Pembrolizumab-associated anti-MDA5 dermatomyositis
in a patient with lung cancer: a first case report
DOI: https://doi.org/https://doi.org/10.57187/s.3513
Antonino Marcello Piliaa,
Lorenzo Salvatia,
Alessia Guidolinb,
Francesca Mazzonic,
Lorenzo Antonuzzoabc,
Paola Parronchiad,
Francesco Liottaad
a Department of Experimental and Clinical
Medicine, University of Florence, Florence, Italy
b Clinical Oncology Unit, Careggi University
Hospital, Florence, Italy
c Medical Oncology, Careggi University
Hospital, Florence, Italy
d Immunology and Cell Therapy Unit, Careggi
University Hospital, Florence, Italy
Summary
We report the first case of anti-melanoma
differentiation-associated gene 5 (MDA5)-positive dermatomyositis as a systemic
immune-related adverse event in a 64-year-old man receiving pembrolizumab to
treat advanced lung cancer. The patient experienced hypothyroidism, myalgia,
skin involvement, dyspnoea and diarrhoea. Laboratory tests revealed raised
inflammatory markers, hypercreatinekinasemia and anti-MDA5 autoantibodies.
Electroneuromyography and pathognomonic signs on physical examination confirmed
the diagnosis of pauci-myopathic dermatomyositis. Pembrolizumab was discontinued
and immunosuppressive therapy led to rapid and progressive improvement, with
complete remission of dermatomyositis. This case report widens the spectrum of
systemic immune-related adverse events associated with pembrolizumab.
Introduction
Immune checkpoint inhibitors have revolutionised
the treatment of many cancers in the last decades. They
bind to programmed death-1 (PD-1), programmed
death ligand-1 (PD-L1) or cytotoxic T-lymphocyte antigen 4,
thereby releasing the brakes that hamper the immune system’s response against
tumours. However, immune checkpoint inhibitor treatment
is frequently associated with the development of
autoimmune toxicities, known as immune-related adverse
events, which are classified according to the
Common Terminology Criteria for Adverse Events (CTCAE) severity scale [1].
Skin and muscle are most frequently involved, although almost any organ can be
affected [2]. Pembrolizumab is an anti-PD-1 monoclonal antibody indicated as
first-line treatment for patients with advanced non-small cell lung cancer whose
tumours express PD-L1 with at least a 50% tumour proportion score [3]. We
report a case of anti-melanoma differentiation-associated gene 5 (MDA5)-positive
dermatomyositis in a patient receiving pembrolizumab for advanced lung cancer. Although
dermatomyositis without autoantibody specificity has been reported sporadically
following treatment with immune checkpoint inhibitors [4, 5], to our knowledge,
this case is the first in the literature to report anti-MDA5-positive
dermatomyositis associated with this monoclonal antibody.
Case description
In May 2021, a 64-year-old man was
diagnosed with stage IV squamous cell lung cancer, with 90% of neoplastic cells
expressing PD-L1. The patient’s medical history included chronic allergic contact
dermatitis since young adulthood and arterial hypertension, which was diagnosed
in 2017 and treated with an angiotensin-converting enzyme inhibitor (ramipril
10 mg once daily). Neither severe acute respiratory syndrome coronavirus 2 nor other
recent respiratory infections were reported. The patient was vaccinated against
coronavirus disease 2019 (COVID-19) with ChAdOx1-S (Vaxzevria) in May 2021,
followed by a booster dose 3 months later. He was an active smoker (4–5
cigarettes per day) with long smoking exposure (65 pack-years).
The patient started first-line
immunotherapy with pembrolizumab (Keytruda®) 200 mg every three weeks on June
21, 2021. After the fourth pembrolizumab dose (800 mg cumulative dose), the
patient had an optimal antitumour response (figures 1 and 2) but developed
hypothyroidism (thyroid-stimulating hormone levels 30.04 mIU/l; normal values
[n.v.] 0.5–5.0 mIU/l). Replacement therapy with levothyroxine 75 mcg per day
was prescribed. Concurrently, the patient reported upper limb myalgia and
muscle weakness, exacerbation of his chronic eczema with new areas of skin
involvement, and moderate dyspnoea on exertion.
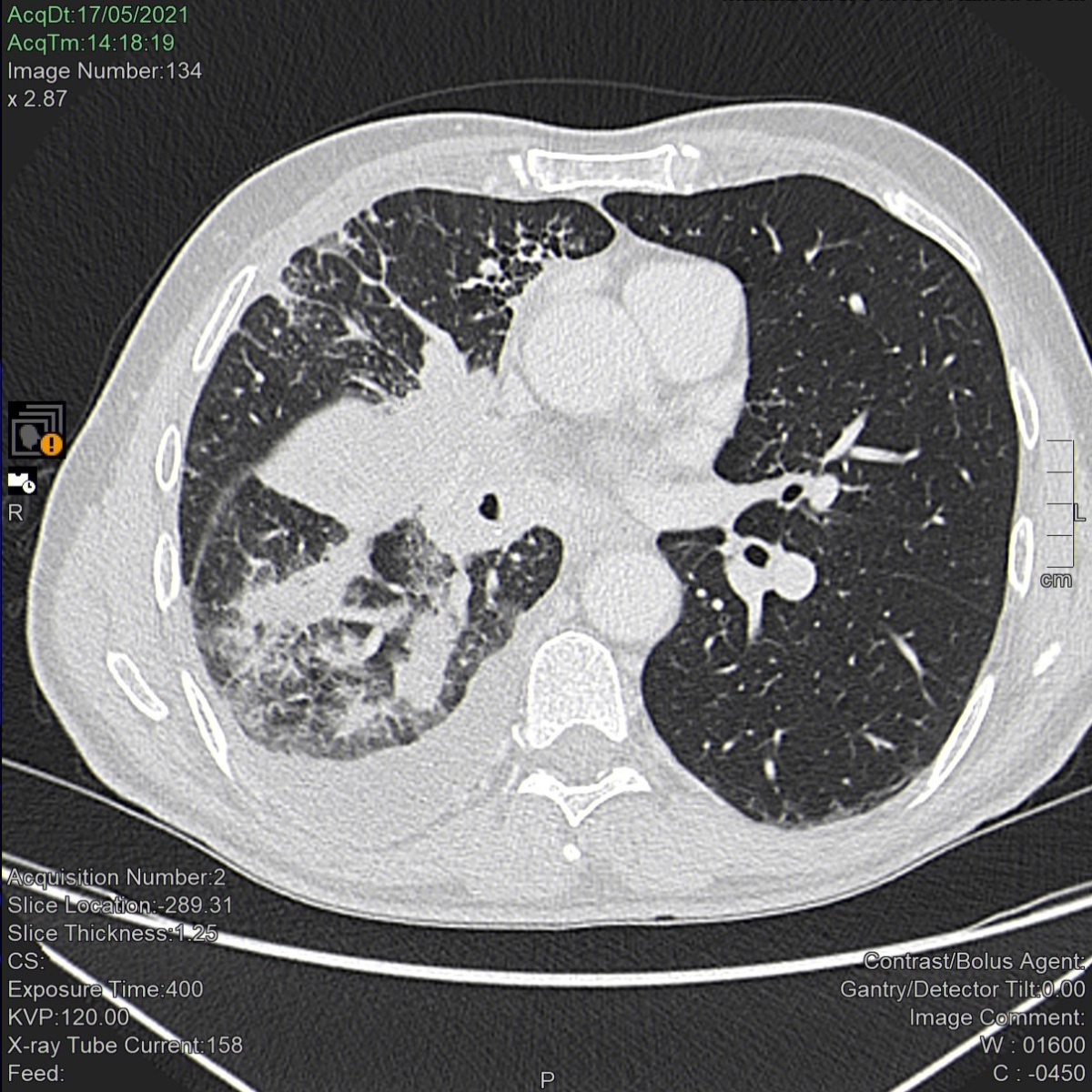
Figure 1Computed tomography scan of the lungs before treatment
with pembrolizumab. A neoplastic mass occupies the right hilum causing
complete stenosis of both the middle lobar bronchus (with complete atelectasis
of the middle lobe) and the segmental apical bronchus of the lower right lobe. The
lower lobar bronchus is reduced in size, parenchymal localizations are visible in
the lower lobe and lymphangitis is apparent in the upper lobe. Pleural effusion
is also apparent.
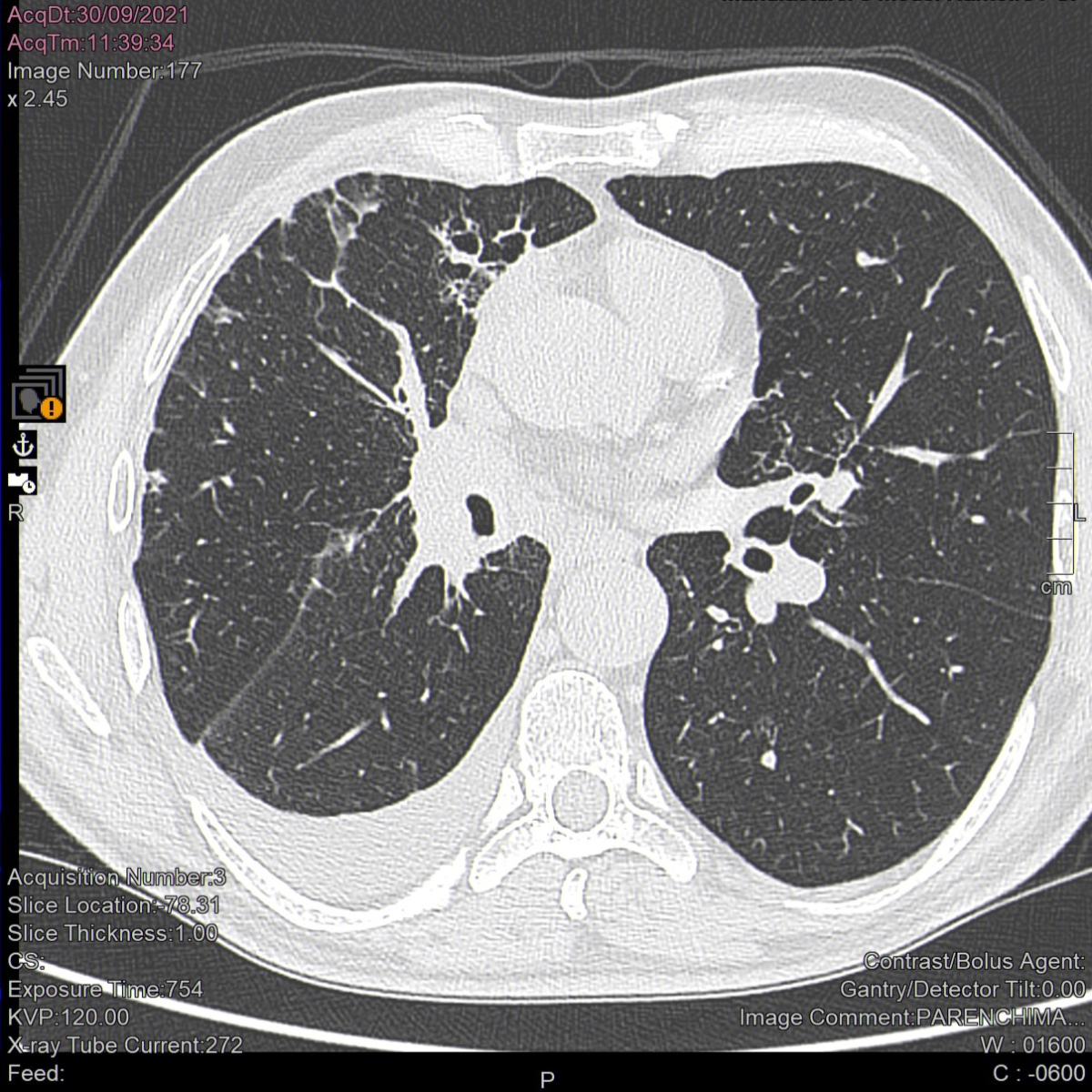
Figure 2Computed tomography scan of the lungs after 3
months of treatment with pembrolizumab. A considerable reduction in the size
of the hilar tissue is apparent 3 months after pembrolizumab initiation. The middle
lobe has re-expanded and the apical segmental bronchus of the lower lobe is newly
patent. Parenchymal localizations have disappeared from the lower lobe and upper
lobe lymphangitis has lessened.
Laboratory tests showed positive inflammation
indexes with high C-reactive protein (1.14 mg/dl; n.v. <0.5 mg/dl) and
fibrinogen (673 mg/dl; n.v. 200–400 mg/dl), and a slight increase in creatine
kinase levels (231 U/l; n.v 25–200 U/l). The patient’s serum was tested for antinuclear
antibodies using HEp-2 cells (Euroimmun, Lübeck, Germany), and showed a highly
positive titre (1/640) with a primary speckled and secondary granular
cytoplasmatic pattern. Extractable nuclear antigen screening for connective
tissue disease was positive (1.1; n.v. 0–1.0) and a Crithidia luciliae
immunofluorescence test (Euroimmun, Lübeck, Germany) was negative for anti-double-stranded
DNA autoantibodies. Based on the clinical picture, a line blot assay was
performed for myositis-specific and myositis-associated antigens (Euroimmun,
Lübeck, Germany) and clear positivity for anti-MDA5 autoantibody was found (34
index). After two months, a second immunoblot assay confirmed this myositis
profile (15 index). Cut-off value: negative ≤5.
Electroneuromyography of all four limbs showed
mild myogenic findings in the proximal upper limbs without signs of myositis and
mild sensory-motor neuropathy in the lower limbs. Skin findings included a
heliotrope rash on the eyelids, nasal bridge and cheeks (figure 3), Gottron’s sign
on
the backs of both hands (figure 4) and the left elbow,
and mechanic’s hands (figure 5). Muscle strength was reduced, and manual muscle
testing found tenderness on abduction of the upper limbs. Auscultation revealed
decreased breath sounds without crepitations. A diagnosis of anti-MDA5-positive
pauci-myopathic dermatomyositis was made. Systemic autoimmune diseases that
develop following immune checkpoint inhibitor treatment and have potentially
life-threatening consequences are classified as grade IV according to the CTCAE
scale and require urgent intervention. Respiratory function tests revealed
moderate non-reversible severe obstructive disease, alveolar hyperinflation and
a moderate reduction of the lungs’ diffusing capacity for carbon monoxide. High-resolution
computed tomography of the chest showed very limited signs of smoking-related
interstitial fibrosis.
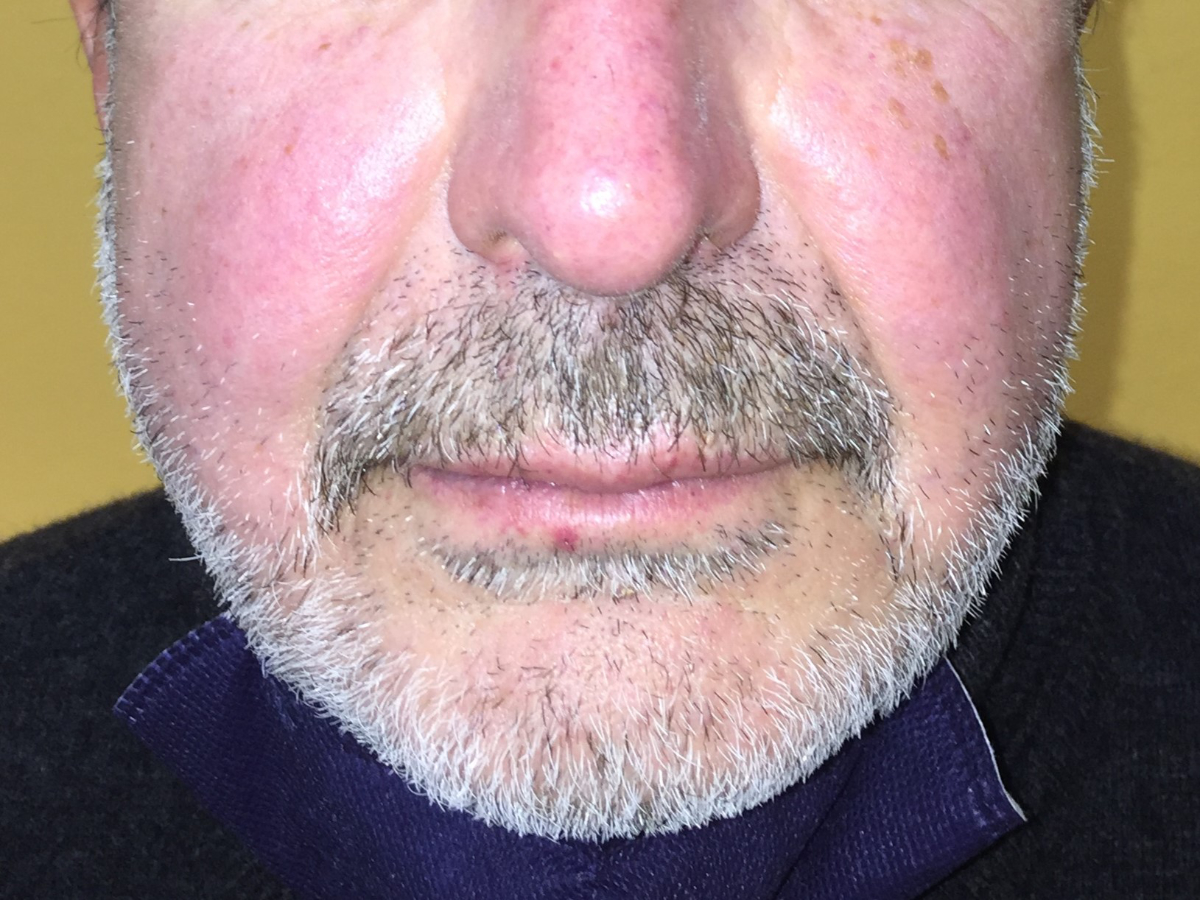
Figure 3Heliotrope rash: bilateral lilac discolouration
of the cheeks and nasal bridge, and telangiectasias on the nose and lower lip.
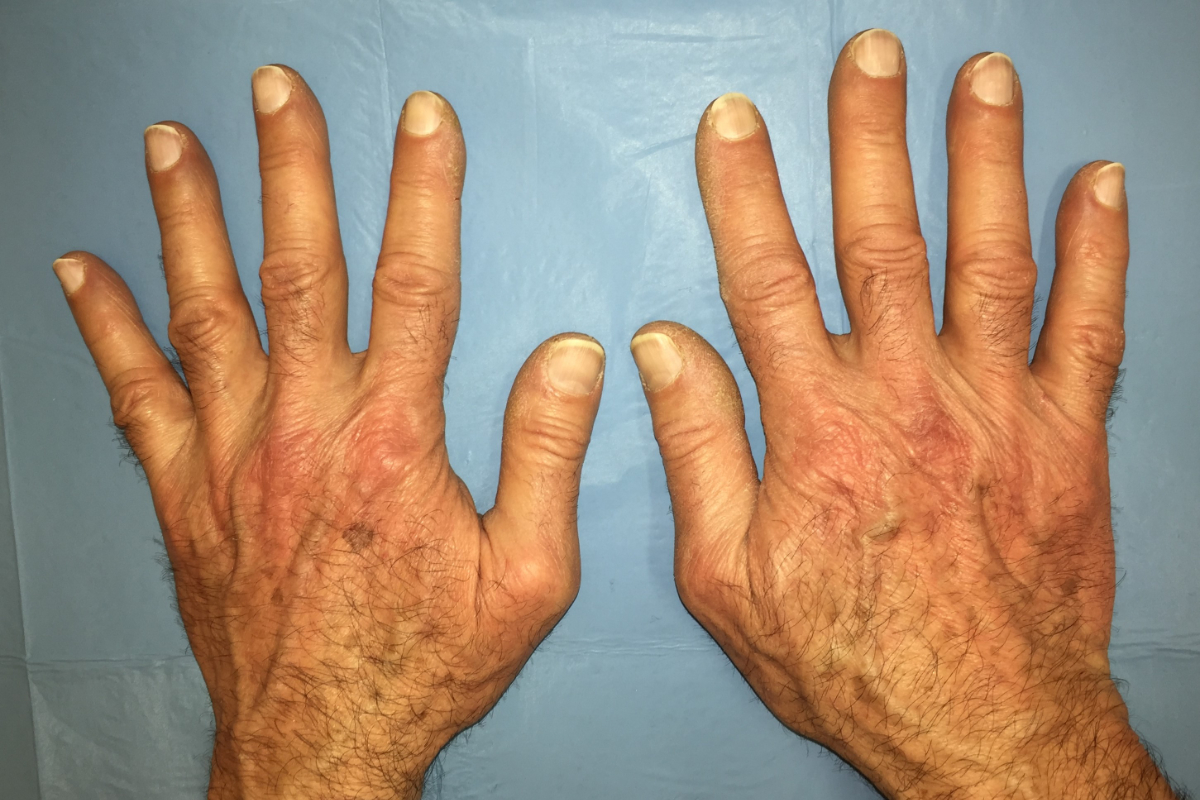
Figure 4Gottron’s sign: symmetrical
erythematous-violaceous macules at the extensor surfaces of the metacarpophalangeal
joints and mild digital clubbing along with hyperkeratosis on the ulnar aspect
of the thumb and radial aspect of the index fingers of both hands.
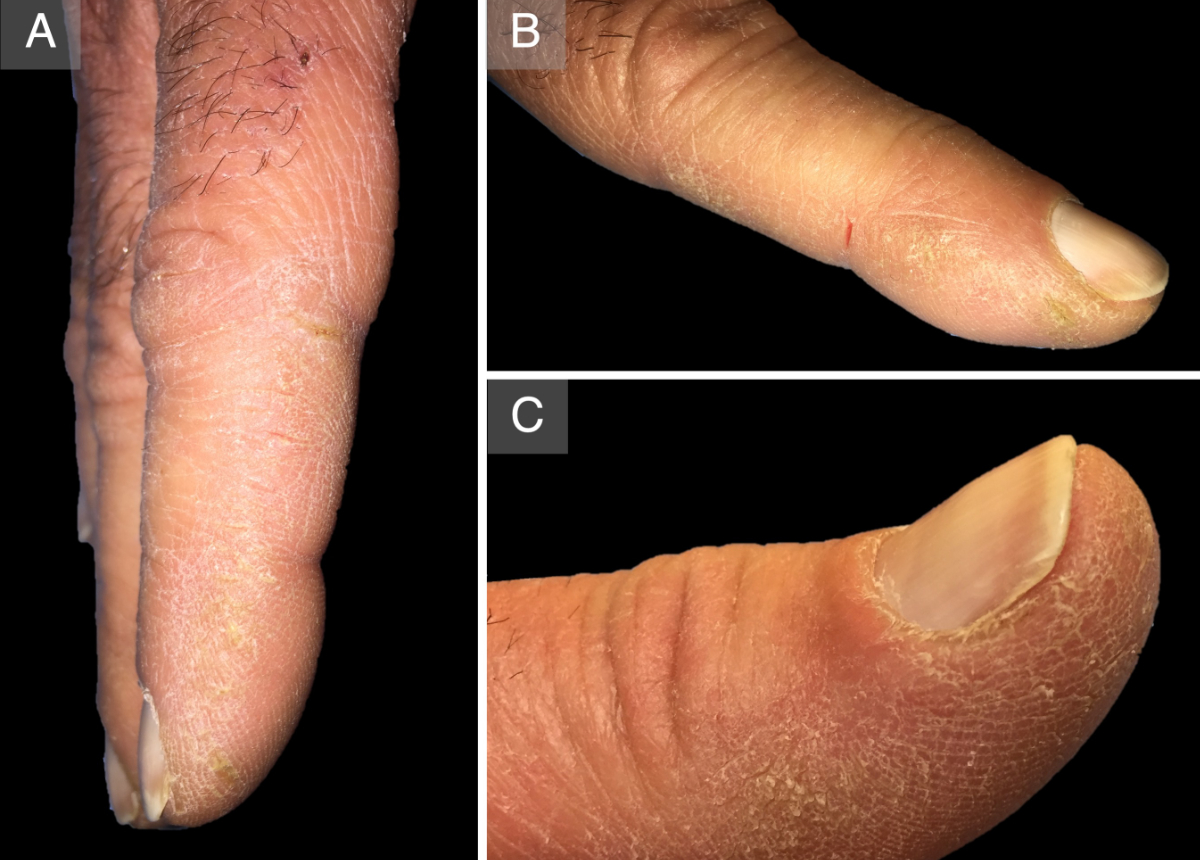
Figure 5Mechanic’s hands. A: Hyperkeratosis on the
distal and lateral aspects of the index finger of the right hand. B: A fissure
on the lateral side of the index finger of the left hand. C: Detail of the
hyperkeratosis on the medial aspect of the thumb of the right hand.
Treatment with pembrolizumab was
temporarily discontinued after December 6, 2021 (1800 mg cumulative dose) and
systemic glucocorticoids (prednisone 0.5 mg/kg daily) were started along with cyclosporin
A 3 mg/kg per day, inhaled fluticasone furoate/vilanterol 92/22 µg
every 24 hours and daily topical application of mometasone furoate 0.1% cream
on the affected skin. The patient was adherent to the recommended treatment and
tolerated it well. After clinical improvement, pembrolizumab was restarted.
There had been a 5-week interruption since the last dose on January 14, 2022. Subsequently,
the patient experienced severe diarrhoea with profound asthenia, severe hypokalaemia
and syncope and required oral budesonide. He received systemic corticosteroids and
a potassium infusion in the emergency department and anti-PD-1 therapy was discontinued
after 9 weeks (March 21, 2022). The cumulative dose of pembrolizumab was
2600 mg. Despite discontinuing pembrolizumab, the patient achieved complete
remission as demonstrated by full-body fluorodeoxyglucose-positron emission
tomography in May 2022. As of November 2023, the patient’s dermatomyositis is controlled
with 5 mg prednisone daily and he receives regular follow-up in both oncology
and immunology clinics.
Discussion
Pembrolizumab-associated immune-mediated
severe skin reactions and myositis have been described in clinical studies and
post-marketing experience [6], but dermatomyositis has rarely been reported. To
date, only 37 cases of pembrolizumab-associated dermatomyositis have been
reported in the European database of suspected adverse drug reactions. Among
the other frequently used immune checkpoint inhibitors, 34 dermatomyositis cases
were reported with nivolumab (Opdivo®), 15 with atezolizumab (Tecentriq®) and seven
with ipilimumab (Yervoy®) [7].
Anti-MDA5-positive dermatomyositis is rare
in Caucasian populations and has unique clinical features with prevalent skin
involvement. It tends to be muscle-sparing (amyopathic) but can lead to rapidly
progressive and life-threatening interstitial lung disease, especially in Asian
patients [8, 9]. Autoantibodies target the cytosolic RNA helicase MDA5, which
is physiologically involved in the antiviral response as it induces type I
interferons. This finding has been associated with the unusual seasonality of
the disease and the upregulation of interferon signalling, which is more common
than in other dermatomyositis variants [10, 11]. More recently, the development
of anti-MDA5-positive dermatomyositis, along with an associated interferon signature,
has been observed within days after vaccination with mRNA-based COVID-19 vaccines
[12, 13]. Although the pathogenic role of anti-MDA5 autoantibodies is unclear,
their levels may be related to severity, treatment resistance and relapse [8].
Patients with classical anti-MDA5-positive dermatomyositis
typically cluster into three subgroups with prevalent involvement of joints
(phenotype 1), skin (phenotype 2), or lung (phenotype 3) [9]. However, our
patient did not fit precisely into any of the proposed phenotypes. Despite the
prevalent skin involvement, he exhibited features of classical dermatomyositis
with heliotrope rash and Gottron’s sign without the palmar papules or necrotic
ulcers, which are related to the skin vasculopathy thought to be associated
with anti-MDA5 autoantibody positivity. Mechanic’s hands, although not
typically present in anti-MDA5 positive dermatomyositis, have also been reported
[14,15]. Our patient’s skin and muscle disease appeared a few months after pembrolizumab
initiation and it was preceded by hypothyroidism. Thyroid dysfunction is quite
common during immune checkpoint inhibitor treatment. However, it rarely involves autoantibody
production, which is not typical of other autoimmune adverse events including dermatomyositis
as well. Furthermore, despite the rarity of anti-MDA5
positive dermatomyositis, no association between these autoantibodies and
malignancy has yet been described. Furthermore, a correlation with COVID-19
vaccination was highly unlikely because of the adenovirus-based platform used
and the time interval between vaccination and the appearance of skin disease.
Thus, the anti-MDA5 autoantibodies in our patient represent a unique finding
temporally connected with anti-PD-1 therapy. Although we did not demonstrate a
direct relationship between pembrolizumab and this systemic autoimmunity, the
absence of antinuclear autoantibodies before treatment initiation is highly
suggestive of a positive correlation.
Anti-MDA5-positive dermatomyositis is treated
with high-dose glucocorticoids and calcineurin inhibitors (cyclosporine A,
tacrolimus); intravenous cyclophosphamide or mycophenolate are options in patients
with aggressive disease or lung involvement [16, 17]. Janus kinase inhibitors like
tofacitinib or ruxolitinib, the anti-CD20 monoclonal antibody rituximab,
plasmapheresis and intravenous immunoglobulins can also be used [18, 19]. In our
patient, a combination of systemic and topical corticosteroids and cyclosporin
A together with provisional interruption of pembrolizumab was sufficient to
achieve complete remission of clinical, imaging and laboratory signs of
anti-MDA5-positive dermatomyositis.
Reinitiating pembrolizumab treatment in our
patient resulted in severe diarrhoea. As this is one of the most common and
lethal side effects of immune checkpoint inhibitors [20], pembrolizumab was
finally discontinued with contrasting findings: a particular sensitivity to
immune checkpoint inhibitor therapy on the one hand, and an excellent antitumoral
response with persistent stability without additional therapy on the other. This
agrees with previous observations that the appearance of immune-related adverse
events is usually associated with a positive anti-tumour response.
The adverse reactions in this case have
been reported to the Italian Medicines Agency and registered in the National
Pharmacovigilance Network (number 951564).
Conclusion
Anti-MDA5 positive dermatomyositis should
be considered as a possible new immune-related adverse event associated with immune
checkpoint inhibitor treatment.
Acknowledgements
The authors thank Dr. Chiara Moroni
(Division of Radiodiagnostic, Careggi University Hospital, Florence, Italy) for
the revision of CT scans.
Informed consent
Patient’s written consent for publication
has been obtained and archived.
Antonino Marcello Pilia
Careggi University Hospital
Largo Brambilla
IT-50134 Florence
antoninomarcello.pilia[at]unifi.it
References
1.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; c2017-2023 [cited
2023 November 04]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
2.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune
Checkpoint Blockade. N Engl J Med. 2018 Jan;378(2):158–68. 10.1056/NEJMra1703481
3.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al.; KEYNOTE-024
Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell
Lung Cancer. N Engl J Med. 2016 Nov;375(19):1823–33. 10.1056/NEJMoa1606774
4.Takatsuki K, Yanagihara T, Egashira A, Ogo N, Yoshizawa S, Sunami S, et al. A Rare
Case of Pembrolizumab-Induced Dermatomyositis in a Patient with Cancer of Unknown
Primary Origin. Am J Case Rep. 2021 Apr;22:e930286. 10.12659/AJCR.930286
5.Kartolo A, Towheed T, Mates M. A case of successful pembrolizumab rechallenge in a
patient with non-small-cell lung cancer and grade 3 dermatomyositis. Immunotherapy.
2021 Apr;13(6):477–81. 10.2217/imt-2020-0309
6.European Medicines Agency. Keytruda Product Information. Annex I. Summary of product
characteristics. [cited 2023 November 04] Available from: https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf
7.European database of suspected adverse drug reactions reports [cited 2023 November
04] Available from: https://www.adrreports.eu/en/index.html
8.Nombel A, Fabien N, Coutant F. Dermatomyositis With Anti-MDA5 Antibodies: Bioclinical
Features, Pathogenesis and Emerging Therapies. Front Immunol. 2021 Oct;12:773352.
10.3389/fimmu.2021.773352
9.Parronchi P, Radice A, Palterer B, Liotta F, Scaletti C. MDA5-positive dermatomyositis:
an uncommon entity in Europe with variable clinical presentations. Clin Mol Allergy.
2015 Nov;13(1):22. 10.1186/s12948-015-0031-y
10.So H, So J, Lam TT, Wong VT, Ho R, Li WL, et al. Seasonal Effect on Disease Onset
and Presentation in Anti-MDA5 Positive Dermatomyositis. Front Med (Lausanne). 2022 Feb;9:837024.
10.3389/fmed.2022.837024
11.Zhang SH, Zhao Y, Xie QB, Jiang Y, Wu YK, Yan B. Aberrant activation of the type I
interferon system may contribute to the pathogenesis of anti-melanoma differentiation-associated
gene 5 dermatomyositis. Br J Dermatol. 2019 May;180(5):1090–8. 10.1111/bjd.16917
12.Holzer MT, Krusche M, Ruffer N, Haberstock H, Stephan M, Huber TB, et al. New-onset
dermatomyositis following SARS-CoV-2 infection and vaccination: a case-based review.
Rheumatol Int. 2022 Dec;42(12):2267–76. 10.1007/s00296-022-05176-3
13.Wu M, Karim M, Ashinoff R. COVID-19 vaccine-associated dermatomyositis. JAAD Case
Rep. 2022 May;23:58–60. 10.1016/j.jdcr.2022.02.023
14.Ganatra K, Aggarwal R, Gupta L. Mechanic’s Hand Heralding Relapse in an Indian Adolescent
with Anti-MDA5 Positive Juvenile Dermatomyositis. Mediterr J Rheumatol. 2022 Jun;33(2):268–70.
10.31138/mjr.33.2.268
15.Kawakami N, Eda H, Nishiura M, Chaya A, Saito F, Namkoong H. A decade of Gottron’s
papules, inverse Gottron’s papules and mechanic’s hand in anti-MDA5-associated interstitial
lung disease with clinically amyopathic dermatomyositis. Oxf Med Case Rep. 2020 Jun;2020(6):omaa039.
10.1093/omcr/omaa039
16.Tsuji H, Nakashima R, Hosono Y, Imura Y, Yagita M, Yoshifuji H, et al. Multicenter
Prospective Study of the Efficacy and Safety of Combined Immunosuppressive Therapy
With High-Dose Glucocorticoid, Tacrolimus, and Cyclophosphamide in Interstitial Lung
Diseases Accompanied by Anti-Melanoma Differentiation-Associated Gene 5-Positive Dermatomyositis.
Arthritis Rheumatol. 2020 Mar;72(3):488–98. 10.1002/art.41105
17.Wu W, Guo L, Fu Y, Wang K, Zhang D, Xu W, et al. Interstitial Lung Disease in Anti-MDA5
Positive Dermatomyositis. Clin Rev Allergy Immunol. 2021 Apr;60(2):293–304. 10.1007/s12016-020-08822-5
18.Wang LM, Yang QH, Zhang L, Liu SY, Zhang PP, Zhang X, et al. Intravenous immunoglobulin
for interstitial lung diseases of anti-melanoma differentiation-associated gene 5-positive
dermatomyositis. Rheumatology (Oxford). 2022 Aug;61(9):3704–10. 10.1093/rheumatology/keab928
19.Chen Z, Wang X, Ye S. Tofacitinib in Amyopathic Dermatomyositis-Associated Interstitial
Lung Disease. N Engl J Med. 2019 Jul;381(3):291–3. 10.1056/NEJMc1900045
20.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects
of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev
Clin Oncol. 2019 Sep;16(9):563–80. 10.1038/s41571-019-0218-0




