
Figure 1Flow diagram: screening and recruitment of patients.
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40119
Proton-pump inhibitors (PPIs) are among the most widely prescribed drug classes in the world [1–3]. A study in Germany found that around 15% of women and 13% of men received a PPI prescription in 2018 [3]. In Switzerland, the annual incidence of PPI prescriptions in adults was 23% in 2017 [4]. PPIs are usually prescribed for the treatment of gastric acid-related diseases such as gastroesophageal reflux disease, dyspepsia, reflux oesophagitis, peptic ulcer disease, hypersecretory conditions and Helicobacter pylori bacterial infections [5–7]. The long-term use of PPIs has been associated with an increased risk of hypocalcaemia, hypomagnesaemia, fractures, Clostridium difficile infections, pneumonia, vitamin B12 malabsorption and gastric pre-malignant lesions [8–13]. The inappropriate prescribing of PPIs leads to unnecessary costs and can be a burden to the healthcare system [1, 14–16]. Studies have shown that around 40–60% of patients who use PPIs have an inappropriate indication [5, 17–19]. The inappropriateness of PPI prescriptions can be verified by the dosage and the reason for use. Inappropriate prescribing of PPIs is often due to off-label indications and prophylaxis, such as corticosteroid and anticoagulant therapy to prevent gastrointestinal bleeding, and stress ulcer prophylaxis in non-intensive care units [5, 20–23]. Many patients during hospital stays receive a PPI prescription that is not discontinued thereafter: although the initial indication is no longer present, the PPI use is maintained [5].
The long-term use of PPIs is rarely necessary, and deprescribing is usually recommended after four to eight weeks [24]. Deprescribing is commonly defined as “the process of withdrawal of an inappropriate medication, supervised by a health care professional with the goal of managing polypharmacy and improving outcomes” [25]. Tapering medications is also part of deprescribing [24]. PPI deprescribing can be carried out by stopping, reducing the dosage or switching to “on-demand” use [24, 26]. Safely deprescribing PPIs can lead to reduced inappropriate polypharmacy while increasing the patient’s overall health status and reducing health care costs [24]. Although academic and media reports have tried to raise awareness and reduce the inappropriate prescription of PPIs, the number of PPI prescriptions has increased in recent years [4, 24, 27–31]. For instance, in 2017, around 23% of Swiss people had at least one PPI prescribed, compared to 20% in 2012 [4].
The Smarter Medicine movement [30] in Switzerland recommends that at least once per year, prescribers should attempt to stop or reduce the dosage of PPIs and evaluate whether at least one indication for use is still present [30]. This movement also recommends that the continuation of PPI use should be discussed with patients, considering the adverse effects and benefits [30]. PPI supplies can only be obtained over the counter in Switzerland for a maximum of two weeks (packages with a maximum of 14 tablets), and long-term prescriptions can only be made by physicians. General practitioners (GPs) usually have a long-term relationship with their patients, gathering knowledge of their medical history, personal characteristics and preferences, thus playing a crucial role in the optimisation of PPI use. Knowing how potentially inappropriate PPI prescriptions evolve over time in primary care settings will help to better understand how they are managed by GPs. We also need to better understand how PPI prescribing evolves in patients after their GPs are part of an awareness campaign. In turn, this will help to tailor deprescribing interventions and optimise the prescribing of PPIs.
We aimed to investigate the prevalence of potentially inappropriate PPI prescriptions in a sample of primary care patients in Switzerland. We also evaluated how GPs managed those patients over 12 months, with the only new strategy implemented being flagging these patients as having potentially inappropriate PPI prescribing. Additionally, we explored which patient characteristics were associated with inappropriate PPI prescriptions and the success of deprescribing.
This observational study was carried out in the canton of Bern in Switzerland. A group of 11 GPs of the same quality circle (meetings in which a small group of GPs reflect together to improve their care practice [32]) was invited to participate. This quality circle takes place around 10 times a year, with each meeting lasting 1.5 hours. Only GPs, and no other health care providers, participated in this quality circle. All the GPs participating in the study were actively practising in the canton of Bern. This quality circle meeting aimed to raise the GPs’ awareness of optimising PPI prescriptions by flagging patients as having a potentially inappropriate PPI prescription. They had not received any guidelines on how to reduce inappropriate PPI prescribing. We used a convenience sampling strategy, in which GPs were asked to use their electronic medical records to screen all patients they had seen before the baseline (June 1, 2021) until they found the first 20 with an active PPI prescription for ≥8 weeks. This consecutive sampling method was chosen to reduce the risk of selection bias. GPs did not receive any compensation for their participation in the study.
The study did not fall within the scope of Swiss human research law because it was initially designed as a quality improvement study. Therefore, a waiver of non-responsibility was obtained from the Ethics Committee of the Canton of Bern (BASEC-Nr: Req-2021-01213).
The inclusion criteria for GPs were attending the quality circle and agreeing in participating in the study. The selection criteria for patients were having been prescribed a PPI commercially available in Switzerland for ≥8 weeks before June 1, 2021, and being patients of one of the 11 GPs participating in the quality circle in which the study took place.
In the first round of data collection at the baseline, the participating GPs assessed their patients’ electronic medical records to identify the first 20 who had an active PPI prescription for ≥8 weeks. They determined the length of PPI prescription by assessing repeated prescriptions in their records. The GPs were asked to report patients’ age, gender, polypharmacy (taking ≥5 medications), name of the PPI’s active substance, daily dose and indication for use. The indication for the PPI prescription was reported by GPs providing information on the following categories to the study team at the baseline: dose too high, no indication, risk of gastrointestinal bleeding (steroids plus anticoagulants; long-term use of non-steroidal anti-inflammatory drugs [NSAIDs]), peptic ulcer or gastroesophageal reflux disease. GPs also had the opportunity to provide additional information as free text. They were responsible for identifying potentially inappropriate PPI prescriptions based on their clinical judgement and the quality circle discussions. Those patients flagged as potentially having an inappropriate PPI prescription (dose too high or no indication) were followed up for one year, and all others were not. In addition, the GPs reported how many patients they had screened to find the first 20 with a PPI prescribed.
In the second round of data collection a year later, the GPs were asked to use their electronic medical records to report the updated status of PPI prescriptions of the same patients who had an inappropriate PPI prescription at baseline and report eventual changes in these prescriptions (e.g., tentative deprescribing of the PPI, return of symptoms, changes in the PPI dosage, whether deprescribing was successful and the reason). They also provided information on whether any attempt to deprescribe the PPI had been followed by a restart of the drug. Cases in which restarting the dose was necessary, but at a reduced dose compared to the baseline, were also considered successful deprescribing. To allow assessing the reasons why deprescribing was not possible, GPs used the information in their patients’ electronic medical records to report this information as free text. They also used these records to determine whether the PPI prescription was still active at the second screening.
In both rounds of data collection, the GPs reported the information using an Excel spreadsheet provided by the research team. Data from the baseline and after one year were gathered and merged using the patient’s identification number. We used age as a whole number and, to standardise the doses of different PPIs, we used pantoprazole as a reference drug. For that, we divided the actual PPI dose by its respective defined daily dose value according to the World Health Organization [33] to calculate a standardisation factor. We then multiplied this standardisation factor for each PPI by the defined daily dose of pantoprazole to obtain the standardised dose of each PPI relative to pantoprazole.
Baseline characteristics are presented as proportion for categorical variables and median and interquartile range (IQR) for continuous variables. The total number of patients that GPs had to screen to find the first 20 with a PPI prescription is reported as mean and range. We calculated absolute standardised differences (ASDs) to assess the balance of clinical and sociodemographic characteristics between those with an inappropriate PPI prescription and those with an appropriate prescription. An ASD of 0 is usually interpreted as a perfect balance between two groups, whereas an ASD of greater than 0.2 is considered an indication of meaningful imbalance [34]. We used multilevel logistic regression to analyse the association between patient characteristics and the frequency of deprescribing. The multi-level logistic regression was adjusted for clustering effects at the GP level (intracluster correlation coefficient [ICC] <0.01). We chose this approach considering that patients of the same GP were likely to be more similar to each other than patients of different GPs. Using a multilevel logistic regression clustered at the GP level can account for clustering effects even with a low ICC. The results remained similar in the sensitivity analysis using multivariable logistic regression. The co-variable selection strategy was based on clinical rationale. We chose the method of complete case analysis for dealing with missing data; therefore, the one patient lost to follow-up was excluded from the analyses. The most common inappropriate indications for PPI and the reasons that deprescribing was not successful were extracted from the free text, coded into themes, and described in numbers and percentages. Patients who had the PPI re-prescribed at a lower dose were considered successful deprescribing. Analyses were performed with Stata 16.1 (StataCorp, College Station, TX, USA), and R version 1.3.1093 was used for the alluvial diagram. A two-sided p-value of 0.05 was considered statistically significant.
In our consecutive sample strategy, GPs had to screen 125 (range: 55 to 224) patients on average to identify the 20 who had a PPI prescription for ≥8 weeks. This means that on average, 15% of the patients had a PPI prescription.Of the participating GPs, five were women, and six were men. All GPs were practising in the canton of Bern in Switzerland. In total, the GPs identified 206 patients who had been prescribed a PPI at the baseline. Ten GPs recruited 20 patients each, and one GP recruited six patients. Of these, 85 patients had a potentially inappropriate PPI prescription and qualified for the one-year follow-up. A slight difference was found in the gender distribution between those with a potentially inappropriate PPI and those with an appropriate prescription (ASD = 0.201). We found no statistically significant difference in the other clinical or sociodemographic characteristics between patients with a potentially inappropriate PPI prescription and those with an appropriate prescription (table 1). We used the CONSORT flow diagram [35] to show the screening and recruitment of participants (figure 1). During the one-year follow-up, one patient died and was thus excluded from the analysis (figure 1). Data on 84 patients were available for the follow-up analysis. At the baseline, of the 206 patients, 109 (53%) were women, the median age was 70 (IQR 59–77) years, 147 (71.4%) had polypharmacy and the median PPI daily dose was 40 mg (IQR 20–40; table 1). The most frequent prescriptions were 138 (67%) of pantoprazole, followed by 30 (15%) of esomeprazole and 22 (11%) of omeprazole.
Table 1Number (percentage) or median (interquartile range) of participants’ characteristics according to the appropriateness of prescription of proton-pump inhibitors at baseline. Categorical variables: number (percentage). Continuous variables: median (interquartile range).
| Total | Inappropriate* | Appropriate | Absolute standardised difference ** | |
| n = 206 (100%) | n = 85 (41.3%) | n = 121 (58.7%) | ||
| Women | 109 (52.9%) | 50 (58.8%) | 59 (48.8%) | 0.201 |
| Age at baseline | 70 (18.0) | 71 (20.0) | 69 (16.0) | 0.163 |
| Polypharmacy (≥5 medications) | 147 (71.4%) | 62 (71.8%) | 85 (71.1%) | 0.015 |
| Daily dose PPI in mg*** | 40 (20.0) | 40 (20.0) | 26.71 (20.0) | 0.136 |
* Inappropriate PPI due to lack of indication or dose too high according to GPs’ classification. 0 missing.
** An absolute standardised difference of 0 indicates a perfect balance between two groups, whereas an absolute standardised difference of greater than 0.2 is typically considered indicative of meaningful imbalance.
*** PPI dose was standardised using pantoprazole as a reference drug.

Figure 1Flow diagram: screening and recruitment of patients.
Regarding the indication for using a PPI, of the 206 patients, 85 (41%) had a potentially inappropriate PPI prescription (55 had no indication for PPI, and 30 had a too-high dose), 82 (40%) had gastroesophageal reflux disease and 22 (11%) had a risk of gastrointestinal bleeding (table 2). In the free text, the most frequently mentioned inappropriate PPI indications were gastritis (n = 13) and the short use of NSAIDs (n = 11), anticoagulants (n = 9) and corticosteroids (n = 7). Figure 2 shows how the inappropriate PPI prescribing progressed over one year. The GPs attempted to stop the PPI for 21 patients, reduce the dose for 16 and increase the dose for one. They reported stopping and then re-prescribing the PPI for 8 patients. During the one-year follow-up, GPs did not change the inappropriate PPI prescription for 55 (65%) patients, whereas deprescribing was successful for 29 (35%) patients. The most common reason that deprescribing an inappropriate PPI was not successful among those 55 patients was a lack of discussion with the patient (23 cases; either because the patient did not come back for another consultation [n = 3] or because the GP did not have time to address the PPI use during the consultation). Other reasons were the PPI becoming indicated over time (n = 8); the presence of symptoms or conditions such as anaemia, nausea, stomach pain and gastritis (n = 6); the unwillingness of the patient to stop (n = 5); hospital or other health care provider recommendation (n = 2) and the return of the patient’s symptoms (n = 1; table 3). Associations between patient characteristics (e.g., age, gender, PPI dose), GP gender, and the success of deprescribing are shown in table 4. The success of deprescribing was associated with neither GP gender (Odds Ratio female = 0.68, 95% CI 0.27 to 1. 68) nor patient characteristics (table 4). No statistical evidence existed for ICC at the GP level (ICC <0.01) in our multi-level logistic regression. The lack of correlation may be due to the small sample size.
Table 2Numbers and percentages of proton-pump inhibitor indications at baseline.
| Indication | Number (percentage) of patients |
| No indication | 55 (26.7%) |
| Dose too high | 30 (14.6%) |
| Gastroesophageal reflux disease | 82 (39.8%) |
| Peptic ulcer | 7 (3.4%) |
| Risk of gastrointestinal bleeding | 22 (10.7%) |
| Gastroesophageal reflux disease and peptic ulcer | 1 (0.5%) |
| Gastroesophageal reflux disease and risk of gastrointestinal bleeding | 6 (2.9%) |
| Peptic ulcer and risk of gastrointestinal bleeding | 3 (1.5%) |
| Total | 206 (100.0%) |
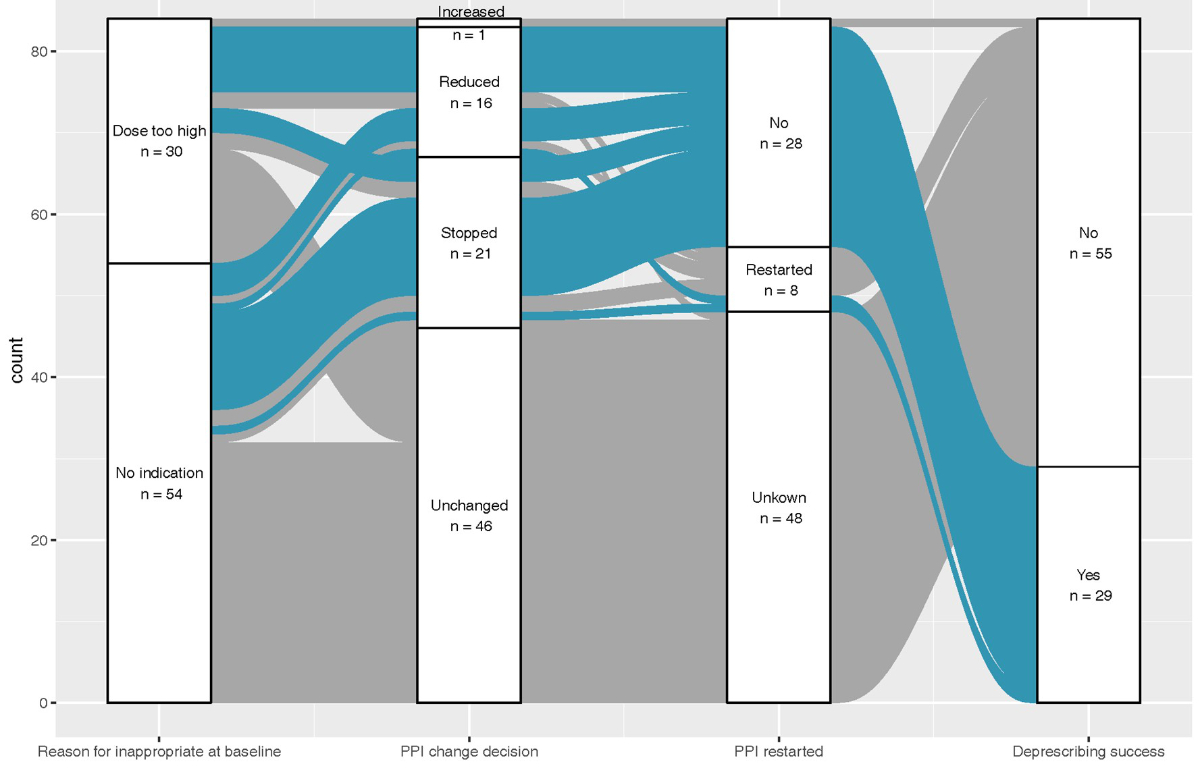
Figure 2Alluvial diagram illustrating the participant flow according to proton-pump inhibitor prescription and successful deprescribing (n = 84).
Table 3Most common reasons that deprescribing proton-pump inhibitors was not successful in patients with an inappropriate proton-pump inhibitor prescription at baseline.
| Reason | Number of patients |
| It was not discussed yet | 23 (42%) |
| Not specified | 10 (18%) |
| Indication changed to appropriate | 8 (15%) |
| Patient was symptomatic* | 6 (11%) |
| Patient did not want to stop | 5 (9%) |
| Hospital or other health care provider recommendation | 2 (4%) |
| Recurrence of symptoms | 1 (2%) |
| Total | 55 (100%) |
* Patient had symptoms and conditions such as anaemia, nausea, stomach pain and gastritis.
Table 4Association between patient characteristics and successful deprescribing of proton-pump inhibitors using a multi-level logistic regression adjusted for clustering effects at GP level. (n = 84; one patient was excluded from the analysis due to loss to follow-up.)
| Crude Odds Ratio (95% CI) | P-value | Adjusted* Odds Ratio (95% CI) | P-value | |
| Age (by 10-year increase) | 1.07 (0.78 to 1.47) | 0.665 | 1.28 (0.89 to 1.90) | 0.212 |
| Female patient (vs male) | 0.66 (0.27 to 1.64) | 0373 | 0.50 (0.19 to 1.34) | 0.170 |
| Polypharmacy ≥5 medications (vs no polypharmacy) | 0.65 (0.24 to 1.72) | 0.385 | 0.52 (0.16 to 1.70) | 0.276 |
| Daily dose PPI in mg (by 1 mg increase) ** | 1.00 (0.97 to 1.02) | 0.605 | 1.00 (0.97 to 1.02) | 0.886 |
| Female GP (vs male) | 0.68 (0.27 to 1.68) | 0.938 | 0.60 (0.23 to 1.57) | 0.297 |
* Multi-level logistic regression adjusted for GP cluster as random effect and covariates in the table.
** Daily PPI doses were standardised using pantoprazole as a reference drug.
In our observational study in primary care settings in Switzerland, 11 GPs consecutively selected 206 patients with a PPI prescription for ≥8 weeks and identified 85 (41%) of these as inappropriate. Of these 85 patients, 26% (55) had no indication, and 15% (30) had a dose too high. Instructing GPs to flag patients with a potentially inappropriate PPI prescription in their electronic medical record resulted in only 35% of these potentially inappropriate prescriptions being discontinued or reduced to a lower dose. In this setup, we found that raising awareness by flagging patients was not enough to improve the appropriateness of PPI use.
The number of inappropriate PPI prescriptions in our study is in line with the results of other studies that found the presence of inappropriate PPI prescribing between 35% and 63% [36–40]. Inappropriate PPI prescribing increased in Switzerland from 13.9% in 2012 to 28% in 2017 [4], and our study indicates that this number might be even higher. Other studies have also reported the lack of an appropriate indication as one of the most common reasons for the potentially inappropriate prescribing of PPIs [37, 39, 41]. In our study, according to GPs, doses were too high for 30 (15%) of the 206 patients with a PPI prescription, similar to a recent study in Iceland, in which 21% of the patients remained in higher-dose treatment after one year [42]. PPIs should be administrated using the lowest dose and for the shortest duration possible [43]. Only a few studies have investigated the appropriateness of PPIs regarding too-high doses, and recommendations are lacking on PPI dose reduction [4, 22]. Inappropriate indications for PPIs have been related to the lack of discontinuation after hospital discharge and automatic renewal of PPI prescriptions without adequate appraisal [19, 43]. Other common reasons for inappropriate prescriptions in our study were gastritis (n = 13) and the short use of NSAIDs (n = 11), anticoagulants (n = 9) and corticosteroids (n = 7), in line with other studies [5, 20-23, 44–47]. Additionally, PPI indications for bariatric surgery, chemotherapy and oesophageal varices were mentioned, although these do not qualify for an appropriate long-term PPI prescription [23, 41]. Women were slightly more likely than men to have an inappropriate PPI prescription (table 1). Female gender has been associated with the use of potentially inappropriate medication in other studies [48–50]. We found no significant association between age, gender, polypharmacy or PPI dose and inappropriate prescribing of PPIs, although other studies have shown that older age and polypharmacy were associated with inappropriate PPI prescription [20, 51, 52]. The lack of these associations could be due to the small number of participants in this study.
In this study, the GPs were simply asked to screen patients who were taking a PPI and flag those who had a potentially inappropriate prescription, not receiving any intervention, although they had a raised awareness of the topic. After the one-year follow-up, inappropriate PPIs were successfully deprescribed in 29 (35%) of the 84 patients with a potentially inappropriate PPI prescription. The GPs did not receive any information on how to involve patients or carry out deprescribing interventions. Other studies have found that involving the patient in the deprescribing attempt is important to reach a higher success rate [53] and that the implementation of behaviour change techniques (BCT) may influence deprescribing outcomes [54, 55]. If patients had been involved in the process and an intervention had focused on BCT, the success rate of deprescribing PPIs may have been higher. Furthermore, our results may have been different if the GPs had received instructions on how to deprescribe PPIs. Current guidelines on deprescribing PPIs suggest incorporating tapering as a crucial part of the process. First reducing the PPI to the lowest effective dose has been recommended, followed by the management of the patient’s symptoms, and only then discontinuing the PPI [24]. In our study, the most mentioned reason that deprescribing an inappropriate PPI was not successful was a lack of discussion with the patient, mostly because the GP did not have time to address the PPI use during the consultation. An unpublished pilot survey by Streit et al. from September to October 2021 with 88 GPs from the German part of Switzerland found that 48 (55%) reported seeing patients with inappropriate PPI prescriptions often or very often but lacked the time to deprescribe PPIs in their everyday clinical practice. In that study, 58% of GPs stated that they would like a guideline on PPI deprescribing, and 26% wanted information material on this topic.
To our knowledge, this is the first study to investigate the prescribing of potentially inappropriate PPIs in Swiss primary care settings using data directly reported by GPs. This overcomes the problems of epidemiological studies where the indication of a PPI is unknown or not recorded or the duration of the prescription might not be clear. A random sample would not have been feasible in our study. Therefore, we chose a simple but feasible consecutive sampling approach to recruit patients with a PPI prescription for ≥8 weeks. The consecutive sampling approach has the advantage of reducing selection bias, limiting the chances of GPs choosing which patients they would like to follow. GPs were responsible for identifying potentially inappropriate PPI prescriptions and too-high doses on their own. This comes with the limitation that the definitions of too-high doses were not standardised and GPs may have interpreted the indications differently. However, this simple self-definition also reflects the usual care in the GPs’ practices. The simplicity of our definition of appropriate and inappropriate PPIs allowed for GPs to quickly browse through their lists of recently consulted patients, but we acknowledge that more sophisticated definitions of inappropriate PPIs exist. This study has a pilot character, with a small sample of 200 patients using PPIs for ≥8 weeks. However, the findings are comparable to larger pharmacoepidemiologic studies that show that inappropriate prescribing is frequent. We only collected information on selected variables; therefore, in the regression analysis, unmeasured confounding cannot be ruled out. Although most GPs throughout Switzerland regularly participate in quality circles, our findings cannot be generalised to other Swiss cantons or other countries because the GPs were part of a specific quality circle in the canton of Bern.
In this small Swiss primary care sample of consecutive patients prescribed a PPI for ≥8 weeks, inappropriate prescription of PPIs was common. Nearly half of the patients taking a PPI had a potentially inappropriate prescription at the baseline. Only raising awareness in GPs by flagging inappropriate PPI prescribing did not result in PPI deprescribing in most patients over 12 months. Inappropriate PPI prescribing is an important issue in Swiss health care that must be addressed and needs more attention from GPs. Further interventions based on BCT are needed to investigate how to successfully deprescribe PPIs in Swiss primary care settings.
The data used and analysed in this study may be made available upon reasonable request.
We thank all GPs of the medix Quality Circle Münsingen for their support in conducting this study. We thank Dr. phil. Kristie Weir for her editorial suggestions.
The study did not receive any funding.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. British Medical Journal Publishing Group; 2008. pp. 2–3.
2. Rane PP, Guha S, Chatterjee S, Aparasu RR. Prevalence and predictors of non-evidence based proton pump inhibitor use among elderly nursing home residents in the US. Res Social Adm Pharm. 2017;13(2):358–63. 10.1016/j.sapharm.2016.02.012
3. Rückert-Eheberg IM, Nolde M, Ahn N, Tauscher M, Gerlach R, Güntner F, et al. Who gets prescriptions for proton pump inhibitors and why? A drug-utilization study with claims data in Bavaria, Germany, 2010-2018. Eur J Clin Pharmacol. 2022 Apr;78(4):657–67. 10.1007/s00228-021-03257-z
4. Muheim L, Signorell A, Markun S, Chmiel C, Neuner-Jehle S, Blozik E, et al. Potentially inappropriate proton-pump inhibitor prescription in the general population: a claims-based retrospective time trend analysis. Therap Adv Gastroenterol. 2021 Apr;14:1756284821998928. 10.1177/1756284821998928
5. Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017 Jan;37:19–24. 10.1016/j.ejim.2016.10.007
6. Savarino V, Marabotto E, Zentilin P, Furnari M, Bodini G, De Maria C, et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev Clin Pharmacol. 2018 Nov;11(11):1123–34. 10.1080/17512433.2018.1531703
7. Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012 Jul;5(4):219–32. 10.1177/1756283X12437358
8. Abraham NS. Proton pump inhibitors: potential adverse effects. Curr Opin Gastroenterol. 2012 Nov;28(6):615–20. 10.1097/MOG.0b013e328358d5b9
9. Deshpande A, Pant C, Pasupuleti V, Rolston DD, Jain A, Deshpande N, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012 Mar;10(3):225–33. 10.1016/j.cgh.2011.09.030
10. Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Official journal of the American College of Gastroenterology| ACG. 2011;106(7):1209-18. 10.1038/ajg.2011.113
11. Song H, Zhu J, Lu D. Long‐term proton pump inhibitor (PPI) use and the development of gastric pre‐malignant lesions. Cochrane Database Syst Rev. 2014(12).
12. Reimer C. Safety of long-term PPI therapy. Best Pract Res Clin Gastroenterol. 2013 Jun;27(3):443–54. 10.1016/j.bpg.2013.06.001
13. Jaynes M, Kumar AB. The risks of long-term use of proton pump inhibitors: a critical review. Ther Adv Drug Saf. 2018 Nov;10:2042098618809927.
14. Ladd AM, Panagopoulos G, Cohen J, Mar N, Graham R. Potential costs of inappropriate use of proton pump inhibitors. Am J Med Sci. 2014 Jun;347(6):446–51. 10.1097/MAJ.0b013e31829f87d5
15. Ahrens D, Chenot JF, Behrens G, Grimmsmann T, Kochen MM. Appropriateness of treatment recommendations for PPI in hospital discharge letters. Eur J Clin Pharmacol. 2010 Dec;66(12):1265–71. 10.1007/s00228-010-0871-9
16. Perren A, Donghi D, Marone C, Cerutti B. Economic burden of unjustified medications at hospital discharge. Swiss Med Wkly. 2009 Jul;139(29-30):430–5.
17. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012 Aug;29(8):681–90. 10.1007/BF03262283
18. Chia CT, Lim WP, Vu CK. Inappropriate use of proton pump inhibitors in a local setting. Singapore Med J. 2014 Jul;55(7):363–6. 10.11622/smedj.2014087
19. Batuwitage BT, Kingham JG, Morgan NE, Bartlett RL. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J. 2007 Jan;83(975):66–8. 10.1136/pgmj.2006.051151
20. Daniels B, Pearson SA, Buckley NA, Bruno C, Zoega H. Long-term use of proton-pump inhibitors: whole-of-population patterns in Australia 2013-2016. Therap Adv Gastroenterol. 2020 Mar;13:1756284820913743. 10.1177/1756284820913743
21. Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009 Mar;301(9):937–44. 10.1001/jama.2009.261
22. Scarpignato C, Gatta L, Zullo A, Blandizzi C; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016 Nov;14(1):179. 10.1186/s12916-016-0718-z
23. Yadlapati R, Kahrilas PJ. When is proton pump inhibitor use appropriate? BMC Med. 2017 Feb;15(1):36. 10.1186/s12916-017-0804-x
24. Farrell B, Pottie K, Thompson W, Boghossian T, Pizzola L, Rashid FJ, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017 May;63(5):354–64.
25. Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015 Dec;80(6):1254–68. 10.1111/bcp.12732
26. Farrell B, Lass E, Moayyedi P, Ward D, Thompson W. Reduce unnecessary use of proton pump inhibitors. BMJ. 2022 Oct;379:e069211. 10.1136/bmj-2021-069211
27. Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med. 2014 Jun;32(6):618–22. 10.1016/j.ajem.2014.03.019
28. Haastrup P, Paulsen MS, Zwisler JE, Begtrup LM, Hansen JM, Rasmussen S, et al. Rapidly increasing prescribing of proton pump inhibitors in primary care despite interventions: a nationwide observational study. Eur J Gen Pract. 2014 Dec;20(4):290–3. 10.3109/13814788.2014.905535
29. Hollingworth S, Duncan EL, Martin JH. Marked increase in proton pump inhibitors use in Australia. Pharmacoepidemiol Drug Saf. 2010 Oct;19(10):1019–24. 10.1002/pds.1969
30. Smarter medicine - Choosing Wisely Switzerland. Top-5-Listen – Ambulante Allgemeine Innere Medizin. Available from: https://www.smartermedicine.ch/. Accessed in 07 May 2023.
31. SRF. Die beliebten Magensäure-Bremser sind nicht ohne. SRF; 2016. Available from: https://www.srf.ch/wissen/gesundheit/die-beliebten-magensaeure-bremser-sind-nicht-ohne. Accessed in 25 May 2023.
32. Rohrbasser A, Kirk UB, Arvidsson E. Use of quality circles for primary care providers in 24 European countries: an online survey of European Society for Quality and Safety in family practice delegates. Scand J Prim Health Care. 2019 Sep;37(3):302–11. 10.1080/02813432.2019.1639902
33. World Health Organization. Defined Daily Dose (DDD) Index. Available on https://www.whocc.no/atc_ddd_index/?code=A02BC. Accessed 2023 May 17.
34. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011 May;46(3):399–424. 10.1080/00273171.2011.568786
35. Hopewell S, Hirst A, Collins GS, Mallett S, Yu LM, Altman DG. Reporting of participant flow diagrams in published reports of randomized trials. Trials. 2011 Dec;12(1):253. 10.1186/1745-6215-12-253
36. Liu Y, Zhu X, Li R, Zhang J, Zhang F. Proton pump inhibitor utilisation and potentially inappropriate prescribing analysis: insights from a single-centred retrospective study. BMJ Open. 2020 Nov;10(11):e040473. 10.1136/bmjopen-2020-040473
37. Choudhry MN, Soran H, Ziglam HM. Overuse and inappropriate prescribing of proton pump inhibitors in patients with Clostridium difficile-associated disease. QJM. 2008 Jun;101(6):445–8. 10.1093/qjmed/hcn035
38. Brandhagen DJ, Pheley AM, Onstad GR, Freeman ML, Lurie N. Omeprazole use at an urban county teaching hospital. J Gen Intern Med. 1995 Sep;10(9):513–5. 10.1007/BF02602404
39. Giannini EG, Crespi M, Djahandideh A, Demarzo MG, Moscatelli A, Bodini G, et al. Appropriateness of proton pump inhibitors treatment in clinical practice: prospective evaluation in outpatients and perspective assessment of drug optimisation. Dig Liver Dis. 2020 Aug;52(8):862–8. 10.1016/j.dld.2020.05.005
40. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):100-4.
41. Nowbahari E, Bigot A, Maillot F, Antier D, Foucault-Fruchard L. Reassessment of inappropriate prescriptions of proton pump inhibitors in elderly in-patients: it’s time to take action. Ann Pharm Fr. 2020 Mar;78(2):150–7. 10.1016/j.pharma.2020.01.001
42. Hálfdánarson ÓÖ, Pottegård A, Björnsson ES, Lund SH, Ogmundsdottir MH, Steingrímsson E, et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Therap Adv Gastroenterol. 2018 May;11:1756284818777943. 10.1177/1756284818777943
43. Dharmarajan TS. The Use and Misuse of Proton Pump Inhibitors: An Opportunity for Deprescribing. J Am Med Dir Assoc. 2021 Jan;22(1):15–22. 10.1016/j.jamda.2020.09.046
44. Munson JC, Wahl PM, Daniel G, Kimmel SE, Hennessy S. Factors associated with the initiation of proton pump inhibitors in corticosteroid users. Pharmacoepidemiol Drug Saf. 2012 Apr;21(4):366–74. 10.1002/pds.2350
45. Lassalle M, Le Tri T, Bardou M, Biour M, Kirchgesner J, Rouby F, et al. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. Eur J Clin Pharmacol. 2020 Mar;76(3):449–57. 10.1007/s00228-019-02810-1
46. Parente F, Cucino C, Gallus S, Bargiggia S, Greco S, Pastore L, et al. Hospital use of acid-suppressive medications and its fall-out on prescribing in general practice: a 1-month survey. Aliment Pharmacol Ther. 2003 Jun;17(12):1503–6. 10.1046/j.1365-2036.2003.01600.x
47. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012 Aug;66(8):767–73. 10.1111/j.1742-1241.2012.02973.x
48. Achterhof AB, Rozsnyai Z, Reeve E, Jungo KT, Floriani C, Poortvliet RK, et al. Potentially inappropriate medication and attitudes of older adults towards deprescribing. PLoS One. 2020 Oct;15(10):e0240463. 10.1371/journal.pone.0240463
49. Nyborg G, Brekke M, Straand J, Gjelstad S, Romøren M. Potentially inappropriate medication use in nursing homes: an observational study using the NORGEP-NH criteria. BMC Geriatr. 2017 Sep;17(1):220. 10.1186/s12877-017-0608-z
50. Fromm MF, Maas R, Tümena T, Gaßmann KG. Potentially inappropriate medications in a large cohort of patients in geriatric units: association with clinical and functional characteristics. Eur J Clin Pharmacol. 2013 Apr;69(4):975–84. 10.1007/s00228-012-1425-0
51. Pottegård A, Broe A, Hallas J, de Muckadell OB, Lassen AT, Lødrup AB. Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol. 2016 Sep;9(5):671–8. 10.1177/1756283X16650156
52. Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010 May;69(5):543–52. 10.1111/j.1365-2125.2010.03628.x
53. Duncan P, Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm Sci Pract. 2017 Jan;24(1):37–42. 10.1136/ejhpharm-2016-000967
54. Isenor JE, Bai I, Cormier R, Helwig M, Reeve E, Whelan AM, et al. Deprescribing interventions in primary health care mapped to the Behaviour Change Wheel: A scoping review. Res Social Adm Pharm. 2021 Jul;17(7):1229–41. 10.1016/j.sapharm.2020.09.005
55. Hansen CR, O’Mahony D, Kearney PM, Sahm LJ, Cullinan S, Huibers CJ, et al. Identification of behaviour change techniques in deprescribing interventions: a systematic review and meta-analysis. Br J Clin Pharmacol. 2018 Dec;84(12):2716–28. 10.1111/bcp.13742