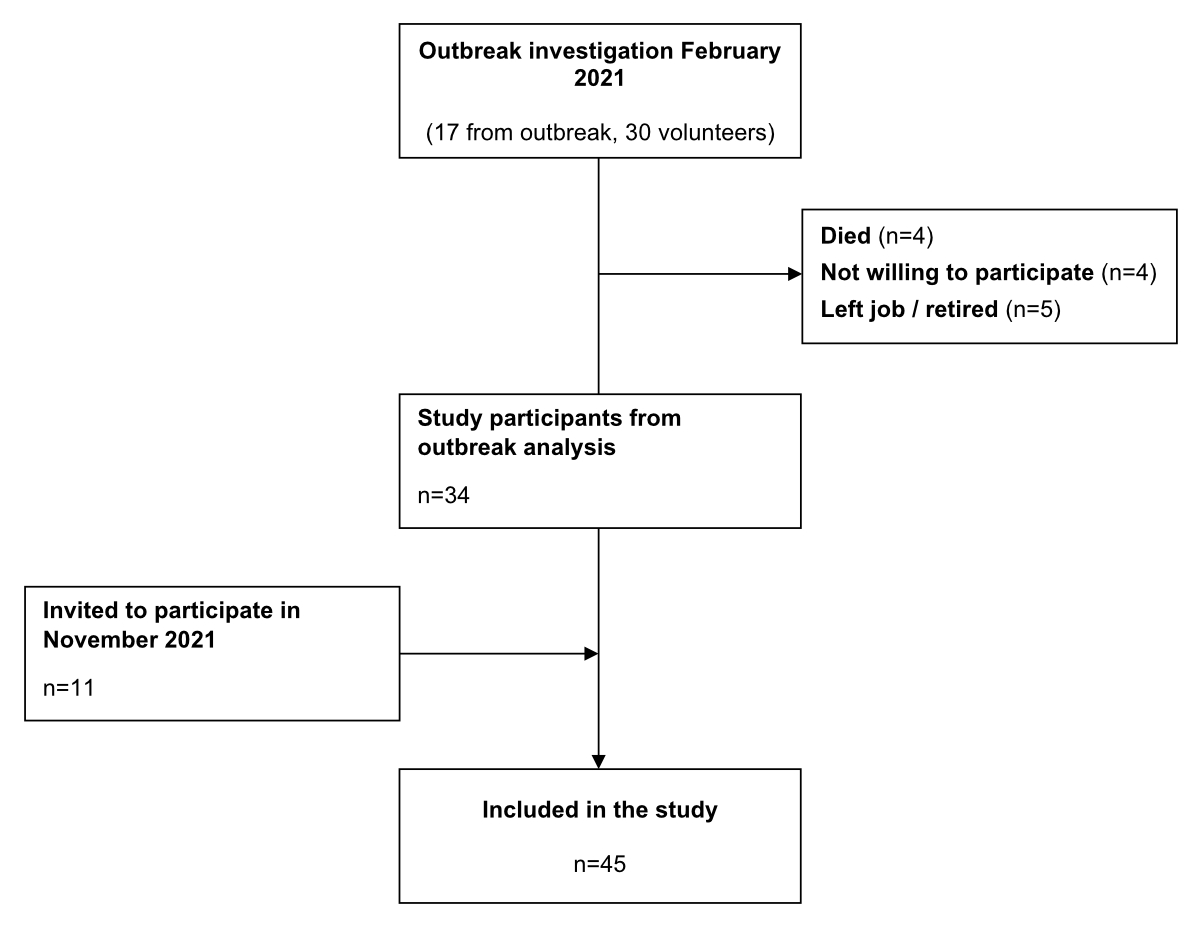
Figure 1Selection of study participants.
DOI: https://doi.org/https://doi.org/10.57187/s.3502
Caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus disease 2019 (COVID-19) pandemic remains a global threat to public health [1]. Since the first outbreak in March 2020 in Switzerland, there have been more than 4.4 million confirmed COVID-19 cases, more than 64,000 COVID-19-related hospitalisations, and more than 14,000 COVID-19-related deaths [2]. With the licensing of two mRNA vaccines (Pfizer-BioNTech and Moderna) in December 2020, the nationwide vaccination campaign has been essential for controlling the spread of the SARS-CoV-2 virus [3–5]. Initial clinical trials found that basic immunisation with two vaccine doses provided >90% protection against SARS-CoV-2 infection and severe COVID-19 for up to four months [6, 7]. As infection rates increased drastically again in June 2021 – about six months after the first vaccination campaign – an Israeli study observed a waning in vaccine response over time [8]. Therefore, a third vaccination (first booster) was approved, which became available in Switzerland in November 2021 for people aged ≥65 years and for high-risk groups [9].
Hybrid immunity – the immune response among vaccinated individuals with previous exposure to SARS-CoV-2 – provides the strongest form of protection [10–12]. For example, a study performed in a French nursing home during the SARS-CoV-2 omicron wave in December 2021 documented breakthrough infections among residents who received three vaccine doses [13]. While several residents with hybrid immunity experienced breakthrough infections, the infection rate was higher among previously naïve residents [13]. Furthermore, a recent study found natural immunity from previous infections more durable yet less effective than vaccine-induced immunity [11]. This finding emphasises the importance of vaccination against SARS-CoV-2 and highlights the necessity of booster vaccine doses for maintaining an optimal immune response. Further vaccinations may be necessary for maintaining protective immunity, particularly among older populations and those with comorbidities who appear more prone to waning immune responses [14].
Nursing homes are critical institutions in the ongoing COVID-19 pandemic due to their high-risk resident population [15]. Similarly, healthcare workers in nursing homes are also at high risk of contracting and transmitting the SARS-CoV-2 virus among themselves and residents due to their close patient contact [16]. Therefore, we studied long-term SARS-CoV-2 antibody responses among residents and healthcare workers of a nursing home in Switzerland between February 2021 and June 2022.
This study was conducted in a two-house nursing home in the canton of Solothurn, Switzerland, with a 93 resident capacity. The nursing home was among the first in the canton to vaccinate healthcare workers and residents. The study participants comprised six residents and 39 healthcare workers. Participants received their first COVID-19 mRNA vaccine dose (Pfizer/BioNTech, BNT162b2) in December 2020 and the second in January 2021. In February 2021, the nursing home experienced an outbreak in one house, which we investigated in a previous study [17]. Consistent with national recommendations, participants received a third vaccination – the first booster – in November and December 2021. As of June 2022, 41 (91%) participants had received basic immunisation with two doses, and 35 (78%) had received booster doses. While most participants received each vaccine dose simultaneously at the given time points, a few had slightly varying vaccine administration dates.
This study enrolled all staff and residents from the outbreak investigation in February 2021 [17] who were alive and willing to participate. Before the third vaccination in November 2021, we invited additional staff and residents to participate in this study. Residents with impaired judgment were not offered participation per ethics committee guidelines. The selection of study participants is shown in figure 1.

Figure 1Selection of study participants.
We developed a standardised questionnaire to obtain basic information, such as sex, age, profession, COVID-19 infection episodes, and vaccination history (supplementary file available for download at https://doi.org/10.57187/s.3502). We entered questionnaire data into REDCap [18, 19]. Data were collected in February 2022 and updated in June 2022 at the end of the study.
We collected four blood samples at different time points for analysis. The first (time point 1) was taken in March 2021 during the outbreak investigation [17]. Three additional blood samples were drawn during follow-up at the following time points: time point 2 in November 2021 (eight months after basic immunisation and before the first booster [third vaccination]), time point 3 in February 2022 (one month after the first booster), and time point 4 in June 2022 (six months after the first booster; figure S1 in the appendix). Blood samples were analysed and stored at the Institute of Medical Virology at the University of Zürich, Switzerland.
We assessed antibody reactivity with ABCORA 2.0, a bead-based, multiplex immunoassay that uses Luminex technology. We measured immunoglobulin G (IgG), A (IgA), and M (IgM) responses to SARS-CoV-2 spike protein subunits (receptor-binding domain [RBD], S1, and S2) and nucleocapsid (N) protein. Seroprofiling with ABCORA 2.0 also allowed for reliable predictions of neutralisation activity against the SARS-CoV-2 Wuhan-Hu-1 strain based on anti-S1 reactivity sum of S1 signal over cutoff (SOC) values for IgG, IgA and IgM (Sum S1) [20, 21].
We defined SARS-CoV-2 infection status as positive in cases with reactive values in N protein serology, documented infection with positive polymerase chain reaction tests, or both.
We used descriptive statistics to describe participants’ characteristics. We compared the predicted neutralisation activity of different immunisation statuses. We assessed potential risk factors for low predicted neutralisation activity using univariate linear regression models with single or multiple predictors. Variables were selected based on previous studies [8, 14, 20, 22] and expert knowledge. For the regression analysis, we log-transformed each participant’s most recent Sum S1 values and exponentiated coefficients to obtain a ratio of means [20]. The ratio of means shows the expected mean Sum S1 value relative to a reference group for each risk factor. We performed all analyses in the R statistical software (version 4.2.2) [23].
The Ethics Committee of Northwestern and Central Switzerland reviewed and approved this study (reference no. 2022-00261). All study participants provided written informed consent.
The median age of the six resident participants was 86 years (range = 54–104). All were female, five suffered from at least one comorbidity, and five smoked tobacco. Three received booster vaccines, one received basic immunisation with two doses, and two were unvaccinated. All resident participants experienced at least one SARS-CoV-2 episode [17]. By the final time point in June 2022, four residents showed hybrid immunity and two (33%) only natural immunity from previous SARS-CoV-2 infections (table 1).
Table 1Epidemiological characteristics of resident and healthcare worker participants from a nursing home in Switzerland (2021–2022).
| Healthcare workers | Residents | Total | ||
| Variable | n = 39 | n = 6 | n = 45 | |
| Sex, n (%) | Female | 32 (82) | 6 (100) | 38 (84) |
| Male | 7 (18) | 0 (0) | 7 (16) | |
| Age (years), median (IQR) | 56 (46–60) | 86 (69–91) | 56 (46–60) | |
| Age (years), n (%) | <70 | 38 (97) | 2 (33) | 29 (64) |
| ≥70 | 1 (3) | 4 (67) | 16 (36) | |
| SARS-CoV-2 vaccination, n (%) | 38 (97) | 4 (67) | 42 (93) | |
| First booster* | 32 (82) | 3 (50) | 35 (78) | |
| No booster** | 6 (15) | 1 (17) | 7 (16) | |
| Unvaccinated | 1 (3) | 2 (33) | 3 (7) | |
| SARS-CoV-2 infection, n (%) | Yes | 29 (74) | 6 (100) | 35 (78) |
| No | 10 (26) | 0 (0) | 10 (22) | |
| Immunisation status, n (%) | Hybrid | 28 (72) | 4 (67) | 32 (71) |
| Vaccination | 10 (26) | 0 (0) | 10 (22) | |
| Natural | 1 (2) | 2 (33) | 3 (7) | |
| Comorbidities (total), n (%) | 12 (31) | 5 (83) | 17 (38) | |
| None | 27 | 1 | 28 | |
| Diabetes | 0 | 1 | 1 | |
| Cardiovascular disease | 2 | 0 | 2 | |
| Chronic lung disease | 4 | 0 | 4 | |
| Hypertonia | 7 | 4 | 11 | |
| Cancer/immunosuppression | 2 | 0 | 2 | |
| Adiposities (BMI>35) | 0 | 3 | 3 | |
| Other | 0 | 2 | 2 | |
| Tobacco smoker, n (%) | Yes | 7 (18) | 5 (83) | 12 (27) |
| No | 32 (82) | 1 (17) | 33 (73) | |
IQR: interquartile range; BMI: body mass index.
* Booster: three vaccine doses.
** No booster: one or two vaccine doses.
The median age of the 39 healthcare worker participants was 56 years (interquartile range [IQR] = 46–60). Seven (18%) were male, and 32 (82%) were female. Fifteen (38%) suffered from at least one comorbidity. Seven (18%) smoked tobacco. Thirty-two (82%) received boosters, six (15%) received either one or two vaccine doses, and one (2%) was unvaccinated. Twenty-nine (74%) experienced one confirmed SARS-CoV-2 episode. By June 2022, 28 (72%) healthcare worker participants showed hybrid immunity, 10 (26%) showed vaccine-induced immunity, and one (2%) showed natural immunity (table 1).
Participants with hybrid immunity from combined vaccination and previous SARS-CoV-2 infections showed the highest predicted neutralisation activities (n = 32; median Sum S1 = 258, IQR = 206–333). We further differentiated between individuals with and without booster doses within the hybrid immunity group. Participants with booster vaccines showed the highest predicted neutralisation activities (n = 25; median Sum S1 = 273, IQR = 224–336). In contrast, those without booster vaccine doses showed lower predicted neutralisation activities (n = 7; median Sum S1 = 208, IQR = 131–258). The group with only natural immunity from previous infections showed the lowest predicted neutralisation activities (n = 3; median Sum S1 = 41, IQR = 29–85). Therefore, amongst the participants who reported a SARS-CoV-2 infection, predicted neutralisation activities tended to increase with the number of vaccine doses (p = 0.077). Among those who received a booster vaccine dose, we found that participants with hybrid immunity showed higher Sum S1 levels than those who did not report a SARS-CoV-2 infection episode. However, these results were not statistically significant (p = 0.77; figure 2).
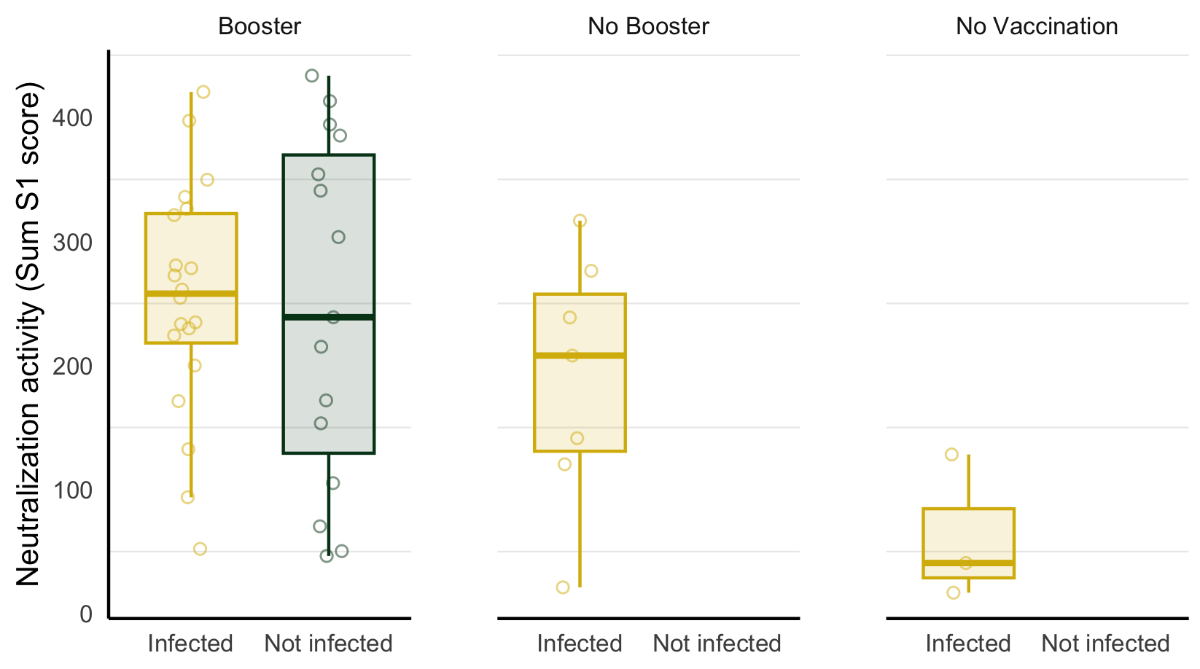
Figure 2Sum S1 levels by SARS-CoV-2 immunisation status in resident and healthcare worker participants from a nursing home. Study participants were differentiated by their number of received SARS-CoV-2 vaccine doses (booster: three vaccine doses; no booster: one or two vaccine doses; and no vaccination) and SARS-CoV-2 infection status. In the boxplots, the middle horizontal line denotes the median, and the lower and upper hinges delineate the interquartile range (IQR), which extends from the 25th (lower hinge) to the 75th (upper hinge) percentile. The upper (lower) whisker extends from the upper (lower) hinge to the highest (lowest) value, at most up to 1.5 times the 75th (25th) percentile.
All participants within the vaccination-only group received booster doses and showed lower Sum S1 levels than both hybrid immunity groups (n = 10; median Sum S1 = 163, IQR = 79–355). The group with only natural immunity from previous infections showed the lowest predicted neutralisation activities (n = 3; median Sum S1 = 41, IQR = 29–85; figure 1).
Longitudinal binding and predicted neutralisation activity
We separately analysed the predicted neutralisation activity based on Sum S1 levels for the four time points among resident and healthcare worker participants. Sum S1 levels showed similar dynamics over time to the IgG response to spike protein S1 (figures 3 and S2). Since results were only available for one participant in the resident group for the first two time points, we could not use them for analysis. In the healthcare worker group, we observed a decrease of 200 in predicted neutralisation activity from time point 1 (after basic immunisation; median Sum S1 = 215, IQR = 122–291) to time point 2 (before the booster vaccination; median Sum S1 = 15, IQR = 9–114; median duration between the first and second time points = 10 months; decrease = 93%, p = 0.001). At time point 3 in February 2022 – shortly after administering the booster dose (median = 2 months) – we observed an increase of 309 in predicted neutralisation activity in the healthcare worker group (median Sum S1 = 324, IQR = 260–372) and a similar increase in the resident group (median Sum S1 = 343, IQR = 125–350). The healthcare worker group showed a further decrease in Sum S1 levels from time points 3 to 4 (median Sum S1 = 239, IQR = 193–318; median = 6 months; decrease = 26%, p = 0.003). A similar decrease was also observed in the resident group; however, the change was nonsignificant (median Sum S1 = 77, IQR = 20–170; median = 6 months; decrease = 88%, p = 0.320; figure 3, table S1 in the appendix).

Figure 3Time-dependent SARS-CoV-2 antibody kinetics and potency in resident and healthcare worker participants from a nursing home. Boxplots showing anti-S1 IgG antibodies (A) and Sum S1 reactivity (B) signal over cutoff (SOC) values representing predicted neutralising potency for healthcare worker and resident participants. Boxplots show antibody levels determined using a multifactorial serological SARS-CoV-2 assay (ABCORA) at time points 1 (March 2021; two months after the second vaccine dose), 2 (November 2021; 10 months after the second vaccine dose), 3 (February 2022; two months after the third vaccine dose), and 4 (June 2022; six months after the third vaccine dose). The complete figure, including IgA and IgM antibody levels, is shown in figure S2 in the appendix.
IgG: immunoglobulin G; HCW: healthcare worker.
We performed a regression analysis of the standardised questionnaire data to identify possible associations between participants’ predicted neutralisation based on the most recent Sum S1 levels and epidemiological characteristics. We found low predicted neutralisation activity associated with age ≥ 70 years (adjusted ratio of means [AM] = 0.5, 95% confidence interval [CI] = 0.2–0.9) and resident status (AM = 0.7, 95% CI 0.3–1.0). Participants who smoked tobacco also had lower predicted neutralisation activity (AM = 0.5, 95% CI 0.3–0.8). We observed a positive association between Sum S1 levels and the number of vaccine doses. Unvaccinated participants showed lower predicted neutralisation activities than those with basic immunisation after one or two vaccine doses (AM = 2.3, 95% CI 0.9–5.9) and those who received a booster vaccine dose (AM = 3.6, 95% CI 1.5–8.8). Positive SARS-CoV-2 infection status also correlated with higher predicted neutralisation activity (AM = 1.5, 95% CI 0.9–2.5; table 2).
Table 2Univariable and multivariable analyses showing associations between participant characteristics and predicted SARS-CoV-2 neutralisation activity (Sum S1) in residents and healthcare workers. Results are presented as the ratio of means of Sum S1.
| Characteristic | Univariable analysis | Multivariable analysis | |||
| Ratio of means (95% confidence interval) | p-value | Adjusted ratio of means (95% confidence interval) | p-value | ||
| Sex | 0.33 | 0.72 | |||
| Male | 1 (reference) | 1 (reference) | |||
| Female | 0.72 (0.37–1.40) | 0.91 (0.52–1.57) | |||
| Age group (years) | 0.002 | 0.019 | |||
| <70 | 1 (reference) | 1 (reference) | |||
| ≥70 | 0.33 (0.16–0.65) | 0.49 (0.19–0.86) | |||
| Study group | 0.003 | 0.037 | |||
| Healthcare workers | 1 (reference) | 1 (reference) | |||
| Residents | 0.36 (0.19–0.69) | 0.69 (0.25–0.96)*** | |||
| Vaccination status | 0.002 | 0.012 | |||
| Unvaccinated | 1 (reference) | 1 (reference) | |||
| No booster** | 3.33 (1.25–8.83) | 2.30 (0.89–5.92) | |||
| Booster* | 4.82 (2.06–11.30) | 3.63 (1.49–8.82) | |||
| SARS-CoV-2 infection | 0.46 | 0.10 | |||
| No | 1 (reference) | 1 (reference) | |||
| Yes | 1.24 (0.70–2.21) | 1.52 (0.92–2.50) | |||
| Comorbidities | 0.64 | 0.80 | |||
| No | 1 (reference) | 1 (reference) | |||
| Yes | 0.89 (0.55–1.45) | 1.05 (0.69–1.61) | |||
| Tobacco smoker | 0.08 | 0.007 | |||
| No | 1 (reference) | 1 (reference) | |||
| Yes | 0.57 (0.31–1.06) | 0.49 (0.29–0.81) | |||
* Booster: three vaccine doses.
** No booster: one or two vaccine doses.
*** Due to the collinearity with the “age group” parameter, the “study group” parameter was omitted from the multivariable analysis. The multivariable values for the “study group” parameter were calculated in a separate analysis without the “age group” parameter.
This follow-up study of healthcare workers and residents in a nursing home in Switzerland analysed the long-term course of their SARS-CoV-2 immune response. We found participants with hybrid immunity from SARS-CoV-2 vaccination and previous SARS-CoV-2 infection showed the highest immunological response. Receiving a booster vaccine dose counteracted waning vaccine-induced immunity to maintain a robust immune response. Nursing home residents showed greater susceptibility to a waning immune response than healthcare workers, putting them at higher risk of SARS-CoV-2 infection.
Study participants with hybrid immunity from three vaccines (first booster) and a previous SARS-CoV-2 infection showed the highest Sum S1 levels, likely due to the combined effects of vaccine-induced and natural immune responses. In contrast, participants with only two vaccination doses and previous SARS-CoV2 infection showed lower predicted neutralisation activities. Nevertheless, they showed higher Sum S1 levels than participants with only vaccine-induced immunity, all of whom received booster vaccine doses. This finding suggests that hybrid immunity provides a more robust immunological response. Our analysis of epidemiological data showed a trend in higher neutralisation levels among participants with positive SARS-CoV-2 infection status, consistent with our immunisation status analysis results. Previous studies also reported that a combination of prior SARS-CoV-2 infection and vaccination was associated with lower infection rates and less severe disease [10, 11]. Some participants reported SARS-CoV-2 infection episodes after their final vaccine dose. Therefore, a time-dependent increase in predicted neutralisation activity must be considered. Nonetheless, our results indicate that frequent re-exposure to SARS-CoV-2, whether through vaccination or infection, maintains high predicted neutralisation activities.
Unvaccinated participants showed the lowest Sum S1 levels, and all reported at least one SARS-CoV-2 infection episode. Despite showing considerably higher Sum S1 levels, all participants with only one or two vaccine doses also contracted SARS-CoV-2 infections, indicating that basic immunisation does not provide a sufficiently strong long-term vaccine-induced immune response to prevent infection. Previous studies described a waning in vaccine-induced immunity over time, leading to a renewed increase in infection rates [8, 14]. Our observations confirm these findings. Clinical trials found infection rates decline after introducing a third vaccination dose (first booster) [22, 24], showing that administering further vaccine doses counteracts waning. In our study, all participants who did not contract SARS-CoV-2 infections received booster vaccine doses. Furthermore, participants with hybrid immunity and booster vaccine doses showed higher predicted neutralisation activities than those without booster doses. These observations indicate that maintaining vaccine-induced immunity with booster doses is crucial for maintaining a high protective immunity level.
Our longitudinal follow-up measurements allowed us to analyse waning immune responses more closely. We found a greater decrease in Sum S1 levels after basic vaccine doses than booster doses. One possible explanation is a longer interval between the second vaccination and time point 2 (median = 10 months) than between the booster vaccine dose and time point 4 (median = 6 months). Another explanation is the increased hybrid immunity throughout this study, which was associated with higher predicted neutralisation levels.
When comparing study groups in our follow-up analysis, we observed a greater waning of immune responses in the resident group than in the healthcare workers group. However, these results were not statistically significant, possibly due to the small sample size. Nevertheless, our regression analysis showed a correlation between older age and low Sum S1 levels. The resident group, most of whom were aged >70 years, also had lower predicted neutralisation activities. The immune system experiences an age-dependent decline [25]. Therefore, older individuals show weaker immune responses with faster decreases in antibody titers, leading to reduced vaccine-induced immunity [26]. Such a phenomenon was previously reported for vaccines against other infectious diseases, such as influenza and varicella [27, 28]. Menni et al. found a more pronounced waning of antibody titers among participants aged >55 years than younger participants after all three SARS-CoV-2 vaccine doses, leading to more severe infections and hospitalisations [14].
Finally, we found an association between smoking and lower predicted neutralisation levels. Recent studies have found a negative association between smoking and SARS-CoV-2 mRNA vaccine-induced antibody responses. Current smokers show lower antibody titers after vaccine administration and a faster decline in antibody levels over time than non-smokers [29, 30]. Furthermore, a Japanese study found that antibody responses declined with increasing cigarette dependence [29].
Our study is limited by its small sample size, which allowed for assessing the response to basic vaccine doses only among healthcare workers and prevented analysing the impact of the number of SARS-CoV-2 infection episodes and comorbidities on predicted neutralisation levels. However, we followed participants over a long period. We also collected samples in a coordinated and standardised manner, allowing for assessing the long-term immune response to basic and booster vaccine doses. Moreover, we used a wide range of reliable serological markers [20, 21].
We performed a long-term study following residents and healthcare workers in a nursing home up to six months after administering a third mRNA vaccine dose against SARS-CoV-2. Our results showed that frequent exposure to SARS-CoV-2 through vaccination and infection resulted in hybrid immunity, providing the most robust immune response. Follow-up measurements showed waning, even after the third vaccine dose, suggesting that repeated booster doses are necessary to maintain optimal protection. We recommend that future vaccine campaigns prioritise individuals with less frequent SARS-CoV-2 exposure and, consequently, weaker immune responses for immunisation. Since our study only included a few participants with more than one SARS-CoV-2 episode, we could not examine whether predicted neutralisation levels increase with the number of previous infections. Therefore, it is unclear whether the strength of hybrid immunity increases with each infection episode. Similarly, whether susceptibility to waning decreases with each additional vaccine dose remains unclear. High-risk populations, such as nursing home residents and older adults, remain particularly vulnerable because of their weakened immune responses. Therefore, they benefit most from SARS-CoV-2 preventive measures and continuous monitoring of their protective immunity.
We thank all residents and healthcare workers at the nursing home and those willing to participate in our study. We are also indebted to the staff at the nursing home for assisting with data collection.
Author contributions: Conception and design: IA, CM, AT, LF. Data collection: LP, IA, CM, KZ, LF. Laboratory analysis: IA, AA, SE, AT. Data analysis: LP, IA, NB. Wrote the first draft of the paper: LP, IA, ME, AT, LF. LP, IA, NB, and LF revised it based on comments from all authors. All authors reviewed and approved the final version of the manuscript.
There was no specific funding for this project. ME, NB, KZ and LF were supported by the National Institute of Allergy and Infectious Diseases through cooperative agreement no. AI069924. ME was supported by special project funding (grant no. 189498) from the Swiss National Science Foundation.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al.; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb;382(8):727–33. 10.1056/NEJMoa2001017
2. Federal Office of Public Health (FOPH). COVID -19 Switzerland. 2021 [cited 2023 February 6 2023]; Available from: https://www.covid19.admin.ch/en/overview
3. Swissmedic. Swissmedic grants authorisation for the first COVID-19 vaccine in Switzerland. 2021 [cited 2023 February 2023]; Available from: https://www.swissmedic.ch/swissmedic/en/home/news/coronavirus-covid-19/covid-19-impfstoff_erstzulassung.html
4. Swissmedic. Swissmedic grants authorisation for the COVID-19 vaccine from Moderna: Second COVID-19 vaccine authorised in Switzerland. 2021 [cited 2021 January]; Available from: https://www.swissmedic.ch/swissmedic/en/home/news/coronavirus-covid-19/zulassung-covid-19-impfstoff-moderna.html
5. Zürcher K, Mugglin C, Egger M, Müller S, Fluri M, Bolick L, et al. Vaccination willingness for COVID-19 among healthcare workers: a cross-sectional survey in a Swiss canton. Swiss Med Wkly. 2021 Sep;151(3738):w30061. 10.4414/SMW.2021.w30061
6. Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021 May;397(10287):1819–29. 10.1016/S0140-6736(21)00947-8
7. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al.; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec;383(27):2603–15. 10.1056/NEJMoa2034577
8. Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med. 2021 Dec;385(24):e85. 10.1056/NEJMoa2114228
9. Federal Office of Public Health (FOPH). Impfempfehlung für mRNA-Impfstoffe gegen Covid-19 (Stand 09.11.21). Federal office of public health 2021.
10. Carazo S, Skowronski DM, Brisson M, Barkati S, Sauvageau C, Brousseau N, et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study. Lancet Infect Dis. 2023 Jan;23(1):45–55. 10.1016/S1473-3099(22)00578-3
11. Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N Engl J Med. 2022 Jul;387(1):21–34. 10.1056/NEJMoa2203965
12. Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023 May;23(5):556–67. 10.1016/S1473-3099(22)00801-5
13. Bruel T, Pinaud L, Tondeur L, Planas D, Staropoli I, Porrot F, et al. Neutralising antibody responses to SARS-CoV-2 omicron among elderly nursing home residents following a booster dose of BNT162b2 vaccine: A community-based, prospective, longitudinal cohort study. EClinicalMedicine. 2022 Jul;51:101576. 10.1016/j.eclinm.2022.101576
14. Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022 Jul;22(7):1002–10. 10.1016/S1473-3099(22)00146-3
15. Smith PW, Bennett G, Bradley S, Drinka P, Lautenbach E, Marx J, et al.; SHEA; APIC. SHEA/APIC guideline: infection prevention and control in the long-term care facility, July 2008. Infect Control Hosp Epidemiol. 2008 Sep;29(9):785–814. 10.1086/592416
16. Gómez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Díaz ZM, Wyssmann BM, et al. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am J Epidemiol. 2021 Jan;190(1):161–75. 10.1093/aje/kwaa191
17. Zurcher K, et al. Alpha variant coronavirus outbreak in a nursing home despite high vaccination coverage: molecular, epidemiological and immunological studies. Clin Infect Dis. 2022;•••:
18. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al.; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019 Jul;95:103208. 10.1016/j.jbi.2019.103208
19. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. 10.1016/j.jbi.2008.08.010
20. Chammartin F, Kusejko K, Pasin C, Trkola A, Briel M, Amico P, et al.; and the Swiss HIV Cohort Study. Determinants of antibody response to severe acute respiratory syndrome coronavirus 2 mRNA vaccines in people with HIV. AIDS. 2022 Aug;36(10):1465–8. 10.1097/QAD.0000000000003246
21. Abela IA, Pasin C, Schwarzmüller M, Epp S, Sickmann ME, Schanz MM, et al. Multifactorial seroprofiling dissects the contribution of pre-existing human coronaviruses responses to SARS-CoV-2 immunity. Nat Commun. 2021 Nov;12(1):6703. 10.1038/s41467-021-27040-x
22. Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021 Dec;398(10316):2093–100. 10.1016/S0140-6736(21)02249-2
23. Team RC (Computing S, editor). R: A language and environment for statistical computing, R.F.f. Vienna, Austria; 2021.
24. Hussein K, Dabaja-Younis H, Szwarcwort-Cohen M, Almog R, Leiba R, Weissman A, et al. Third BNT162b2 Vaccine Booster Dose against SARS-CoV-2-Induced Antibody Response among Healthcare Workers. Vaccines (Basel). 2022 Oct;10(10):1741. 10.3390/vaccines10101741
25. Crooke, S.N., et al., Immunosenescence and human vaccine immune responses. Immunity & ageing : I & A, 2019. 16: p. 25-25. 10.1186/s12979-019-0164-9
26. Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008 Apr;46(7):1078–84. 10.1086/529197
27. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006 Feb;24(8):1159–69. 10.1016/j.vaccine.2005.08.105
28. Lelic A, Verschoor CP, Lau VW, Parsons R, Evelegh C, Bowdish DM, et al. Immunogenicity of Varicella Vaccine and Immunologic Predictors of Response in a Cohort of Elderly Nursing Home Residents. J Infect Dis. 2016 Dec;214(12):1905–10. 10.1093/infdis/jiw462
29. Mori Y, Tanaka M, Kozai H, Hotta K, Aoyama Y, Shigeno Y, et al. Antibody response of smokers to the COVID-19 vaccination: evaluation based on cigarette dependence. Drug Discov Ther. 2022 May;16(2):78–84. 10.5582/ddt.2022.01022
30. Ferrara P, Ponticelli D, Agüero F, Caci G, Vitale A, Borrelli M, et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health. 2022 Feb;203:97–9. 10.1016/j.puhe.2021.12.013
Table S1Median Sum S1 and IgG S1 levels in the healthcare worker and resident groups at the four time points. Values for the first (March 2021) and second (November 2021) time points in the resident group are in brackets since they were not used for analysis due to the small sample size.
| Time point | Sum S1: HCW | Sum S1: Residents | IgG S1: HCW | IgG S1: Residents |
| March 2021 | 215 | (354) | 200 | (325) |
| November 2021 | 15 (93% decrease) | (173) | 15 (92% decrease) | (163) |
| February 2022 | 324 | 343 | 321 | 338 |
| June 2022 | 239 (26% decrease) | 77 (88% decrease) | 231 (18% decrease) | 73 (79% decrease) |
HCW: healthcare workers; IgG: immunoglobulin G.
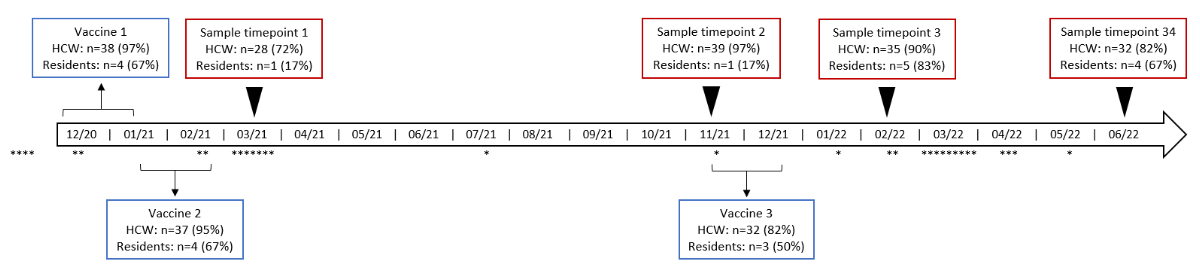
Figure S1Timeline of events. The red boxes show the four time points at which blood samples were drawn, and the blue boxes show the time periods in which vaccines were administered. Also shown are the times at which positive SARS-CoV-2 tests were recorded according to the standardised questionnaire.
HCW: healthcare workers; n: number of participants; *: positive SARS-CoV-2 test. HCW: n = 39; residents: n = 6.
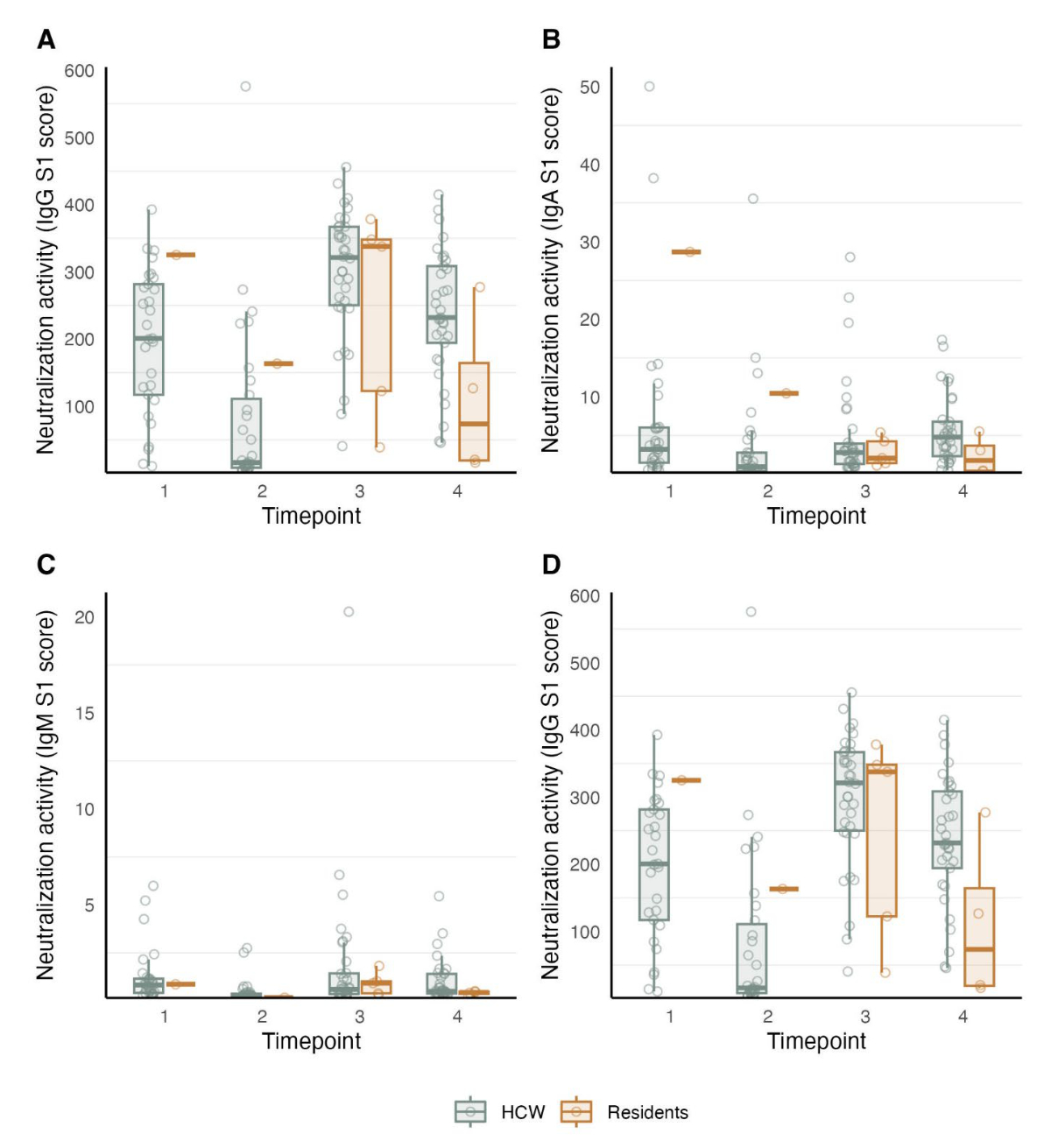
Figure S2Boxplots showing ABCORA-derived anti-S1 IgG antibodies (A), anti-S1 IgA antibodies (B), anti-S1 IgM antibodies (C), and Sum S1 reactivity signal over cutoff (SOC) values representing predicted neutralising activity (D) at time point 1 (March 2021; two months after the second vaccine dose), 2 (November 2021; 10 months after the second vaccine dose), 3 (February 2022; two months after the third vaccine dose), and 4 (June 2022; six months after the third vaccine dose) in the healthcare worker and resident groups.
IgG: immunoglobulin G; IgA: Immunoglobulin A; IgM: Immunoglobulin M; HCW: healthcare worker.