty), mechanical
mitral valve replacement (n = 2) and mechanical aortic valve replacement (n = 1).
Before implantation, 60 (84%) patients were classified as
INTERMACS profiles 2–4, without differences between groups.
Table 1Baseline characteristics of the
study population. Categorical variables are presented as counts (percentages)
and continuous variables as medians (interquartile ranges).
|
All patients |
Bridge to transplantation |
Destination therapy |
p value |
|
n = 71 |
n = 51 |
n = 20 |
| Clinical characteristics |
Age
in years |
58
(52–69) |
53
(47–60) |
71
(69–74) |
<0.001 |
| Male
sex |
63
(89%) |
47
(92%) |
16
(80%) |
0.21 |
| Body
surface area, in m2 |
2.0
(1.8–2.1) |
2.0
(1.8–2.1) |
1.9
(1.8–2.1) |
0.42 |
| Diabetes
mellitus |
18
(25%) |
14
(28%) |
4
(20%) |
0.76 |
| History
of stroke |
11
(16%) |
6
(12%) |
5
(25%) |
0.28 |
| Previous
cardiac surgery |
10
(14%) |
6
(12%) |
4
(20%) |
0.45 |
| INTERMACS
profiles |
|
|
|
|
0.90 |
| 1 |
9
(13%) |
7
(14%) |
2
(10%) |
|
| 2 |
14
(20%) |
10
(20%) |
4
(20%) |
|
| 3 |
21
(29%) |
14
(27%) |
7
(35%) |
|
| 4 |
25
(35%) |
18
(35%) |
7
(35%) |
|
| 5 |
2
(3%) |
2
(4%) |
0 |
|
| Haemodynamics |
LVEF,
in % |
22
(17–28) |
22
(16–29) |
22
(18–28) |
0.27 |
| LVEDD,
in mm |
[n = 61/71]: 66 (59–70) |
[n = 42/51]: 65 (58–70) |
[n = 19/20]: 69 (63–71) |
0.30 |
| MAP,
in mm Hg |
[n = 50/71]: 79 (68–88) |
[n = 37/51]: 72 (66–85) |
[n = 13/20]: 87 (77–95) |
0.17 |
| CVP,
in mm Hg |
[n = 47/71]: 10 (6–12) |
[n = 31/51]: 11 (7–12) |
[n = 16/20]: 8 (6–12) |
0.79 |
| PCWP,
in mm Hg |
[n = 54/71]: 23 (19–32) |
[n = 37/51]: 25 (19–32) |
[n = 17/20]: 22 (17–32) |
0.57 |
| PVR, in Woods units |
[n = 54/71]: 2.4 (1.5–3.0) |
[n = 37/51]: 2.4 (1.4–3.0) |
[n = 17/20]: 2.2 (1.6–3.8) |
0.02 |
| CI, in l/m2/min |
[n = 54/71]: 2.2 (1.9–2.5) |
[n = 37/51]: 2.2 (1.9–2.7) |
[n = 17/20]: 2.2 (1.8–2.3) |
0.65 |
| Laboratory |
Sodium, in mmol/l |
139 (136–141) |
139 (136–141) |
138 (131–140) |
0.59 |
| Creatinine, in µmol/l |
116 (95–160) |
107 (88–131) |
162 (139–193) |
<0.001 |
| Blood urea nitrogen, in mmol/l |
[n = 70/71]: 9 (6–13) |
[n = 50/51]: 7 (5–11) |
[n = 20/20]: 13 (9–17) |
<0.001 |
| White blood cell count, in 109/l |
7.8 (6.2–10.4) |
8.1 (6.5–10.5) |
6.6 (6.0–10.3) |
0.14 |
| Platelet count, in 109/l |
218 (166–262) |
222 (178–269) |
192 (149–255) |
0.20 |
| Haematocrit, in % |
35 (31–40) |
35 (32–40) |
33 (29–40) |
0.31 |
| Total bilirubin, in µmol/l |
[n = 70/71]: 12 (9–19) |
[n = 50/51]: 13 (9–21) |
[n = 20/20]: 11 (8–16) |
0.39 |
| Aspartate
aminotransferase, in U/l |
28 (19–54) |
27 (19–60) |
29 (18–43) |
0.40 |
| Alanine transaminase, in U/l |
31 (21–69) |
34 (23–89) |
22 (17–36) |
0.009 |
| Albumin, in g/l |
[n = 65/71]: 36 (29–41) |
[n = 46/51]: 36 (31–41) |
[n = 19/20]: 35 (26–40) |
0.32 |
| Lactates, in mmol/l |
[n = 70/71]: 1.1 (1.0–1.5) |
[n = 50/51]: 1.1 (1.0–1.9) |
[n = 20/20]: 1.0 (1.0–1.2) |
0.17 |
| Pre-implantation support |
In intensive care unit |
25 (35%) |
20 (39%) |
5 (25%) |
0.29 |
| Preoperative mechanical ventilation |
16 (23%) |
13 (26%) |
3 (15%) |
0.53 |
| Preoperative MCS |
IABP |
5 (7%) |
5 (10%) |
0 |
0.31 |
| ECLS / CentriMag |
10 (14%) |
9 (18%) |
1 (5%) |
0.26 |
| Haemofiltration / dialysis |
8 (11%) |
4 (8%) |
4 (20%) |
0.21 |
Baseline haemodynamics, laboratory and
pre-implantation support data of the study population are listed in table 1. Median
left ventricular ejection fraction, end-diastolic diameter and cardiac index
were, respectively, 22 (17–28)%,
66 (59–70) mm and 2.2 (1.9–2.5) l/m2/min. Patients in the BTT group
had lower pulmonary vascular resistance than those in the DT group (2.2 [1.6–3.8]
WU vs 2.4 [1.4–3.0] WU, p = 0.02). Most patients had chronic renal failure and 20
(28.2%) had creatinine levels ≥150 µmol/l. Patients in
the DT group had more advanced chronic renal failure than those in the BTT
group, as shown by higher creatinine values (respectively, 162 [139–193] µmol/l vs
107 [88–131] µmol/l, p <0.001) and urea values
(respectively, 13 [9–17] mmol/l vs 7 [5–11] mmol/l, p <0.001). Hepatic
cholestasis and/or hepatitis (defined as total bilirubin, aspartate
aminotransferase or alanine transaminase values ≥3 upper limit of normal) was present
in 29 (40.8%) patients, with
lower alanine transaminase values in the DT group (34 [23–89] U/l vs 22 [17–36]
U/l, p = 0.009). Regarding pre-implantation support, no difference was observed
between groups.
Intraoperative data
Intraoperative data are presented in table
2. Most patients (n = 60, 84%) were implanted by median sternotomy, four (6%)
underwent a left anterolateral thoracotomy and seven (10%) were implanted by
double mini-thoracotomy. The
median cardiopulmonary bypass time was 64 (56–81) minutes and aortic crossclamp was
performed in four
patients because of concomitant cardiac procedures (aortic valve replacement in
three and outflow graft anastomosis in one). There was no difference in intraoperative
data between
groups.
Table 2Intraoperative data. Categorical variables are presented as counts (percentages) and
continuous variables as medians (interquartile ranges).
| |
All patients |
Bridge to transplantation |
Destination therapy |
p value |
| n = 71 |
n = 51 |
n = 20 |
|
| Incision |
Median
sternotomy |
60
(84%) |
43
(84%) |
17
(85%) |
>0.99 |
| Left
thoracotomy |
4
(6%) |
2
(4%) |
2
(10%) |
0.31 |
| Double
mini-thoracotomy |
7
(10%) |
6
(12%) |
1
(5%) |
0.66 |
| CPB
time, in min |
64
(56–81) |
62
(54–78) |
71
(57–88) |
0.18 |
| Aortic
crossclamp |
4
(6%) |
3
(6%) |
1
(5%) |
>0.99 |
| Duration of aortic crossclamp, in min |
41
(29–55) |
45
(33–55) |
36
(–) |
>0.99 |
| Left ventricular assist device outflow |
Ascending aorta |
67
(94%) |
49
(96%) |
18
(90%) |
0.31 |
| Descending
thoracic aorta |
4
(6%) |
2
(4%) |
2
(10%) |
– |
| Concomitant
cardiac procedures |
AV
replacement |
3
(4%) |
2
(4%) |
1
(5%) |
>0.99 |
| Perioperative
ASD closure |
4
(6%) |
3
(6%) |
1
(5%) |
>0.99 |
Outcomes
Patient survival
The median follow-up duration was 18.3
months (IQR: 7.5–33.9 months). Among the 71 patients included, 13 (18.3%) died,
5/51 (9.8%) in the BTT group and 8/20 (40%) in the DT group. The overall patient
survival rates on HeartMate 3 support were (mean ± standard error): 89.4 ± 3.8% [95%
CI: 78.9–94.8%]
at 1-year and 86.8 ± 4.5% [95% CI: 74.9–93.3%] at 2-year follow-up (figure 2). In
the BTT group, patient survival rates at 1-year and 2-year were 91.3 ± 4.2% [95%
CI: 78.2–96.7%] and 87.3 ± 5.6% [95% CI: 71–94.8%] respectively. In the DT group,
patient survival rates at 1-year and 2-year were similar at 84.7 ± 8.1% [95% CI:
59.7–94.8%]. At 5-year follow-up, patients in the BTT group had numerically
better survival on HeartMate 3 support than those in the DT group [87.3 ± 5.6%
(95% CI: 71–94.8%) vs 49.4 ± 15.1% (95% CI: 19.2–74%)] (figure 3).
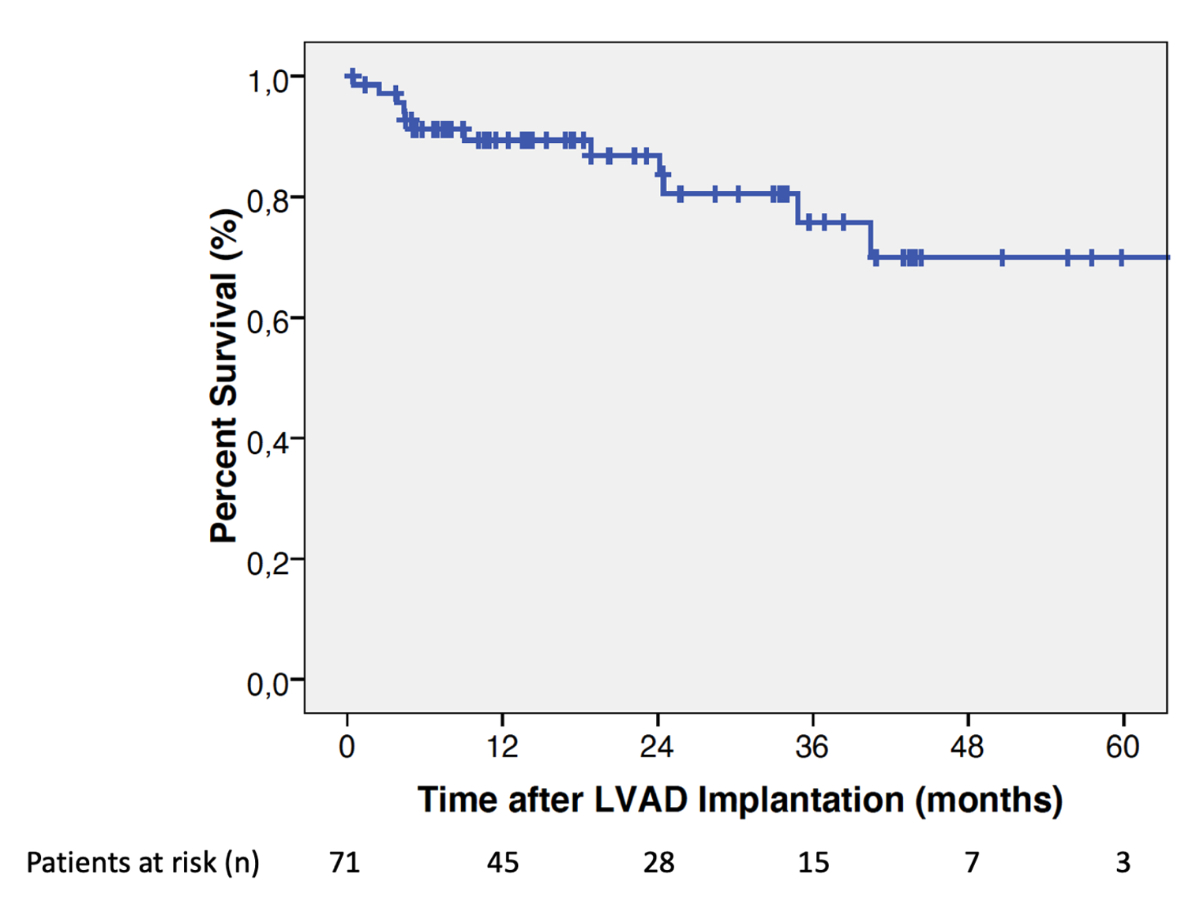
Figure 2Overall estimates of patient
survival after HeartMate 3 left ventricular assist device (LVAD)
implantation.
A total of 30 (42.2%) patients underwent
LVAD explantation: 29 (41%) had heart transplantation and one (1.4%) had device
weaning for recovery from peripartum cardiomyopathy. The overall median
duration of postoperative mechanical ventilation was 3 (1–3) days without
difference between groups. Patients in the BTT group tended to have a shorter
intensive care unit stay (6 [4–18] days vs 13 [5–40] days, p = 0.09) and a
shorter mean hospital stay (29 [22–66] days vs 69 [25–113] days, p = 0.05).

Figure 3Estimates of patient survival after
HeartMate 3 LVAD implantation by study group. BTT: bridge to
transplantation; DT: destination therapy;
LVAD: left ventricular assist device.
Adverse events
Adverse events observed during support are listed
in table 3. We did not identify any cases of pump thrombosis or technical
malfunction during follow-up.
Table 3Adverse
events observed during mechanical circulatory support. Categorical
variables are presented as counts (percentages) and continuous variables as
medians (interquartile ranges).
|
All patients |
Bridge to transplantation |
Destination therapy |
p value |
| n = 71 |
n = 51 |
n = 20 |
|
| RVAD support |
Temporary
RVAD support |
18
(25%) |
10
(20%) |
8
(40%) |
0.13 |
| – Duration
of RVAD support, in days |
8
(6–9) |
8
(4–9) |
7
(6–8) |
0.34 |
| Durable
RVAD support |
0 |
0 |
0 |
– |
| Renal
dysfunction requiring temporary dialysis |
12
(17%) |
6
(12%) |
6
(30%) |
0.08 |
| Pump
thrombosis |
0 |
0 |
0 |
– |
| Technical
malfunction |
0 |
0 |
0 |
– |
| Bleeding |
Any bleeding event [/ patient/year] |
35 (50%) [0.27] |
21 (42%) [0.24] |
14 (70%) [0.30] |
0.06 |
| Bleeding requiring surgery |
23 (32%) |
14 (28%) |
9 (45%) |
0.17 |
| GI bleeding [/patient/year] |
14 (20%) [0.11] |
8 (16%) [0.09] |
6 (30%) [0.13] |
0.20 |
| Infection |
VAD-specific [/patient/year] |
|
50 (70%) [0.38] |
36 (71%) [0.41] |
14 (70%) [0.31] |
0.45 |
| Driveline culture >0 |
48 (68%) |
34 (67%) |
14 (70%) |
– |
| Driveline surgical |
10 (14%) |
6 (12%) |
4 (20%) |
– |
| Pump |
2 (3%) |
2 (4%) |
0 |
– |
| VAD-related (mediastinitis) |
4 (6%) |
2 (4%) |
2 (10%) |
0.31 |
| Neurological
complications |
TIA /
Ischaemic stroke [/patient/year] |
8 (12%) [0.06] |
4 (8%) [0.05] |
4 (22%) [0.09] |
0.19 |
| Disabling
ischaemic stroke |
1 (1.4%) |
1 (2%) |
0 |
– |
| Ischaemic stroke-related death |
1 (1.4%) |
1 (2%) |
0 |
– |
| Haemorrhagic stroke |
0 |
0 |
0 |
– |
| Post-traumatic IC bleeding
[/patient/year] |
2 (3%) [0.02] |
0 [0] |
2 (10%) [0.04] |
0.08 |
A temporary right ventricle support device
was implanted in a quarter of patients during the initial left ventricular
assist device implantation procedure. The need for right ventricle support tended
to be higher in the DT group with 8 out of 20 patients (40%) requiring support
compared to 10 out of 51 patients (20%) in the BTT group (p = 0.13). All
patients who received temporary right ventricle support were successfully
weaned after a median support time of 8 (6–9) days without any differences between
groups, and none of
them required a permanent RV device. No instance of severe right ventricle
dysfunction was observed during the follow-up period.
A total of 12 patients (17%) required immediate
postoperative dialysis. Patients in the DT group tended to have more perioperative
renal dysfunction requiring dialysis than those in the BTT group (12% vs 30%, p =
0.08).
All patients were weaned off dialysis prior to hospital discharge.
Bleeding complications were observed in
half of patients and predominantly occurred during the perioperative period. A
total of 23 (32%) patients required surgical re-exploration, which confirmed
that bleeds were related neither to the inflow or outflow sutures nor to the
pump connections. Non-surgical bleeding occurred in 12 (16.9%) patients and
were mainly of gastrointestinal origin (n = 9/12, 75%). Patients in the DT
group displayed a higher tendency for bleeding complications compared to those
in the BTT group (70% vs 42% p = 0.06).
A VAD-specific infection was diagnosed in
50 (70%) patients: 48/50 (96%) had a driveline infection and 2/50 (4%) had a pump
infection confirmed by surgical or percutaneous CT-guided drainage. Among
patients with driveline infection, 38 (79.2%) were successfully treated using
local wound care and culture-directed antibiotic therapy, while 10 (20.8%)
required surgical debridement and relocation of the driveline exit site. The
two patients with a pump infection were placed on the emergency transplantation
list and ultimately underwent successful transplantation, without
post-transplant mediastinal infections. There were no differences between
groups regarding infection-related adverse events.
Ischaemic neurological complications
(stroke or transient ischaemic attack) occurred in 8 (12%) of the study
population, including one patient with a fatal ischaemic stroke in the BTT
group. No spontaneous haemorrhagic stroke occurred. The rate of neurological
complications was similar in both groups.
Discussion
The main results of this study are: (1) Patients
with advanced heart failure supported with a HeartMate 3 LVAD device had a
favourable 2-year patient survival rate; (2) Adverse events remain high, mainly
due to driveline infections and bleeding; and (3) Patients with LVAD-DT
experience worse outcomes and more adverse events than those with LVAD-BTT.
We reported our 7-year experience with the HeartMate 3
LVAD used as BTT or DT through a single-centre study. Despite a retrospective observational
design, all patients were consecutively included and were representative of
real-world conditions. Indeed, our population study shares comparable
characteristics at baseline to those of previously published studies, with a
clear majority (84%) having a pre-implant INTERMACS clinical profile of 2–4.
This is in line with the ELEVATE registry [18] and the CE Mark study [19], in
which 88% and 92% of patients, respectively, were in the same INTERMACS class. Post-implantation
patient survival rates in our population were 89.4% at 1-year and 86.8% at 2-year
follow-up. These results are quite favourable and comparable to patient survival
observed in the MOMENTUM 3 trial (86.6% and 79% in the HeartMate 3
group at 1- and 2-year follow-up, respectively) (20),
the CE Mark study (81% and 74% at 1- and 2-year follow-up, respectively) (19) and
the ELEVATE registry (83.4% at 2-year
follow-up) [18].
Although we observed satisfactory patient
survival, the incidence of adverse events remained substantial. Infection-related
adverse events were frequent with 70% of patients experiencing VAD-specific
infections. Primarily related to driveline infections, this highlights the
importance of rigorous exit site care and meticulous hygiene practices to
prevent this adverse event. However, the majority of these infections were
successfully treated using local wound care and culture-directed antibiotic
therapy, emphasising the benefits of early detection and aggressive management
of driveline infections. The development of durable and exclusively internal
heart pumps, eliminating the need for a driveline, would be a major step
forward in this regard. Bleeding complications were also common with half of
the patients experiencing bleeding events, mostly during the perioperative
period. Bleeding risk remains a major clinical challenge after HeartMate 3
LVAD implantation, notably because of the high risk of gastrointestinal
bleeding during follow-up [6, 18, 19]. While the aetiology of bleeding
complications associated with left ventricular assist device is acknowledged to
be multifactorial, the combined use of and antiplatelet agents is a well-known
risk factor. This antithrombotic regimen continues to be recommended [21] even for
state-of-the-art devices such as
the HeartMate 3, despite advances in device engineering lowering the risk
of pump thrombosis and improving haemocompatibility profiles compared with
axial-flow pumps [20]. Nevertheless,
the recent ARIES-HM3 trial [22] showed that in
patients with advanced heart failure supported with HeartMate 3 LVAD and
anticoagulated with a vitamin K antagonist, the placebo was noninferior to
daily aspirin with respect to the composite endpoint of bleeding and thrombotic
events at 1 year. It therefore seems
reasonable to assume that the optimal antithrombotic treatment regimen in HeartMate 3
patients should be based on anticoagulation alone. This is more so true for HeartMate 3
LVAD patients with advanced heart failure due to cardiomyopathy of non-ischaemic
origin. In addition, some small observational studies have shown that the use
of a direct oral anticoagulant appears to be safe and could reduce the risk of
bleeding compared with vitamin K antagonists in left ventricular assist device
patients [23, 24]. Nevertheless, in the absence of a randomised controlled
phase 3 trial, the standard of care remains vitamin K antagonists. The results
of the ongoing DOAC LVAD Phase 2 study [25] will provide further information on
the safety and feasibility of anticoagulant therapy with apixaban in HeartMate 3
patients. Notably, we have not identified any cases of pump thrombosis or
technical malfunction during follow-up, suggesting the reliability and
durability of the HeartMate 3 LVAD.
Regarding outcomes, the BTT group showed
higher patient survival rates at 5-year follow-up than the DT group. Given that
a pre-transplant continuous-flow mechanical circulatory support strategy with
subsequent orthotopic heart transplantation provides post-transplant outcomes
not different to those of direct heart transplantation [26], HeartMate 3
implantation as bridge to transplantation is a valid option in this high-risk
population. We also observed a disparity between the two study groups in terms
of the occurrence of adverse events: patients in the DT group experienced a
higher incidence of adverse events than patients in the BTT group, which is
consistent with other published data [27]. This difference may be attributed to
the fact that patients in the BTT group are generally in a relatively better
clinical state at the time of LVAD implantation, as they are considered
suitable candidates for heart transplantation. In contrast, patients in the DT
group have a more complex clinical profile characterised by older age, a higher
prevalence of comorbidities and a more advanced stage of heart failure. These
factors predispose them to poorer outcomes than patients implanted as bridge to
transplantation. Indeed, clinical frailty has been associated with prolonged
time to extubation, extended hospital stays and increased long-term mortality
in LVAD implantation when compared to non-frail individuals [28]. The challenge
is to optimise the selection of patients with a pre-implantation destination
therapy strategy by limiting LVAD implantation to those whose comorbidities and
frailty could improve after LVAD haemodynamic restoration. As previously
reported by Cain et al. [29], it is necessary to identify two types of frailty
in LVAD-DT eligible patients: the “LVAD-responsive frailty”, which may improve
with ventricular assistance and does not represent a barrier to LVAD
implantation, and “LVAD-independent frailty”, which may persist despite LVAD
implantation and for which this procedure should be avoided.
Limitations
The main limitations of this
hypothesis-generating study are the relatively small sample size and its
retrospective observational design. Therefore, the results of statistical
analyses should be interpreted with caution. By definition, there is a misbalance
favouring patient survival on HeartMate 3 support in the BTT group over
the DT group, related to the competing risk of heart transplantation (exposure
to HeartMate 3 support was lower for patients in the BTT group than for
those in the DT group). All implant procedures were exclusively conducted at a
single institution, potentially impacting the generalisability of our findings
and their susceptibility to institutional biases.
Conclusion
Although HeartMate 3 LVAD demonstrates
favourable survival in patients with advanced heart failure, patients with
LVAD-DT have a poorer prognosis compared to those with LVAD-BTT. A careful
selection of patients eligible for an LVAD-DT and diligent post-implantation
management are essential to improve outcomes and limit the occurrence of
adverse events which remain high in this population.
Dr John Kikoïne
Lausanne University Hospital
Rue du Bugnon 46
CH-1003 Lausanne
john.kikoine[at]chuv.ch
References
1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007 Sep;93(9):1137–46.
doi:10.1136/hrt.2003.025270
2. Roger VL. Epidemiology of heart failure. Circ Res. 2013 Aug;113(6):646–59. doi:10.1161/CIRCRESAHA.113.300268
3. Smeets M, Vaes B, Mamouris P, Van Den Akker M, Van Pottelbergh G, Goderis G, et al. Burden
of heart failure in Flemish general practices: a registry-based study in the Intego
database. BMJ Open. 2019 Jan;9(1):e022972. doi:10.1136/bmjopen-2018-022972
4. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESC Scientific
Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic
heart failure. Eur Heart J. 2021 Sep;42(36):3599–726. doi:10.1093/eurheartj/ehab368
5. Pinney SP, Anyanwu AC, Lala A, Teuteberg JJ, Uriel N, Mehra MR. Left Ventricular Assist
Devices for Lifelong Support. J Am Coll Cardiol. 2017 Jun;69(23):2845–61. doi:10.1016/j.jacc.2017.04.031
6. Mehra MR, Goldstein DJ, Cleveland JC, Cowger JA, Hall S, Salerno CT, et al. Five-Year
Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular
Assist Devices in the MOMENTUM 3 Randomized Trial. JAMA. 2022 Sep;328(12):1233–42.
doi:10.1001/jama.2022.16197
7. Hullin R, Meyer P, Yerly P, Kirsch M. Cardiac Surgery in Advanced Heart Failure. J
Clin Med. 2022 Jan;11(3):773. doi:10.3390/jcm11030773
8. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, et al.; International
Society for Heart Lung Transplantation (ISHLT) Infectious Diseases, Pediatric and
Heart Failure and Transplantation Councils. The 2016 International Society for Heart
Lung Transplantation listing criteria for heart transplantation: A 10-year update.
J Heart Lung Transplant. 2016 Jan;35(1):1–23. doi:10.1016/j.healun.2015.10.023
9. Nowacka A, Hullin R, Tozzi P, Barras N, Regamey J, Yerly P, et al. Short-term single-centre
experience with the HeartMate 3 left ventricular assist device for advanced heart
failure. Eur J Cardiothorac Surg. 2020 Sep;58(3):511–8. doi:10.1093/ejcts/ezaa075
10. Schmitto JD, Molitoris U, Haverich A, Strueber M. Implantation of a centrifugal pump
as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy
combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg. 2012 Feb;143(2):511–3.
doi:10.1016/j.jtcvs.2011.07.046
11. Pfister R, Tozzi P, Hullin R, Yerly P, Jahns FP, Prêtre R et al. HeartMate 3 implantation
via left antero-lateral thoracotomy to avoid resternotomy in high risk patients. Multimed
Man Cardiothorac Surg. 2018 Apr;2018 doi:10.1510/mmcts.2018.026
12. Hanke JS, Krabatsch T, Rojas SV, Deniz E, Ismail I, Martens A, et al. In Vitro Evaluation
of Inflow Cannula Fixation Techniques in Left Ventricular Assist Device Surgery. Artif
Organs. 2017 Mar;41(3):272–5. doi:10.1111/aor.12735
13. Schibilsky D, Benk C, Haller C, Berchtold-Herz M, Siepe M, Beyersdorf F, et al. Double
tunnel technique for the LVAD driveline: improved management regarding driveline infections.
J Artif Organs. 2012 Mar;15(1):44–8. doi:10.1007/s10047-011-0607-3
14. Lenoir M, Quessard A, N’guyen A, Kirsch M. Simplified temporary right ventricular
support after implantation of a left ventricular assist device. Heart Surg Forum.
2013 Jun;16(3):E152–4. doi:10.1532/HSF98.20131003
15. Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, et al. INTERMACS
profiles of advanced heart failure: the current picture. J Heart Lung Transplant.
2009 Jun;28(6):535–41. doi:10.1016/j.healun.2009.02.015
16. Kormos RL, Antonides CF, Goldstein DJ, Cowger JA, Starling RC, Kirklin JK, et al. Updated
definitions of adverse events for trials and registries of mechanical circulatory
support: A consensus statement of the mechanical circulatory support academic research
consortium. J Heart Lung Transplant. 2020 Aug;39(8):735–50. doi:10.1016/j.healun.2020.03.010
17. Hannan MM, Husain S, Mattner F, Danziger-Isakov L, Drew RJ, Corey GR, et al.; International
Society for Heart and Lung Transplantation. Working formulation for the standardization
of definitions of infections in patients using ventricular assist devices. J Heart
Lung Transplant. 2011 Apr;30(4):375–84. doi:10.1016/j.healun.2011.01.717
18. Zimpfer D, Gustafsson F, Potapov E, Pya Y, Schmitto J, Berchtold-Herz M, et al. Two-year
outcome after implantation of a full magnetically levitated left ventricular assist
device: results from the ELEVATE Registry. Eur Heart J. 2020 Oct;41(39):3801–9. doi:10.1093/eurheartj/ehaa639
19. Schmitto JD, Pya Y, Zimpfer D, Krabatsch T, Garbade J, Rao V, et al. Long-term evaluation
of a fully magnetically levitated circulatory support device for advanced heart failure-two-year
results from the HeartMate 3 CE Mark Study. Eur J Heart Fail. 2019 Jan;21(1):90–7.
doi:10.1002/ejhf.1284
20. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et al.; MOMENTUM
3 Investigators. A Fully Magnetically Levitated Left Ventricular Assist Device - Final
Report. N Engl J Med. 2019 Apr;380(17):1618–27. doi:10.1056/NEJMoa1900486
21. Saeed D, Feldman D, Banayosy AE, Birks E, Blume E, Cowger J, et al. The 2023 International
Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support:
A 10- Year Update. J Heart Lung Transplant. 2023 Jul;42(7):e1–222. doi:10.1016/j.healun.2022.12.004
22. Mehra MR, Netuka I, Uriel N, Katz JN, Pagani FD, Jorde UP, et al.; ARIES-HM3 Investigators.
Aspirin and Hemocompatibility Events With a Left Ventricular Assist Device in Advanced
Heart Failure: The ARIES-HM3 Randomized Clinical Trial. JAMA. 2023 Dec;330(22):2171–81.
doi:10.1001/jama.2023.23204
23. Meredith T, Schnegg B, Hayward C. The use of direct oral anticoagulants in patients
with ventricular assist devices: is there hope for Factor Xa inhibition? Artif Organs.
2021 May;45(5):E123–9. doi:10.1111/aor.13848
24. Whitehouse KR, Avula D, Kahlon T, Costelle D, Dunbar-Matos C, Pahwa S, et al. Apixaban:
Alternative Anticoagulation for HeartMate 3 Ventricular Assist Device. ASAIO J. 2022 Mar;68(3):318–22.
doi:10.1097/MAT.0000000000001650
25. Dimond M, Looby M, Shah B, Sinha SS, Isseh I, Rollins AT, et al. Design and Rationale
for the Direct Oral Anticoagulant Apixaban in Left Ventricular Assist Devices (DOAC
LVAD) Study. J Card Fail. 2023 Nov;S1071-9164(23)00863-1
26. Hullin R, Abdurashidova T, Pitta-Gros B, Schukraft S, Rancati V, Lu H, et al. Post-transplant
survival with pre-transplant durable continuous-flow mechanical circulatory support
in a Swiss cohort of heart transplant recipients. Swiss Med Wkly. 2023 Dec;153(12):3500.
doi:10.57187/s.3500
27. Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J, Ransom J, et al. Association
of Clinical Outcomes With Left Ventricular Assist Device Use by Bridge to Transplant
or Destination Therapy Intent: The Multicenter Study of MagLev Technology in Patients
Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) Randomized
Clinical Trial. JAMA Cardiol. 2020 Apr;5(4):411–9. doi:10.1001/jamacardio.2019.5323
28. Tse G, Gong M, Wong SH, Wu WK, Bazoukis G, Lampropoulos K, et al.; International Health
Informatics Study (IHIS) Network. Frailty and Clinical Outcomes in Advanced Heart
Failure Patients Undergoing Left Ventricular Assist Device Implantation: A Systematic
Review and Meta-analysis. J Am Med Dir Assoc. 2018 Mar;19(3):255–261.e1. doi:10.1016/j.jamda.2017.09.022
29. Cain MT, Firstenberg MS, Cleveland JC Jr. Heart Transplant and Ventricular Assist:
Cardiac Surgery and Heart Failure Perspective. US Cardiol. 2021 Sep;15:e16. doi:10.15420/usc.2021.11


