Post-transplant survival with pre-transplant durable continuous-flow mechanical circulatory
support in a Swiss cohort of heart transplant recipients
DOI: https://doi.org/https://doi.org/10.57187/s.3500
Roger Hullina,
Tamila Abdurashidovaa,
Barbara Pitta-Grosa,
Sara Schukrafta,
Valentina Rancatib,
Henri Lua,
Anouck Zurbuchena,
Carlo Marcuccib,
Zied Ltaiefc,
Karl Lefold,
Christoph Hubere,
Manuel Pascuald,
Piergiorgio Tozzif,
Philippe Meyere,
Matthias Kirschf
a Cardiology, Cardiovascular Department, University
Hospital Lausanne, University of Lausanne, Lausanne, Switzerland
b Anesthesiology, Surgical Department, University
Hospital Lausanne, University of Lausanne, Lausanne, Switzerland
c Intensive Care Department, University
Hospital Lausanne, University of Lausanne, Lausanne, Switzerland
d Solid Organ Transplantation Center, University
Hospital Lausanne, University of Lausanne, Lausanne, Switzerland
e Cardiac Surgery, Cardiovascular
Department, University Hospital Lausanne, University of Lausanne, Lausanne,
Switzerland
f Cardiology, Department of Medical
Specialties and Cardiovascular Surgery, Department of Surgery, Geneva
University Hospital, University of Geneva, Geneva, Switzerland
Summary
BACKGROUND:
Worldwide, almost half of all heart transplantation candidates arrive today at their
transplant operation with durable continuous-flow mechanical circulatory support
(CF-MCS). This evolution is due to a progressive increase of waiting list time
and hence an increased risk of haemodynamic worsening. Longer duration of CF-MCS
is associated with a higher risk of device-related complications with potential
adverse impact on post-transplant outcome as suggested by recent results from
the United Network of Organ Sharing of the United States.
METHODS: A
2-centre Swiss heart transplantation programme conducted a retrospective observational
study of consecutive patients of theirs who underwent a transplant in the
period 2008–2020. The primary aim was to determine whether post-transplant
all-cause mortality is different between heart transplant recipients without or
with pre-transplant CF-MCS. The secondary outcome was the acute cellular
rejection score within the first year post-transplant.
RESULTS: The
study participants had a median age of 54 years; 38/158 (24%) were females. 53/158
study participants (34%) had pre-transplant CF-MCS with a median treatment duration
of 280 days. In heart transplant
recipients with pre-transplant CF-MCS, the prevalence of ischaemic
cardiomyopathy was higher (51 vs 32%; p = 0.013), the left ventricular ejection
fraction was lower (20 vs 25; p = 0.047) and pulmonary vascular resistance was higher
(2.3 vs 2.1
Wood Units; p = 0.047).
Over the study period, the proportion of heart transplant recipients with pre-transplant
CF-MCS and the duration of pre-transplant CF-MCS treatment increased (2008–2014
vs 2015–2020: 22% vs 45%, p = 0.009; increase of treatment days per year: 34.4 ± 11.2
days, p = 0.003; respectively). The primary and secondary outcomes
were not different between heart transplant recipients with pre-transplant
CF-MCS or direct heart transplantation (log-rank p = 0.515; 0.16 vs 0.14, respectively;
p = 0.81).
CONCLUSION:
This data indicates that the strategy of pre-transplant CF-MCS with subsequent orthotopic
heart transplantation provides post-transplant outcomes not different to direct
heart transplantation despite the fact that the duration of pre-transplant
assist device treatment has progressively increased.
Introduction
Modern medical
therapy has significantly prolonged the survival of heart failure patients resulting
in a large increase in the number of patients living with advanced-stage heart
failure [1, 2]. To date, orthotopic heart
transplantation has remained the treatment of choice for selected patients with
advanced heart failure. Intensive efforts have increased the availability of
donor hearts and the number of heart transplants performed worldwide [3, 4]. Nonetheless,
the overall donor heart
supply still falls short of demand [5], resulting in
prolonged waiting list time. In Switzerland, the mean waiting list time increased
from 181 to 307 days at a regional heart transplantation centre from the period
1987–1999 to 2011–2018 [6].
A longer waiting
list time carries the risks of worsening central haemodynamics, progressive
deterioration of end-organ function and increased waiting list mortality [7, 8]. For
a long time, heart
transplantation under urgent status remained the only option for shortening the
waiting list time. In recent years, long-term continuous-flow mechanical
circulatory support (CF-MCS) has been shown to improve haemodynamics, to decrease
end-organ dysfunction [9] and to reduce waiting
list mortality of heart transplant candidates [7, 8]. This may explain why the number
of
heart transplant candidates with a bridge to transplant by means of durable
CF-MCS has increased worldwide, as reflected by the International Society of
Heart and Lung Transplantation (ISHLT) registry demonstrating that 42.9% of heart
transplant recipients had pre-transplant long-term CF-MCS in the years 2011–2018
[4, 10, 11].
However, studies
investigating post-transplant survival of heart transplant recipients with pre-transplant
CF-MCS have provided heterogeneous results, with some reports suggesting similar
survival when compared with direct heart transplantation [12, 13] and others indicating
inferior outcomes
[14–18]. In October 2018, the US national
heart organ allocation system changed priorities resulting in prolonged CF-MCS
duration and inferior post-transplant outcomes of these patients when compared to
direct heart transplantation [16, 17]. Different priorities of national allocation
systems for heart
transplantation can therefore explain the inhomogeneous results of post-transplant
survival reported for pre-transplant CF-MCS patients and prolonged duration of
CF-MCS may therein play a role [14–17]. The heterogeneous results may also relate
to the different CF-MCS
types that have been implanted in recent years, given that the HeartMate II®,
the HVAD® and the HeartMate 3® differ with respect to the incidence of device-related
complications [19, 20]. And this difference may have been accentuated by varying implantation
rates across different world regions. Finally, dissimilarity of the post-transplant
care protocol and the annual rate of locally performed transplant operations
may also affect post-transplant outcome [3, 4].
In
Switzerland, the national donor heart allocation system has remained largely
unchanged since 2007. In particular, a high-urgency status has always been limited
to approximately 30% of the annual number of all heart transplants. Given that the
pre- and post-transplant follow-up protocols for our regional heart transplant cohort
has not changed significantly since 2008, the present observational study
compares against this stable background post-transplant survival in patients
without or with pre-transplant CF-MCS both for the total of all CF-MCS devices
implanted and for each CF-MCS type separately.
Methods
Study inclusion
and exclusion criteria
The inclusion
criteria for this observational study were: transplant operation carried out
between 1 January 2008 and 31 December 2020; and consent provided by the heart transplant
recipient. The study excluded patients undergoing retransplantation and
patients who had received extra- or paracorporeal ventricular support pre-transplant.
Study population
This 2-centre
observational study included 175 consecutive patients who underwent 176 heart transplant
operations in the years 2008 to 2020. Study participants were all under follow-up
by the Heart Transplantation Programme of Suisse Romande established at the University
Hospitals of Lausanne and of Geneva. Addition of patients to the waiting list of
heart transplant candidates was decided in regularly scheduled joint sessions
of the dedicated multidisciplinary teams at both sites, as described elsewhere [21].
Each centre provided pre-transplant
and post-transplant care to their local patients [21]. In contrast, the transplant
surgery and immediate postoperative follow-up were always carried out at the University
Hospital of Lausanne.
Four of 175
heart transplant recipients were excluded from the final analysis because retransplantation
of heart transplant recipients with transplant operation before 2008 precluded
these patients from pre-transplant CF-MCS. Likewise, a retransplantation of a heart
transplant recipient with first transplant within the study period was not
considered and censored as a primary outcome event. Of the remaining 171 heart transplant
recipients, 13 were excluded because pre-transplant long-term MCS support used extra-
or para-corporeal devices (figure S1 in the appendix).
Acquisition of
anthropometric, biological, clinical and outcome data
Patient characteristics
were collected from the electronic health reports of individual patients at the
Lausanne and Geneva University Hospitals (TA, AZ). Data accuracy was confirmed
by revisiting all patients’ data, which demonstrated 98.6% correctness (TA). Donor-specific
characteristics were extracted from the Swisstransplant organ allocation system
(KL). The acute cellular rejection score was calculated from corresponding data
extracted from the Swiss Transplant Cohort study (BPG). Comprehensive
transthoracic echocardiography was always acquired on GE Healthcare machines by
board-certified cardiologists. LVEF was quantitatively assessed using the biplane
Simpson method [22]. All-cause mortality (ACM) was collected from
local documentation and confirmed by death dates extracted from the Swiss
registry of deaths up to the censor date 31 December 2021 on access date 26
March 2022.
Outcomes
Heart transplant
recipients were separated into two groups as a function of pre-transplant CF-MCS.
The primary
outcome compares the post-transplant survival time
of primary heart transplant recipients with that of heart transplant recipients
with pre-transplant CF-MCS.
The secondary
outcome compares the acute cellular rejection scores in the first post-operative
year [6, 21].
Statistical
analysis
Statistical
analysis was performed using SPSS BASE 17.0 statistical software (SPSS Inc.,
Chicago, IL, USA). Categorical variables were expressed as percentages and
compared using Pearson’s Chi-square or Fisher’s exact test (when expected n ≤5).
Continuous variables were expressed as medians and interquartile ranges (IQR). The
groups were compared using the nonparametric Mann-Whitney test to avoid the
assumption of normal distribution of variables. Correlation between two
continuous variables was tested using the nonparametric Spearman correlation
test.
The mean rejection score within the first
year post-transplant was calculated as the sum of the rejection grade of all
endomyocardial biopsies taken during the first year post-transplant divided by
the number of procurements [21]. Endomyocardial biopsies were graded for
acute cellular rejection based on the 2004 ISHLT score.
Simple linear regression analysed the change
of the duration of pre-transplant CF-MCS time as a function of the year of CF-MCS
implantation after checking the data for linearity.
Survival data was analysed with standard Kaplan-Meier
actuarial techniques for estimation of survival probabilities ± standard
deviation and were compared using the log-rank test.
A two-tailed p value <0.05 was considered to
indicate statistical significance.
Ethics approval
The
protocol was approved by the local research ethics committee (CER-VD 2022-00562,
CER VD 2019-704); the study was conducted in accordance with the Declaration of
Helsinki and complies with the ISHLT Ethics Statement.
Results
Characteristics of
heart transplant recipients with or without pre-transplant CF-MCS support
Table 1
shows that most baseline characteristics were not different between patients
with direct heart transplantation (n = 105) or heart transplant with pre-transplant
CF-MCS (n = 53). There was no difference for non-white ethnicity (11 vs 11%; p
= 0.99), median age (overall: 53.7 years; 53.7 vs 54.5 years; p = 0.17) or sex (overall:
24% were females; 27 vs 19%; p = 0.33). In pre-transplant CF-MCS patients, median
body mass index (BMI) (27.1 vs 24.8; p = 0.004) and body surface index (BSA)
were higher, and the prevalence of smoking history (71 vs 44%; p = 0.001) and of
dyslipidaemia (60 vs 38%; p = 0.006) were higher.
Table 1Characteristics of heart transplant recipients without or with pre-transplant continuous-flow
mechanical circulatory support (CF-MCS) treatment on waitlisting. Categorical
values are presented as absolute numbers (n) and percentages; continuous values
are presented as medians.
| |
|
All
patients (n = 158) |
No pre-transplant
CF-MCS (n = 105) |
Pre-transplant
CF-MCS (n = 53) |
p value |
| Demographics |
Non-white
ethnicity |
17 (11) |
11 (11) |
6 (11) |
0.99 |
| Age, in years |
53.7 |
53.7 |
54.5 |
0.17 |
| Sex,#females/#
males (% females) |
38/120
(24) |
28/77
(27) |
10/43
(19) |
0.33 |
| Anthropometrics |
BMI |
25.6 |
24.8 |
27.1 |
0.004 |
| BSA, in m2 |
1.88 |
1.87 |
1.96 |
0.018 |
| Pre-transplant
risk factors |
Smoking |
83 (53) |
46 (44) |
37 (71) |
0.001 |
| COPD |
15 (10) |
8 (8) |
7 (13) |
0.26 |
| Hypertension
|
62 (40) |
35 (33) |
27 (52) |
0.03 |
| Diabetes
mellitus |
34 (22) |
20 (19) |
14 (26) |
0.29 |
| Dyslipidaemia
|
71 (45) |
39 (38) |
32 (60) |
0.006 |
| Dialysis |
4 (2.5) |
3 (2) |
1 (2) |
1.0 |
Table 2
shows that the proportion of heart transplant patients with ischaemic
cardiomyopathy was higher in heart transplant recipients with pre-transplant
CF-MCS (51 vs 32%, p = 0.013). The percentage of patients with mixed or
acquired cardiomyopathy was not different between groups. Seventeen direct heart
transplant recipients presented a cardiac pathology unfavourable for CF-MCS
treatment such as hypertrophic cardiomyopathy, arrhythmogenic right ventricular
cardiomyopathy, severe left ventricular noncompaction or Danon’s disease. The proportion
of transplant operations after pre-transplant CF-MCS increased from the period 2008–2014
to 2015–2020 (22 vs 45%; p = 0.009) (table S1 in the appendix).
Table 2Aetiology
of end-stage heart failure on waitlisting. Categorical
values are presented as absolute numbers (n) and percentages.
| Aetiology |
No pre-transplant
CF-MCS (n = 105) |
Pre-transplant
CF-MCS (n = 53) |
p value |
| Ischaemic
heart disease |
34 (32) |
27 (51) |
0.013 |
| Congenital
heart disease |
12 (11) |
2 (4) |
0.11 |
| Primary cardiomyopathies |
| Genetic |
Hypertrophic
cardiomyopathy |
8 (8) |
0 (0) |
0.002 |
| ARVC |
4 (4) |
0 (0) |
|
| Left ventricular
noncompaction |
3 (2) |
0 (0) |
|
| Glycogen storage
disease (Danon) |
2 (2) |
0 (0) |
|
| Mixed |
Dilated cardiomyopathy |
24 (23) |
18 (34) |
0.28 |
| Restrictive
cardiomyopathy |
3 (3) |
0 (0) |
| Acquired |
Inflammatory
(myocarditis) |
3 (3) |
3 (6) |
0.38 |
| Secondary
cardiomyopathies |
Infiltrative |
3 (3) |
0 (0) |
0.24 |
| Inflammatory
(sarcoidosis) |
2 (2) |
0 (0) |
| Consequence
of cancer therapy/Toxicity |
4 (4) |
1 (2) |
| Neuromuscular |
1 (1) |
0 (0) |
| Valvular heart
disease |
2 (2) |
2 (4) |
| Total |
105
(100) |
53 (100) |
|
Table 3
shows that on waitlisting the percentage of patients presenting with NYHA class
IV was not different between groups (25 vs 30%; p = 0.56); median left
ventricular ejection fraction (LVEF) was lower in pre-transplant CF-MCS than in
direct heart transplant recipients (20.0 vs 25.0; p = 0.001); median PVR was
higher in pre-transplant CF-MCS patients (2.3 vs 2.1 Wood Units; p = 0.047). In
both groups, the proportion of patients on automated intra-cardiac
defibrillator (AICD) treatment was high (68% vs 66%; p = 0.86), and many
patients were on resynchronisation therapy (pre-transplant CF-MCS vs direct heart
transplant (40% vs 32%; p = 0.40). At transplantation, heart transplant candidates
with pre-transplant CF-MCS had lower levels of creatinine, urea and haemoglobin
while ASAT, ALAT and bilirubin levels were not different (table S2 in the
appendix).
Table 3Functional characteristics and device treatment on waitlisting. Categorical
values are presented as absolute numbers (n) and percentages; continuous values
are presented as medians.
| |
All
patients (n = 158) |
No pre-transplant
CF-MCS (n = 105) |
Pre-transplant
CF-MCS (n = 53) |
p value |
| NYHA
class IV |
39 (27) |
25 (25) |
14 (30) |
0.56 |
| VO2
max, in ml O2·kg-1·min-1 |
13.0 |
13.4 |
12.6 |
0.17 |
| LVEF (%) |
22.0 |
25.0 |
20.0 |
0.001 |
| PVR (Wood
Units) |
2.2 |
2.1 |
2.3 |
0.047 |
| Heart
rate ≥100 bpm |
13 (9) |
9 (9) |
4 (10) |
0.98 |
| CRT |
55 (35) |
34 (32) |
21 (40) |
0.40 |
| AICD |
106 (67) |
71 (68) |
35 (66) |
0.86 |
Table 4 shows
the relative proportions of the 3 different CF-MCS types implanted in the
period 2008–2020: the Abbott HeartMate II® (n = 13), the HeartWare HVAD® (n = 13)
and the Abbott HeartMate 3® (n = 27). One patient had
biventricular long-term mechanical continuous support (LT-MCS) using the HVAD® device. Heart transplant recipients
with pre-transplant CF-MCS overall had a median duration of 280.0 days on the
device; the median duration of treatment was not significantly different
between the 3 different continuous flow (CF) LT-MCS types implanted (HeartMate II® vs HVAD® vs HeartMate 3®: 226.0 vs 232.0 vs 329.0 days; p = 0.186).
However, the median duration of treatment increased progressively during the
study period (slope linear regression: 34.4 ± 11.2 days/year, p = 0.003) (figure 1).
Table 4Characteristics of continuous-flow mechanical circulatory support (CF-MCS) at
time of transplant operation. Categorical
values are presented as absolute numbers (n) and percentages; continuous values
are presented as medians.
| Implanted
CF-MCS |
53 (100%) |
| HeartMate
II® |
13 |
| HVAD® |
13 |
| HeartMate
3® |
27 |
| Left ventricular
CF-MCS / biventricular CF-MCS |
52 (98%)
/ 1 (2%) |
| Duration
of CF-MCS (any CF device) |
280.0
days (range: 36– 1343 days; sum: 19,908 patient-days) |
| Duration,
by CF-MCS type |
HeartMate
II® |
226.0
days (range: 36–567 days) |
| HVAD® |
232.0
days (range: 87–917 days) |
| HeartMate
3® |
329.0
days (range: 42–1343 days) p = 0.186 |
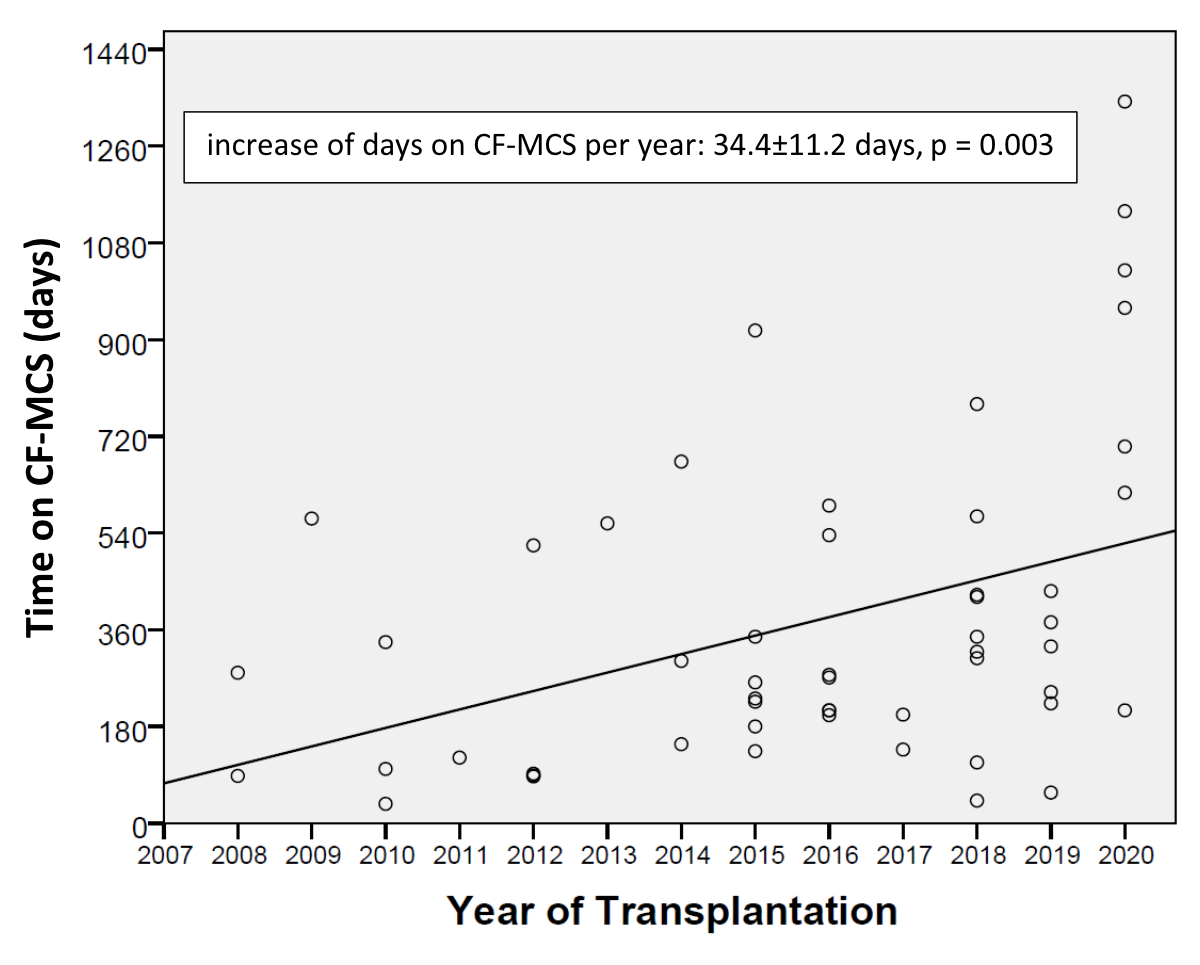
Figure 1Increase in the duration of continuous-flow mechanical circulatory
support as a function of the year
of transplant operation.
The beta slope of the regression line represents the change in the outcome
(duration on support in days) with unit change in the predictor (year of ventricular
assist device implantation).
Table 5
shows that the groups did not differ with respect to donor age, recipient/donor
sex mismatch or the proportion of heart transplants under urgent status. Furthermore,
cold ischaemic time and transport distance were not different; transport
distance was significantly correlated with cold ischaemic time (r = 0.680; p = 0.0001).
A total of 30.4% heart transplant patients without pre-transplant CF-MCS had
previous cardiac surgery. The median length of the post-transplant stay in
hospital was not different between groups (direct heart transplant vs
pre-transplant CF-MCS: 33 vs 33 days; p = 0.28). No pre-transplant CF-MCS patient
had a combined heart and kidney transplant operation.
Table 5Parameters
of transplant surgery known to be associated with post-surgical outcome. Categorical
values are presented as absolute numbers (n) and percentages; continuous values
are presented as medians.
| |
All
patients (n = 158) |
No pre-transplant
CF-MCS (n = 105) |
Pre-transplant
CF-MCS (n = 53) |
p value |
| Previous cardiac surgery |
85 (54%) |
32 (31%) |
53 (100%) |
<0.0001 |
| High-urgency
status |
29 (18%) |
19 (18%) |
10 (19%) |
0.90 |
| Time on urgent list (days) |
21.0 |
11.5 |
47.5 |
0.003 |
| Transport
distance (km) |
55.0 |
55.0 |
50.0 |
0.26 |
| Cold ischaemic
time (minutes) |
162.6 |
170.0 |
151.8 |
0.14 |
| Donor age
(years) |
47.0 |
47.0 |
49.0 |
0.37 |
| Recipient/donor
sex mismatch |
64 (41%) |
48 (46%) |
16 (30%) |
0.06 |
| Heart +
kidney transplantation |
4 (2.5%) |
4 (4%) |
0 (0%) |
0.15 |
| Length of stay after heart transplantation (days) |
33.0 |
33.0 |
33.0 |
0.28 |
The mean post-transplant
follow-up time was 42.4 months for all study participants and not different
between patients without or with pre-transplant CF-MCS (38.3 vs 45.9; p = 0.71).
Furthermore, the mean rejection score within the first year post-transplant was
not different (0.16 vs 0.14; p = 0.81).
Figure 2 represents
the Kaplan-Meier estimates of overall post-transplant survival, which were
88.9 ± 2.5% for the first year post-transplant and 87.3 ± 2.7%, 83.8 ± 3.3% and
77.8 ± 4.7% for 3-, 5- and 10-year survival, respectively.
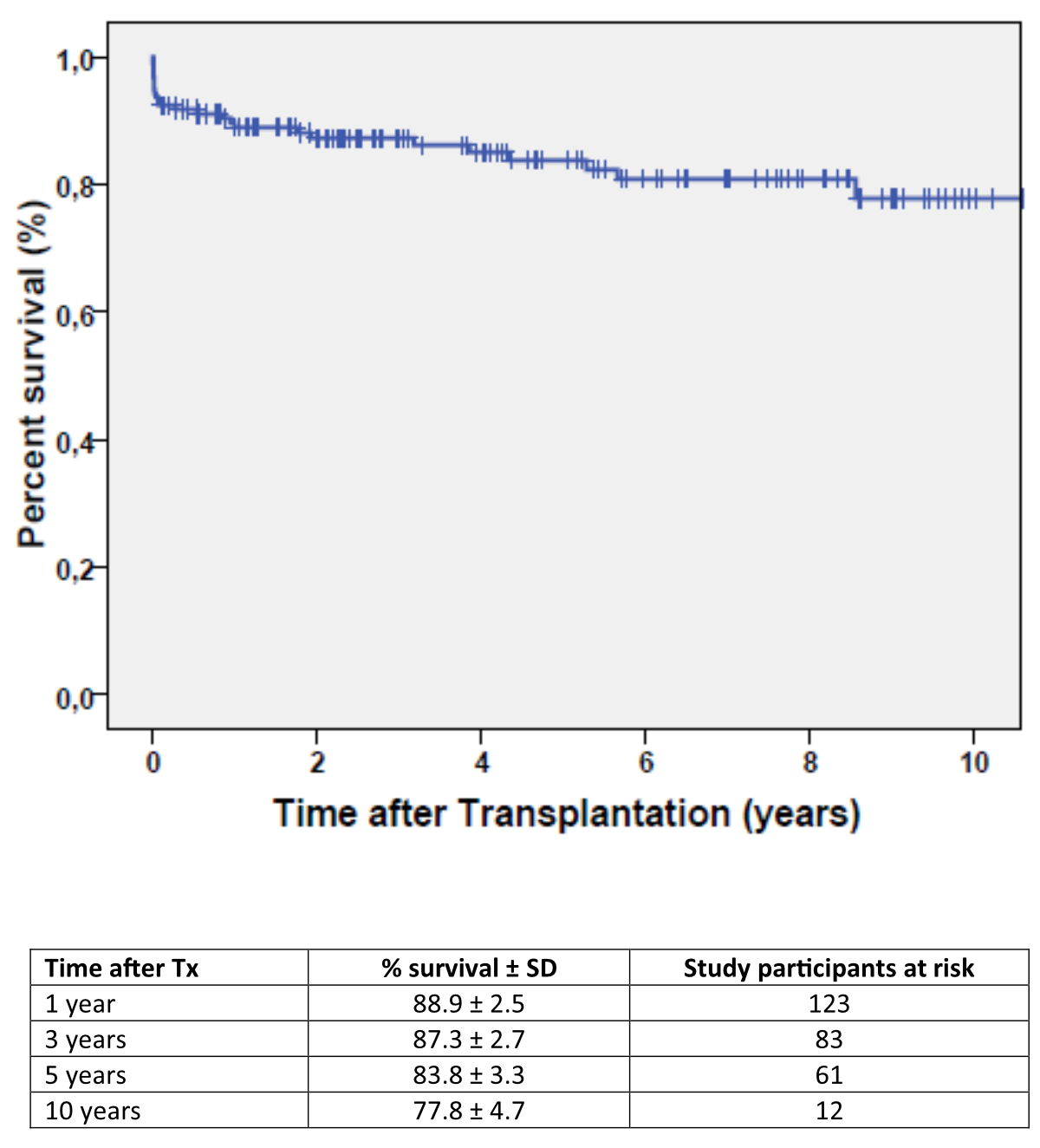
Figure 2Overall survival of the 158 heart transplant recipients.
Survival was
not different when Kaplan-Meier estimates compared heart transplant recipients
with or without pre-transplant CF-MCS (log-rank p = 0.515) (figure 3).

Figure 3Kaplan-Meier estimates of survival after heart transplantation comparing direct
heart transplant patients (n = 105) with pre-transplant continuous-flow mechanical
circulatory support (CF-MCS) patients (n = 53). Colour code: Blue line, post-transplant
survival with direct heart transplant; Green line, post-transplant survival
with pre-transplant CF-MCS. Heart transplant recipients without pre-transplant ventricular
assist
device (n = 105)
had 19 events during the observation period while heart transplant recipients
with pre-transplant mechanical circulatory support had 7 events (log-rank, p = 0.515).
Results are expressed as percent survival ± standard deviation.
No
significant difference was evident when survival was compared between heart transplant
recipients without or with CF-MCS with the former HeartMate II® or HVAD® devices, or with the current HeartMate
3® (log-rank p = 0.681) (figure 4).
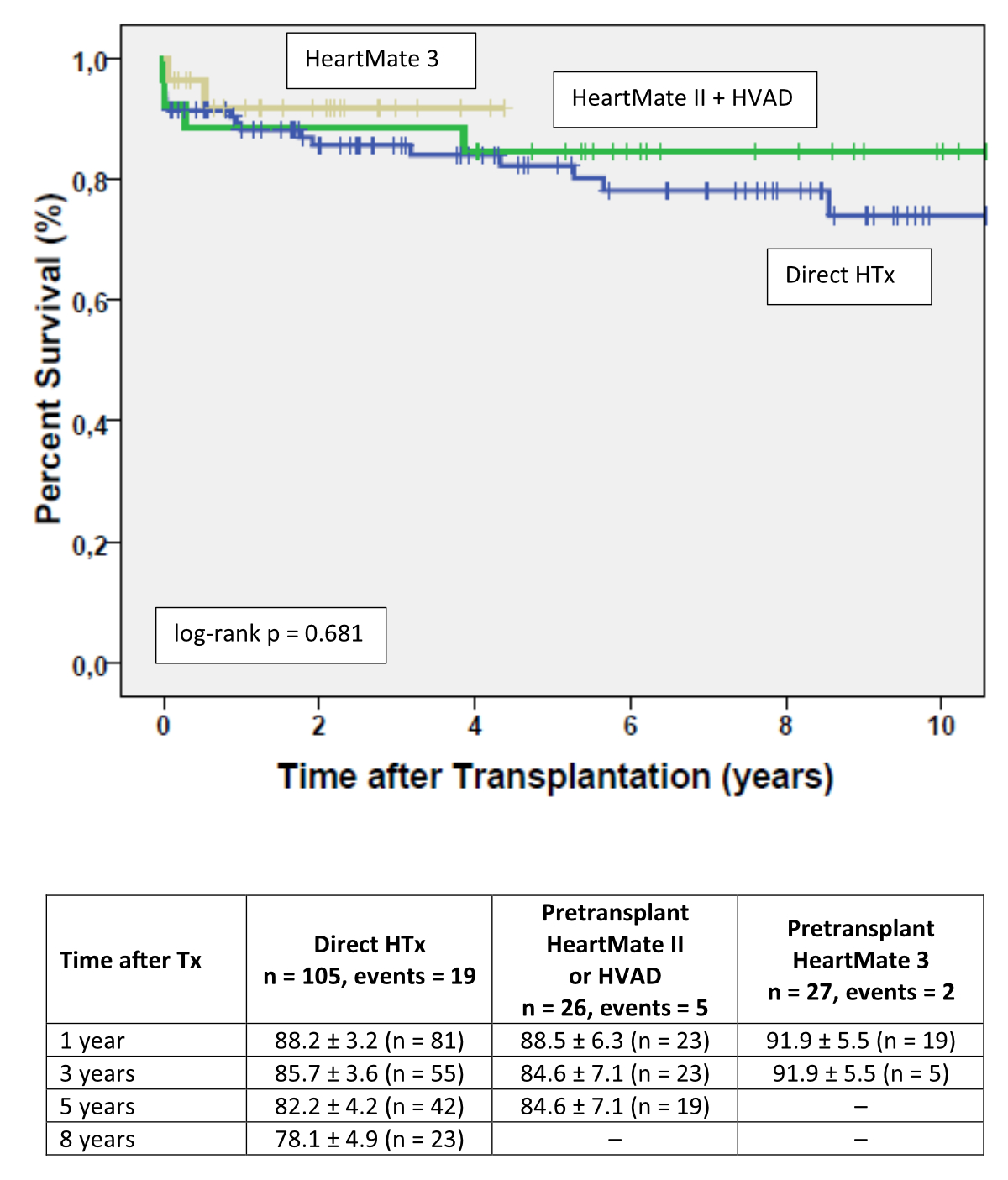
Figure 4Kaplan-Meier estimates of survival after heart transplantation comparing direct
heart transplant recipients (n = 105) with heart transplant recipients with pre-transplant
HeartMate II® or HVAD® (n = 26), or HeartMate 3® (n = 27).Colour code: Blue line, post-transplant survival with
direct heart transplantation; Green line, post-transplant survival with pre-transplant
Heart Mate II® or HVAD®; Yellow line, post-transplant survival with pre-transplant
HeartMate 3®. Survival after heart transplantation in patients without prior
CF-MCS support and those with HeartMate 2® or HVAD®, and those with HeartMate 3®. Results are expressed as percent
survival ± standard deviation.
Discussion
In this regional
cohort of heart transplant recipients, one-third arrived at transplant surgery
while on CF-MCS. Long-term post-transplant survival was not significantly different
between heart transplant recipients with or without pre-transplant CF-MCS. During
the observation period, the median time on CF-MCS increased progressively and
was longest in the HeartMate 3® group. Post-transplant survival was
not significantly different between device type groups but visually superior in
patients with the HeartMate 3® despite their longer duration on
CF-MCS.
Heart transplantation still
remains the long-term treatment of choice for advanced heart failure patients
and a major reason is the 10-year survival of up to 75% [4]. From 2009 to
2018, the worldwide number of heart transplant candidates increased substantially
due to an increasing proportion of heart transplant candidates aged 65 years and
older [23]. Advanced heart failure patients in this age group
are usually considered candidates for destination therapy [24]. In fact, for
the 25,551 CF-MCS implantations for the period 2010–2019 documented by the INTERMACS
registry, the mean age was 57 years and 50.4% of these implantations were intended
as destination therapy [25]. However, the 5-year survival for CF-MCS treatment
was only 43.3% in these patients [25], falling short of reported post-transplant 10-year
survival of heart transplant recipients documented by the ISHLT registry [4]. This
may explain
the preference for heart transplantation waitlisting particularly when advanced
heart failure presents as monopathology [24]. However, this
attitude has worsened the worldwide imbalance between the number of heart
transplantation candidates and the number of available donor hearts explaining
the worldwide increase in waiting list time [5, 6, 26].
Today’s clinical
importance of pre-transplant CF-MCS is evident from various registries: the
INTERMACS registry shows that almost 50% of all CF-MCS implantations are intended
as either a bridge to transplant or a bridge to candidacy for heart
transplantation [25]; the ISHLT
registry documents that 42.9% of all heart
transplantation recipients arrived at transplant surgery with pre-transplant
MCS [4]; the United Network of Organ Sharing (UNOS)
registry reported a proportion of 37.3% heart transplantation recipients with
pre-transplant MCS [26]; European
or Asian centres specify pre-transplant CF-MCS in 22% to 47% of their local heart
transplantation recipient cohorts [27–30]. In accordance, the
present cohort indicates pre-transplant CF-MCS in 33.5% of all heart
transplantation recipients.
Heart transplantation recipients on bridge to
transplant were older and more overweight in the present study population;
furthermore, LVEF was lower and pulmonary vascular resistance on waitlisting was
higher suggesting that heart failure was more advanced. These findings
correspond to reports from other cohorts [27–30] and match the profile of waiting
list patients
implanted with a HeartMate II® device or the HVAD® in the
pivotal studies [31, 32]. However, 51% of the bridge-to-transplant patients in the
present study population had end-stage heart failure of ischaemic origin while
this proportion was lower elsewhere [29, 30]. This observation may relate to the decrease
of waitlisted
heart transplant candidates with cardiomyopathy of non-ischaemic origin as reported
previously from our cohort [6]. However, we cannot exclude a selection bias since
patients with heart failure of non-ischaemic origin
are prone to a higher incidence of early postoperative right heart failure
after CF-MCS implantation [33] and therefore more often considered for urgent
transplant surgery [24, 34].
In view of this large
use of pre-transplant CF-MCS, post-transplant survival is an important point of
interest. In the UNOS registry, heart transplantation recipients with pre-transplant
CF-MCS and transplant surgery in the years 2007 to 2017 presented a minor increase
in 5-year mortality when compared with direct heart transplantation [35]. This
increase resulted from a 2% higher upfront mortality within the first 3 post-transplant
months which was related with redo sternotomy and device explantation [35]. Since
long-term survival conditional on 90-day survival was in this analysis similar between
direct heart transplantation and heart transplantation with pre-transplant
CF-MCS, the authors of this study argued that live years gained with MCS should
counterbalance the small increase of mortality early after MCS implantation [35].
The effect
of redo sternotomy and device explantation on post-transplant mortality was no
longer present in another analysis of the UNOS database restricted to the years
2015–2018. This analysis also included heart transplantation recipients with
pre-transplant HeartMate3® support (n = 177) in addition to patients with pre-transplant
HeartMate II® (n = 881) or HVAD® support (n = 920).
In detail, 6-month post-transplant mortality was not different between direct heart
transplantation and heart transplantation with pre-transplant CF-MCS and
similarly not different between either CF LT-MCS type. However, survival was
numerically best in heart transplantation recipients with pre-transplant
HeartMate 3® support [36]. In
accordance, no difference in post-transplant
1-year survival was reported by Alwair et al. comparing heart
transplantation recipients arriving at transplant operation with HVAD® or HeartMate 3® support before the
change of the national donor heart allocation in 2018 [37]. The present
study is in accordance with these results, expanding the observations discussed
above by the first report on 3-year post-transplant survival for heart
transplantation recipients on pre-transplant HeartMate 3® device treatment.
The above-mentioned
results were obtained in US heart transplantation recipients with transplant
operation before October 2018 when heart transplantation candidates with
durable CF-MCS (26) were prioritised by the national allocation system [12]. The duration
of CF-MCS was shorter in US heart transplantation recipients before October 2018
and longer thereafter [16, 17]. In contrast, the donor heart allocation algorithms
of
Eurotransplant and of Switzerland prioritise pre-transplant CF-MCS patients
only when severe device complications warrant urgent transplant surgery, which
can explain the longer waiting list time documented for heart transplantation
candidates with pre-transplant CF-MCS by the EUROMACS registry [38] or the
present study. Longer duration of CF LT-MCS treatment, however, is associated
with a significant increase in device-related complications such as pump-related
infection, gastrointestinal bleeding or stroke which occur in 20–25% of
patients surviving the first post-implantation year [39]. CF-MCS-related
complications prolong hospitalisation after transplant operation [40] but have also
been associated with worse outcomes in heart transplantation recipients with
>1 year of pre-transplant CF-MCS [13, 14, 41], especially when pump exchange had been
required prior to transplant
surgery [42]. In contrast, post-transplant mortality was in the
present study population not different between heart transplantation recipients
with direct transplant operation or pre-transplant CF-MCS. Of note, survival
was numerically best with pre-transplant HeartMate 3® similar to
other reports [36] despite having the longest duration of CF-MCS.
Reasons for these favourable results are manifold and may relate to the low
complication rate of the HeartMate 3® device [43]; however, local care of heart
transplantation candidates with or without CF LT-MCS and post-transplant
follow-up of patients in a Swiss medium size-volume centre may explain the overall
excellent outcomes as well [44, 45].
Limitations
This
observational study reports post-transplant outcomes from a Swiss medium-volume
heart transplantation centre with a median annual caseload of 13.5 heart
transplant operations/year for the period 2008–2020. Furthermore, three
different CF-MCS types were implanted during the study period, which may have impacted
on the results if numbers had been larger. In addition, the small numbers
predispose to bias since patient selection for heart transplantation waitlisting
may not have been representative and decision-making on when to implant
long-term CF MCS was not standardised but based on clinical appraisal. Furthermore,
change in perioperative and postoperative management during the study period may
have impacted on outcomes. These considerations may limit broad applicability
of the study results; however, the clinical characteristics of heart
transplantation candidates are compatible with current indications as reported
previously [3, 6] and the decision for CF-MCS was in
concordance with the EACTS expert consensus on long-term MCS [46]. Since these results
were obtained on the background of the Swiss
national donor heart allocation algorithm, we cannot exclude that these results
are not applicable to countries with other allocation algorithms.
Conclusion
The lack of
a difference in post-transplant survival between patients with direct heart transplant
and those with pre-transplant CF-MCS is encouraging and indicates that a bridge-to-transplant
strategy is a valid option in patients with haemodynamic compromise while on
the waiting list for heart transplantation in Switzerland. This result warrants
further study in a larger Swiss national cohort since confirmation would
provide additional evidence in favour of the equity of the current Swiss donor
heart allocation policy.
Roger
Hullin MD
Associate
Professor
Severe
Heart Failure and Heart Transplantation
Cardiology, Cardiovascular
Department
University
Hospital Lausanne
University
of Lausanne
Rue du
Bugnon 46
CH-1011
Lausanne
roger.hullin[at]chuv.ch
References
1. Jones NR, Roalfe AK, Adoki I, Hobbs FD, Taylor CJ. Survival of patients with chronic
heart failure in the community: a systematic review and meta-analysis. Eur J Heart
Fail. 2019 Nov;21(11):1306–25. 10.1002/ejhf.1594
2. Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, et al. Advanced
heart failure: a position statement of the Heart Failure Association of the European
Society of Cardiology. Eur J Heart Fail. 2018 Nov;20(11):1505–35. 10.1002/ejhf.1236
3. Lund LH, Edwards LB, Dipchand AI, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al.;
International Society for Heart and Lung Transplantation. The registry of the International
Society for Heart and Lung Transplantation: thirty-third adult heart transplantation
report - 2016; focus theme: primary diagnostic indications for transplant. J Heart
Lung Transplant. 2016 Oct;35(10):1158–69. 10.1016/j.healun.2016.08.017
4. Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr, Hsich E, et al.; International
Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant
Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth
adult heart transplantation report - 2019; focus theme: Donor and recipient size match.
J Heart Lung Transplant. 2019 Oct;38(10):1056–66. 10.1016/j.healun.2019.08.004
5. Weiss J, Beyeler F, Immer FF, Swisstransplant H; Swisstransplant Heart Working Group
Stah. Heart allocation and transplantation in Switzerland since the introduction of
the Swiss Organ Allocation System (SOAS). Swiss Med Wkly. 2014 Nov;144:w14057. 10.4414/smw.2014.14057
6. Zurbuchen A, Tozzi P, Regamey J, Abdurashidova T, Meyer P, Lefol K, et al. Has the
Profile of Heart Transplantation Recipients changed within the last 3 decades? An
analysis from the Lausanne Heart Transplantation Center. Swiss Med Wkly. 2022;152:w30108.
10.4414/SMW.2022.w30108
7. Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al.; HeartMate
II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart
transplantation. N Engl J Med. 2007 Aug;357(9):885–96. 10.1056/NEJMoa067758
8. Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, et al.; HeartWare
Bridge to Transplant ADVANCE Trial Investigators. HeartWare ventricular assist system
for bridge to transplant: combined results of the bridge to transplant and continued
access protocol trial. J Heart Lung Transplant. 2013 Jul;32(7):675–83. 10.1016/j.healun.2013.04.004
9. Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, et al. Prevalence and prognostic
importance of changes in renal function after mechanical circulatory support. Circ
Heart Fail. 2014 Jan;7(1):68–75. 10.1161/CIRCHEARTFAILURE.113.000507
10. Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Fifth
INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory
support patients. J Heart Lung Transplant. 2013 Feb;32(2):141–56. 10.1016/j.healun.2012.12.004
11. Ciarka A, Edwards L, Nilsson J, Stehlik J, Lund LH. Trends in the use of mechanical
circulatory support as a bridge to heart transplantation across different age groups.
Int J Cardiol. 2017 Mar;231:225–7. 10.1016/j.ijcard.2016.10.049
12. Moonsamy P, Axtell AL, Ibrahim NE, Funamoto M, Tolis G, Lewis GD, et al. Survival
after heart transplantation in patients bridged with mechanical circulatory support.
J Am Coll Cardiol. 2020 Jun;75(23):2892–905. 10.1016/j.jacc.2020.04.037
13. Zhang B, Guo S, Ning J, Li Y, Liu Z. Continuous-flow left ventricular assist device
versus orthotopic heart transplantation in adults with heart failure: a systematic
review and meta-analysis. Ann Cardiothorac Surg. 2021 Mar;10(2):209–20. 10.21037/acs-2020-cfmcs-fs-197
14. Fukuhara S, Takeda K, Polanco AR, Takayama H, Naka Y. Prolonged continuous-flow left
ventricular assist device support and posttransplantation outcomes: A new challenge.
J Thorac Cardiovasc Surg. 2016 Mar;151(3):872–880.e5. 10.1016/j.jtcvs.2015.10.024
15. Takeda K, Takayama H, Kalesan B, Uriel N, Colombo PC, Jorde UP, et al. Outcome of
cardiac transplantation in patients requiring prolonged continuous-flow left ventricular
assist device support. J Heart Lung Transplant. 2015 Jan;34(1):89–99. 10.1016/j.healun.2014.09.007
16. Uriel MH, Clerkin KJ, Takeda K, Naka Y, Sayer GT, Uriel N, et al. Bridging to transplant
with HeartMate 3 left ventricular assist devices in the new heart organ allocation
system: an individualized approach. J Heart Lung Transplant. 2022;000:1–10.
17. Mullan CW, Chouairi F, Sen S, Mori M, Clark KA, Reinhardt SW, et al. Changes in use
of left ventricular assist devices as bridge to transplantation with new heart allocation
policy. JACC Heart Fail. 2021 Jun;9(6):420–9. 10.1016/j.jchf.2021.01.010
18. Truby LK, Farr MA, Garan AR, Givens R, Restaino SW, Latif F, et al. Impact of bridge
to transplantation with continuous-flow left ventricular assist devices on posttransplantation
mortality. Circulation. 2019 Aug;140(6):459–69. 10.1161/CIRCULATIONAHA.118.036932
19. Mihalj M, Heinisch PP, Schober P, Wieser M, Martinelli M, de By TM, et al. Third-generation
continuous-flow left ventricular assist devices: a comparative outcome analysis by
device type. ESC Heart Fail. 2022 Oct;9(5):3469–82. 10.1002/ehf2.13794
20. Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr, Yuzefpolskaya M, Salerno C, et al.;
MOMENTUM 3 Investigators. for the MOMENTUM 3 investigators. Two-year outcomes with
a magnetically levitated cardiac pump in heart failure. N Engl J Med. 2018 Apr;378(15):1386–95.
10.1056/NEJMoa1800866
21. Schmidhauser M, Regamey J, Pilon N, Pascual M, Rotman S, Banfi C, et al. The impact
of multidisciplinary care on early morbidity and mortality after heart transplantation.
Interact Cardiovasc Thorac Surg. 2017 Sep;25(3):384–90. 10.1093/icvts/ivx151
22. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations
for cardiac chamber quantification by echocardiography in adults: an update from the
American Society of Echocardiography and the European Association of Cardiovascular
Imaging. Eur Heart J Cardiovasc Imaging. 2015 Mar;16(3):233–70. 10.1093/ehjci/jev014
23. Colvin M, Smith JM, Hadley N, Skeans MA, Uccellini K, Goff R, et al. OPTN/SRTR 2018
Annual Data Report: heart. Am J Transplant. 2020 Jan;20 Suppl s1:340–426. 10.1111/ajt.15676
24. Hullin R, Meyer P, Yerly P, Kirsch M. Cardiac surgery in advanced heart failure. J
Clin Med. 2022 Jan;11(3):773–90. 10.3390/jcm11030773
25. Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, et al. The Society
of Thoracic Surgeons Intermacs 2020 Annual Report. Ann Thorac Surg. 2021 Mar;111(3):778–92.
10.1016/j.athoracsur.2020.12.038
26. Truby LK, Garan AR, Givens RC, Takeda K, Takayama H, Trinh PN, et al. Ventricular
assist device utilization in heart transplant candidates. Circ Heart Fail. 2018 Apr;11(4):e004586.
10.1161/CIRCHEARTFAILURE.117.004586
27. Nelson LM, Rossing K, Boesgaard S, Møller-Sørensen H, Møller CH, Gustafsson F, et
al. Three decades of heart transplantation: experience and long-term outcome. Scand
Cardiovasc J. 2022 Dec;56(1):65–72. 10.1080/14017431.2022.2061726
28. Immohr MB, Mehdiani A, Albert A, Boettger C, Dalyanoglu H, Scheiber D, et al. Heart
transplantation in patients with ventricular assist devices: impacts of the implantation
technique and support duration. J Card Surg. 2020 Feb;35(2):352–9. 10.1111/jocs.14392
29. Carrozzini M, Bejko J, Gambino A, Tarzia V, Lanera C, Gregori D, et al. Results of
new-generation intrapericardial continuous flow left ventricular assist devices as
a bridge-to-transplant. J Cardiovasc Med (Hagerstown). 2018 Dec;19(12):739–47. 10.2459/JCM.0000000000000721
30. Wong KL, Ho KL, Lee OJ, Lun KS, Bhatia I, Tam WY, et al. Emerging roles of left ventricular
assist device therapy as bridge to transplant in an Asian city with scarce heart transplant
donor. J Thorac Dis. 2021 Oct;13(10):5717–30. 10.21037/jtd-21-298
31. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al.; HeartMate
II Investigators. Advanced heart failure treated with continuous-flow left ventricular
assist device. N Engl J Med. 2009 Dec;361(23):2241–51. 10.1056/NEJMoa0909938
32. Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, et al.; HeartWare
Bridge to Transplant ADVANCE Trial Investigators. HeartWare ventricular assist system
for bridge to transplant: combined results of the bridge to transplant and continued
access protocol trial. J Heart Lung Transplant. 2013 Jul;32(7):675–83. 10.1016/j.healun.2013.04.004
33. Løgstrup BB, Nemec P, Schoenrath F, Gummert J, Pya Y, Potapov E, et al. Heart failure
etiology and risk of right heart failure in adult left ventricular assist device support:
the European Registry for Patients with Mechanical Circulatory Support (EUROMACS).
Scand Cardiovasc J. 2020 Oct;54(5):306–14. 10.1080/14017431.2020.1781239
34. Hullin R. Heart transplantation: current practice and outlook to the future. Swiss
Med Wkly. 2014 Aug;144:w13977. 10.4414/smw.2014.13977
35. Suarez-Pierre A, Lui C, Zhou X, Crawford TC, Fraser CD 3rd, Giuliano K, et al. Early
outcomes after heart transplantation in recipients bridged with a HeartMate 3 device.
Ann Thorac Surg. 2019 Aug;108(2):467–73. 10.1016/j.athoracsur.2019.01.084
36. Suarez-Pierre A, Zhou X, Fraser CD 3rd, Grimm JC, Crawford TC, Lui C, et al. Survival
and functional status after bridge-to-transplant with a left ventricular assist device.
ASAIO J. 2019;65(7):661–7. 10.1097/MAT.0000000000000874
37. Alwair H, Whitehouse K, Slaughter MS, Trivedi JR. A tale of two centrifugal-flow ventricular
assist devices as bridge to heart transplant. Ann Thorac Surg. 2022 Mar;113(3):757–62.
10.1016/j.athoracsur.2021.03.093
38. de By TM, Schoenrath F, Veen KM, Mohacsi P, Stein J, Alkhamees KM, et al. The European
Registry for patients with mechanical circulatory support of the European Association
for Cardio-Thoracic Surgery: third report. Eur J Cardiothorac Surg. 2022 Jun;62(1):ezac032.
10.1093/ejcts/ezac032
39. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, et al. The
Society of Thoracic Surgeons Intermacs database annual report: evolving indications,
outcomes, and scientific partnerships. J Heart Lung Transplant. 2019 Feb;38(2):114–26.
10.1016/j.healun.2018.11.013
40. Immohr MB, Boeken U, Mueller F, Prashovikj E, Morshuis M, Böttger C, et al. Complications
of left ventricular assist devices causing high urgency status on waiting list: impact
on outcome after heart transplantation. ESC Heart Fail. 2021 Apr;8(2):1253–62. 10.1002/ehf2.13188
41. Hariri IM, Dardas T, Kanwar M, Cogswell R, Gosev I, Molina E, et al. Long-term survival
on LVAD support: device complications and end-organ dysfunction limit long-term success.
J Heart Lung Transplant. 2022 Feb;41(2):161–70. 10.1016/j.healun.2021.07.011
42. Suarez-Pierre A, Zhou X, Lui C, Grimm JC, Hsu S, Choi CW, et al. Impact of left ventricular
assist device exchange on outcomes after heart transplantation. Ann Thorac Surg. 2020 Jan;109(1):78–84.
10.1016/j.athoracsur.2019.05.038
43. Goldstein DJ, Meyns B, Xie R, Cowger J, Pettit S, Nakatani T, et al. Third Annual
Report From the ISHLT Mechanically Assisted Circulatory Support Registry: A comparison
of centrifugal and axial continuous-flow left ventricular assist devices. J Heart
Lung Transplant. 2019 Apr;38(4):352–63. 10.1016/j.healun.2019.02.004
44. Tozzi P, Nowacka A, Hullin R, Yerly P, Kirsch M. The role of Heart Failure Team in
managing mechanical circulatory support in a Swiss low-volume institution. Heart Surg
Forum. 2018 Jun;21(4):E257–62. 10.1532/hsf.1979
45. Pettit SJ, Jhund PS, Hawkins NM, Gardner RS, Haj-Yahia S, McMurray JJ, et al. How
small is too small? A systematic review of center volume and outcome after cardiac
transplantation. Circ Cardiovasc Qual Outcomes. 2012 Nov;5(6):783–90. 10.1161/CIRCOUTCOMES.112.966630
46. Potapov EV, Antonides C, Crespo-Leiro MG, Combes A, Färber G, Hannan MM, et al. 2019
EACTS Expert Consensus on long-term mechanical circulatory support. Eur J Cardiothorac
Surg. 2019 Aug;56(2):230–70. 10.1093/ejcts/ezz098
Appendix: supplementary figure and tables
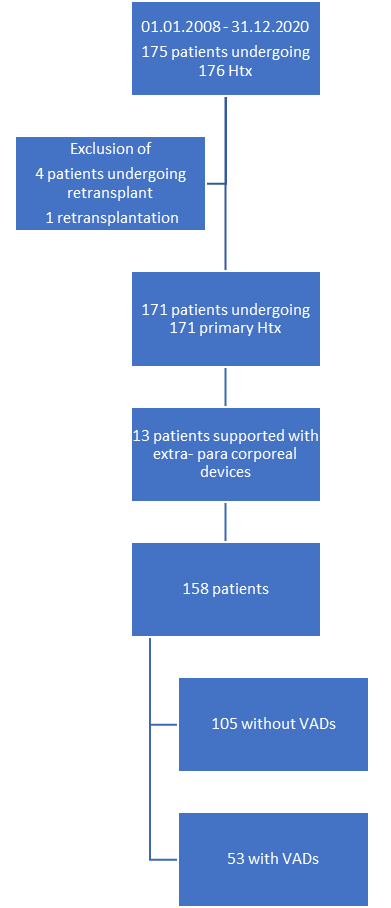
Figure S1Study population with heart transplantation from 1 January 2008 to 31 December 2020
and minimal follow-up of 12 months. Htx: heart transplantation; VAD: ventricular assist
device.
Table S1Number of heart transplants performed during
the study period with proportion of patients with pre-transplant CF-MCS.
| |
Heart
transplants performed |
No pre-transplant CF-MCS |
Pre-transplant CF-MCS |
% of patients
with CF-MCS |
| 2008 |
6 |
4 |
2 |
33% |
| 2009 |
5 |
4 |
1 |
20% |
| 2010 |
12 |
9 |
3 |
25% |
| 2011 |
11 |
10 |
1 |
9% |
| 2012 |
13 |
9 |
4 |
31% |
| 2013 |
11 |
10 |
1 |
9% |
| 2014 |
11 |
8 |
3 |
27% |
| 2015 |
11 |
4 |
7 |
64% |
| 2016 |
13 |
6 |
7 |
54% |
| 2017 |
7 |
5 |
2 |
29% |
| 2018 |
20 |
11 |
9 |
45% |
| 2019 |
19 |
13 |
6 |
31% |
| 2020 |
19 |
12 |
7 |
37% |
| Total |
n = 158 |
n = 105 |
n = 53 |
34% |
Table S2Pre-transplant laboratory values. Continuous
values are presented as medians.
| |
All
patients (n = 158) |
No pre-transplant
CF-MCS (n = 105) |
Pre-transplant
CF-MCS (n = 53) |
p value |
| |
|
|
|
|
| Creatinine (μmol/l) |
101.0 |
104.0 |
95.0 |
0.03 |
| Urea (mmol/l) |
8.0 |
8.4 |
6.4 |
0.0001 |
| Haemoglobin (g/l) |
129.0 |
131.0 |
126.0 |
0.04 |
| Leucocytes
(109/l) |
7.6 |
7.6 |
7.7 |
0.97 |
| Platelets
(109/l) |
214.5 |
218.0 |
200.0 |
0.80 |
| Total bilirubin
(μmol/l) |
10.0 |
11.0 |
7.5 |
0.07 |
| ASAT
(U/l) |
30.0 |
30.0 |
28.0 |
0.15 |
| ALAT
(U/l) |
28.0 |
27.5 |
28.0 |
0.65 |
| Serum albumin
(g/l) |
30.0 |
31.0 |
25.5 |
0.42 |
| Serum iron
(μmol/l) |
13.3 |
14.0 |
12.6 |
0.06 |
| CRP
(mg/l) |
6.0 |
6.0 |
6.5 |
0.68 |




