Evolution of
humoral immune response to SARS-CoV-2 mRNA vaccine in liver transplant
recipients – a longitudinal study
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40118
Isabella C. Schoepfabc,
Carlotta
Riebensahmb,
Chiara Becchettiad,
Valentine Blasera,
Céline V. Unternährera,
Vanessa Banza,
Cédric Hirzelb,
Franziska
M. Suter-Rinikere,
Annalisa
Berzigottiaf
a Department
of Visceral Surgery and Medicine, Inselspital, Bern University Hospital,
University of Bern, Bern, Switzerland
b Department
of Infectious Diseases, Inselspital, Bern University Hospital, University of
Bern, Bern, Switzerland
c University
Department of Medicine and Infectious Diseases Service, Kantonsspital
Baselland, University of Basel, Bruderholz, Switzerland
d Hepatology
and Gastroenterology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy
e Institute
for Infectious Diseases, University of Bern, Bern, Switzerland
f Department
of Biomedical Research, University of Bern, Bern, Switzerland
Summary
BACKGROUND AND AIM: Liver transplant recipients show suboptimal
vaccine-elicited immune responses to severe acute respiratory coronavirus 2
(SARS-CoV-2) vaccination. This study aimed to assess real-world data on SARS-CoV-2
antibodies after the second and third SARS-CoV-2 vaccination in liver
transplant recipients in Switzerland.
METHODS: We enrolled liver transplant recipients
who attended regular follow-up visits between 01/07/2021 and 30/04/2022 at the
outpatient clinic of the Department of Visceral Surgery and Medicine at Bern
University Hospital, Switzerland. Following the
Swiss Federal Office of Public Health recommendations, we measured SARS-CoV-2
anti-spike IgG antibodies in 117 liver transplant recipients ≥4 weeks after the
second SARS-CoV-2 mRNA vaccination from 07/2021–04/2022. In case of antibody
levels of <100 AU/ml, patients received a third vaccination and antibodies
were re-measured. Patients with antibody levels of >100 AU/ml were defined
as “responders”, those with 12–100 AU/ml as “partial responders” and those with
<12 AU/ml as “non-responders”.
RESULTS: After
two vaccinations, 36/117 (31%) were responders, 42/117 (36%) were partial
responders and 39/117 (33%) were non-responders. The humoral immune response
improved significantly after the third vaccination, resulting in 31/55 (56%)
responders among the previous partial or non-responders. A total of 26 patients
developed COVID-19, of whom two had a moderate or severe course (both
non-responders after three doses).
DISCUSSION: One
third of liver transplant recipients showed an optimal response following two
vaccinations; a third dose achieved a complete antibody response in more than half
of partial and non-responders. We observed only one severe
course of COVID-19 and no deaths from COVID-19 in the vaccinated liver
transplant recipients.
Introduction
Liver
transplant recipients are considered a vulnerable population in the setting of
the coronavirus disease 2019 (COVID-19) pandemic [1–3]. Determining the best
vaccination strategy is thus essential to ensure optimal protection against
COVID-19 in this population. In Switzerland, the two severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) messenger ribonucleic acid (mRNA) vaccines
BNT162b2 (Comirnaty®, Pfizer-BioNTech) [4] and mRNA-1273 (Spikevax®, Moderna) [5] have been licensed since December 2020 and January 2021,
respectively. Both vaccines have a high efficacy in preventing severe COVID-19 [6,
7] in
non-immunocompromised individuals. However, the immunogenicity of these
vaccines is reduced in immunosuppressed individuals [8–10].
In the
general population, the humoral immune response following COVID-19 [11] or
SARS-CoV-2 mRNA vaccine [12] has been assessed by different types of assays,
mainly SARS-CoV-2 anti-spike IgG antibodies. In organ transplant patients,
suboptimal immunogenicity was observed after the administration of two doses of
SARS-CoV-2 vaccine [8,13], with vaccine breakthroughs reported [14]. This
prompted public health authorities in Switzerland and other countries to adjust
their vaccination strategies for this population. In July 2021, the Swiss Federal
Office of Public Health (FOPH) recommended
measuring the SARS-CoV-2 anti-spike IgG antibodies as a surrogate of humoral
immune response in all immunocompromised individuals 4 weeks after the second
vaccination with a SARS-CoV-2 mRNA vaccine. In case of absent antibodies, or
antibody titres not in a clear positive range, a third vaccine dose and a
repeated measurement of SARS-CoV-2 anti-spike IgG antibodies were recommended.
To date, limited data are available on the immunogenicity of the SARS-CoV-2
mRNA vaccine in the liver transplant population after the second and third doses
of SARS-CoV-2 vaccine [15–20], mostly from small, cross-sectional studies [15–19].
We evaluated the SARS-CoV-2 anti-spike IgG antibody responses after the second
dose of SARS-CoV-2 mRNA vaccine in 117 well-characterised liver transplant
recipients in Switzerland and after a third dose in 55 with no or partial
response after the second.
Methods
Study design
We enrolled
liver transplant recipients who attended regular follow-up visits between 01/07/2021
and 30/04/2022 at the outpatient clinic of the Department of Visceral Surgery
and Medicine at Bern University Hospital, Switzerland. We included liver
transplant recipients who were aged 18 years or older and had provided written
general consent to the Inselspital. The exclusion criteria were a lack of
general informed consent, liver transplant recipients who declined SARS-CoV-2
antibody testing and those younger than 18 years. We measured the SARS-CoV-2
anti-spike IgG antibodies of 117 patients ≥4 weeks after the second dose of a
SARS-CoV-2 mRNA vaccine. Liver transplant
recipients with antibody levels of <100 AU/ml (equivalent to <550 binding
antibody units/ml) received a third dose of a SARS-CoV-2 mRNA vaccine, and the SARS-CoV-2
anti-spike IgG antibody measurement was repeated ≥4 weeks after the third dose
(figure 1). The
Swiss FOPH recommendations changed on 04/11/2021, recommending a third dose of
SARS-CoV-2 mRNA vaccine for all immunocompromised persons, regardless of the
SARS-CoV-2 antibody levels. Similarly, routine measurement of antibodies was no
longer recommended. However, in individual cases, antibody measurements were
done later, and we included these additional data in our study.
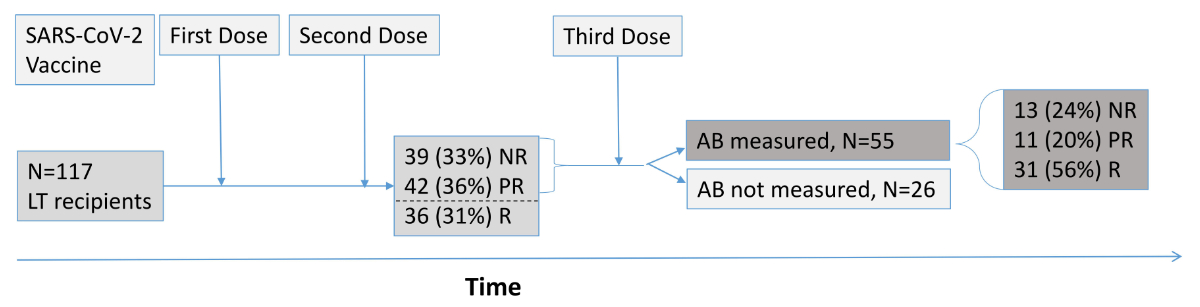
Figure 1Study population. Flowchart of 117 liver transplant (LT) recipients with
SARS-CoV-2 anti-spike IgG antibody testing >4 weeks after the second dose of
a SARS-CoV-2 mRNA vaccine. A total of 81 LT recipients with antibody levels of <100
AU/ml (equivalent to <550 binding antibody units/ml) received a third dose
of a SARS-CoV-2 mRNA vaccine, and SARS-CoV-2 anti-spike IgG antibody
measurement was repeated in 55 LT recipients >4 weeks after the third dose.
AB:
antibodies; NR: non-responder; PR: partial responder; R: responder; SARS-CoV-2:
severe acute respiratory syndrome coronavirus 2.
We defined patients with antibody
concentrations under the assay
detection limit of 12 AU/ml (equivalent to 33.8 BAU/ml) as
“non-responders”, those with 13–100 AU/ml (equivalent to 33.8–550 BAU/ml) as
“partial responders” and those with >100 AU/ml (equivalent to >550
BAU/ml) as “responders” to the vaccination.
The study
was conducted in accordance with the Declaration of Helsinki and Good Clinical
Practice. All patients gave informed consent by signing the general informed
consent. The Bern Cantonal Ethics Committee (KEK 2022-00309) approved the study.
SARS-CoV-2
anti-spike IgG immunoassays
Serum
samples were analysed by chemiluminescent immunoassay technology (LIAISON®
SARS-CoV-2, Diasorin, Saluggia [VC], Italy) according to the manufacturer’s
instructions on the LIAISON® XL Analyzer. IgG antibodies against S1/S2 antigens
of SARS-CoV-2 were detected in a semi-quantitative assay with a lower limit of
detection of 0.3 AU/ml (arbitrary units/ml) and an upper limit for quantitative
evaluation of 400 AU/ml at the Institute for Infectious Diseases, University of
Bern.SARS-CoV-2 anti-spike IgGantibody measurements performed in an
external laboratory (n=32) were also considered in the analysis when reported
in BAU/ml. For better comparability with the externally performed measurements,
we analysed the serum samples a second time by LIAISON® SARS-CoV-2 TrimericS
IgG assay, where the values are given in BAU/ml with a range between 4.81 and 2,080
BAU/ml. A concentration of 2,080 BAU/ml is the upper limit of antibody
quantification without dilution of the serum. Therefore, all values of >2,080
BAU/ml were assigned to 2,080 BAU/ml. Since the conversion factor for the
DiaSorin S1/2 IgG assay (reported in AU/ml) to BAU per millilitre is not
provided by the manufacturer, we determined the conversion factor by performing
the LIAISON® SARS-CoV-2 TrimericS IgG assay in addition to the DiaSorin S1/2
IgG assay (figure S1 in the appendix; conversion factor: 5.5). This was comparable
to the conversion factor
reported in another report [21]. A cut-off of >100 AU/ml (equivalent to
>550 BAU/ml) for clear positive results and a cut-off of >12 AU/ml
(equivalent to <33.8 BAU/ml) for minimal positive results were used. Therefore,
all values of <33.8 BAU/ml were assigned to 33.8 BAU/ml.
Statistical
analysis
Continuous
baseline variables are reported as medians and interquartile ranges (IQRs), and
categorical variables are reported as frequencies. Differences between
categorical characteristics of responders versus partial and non-responders
were investigated using Fisher’s exact test, and the Wilcoxon rank-sum test was
applied for continuous variables. The analyses were performed using Stata/SE version
16.0
(StataCorp, College Station, TX, USA), and a statistical significance level of
5% was used throughout. GraphPad
Prism (GraphPad Software 9.4.1) and PowerPoint 2019 were used to create the
figures.
Results
Patients
Out of 119
liver transplant recipients who regularly attended our outpatient clinic, we excluded
two who declined SARS-CoV2 antibody testing. The analyses are based on 117 liver
transplant recipients, the clinical characteristics of whom are shown in table 1.
Of the liver transplant recipients, 46
(39.3%) received the mRNA-1273 vaccine, and 57 (58.7%) received the BNT16b2
vaccine. In 14 patients (12%), no information was available on the vaccine
type (mRNA-1273 or BNT16b2). After two doses of vaccination, partial and
non-responders were older (p <0.01) than responders.
Table 1Characteristics of 117 liver transplant (LT) recipients at baseline (defined as
the last routine visit after the second COVID-19 vaccine and before or at the
first SARS-CoV-2 antibody measurement).
| |
Study population (n = 117) |
Vaccine responders* (n = 36) |
Partial/non-responders** (n = 81) |
p-value |
| Age, years (median [IQR]) |
62 (53–69) |
55 (44–64) |
64 (58–70) |
<0.01 |
| Female sex, n (%) |
37 (31.6) |
16 (36.4) |
21 (28.8) |
0.31 |
| BMI, kg/m(median [IQR]) |
25.1 (23.0–29.0) |
25.5 (23.5–28.0) |
25.1 (22.9–30.0) |
0.99 |
| Time since
transplantation, months (median [IQR]) |
48 (24–149) |
81 (33–174) |
44 (23–143) |
0.11 |
| Acute rejection during
the year before vaccination, n (%) |
3 (2.6) |
1 (2.8) |
2 (2.5) |
0.67 |
| Transplanted organ, n
(%) |
|
|
|
0.64 |
| – Liver |
113 (96.6) |
35 (97.2) |
78 (96.3) |
|
| – Combined liver and
kidney |
4 (3.4) |
1 (2.8) |
3 (3.7) |
|
| Type of
immunosuppressive therapy, n (%) |
|
|
|
|
| – Calcineurin inhibitors
(tacrolimus, cyclosporin) |
76 (65.0) |
22 (61.1) |
54 (66.7) |
0.35 |
| – Antimetabolites (mycophenolate
mofetil, azathioprine) |
34 (29.1) |
9 (25.0) |
25 (30.9) |
0.34 |
| – mTor inhibitors (sirolimus,
everolimus) |
47 (40.2) |
15 (41.7) |
32 (39.5) |
0.49 |
| – Steroids |
5 (4.3) |
1 (2.8) |
4 (4.9) |
0.51 |
| Regimen of
immunosuppressive therapy, n (%) |
|
|
|
0.61 |
| – Monotherapy |
76 (65.0) |
26 (72.2) |
50 (61.7) |
|
| – Dual therapy |
37 (31.6) |
9 (25.0) |
28 (34.6) |
|
| – Triple therapy |
4 (3.4) |
1 (2.8) |
3 (3.7) |
|
| Vaccine type, n (%) *** |
|
|
|
0.88 |
| – mRNA-1273 |
46 (39.3) |
13 (36.1) |
33 (40.7) |
|
| – BNT16b2 |
57 (58.7) |
19 (52.7) |
38 (46.9) |
|
| Median leukocyte count
(/mm) [IQR] |
5.6 (4.4–7.0) |
5.5 (4.6–6.6) |
5.6 (4.4–7.0) |
0.86 |
| Median lymphocyte count
(/mm) [IQR] |
1.4 (0.9–1.9) |
1.7 (1.1–2.2) |
1.3 (0.9–1.7) |
0.08 |
| COVID-19, n (%) |
26 (22.2) |
11 (30.6) |
15 (18.5) |
0.12 |
| – COVID-19 severity, n (%) **** |
|
|
|
|
| – Mild |
24 (92.3) |
11 (100.0) |
13 (86.7) |
|
| – Moderate |
1 (3.8) |
0 |
1 (7.1) |
|
| – Severe |
1 (3.8) |
0 |
1 (7.1) |
|
| – Death due to COVID-19 |
0 |
0 |
0 |
|
SARS-CoV-2
anti-spike IgG antibody level after second vaccine dose
The median
anti-SARS-CoV-2 anti-spike IgG antibody concentration was 177 (IQR 33.8–780)
BAU/ml. We observed 36/117 (31%) responders, 42 partial responders (36%; median
197; IQR 129–305) and 39 non-responders (33%; figures 2 and 3A).
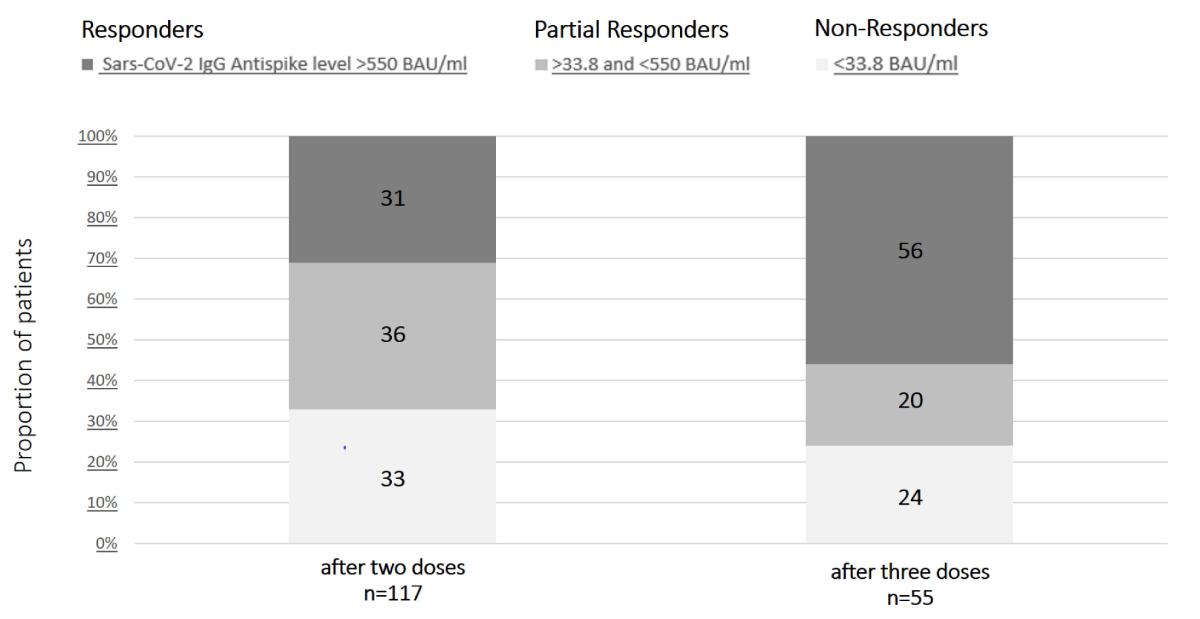
Figure 2Distribution
of liver transplant (LT) recipients according to SARS-CoV-2 anti-spike IgG antibody
levels after the second and third SARS-CoV-2 vaccines. Among the 117 LT
recipients who were tested ≥4 weeks after the second dose, 36 (31%) had SARS-CoV-2
anti-spike IgG antibody concentrations of ≥550 BAU/ml. Among the 55 LT
recipients who were retested at ≥4 weeks after the third dose, 31 (56%) had
SARS-CoV-2 anti-spike IgG antibody concentrations of ≥550 BAU/ml. BAU: binding
antibody units; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
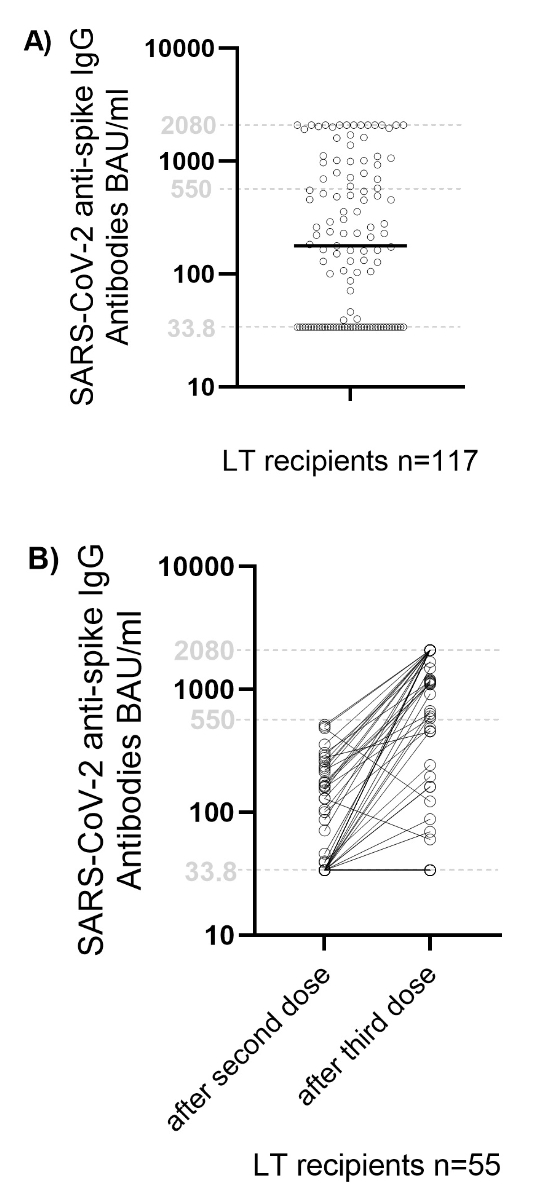
Figure 3Immune responses
after SARS-CoV-2 vaccination in liver transplant (LT) recipients. Figure (A) shows
the SARS-CoV-2 anti-spike IgG antibody levels in the whole study
population (117 LT recipients) after the second dose. Each point represents an
individual patient, and horizontal lines indicate the median. The dotted lines
indicate the threshold values of 33.8 BAU/ml and 550 BAU/ml, and the black line
indicates the median. Values below the detection limit are plotted on the
dotted line at 33.7 BAU/ml, and values above the detection limit are plotted on
the dotted line at 2,080 BAU/ml. Figure (B) figure shows
SARS-CoV-2 anti-spike IgG antibody levels after the second and third doses of
SARS-CoV-2 vaccine in the 55 LT recipients who were retested at ≥4 weeks after the
third vaccination. Again, each point represents an individual patient’s values.
Values below the detection limit are plotted on the dotted line at 33.8 BAU/ml,
and values above the detection limit are plotted on the dotted line at 2,080
BAU/ml.
BAU: binding antibody units; SARS-CoV-2:
severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2
anti-spike IgG antibody level after third vaccine dose
Of 81 partial
and non-responders, 79 received a third dose. Antibody measurements were
available for 55/81. Four or more weeks after the third dose, SARS-CoV-2
anti-spike IgG antibodies were detected in 42/55 patients (76%; 31/55
responders, 11/55 partial responders). Thirteen patients (24%) remained
non-responders (figures 2 and 3B). The median
SARS-CoV-2 anti-spike IgG antibody concentration was 668 (IQR 60.1–2080)
BAU/ml.
The
proportion of patients classified as responders increased from 31% (36/117)
after two vaccines to 56% (31/55) after administering a third vaccine dose to
previous partial and non-responders. We observed a downward immune response in
two liver transplant recipients at the second antibody measurement compared
with the first antibody measurement (figure 3B). This might be explained by
changes in the immunosuppressive therapy (intensification at the second
measurement in one case; reduction of immunosuppressants at the first
measurement in the other case).
COVID-19
Of the 117 liver
transplant recipients, 26 (22%) were diagnosed with COVID-19. Among them, five
were diagnosed before the third vaccination, two before any vaccination, one
after the first vaccination and two after the second vaccination (clinical
characteristics: table S1 in the appendix). Twenty-one patients were diagnosed
after the third vaccination (clinical characteristics: table S2 in the appendix),
during the Omicron wave of early 2022.
Among the
21 patients who developed COVID-19 after the third dose of SARS-CoV-2 vaccine
(median time from third vaccination to COVID-19: 4 months [IQR 2–5]; table S2), 11
(52%) were
non-responders, four (19%) were partial responders and six (29%) were
responders after the second dose of SARS-CoV-2 vaccine. Of the 10 patients who
were retested after the third dose, four (40%) were non-responders, three (30%)
were partial responders and three (30%) were responders.
Among the
26 patients who developed COVID-19, only one had severe COVID-19 (admitted to an
intensive care unit) in April 2022, one had moderate COVID-19 (admission to a general
ward) in February 2022, and 24 had a mild infection, i.e. asymptomatic or with
mild symptoms (cough, sore throat, fever) treated in an outpatient setting.
Both patients with severe or moderate COVID-19 had no detectable antibodies
after the second and third SARS-CoV-2 vaccinations. The patients with moderate
and severe courses of COVID-19 were undergoing dual immunosuppressive therapy
(cyclosporine and mycophenolate mofetil) and triple immunosuppressive therapy
(tacrolimus, mycophenolate mofetil and prednisone), respectively, at the time of
the first SARS-CoV-2 antibody measurement. As additional predisposing factors
for a moderate or severe COVID-19 course, the patient with moderate COVID-19
had the following factors according to the FOPH criteria list: age >65
years, hypertensive cardiopathy and multiple cardiovascular risk factors
(diabetes mellitus, arterial hypertension, past nicotine use with cumulative 72
package years). The patient with severe COVID-19 had dual organ transplantation
(kidney and liver) and two cardiovascular risk factors (arterial hypertension,
past nicotine use with cumulative 15 package years). Neither
of the two patients with moderate or severe COVID-19 has developed cirrhosis of
the graft to date.No death was
registered due to COVID-19 among the liver transplant recipients during the
observation period.
Discussion
Our study
evaluating the SARS-CoV-2 anti-spike IgG antibody responses after the second
dose of SARS-CoV-2 mRNA vaccine in 117 liver transplant recipients, and after a
third dose in 55 of them with a partial or no response after the second, showed
three major findings. First, one third of patients did not develop
vaccine-elicited SARS-CoV-2 anti-spike IgG antibodies after two doses of a SARS-CoV-2
mRNA vaccine. The humoral immune response improved significantly after the
third vaccination: more than half of the previous partial and non-responders
developed SARS-CoV-2 anti-spike IgG antibodies in a clear positive range. Our
findings are confirmed by a recent study that observed a significantly improved
immune response in liver transplant recipients after a third dose of mRNA
SARS-CoV-2 vaccination [22]. Second, despite three vaccine doses, 24% of liver
transplant recipients did not develop detectable vaccine-elicited antibodies.
This is clinically relevant because these patients might qualify for COVID-19
prophylaxis with monoclonal antibodies or early antiviral therapy (i.e. remdesivir
or nirmatrelvir
and ritonavir) to prevent a severe course of COVID-19. Furthermore, maintaining
surveillance while systematically implementing preventative measures is
essential for patients with an absent humoral immune response. Third, we
observed only one severe course of COVID-19 and no deaths from COVID-19 in our
vaccinated liver transplant recipients. Interestingly, the patient who
developed severe COVID-19 was a non-responder to the three vaccine doses.
The strengths
of our study include the adequate number of well-characterised liver transplant
recipients, as well as the measurement of SARS-CoV-2 anti-spike IgG antibodies
at multiple time points in the same patients. However, we would like to
highlight some limitations of this cohort study. We only measured the humoral
immune response by analysing SARS-CoV-2 anti-spike IgG antibody levels and did
not investigate the T-cell-mediated cellular immune response after SARS-CoV-2
vaccination. We did not measure neutralising antibodies but used anti-spike
antibodies as a surrogate [23]. Recent studies have found that individuals
boosted with mRNA vaccines exhibited potent neutralisation of Omicron in the
general population, suggesting an enhanced cross-reactivity of neutralising
antibody responses [24]. However, recent studies in transplant recipients have shown
a suboptimal antibody response against the SARS-CoV-2 Omicron variant after a
third dose of mRNA vaccine [25, 26]. Therefore, future recommendations for an
antibody-driven vaccination strategy for immunosuppressed patients should
account for potential differences in inducing neutralising immunity against new
variants. Our cutoff of 550 BAU/ml for consideration as a partial responder and
receiving a re-vaccination was chosen arbitrarily. However, another study showed
that SARS-CoV-2 anti-spike IgG antibodies of 550 BAU/ml are >80% protective
in preventing symptomatic COVID-19 [27].
In our study, only monovalent SARS-CoV-2 booster vaccines were administered. Therefore,
the results should be cautiously extrapolated to bivalent SARS-CoV-2 booster
vaccines. The immune response following mRNA vaccination may vary among
different groups of organ transplant recipients. In a recent study [28], the antibody
response after the third SARS-CoV-2 vaccination was compared in different
groups of solid organ transplant recipients (liver, kidney, lung, heart,
combined). Liver recipients but none of the other groups were positively
associated with an antibody response in multivariable analysis. Therefore, the
results of our study regarding liver transplant recipients should be cautiously
extrapolated to other solid organ transplant recipients.
In
conclusion, our results underline that a strategy based on antibody measurement
after two doses of vaccination, and subsequent administration of a third dose
in patients with an absent or partial response, effectively elicited antibody
response in the vast majority of patients, with 24% lacking a response after
three doses. Knowledge of an inadequate humoral immune response may provide an
early opportunity to implement prophylaxis and prevention measures in liver
transplant recipients at high risk of severe COVID-19.
Data availability
statement
The data
that support the findings of this study are not publicly available because they
contain information that could compromise the privacy of research participants
but are available from Annalisa Berzigotti (corresponding author) in
consultation with the Bern Ethics Committee’s rules and regulations.
Acknowledgments
Author contributions: Isabella C. Schoepf and Carlotta
Riebensahm: literature search, data curation, formal analysis, writing -
original draft; Chiara Becchetti: data interpretation, writing - review and
editing; Valentine Blaser, Céline V. Unternährer: data collection; Vanessa
Banz: writing - review and editing; Cédric Hirzel: conceptualisation, writing -
review and editing; Franziska M. Suter-Riniker: resources, methodology;
Annalisa Berzigotti: conceptualisation, supervision, writing- review and
editing, access and verification of the underlying data
Annalisa Berzigotti
Inselspital, Bern University
Hospital
BHH D115
Freiburgstrasse 7
CH-3010 Bern
annalisa.berzigotti[at]insel.ch
References
1. Becchetti C, Gschwend SG, Dufour JF, Banz V. Covid-19 in liver transplant recipients:
A systematic review. J Clin Med. 2021 Sep;10(17):4015. 10.3390/jcm10174015
2. Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, et al.; COVID-LT
group. COVID-19 in an international European liver transplant recipient cohort. Gut.
2020 Oct;69(10):1832–40. 10.1136/gutjnl-2020-321923
3. Guarino M, Cossiga V, Loperto I, Esposito I, Ortolani R, Fiorentino A, et al. COVID-19
in liver transplant recipients: incidence, hospitalization and outcome in an Italian
prospective double-centre study. Sci Rep. 2022 Mar;12(1):4831. 10.1038/s41598-022-08947-x
4. Swissmedic. Swissmedic erteilt Zulassung für den ersten COVID-19-Impfstoff in der
Schweiz. https://www.swissmedic.ch/swissmedic/de/home/news/coronavirus-covid-19/covid-19-impfstoff_erstzulassung.html [accessed: 30 January 2021].
5. Swissmedic. Swissmedic erteilt die Zulassung für den COVID-19 Impfstoff von Moderna.
https://www.swissmedic.ch/swissmedic/de/home/news/coronavirus-covid-19/zulassung-covid-19-impfstoff-moderna.html [accessed: 30 January 2021].
6. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al.; C4591001
Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N
Engl J Med. 2020 Dec;383(27):2603–15. 10.1056/NEJMoa2034577
7. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al.; COVE Study Group.
Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb;384(5):403–16.
10.1056/NEJMoa2035389
8. Boyarsky BJ, Werbel WA, Avery RK, Tobian AA, Massie AB, Segev DL, et al. Antibody
Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients.
JAMA. 2021 Jun;325(21):2204–6. 10.1001/jama.2021.7489
9. Rabinowich L, Grupper A, Baruch R, Al. E. Low immuno-genicity to SARS-CoV-2 vaccination
among liver transplant recipients. J Hepatol 2021;10: 1016/j.
10. Marion O, Del Bello A, Abravanel F, Couat C, Faguer S, Esposito L, et al. Safety and
immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ
transplants. Ann Intern Med. 2021 Sep;174(9):1336–8. 10.7326/M21-1341
11. Wei J, Matthews PC, Stoesser N, Maddox T, Lorenzi L, Studley R, et al.; COVID-19 Infection
Survey team. Anti-spike antibody response to natural SARS-CoV-2 infection in the general
population. Nat Commun. 2021 Oct;12(1):6250. 10.1038/s41467-021-26479-2
12. Faro-Viana J, Bergman ML, Gonçalves LA, Duarte N, Coutinho TP, Borges PC, et al. Population
homogeneity for the antibody response to COVID-19 BNT162b2/Comirnaty vaccine is only
reached after the second dose across all adult age ranges. Nat Commun. 2022 Jan;13(1):140.
10.1038/s41467-021-27761-z
13. Marion O, Del Bello A, Abravanel F, Couat C, Faguer S, Esposito L, et al. Safety and
immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ
transplants. Ann Intern Med. 2021 Sep;174(9):1336–8. 10.7326/M21-1341
14. Caillard S, Chavarot N, Bertrand D, Kamar N, Thaunat O, Moal V, et al.; French Society
of Transplantation. Occurrence of severe COVID-19 in vaccinated transplant patients.
Kidney Int. 2021 Aug;100(2):477–9. 10.1016/j.kint.2021.05.011
15. Herrera S, Colmenero J, Pascal M, Escobedo M, Castel MA, Sole-González E, et al. Cellular
and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart
transplant recipients. Am J Transplant. 2021 Dec;21(12):3971–9. 10.1111/ajt.16768
16. Strauss AT, Hallett AM, Boyarsky BJ, Ou MT, Werbel WA, Avery RK, et al. Antibody Response
to Severe Acute Respiratory Syndrome-Coronavirus-2 Messenger RNA Vaccines in Liver
Transplant Recipients. Liver Transpl. 2021 Dec;27(12):1852–6. 10.1002/lt.26273
17. Cholankeril G, Al-Hillan A, Tarlow B, Abrams D, Jacobs JS, Flores NP, et al. Clinical
Factors Associated With Lack of Serological Response to SARS-CoV-2 Messenger RNA Vaccine
in Liver Transplantation Recipients. Liver Transpl. 2022 Jan;28(1):123–6. 10.1002/lt.26351
18. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an
mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021 Aug;385(7):661–2.
10.1056/NEJMc2108861
19. Del Bello A, Abravanel F, Marion O, Couat C, Esposito L, Lavayssière L, et al. Efficiency
of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid
organ transplant recipients. Am J Transplant. 2022 Jan;22(1):322–3. 10.1111/ajt.16775
20. Kamar N, Abravanel F, Marion O, Esposito L, Hebral AL, Médrano C, et al. Anti-SARS-CoV-2
spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2
vaccine in a large cohort of solid organ transplant patients. Am J Transplant. 2022 May;22(5):1467–74.
10.1111/ajt.16950
21. Rashidi-Alavijeh J, Frey A, Passenberg M, Korth J, Zmudzinski J, Anastasiou OE, et
al. Humoral Response to SARS-Cov-2 Vaccination in Liver Transplant Recipients-A Single-Center
Experience. Vaccines (Basel). 2021 Jul;9(7):738. 10.3390/vaccines9070738
22. Davidov Y, Indenbaum V, Tsaraf K, Cohen-Ezra O, Likhter M, Ben Yakov G, et al. A third
dose of the BNT162b2 mRNA vaccine significantly improves immune responses among liver
transplant recipients. J Hepatol. 2022 Sep;77(3):702–9. 10.1016/j.jhep.2022.03.042
23. Mahmoud SA, Ganesan S, Naik S, Bissar S, Zamel IA, Warren KN, et al. Serological Assays
for Assessing Postvaccination SARS-CoV-2 Antibody Response. Microbiol Spectr. 2021 Oct;9(2):e0073321.
10.1128/Spectrum.00733-21
24. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. mRNA-based
COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron
variant. Cell. 2022 Feb;185(3):457–466.e4. 10.1016/j.cell.2021.12.033
25. Kumar D, Hu Q, Samson R, Ferreira VH, Hall VG, Ierullo M, et al. Neutralization against
Omicron variant in transplant recipients after three doses of mRNA vaccine. Am J Transplant.
2022 Aug;22(8):2089–93. 10.1111/ajt.17020
26. Al Jurdi A, Gassen RB, Borges TJ, Lape IT, Morena L, Efe O, et al. Suboptimal antibody
response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney
transplant recipients. Kidney Int. 2022 Jun;101(6):1282–6. 10.1016/j.kint.2022.04.009
27. Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al.; Oxford COVID Vaccine
Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2
infection. Nat Med. 2021 Nov;27(11):2032–40. 10.1038/s41591-021-01540-1
28. Balsby D, Nilsson AC, Möller S, et a. Determinants of Antibody Response to a Third
SARS-CoV-2 mRNA Vaccine Dose in Solid Organ Transplant Recipients:Results from the
Prospective Cohort Study COVAC-Tx Vaccines 2022;10(565):1-7.
Appendix
The appendix is available in the pdf version of the article.


