Haemoglobin thresholds for transfusion: how are we doing in the era of Choosing Wisely?
A retrospective cohort study
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40132
Phyranavy Jeganathan-Udayakumara,
Nicole Tochtermanna,
Thomas Becka,
Maria M. Wertliab*,
Christine Baumgartnera*
a Department of General Internal
Medicine, Inselspital, Bern University Hospital, University of Bern, Bern,
Switzerland
b Department of Internal Medicine, Kantonsspital Baden, Baden,
Switzerland
* These authors contributed equally as co-last authors to this manuscript
Summary
INTRODUCTION: Clinical practice guidelines
and the Choosing Wisely initiative launched in 2012 recommend a haemoglobin (Hb)
threshold of 70–80 g/lfor red blood cell (RBC) transfusions in stable hospitalised
patients. Data on transfusion
practices and their trends in medical inpatients are limited. To address
this gap, we investigated transfusion practices and their trends in general internal
medicine
and other clinics.
METHODS: This retrospective
cohort study analysed data from all hospitalisations with RBC transfusions at a
Swiss university hospital between 2012 and 2019. We included all first
transfusion episodes if pretransfusion Hb was available. The primary endpoint
was mean pretransfusion Hb; secondary endpoints included potentially inadequate
transfusions (i.e., transfusions at Hb ≥80 g/l) and receipt of a single RBC unit.
Trends in mean pretransfusion Hb
over time were estimated using generalised estimating equations, and risk
factors for potentially inadequate transfusions were identified using
multivariable adjusted generalised estimating equations models.
RESULTS: Of 14,598 hospitalisations with RBC transfusions,
1980 (13.6%) were discharged from general internal medicine. From 2012 to 2019,
mean pretransfusion Hb decreased from 74.0 g/l to 68.8 g/l in general internal medicine
(mean annual decrease –0.76 g/l, 95% confidence interval
[CI] –0.51 to –1.02) and from 78.2 g/l to 72.7 g/l in other clinics (mean annual decrease
–0.69, 95% CI –0.62
to –0.77; p for interaction 0.53). The overall proportion of potentially inadequate
transfusions was 17.8%
in general internal medicine and 24.1% in other clinics (p <0.001) and decreased over
the study
period from 26.9% to 5.5% in general internal medicine and from 37.0% to 15.2% in
other clinics. In
contrast, the proportion of cases receiving a single RBC unit increased (39.5%
to 81.4% in general internal medicine, 42.7% to 66.1% in other clinics). Older age
(adjusted odds
ratio [aOR] 1.45, 95% CI 1.32–1.58 for ≥65 vs <65 years), having surgery (aOR 1.24,
95% CI 1.14–1.36), acute haemorrhage (aOR 1.16,
95% CI 1.02–1.33), chronic heart failure (aOR 1.17, 95% CI 1.04–1.32), ischaemic
heart diseases (aOR 1.27, 95% CI 1.15–1.41), chronic pulmonary diseases (aOR
1.24, 95% CI 1.08–1.42), malignancy (aOR 1.11, 95% CI 1.01–1.21), and rheumatic
disease (aOR 1.27, 95% CI 1.01–1.59) were risk factors for potentially
inadequate transfusions.
CONCLUSIONS: More restrictive transfusion
practices were adopted in general internal medicine and other clinics over time,
suggesting that guideline recommendations and the Choosing Wisely initiative may
have been increasingly followed. Interventions to reduce potentially inadequate transfusions
should target providers who care for older patients and those with surgery or chronic
cardiac and pulmonary diseases.
Introduction
Red blood cell (RBC) transfusions are a common and potentially life-saving procedure
to treat symptomatic anaemia or haemorrhage [1, 2]. Worldwide, 120 million units of
blood are donated annually, with differing transfusion practices across regions [3].
Even though transfusion-related infection rates have decreased in past decades [4],
a risk remains for adverse events, such as transfusion-associated circulatory overload
or haemolytic transfusion reactions [4, 5]. Moreover, RBC transfusions are related
to substantial costs, varying internationally from $500 to $1200 US per transfused
unit [6, 7]. Therefore, providers should assess the risks and benefits before performing
RBC transfusions.
Besides clinical evidence for acute bleeding, the absolute haemoglobin (Hb) value
is the main trigger for RBC transfusions. Various studies have shown that a transfusion
threshold of Hb <70 g/l (restrictive transfusion strategy) is not related to an increase
in mortality and leads to lower use of transfusions compared with a threshold of Hb
<100 g/l (liberal transfusion strategy) [8–11]. These findings have resulted in recommendations
for a restrictive transfusion strategy by clinical practice guidelines as well as
the Choosing Wisely® initiative launched in 2012 in the United States [12]: administration of RBC transfusions
in hemodynamically stable, non-bleeding patients is not recommended if Hb is >70 g/l
[1, 13]. Similarly, the Swiss Society for General Internal Medicine launched the Smarter
Medicine initiative in 2014, emphasising that only the minimum amount of RBCs should
be transfused to treat symptomatic anaemia and providers should target a safe Hb level
of 70 g/l in stable non-cardiac patients [14].
Most of the studies investigating transfusion trends in the era of Choosing Wisely® were conducted in intensive care units or surgical settings, and less is known about
transfusion practices in the heterogeneous population of medical inpatients [15–17].
Thus, the overall goal of our study was to analyse transfusion practices and their
trends over time in general internal medicine compared to other clinics.
Materials and methods
Study design and setting
We conducted a retrospective cohort study using data from a high-volume university
hospital in Switzerland, which provides care for 44,000 inpatients annually. Clinical
coverage is provided by post-graduate medical education trainees (i.e., resident physicians)
under the supervision of attending physicians in most units [18]. Therefore, orders
for laboratory tests and procedures, such as RBC transfusions, are made at the discretion
of the attending physicians or their delegates. In 2014, following the publication
of the Choosing Wisely® and the Swiss Smarter Medicine recommendations, the following systematic educational
interventions were implemented in the general internal medicine department: resident
training, smart cards used with computers during ward rounds, and skills training.
Residents and attending training was repeated biannually or in case of adverse events
related to transfusion. These trainings were not consistently conducted in all other
departments.
Due to the nature of the study, it was exempted from ethical approval (Cantonal Ethics
Committee Bern, Req-2020-01226). Anonymised data were used for this study, and informed
consent was not necessary. This retrospective cohort study was not registered in a
trial registry platform, and no study protocol has been published.
Study population
We included
all hospitalisations of adults at the Bern University Hospital who received ≥1 RBC
transfusion between January 2012 and December 2019. Patients <18 years of
age and outpatients were excluded. Transfusions were identified from billing
data with a Swiss Operations Classification (CHOP) code for RBC transfusion
(99.04.xx). CHOP codes were originally based on procedure codes from the
International Classification of Diseases, 9th revision, Clinical
Modification (ICD-9-CM) and modified to code medical procedures in Switzerland [19].
To identify the timing of RBC
transfusions, we restricted the main analysis to patients who (in addition to
the CHOP code) had a medication order with a time stamp for erythrocyte blood
products (ATC code B05AX01) as identified from the electronic medical records. ATC
codes were not available for transfusions administered
in the emergency department, intensive care unit, or operating rooms, so these
transfusions were not captured in our analysis.
Outcomes
The primary
outcome was the transfusion threshold (i.e., the mean pretransfusion Hb value before
the first RBC transfusion episode after hospital admission). Hb values and the
time point of measurement were identified from electronic laboratory records. Secondary
outcomes were the proportion of potentially inadequate transfusions (first defined
as any RBC transfusion at Hb ≥80 g/l, and second using a more restrictive
threshold of Hb ≥70 g/l) during the first transfusion episode of hospitalisation,
the number of RBC units ordered during the first transfusion episode and the
full hospitalisation, and the proportion of cases receiving a single RBC unit
during the first transfusion episode. The first transfusion episode was defined
as the period between the first administration of 1 RBC unit and the next Hb
measurement. Additional secondary outcomes were the posttransfusion Hb value
after the first transfusion and the minimal Hb during hospitalisation.
Covariates
For every
hospitalisation, we collected information on patients’ demographics, year and
type (emergency vs elective) of hospital admission, comorbidities based on
ICD-10 codes, transfer to intermediate or intensive care, length of hospital
stay, and death during hospitalisation. We also calculated the Charlson
comorbidity index, a validated measure to predict mortality in patients with
multiple comorbidities [20]. For every hospitalisation, the
department in charge (defined as the discharge unit) was identified and
assigned to either the general internal medicine or other clinic group. For
patients who were transferred between departments, the department in charge was
defined as the department from which the patient was discharged.
Statistical
analysis
Clinical
characteristics and outcomes of cases discharged from general internal medicine
and other clinics were compared using the Student’s t-test, Wilcoxon rank sum
test, or chi-squared test, as appropriate.
Unadjusted
trends over time in mean pretransfusion Hb values, the proportion of
potentially inadequate transfusions at Hb ≥80 g/l, and the proportion of cases
receiving a single RBC unit were estimated using generalised estimating equations
with an exchangeable correlation structure and robust
standard errors. This analysis accounted for the within-patient correlation because
a case can be hospitalised repeatedly. An interaction term between department (i.e.,
general internal medicine vs other clinics) and year was included to
investigate whether trends differed between departments. Cases without
measurement of Hb before the first transfusion were not considered for the
primary outcome.
To investigate
risk factors for potentially inappropriate transfusions (i.e., transfusions at
Hb ≥80 g/l), we employed a multivariable regression model using generalised
estimating equations (with a binomial distributional family, a logit link, an exchangeable
correlation structure, and robust standard errors) to account for
within-patient correlation. Age, sex, type of admission, discharge clinic (general
internal medicine vs other clinics), surgical procedures, and comorbidities (as
listed in table 1) were included as predictors in the model.
We conducted sensitivity analyses broadly excluding cases to whom higher Hb thresholds
for RBC transfusion may apply: patients with an ICD-10 diagnosis code for acute coronary
syndrome and preexisting cardiovascular disease (defined as a history of myocardial
infarction, peripheral vascular disease, or cerebrovascular disease), hypovolemic
and traumatic shock, or those undergoing surgery [1, 11, 21]. Finally, we conducted
a sensitivity analysis including all cases with a CHOP code for RBC transfusion irrespective
of the presence of an ATC code to identify all hospitalised patients who received
at least one RBC transfusion during the study period. For these cases, we analysed
their minimal Hb during their hospital stay because identification of a pretransfusion
Hb was not possible due to missing information on the timing of the RBC transfusion
in patients without an ATC code.
Given
the small proportion of missing data (i.e., data on the primary
outcome were not available for 180 cases, representing 1.2% of the overall
sample size),
we performed complete case analyses. Two-sided p-values of 0.05 were considered
statistically significant. All statistical analyses were conducted with Stata
statistical software, release 16 (Stata Corporation, College Station, TX, USA).
Results
We included
14,598 hospitalisations with RBC transfusions, representing 10,609 unique
patients (figure S1). Of all cases, 1980 (13.6%) were discharged from general
internal medicine. Characteristics of cases are presented in table 1. The median
age was 66 years (interquartile range [IQR] 55–75 years), and 44.7% were women.
Compared to cases from other clinics, those discharged from general internal medicine
were older (median
age 72 vs 66 years, p <0.001) and more likely to be emergency admissions (76.4%
vs 39.5%, p <0.001), whereas the proportion of intensive care unit admissions
was lower (21.1% vs 28.7%, p <0.001). Patients from general internal medicine tended
to have more comorbidities
but were less likely to have surgical procedures than those from other clinics.
The median length of stay was 12 days and in-hospital death occurred in 760
patients (5.2%) (table 1).
Table 1Characteristics
of cases discharged from general internal medicine or other clinics. Numbers
are presented in n (%) unless indicated otherwise. Characteristics were
compared using the Wilcoxon rank sum test for continuous variables or the
chi-squared test for categorical variables. Values were missing for the
Charlson comorbidity index (n = 861).
| |
All cases |
General internal medicine |
Other clinics |
p-value |
| n = 14,598 |
n = 1980 |
n = 12,618 |
| Age in years, median
(IQR) |
66 (55–75) |
72 (62–81) |
66 (54–74) |
<0.001 |
| Female sex |
6534 (44.7) |
833 (42.1) |
5701 (45.2) |
0.010 |
| Emergency admission |
6496 (44.5) |
1512 (76.4) |
4984 (39.5) |
<0.001 |
| Intensive care unit admission* |
4038 (27.7) |
417 (21.1) |
3621 (28.7) |
<0.001 |
| Surgical procedure |
8756 (60.0) |
634 (32.0) |
8122 (64.4) |
<0.001 |
| Comorbidities |
Acute haemorrhage** |
2318 (15.9) |
483 (24.4) |
1835 (14.5) |
<0.001 |
| Hypovolemic and traumatic shock |
361 (2.5) |
42 (2.1) |
319 (2.5) |
0.28 |
| Coagulation defects*** |
5320 (36.) |
740 (37.4) |
4520 (36.3) |
0.36 |
| Peripheral vascular disease |
2491 (17.1) |
367 (18.5) |
2124 (16.8) |
0.061 |
| Cerebrovascular disease |
845 (5.8) |
185 (9.3) |
660 (5.2) |
<0.001 |
| Ischaemic heart disease |
3353 (23.0) |
524 (26.5) |
2829 (22.4) |
<0.001 |
| Chronic heart failure |
2115 (14.5) |
597 (30.2) |
1518 (12) |
<0.001 |
| Chronic pulmonary disease |
1317 (9.0) |
271 (13.7) |
1046 (8.3) |
<0.001 |
| Dementia |
366 (2.5) |
146 (7.4) |
220 (1.7) |
<0.001 |
| Diabetes mellitus |
3074 (21.1) |
503 (25.4) |
2571 (20.4) |
<0.001 |
| Peptic ulcer disease |
340 (2.3) |
76 (3.8) |
264 (2.1) |
<0.001 |
| Any malignancy |
6088 (41.7) |
613 (31.0) |
5475 (43.4) |
<0.001 |
| Liver disease |
1220 (8.4) |
241 (12.2) |
979 (7.8) |
<0.001 |
| Renal disease |
4390 (30.1) |
850 (42.9) |
3540 (28.1) |
<0.001 |
| Rheumatic disease |
401 (2.8) |
60 (3) |
341 (2.7) |
0.407 |
| Charlson Comorbidity Index,
median (IQR)**** |
5 (3–8) |
6 (4–8) |
5 (3–7) |
<0.001 |
| Length of stay in days, median
(IQR) |
12 (7–21.3) |
10.9 (6.7–19.8) |
12.1 (7–21.8) |
<0.001 |
| In-hospital death |
760 (5.2) |
154 (7.8) |
606 (4.8) |
<0.001 |
The first
transfusion was administered a median of 72.5 hours after admission (IQR 28.1–166
hours), and the median time between Hb measurement and first transfusion was
3.9 hours (IQR 2.1–6.9 hours). Among the 14,418 (98.8%) cases with available
data for Hb prior to the first RBC transfusion, the mean pretransfusion Hb was 74.9
g/l (standard deviation [SD] 9.1 g/l) and was lower in cases from general
internal medicine compared to those from other clinics (72.6 g/l, SD 9.8 g/l vs
75.2 g/l, SD 9.0 g/l, p <0.001), as was the proportion of potentially inadequate
transfusions for both transfusion thresholds (table 2). The proportion of a
first transfusion at Hb ≥80 g/l was 17.8% in general internal medicine and 24.1%
in other clinics (p <0.001) and 65.8% vs 75.9%, respectively, at Hb ≥70 g/l (p
<0.001). A median of 1 RBC unit was ordered during the first transfusion
episode, and 54.2% of all transfused patients received a single RBC unit. The
proportion of patients receiving a single RBC unit was higher in general
internal medicine compared to other clinics (60.9% vs 53.1%, p <0.001) (table
2).
Table 2Primary and
secondary outcomes of cases discharged from general internal medicine and other
clinics. Outcomes
were compared using the Student’s t-test or Wilcoxon rank sum test for continuous
variables or the chi-squared test for categorical variables, as appropriate.
Values were missing for pretransfusion Hb prior to the first transfusion in 180
cases, mean posttransfusion Hb after the first transfusion in 303 cases,
potentially inadequate transfusions at Hb ≥80 g/l and Hb ≥70 g/l in 180 cases,
RBC units ordered during 1st transfusion episode in 311 cases, RBC
units transfused during hospitalisation in 3 cases, and transfusion of a single
RBC unit during the first transfusion episode in 311 cases.
| |
All cases |
General internal medicine |
Other clinics |
p-value |
| n = 14,598 |
n = 1980 |
n = 12,618 |
| Primary outcome |
|
|
|
|
| Pretransfusion Hb (g/l) prior to 1st
transfusion, mean (SD) |
74.9 (9.1) |
72.6 (9.8) |
75.2 (9.0) |
<0.001 |
| Secondary outcomes |
|
|
|
|
| Posttransfusion Hb (g/l) after 1st
transfusion, mean (SD) |
86.9 (12.5) |
84.5 (13.0) |
87.3 (12.4) |
<0.001 |
| Potentially inadequate transfusions at Hb ≥80
g/l, n (%) |
3358 (23.3) |
347 (17.8) |
3011 (24.1) |
<0.001 |
| Potentially inadequate transfusions at Hb ≥70
g/l, n (%) |
10,743 (74.5) |
1284 (65.8) |
9459 (75.9) |
<0.001 |
| RBC units ordered during 1st
transfusion episode, median (IQR) |
1 (1–2) |
1 (1–2) |
1 (1–2) |
<0.001 |
| RBC units transfused during hospitalisation,
median (IQR) |
2 (1–3) |
2 (1–3) |
2 (1–3) |
<0.001 |
| Cases with transfusion of a single RBC unit
during the first transfusion episode, n (%) |
7742 (54.2) |
1177 (60.9) |
6565 (53.1) |
<0.001 |
| Sensitivity analysis excluding cases in whom
higher Hb thresholds may apply* |
n = 3910 |
n = 812 |
n = 3098 |
|
| Pretransfusion Hb (g/l) prior to 1st
transfusion, mean (SD) |
73.0 (9.6) |
70.7 (10.6) |
73.7 (9.3) |
<0.001 |
| Potentially inadequate transfusions at Hb ≥80
g/l, n (%) |
705 (18.0) |
101 (12.4) |
604 (19.5) |
<0.001 |
| Potentially inadequate transfusions at Hb ≥70
g/l, n (%) |
2581 (66.0) |
566 (57.4) |
2115 (68.3) |
<0.001 |
| Sensitivity analysis including all cases with
a CHOP code for RBC transfusion |
n = 28,150 |
n = 2790 |
n = 25,360 |
|
| Minimal Hb during hospitalisation (g/l), mean
(SD) |
73.2 (10.7) |
69.7 (10.7) |
73.6 (10.6) |
<0.001 |
RBC transfusion
practice over time
Over the
observed period, the mean pretransfusion Hb decreased from 74.0 g/l to 68.8 g/l
in general internal medicine (mean annual decrease –0.76 g/l, 95% confidence
interval [CI] –0.51 to –1.02) and from 78.2 g/l to 72.7 g/l in other clinics
(mean annual decrease –0.69, 95% CI –0.62 to –0.77) (figure 1).

Figure 1Mean haemoglobin value prior to transfusion over
time in cases discharged from general internal medicine compared to those
discharged from other clinics. The Smarter Medicine recommendations endorsing a
more restrictive haemoglobin threshold of 70 g/l in the majority of non-cardiac
patients were published in 2014. Data on haemoglobin prior to the first
transfusion were missing in 180 (1.2%) cases (general internal medicine n = 53,
other clinics n = 127).
The
decrease was similar in general internal medicine and other clinics (p for
interaction = 0.53). Similarly, the number of potentially inadequate transfusions
at Hb ≥ 80 g/l decreased from 26.9% to 5.5% in general internal medicine (mean annual
decrease =
3.0%, 95% CI 2.3–3.8) and from 37.0% to 15.2% in other clinics (mean annual
decrease 3.3%, 95% CI 2.9–3.6) (figure 2 and S2).

Figure 2Potentially
inadequate transfusions over time. Potentially inadequate transfusions were
defined as red blood cell transfusions at haemoglobin (Hb) ≥80 g/l. Data on
potentially inadequate transfusions were missing in 180 (1.2%) cases (general internal
medicine n = 53, other clinics n = 127).
The proportion
of patients who received a single RBC unit during the first transfusion episode
increased from 39.5% to 81.4% among general internal medicine patients (mean
annual increase 5.8%, 95% CI 5.0–6.7) and from 42.7% to 66.1% among patients discharged
from other clinics (mean annual increase 3.6%, 95% CI 3.2–4.1) (figure 3). Similarly,
the proportion of patients receiving a
single RBC unit during the overall hospitalisation increased from 22.2% in 2012
to 39.9% in 2019 (figure S3).

Figure 3Transfusion of
a single red blood cell (RBC) unit during the first transfusion episode over
time. A transfusion episode was defined as the time period between the first
administration of 1 RBC unit and the next haemoglobin measurement. Data on the number
of RBC units transfused during the first transfusion episode were missing in
311 (2.1%) cases (general internal medicine n = 47, other clinics n = 264).
Risk factors for potentially inadequate
transfusions
Several
factors were associated with a higher risk of receiving potentially inadequate
transfusions at Hb ≥80 g/l in multivariable analyses (table 3), including older
age (adjusted odds ratio [aOR] 1.45, 95% CI 1.32–1.58 for ≥65 vs <65 years),
having surgical procedures (aOR 1.24, 95% CI 1.14–1.36), acute haemorrhage (aOR
1.16, 95% CI 1.02–1.33), chronic heart failure (aOR 1.17, 95% CI 1.04–1.32),
ischaemic heart diseases (aOR 1.27, 95% CI 1.15–1.41), chronic pulmonary
diseases (aOR 1.24, 95% CI 1.08–1.42), any malignancy (aOR 1.11, 95% CI 1.01–1.21),
and rheumatic disease (aOR 1.27, 95% CI 1.01–1.59). Conversely, a lower risk of
potentially inadequate transfusions was associated with having a coagulation
disorder (aOR 0.66, 05% CI 0.60–0.72), peripheral vascular disease (aOR 0.73,
95% CI 0.65–0.82), liver disease (aOR 0.66, 95% CI 0.56–0.79), and being discharged
from general internal medicine compared to other clinics (aOR 0.68, 95% CI 0.60–0.78)
(table 3).
Table 3Risk
factors for potentially inadequate transfusions at haemoglobin values of ≥80
g/l.
Of the
study population, 180 cases had missing values on pretransfusion Hb and were excluded
from this analysis. All variables shown
in this table were included in the model.
| |
Multivariable adjusted analysis |
| Characteristic |
Adjusted odds ratio |
95% confidence interval |
p-value |
| Age ≥65 years |
1.45 |
1.32–1.58 |
<0.001 |
| Female sex |
1.02 |
0.93–1.11 |
0.62 |
| Emergency admission |
0.93 |
0.85–1.01 |
<0.08 |
| Discharge from general internal medicine |
0.69 |
0.60–0.78 |
<0.001 |
| Surgical procedure |
1.24 |
1.14–1.36 |
<0.001 |
| Acute haemorrhage |
1.16 |
1.02–1.33 |
0.021 |
| Hypovolemic/traumatic shock |
0.89 |
0.67–1.18 |
0.41 |
| Coagulation disorders |
0.66 |
0.60–0.72 |
<0.001 |
| Peripheral vascular disease |
0.73 |
0.65–0.82 |
<0.001 |
| Cerebrovascular disease |
0.97 |
0.82–1.15 |
0.74 |
| Chronic heart failure |
1.17 |
1.04–1.32 |
0.011 |
| Ischaemic heart disease |
1.27 |
1.15–1.41 |
<0.001 |
| Chronic pulmonary disease |
1.24 |
1.08–1.42 |
0.002 |
| Dementia |
1.21 |
0.95–1.54 |
0.13 |
| Diabetes mellitus |
1.01 |
0.91–1.12 |
0.82 |
| Peptic ulcer disease |
0.92 |
0.69–1.22 |
0.56 |
| Any malignancy |
1.11 |
1.01–1.21 |
0.023 |
| Liver disease |
0.66 |
0.56–0.79 |
<0.001 |
| Renal disease |
0.94 |
0.85–1.03 |
0.20 |
| Rheumatic disease |
1.27 |
1.01–1.59 |
0.038 |
Results from
sensitivity analyses
In a
sensitivity analysis of 3910 cases excluding patients for whom higher Hb
thresholds for RBC transfusion may apply, the mean pretransfusion Hb was lower
overall (73.0 g/l, SD 9.6 g/l) and remained lower in cases from general
internal medicine compared to cases from other clinics (70.7 g/l, SD 10.6 g/l vs
73.7 g/l, SD 9.3 g/l, p <0.001) (table 2). Similarly, potentially inadequate
first transfusions were lower at both thresholds when compared to the original
sample (table 2).
In a second
sensitivity analysis including all 28,150 cases (representing 21,241 unique
patients; characteristics in table S1) who received at least one RBC unit transfusion
during the study period irrespective of the presence of an ATC code and had at
least one Hb measurement available, mean minimal Hb during their hospitalisation
was 73.2 g/l (SD 10.7 g/l) (table 2). The mean minimal Hb was lower in cases discharged
from general internal medicine compared to those from other clinics (69.7 g/l, SD
10.7 vs 73.6 g/l, SD 10.6, p <0.001) (table 2). From 2012 to 2019, the
minimal Hb decreased from 71.5 g/l to 66.9 g/l in general internal medicine (mean
annual decrease
0.67 g/l, 95% CI 0.50–0.85), and from 75.9 g/l to 70.9 g/l in other clinics
(mean annual decrease 0.62 g/l, 95% CI 0.56–0.68) (figure S4).
Discussion
In this
study, we observed an almost linear decrease in the transfusion threshold over 7
years in all departments, which may indicate an increased awareness of the
Choosing Wisely® recommendations and improved
adherence to national and international guidelines across the board. However, the
transfusion threshold and the proportion of potentially inadequate transfusions
in general internal medicine wards remained significantly lower compared to
other clinics over the study period, suggesting an impact of the efforts to systematically
promote the Choosing Wisely® recommendations. Although the
number of potentially inadequate transfusions and the number of transfused RBC
units during the first transfusion episode decreased during the study period,
potentially inadequate RBC transfusions were observed in up to one-fifth of all
cases, particularly in older patients, those with surgery, chronic pulmonary,
or ischaemic heart disease.
Randomised trials found no increase in mortality and morbidity with a restrictive
transfusion threshold (Hb = 70–80 g/l) compared to a more liberal threshold (Hb =
90–100 g/l) [10, 11, 22]. Based on these findings, guidelines from the American Association
of Blood Banks (AABB) and the European Society of Intensive Care Medicine (ESICM)
recommend a restrictive transfusion strategy with Hb thresholds of 70 g/l in stable,
hospitalised patients [1, 23, 24]. Initiatives to reduce low-value care, such as Choosing
Wisely® or the Swiss equivalent Smarter Medicine, endorse not transfusing above an Hb level
of 70–80 g/l in hemodynamically stable, non-bleeding patients without signs of inadequate
tissue oxygenation, and advise administering a single RBC unit as the standard in
such patients [12, 14].
Previous studies have analysed RBC transfusion practices and their trends over time
[25–28]. Similar to our results, the pretransfusion Hb value among 468 patients hospitalised
in 2012 and 2013 in internal medicine wards of a Swiss regional hospital was 73.0
g/l with higher thresholds observed in surgical units [26]. In another small regional
hospital in Switzerland, 63% of 400 RBC transfusions in acutely ill inpatients were
administered at an Hb level <70 g/l and 9.7% at a level >80 g/l in 2016. The investigators
identified several targets to improve patient blood management, including the implementation
of local transfusion guidelines and systematic training of medical trainees regarding
a patient-centred restrictive transfusion policy, especially in the surgery department
[29]. As a result of educational and quality improvement projects, a significant decline
in mean pretransfusion Hb levels over time was observed in a large retrospective cohort
of >60,000 inpatients at Kaiser Permanente Northern California hospitals between 2009
and 2013 [30]. Similar to our study, pretransfusion Hb thresholds were slightly lower
in medical compared to surgical inpatients. Given that this study was conducted in
a private nonprofit healthcare organisation in the United States, the data may not
be directly comparable to ours (e.g., because economic pressure may play a more important
role in private hospitals or patient care is organised differently). A nationwide
study in the United States showed that the proportion of hospitalised patients receiving
at least one RBC transfusion decreased from 2011 to 2014 [27], and the 2020 Swiss
hemovigilance report demonstrated a reduction in the absolute number of RBC transfusions
administered in Switzerland since 2015 [31]. Even though physicians and educators
continue to struggle with how to implement Choosing Wisely® recommendations to reduce low-value care worldwide [32], the overall body of evidence
indicates that a more restrictive RBC transfusion strategy has been slowly adopted
over the last decade in various clinical settings, similar to our institution [30].
This trend may reflect not only the growing awareness of the recommendations and the
solid body of evidence on the safety of restrictive transfusion thresholds but may
also result from the development and spread of dedicated quality improvement initiatives
and patient blood management programmes [33, 34].
Despite the trend towards more restrictive transfusions practices, up to 60% of transfusions
are administered above the recommended Hb level of 70–80 g/l [6, 35, 36]. The substantially
lower proportion of potentially inadequate transfusions in general internal medicine
compared to other clinics in our study may be due to differences in the patient population
(e.g. fewer critically ill patients). However, it may also indicate that systematic
education of the Choosing Wisely® recommendations as performed in our general internal medicine department and peer
behaviour influence clinical practice, although the Choosing Wisely® and Smarter Medicine initiatives may have had only a minor effect on the overall
decrease of the transfusion threshold as also observed in other clinics. Even though
restrictive transfusion thresholds may not be adequate in all clinical situations,
such as major bleeding or acute ischaemic events [1, 21], these exceptions apply to
a minority of patients in our cohort, suggesting that there remains room for improvement
in transfusion behaviour. This is particularly true for transfusion decisions in older
individuals, surgical patients, and those with chronic cardiovascular and pulmonary
disease or malignancy, as these factors predict transfusions at inappropriately high
Hb levels of >80 g/l as found in our study as well as others [6, 15, 37]. These findings
may reflect the uncertainty of physicians regarding recommendations in these subgroups
and particularly for transfusions in the range of Hb levels between 70 g/l and 90
g/l. The Hb threshold for transfusion in patients with preexisting cardiovascular
disease and those undergoing orthopaedic or cardiac surgery as recommended by the
American Association of Blood Banks guidelines is 80 g/l, and thus higher than the
threshold of 70 g/l in hospitalised patients without these characteristics [1]. In
patients with cancer and haematological malignancy, anaemia due to factors such as
bone marrow infiltration, inflammation, or treatment side effects is particularly
common [38]. However, transfusion recommendations do not differ for this particular
population [1, 23, 38], as there is insufficient evidence demonstrating a potential
benefit of a more liberal transfusion strategy for patients with malignancy compared
to those without [11]. Continuing efforts are needed to support practising physicians
by adhering to current guideline recommendations [39]. For example, computerised decision
support using a smartphone app with the provision of evidence-based recommendations
can improve adherence to transfusion guidelines [40]. Other interventions that can
reduce the overuse of RBC transfusions among inpatients include education, combined
with alerts in the electronic health record system [41, 42], and audit and feedback
[43], mostly implemented in multifaceted interventions [44, 45]. In addition, the
impact of current restrictive transfusions policies on patient-centred outcomes and
length of stay should be further investigated.
A strength
of this study was its large sample size and the variety of cases from various
departments. We only analysed the first RBC transfusion episode of each
hospitalisation because
subsequent transfusions thresholds may be influenced by the response to
transfusions in each case. Notably, generalised estimating equations is a population
average model,
and thus the results (e.g., change in mean pretransfusion haemoglobin per year)
can be interpreted across all patients observed. Compared to random effect (or
mixed) models, population average models are less prone to biased estimates
because they do not require untestable assumptions on the underlying
data-generating distribution [46]. However, the study has some
limitations. First, due to the use of retrospective data from electronic health
records, we did not have complete information on the patient’s clinical status.
Thus, we cannot make definite judgements about the appropriateness of the pretransfusion
Hb thresholds in individual cases. Second, we did not have data on the timing
of RBC transfusions and pretransfusion Hb for all cases in our hospital because
data from the emergency department, intensive care unit, or operating rooms are
captured in separate systems. Consequently, our findings are not generalisable to
these settings. Third, we compared patients
discharged from general internal medicine vs other clinics, although this may
not have been the place where the RBC transfusions occurred. However, most
in-hospital transfers occur between either the emergency department, operating
rooms, or the intensive care units and the wards (rather than between wards),
so it is unlikely that a relevant proportion of RBC transfusions investigated
in this study were administered outside of the discharge clinic. Fourth, our
results arise from a single-centred study conducted at a university hospital. Thus,
our findings and conclusions may not represent the transfusion practices from
other regions or smaller non-university hospitals, as differences in the availability
of resources and patient populations among different healthcare settings can
influence transfusion practices. Finally, we did not explore the outcomes of the transfused
patients;
therefore, the effect of transfusions at various Hb thresholds on
patient-relevant endpoints is unknown. However, while transfusion thresholds decreased
over the study period, in-hospital mortality in our institution did not increase.
Conclusion
Pretransfusion Hb thresholds have decreased and the proportion of
patients receiving only one RBC unit during their first transfusion episode has
increased over the last decade in general internal medicine and other clinics in
our institution. Significantly lower Hb thresholds and a lower proportion of potentially
inadequate transfusion in general internal medicine wards compared to other
wards may suggest that continuous education on Choosing Wisely® recommendations influences clinical
practice, although other factors such as economic pressure may play a role. Potentially
inadequate RBC transfusions in up to one-fifth of cases indicate room for
improvement. Risk factors for transfusions at Hb levels of >80 g/l included older
age, surgery, chronic pulmonary and ischaemic heart disease, and malignancy.
Thus, interventions to further improve adherence with transfusion
recommendations should primarily target physicians caring for these patient populations.
While the focus of
this study was primarily on transfusion thresholds and the number of RBC units
transfused, individualised medicine remains important; this is also reflected
by recommendations to consider individual symptoms of anaemia when deciding on
RBC transfusions [14].
Open science
The data
analysed for the current study are not publicly available because this study
was exempted from ethical approval. Data may be shared with researchers for
reasonable scientific purposes on request if an ethical committee has approved the
use. For data access and requests for the
analytical code, external researchers can contact the corresponding author.
Acknowledgments
We thank
Jeremy Koch and Livia V. Grimm for their help with data extraction.
Author contributions: All authors participated in the
research and preparation of the manuscript. Study concept and design: CB, MMW;
Interpretation of study results: PJU, NT, CB, MMW, TB; Drafting of the
manuscript: PJU, CB; Critical revision of the manuscript: PJU, NT, CB, TB, MMW;
Statistical analyses: PJU, CB; Study supervision: CB, MMW.
Christine Baumgartner, MD, MAS
Department of General Internal Medicine
Inselspital / Bern
University Hospital
Freiburgstrasse
CH-3010 Bern
christine.baumgartner[at]insel.ch
References
1. Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical Practice
Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA.
2016 Nov;316(19):2025–35. 10.1001/jama.2016.9185
2. Sharma S, Sharma P, Tyler LN. Transfusion of blood and blood products: indications
and complications. Am Fam Physician. 2011 Mar;83(6):719–24.
3. Blood transfusion safety - World Health Organization. September 21, 2021]; Available
from: https://www.who.int/health-topics/blood-transfusion-safety/
4. Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood.
2008 Oct;112(7):2617–26. 10.1182/blood-2008-07-077370
5. de Bruin S, Scheeren TW, Bakker J, van Bruggen R, Vlaar AP; Cardiovascular Dynamics
Section and Transfusion Guideline Task Force of the ESICM. Transfusion practice in
the non-bleeding critically ill: an international online survey-the TRACE survey.
Crit Care. 2019 Sep;23(1):309. 10.1186/s13054-019-2591-6
6. Soril LJ, Noseworthy TW, Stelfox HT, Zygun DA, Clement FM. A retrospective observational
analysis of red blood cell transfusion practices in stable, non-bleeding adult patients
admitted to nine medical-surgical intensive care units. J Intensive Care. 2019 Apr;7(1):19.
10.1186/s40560-019-0375-3
7. Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based
costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010 Apr;50(4):753–65.
10.1111/j.1537-2995.2009.02518.x
8. Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al. Health care-associated
infection after red blood cell transfusion: a systematic review and meta-analysis.
JAMA. 2014 Apr;311(13):1317–26. 10.1001/jama.2014.2726
9. Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal
transfusion strategy for red blood cell transfusion: systematic review of randomised
trials with meta-analysis and trial sequential analysis. BMJ. 2015 Mar;350 mar24 9:h1354.
10.1136/bmj.h1354
10. Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter,
randomized, controlled clinical trial of transfusion requirements in critical care.
Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials
Group. N Engl J Med. 1999 Feb;340(6):409–17. 10.1056/NEJM199902113400601
11. Carson JL, Stanworth SJ, Dennis JA, Trivella M, Roubinian N, Fergusson DA, et al. Transfusion
thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev. 2021 Dec;12(12):CD002042.
12. Wisely C. American Association of Blood Banks. Five Things Physicians and Patients
Should Question. [December 6, 2022]; Available from https://www.choosingwisely.org/societies/american-association-of-blood-banks/
13. Murphy MF, Wallington TB, Kelsey P, Boulton F, Bruce M, Cohen H, et al.; British Committee
for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the clinical
use of red cell transfusions. Br J Haematol. 2001 Apr;113(1):24–31. 10.1046/j.1365-2141.2001.02701.x
14. Smarter Medicine. Stationäre Allgemeine Innere Medizin. [December 6, 2022]; Available
from https://www.smartermedicine.ch/de/top-5-listen/stationaere-allgemeine-innere-medizin-2016
15. Rahimi-Levene N, Ziv-Baran T, Peer V, Golik A, Kornberg A, Zeidenstein R, et al. Hemoglobin
transfusion trigger in an internal medicine department - A “real world” six year experience.
PLoS One. 2018 Mar;13(3):e0193873. 10.1371/journal.pone.0193873
16. Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al.; FOCUS
Investigators. Liberal or restrictive transfusion in high-risk patients after hip
surgery. N Engl J Med. 2011 Dec;365(26):2453–62. 10.1056/NEJMoa1012452
17. Vincent JL, Jaschinski U, Wittebole X, Lefrant JY, Jakob SM, Almekhlafi GA, et al.;
ICON Investigators. Worldwide audit of blood transfusion practice in critically ill
patients. Crit Care. 2018 Apr;22(1):102. 10.1186/s13054-018-2018-9
18. von Babo M, Chmiel C, Müggler SA, Rakusa J, Schuppli C, Meier P, et al. Transfusion
practice in anemic, non-bleeding patients: cross-sectional survey of physicians working
in general internal medicine teaching hospitals in Switzerland. PLoS One. 2018 Jan;13(1):e0191752.
10.1371/journal.pone.0191752
19. Schweizerische Operationsklassifikation (CHOP), Systematisches Verzeichnis – Version
2020 April 3, 2021]; Available from: https://www.bfs.admin.ch/bfs/de/home/statistiken/kataloge-datenbanken/publikationen.assetdetail.9286150.html
20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic
comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
10.1016/0021-9681(87)90171-8
21. Docherty AB, O’Donnell R, Brunskill S, Trivella M, Doree C, Holst L, et al. Effect
of restrictive versus liberal transfusion strategies on outcomes in patients with
cardiovascular disease in a non-cardiac surgery setting: systematic review and meta-analysis.
BMJ. 2016 Mar;352:i1351. 10.1136/bmj.i1351
22. Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al.; TRISS
Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin
threshold for transfusion in septic shock. N Engl J Med. 2014 Oct;371(15):1381–91.
10.1056/NEJMoa1406617
23. Blood transfusion. NICE guideline. [December 6, 2022]; Available at https://www.nice.org.uk/guidance/ng24/resources/blood-transfusion-pdf-1837331897029
24. Vlaar AP, Oczkowski S, de Bruin S, Wijnberge M, Antonelli M, Aubron C, et al. Transfusion
strategies in non-bleeding critically ill adults: a clinical practice guideline from
the European Society of Intensive Care Medicine. Intensive Care Med. 2020 Apr;46(4):673–96.
10.1007/s00134-019-05884-8
25. Netzer G, Liu X, Harris AD, Edelman BB, Hess JR, Shanholtz C, et al. Transfusion practice
in the intensive care unit: a 10-year analysis. Transfusion. 2010 Oct;50(10):2125–34.
10.1111/j.1537-2995.2010.02721.x
26. Surial B, Burkhart A, Terliesner N, Morgenthaler M, Bächli E. Adherence to transfusion
guidelines: are we prepared for the Smarter Medicine or Choosing Wisely initiative? Swiss
Med Wkly. 2015 Jan;145:w14084. 10.4414/smw.2015.14084
27. Goel R, Chappidi MR, Patel EU, Ness PM, Cushing MM, Frank SM, et al. Trends in Red
Blood Cell, Plasma, and Platelet Transfusions in the United States, 1993-2014. JAMA.
2018 Feb;319(8):825–7. 10.1001/jama.2017.20121
28. Roubinian NH, Murphy EL, Mark DG, Triulzi DJ, Carson JL, Lee C, et al. Long-Term Outcomes
Among Patients Discharged From the Hospital With Moderate Anemia: A Retrospective
Cohort Study. Ann Intern Med. 2019 Jan;170(2):81–9. 10.7326/M17-3253
29. Patient Blood Management am Kleinspital - Swiss Medical Forum. [July 9, 2023]; Available
from https://medicalforum.ch/de/detail/doi/smf.2020.08505
30. Roubinian NH, Escobar GJ, Liu V, Swain BE, Gardner MN, Kipnis P, et al.; NHLBI Recipient
Epidemiology and Donor Evaluation Study (REDS-III). Trends in red blood cell transfusion
and 30-day mortality among hospitalized patients. Transfusion. 2014 Oct;54(10 Pt 2):2678–86.
10.1111/trf.12825
31. Swissmedic, Haemovigilance Jahresbericht 2020, July 2020. [December 6, 2022]; Available
from https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/marktueberwachung/haemovigilance/haemovigilance-publications-events/haemovigilance-report-2020.html
32. Baron RJ, Lynch TJ, Rand K. Lessons From the Choosing Wisely Campaign’s 10 Years of
Addressing Overuse in Health Care. JAMA Health Forum. 2022 Jun;3(6):e221629. 10.1001/jamahealthforum.2022.1629
33. Meybohm P, Richards T, Isbister J, Hofmann A, Shander A, Goodnough LT, et al. Patient
Blood Management Bundles to Facilitate Implementation. Transfus Med Rev. 2017 Jan;31(1):62–71.
10.1016/j.tmrv.2016.05.012
34. Verdecchia NM, Wisniewski MK, Waters JH, Triulzi DJ, Alarcon LH, Yazer MH. Changes
in blood product utilization in a seven-hospital system after the implementation of
a patient blood management program: A 9-year follow-up. Hematology. 2016 Sep;21(8):490–9.
10.1080/10245332.2015.1112496
35. Seitz KP, Sevransky JE, Martin GS, Roback JD, Murphy DJ. Evaluation of RBC Transfusion
Practice in Adult ICUs and the Effect of Restrictive Transfusion Protocols on Routine
Care. Crit Care Med. 2017 Feb;45(2):271–81. 10.1097/CCM.0000000000002077
36. Sadana D, Kummangal B, Moghekar A, Banerjee K, Kaur S, Balasubramanian S, et al. Adherence
to blood product transfusion guidelines-An observational study of the current transfusion
practice in a medical intensive care unit. Transfus Med. 2021 Aug;31(4):227–35. 10.1111/tme.12771
37. Peyrony O, Gamelon D, Brune R, Chauvin A, Ghazali DA, Yordanov Y, et al. Red Blood
Cell Transfusion in the Emergency Department: An Observational Cross-Sectional Multicenter
Study. J Clin Med. 2021 Jun;10(11):2475. 10.3390/jcm10112475
38. Patient blood management in hematology and oncology. [July 9, 2023]; Available at
https://www.aabb.org/docs/default-source/default-document-library/resources/pbm-in-hematology-and-oncology.pdf?sfvrsn=21725df9_2
39. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians
follow clinical practice guidelines? A framework for improvement. JAMA. 1999 Oct;282(15):1458–65.
10.1001/jama.282.15.1458
40. Watson P, Watson D, Dhesi A, New HV, Davidson A, Armstrong R, et al. Improving blood-prescribing
decisions: evaluating the efficacy of a blood guidelines app. Transfus Med. 2020 Dec;30(6):485–91.
10.1111/tme.12736
41. Zuckerberg GS, Scott AV, Wasey JO, Wick EC, Pawlik TM, Ness PM, et al. Efficacy of
education followed by computerized provider order entry with clinician decision support
to reduce red blood cell utilization. Transfusion. 2015 Jul;55(7):1628–36. 10.1111/trf.13003
42. Rothschild JM, McGurk S, Honour M, Lu L, McClendon AA, Srivastava P, et al. Assessment
of education and computerized decision support interventions for improving transfusion
practice. Transfusion. 2007 Feb;47(2):228–39. 10.1111/j.1537-2995.2007.01093.x
43. Delaforce A, Duff J, Munday J, Hardy J. Overcoming barriers to evidence-based patient
blood management: a restricted review. Implement Sci. 2020 Jan;15(1):6. 10.1186/s13012-020-0965-4
44. Garrioch M, Sandbach J, Pirie E, Morrison A, Todd A, Green R. Reducing red cell transfusion
by audit, education and a new guideline in a large teaching hospital. Transfus Med.
2004 Feb;14(1):25–31. 10.1111/j.0958-7578.2004.00476.x
45. Tinmouth A, Macdougall L, Fergusson D, Amin M, Graham ID, Hebert PC, et al. Reducing
the amount of blood transfused: a systematic review of behavioral interventions to
change physicians’ transfusion practices. Arch Intern Med. 2005 Apr;165(8):845–52.
10.1001/archinte.165.8.845
46. Hubbard AE, Ahern J, Fleischer NL, Van der Laan M, Lippman SA, Jewell N, et al. To
GEE or not to GEE: comparing population average and mixed models for estimating the
associations between neighborhood risk factors and health. Epidemiology. 2010 Jul;21(4):467–74.
10.1097/EDE.0b013e3181caeb90
Appendix: Supplementary table and figures
Table S1Characteristics
of all cases with an RBC transfusion in the study period (irrespective of the
presence of an ATC code for RBC transfusions). Numbers are
presented in n (%), unless indicated otherwise. Characteristics were compared
using the Wilcoxon rank sum test for continuous variables or the chi-squared
test for categorical variables.
| |
All patients |
General internal medicine |
Other clinics |
p-value |
| n = 28,150 |
n = 2790 |
n = 25,360 |
| Age in years, median
(IQR) |
67 (56–76) |
73 (62–81) |
67 (55–75) |
<0.001 |
| Female sex |
12,313 (43.7) |
1215 (43.6) |
11,098 (48.8) |
0.829 |
| Emergency admission |
12,034 (42.8) |
2124 (76.1) |
9910 (39.1) |
<0.001 |
| Intensive care unit admission* |
12,534 (44.5) |
848 (30.4) |
11,686 (46.1) |
<0.001 |
| Surgical procedure |
20,175 (71.7) |
1029 (36.9) |
19,146 (75.5) |
<0.001 |
| Comorbidities |
Acute haemorrhage** |
4632 (16.5) |
698 (25) |
3934 (15.5) |
<0.001 |
| Hypovolemic and traumatic shock |
1277 (4.5) |
101 (3.6) |
1176 (4.6) |
0.014 |
| Coagulation defects*** |
9690 (34.4) |
1048 (37.6) |
8642 (34.1) |
<0.001 |
| Peripheral vascular disease |
5926 (21.1) |
537 (19.3) |
5389 (21.3) |
0.014 |
| Cerebrovascular disease |
2595 (9.2) |
325 (11.7) |
2270 (9) |
<0.001 |
| Ischaemic heart disease |
8616 (30.6) |
773 (27.7) |
7843 (30.9) |
<0.001 |
| Chronic heart failure |
5046 (17.93) |
833 (29.9) |
4213 (16.6) |
<0.001 |
| Chronic pulmonary disease |
2671 (9.5) |
378 (13.6) |
2293 (9) |
<0.001 |
| Dementia |
671 (2.4) |
208 (7.5) |
463 (1.8) |
<0.001 |
| Diabetes mellitus |
5848 (20.8) |
709 (25.4) |
5139 (20.3) |
<0.001 |
| Peptic ulcer disease |
653 (2.3) |
121 (4.3) |
532 (2.1) |
<0.001 |
| Any malignancy |
8323 (29.6) |
777 (27.9) |
7546 (29.8) |
0.036 |
| Liver disease |
2177 (7.7) |
330 (11.8) |
1847 (7.3) |
<0.001 |
| Renal disease |
7976 (28.3) |
1164 (41.7) |
6812 (26.9) |
<0.001 |
| Rheumatic disease |
698 (2.5) |
78 (2.8) |
620 (2.4) |
0.258 |
| Charlson Comorbidity Index,
median (IQR)**** |
5 (3–7) |
6 (4–8) |
5 (3–7) |
<0.001 |
| Length of stay in days, median
(IQR) |
11 (6.8–19) |
10.6 (6.1–19) |
11 (6.9–19) |
0.037 |
| In-hospital death |
2272 (8.1) |
345 (12.4) |
1927 (7.6) |
<0.001 |
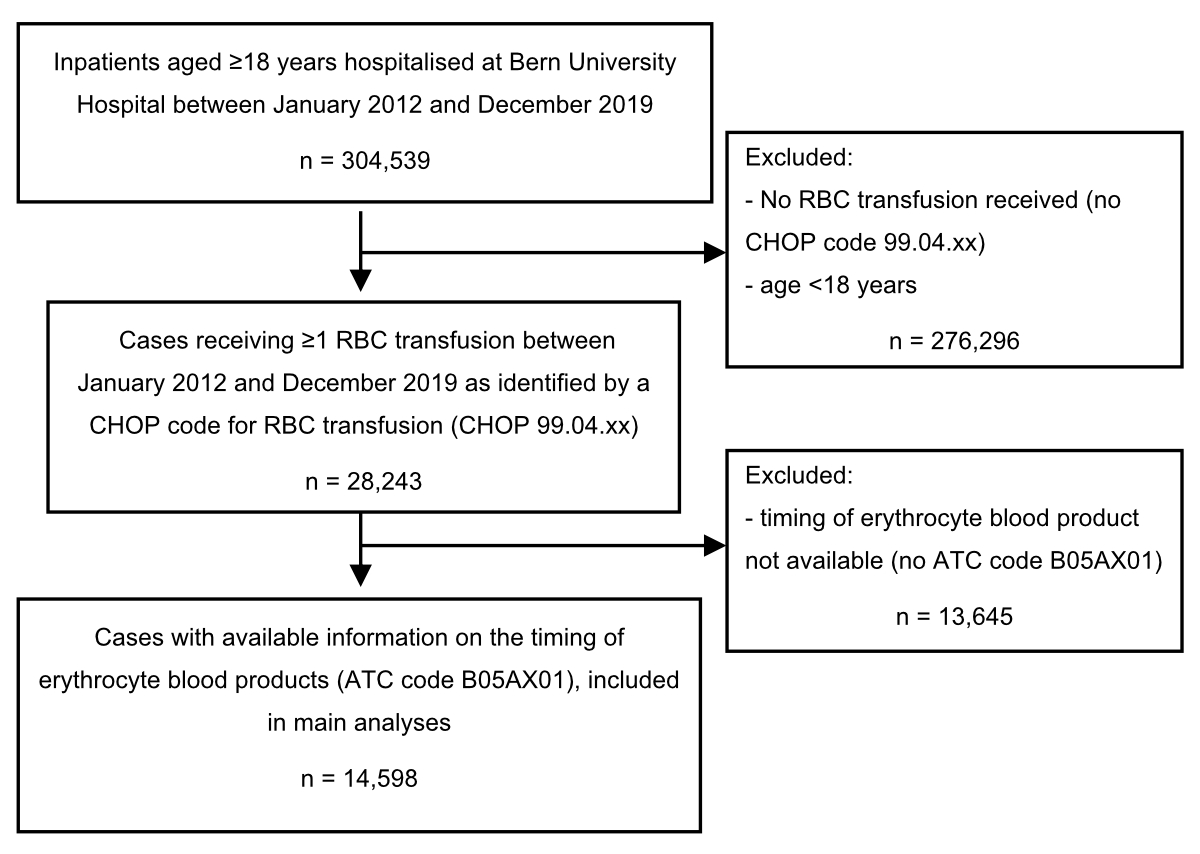
Figure S1Flow chart. ATC
codes were not available for transfusions administered in the emergency
department, intensive care unit, or operating rooms.
ATC: anatomical therapeutical chemical; CHOP: Swiss Operations
Classification; RBC: red blood cell

Figure S2Proportion of red
blood cell transfusions at different haemoglobin (Hb) thresholds. Data on haemoglobin
thresholds were missing in 180 (1.2%) cases (general internal medicine n = 53,
other clinics n = 127).

Figure S3Number of red
blood cell units during hospitalisation over time among all cases receiving a red
blood cell transfusion. Data were missing in 3 cases (general internal medicine
n = 0, other clinics n = 3).

Figure S4Minimal haemoglobin
(Hb) during hospitalisation over time amongall patients receiving a red blood cell
transfusion during the study period
from 2012 to 2019. Data on minimal haemoglobin were missing for 93 of the
28,243 cases with a Swiss Operations
Classification code for red blood cell transfusion (0.3%).






