Management of biliary obstruction in patients with newly diagnosed
alveolar echinococcosis: a Swiss retrospective cohort study
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40116
Sandra Müllerab,
Soleen Ghafoorc,
Cordula Meyer zu Schwabedissena,
Felix Grimmd,
Fritz Ruprecht Murraye,
Lars Husmannf,
Nadine Stanekg,
Peter Deplazesd,
Christoph Schlaga,
Andreas E. Kremerah,
Christoph Gublere,
Cäcilia S. Reinerc,
David
Semelab,
Beat Müllhauptah,
Ansgar Deibelah
a Department
of Gastroenterology and Hepatology, University Hospital Zurich, Zurich,
Switzerland
b Department
of Gastroenterology and Hepatology, Cantonal Hospital St. Gallen, St. Gallen,
Switzerland
c Institute
of Diagnostic and Interventional Radiology, University Hospital Zurich, Zurich,
Switzerland
d Institute
of Parasitology, University Zurich, Zurich, Switzerland
e Department
of Gastroenterology and Hepatalogy, Stadtspital Zurich, Zurich, Switzerland
f Department
of Nuclear Medicine, University Hospital Zurich, Zurich, Switzerland
g Department
of Gastroenterology and Hepatology, Luzerner Kantonsspital, Luzern, Switzerland
h Swiss
HPB (Hepato-Pancreato-Biliary) Center, University Hospital Zurich, Zurich,
Switzerland
Summary
BACKGROUND AND STUDY AIMS: Alveolar echinococcosis, an
orphan zoonosis affecting the liver, is of increasing concern worldwide. Most
symptomatic cases present at an advanced and inoperable stage, sometimes with
biliary obstruction prompting biliary tract interventions. These are, however,
associated with a high risk of infectious complications. The aim of this
retrospective study was to compare the effectiveness and safety of conservative
and interventional treatment approaches in patients with newly diagnosed alveolar
echinococcosis and biliary obstruction.
PATIENTS AND METHODS: Alveolar echinococcosis patients
treated at two referral centres in Switzerland, presenting with hyperbilirubinaemia
(total bilirubin >1.5 Upper Limit of Normal) at diagnosis were included,
unless another underlying aetiology, i.e. common bile duct stones or
decompensated cirrhosis, was identified. Patients were divided into two groups,
according to whether they initially received a biliary tract intervention. The
primary endpoint was normalisation of bilirubin levels within a 6-month period.
Secondary endpoints included, among others, the occurrence of early and late
biliary complications, the need for biliary tract interventions during
follow-up and overall duration of hospital stays for treatment initiation and for
biliary complications.
RESULTS: 28 patients were included in this study, of whom 17
received benzimidazole therapy alone and 11 additionally received a biliary
tract intervention. Baseline characteristics did not differ between groups. All
but one patient in each group achieved the primary endpoint (p=0.747). Biliary
tract intervention was associated with faster laboratory improvement (t1/2
1.3 vs 3.0 weeks), but also with more frequent early biliary
complications (7/11 vs 1/17, p=0.002) and longer initial hospital stay (18 days
vs 7 days, p=0.007).
CONCLUSION: Biliary
obstruction in patients with newly diagnosed alveolar echinococcosis can be
treated effectively with benzimidazole therapy alone. Biliary tract
intervention, on the other hand, is associated with a high complication rate
and should probably be reserved for patients with insufficient response to
benzimidazole therapy.
Introduction
Alveolar echinococcosis is an orphan zoonosis caused by the
larval stage of Echinococcus multilocularis, the fox tapeworm, which is
endemic across large parts of the northern hemisphere [1]. Infection occurs accidentally
through ingestion of E. multilocularis eggs, which hatch in the
intestine and migrate as oncospheres mainly to the liver [2]. There, the
oncospheres transform into metacestodes and cause a silently progressing
hepatic disease with infiltrative growth mimicking a malignant tumour [1]. In
some patients, the parasitic tumour invades and occludes bile ducts and blood
vessels as well as neighbouring organs [1]. Consequently, obstructive jaundice,
cholangitis, secondary Budd-Chiari syndrome and portal vein thrombosis with or
without portal hypertension and its complications can occur [3]. Staging of the
disease is done through the PNM classification, similarly to TNM staging of
solid malignancies [4].
Without adequate treatment, 90% of alveolar echinococcosis
patients die within 10 years [5]. A cure is only achieved by R0 resection followed
by two years of benzimidazole treatment or, rarely, by liver transplantation
[6]. However, since patients frequently present at an advanced stage, R0
resection is possible only in 20–50% of cases [6]. Inoperable alveolar
echinococcosis requires long-term treatment with benzimidazoles, which is
generally well tolerated and has been shown to be highly effective at stopping
disease progression [7]. Parameters associated with disease activity are anti-Em18
(formerly called anti-EmII/3-10) antibody levels and perilesional fluorodeoxyglucose
(FDG)-uptake on PET/CT imaging [8–9]. Possible drug-related side effects
include myelosuppression, hair loss, gastrointestinal symptoms, elevated liver
enzymes and in rare cases acute liver injury [5, 8]. Today, therapy discontinuation
can be considered in highly selected inoperable patients [10–12].
Alveolar echinococcosis is of increasing concern in Europe
and other endemic areas, where incidences have been rising since the turn of
the millennium [13–16]. Different explanations for this phenomenon have been
discussed elsewhere [17–18]. One possible explanation is a more susceptible
population, as an increase in patients with a concomitant
immunosuppression-associated condition has been observed since the 2000s decade
[14]. However, the impact of these immunosuppression-associated conditions seems insufficient
to explain the
steady increase in new cases per year when analysed with a linear model over
the past two decades [15]. More likely, the habitat expansion of a growing fox
population into European cities leads to an increasingly E. multilocularis
egg-contaminated environment in densely populated areas and in turn to
increasing infections in humans [19–20].
Biliary obstruction in alveolar echinococcosis is common and
can range from slightly increased laboratory cholestasis parameters to overtly
jaundiced patients, which constitute about 1/10 of all or 1/5 of symptomatic alveolar
echinococcosis patients [21–22]. Usually, symptomatic obstruction of the large-
and medium-sized bile ducts leading to jaundice and/or pruritus is considered
for treatment with a biliary tract intervention, either an endoscopic
retrograde cholangiopancreatography (ERCP) with placement of biliary drains or,
when retrograde cannulation of the bile duct is not possible, percutaneous
transhepatic cholangiography and drainage (PTCD) or EUS-guided intervention.
There are no uniform criteria to determine when a biliary tract intervention
should or should not be performed, especially in asymptomatic patients with
slight hyperbilirubinaemia. Previous studies have shown the feasibility of
biliary tract intervention in patients with biliary obstruction due to alveolar
echinococcosis, newly diagnosed or under established benzimidazole treatment [23–25].
Yet these interventions are associated with frequent complications including
cholangitis, post-ERCP pancreatitis and/or biliary tract or intestinal
perforation and often require multiple interventions over an extended period of
time [24–25]. Due to similar experience at our departments, a different
approach was sought for newly diagnosed, previously untreated alveolar
echinococcosis patients and for the past two decades an increasing number of
patients were treated with benzimidazole therapy alone, even in the setting of
severe cholestasis. The rationale being that treatment would reduce activity of
the parasitic lesion, perilesional inflammation and consequently the pressure
on the obstructed bile ducts, ultimately restoring bile flow.
The aim of this study was to retrospectively compare
interventional and conservative treatment approaches in patients with newly
diagnosed alveolar echinococcosis treated at our departments over the past two
decades. Of particular interest were the baseline disease characteristics,
including alveolar echinococcosis stage, level of biliary obstruction,
bilirubin levels and presence of pruritus as well as treatment-associated
outcomes, such as decrease in bilirubin levels, early complications due to
biliary tract intervention and late recurrence of cholestasis or occurrence of
secondary biliary complications.
Methods
Study population and design
A retrospective cohort study of patients with alveolar
echinococcosis was undertaken at the two participating study centres. All
patients treated for alveolar echinococcosis at the University Hospital Zurich
since 01/2000 and at the Cantonal Hospital St Gallen since 01/2010 were
reviewed for study inclusion. The chosen dates reflect the implementation of
electronic hospital information systems at the participating centres allowing easy
identification of patients and optimal data quality. Both centres are tertiary
referral centres, therefore patients may have presented and initially been
treated at another clinic beforehand. All relevant medical data from external
sources, including reports of biliary tract interventions and imaging data,
were validated and included in this study. All patients presenting at diagnosis
with a bilirubin level ≥32 µmol/l (≥1.5 ULN [Upper Limit of Normal]) and
suspected biliary obstruction due to alveolar echinococcosis were included.
Patients with hyperbilirubinaemia due to a cause other than alveolar
echinococcosis, i.e. liver disease including acute viral, alcoholic, autoimmune
or drug-induced hepatitis, decompensated cirrhosis, vascular liver disease and
inborn errors of bilirubin metabolism were excluded, as were those with an
independent indication for ERCP, such as concomitant common bile duct stones,
biliary pancreatitis, cholangitis and biliary obstruction due to biliary or
pancreatic malignancy. The cohort was then divided into two groups, depending
on whether patients received a biliary tract intervention (ERCP/PTCD) within 14
days of starting benzimidazole therapy (group A) or were treated with
benzimidazole therapy alone (group B). Treatment initiation was defined as the
date of biliary tract intervention or the start of benzimidazole therapy – whichever
occurred first. The
follow-up period lasted from treatment initiation until surgical resection,
last contact or study closure in March 2022.
Analysis parameters included age at diagnosis and sex,
parameters of cholestasis including total bilirubin levels, pruritus and
evidence of bile duct dilation on cross-sectional imaging, as well as
parameters of biliary tract or cyst infection including CRP levels and reported
fever. Collected data regarding medical treatment included initiation of
benzimidazole therapy, benzimidazole type and initial maintenance dose.
Characteristics of the first biliary tract intervention of interest were
modality (ERCP vs PTCD), papillotomy (conventional vs precut), biliary tract
manipulation by balloon dilation or brush cytology, potential insertion of
drains and their size, prophylactic use of antibiotic therapy.
Diagnosis of alveolar echinococcosis and disease activity
Diagnosis of alveolar echinococcosis was classified
according to WHO criteria as ‘possible’, ‘probable’ or ‘definitive’ [4]. To
verify the diagnosis, all patients had serological testing for alveolar
echinococcosis consisting of a combination of anti-EgP, anti-EgHF, anti-Em2+
and anti-EmG11 enzyme-linked immunosorbent assay (ELISA), as well as anti-AgB
Western blot or EITB [26]. Anti-EmII/3-10 or anti-Em18 antibody ELISA results were
used in this study as parameters of parasite viability [8, 10–11]. However,
only after publication of the initial study in 2004, showing their usefulness
in this regard, were these tests routinely used in our departments.
Additionally, if available, PET/CT imaging at any time during follow-up was
used to determine whether patients had active disease [9, 27].
Analysis of cross-sectional imaging
Baseline cross-sectional images (contrast-enhanced CT, MRI
and PET/CT) at the time of the initial diagnosis were reviewed by a board-certified
radiologist specialised in abdominal imaging (SG, 7 years of experience in
cross-sectional imaging). The alveolar echinococcosis was staged according to
the WHO PNM classification system [4]. In addition, the level of the biliary
obstruction caused by the lesion was categorised based on imaging as follows:
at the level of the common bile duct, at the level of the hepatic hilum, at the
level of the right/left hepatic duct, or segmental/at the level of the
secondary biliary radicals.
Definition of study endpoints
The primary endpoint was defined as normalisation of
bilirubin levels within a 6-month period after treatment initiation. Secondary
endpoints were the decrease in total bilirubin levels over a 6-month period
after treatment initiation; possible discontinuation of benzimidazole therapy
due to side effects; number of biliary tract interventions and duration of
initial biliary stent placement; occurrence and type of early biliary
complications; occurrence and type of late biliary complications during the
entire follow-up period, including type of and time to complication; need for
biliary tract interventions during follow-up, including time to intervention; the
overall duration of hospital stays for treatment initiation and for biliary
complications; curative surgical resection during follow-up and time to
surgery.
Early biliary complications were defined as the occurrence
of any of the following within 30 days after the biliary tract intervention or the
start of benzimidazole therapy: cholangitis, pancreatitis, post-papillotomy
bleeding, small bowel perforation, stent dysfunction or death. Late biliary
complications were defined as the occurrence of any of the following beyond 30
days after biliary tract intervention or start of benzimidazole therapy:
recurrent cholestasis with or without cholangitis, secondary sclerosing
cholangitis or biliary cirrhosis.
Endpoints were assessed in an intention-to-treat manner, meaning
that associations were sought with the treatment groups defined earlier.
Statistical analysis
The statistical analysis was performed using the appropriate
test in Graphpad Prism 8 (GraphPad Software, Inc.; Boston, MA, USA). To compare
unpaired, numerical data the Mann-Whitney U-test was used. For unpaired,
categorical data the Fisher’s exact or Chi-square test was applied. Missing
data were interpreted as “missing completely at random”. A two-tailed p-value
<0.05 was regarded as statistically significant. Additionally, a one-phase
decay model was used to determine the average half-life of bilirubin after
treatment initiation. The plateau was set to lie between 0 and 21 µmol/l, the upper
limit of normal for bilirubin.
Ethical considerations
Ethical approval for this study was obtained from the local
ethics committee in Zurich (Kantonale Ethikkomission Zürich, BASEC 2021-01136).
Patients followed up at our clinics provided written informed consent either
through participation in the Eastern Switzerland Echinococcosis Cohort Study
(BASEC: 2020-00495) – which also
includes deceased patients for whom written consent was waived – or by providing General
Consent.
This study is reported following the Strengthening the
Reporting of Observational Studies in Epidemiology (STROBE) Statement Checklist.
Results
Study population
Of patients treated for alveolar echinococcosis, 227 at the University Hospital
Zurich and 45 patients at the Cantonal Hospital St Gallen, 33 met inclusion
criteria by presenting with bilirubin levels of ≥32 µmol/l (≥1.5 ULN) at first
presentation (figure 1). Of these 33, 5 were excluded due to other reasons for
hyperbilirubinaemia, including common bile duct stones (n = 3), biliary
pancreatitis (n = 1) and decompensated liver cirrhosis (n = 1, figure 1). In 11
patients, biliary tract intervention was performed (group A), while 17 patients
were managed conservatively with benzimidazole therapy alone (group B, figure
1). In 19 patients, alveolar echinococcosis was diagnosed as ‘probable’
according to WHO criteria by imaging and positive serology, and in 9 patients
by histopathology, corresponding to a ‘definitive’ diagnosis according to WHO
criteria.
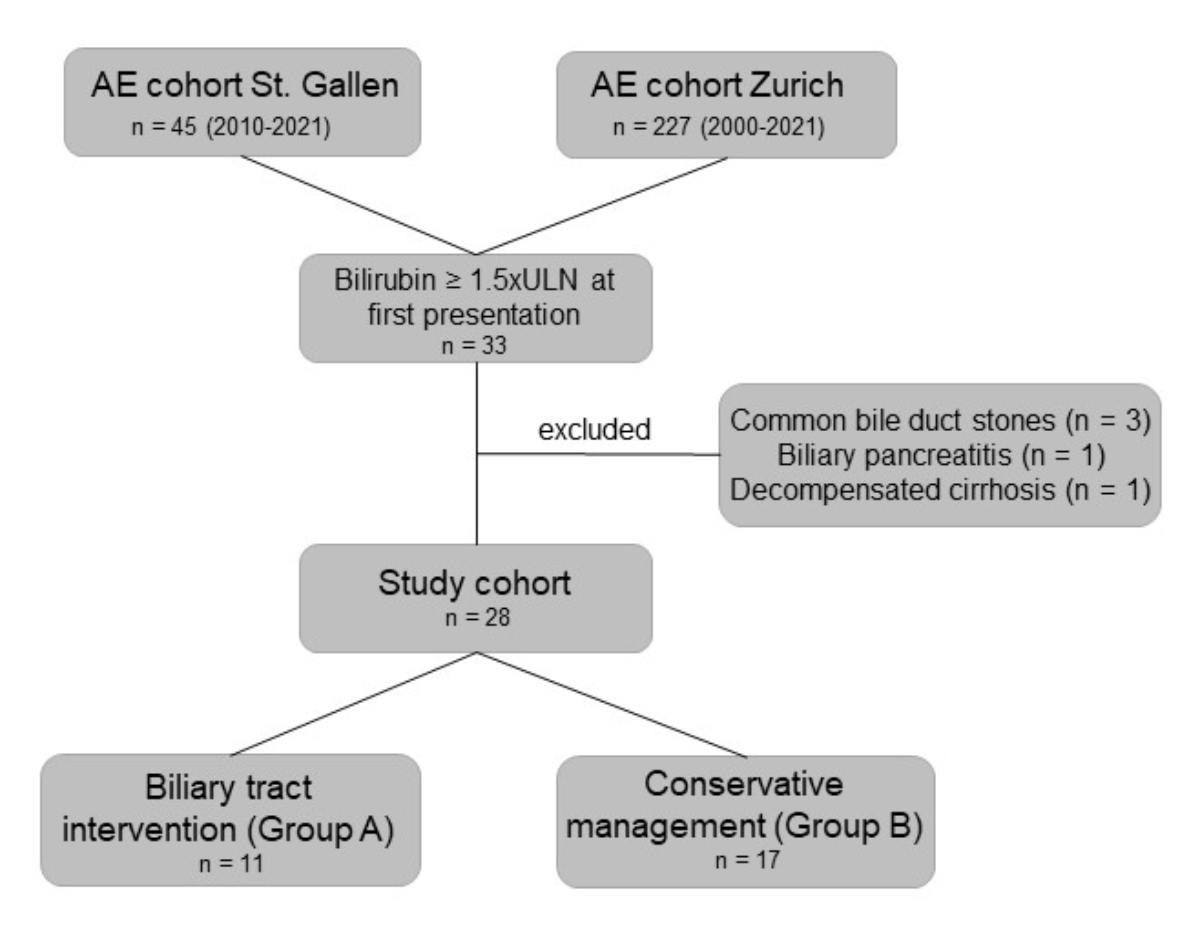
Figure 1Study flowchart. The chart depicts patients who were screened at both centres.
33 patients had hyperbilirubinaemia at initial presentation. 5 of them were
excluded due to other reasons of cholestasis or endoscopic retrograde
cholangiography (ERC) indication, leaving 28 patients for final analysis. 11
received a biliary tract intervention (group A), while 17 patients were treated
with benzimidazole therapy alone (group B).AE: alveolar echinococcosis; ULN: upper
limit of normal.
Baseline patient characteristics including image analysis
At baseline, there were no significant differences regarding
age at diagnosis, sex, PNM classification, alveolar echinococcosis stage (early
vs late), level of biliary obstruction and presence of dilated intrahepatic
bile ducts on cross-sectional imaging, bilirubin levels, reported pruritus, CRP
levels and reported fever. There was a numerical trend for less advanced local
liver involvement (‘P’ classification) and early alveolar echinococcosis stage
in group A (table 1). Either positive anti-Em18 (-EmII/3-10) serology or
increased FDG uptake on PET/CT indicating active alveolar echinococcosis was
seen in all but three patients in group A (table 1). In these three patients,
serological markers assessing parasite viability were either not assessed (n =
2) or negative (n = 1); none of the three had had PET/CT scanning.
Table 1This table indicates the baseline characteristics of
patients of both groups in regard to alveolar echinococcosis disease, biliary
obstruction and infectious complication before initiation of any therapy. There
were no significant differences between the groups. For the purpose of
comparison, level of biliary obstruction was assessed by review of
cross-sectional imaging in both groups.
| |
Group
A (intervention): n = 11 |
Group
B (conservative): n = 17 |
p
value (univariate) |
| Age at diagnosis, median (range) |
54 years (28–74) |
47 years (23–73) |
0.131 |
| Female sex, n (%) |
4 (36.4) |
11 (64.7) |
0.246 |
| PNM classification (n, %) |
0.059 |
|
P2 |
3 (27.3) |
– |
|
| P3 |
4 (45.5) |
9 (52.9) |
|
| P4 |
3 (18.2) |
8 (47.1) |
|
| Missing |
1 (9.1)* |
|
|
| Alveolar echinococcosis stage
(n, %) |
0.055 |
|
I–II |
2 (18.2) |
0 (0) |
|
| III–IV |
8 (90.9) |
17 (100) |
|
| Missing |
1 (9.1)* |
|
|
| Level of biliary obstruction (n,
%) |
0.493 |
|
Common bile duct |
2 (18.4) |
1 (5.9) |
|
| Hilar/bifurcation |
7 (63.6) |
13 (76.5) |
|
| Right/left hepatic branch |
1 (9.1) |
3 (17.6) |
|
| Missing |
1 (9.1)* |
|
|
| Intrahepatic bile duct dilation
on imaging, n (%) |
10 (90.9) |
16 (94.1) |
0.999 |
| Missing: 1 (9.1)* |
|
|
| Positive serological
and/or PET viability parameters, n (%) |
8 (72.7) |
17 (100) |
0.346 |
| Missing: 2 (18.4) |
|
|
| Bilirubin, median (range) |
110 µmol/l (58–456) |
95 µmol/l (32–311) |
0.279 |
| Pruritus, n (%) |
6 (54.5) |
11 (64.7) |
0.701 |
| CRP, median (range) |
10 mg/l (5–28) |
10 mg/l (2–31) |
0.961 |
| Missing: 1 (9.1) |
Missing: 5 (29.4) |
|
| Fever, n (%) |
0 (0) |
0 (0) |
0.999 |
Biliary tract intervention and benzimidazole therapy
In group A, nine patients initially underwent endoscopic
retrograde cholangiography (ERC) and two patients had PTCD a priori (figure 1, table
2). Patients receiving ERC were treated with sphincterotomy and placement of
plastic drains (7–11 Fr) or a metal stent (10 mm) in one patient (table 2).
Additional endoluminal balloon dilation and/or brush cytology was performed in
five of these patients (table 2). The two patients receiving PTCD had no
endoluminal manipulation and drain sizes were 8.5 and 12 Fr (table 2).
Antibiotic prophylaxis was administered in 6/11 patients (table 2). Antibiotic
agents used were amoxicillin/clavulanic acid and ceftriaxone (table 2). Biliary
drainage was in situ for a median of 74.5 days and patients required a median
of three interventions (table 3). Four patients had a biliary stent in situ until
surgical resection of the alveolar echinococcosis lesion (31 days, 48 days, 63
days) or death (20 days). Two patients required prolonged interventional
treatment (8 months and 14 months), of which the latter had the biliary stent
in place at last follow-up. In all but one patient of group A, benzimidazole
therapy was started in addition during follow-up (table 3). The median time
delta was 5 days, but in two was as late as 85 and 140 days after biliary tract
intervention (table 3).
Table 2Characteristics of the first biliary tract intervention. Shown are the individual
characteristics of biliary tract
intervention in patients of the group that had early interventional therapy of
the biliary obstruction caused by newly diagnosed alveolar echinococcosis.
| ID |
Modality |
Endoscopic papillotomy |
Brush cytology sampling |
Balloon dilation of stenosis |
Stent material |
Stent size |
Antibiotic prophylaxis |
Early complication(s) |
| 1 |
ERC |
Conv. |
Yes |
8 mm |
Metal |
10 mm |
Ceftriaxone |
Ascending cholangitis / pancreatitis |
| 2 |
ERC |
Conv. |
No |
10 mm |
Plastic |
10 Fr |
Amoxicillin / clavulanic acid |
Ascending cholangitis |
| 3 |
ERC |
Conv. |
No |
15 mm |
Plastic |
7 Fr |
Ceftriaxone |
Ascending cholangitis / pancreatitis |
| 4 |
PTCD |
n.a. |
No |
No |
Plastic |
12 Fr |
Amoxicillin / clavulanic acid |
Ascending cholangitis |
| 5 |
ERC |
Conv. |
No |
No |
Plastic |
11 Fr |
No |
None |
| 6 |
ERC |
Precut |
No |
No |
Plastic |
7 Fr |
Ceftriaxone |
Ascending cholangitis |
| 7 |
ERC |
Conv. |
No |
No |
Plastic |
8.5 Fr |
No |
None |
| 8 |
ERC |
Conv. |
Yes |
No |
Plastic |
10 Fr |
No |
Ascending cholangitis |
| 9 |
ERC |
Conv. |
Yes |
No |
Plastic |
10 Fr |
No |
None |
| 10 |
PTCD |
n.a. |
No |
No |
Plastic |
8.5 Fr |
Ceftriaxone |
None |
| 11 |
ERC |
Conv. |
No |
No |
Plastic |
9 Fr |
No |
Ascending cholangitis |
In group B, all patients received benzimidazole therapy (table
3). The drug of first choice was albendazole in both groups and the median initial
daily maintenance dose did not differ significantly between groups (group A 600 mg,
group B 400 mg; p=0.870). Only one patient in group B experienced side
effects from albendazole treatment, requiring a switch to mebendazole. No
patient had severe side effects leading to cessation of benzimidazole
altogether (table 3).
Table 3Secondary endpoints. Patients in the biliary tract intervention group exhibited
significantly more early complications and were hospitalised for a longer
duration for initial treatment. In regard to late complications, there were no
statistically significant differences between groups; however the number of
patients was small in both groups.
| |
Group A (intervention): n = 11 |
Group B (conservative): n = 17 |
p value (univariate) |
| Follow-up, median (range) in months |
12 (1–122) |
50 (4–211) |
0.179 |
| Benzimidazole treatment, n (%) |
10 (90.9) |
17 (100) |
n.a. |
| |
Time delta to biliary tract intervention, median (range) |
5 days (-4–140) |
n.a. |
|
| Discontinuation due to adverse drug reaction, n (%) |
0 (0) |
0 (0) |
|
| Initial biliary tract intervention, n (%) |
11 (100) |
0 (0) |
n.a. |
| |
Duration of stent placement, median (range) |
74.5 days (8–436) |
n.a. |
|
| Number of interventions, median (range) |
3 (1–5) |
n.a. |
|
| Early complications, n (%) |
7 (63.6) |
1 (5.9) |
0.002* |
| |
Cholangitis, n |
7 |
1b |
|
| Pancreatitis, n |
2 |
n.a. |
|
| Death, n |
1a |
0 |
|
| Late complications, n (%) |
4 (36.4) |
4 (23.5) |
0.672 |
| |
Recurrent cholestasis, n |
2 |
2 |
|
| Cholangitis, n |
2 |
2c |
|
| Secondary sclerosing cholangitis or biliary cirrhosis, n |
0 |
0 |
|
| Death, n |
0 |
0 |
|
| Biliary tract intervention for late complications, n (%) |
4 (36.4) |
3 (17.6) |
0.381 |
| |
Time to intervention |
4, 5, 12, 76 months |
7, 65, 95 months |
|
| Duration of stent placement |
113, 333, 455, 1602 days |
3, 158, 249 days |
|
| Number of interventions, n |
4, 5, 12, 14 |
1, 5, 7 |
|
| Hospitalisation days |
|
|
|
| |
At initial treatment, median (range) |
18 days (7–107),
n = 10 |
7 days (2–15),
n = 6 |
0.007* |
| During follow-up, median (range) |
43 days (13–117),
n = 3 |
23.5 days (13–26),
n = 4 |
0.486 |
| Surgical resection, n (%) |
3 (27.3) |
3 (17.6) |
0.368 |
| |
Time to surgery |
1, 2, 3 months |
4, 5, 7 months |
|
Primary endpoint
All but one patient in each group achieved the primary
endpoint, i.e. normalisation of bilirubin levels within a 6-month period after
treatment initiation (p = 0.747, figure 2). The one patient in group A had their biliary
drain removed after 8 days due to cholangitis and reinserted after 3 months (figure
2). The one patient in group B received PTCD and shortly thereafter surgical
resection of the alveolar echinococcosis lesion at seven months of follow-up (table
3).
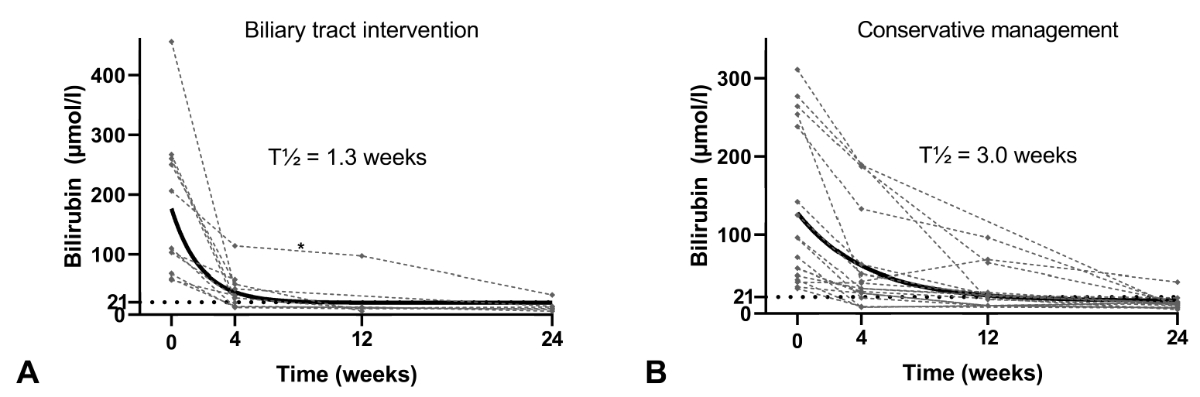
Figure 2Bilirubin levels during follow-up.
The figure shows bilirubin levels of both groups over a period
of six months following treatment initiation. There was no statistically
significant difference between the two groups regarding the primary endpoint.
Patients in group A (left) exhibited fast resolution of hyperbilirubinaemia
with a calculated half-life of 1.3 weeks. * This patient’s biliary drain was removed
after eight days due to cholangitis and reinserted after 3 months.
Patients in group B (right) had a slower resolution of hyperbilirubinaemia
with a calculated half-life of 3.0 weeks. However, all but one
patient in this group achieved the primary endpoint.
Secondary endpoints
The follow-up period at a study centre – as defined for this study – of patients in
group A reached a
median of 12 months and that of patients in group B a median of 50 months (table
3). In group A, 10/11 patients (91%) showed fast resolution of hyperbilirubinaemia,
with a calculated bilirubin half-life of 1.3 weeks ≈ 9 days (figure 2). Early
biliary complications were reported in 7/11 patients (64%), seven had
cholangitis within 30 days of intervention and in two post-ERCP pancreatitis
was additionally reported (tables 2 and 3). Concordantly, 10/11 patients (91%)
were hospitalised at treatment initiation and the median duration of hospital
stay was significantly longer compared to group B (table 3). The one patient
who did not receive benzimidazole therapy at all experienced cholangitis with
septic shock and multiorgan failure following ERC and required ICU admission. However,
he refused life support with dialysis and mechanical ventilation and ultimately
died within one month (table 3).
Patients in group B showed a slower resolution of
hyperbilirubinaemia, with a calculated bilirubin half-life of 3.0 weeks ≈ 21
days (figure 2). A significant regression of intrahepatic bile duct dilation
could be observed on magnetic resonance cholangiopancreatography (MRCP), when
performed (figure 3). In this group, only one early biliary complication was
reported (table 3). This patient had cholangitis that resolved with antibiotic
therapy alone (table 3). Only 6/17 patients (35%) were hospitalised at
treatment initiation and the median duration of the hospital stay was
significantly shorter when compared to group B (table 3). Two patients in this
group received surgical resection of the alveolar echinococcosis lesion after
resolution of hyperbilirubinaemia after four and five months of follow-up (table
3).
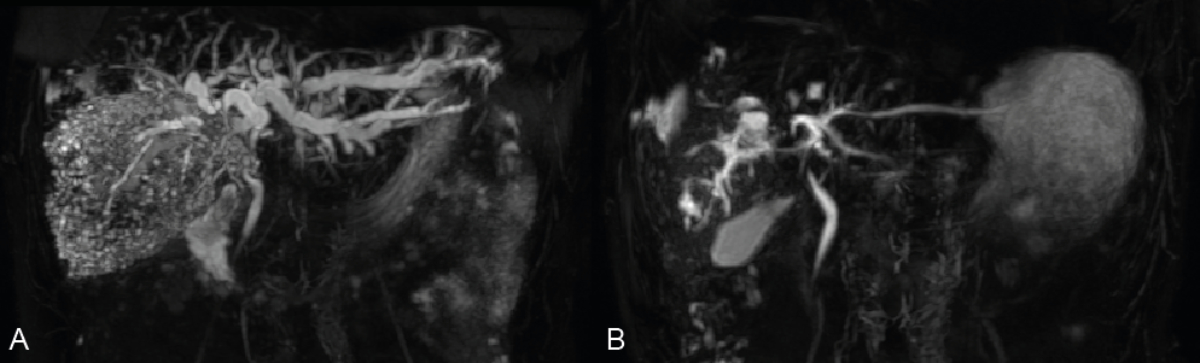
Figure 3Representative magnetic resonance cholangiopancreatography
(MRCP) of a patient treated with benzimidazole therapy alone (group B). The
left MRCP image indicates the status before benzimidazole initiation (A) while
the right panel illustrates the biliary tract following six months of therapy
(B). Note the striking decrease in bile duct dilation. Bilirubin levels were
227 µmol/l at initial presentation and had completely resolved (10 µmol/l) by
six months.
Late biliary complications, consisting of recurrent
cholestasis or cholangitis, occurred in four patients of group A (36%) and four
patients of group B (24%, table 3). In group A, three patients received a repeat
biliary tract intervention during long-term follow-up and required hospitalisation
with a median duration of 43 days (table 3). Three patients in group B (18%)
required a biliary tract intervention during follow-up (table 3). In two
patients, cholangitis could be treated by antibiotic therapy alone. Four
patients required hospitalisation with a median duration of 23.5 days (table
3). In both groups, biliary tract intervention during follow-up required
long-term placement of biliary drains (table 3). Secondary sclerosing
cholangitis, biliary cirrhosis or death during the defined follow-up period for
late biliary complications was not reported in either group (table 3).
Discussion
The primary aim of the study was to compare two different
approaches – conservative vs interventional – for the treatment of biliary
obstruction in newly diagnosed alveolar echinococcosis patients. At baseline, the
two groups did not show significant differences in disease severity, in
particular regarding the extent of cholestasis. In both groups, the primary
endpoint – normalisation of the bilirubin level within a 6-month period after
treatment initiation – could be achieved in all but one patient each, which was
not statistically different. While symptomatic hyperbilirubinaemia resolved
faster after biliary tract intervention (t1/2 1.3 vs 3.0 weeks),
there was also an association with more early, intervention-associated
complications, mainly cholangitis and pancreatitis. Consequently, these
patients required longer hospitalisation at treatment initiation (median 18
days) and one patient died due to the resulting septic shock with multiorgan
failure. On the other hand, patients treated conservatively (benzimidazole
therapy alone) demonstrated slower regression of hyperbilirubinaemia but only a
single early biliary complication. Late biliary complications did not occur
more frequently compared to the intervention group.
The major limitations of this study include its
retrospective design, which entails comparison on the basis of non-uniform
decision criteria, as well as the small sample size and long inclusion period.
There is also the risk of selection bias in the study’s patient population.
While success with conservative management in individual alveolar
echinococcosis patients led to abandonment of the interventional approach in
most patients who were diagnosed and treated at the two participating tertiary
referral hospitals during the last two decades, a substantial number of
patients (7) had their initial biliary tract intervention at secondary care
hospitals and were referred shortly thereafter. It cannot be excluded that alveolar
echinococcosis patients with biliary obstruction at initial presentation and an
uneventful course after biliary tract intervention were not referred by these
secondary care hospitals. However, this is highly unlikely as the two centres
involved in the study are the reference centres for alveolar echinococcosis
cases in Eastern Switzerland.
Furthermore, significant changes have been made in patient
management during biliary tract intervention over the observed period. In
particular, the prophylactic use of NSAIDs for post-ERCP pancreatitis was
established [28]. The date of biliary tract intervention, however, was not
relevant in group A, as these patients were spread evenly across the
observation period with three having had interventions as recently as 2020 and
2021. Unfortunately, the use of NSAID prophylaxis could not be assessed due to
inconsistent reporting.
There are only a few studies that have reported the
complication rate after biliary intervention in alveolar echinococcosis
patients. In a small German series of seven patients treated with ERC, two
(29%) developed post-ERC cholangitis [24]. However, only two patients in this
group had had no prior ERC [24]. Similarly, in a European survey, complications
were reported in 22 of 129 (17%) biliary tract interventions [25]. However,
these 129 interventions were carried out in only 38 patients who hence received
repeated interventions [25].
Further limitations to the methodology of this study include
the lack of assessment of pruritic symptoms during follow-up, especially in the
conservative treatment group. This was due to the inconsistent reporting of
such symptoms by physicians. Our clinical experience is that patients’ response
to these symptoms correlate with the decrease in bilirubin levels seen
following treatment initiation, with most patients reporting a substantial
improvement of pruritus within 4 weeks. Another limitation is the assessment of
the level of biliary obstruction by review of cross-sectional imaging, which may
differ slightly compared to retrograde cholangiography. The rationale here was
to have a method that allows comparison between the groups. Inherently,
radiologists report the level of biliary obstruction according to the antegrade
flow of bile, while endoscopists interpret the retrograde filling of the bile
ducts with contrast medium during ERC.
In conclusion, the study shows that biliary obstruction in
patients with newly diagnosed alveolar echinococcosis can be treated
effectively with benzimidazole therapy alone. This approach was associated with
fewer early, treatment-associated biliary complications compared to upfront
biliary tract intervention, whereas the frequency of late biliary complications
did not differ between groups. While true treatment superiority with regard to
efficacy and safety can only be assessed in a randomised controlled trial, such
data is unlikely to be procured in the near future due to the low incidence of alveolar
echinococcosis. Based on our preliminary data, we recommend reserving biliary
tract intervention for patients who do not respond sufficiently to
benzimidazole therapy alone within six months or develop recurrent cholestasis
/ cholangitis while already on established benzimidazole therapy.
Data availability statement
Data will be made available, if approved by the Ethics
Committee Zurich (contact via info.kek[at]kek.zh.ch).
Acknowledgments
This
project was supported by the Fontana Foundation.
Author contributions: AD and BM conceived the study. AD
obtained ethical approval, designed and supervised the study. SM and AD
retrieved retrospective data, performed data analysis and wrote the manuscript
together. SG reviewed cross-sectional imaging of patients. CMzS recruited and
consulted alveolar echinococcosis patients for the Zurich Echinococcosis Cohort
Study. FG supervised and interpreted alveolar echinococcosis serology. SG, FG,
CMzS, FRM, LH, NS, PD, CS, AEK, CG, CSR, DS and BM critically revised the
manuscript for important intellectual content. All authors approved the final
version of the manuscript.
Dr. med. Ansgar Deibel
Department of Gastroenterology and Hepatology
University Hospital Zurich
Raemistrasse 100
CH-8091 Zurich
rudolfansgar.deibel[at]usz.ch
References
1. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis,
a zoonosis of increasing concern. Clinical microbiology reviews. 2004;17(1):107-35.
Epub 2004/01/17. doi: 10.1128/cmr.17.1.107-135.2004. PubMed PMID: 14726458; PubMed Central PMCID: PMCPMC321468. 10.1128/CMR.17.1.107-135.2004
2. Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, et al. Ecology and
Life Cycle Patterns of Echinococcus Species. Adv Parasitol. 2017;95:213-314. Epub
20170106. doi: 10.1016/bs.apar.2016.11.002. PubMed PMID: 28131364.
3. Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C, et al. The
Echinococcoses: Diagnosis, Clinical Management and Burden of Disease. Adv Parasitol.
2017;96:259–369. 10.1016/bs.apar.2016.09.006
4. Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, et al. WHO classification of
alveolar echinococcosis: principles and application. Parasitol Int. 2006;55 Suppl:S283–7.
10.1016/j.parint.2005.11.041
5. Ammann RW, Hoffmann AF, Eckert J. [Swiss study of chemotherapy of alveolar echinococcosis—review
of a 20-year clinical research project]. Schweiz Med Wochenschr. 1999 Feb;129(8):323–32.
6. Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for
the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta
Trop. 2010 Apr;114(1):1–16. 10.1016/j.actatropica.2009.11.001
7. Torgerson PR, Schweiger A, Deplazes P, Pohar M, Reichen J, Ammann RW, et al. Alveolar
echinococcosis: from a deadly disease to a well-controlled infection. Relative survival
and economic analysis in Switzerland over the last 35 years. J Hepatol. 2008 Jul;49(1):72–7.
10.1016/j.jhep.2008.03.023
8. Ammann RW, Renner EC, Gottstein B, Grimm F, Eckert J, Renner EL; Swiss Echinococcosis
Study Group. Immunosurveillance of alveolar echinococcosis by specific humoral and
cellular immune tests: long-term analysis of the Swiss chemotherapy trial (1976-2001).
J Hepatol. 2004 Oct;41(4):551–9. 10.1016/j.jhep.2004.06.015
9. Reuter S, Schirrmeister H, Kratzer W, Dreweck C, Reske SN, Kern P. Pericystic metabolic
activity in alveolar echinococcosis: assessment and follow-up by positron emission
tomography. Clin Infect Dis. 1999 Nov;29(5):1157–63. 10.1086/313438
10. Ammann RW, Stumpe KD, Grimm F, Deplazes P, Huber S, Bertogg K, et al. Outcome after
Discontinuing Long-Term Benzimidazole Treatment in 11 Patients with Non-resectable
Alveolar Echinococcosis with Negative FDG-PET/CT and Anti-EmII/3-10 Serology. PLoS
neglected tropical diseases. 2015;9(9):e0003964. Epub 2015/09/22. doi: 10.1371/journal.pntd.0003964. PubMed PMID: 26389799; PubMed Central PMCID: PMCPMC4577091.
11. Deibel A, Stocker D, Meyer Zu Schwabedissen C, Husmann L, Kronenberg PA, Grimm F,
et al. Evaluation of a structured treatment discontinuation in patients with inoperable
alveolar echinococcosis on long-term benzimidazole therapy: A retrospective cohort
study. PLoS neglected tropical diseases. 2022;16(1):e0010146. Epub 20220128. doi:
10.1371/journal.pntd.0010146. PubMed PMID: 35089933; PubMed Central PMCID: PMCPMC8827419.
12. Caoduro C, Porot C, Vuitton DA, Bresson-Hadni S, Grenouillet F, Richou C, et al. The
role of delayed 18F-FDG PET imaging in the follow-up of patients with alveolar echinococcosis.
Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54(3):358-63.
Epub 2013/01/11. doi: 10.2967/jnumed.112.109942. PubMed PMID: 23303963.
13. Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, et al. Human
alveolar echinococcosis after fox population increase, Switzerland. Emerging infectious
diseases. 2007;13(6):878-82. Epub 2007/06/08. doi: 10.3201/eid1306.061074. PubMed PMID: 17553227; PubMed Central PMCID: PMCPMC2792858.
14. Chauchet A, Grenouillet F, Knapp J, Richou C, Delabrousse E, Dentan C, et al.; FrancEchino
Network. Increased incidence and characteristics of alveolar echinococcosis in patients
with immunosuppression-associated conditions. Clin Infect Dis. 2014 Oct;59(8):1095–104.
10.1093/cid/ciu520
15. Deibel A, Meyer zu Schwabedissen C, Husmann L, Grimm F, Deplazes P, Reiner CS, et
al. Characteristics and Clinical Course of Alveolar Echinococcosis in Patients with
Immunosuppression-Associated Conditions: A Retrospective Cohort Study. Pathogens.
2022;11(4):441. PubMed PMID: doi:10.3390/pathogens11040441.
16. Paternoster G, Boo G, Wang C, Minbaeva G, Usubalieva J, Raimkulov KM, et al. Epidemic
cystic and alveolar echinococcosis in Kyrgyzstan: an analysis of national surveillance
data. Lancet Glob Health. 2020 Apr;8(4):e603–11. 10.1016/s2214-109x(20)30038-3 10.1016/S2214-109X(20)30038-3
17. Gottstein B, Deplazes P. Alveolar echinococcosis: what triggers emergence in North
America, Central Europe and Asia? Curr Opin Infect Dis. 2021 Oct;34(5):440–6. 10.1097/qco.0000000000000765 10.1097/QCO.0000000000000765
18. Bresson-Hadni S, Spahr L, Chappuis F. Hepatic Alveolar Echinococcosis. Seminars in
liver disease. 2021;41(3):393-408. Epub 20210623. doi: 10.1055/s-0041-1730925. PubMed PMID: 34161992.
19. Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanization
of Echinococcus multilocularis. Trends Parasitol. 2004 Feb;20(2):77–84. 10.1016/j.pt.2003.11.011
20. Romig T, Thoma D, Weible AK. Echinococcus multilocularis—a zoonosis of anthropogenic
environments? J Helminthol. 2006 Jun;80(2):207–12. 10.1079/joh2006347 10.1079/JOH2006347
21. Grüner B, Kern P, Mayer B, Gräter T, Hillenbrand A, Barth TE, et al. Comprehensive
diagnosis and treatment of alveolar echinococcosis: A single-center, long-term observational
study of 312 patients in Germany. GMS infectious diseases. 2017;5:Doc01. Epub 2017/01/06.
doi: 10.3205/id000027. PubMed PMID: 30671323; PubMed Central PMCID: PMCPMC6301735.
22. Piarroux M, Piarroux R, Giorgi R, Knapp J, Bardonnet K, Sudre B, et al. Clinical features
and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a
survey in 387 patients. J Hepatol. 2011 Nov;55(5):1025–33. 10.1016/j.jhep.2011.02.018
23. Ozturk G, Polat KY, Yildirgan MI, Aydinli B, Atamanalp SS, Aydin U. Endoscopic retrograde
cholangiopancreatography in hepatic alveolar echinococcosis. J Gastroenterol Hepatol.
2009 Aug;24(8):1365–9. 10.1111/j.1440-1746.2009.05877.x
24. Stojkovic M, Junghanss T, Veeser M, Weber TF, Sauer P. Endoscopic Treatment of Biliary
Stenosis in Patients with Alveolar Echinococcosis--Report of 7 Consecutive Patients
with Serial ERC Approach. PLoS neglected tropical diseases. 2016;10(2):e0004278. Epub
2016/02/26. doi: 10.1371/journal.pntd.0004278. PubMed PMID: 26910822; PubMed Central PMCID: PMCPMC4766234.
25. Ambregna S, Koch S, Sulz MC, Grüner B, Öztürk S, Chevaux JB, et al. A European survey
of perendoscopic treatment of biliary complications in patients with alveolar echinococcosis.
Expert Rev Anti Infect Ther. 2017 Jan;15(1):79–88. 10.1080/14787210.2017.1252260
26. Schweiger A, Grimm F, Tanner I, Müllhaupt B, Bertogg K, Müller N, et al. Serological
diagnosis of echinococcosis: the diagnostic potential of native antigens. Infection.
2012 Apr;40(2):139–52. 10.1007/s15010-011-0205-6
27. Reuter S, Grüner B, Buck AK, Blumstein N, Kern P, Reske SN. Long-term follow-up of
metabolic activity in human alveolar echinococcosis using FDG-PET. Nucl Med (Stuttg).
2008;47(4):147–52. 10.3413/nukmed-0139
28. Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, et
al. Endoscopic biliary stenting: indications, choice of stents, and results: European
Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October
2017. Endoscopy. 2018;50(9):910-30. Epub 20180807. doi: 10.1055/a-0659-9864. PubMed PMID: 30086596.


