Hospital incidence, mortality, and gender disparities in patients treated for type
A aortic dissections in Switzerland – a secondary data analysis of Swiss DRG statistics
DOI: https://doi.org/https://doi.org/10.57187/s.3499
Lorenz Meulia,
Benedikt Reutersberga,
Petar Risteskib,
Omer
Dzemalib,
Alexander
Zimmermanna
a Department
of Vascular Surgery, University Hospital Zurich, Zurich, Switzerland
b Department of
Cardiac Surgery, University Hospital Zurich, Zurich, Switzerland
Summary
AIMS OF THE
STUDY: The incidence of type A aortic dissection (TAAD) has increased in
several countries in recent decades, but epidemiological data for Switzerland
are lacking. Furthermore, there are conflicting data regarding a gender-disparity
with higher type A aortic
dissection mortality in women. This study analysed sex-specific
hospital incidence and in-hospital mortality rates of TAAD in Switzerland.
METHODS: This
study is a secondary data analysis of case-related hospital discharge data from
the Swiss Federal Statistical Office for 2009–2018. Cases that were hospitalised
and surgically treated for type A aortic dissection were included in this
analysis. Standardised incidence rates were calculated using the European
standard population in 2013. All-cause in-hospital mortality rates were
calculated as raw values and standardised for age, sex, and the van Walraven
comorbidity score.
RESULTS: A
total of 2117 participants were included in this study, of whom 67.1% were
male. The age-standardised cumulative hospital incidence for type A aortic
dissection treatment was 3.5 per 100,000 (95% CI: 3.3–3.7) for men and 1.7 (1.6–1.8)
per 100,000 for women (p <0.001). The
incidence rates increased in both sexes during the observed decade. The adjusted mortality
rates for treatment of TAAD
decreased from 27.6% (26.7–28.5%) in 2009 to 18.5% (17.9–19.1%) in 2018
in women, and they decreased from 19.0% (18.4–19.6%) to 12.3% (11.9–12.7%) in the
same period in men. Multivariable logistic regression analysis
revealed that female sex was significantly associated with higher mortality,
with an odds ratio of 1.39 (1.07–1.79) (p = 0.012).
CONCLUSIONS:
Hospital incidence rates for the treatment of type A
aortic dissection increased in both sexes over the observed decade. The
mortality rate was significantly higher in women than it was in men, but it decreased
in both sexes. TAAD remains a cardiovascular emergency with a high mortality
rate even after emergency surgery.
Introduction
Aortic
dissection is a life-threatening condition caused by an intimal flap separating
the aortic lumen into a true lumen and a false lumen. This can cause
malperfusion to aortic branches as the dissection extends. The original
Stanford classification from 1970 distinguishes type A aortic dissection (TAAD),
which involves the ascending aorta, from type B aortic dissection (TBAD), which
involves the aorta distal to the left subclavian artery [1]. TAAD and TBAD can distally
extend to the iliac
and sometimes the femoral arteries. Aortic dissections of the arch without the
involvement of the ascending aorta were not reflected by this classification.
The European Association for Cardio-Thoracic Surgery and the European Society
for Vascular Surgery classifies these pathologies as "non-A-non-B
dissections" [2].
While type
B aortic dissection can often be treated conservatively by blood pressure
control and radiological monitoring of the dissection, type A aortic dissection
typically requires urgent cardiac surgery. This is because the dissection can
lead to aortic rupture, coronary artery malperfusion, acute aortic valve
regurgitation, cardiac tamponade, and stroke [3]. Surgery for type A aortic dissection
aims to
replace the ascending aorta with a synthetic graft and thereby seal the
dissection membrane to restore normal blood flow and prevent rupture. This is
typically performed via sternotomy with extracorporeal circulation and
hypothermic circulatory arrest to perform an open distal anastomosis. Techniques
differ depending on the extent of dissection and the surgeon’s expertise.
Although these procedures carry a risk of stroke and bleeding, they are highly
effective in preventing death from type A aortic dissection [3].
A secondary
analysis of diagnosis-related group (DRG) data on the epidemiology of aortic
dissections in Germany revealed relevant information that might help further
improve treatment quality [4]. Such
longitudinal, nationwide epidemiological data on type A aortic dissection are
lacking for Switzerland. Therefore, this study analysed the hospital incidence,
treatment details, and all-cause in-hospital mortality of the treatment of type
A aortic dissection in Switzerland between 2009 and 2018 using case-related
hospital discharge data from the Swiss Federal Statistical Office (SFSO) to gain
a better understanding of this pathology in Switzerland.
Materials and
methods
This was a
secondary data analysis of case-related hospital discharge data from the Swiss
Federal Statistical Office. The detailed methodological approach for
using this data was described in a previous publication on abdominal aortic
aneurysms [5]. In brief, every Swiss facility that treats
inpatients is required to report all hospital admissions to the SFSO on an
annual basis. Among others, variables include age; sex; up to 50 diagnosis
codes; up to 100 procedure codes; information on discharge, including all-cause
in-hospital mortality; information on the location before admission and type of
admission; information on the insurance class, time to treatment, total length
of stay, and length of stay in the intensive care unit (ICU); and duration of
ventilation. Diagnoses were recorded according to the 10th revision
of the International Classification of Diseases (ICD-10), and procedures were recorded
according to the Swiss classification of surgical interventions (CHOP). The
current CHOP code list is available online at https://bit.ly/3zYViv6 (last access on 22.11.2023).
Because of personal
data protection regulations, no unique identifiers were available for patients,
and the exact institution numbers were encoded. Thus, the data do not allow the
identification of patients with reinterventions during new hospital admissions.
The
analysis of this anonymised dataset did not require ethical approval (waived by
the local ethics board: BASEC-Nr. Req-2021-01010). This study is reported in
accordance with the STrengthening the Reporting of OBservational studies in
Epidemiology (STROBE) statement [6]. For legal reasons, the
data are not publicly available, but they can be requested directly from the SFSO.
Inclusion and
exclusion criteria
The ICD-10
distinguishes between thoracic, thoraco-abdominal, and abdominal aortic
dissections. Furthermore, for each anatomical region, it differentiates between
ruptured and non-ruptured dissections; thus, the Stanford classification is not
directly reflected in the ICD-10 codes. Therefore, only cases with a
combination of a corresponding ICD-10 diagnosis code, a CHOP procedure code for
aortic replacement, and a CHOP procedure code for extracorporeal circulation
were included.
The ICD-10
codes used were I71.00, I71.01, I71.03, I71.04, I71.05, and I71.07, including
all ruptured and non-ruptured thoracic- or thoraco-abdominal aortic dissections.
The CHOP codes for resection of the thoracic aorta with replacement were
38.45.00, 38.45.10-14, 38.45.19, 38.45.20, and 38.45.29. Of note, the CHOP code for
the use of a hybrid prosthesis
(38.45.14) has only been available since 2011. A hybrid procedure generally
means a surgical graft replacement of the aortic arch with stent-grafting of
the descending thoracic aorta using a covered stent, known as the "frozen elephant
trunk" procedure [7]. The stent graft in the
descending aorta serves as a landing zone for subsequent endovascular graft
placements if the thoraco-abdominal aorta requires further treatment. The CHOP codes
for extracorporeal
circulation were 39.61.00, 39.61.1, 39.61.10-15, 39.61.2, 39.61.21-26, and
39.61.99. The details of the CHOP codes and ICD codes used are available in the
supplementary material (see appendix 1).
Statistical analysis
Baseline
characteristics and treatment outcomes were stratified by sex. For descriptive
analyses, the mean and standard deviation (SD) are reported for continuous
variables with approximately normal distributions. For variables with skewed
distributions, the median and quartiles (Q1, Q3) are reported. Continuous variables
were compared with Student’s t-test if they were normally
distributed or the Mann-Whitney U test if they had a skewed distribution. For
categorical variables, frequencies and percentages are presented. The variables
were compared using Pearson’s Chi2 test.
To allow
international comparison of the epidemiological data, age-standardised all-cause
in-hospital mortality rates were calculated. Age-standardised mortality rates
are weighted averages of the age-specific mortality rates per 100,000 persons,
where the weight is the proportion of persons in each age group of the standard
population. The standard European population from 2013 with 5-year bins was
used as a reference to calculate age-standardised cumulative incidence rates
for the Swiss population using SFSO data [8–10]. Because the age distribution of the
2009 Swiss
population was not available, the 2010 age distribution was used for 2009. Logit
Wald 95% confidence intervals (95% CIs) for the directly age-standardised
estimates were adjusted as suggested by Altman et al. but on the logit scale [11].
Sex-specific all-cause in-hospital mortality
rates were calculated as raw rates and standardised for age, year of treatment,
and a sum score of weighted Elixhauser ICD-10 diagnosis groups according to van
Walraven [12].
To analyse
the association between sex and all-cause in-hospital mortality, a
multivariable logistic regression model was built for the overall cohort. The
variables sex (factor), age (continuous), type of admission (factor), van
Walraven comorbidity score (continuous), insurance class (factor), hospital
level (factor), and year of treatment (factor) were included in this model to
adjust for potential confounding. Regression coefficients were presented as
odds ratios (ORs) with corresponding 95% CIs.
No
statistical methods to handle missing data were needed because no missing
values were present in any variables of the primary analyses. All analyses were
performed with R version 4.2.3 on macOS 12.5.1 [13]. A complete list of all R
packages used with version details is available in the supplementary material (see
appendix 2). The R code can be shared upon request. All p-values
were two-sided with an α-level of 5%.
Results
From
01.01.2009 to 31.12.2018, 10,020 individuals were hospitalised with ruptured or
non-ruptured thoracic or thoraco-abdominal aortic dissections as the primary or
secondary diagnosis. Cases without surgical treatment of the aortic dissection involving
the use of extracorporeal circulation were excluded (n = 7903). A total of 2117
individuals were surgically treated for aortic dissection with the use of extracorporeal
circulation and included in this study (figure 1).

Figure 1Patient flow. The total dataset contained all hospitalisations in the Swiss population
from 2009 to 2018. ICD
= International Classification of Diseases (version 10); CHOP = Swiss medical procedural
classification; ECC = extracorporeal circulation; TAAD = type A aortic dissection.
Table 1
summarises the baseline characteristics of the study cohort stratified by sex. Males
accounted for 67.1% of patients, and the overall median age was 65 years (Q1 to Q3:
55–73). Females were significantly older than males, with a median age of 71 (61–77)
years versus 62 (53–71) years, p <0.001. Females had significantly
more diagnoses of cerebrovascular disease and arterial hypertension (p = 0.010).
The other comorbidities were similar between men and women. The van Walraven
Score steadily increased from 2009 to 2018 in both
sexes (figure 2).
Table 1Baseline characteristics. Continuous variables
are presented as medians and quartiles (Q1 to Q3). Counts are presented with
percentages in parentheses. Definitions of the comorbidities are available in
the supplementary material (appendix).
|
Male |
Female |
Total |
|
| Variable |
n = 1420 |
n = 697 |
n = 2117 |
p-Value |
| Age, years |
62 (53–71) |
71 (61–77) |
65 (55–73) |
<0.001 |
| van Walraven score |
13 (3–19) |
13 (3–20) |
13 (3–19) |
0.257 |
| Coronary artery disease |
318 (22.4) |
142 (20.4) |
460 (21.7) |
0.289 |
| Chronic heart failure |
210 (14.8) |
125 (17.9) |
335 (15.8) |
0.062 |
| Cerebrovascular disease |
268 (18.9) |
165 (23.7) |
433 (20.5) |
0.010 |
| Arterial hypertension |
712 (50.1) |
391 (56.1) |
1103 (52.1) |
0.010 |
| Chronic pulmonary disease |
126 (8.9) |
77 (11.0) |
203 (9.6) |
0.110 |
| Diabetes mellitus |
62 (4.4) |
42 (6.0) |
104 (4.9) |
0.097 |
| Chronic kidney disease |
211 (14.9) |
96 (13.8) |
307 (14.5) |
0.505 |
| Cancer |
13 (0.9) |
7 (1.0) |
20 (0.9) |
0.843 |
| Obesity |
52 (3.7) |
29 (4.2) |
81 (3.8) |
0.574 |
| Connective tissue disease |
22 (1.5) |
18 (2.6) |
40 (1.9) |
0.101 |
| Location before admission |
|
|
|
0.898 |
| – Acute care hospital |
694 (48.9) |
332 (47.6) |
1026 (48.5) |
|
| – Home |
688 (48.5) |
344 (49.4) |
1032 (48.7) |
|
| – Nursing home |
5 (0.4) |
2 (0.3) |
7 (0.3) |
|
| – Other |
33 (2.3) |
19 (2.7) |
52 (2.5) |
|
| Year |
|
|
|
0.995 |
| – 2009 |
108 (7.6) |
52 (7.5) |
160 (7.6) |
|
| – 2010 |
120 (8.5) |
60 (8.6) |
180 (8.5) |
|
| – 2011 |
120 (8.5) |
57 (8.2) |
177 (8.4) |
|
| – 2012 |
138 (9.7) |
64 (9.2) |
202 (9.5) |
|
| – 2013 |
150 (10.6) |
83 (11.9) |
233 (11.0) |
|
| – 2014 |
158 (11.1) |
79 (11.3) |
237 (11.2) |
|
| – 2015 |
150 (10.6) |
68 (9.8) |
218 (10.3) |
|
| – 2016 |
151 (10.6) |
80 (11.5) |
231 (10.9) |
|
| – 2017 |
157 (11.1) |
74 (10.6) |
231 (10.9) |
|
| – 2018 |
168 (11.8) |
80 (11.5) |
248 (11.7) |
|

Figure 2Comorbidities (van Walraven score). Comorbidities are summarised using a
sum score of weighted Elixhauser ICD-10 diagnosis groups according to van
Walraven [12]. The score
is calculated from grouped ICD-10 diagnoses per patient and weighted on the basis
of the association of each category and mortality. The boxplots are stratified
by sex. The whisker extends from the hinge to the largest and the smallest
value, no greater than 1.5 × the inter-quartile range.
Outlying points are not plotted to increase readability.
Epidemiology
The overall
age-standardised hospital incidence rates for type A aortic dissection treatment
was 3.5 per 100,000 (95% CI: 3.3–3.7) in men and 1.7 per 100,000 (1.6–1.8) in women
(p <0.001). The incidence rates increased in both sexes
during the observed decade; for men, the incidence increased from 2.9 (2.4–3.5) per
100,000 in 2009 to 3.9 (3.4–4.6) per 100,000 in 2018, and for women,
the incidence increased from 1.4 (1.1–1.9) per 100,000 in 2009 to 2.0 (1.6–2.4) per
100,000 in 2018 (figure 3).

Figure 3Incidence of type A aortic dissection. Age-standardised incidence rates
stratified by sex and presented with 95% confidence intervals for each year.
Treatment specifications
Table 2
summarises the treatment specifications of the study cohort stratified by sex. The
vast majority of the 2117
cases with type A aortic dissection were treated at major hospitals. These
included university hospitals (76.6%) and major non-university hospitals
(17.1%). The remaining 6.3% of cases (133 patients) were treated at small
hospitals, including specialty clinics. In 38 of the
2117 cases (1.8%), a hybrid procedure was performed to treat the TAAD.
Table 2Treatment specifications.
Continuous variables are presented by median and quartiles (Q1 to Q3). Counts are
presented with percentages in parentheses.
|
Male |
Female |
Total |
|
| Variable |
n = 1420 |
n = 697 |
n = 2117 |
p-Value |
| Type of hospital |
|
|
|
0.356 |
| – University hospital |
1096 (77.2) |
525 (75.3) |
1621 (76.6) |
|
| – Major hospital |
232 (16.3) |
131 (18.8) |
363 (17.2) |
|
| – Other |
92 (6.5) |
41 (5.9) |
133 (6.3) |
|
| Treatment |
|
|
|
0.116 |
| – Open repair |
1390 (97.9) |
689 (98.9) |
2079 (98.2) |
|
| – Hybrid repair |
30 (2.1) |
8 (1.1) |
38 (1.8) |
|
| Packed red blood cells |
|
|
|
<0.001 |
| – 0 |
602 (42.4) |
212 (30.4) |
814 (38.5) |
|
| – 1–5 |
433 (30.5) |
252 (36.2) |
685 (32.4) |
|
| – >5 |
385 (27.1) |
233 (33.4) |
618 (29.2) |
|
| Fresh frozen plasma |
|
|
|
0.445 |
| – 0 |
1222 (86.1) |
613 (87.9) |
1835 (86.7) |
|
| – 1–5 |
103 (7.3) |
46 (6.6) |
149 (7.0) |
|
| – >5 |
95 (6.7) |
38 (5.5) |
133 (6.3) |
|
| Platelet transfusion |
|
|
|
0.539 |
| – 0 |
1291 (90.9) |
643 (92.3) |
1934 (91.4) |
|
| – 1–5 |
104 (7.3) |
42 (6.0) |
146 (6.9) |
|
| – >5 |
25 (1.8) |
12 (1.7) |
37 (1.7) |
|
| Length of stay ICU, h |
64.5 (27–157) |
78 (32–165) |
68 (29–158) |
0.143 |
| Length of stay, d |
12 (9–19) |
13 (9–20) |
12 (9–19) |
0.654 |
Females
required red blood cell transfusions more frequently than males (p <0.001). The
total length of stay in the ICU was 68 hours (29–158), whereas the
total length of hospital stay was 12 days (9–19). Both durations were
similar in men and women. However, 16.7% of all patients were transferred to
another acute care hospital after surgical treatment. The data reflect only the
time until discharge from the hospital where the surgical treatment was
performed, and therefore, they do not reflect the total length of stay until
discharge from any subsequent inpatient treatments.
Treatment outcomes
The
raw all-cause
in-hospital mortality rate for surgically treated type
A aortic dissection was 15.2% (95% CI: 13.7–16.8%). The raw all-cause
in-hospital mortality rates were lower in men (13.0% [11.4–14.9%]) than in women (19.7%
[16.9–22.8%]), as shown
in table 3.
The
adjusted all-cause
in-hospital mortality rates for type A aortic
dissection treatment decreased from 19.0% (95% CI: 18.4–19.6%) in 2009 to
12.3% (11.9–12.7%) in 2018 in men and from 27.6% (26.7–28.5%) in 2009 to
18.5% (17.9–19.1%) in 2018 in women, see figure 4. These decreases were not statistically
significant for men (p = 0.082) or women (p = 0.081). However, mortality was
significantly lower in 2014–2016 compared with the reference year 2009. These
findings are also reflected in figure 4, in which the smoothed adjusted
survival curves for both sexes drop slightly in these years.
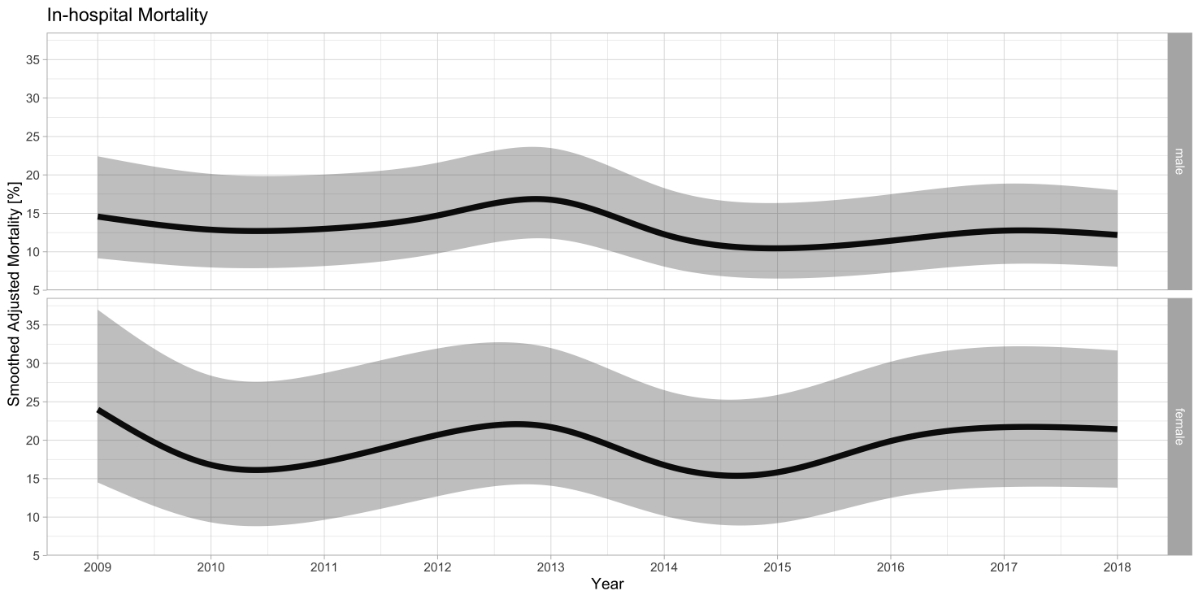
Figure 4Smoothed and adjusted all-cause in-hospital mortality of patients treated for type
A aortic dissection. Smoothed
adjusted all-cause in-hospital mortality rates of patients surgically treated
for type A aortic dissection stratified by sex with the corresponding 95%
confidence interval. The mortality rates were adjusted for age, year of
treatment, and the Elixhauser comorbidity score.
Figure
5 presents the results of the regression analysis of all-cause in-hospital mortality.
Female sex was significantly associated with higher mortality (OR: 1.39, 95% CI:
1.07–1.79, p = 0.012). Transfer from another acute care hospital was
associated with a decreased mortality (OR: 0.72 [0.56–0.93], p = 0.012).
Likewise, private insurance class was associated with decreased mortality (OR:
0.60 [0.43–0.81], p = 0.001.
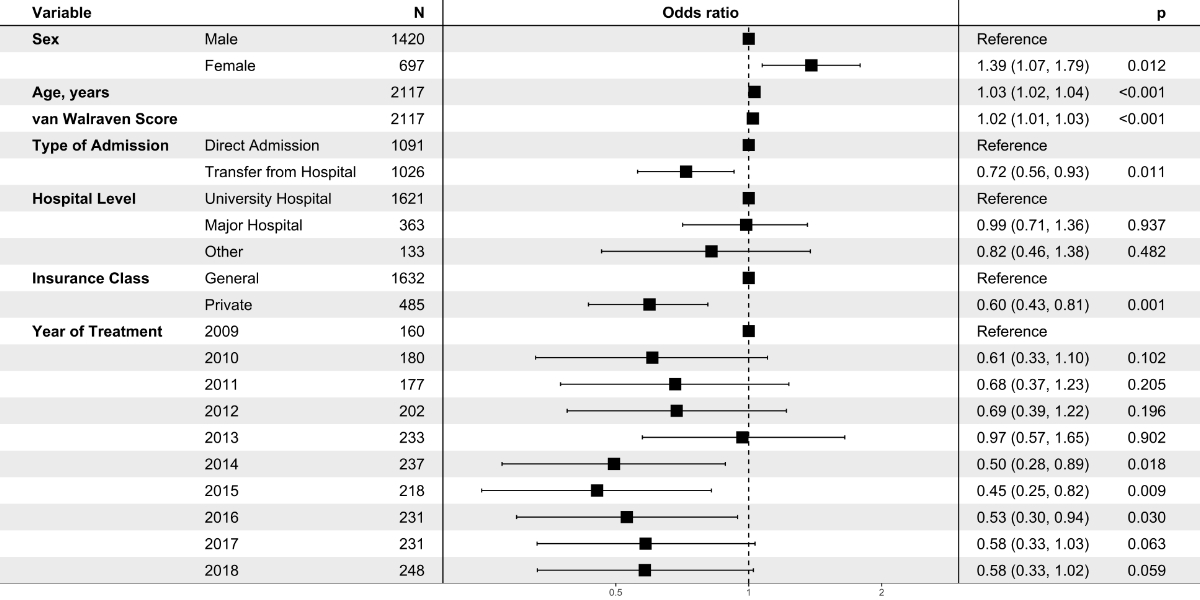
Figure 5Multivariable logistic regression model of
all-cause in-hospital mortality. A total of 2117 cases with type A aortic
dissection and 322 hospital deaths were recorded in the Swiss Federal
Statistical Office between 2009 and 2018. Data are presented as odds ratios
with corresponding 95% confidence intervals. For continuous variables (i.e.,
age in years and van Walraven score), odds ratios are given per one-unit increase
in the variable.
Table
3 shows other relevant diagnoses during the hospital stay, including myocardial
infarction, stroke, paraplegia, acute mesenteric ischemia, renal failure, and
limb ischemia. The rates of these complications did not differ between men and
women.
Table 3Hospital treatment outcomes. These are
unadjusted raw counts presented with percentages in parentheses. All
comparisons are descriptive and unadjusted. The variable "destination after discharge"
does not include
deceased patients; the percentage was calculated for the overall cohort.
|
Male |
Female |
Total |
|
| Variable |
n = 1420 |
n = 697 |
n = 2117 |
p-Value |
| Myocardial infarction |
64 (4.5%) |
33 (4.7%) |
97 (4.6%) |
0.814 |
| Acute stroke |
50 (3.5%) |
37 (5.3%) |
87 (4.1%) |
0.052 |
| Acute paraplegia |
34 (2.4%) |
13 (1.9%) |
47 (2.2%) |
0.437 |
| Acute mesenteric ischemia |
61 (4.3%) |
33 (4.7%) |
94 (4.4%) |
0.645 |
| Large bowel resection |
14 (1.0%) |
13 (1.9%) |
27 (1.3%) |
0.090 |
| Small bowel resection |
10 (0.7%) |
7 (1.0%) |
17 (0.8%) |
0.467 |
| Acute renal failure |
55 (3.9%) |
24 (3.4%) |
79 (3.7%) |
0.624 |
| Dialysis (cvvHD) |
147 (10.4%) |
83 (11.9%) |
230 (10.9%) |
0.280 |
| Acute limb ischemia |
76 (5.4%) |
45 (6.5%) |
121 (5.7%) |
0.304 |
| Lower leg fasciotomy |
25 (1.8%) |
6 (0.9%) |
31 (1.5%) |
0.105 |
| Major amputation |
2 (0.1%) |
0 (0.0%) |
2 (0.1%) |
0.322 |
| Destination after discharge |
|
|
|
<0.001 |
| – Home |
267 (18.8%) |
82 (11.8%) |
349 (16.5%) |
|
| – Nursing home |
6 (0.4%) |
6 (0.9%) |
12 (0.6%) |
|
| – Other |
21 (1.5%) |
7 (1.0%) |
28 (1.3%) |
|
| – Rehabilitation |
711 (50.1%) |
341 (48.9%) |
1052 (49.7%) |
|
| – Acute care hospital |
230 (16.2%) |
124 (17.8%) |
354 (16.7%) |
|
| All-cause raw in-hospital mortality |
185 (13.0%) |
137 (19.7%) |
322 (15.2%) |
<0.001 |
Discussion
This is the
first study to demonstrate the nationwide hospital incidence of type A aortic
dissection treatment in Switzerland. We used case-related hospital discharge
data to estimate the actual incidence and treatment outcomes of patients
treated for type A aortic dissection.
The
epidemiological metrics are comparable to international data [4, 14]. The overall
incidence
of TAAD might be approximately twice as high because the prehospital death rate
is estimated to be approximately 50%, and TAAD has been identified as one of
the leading causes of out-of-hospital death [15, 16]. The excellent prehospital
care in Switzerland, including the broad availability of helicopter emergency
medical services and short travel distances, may result in a lower prehospital
death rate [17].
In
line with previous publications, we observed an increase in the incidence of type
A aortic dissection treatment during the observed decade [4, 15, 18]. This increase
might be attributable
to the demographic change in Switzerland (i.e., the ageing population); the
broader availability and use of computed tomography in emergency departments,
presumably leading to an increased detection rate; and/or a decrease in the
rate of older adults turning down treatment.
Furthermore,
the rate of hybrid procedures (i.e., procedures using a hybrid prosthesis,
specifically the frozen elephant trunk procedure) gradually increased after 2011,
when the first two procedures were coded. Since then, the proportion steadily
increased to 4.4% of cases (11 of 248 cases) in 2018.
Gender-specific outcomes
This study identified
a difference in treatment outcomes between men and women. The multivariable
regression model showed that female sex was associated with a significantly
higher mortality rate (OR: 1.39). An international registry-based study comprising
data from 58 large referral centres in 13 countries also reported a higher
mortality in females [19]. They found higher rates of organ malperfusion, shock, and
altered consciousness for women compared to men. A possible explanation for this
difference is that aortic dissection presents later in women than in men in
Switzerland. Table 3 shows that the rate of malperfusion for several organ
systems (i.e., the brain, heart, intestines, and limbs) was higher in women.
However, none of the observed differences reached statistical significance.
Interestingly, a cohort study conducted at the Sakakibara Heart Institute in
Japan reported that the time from the onset of symptoms to operation was
similar in both sexes, and mortality did not differ between sexes in their
cohort [20].
By
contrast, other studies have shown no differences in survival between sexes: A
recently published study on gender-specific outcomes in type A aortic
dissection analysed German registry data and found no difference in mortality
between men and women [21]. Furthermore, a comparable analysis of German DRG statistics
reported
a tendency towards higher all-cause in-hospital mortality in women, but the
difference in mortality between men and women was not significant [4]. Neither of
these studies reported the time from the onset of symptoms to
operation. Thus, it remains unclear why the adjusted mortality rates were
higher in women than in men in Switzerland.
In line
with all the studies discussed above, women were, on average, significantly
older than men at the time of type A aortic dissection [4, 19, 21]. In our study,
the age difference was 9 years.
This was included in the regression model, but further adjustments for
functional capacity parameters were not possible with these administrative
data. In addition to possible confounding from unmeasured variables such as
time-delay for treatment, gender-specific factors might lead to higher
mortality.
Gender-specific
differences in treatment outcomes have been described for the treatment of
ruptured abdominal aortic aneurysms [5, 22]. A systematic review in this field showed
that
women treated for abdominal aortic aneurysms had higher rates of transfusion
and pulmonary and bowel complications compared with men [22]. The authors concluded
that these factors might
be causes for the observed outcome disparities and could thus be targets for
quality improvement. In the present study, women had significantly higher
transfusion rates than men. This could be due to higher peri-interventional
blood loss or more post-procedural bleeding complications. Further exploratory
studies are needed to confirm and elucidate these findings.
Mortality
Mortality
rates tended to decline in the observed decade, but type A aortic dissection
remains a fatal cardiovascular emergency. In addition to the discussed
association between gender and all-cause in-hospital mortality, age, van
Walraven comorbidity Score, interhospital transfer, and insurance class were
significantly associated with mortality in the multivariable regression model. For
each decade of increasing age, the probability of in-hospital mortality
increased (OR: 1.34). This observed association seems plausible and has been
previously reported [4].
The
association between comorbidity scores obtained from administrative data (i.e.,
the Elixhauser score or the van Walraven score) and all-cause in-hospital
mortality has been reported previously and was confirmed in this study. The
inclusion of this score is an attempt to adjust for comorbidities. However, in
several populations, these scores increased over time; this may be due to an
increase in documentation and coding of comorbidities rather than an actual
increase in comorbidities [4, 5, 23, 24]. Thus, its value as an estimation of the
disease burden is questionable.
The
regression analysis also revealed an association between the type of admission
and all-cause in-hospital mortality. Patients transferred from other acute care
hospitals had lower mortality than patients who directly presented at the
treating institution. A similar picture was recently presented for the
treatment of ruptured abdominal aortic aneurysm in Switzerland [25]. Several factors
must be considered when
interpreting these findings. First, the turn-down rate might be different in
hospitals that do not surgically treat type A aortic dissection compared with
those that do. This might lead to systematic differences between referred
patients and patients who were directly admitted and treated. Second, the
transfer of patients with cardiovascular emergencies between hospitals could
lead to an increase in mortality and a selection of patients who are more haemodynamically
stable and actually reach the hospital for surgical treatment [26]. In Switzerland,
the transfer of patients with
cardiovascular emergencies between hospitals is generally fast, and no deaths
were observed in an analysis of data from the largest helicopter emergency
medical service provider [17]. However, data on haemodynamic parameters are
not available in these datasets, nor is information on deaths during
interhospital transfers.
The
regression analysis also showed that private insurance was associated with a
lower mortality rate than nonprivate insurance (OR: 0.60). The
authors hypothesise that insurance class is a surrogate parameter for better
socioeconomic status and thus a better risk profile rather than an indicator of
different treatment for this cohort. Similar results have been shown for abdominal
aortic aneurysm treatment in Switzerland [25]. Another explanation for this finding
could be
the fact that patients with relevant comorbidities are less likely to be
accepted by private insurance. However, none of these interpretations can be
verified.
Limitations
This
analysis has several limitations. The data are not clinical but administrative.
The main strengths and limitations of statutorily collected routine data are
well-known [23]. For aortic dissections, the ICD coding does
not directly allow differentiation between type A and type B Stanford
dissections. The combination of ICD coding and procedural coding allows the
identification of cases surgically treated with type A aortic dissection.
Nevertheless, the proportion of palliative treatment of type A aortic
dissection cannot be estimated, as these cases cannot be distinguished from
conservatively treated type B aortic dissection. In addition, some cases with
complicated type B aortic dissection in this cohort may have been surgically
treated with extracorporeal circulation.
For this
specific analysis, the main advantage compared with registry data is the
presumably complete coverage of the Swiss population. The main limitations are
as follows:
First, the
risk of bias due to coding errors cannot be ruled out. For hard outcomes, such
as all-cause in-hospital mortality, the risk is presumably low.
Second, the
data allow non-independent observations of patients treated twice for the same
ICD code, which leads to double counting and distorts the incidence rates. The
proportion of patients remains unknown but is likely to be negligible.
Third,
comparison of mortality rates with international data is limited because adjustments
were only possible for age, gender, and the van Walraven comorbidity index; these
adjustments neither reflect the cardiovascular risk profile nor the functional
capacity of the treated individuals.
Fourth, the
data do not allow a distinction to be made between persons with permanent
residence in Switzerland and persons with non-permanent residence (e.g.,
travellers, tourists, etc.). Therefore, the actual incidence rates for permanent
residents of Switzerland may be slightly different.
Implications
for research and practice
This study revealed
an unexplained gender difference in type A aortic dissection treatment outcomes
in Switzerland, with higher mortality in women. This finding should be further
investigated using clinical data to explore, identify, and ultimately address
possible deficiencies in the treatment of women with type A aortic dissection.
Conclusion
Hospital
incidence rates for the treatment of type A aortic dissection increased in both
sexes over the observed decade. The adjusted all-cause in-hospital mortality
rates decreased in both sexes, but the mortality rate was significantly higher
in women than in men. This unexplained gender difference in type A aortic
dissection treatment outcomes warrants further exploration. Women received more
blood products, possibly indicating an increased risk of bleeding complications
in this population.
Acknowledgments
We thank
Mr. Klaus Steigmiller for his contribution to the statistical analysis of the
dataset.
Author contributions: LM: Study conception and design,
statistical analysis, interpretation of the analysis, writing of the
manuscript, agreed to the publication of this manuscript and to be named as an
author. BR: Study conception and design, interpretation of the analysis, revision
of the manuscript, agreed to the publication of this manuscript and to be named
as an author. PR: Interpretation of the analysis,
revision of the manuscript, agreed to the publication of this manuscript and to
be named as an author. OD:
Interpretation of the
analysis, revision of the manuscript, agreed to the publication of this
manuscript and to be named as an author. AZ: Study conception and design,
interpretation of the analysis, revision of the manuscript, agreed to the
publication of this manuscript and to be named as an author.
Prof. Dr. med. Alexander Zimmermann, MHBA FEBVS
Department of Vascular Surgery
University Hospital Zurich (USZ)
University of Zurich (UZH)
Raemistrasse 100
CH-8091 Zurich
alexander.zimmermann[at]usz.ch
References
1. Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute
aortic dissections. Ann Thorac Surg. 1970 Sep;10(3):237–47. 10.1016/S0003-4975(10)65594-4
2. Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T, et al. Editor’s
Choice - Current Options and Recommendations for the Treatment of Thoracic Aortic
Pathologies Involving the Aortic Arch: An Expert Consensus Document of the European
Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular
Surgery (ESVS). Eur J Vasc Endovasc Surg. 2019 Feb;57(2):165–98. 10.1016/j.ejvs.2018.09.016
3. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al.; The
Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society
of Cardiology (ESC). 2014 ESC Guidelines on the diagnosis and treatment of aortic
diseases: document covering acute and chronic aortic diseases of the thoracic and
abdominal aorta of the adult. Eur Heart J. 2014 Nov;35(41):2873–926. 10.1093/eurheartj/ehu281
4. Reutersberg B, Salvermoser M, Trenner M, Geisbüsch S, Zimmermann A, Eckstein HH, et
al. Hospital Incidence and In-Hospital Mortality of Surgically and Interventionally
Treated Aortic Dissections: Secondary Data Analysis of the Nationwide German Diagnosis-Related
Group Statistics From 2006 to 2014. J Am Heart Assoc. 2019 Apr;8(8):e011402. 10.1161/JAHA.118.011402
5. Meuli L, Menges AL, Steigmiller K, Kuehnl A, Reutersberg B, Held U, et al. Hospital
incidence and mortality of patients treated for abdominal aortic aneurysms in Switzerland
- a secondary analysis of Swiss DRG statistics data. Swiss Med Wkly. 2022 Jun;152(25–26):w30191.
10.4414/SMW.2022.w30191
6. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)
Statement: guidelines for reporting observational studies. Int J Surg. 2014 Dec;12(12):1495–9.
10.1016/j.ijsu.2014.07.013
7. Suto Y, Yasuda K, Shiiya N, Murashita T, Kawasaki M, Imamura M, et al. Stented elephant
trunk procedure for an extensive aneurysm involving distal aortic arch and descending
aorta. J Thorac Cardiovasc Surg. 1996 Nov;112(5):1389–90. 10.1016/S0022-5223(96)70157-5
8. https://www.pxweb.bfs.admin.ch/pxweb/de/px-x-0102010000_101/-/px-x-0102010000_101.px. Federal Statistical Office - Permanent and non-permanent resident population by
institutional units, citizenship (category), sex and age. 2020.
9. Statistik der stationären Betriebe des Gesundheitswesens. Krankenhaustypologie [Internet].
Statistik der stationären Betriebe des Gesundheitswesens. Krankenhaustypologie. 2013.
9 p. (2006). Available from: https://www.bfs.admin.ch/hub/api/dam/assets/16987. 2013.
10. https://www.bfs.admin.ch/bfs/en/home/statistics/population.assetdetail.14367975.html. Federal Statistical Office - Population Data. 2020.
11. Altman D, Machin D, Bryant T, Gardner M. Statistics with Confidence: Confidence Intervals
and Statistical Guidelines, 2nd Edition [Internet]. 2000 [cited 2021 Dec 5]. Available
from: https://eprints.soton.ac.uk/393017/
12. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser
comorbidity measures into a point system for hospital death using administrative data.
Med Care. 2009 Jun;47(6):626–33. 10.1097/MLR.0b013e31819432e5
13. R Core Team. (2013). R: A language and environment for statistical computing. URL
http://www.R-project.org/. n.d. Vienna, Austria: R Foundation for Statistical Computing; 2013.
14. Gudbjartsson T, Ahlsson A, Geirsson A, Gunn J, Hjortdal V, Jeppsson A, et al. Acute
type A aortic dissection - a review. Scand Cardiovasc J. 2020 Feb;54(1):1–13. 10.1080/14017431.2019.1660401
15. Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM; Oxford Vascular
Study. Population-based study of incidence and outcome of acute aortic dissection
and premorbid risk factor control: 10-year results from the Oxford Vascular Study.
Circulation. 2013 May;127(20):2031–7. 10.1161/CIRCULATIONAHA.112.000483
16. Moriwaki Y, Tahara Y, Kosuge T, Suzuki N. Etiology of out-of-hospital cardiac arrest
diagnosed via detailed examinations including perimortem computed tomography. J Emerg
Trauma Shock. 2013 Apr;6(2):87–94. 10.4103/0974-2700.110752
17. Meuli L, Zimmermann A, Menges AL, Tissi M, Becker S, Albrecht R, et al. Helicopter
emergency medical service for time critical interfacility transfers of patients with
cardiovascular emergencies. Scand J Trauma Resusc Emerg Med. 2021 Dec;29(1):168. 10.1186/s13049-021-00981-4
18. Clouse WD, Hallett JW Jr, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, et al. Acute
aortic dissection: population-based incidence compared with degenerative aortic aneurysm
rupture. Mayo Clin Proc. 2004 Feb;79(2):176–80. 10.4065/79.2.176
19. Huckaby LV, Sultan I, Trimarchi S, Leshnower B, Chen EP, Brinster DR, et al. Sex-Based
Aortic Dissection Outcomes From the International Registry of Acute Aortic Dissection.
Ann Thorac Surg. 2022 Feb;113(2):498–505. 10.1016/j.athoracsur.2021.03.100
20. Fukui T, Tabata M, Morita S, Takanashi S. Gender differences in patients undergoing
surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg. 2015 Sep;150(3):581–7.e1.
10.1016/j.jtcvs.2015.06.031
21. Rylski B, Georgieva N, Beyersdorf F, Büsch C, Boening A, Haunschild J, et al.; German
Registry for Acute Aortic Dissection Type A Working Group of the German Society of
Thoracic, Cardiac, and Vascular Surgery. Gender-related differences in patients with
acute aortic dissection type A. J Thorac Cardiovasc Surg. 2021 Aug;162(2):528–535.e1.
10.1016/j.jtcvs.2019.11.039
22. Pouncey AL, David M, Morris RI, Ulug P, Martin G, Bicknell C, et al. Editor’s Choice
- Systematic Review and Meta-Analysis of Sex Specific Differences in Adverse Events
After Open and Endovascular Intact Abdominal Aortic Aneurysm Repair: Consistently
Worse Outcomes for Women. Eur J Vasc Endovasc Surg. 2021 Sep;62(3):367–78. 10.1016/j.ejvs.2021.05.029
23. Trenner M, Eckstein HH, Kallmayer MA, Reutersberg B, Kühnl A. Secondary analysis of
statutorily collected routine data. Gefasschirurgie. 2019 May;24(3):220–7. 10.1007/s00772-019-0524-y
24. Kühnl A, Erk A, Trenner M, Salvermoser M, Schmid V, Eckstein HH. Incidence, Treatment
and Mortality in Patients with Abdominal Aortic Aneurysms. Deutsches Aerzteblatt Online.
2017 Jun 5;
25. Meuli L, Menges AL, Stoklasa K, Steigmiller K, Reutersberg B, Zimmermann A. Inter-hospital
transfer of patients with ruptured abdominal aortic aneurysm in Switzerland. Eur J
Vasc Endovasc Surg. 2022 Dec.
26. Mell MW, Wang NE, Morrison DE, Hernandez-Boussard T. Interfacility transfer and mortality
for patients with ruptured abdominal aortic aneurysm. J Vasc Surg. 2014 Sep;60(3):553–7.
10.1016/j.jvs.2014.02.061
Appendices
Appendix 1: CHOP codes and ICD codes used.
Appendix 2: Complete list of all R
packages used with version details.
The appendices are available for download as separate files at https://doi.org/10.57187/s.3499.




