Contact tracing for COVID-19 in a Swiss canton:
analysis of key performance indicators
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40112
Leonie Herona,
Catrina Mugglina,
Kathrin Zürchera,
Erich Brumannb,
Bettina
Keune-Dübib,
Nicola Lowa,
Lukas Fennera
a Institute of
Social and Preventive Medicine, University of Bern, Bern, Switzerland
b Cantonal Physician’s Office, Canton of Solothurn, Solothurn, Switzerland
Summary
BACKGROUND: Contact tracing (CT) has played
an important role in strategies to control COVID-19. However, there is limited
evidence on the performance of digital tools for CT and no consensus on which
indicators to use to monitor their performance. We aimed to describe the system
and analyse outcomes of CT with a partially automated workflow in the Swiss
canton of Solothurn, using key performance indicators (KPIs).
METHODS: We describe the process of CT used
in the canton of Solothurn between November 2020 and February 2022, including
forward and backward CT. We developed 16 KPIs representing CT structure (S1–2),
process (P1–11) and outcome (O1–3) based on previous literature to analyse the
relative performance of CT. We report the changes in the indicators over waves
of SARS-CoV-2 infections caused by several viral variants.
RESULTS: The CT team in Solothurn processed
57,363 index cases and 71,809 contacts over a 15-month period. The CT team
successfully contacted 99% of positive cases within 24 hours (KPI P7) throughout
the pandemic and returned almost all test results on the same or next day (KPI
P6), before the delta variant emerged. Three-quarters of contacts were notified
within 24 hours of the CT interview with the index (KPI P8) before the emergence
of the alpha, delta and omicron variants, when the proportions decreased to
64%, 36% and 54%, respectively. The percentage of new symptomatic cases tested
and interviewed within 3 days of symptom onset was high at >70% (KPI P10) and
contacts started quarantine within a median of 3 days of index case symptom
onset (KPI P3). About a fifth of new index cases had already been in quarantine
by the time of their positive test (KPI O1), before the delta variant emerged.
The percentage of index cases in isolation by day of testing remained at almost
100% throughout the period of analysis (KPI O2).
CONCLUSIONS: The CT in Solothurn used a
partially automated workflow and continued to perform well throughout the
pandemic, although the relative performance of the CT system declined at higher
caseloads. CT remains an important tool for controlling the spread of
infectious diseases, but clearer standards should improve the performance, comparability
and monitoring of infection in real time as part of pandemic preparedness
efforts.
Introduction
Contact tracing (CT) has been an important
part of strategies to reduce the spread of severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) infections and control the coronavirus disease 2019
(COVID-19) pandemic [1–2]. Forward CT involves identifying contact persons whom the
index case might
have infected, whereas backward CT involves finding the source of the infection
of the index case. Backward tracing appears to be more effective for
controlling COVID-19, based on the principle that the person who infected the
index is likely to have had more contacts than the index themself [3–4]. In Japan,
public health experts have
suggested that forward CT is only feasible below a 7-day incidence threshold of
15 cases per 100,000 people (Hitoshi Oshitani, personal communication, 7
February 2022). The ‘test-trace-isolate-quarantine’ strategy, which includes CT
activities, can break chains of transmission [5]. During the COVID-19 pandemic, authorities
were under pressure to quickly
establish CT systems and deal with surges in cases. Although public health
authorities quickly trained individuals to join CT teams [6], there was a great need
to incorporate a
variety of digital tools into CT systems to reduce the high workload of tracers
and make CT more efficient [7].
There is limited evidence on the
performance of digital outbreak response tools [8].
According to the World Health Organization (WHO), outbreak response tools [9] are
workflows that facilitate data entry
and automated communication with cases and contacts, particularly when
caseloads are high. They are also known as partly automated CT systems [8] as some
parts of the system are automated,
e.g. messages are sent automatically, whereas other tasks need to be done by a
human, e.g. the index case providing contact details in an online form or an
in-person interview. These digital systems are distinct from proximity tracing
applications [10], which are used as
complementary tools to notify users that they have been in close physical
proximity to an infected person [9].
Several key performance indicators (KPIs)
have been used to evaluate the performance of CT for COVID-19 [11–12] and can be categorised
according to
whether they measure the structure, process or outcome of a public health
intervention like CT [13]. According to
Swiss law, the cantonal physician’s office in Solothurn was responsible for implementing
CT and they developed a partially automated CT workflow in their canton, which
was introduced after the first wave of the COVID-19 pandemic. The objectives of
the present study were 1) to describe the CT system developed by the Swiss
canton of Solothurn and 2) to describe KPIs to analyse CT outcomes between the
implementation of new CT software on 15 November 2020 (onset of the second
wave) and 2 February 2022, at which point the Federal Office of Public Health (FOPH)
of Switzerland ended the requirement to isolate [14].
Methods
We used CT data that were routinely
collected between 15 November 2020 and 2 February 2022. More details about the
canton of Solothurn, definitions and CT practices can be found in supplementary
file 1 and in a previous analysis [15]. We
did not prepare a statistical analysis plan in advance.
Context
Solothurn is a mid-sized canton in
Switzerland with a population of approximately 280,000 [16]. The official language
is German and three quarters (213,800;
76%) of the population are Swiss, which is similar to Switzerland as a whole (supplementary
table 1). Over the course of the COVID-19 pandemic, the Swiss government and
the canton of Solothurn imposed several measures to reduce transmission of
SARS-CoV-2 including restrictions on gatherings, mask mandates, school closures,
travel quarantines, but also the provision of contact data based on the
Epidemics Act law and FOPH directives [14, 17].
As of December 2022, 139,717 SARS-CoV-2 infections had been confirmed in
Solothurn and 379 people reported to have died of COVID-19 [18]. The epidemiological
landscape has changed
with the emergence of new SARS-CoV-2 variants of concern (VOC) [19], and population
immunity through infection
and vaccines, licensed in Switzerland since December 2020.
Contact tracing practices and data collection system
Definitions
We defined an index case as an individual
who tested positive for SARS-CoV-2 by a polymerase chain reaction (PCR) test or
a positive rapid antigen test after their introduction at the end of 2020 within
the testing criteria (adult within four days of symptom onset, non-healthcare
worker, non-vulnerable person) with legal residency in the Swiss canton of
Solothurn. Index cases were required to isolate for 10 days either from the
date of the positive test result or the date of symptom onset. Close contacts
were defined as Solothurn residents who had either spent at least 15 minutes
with an index case at a distance of less than 1.5 metres without wearing a face
mask or were living in the same household as the index case in the two days before
testing positive [14].
Contact tracing practices and digital
workflow
The CT team collected data from index cases
and contacts through in-depth telephone interviews and the collation of
self-reported information from online forms. After the first epidemic wave in
spring 2020, the public health authorities of the canton of Solothurn
implemented a process-oriented CT software “Straatos” at the beginning of the
second epidemic wave in October 2020 [20–21]
(see supplementary file 1 for details). Index cases received a link via text
message to an online form after testing positive (supplementary files 1 and 2). Index
cases reported their age, sex, place of residence, symptoms, date of symptom
onset, potential source of infection, vaccination status, close contacts, and
places visited before symptom onset or positive test if asymptomatic. The CT
system then automatically emailed the index case an ‘administrative order’,
digitally signed by the cantonal physician, which ordered the individual to
isolate, based on the Epidemics Act [17].
The CT staff telephoned index cases within one day of their positive test
result to confirm their responses to the online form, whenever workload allowed
it (see contingency planning in supplementary file 3). Afterwards, the contact
tracer would discuss the information provided with another member of staff. The
index cases received an automated text message at the end of isolation on day
ten (to 12 January 2022) or on day five (from 13 January 2022) to report any
symptoms online. There was a three-step ‘traffic light’ system with levels one
(indicating a low caseload and high staff availability) to four (indicating high
caseload and low staff availability) to determine the extent to which staff
would individually contact the index cases and contacts, check vaccination
documents and test orders, and implement CT in institutional and business
settings (supplementary file 3). The levels were not objectively defined;
usually, the level increased at the beginning of a wave of infection. For
example, at levels one and two, the CT staff contacted the index cases on days
six and ten. However, at level three they only contacted them on these days
when there were sufficient resources and at level four they did not contact
them on these days.
Close contacts identified through forward
CT received a text message with a link to an online form (supplementary file 2). The
text of the
message instructed them to quarantine at home and get tested five days after
their last contact with the index case. Contacts reported their age, sex,
vaccination status, workplace or school, and email address. Contacts were also
emailed an ‘administrative order’ [17],
which included information on what to do if they developed symptoms, current
recommendations by the FOPH, links to the FOPH website, and a telephone number
and email address if they needed more information. The contacts were called by
telephone in the first two days of quarantine and at the end of the quarantine,
depending on the caseload. Contacts received an
automated text message on day five of quarantine to remind them to get tested. At
the onset of the omicron wave (27 December 2021), the workload was judged to be
too high to continue to call contacts at the start of the quarantine period by
telephone. From January 2021, contacts could leave quarantine on day 7 if they
tested negative for SARS-CoV-2. After this date, contacts were only telephoned
at the end of quarantine in exceptional circumstances.
The CT team in Solothurn started backward
tracing (in addition to forward tracing) on 15 November 2020. Index cases
reported locations and events visited in the 10 days prior to testing positive
using an online form (supplementary table 2 and file 1) and provided data were reviewed
during the telephone interview. The CT software automatically identified index
patients who could belong to a cluster because of attendance at the same event,
bar, school, nursing home, or sharing a residential building (supplementary
file 1). When staff capacity allowed, the CT team contacted the venues at which
index cases had reported visiting in the ten days before infection. Based on
this information, a mobile testing team (run by the two cantonal testing centres)
was sent out.
Key performance indicators
We defined 16 KPIs: 2 for the structure, 11
for processes and 3 for outcomes of CT (supplementary table 3) [13]. The KPIs were
based on previous research [11, 22] and an unpublished list of COVID CT
indicators that had been discussed early in the pandemic [12]. ‘Structure’ indicators
relate to the
setting, including human resources and equipment in a CT context. They included
quantification of the proportion of individuals using the Swiss Covid digital
tracing application, used to notify individuals when they had been in close
proximity to a positive case [23], and
the capacity of the CT workforce over time, measured as the number of full-time
equivalent (FTE) staff. “Process” indicators measured the speed and
completeness of investigating, testing and CT. “Outcomes” of CT refer to
measures that should indicate whether a chain of transmission could have been
broken. Most (13) KPIs for COVID-19 CT had associated targets (supplementary
table 3) [11–12, 22]. Index cases without
a case date (date of first contact with the index case) were removed. Dates
that were 25 days before or after the automatically generated case date were
removed if we deemed them likely due to a typographical error.
Statistical analysis
We describe the level of engagement with CT
in Solothurn at each stage in a cascade of CT processes. We also describe the
characteristics of the index cases and compare them with Swiss national data
(from the FOPH). We report their reported source of infection, activities or
locations where they spent time in the ten days before testing positive, their
number of close contacts and the characteristics of the close contacts. Several
SARS-CoV-2 variants were circulating at the same time; we have indicated the
dominant viral variant based on national testing data (supplementary file 1). We
calculate KPIs by period of dominant viral variant and plot KPIs against the
incidence rate of SARS-CoV-2 over the entire period. All analyses were
conducted using R (version 3.5.1). The analysis code is available at https://github.com/leonieheron.
Ethics statement
In accordance with the Swiss Epidemics Act,
informed consent is not required for the collection or processing of personal data
in the context of outbreak investigations and containment of infectious
diseases. We obtained approval from the Ethics Committee of Northwestern and
Central Switzerland (EKNZ, reference nº 2022-00261, www.swissethics.ch) to
analyse CT data and publish only anonymised data.
Results
Description of contact tracing
Over sixty thousand (n = 60,378) positive
tests were recorded in the canton of Solothurn from 15 November 2020 to 2 February
2022 (figure
1). The CT team contacted almost all positive cases (95%, n = 57,363) by text
message through the CT software and, if workload allowed, additionally by phone.
The remaining 5% of cases came from nursing homes. Nursing home residents were
called individually (via the health service of the nursing home), and these
calls were documented outside of the CT system in November and December 2020. A
total of 57,360 individuals (all but 3) submitted the online form. The index
cases reported 71,809 contacts, all of whom were contacted by the CT team. The
majority of contacts (93%, n = 66,934) submitted
an online form. Only 66,261 of the 71,809 (92%) contacts reported by the index
cases completed an online form. 5548 contacts were not
linked to any index cases, most of them came from outside the canton (n = 4481).
The other contacts (n = 1167) were either referred by the FOPH
because they were on a flight with a confirmed infection aboard or the reason was
unknown. Nine percent (n = 5830) of the contacts who completed the
online form later tested positive themselves and became index cases.
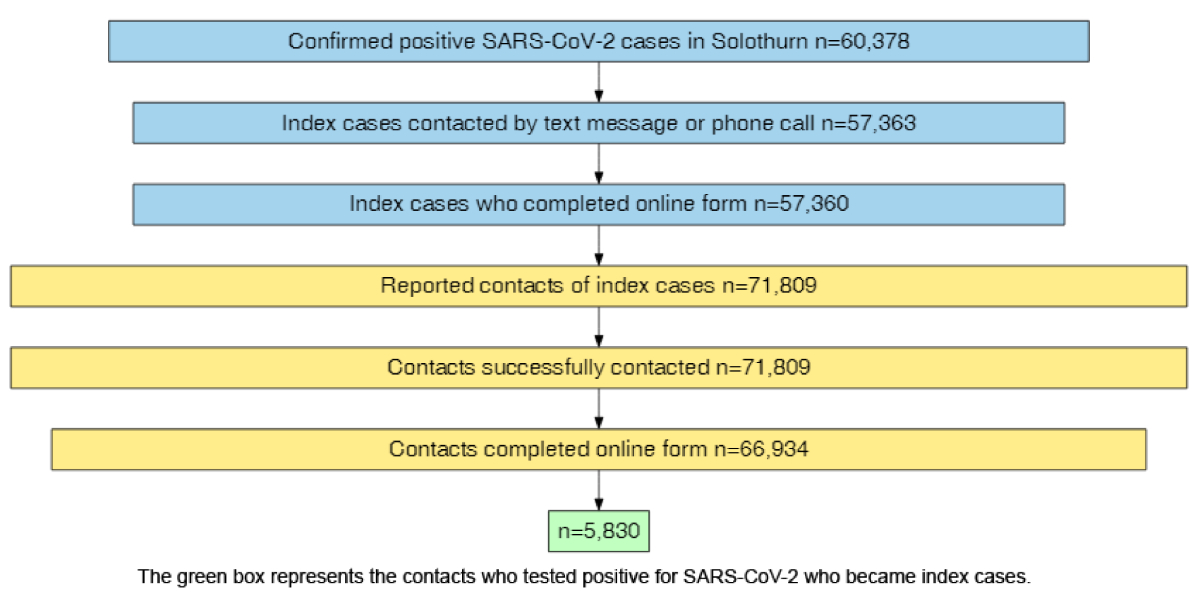
Figure 1Contact tracing cascade in Solothurn from 15
November 2020 to 2 February 2022 (SARS-CoV-2 index cases and contacts). Boxes are
sized in proportion to the
number of individuals at each stage, except for the final box which is as
narrow as the text allows.
Overall, the distribution of index cases
according to age and sex was similar to that of the general population in Switzerland
(table 1, supplementary table 1). The proportion of children (aged 0–17 years) in
CT increased from 7% (n = 503) before the periods of VOCs to 30% and
27%, respectively, when delta and omicron were the dominant VOCs. The
increasing proportion of children in CT partly reflects relaxations in COVID-19
measures in Swiss schools, which were replaced with the repeated testing of
students in June 2021, and most children were not vaccinated, meaning that they
were not exempt from quarantine. The symptoms most commonly reported by the index
cases were cough (46%), runny nose (39%) and sore throat (34%) although the
distribution of symptoms changed as new viral variants emerged (table 1). Loss
of taste or smell was more common in the period before the alpha VOC emerged (23%)
compared with 12% overall. In the period during which alpha was the dominant
VOC, relatively more people reported no symptoms (27% compared with 18%
overall). During the period when delta was the dominant VOC, more people
reported a cough or a runny nose (49% and 43% compared with 46% and 39%
overall). Finally, while omicron was the dominant VOC, a sore throat was
relatively more common (39% compared with 34% overall).
The proportion of vaccinated individuals
increased over time. By the omicron period, 48% of all index cases had been
vaccinated with at least two doses. While only 0.1% (n = 65) and 1.3%
(n = 737) of the age and gender data were missing, respectively, 7.1%
(n = 4062) of the data on symptoms were missing. If participants did
not answer the question on vaccination status, we assumed that they had not
been vaccinated.
Table 1Characteristics of index cases referred to
contact tracing in Solothurn from 15 November 2020 to 2 February 2022 by periods
of different viral dominance.
| Variable |
Level |
Pre-VOC |
Alpha |
Delta |
Omicron |
Entire period |
| 15 Nov 20 – 7
Feb 21* |
8 Feb 21 – 27
Jun 21 |
28 Jun 21 – 26
Dec 21 |
27 Dec 21 – 2 Feb 22 |
15 Nov 20 – 2
Feb 22 |
| Total |
|
7494 |
4964 |
15,609 |
29,296 |
57,363 |
| Age, years |
0–17 |
503
(7%) |
838
(17%) |
4626
(30%) |
7856
(27%) |
13,823
(24%) |
| 18–29 |
1547
(21%) |
1000
(20%) |
2745
(18%) |
6070
(21%) |
11,362
(20%) |
| 30–39 |
1292
(17%) |
879
(18%) |
2582
(17%) |
5355
(18%) |
10,108
(18%) |
| 40–49 |
1116
(15%) |
771
(16%) |
2233
(14%) |
4342
(15%) |
8462
(14%) |
| 50–59 |
1385
(18%) |
749
(15%) |
1674
(11%) |
3325
(11%) |
7133
(12%) |
| 60–69 |
914
(12%) |
440
(9%) |
961
(6%) |
1524
(5%) |
3839
(7%) |
| 70–79 |
463
(6%) |
189
(4%) |
478
(3%) |
492
(2%) |
1622
(3%) |
| ≥80 |
271
(4%) |
91
(2%) |
300
(2%) |
287
(1%) |
949
(2%) |
| Gender |
Female |
3495
(51%) |
2373
(48%) |
7695
(49%) |
13,748
(47%) |
27,311
(48%) |
| Male |
3295
(49%) |
2572
(52%) |
7846
(50%) |
14,085
(48%) |
27,798
(49%) |
| Other/unknown |
0
(0%) |
5
(0%) |
57
(0%) |
1455
(5%) |
1517
(3%) |
| Symptoms** |
Cough |
3263
(44%) |
2082
(42%) |
7632
(49%) |
13,175
(45%) |
26,152
(46%) |
| Runny nose |
2701
(37%) |
1571
(32%) |
6662
(43%) |
11,499
(39%) |
22,433
(39%) |
| Sore throat |
2088
(28%) |
1365
(28%) |
4714
(30%) |
11,360
(39%) |
19,527
(34%) |
| Fever |
2253
(31%) |
1369
(28%) |
5271
(34%) |
9714
(33%) |
18,607
(33%) |
| Sweating |
1914
(26%) |
1271
(26%) |
4308
(28%) |
8751
(30%) |
16,244
(28%) |
| General malaise |
1422
(19%) |
804
(16%) |
2721
(17%) |
5276
(18%) |
10,223
(18%) |
| Other symptoms |
1799
(24%) |
1050
(21%) |
2737
(18%) |
4424
(15%) |
10,010
(18%) |
| Loss of taste or smell |
1698
(23%) |
632
(13%) |
2673
(17%) |
1988
(7%) |
6991
(12%) |
| Nausea |
765
(10%) |
493
(10%) |
1736
(11%) |
3335
(11%) |
6329
(11%) |
| Diarrhoea |
692
(9%) |
416
(8%) |
1283
(8%) |
2146
(7%) |
4537
(8%) |
| Difficulty breathing or shortness of breath |
570
(8%) |
316
(6%) |
902
(6%) |
1586
(5%) |
3374
(6%) |
| Elevated heart rate |
200
(3%) |
83
(2%) |
285
(2%) |
491
(2%) |
1059
(2%) |
| Pneumonia |
36
(0%) |
17
(0%) |
89
(1%) |
34
(0%) |
176
(0%) |
| Acute respiratory distress |
40
(1%) |
8
(0%) |
48
(0%) |
40
(0%) |
136
(0%) |
| Oxygen required |
4
(0%) |
7
(0%) |
6
(0%) |
0
(0%) |
17
(0%) |
| Respiratory failure |
6
(0%) |
3
(0%) |
1
(0%) |
0
(0%) |
10
(0%) |
| No symptoms reported |
1239
(17%) |
1313
(27%) |
3257
(21%) |
3800
(15%) |
9609
(18%) |
| Vaccination status*** |
Vaccinated (2 doses) |
9
(0%) |
233
(5%) |
4246
(27%) |
14,143
(48%) |
18,631
(33%) |
Backward contact tracing
Eleven thousand (n = 11,072, 19%)
index cases reported at least one group activity in the ten days before symptom
onset or positive test (table 2). Most cases (n = 46,288; 81%) did
not report any activities. Of those who did, most reported only one activity
(67%) although the maximum was 23. The most common activities reported in the
last 10 days were being at a fitness centre or doing sports, private parties,
working, or dining with others at home or in a restaurant. During the period
when omicron was the dominant VOC, a much higher proportion of people did not
report any information on activities. Out of the index cases who reported
attending a certain location, 30% reported that they knew of a positive case
who had also attended. The proportion increased as the pandemic continued: in 13%
(n = 430) of venues visited and reported by index cases, the index
case knew of a positive case in attendance during the pre-VOC period, 8% (n = 284)
in the period during which alpha was the dominant VOC, 34% (1139) while the
delta VOC was dominant, and 45% (n = 1516) while the omicron VOC was
dominant. Most index cases reported their likely source of infection (91%, n = 52,350).
The most common responses were family or friends (39%), school (13%), work
(9%), or shopping/public transport (5%). Fourteen percent of respondents could
not classify their source of infection and in one-fifth the information was missing.
Table 2Activities reported in the ten days prior
to a positive SARS-CoV-2 test by individuals in Solothurn from 15 November 2020
to 2 February 2022 by time period of different viral dominance.
| Activity* |
Pre-VOC |
Alpha |
Delta |
Omicron |
Total |
| Fitness
centre / sports |
792
(4%) |
1005
(8%) |
2612
(7%) |
2203
(5%) |
6612
(6%) |
| Private
party |
1491
(7%) |
442
(3%) |
1677
(5%) |
2213
(5%) |
5823
(5%) |
| Work |
1743
(8%) |
956
(7%) |
1705
(5%) |
1339
(3%) |
5743
(5%) |
| Dining
with friends (home) |
1494
(7%) |
679
(5%) |
1257
(3%) |
2159
(5%) |
5589
(5%) |
| Dining at
a restaurant |
817
(4%) |
87
(1%) |
1076
(3%) |
1291
(3%) |
3271
(3%) |
| Bar / club |
113
(1%) |
1
(0%) |
591
(2%) |
1027
(2%) |
1732
(1%) |
| School |
193
(1%) |
199
(2%) |
394
(1%) |
253
(1%) |
1039
(1%) |
| Sporting
event (spectator) |
3
(0%) |
21
(0%) |
366
(1%) |
386
(1%) |
776
(1%) |
| Choir/singing |
10
(0%) |
15
(0%) |
348
(1%) |
71
(0%) |
444
(0%) |
| Foreign
travel |
30
(0%) |
8
(0%) |
125
(0%) |
0
(0%) |
163
(0%) |
| Public
event |
3
(0%) |
0
(0%) |
0
(0%) |
0
(0%) |
3
(0%) |
| Other |
3220
(15%) |
2731
(21%) |
8343
(23%) |
2611
(6%) |
16,905
(14%) |
| Missing |
11,711
(54%) |
7065
(54%) |
18,212
(50%) |
33,806
(71%) |
70,794
(60%) |
| Total |
21,620
(100%) |
13,209
(100%) |
36,706
(100%) |
47,359
(100%) |
118,894
(100%) |
Self-reported source of infection
correlated with the types of activities reported in the ten days prior to
testing positive (supplementary table 2).
Key performance indicators
Figure 2 shows results for four main KPIs,
two for process (P6, percentage of test results returned on same or next day; P8,
percentage of contacts notified on same or next day after interview with index)
and two for outcomes (O1, percentage of new cases who were already in
quarantine by time of positive test, i.e. new cases who were previously
identified as contacts; O2, percentage of index cases in isolation by day of testing).
The missing data for each KPI are outlined in supplementary table 5. Less than
1% of the data were missing for almost all KPIs, except for structure KPI S1
and process KPI P5 (7% and 5% missing data, respectively).
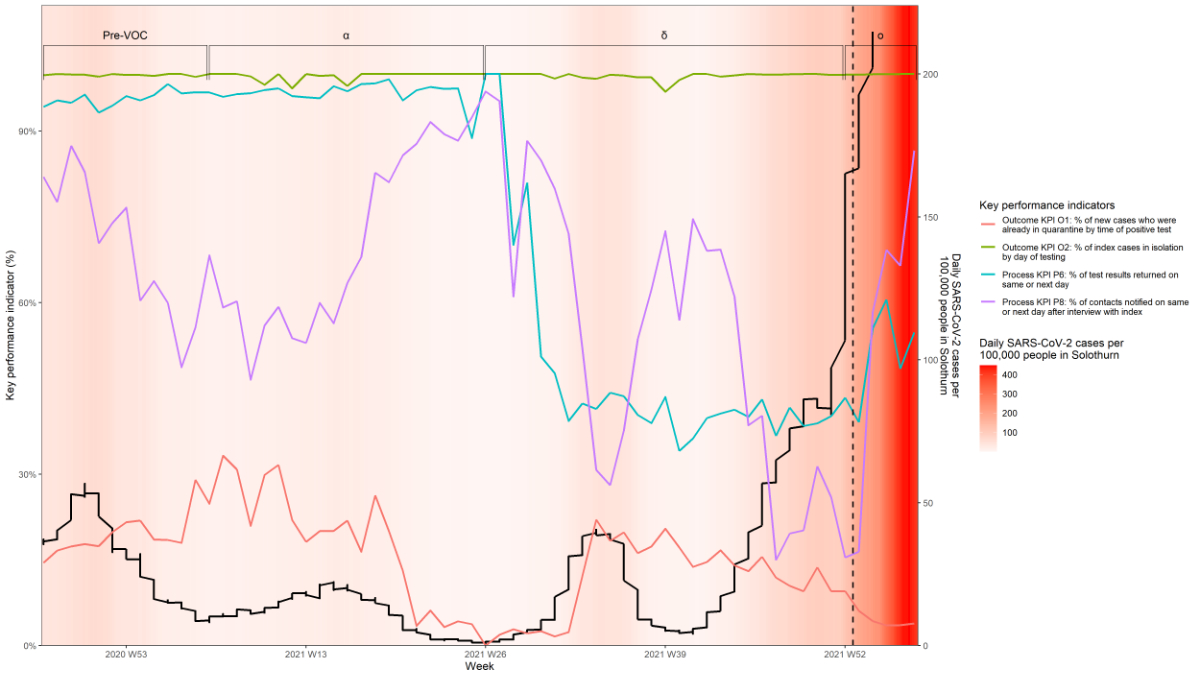
Figure 2Process and outcome key performance
indicators of contact tracing during the COVID-19 pandemic in the canton of Solothurn.The
coloured solid lines indicate the
percentage of the key performance indicators over time. The thicker black line shows
the change in SARS-CoV2 caseload. The Greek symbols α, δ and ο indicate the periods
during which the alpha,
delta and omicron SARS-CoV-2 variants of concern (VOCs), respectively, were
dominant. The dashed line indicates the point at which the system contacted
individuals automatically (1 January 2022).
Structure indicators
The CT workforce changed over time in
response to the changing incidence rate of SARS-CoV-2 infection. The full-time
equivalents of contact tracers increased from 35.4 to 36.3, decreased to 34.2 while
delta was the dominant VOC, and then increased to 42.1 during the omicron wave
(structure KPI S2, figure 3, supplementary table 4). Less than a third (28%) of
index cases reported that they had installed the Swiss Covid app on their
smartphone (structure KPI S1, supplementary table 4).
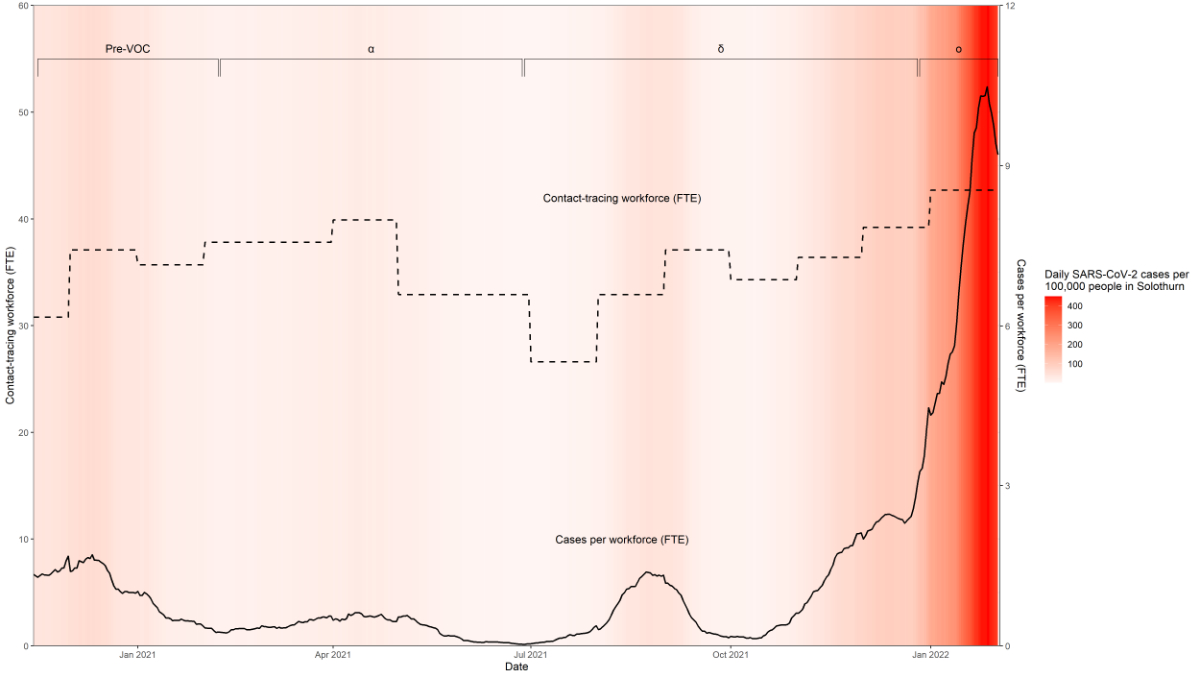
Figure 3SARS-CoV-2 infections and the contact tracing
workforce in the canton of Solothurn from 15 November 2020 to 2 February 2022, inclusive.
The graph is shaded red according to the
SARS-CoV-2 caseload. The dashed line indicates the contact-tracing workforce in
full-time equivalent (FTE) and the solid line indicates the cases per
workforce. The Greek symbols α, δ
and ο indicate the periods during which the alpha, delta and omicron SARS-CoV-2
variants of concern (VOCs), respectively, were dominant.
Process indicators
The process KPIs indicated that performance
changed over the course of the pandemic. During the first waves of the pandemic
and the period during which the alpha VOC was dominant, almost all test results
were returned on the same or next day (process KPI P6, figure 2, supplementary
table 4). The time taken to return PCR test results increased while delta was
the dominant VOC and only partially recovered afterwards (process KPI P2, supplementary
table 4 and supplementary figure 1B). Similarly, almost three-quarters of contacts
were notified within 24 hours of the CT interview by phone with the index
before the emergence of viral variants (process KPI P8, figure 2, supplementary
table 4). While the alpha, delta and omicron VOCs were dominant, the
proportions decreased to 64%, 36% and 54%. The days taken between the lab
processing the result and the subsequent isolation of the index case also
appeared to increase while delta was the dominant VOC (process KPI P4, supplementary
figure 1D).
Nevertheless, the CT team successfully
contacted almost all positive cases within 24 hours (99%) throughout the
pandemic (process KPI P7, supplementary table 4). On average, at least 70% of
new cases were tested and interviewed within 3 days of symptom onset across the
entire period (process KPI P10). The contacts began quarantine a median of 3
days after the index case began experiencing symptoms (process KPI P3, supplementary
table 4, supplementary figure 1C). This time period includes the time it takes
to test the index case after symptom onset, the time to receive the results, and
the time for CT and quarantine orders to be sent to contacts.
Index cases reported between 0 and 69 close
contacts, although most did not report any (52%, n=29,754, process KPI P11).
Two-thirds (65%) of index cases did not report any contacts in the pre-VOC
period and the proportion steadily decreased to 40% in the omicron period (process
KPI P9, figure 4). Of those who did report contacts, the median number (interquartile
range [IQR]) of contacts for each period were 2 (1–4) for the pre-VOC periods
and while alpha was the dominant VOC and 2 (1–3) for the periods during which
delta and omicron were the dominant VOCs.

Figure 4Number of contacts reported by SARS-CoV-2
index cases in Solothurn from 15 November 2020 to 2 February 2022. The
horizontal bars indicate the median, the box indicates the interquartile range
and the dashed lines indicate the minimum and maximum values, with outliers
removed.
Outcome indicators
Overall, the percentage of index cases in
isolation by day of testing remained at almost 100% throughout the period of
analysis (outcome KPI O2, figure 2). However, the percentage of new cases
already in quarantine by time of positive test, i.e. new cases who had
previously been identified as contacts (outcome KPI O1) indicated that the CT
system may have become less efficient as the pandemic continued. In the pre-VOC
period and while alpha was the dominant VOC, about a fifth of index cases were already
in quarantine by the time of the positive test (outcome KPI O1, figure 2, supplementary
table 4). This percentage was highest (over 30% weekly) in February–March 2021
and lowest (<5% weekly) during May–August 2021. In the following waves, only
13% and 5% of index cases were already in quarantine (outcome KPI O1, supplementary
table 4).
Nine percent of contacts tested positive
for SARS-CoV-2 overall (outcome KPI O3, supplementary table 4). The proportion
of contacts who tested positive for SARS-CoV-2 was highest during the period
when alpha was the dominant VOC (13%) and lowest in the omicron wave (6%). From
31 May 2022 contacts who had been vaccinated or had tested positive in the 10–180
days before contact were exempt from quarantine and not under any obligation to
get tested.
Discussion
The CT system in Solothurn used a partially
automated workflow, which likely broke some chains of SARS-CoV-2 infection
between 15 November 2020 and 2 February 2022, based on the high percentage of
test results returned on the same or next day (KPI P6), the high percentage of
contacts notified within 24 hours of the CT interview with the index (KPI P8), the
high percentage of index cases in isolation by day of testing (KPI O2) and
other indicators.
The two main process KPIs indicated that
the system was faster during the earlier stages of the pandemic: more than 95%
and 97% of test results were returned on the same or the next day during the
period before the VOCs and while alpha was the dominant VOC (process KPI P6).
However, during the periods when delta and omicron were the dominant VOCs, the
percentages dropped to 41% and 52%, highlighting the burden placed on the overall
system (testing and CT) during periods of very high caseloads. Furthermore, a
higher proportion of contacts was notified on the same day or next day after
the interview with the index (process KPI P8) during the pre-VOC and alpha
phases compared with the delta and omicron phases.
Interpretation of CT performance takes into
consideration that the targets were proposed early in the COVID-19 pandemic and
may not have been validated. Of the 16 KPIs that we assessed, 13 had at least
one suggested target in the literature (supplementary table 3) [11–12, 22]. Five KPIs
achieved the suggested
targets overall and during each of the periods of different viral variants
(process KPIs P1–3, P5 and P7; supplementary table 4, supplementary
figure 1) and two had mixed results during the epidemic waves (process KPIs P4
and P6). The process KPIs P1–3 and P5 quantified the time taken between symptom
onset, testing, lab results, isolation of the index and quarantine of the
contact: all remained within the suggested targets of less than or equal to
between one and five days. Process KPIs P4 and P6 were the only KPIs that varied
widely from the suggested targets of ≤1 day and 90–100%, respectively. The median
number of days between index test results and isolation was over one overall
but remained at the target of one day for three out of four periods (process KPI
P4).
Some KPIs that did not meet suggested
targets indicate challenges at the testing laboratory and the CT workforce when
delta and omicron were the dominant VOCs. Six KPIs were below the targets,
according to the limited literature (structure KPI S2, process KPIs P8–10 and
outcome KPIs O1 and O3). The caseload changed considerably during this period,
so it was not clear what caused the test results to be delivered more slowly
(process KPI P6). The number of people working in the CT team was below the
suggested target of 30 tracers per 100,000 population, which corresponds to 64
FTE in Solothurn [11] (structure KPI S2).
However, the number of tracers needed is debated and in addition to caseload depends
on the degree of CT automation and team organisation, the level of personal
contact required for interviews and telephone calls, and the availability of
office space and funding. Less than 80% of contacts were notified on the same
day or day after the interview with the index (process KPI P8), but the proportions
were higher in the pre-VOC and alpha phases. More than 25% of cases did not
report any contacts (process KPI P9), most likely due to the rising reluctance
of the population to report contacts and the high volume of cases that
restricted the ability of the CT team to contact index cases to verify
information. Ultimately, it can be argued that above a certain number of cases
per day and population, and particularly in the context of highly transmissible
SARS-CoV-2 VOCs such as omicron, forward CT is no longer feasible. This is
supported by a recent genomic analysis of sequences obtained in 2020, which
found that CT likely slowed transmission during the summer of 2020, when there
were few cases, but not during the second wave in autumn/winter
2020-2021, when there was a very high number of cases [24]. Therefore, public health
experts support
focusing on cluster identification and outbreak investigations when cases are rising
[3–4].
The outcome indicators are closest to
measuring the containment of COVID-19 by CT activities. The main outcome KPI O2
which indicated the percentage of index cases in isolation by day of testing
remained high throughout the pandemic (almost 100% in all time periods),
whereas the proportion of new cases who were already in quarantine by the time
of the positive test (outcome KPI O1) was relatively low (range 5–22%). The
target of 80% has been suggested [12],
but not validated. We observed that less than 80% of new cases were already in
quarantine by the time of the positive test. It is feasible that many residents
would leave the canton on an almost daily basis for work or education. Contacts
who were resident in other regions of Switzerland were under the jurisdiction
of a separate canton and thus it was not possible to link many index cases and
contacts. Therefore, we expect that the percentage may be misleadingly low
because the data were not linked with other regions in Switzerland.
Furthermore, according to the suggested target, we observed that 9% of contacts
overall tested positive for SARS-CoV-2, higher than a target proportion of
<1% (outcome KPI O3). However, this target is not validated and may result
in a larger number of contacts being quarantined.
Contact tracers in other countries reported
similar findings to ours. Contact tracers in the United States and Spain found that
the performance of CT was
worse while caseloads were higher [25–26].
The US team cited overwhelmed staff, unable to conduct thorough interviews due
to time pressure, as a possible reason [25].
They also found that different viral dynamics in new variants complicated CT
because of increased transmissibility and possibly shorter incubation period [25].
The Catalonian team responded by
increasing their workforce [26]. They
promote the use of constant monitoring using KPIs to allow for regular
evaluation of the CT system and the epidemiological situation [26]. In another US
study, contact tracers in
New York were able to use their data to confirm that there was more SARS-CoV-2
transmission at known places of interest in the city [27]. Some reports on CT highlighted
areas where we might have
improved data collection. For example, the US team collected objective data on
test results from contacts to monitor the contacts’ outcomes: the prevalence
ratio of SARS-CoV-2 infection was much higher in contacts compared with non-contacts
when viral transmission was higher in the community [25]. In Taiwan, they benefitted
from centralised digital tools, with
a unique nationwide CT platform, linking various data sources, including
information from telephone companies, and using a smartphone-based real-time
locating system to track contacts [7]. The
CT team reported a subsequent increase in self-reported updates of health
status from 22.5% to 61.5% via automatic text message or web applications in
Taiwan during the COVID-19 pandemic (7), reducing the pressure on the CT
workforce.
Our study provides an overview of a new digitised
CT system established in an urgent and ever-changing real-life situation and
proposes a set of important KPIs for evaluation purposes. To our knowledge, we
present the first analysis to transparently report the performance of a CT
workflow in Switzerland. The strengths of this analysis were the availability
of CT data over a long period of 15 months, nearly the entire period of CT in
the canton of Solothurn. The dataset includes the CT workforce data and covers
periods of different viral VOCs. However, the study is limited by data that are
self-reported, missing or not requested. For example, we noticed that people
became less willing to disclose information on infection sources and did not
report activities as readily as the pandemic progressed. In contrast, index
cases reported more close contacts in the later stages, but that may be because
most of the close contacts had recovered or had been vaccinated and were
therefore no longer ‘at risk’ of being quarantined (vaccinated and recovered
persons could be exempted from quarantine from 31 May 2021). Reporting morale likely
decreased and many index cases decided to circumvent the system by notifying
friends and family themselves. A further limitation is that the CT system only covers
the canton of Solothurn, which may not reflect other cantons and other CT
workflows. However, the canton of Solothurn is a mid-sized canton, with demographics
representative of Switzerland. We did not have access to data from other Swiss
cantons for comparison.
CT is a useful tool for reducing the
transmission of infectious diseases when the incidence rate is below a
manageable threshold. This description of CT in a Swiss canton illustrates how partial
automation of CT contributed to real-time monitoring and surveillance of
SARS-CoV-2 throughout the pandemic. The findings support the use and monitoring
of CT data to inform modelling studies and public health measures in real time.
Sudden exponential increases in caseloads challenged the system although automation
ensured that CT could continue. Our study also shows the need for standardised
benchmarks across CT to facilitate cross-region and cross-border cooperation,
taking into account the resource level of the country, which will require an
electronic data capture system to allow real-time data extraction. Adding
genomics data to the CT workflow might further support and advance
identification of transmission clusters [15, 24,
28]. Digitised
CT workflows can be used for other existing or re-emerging infectious diseases
such as measles or mpox, but also for emerging infections in the years to come [28].
In the context of pandemic preparedness,
we believe that periods of relatively low transmission provide a good
opportunity to analyse and compare CT performance, to improve the systems and to
reach a consensus on targets. Modelling studies could help public health
authorities to understand where to focus attention to maximise interruption of
transmission. Efficient CT systems should automate workflows as far as possible
to adapt to high incidence rates, save on human resources, and generate KPIs to
monitor the system’s performance.
Acknowledgments
We would like to thank all persons who
contributed data to this study. We are also indebted to the cantonal
physician’s office of the canton of Solothurn and the contact tracing team for
their diligent daily work and the immense efforts to continuously improve the
CT workflow.Authors'
contributions: Conception and design: LH, CM, NL,
LF. Contact tracing data collection: CM, EB, BD, LF. Statistical analysis: LH,
CM. Wrote the first draft of the paper and revised it based on comments from
all authors: LH, CM, NL, LF. All authors reviewed and approved the final version
of the manuscript.
Prof. Lukas Fenner
Institute of
Social and Preventive Medicine
University
of Bern
Mittelstrasse 43
CH-3012
Bern
lukas.fenner[at]unibe.ch
and
Dr Bettina
Keune-Dübi
Kantonsärztlicher
Dienst
Gesundheitsamt Kanton Soloturn
Riedholzpl. 3
CH-4509 Solothurn
bettina.keune[at]ddi.so.ch
References
1. Aleta A, Martín-Corral D, Pastore Y Piontti A, Ajelli M, Litvinova M, Chinazzi M,
et al. Modelling the impact of testing, contact tracing and household quarantine on
second waves of COVID-19. Nat Hum Behav. 2020 Sep;4:964–71. 10.1038/s41562-020-0931-9
2. Kucharski AJ, Klepac P, Conlan AJ, Kissler SM, Tang ML, Fry H, et al.; CMMID COVID-19
working group. Effectiveness of isolation, testing, contact tracing, and physical
distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical
modelling study. Lancet Infect Dis. 2020 Oct;20(10):1151–60. 10.1016/S1473-3099(20)30457-6
3. Kojaku S, Hébert-Dufresne L, Mones E, Lehmann S, Ahn YY. The effectiveness of backward
contact tracing in networks. Nat Phys. 2021 May;17:652–8. 10.1038/s41567-021-01187-2
4. Raymenants J, Geenen C, Thibaut J, Nelissen K, Gorissen S, Andre E. Empirical evidence
on the efficiency of backward contact tracing in COVID-19. Nat Commun. 2022 Aug;13(1):4750.
10.1038/s41467-022-32531-6
5. Ashcroft P, Lehtinen S, Bonhoeffer S. Test-trace-isolate-quarantine (TTIQ) intervention
strategies after symptomatic COVID-19 case identification. PLoS One. 2022 Feb;17(2):e0263597.
10.1371/journal.pone.0263597
6. Koetter P, Pelton M, Gonzalo J, Du P, Exten C, Bogale K, et al. Implementation and
Process of a COVID-19 Contact Tracing Initiative: Leveraging Health Professional Students
to Extend the Workforce During a Pandemic. Am J Infect Control. 2020 Dec;48(12):1451–6.
10.1016/j.ajic.2020.08.012
7. Jian SW, Cheng HY, Huang XT, Liu DP. Contact tracing with digital assistance in Taiwan’s
COVID-19 outbreak response. Int J Infect Dis. 2020 Dec;101:348–52. 10.1016/j.ijid.2020.09.1483
8. Braithwaite I, Callender T, Bullock M, Aldridge RW. Automated and partly automated
contact tracing: a systematic review to inform the control of COVID-19. Lancet Digit
Health. 2020 Nov;2(11):e607–21. 10.1016/S2589-7500(20)30184-9
9. World Health Organization. Digital tools for COVID-19 contact tracing: annex: contact
tracing in the context of COVID-19, 2 June 2020 [Internet]. World Health Organization;
2020 [cited 14 December 2022]. Available from: https://apps.who.int/iris/handle/10665/332265
10. Daniore P, Ballouz T, Menges D, von Wyl V. The SwissCovid Digital Proximity Tracing
App after one year: were expectations fulfilled? Swiss Med Wkly. 2021 Sep;151(35-36):w30031.
10.4414/SMW.2021.w30031
11. Vogt F, Kurup KK, Mussleman P, Habrun C, Crowe M, Woodward A, et al. Contact tracing
indicators for COVID-19: rapid scoping review and conceptual framework. PLoS One.
2022 Feb;17(2):e0264433. 10.1371/journal.pone.0264433
12. Frieden T. COVID Contact Tracing Indicator list for consideration [Unpublished work].
2020.
13. Donabedian A. The quality of care. How can it be assessed? JAMA. 1988 Sep;260(12):1743–8.
10.1001/jama.1988.03410120089033
14. Federal Office of Public Health FOPH. Coronavirus: Measures and ordinances [Internet].
Federal Office of Public Health FOPH; 2022 [cited September 20 2022]. Available from:
https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/massnahmen-des-bundes.html
15. Anderegg N, Schwab T, Borcard L, Mugglin C, Keune-Dübi B, Ramette A, et al. Population-based
SARS-CoV-2 whole genome sequencing and contact tracing during the COVID-19 pandemic
in Switzerland. J Infect Dis. 2023;228(3):251-260. 10.1093/infdis/jiad074
16. Kanton Solothurn. Bevölkerungszahlen [Internet]. Kanton Solothurn; 2022 [cited 20
September 2022]. Available from: https://so.ch/verwaltung/finanzdepartement/amt-fuer-finanzen/statistikportal/bevoelkerung/bevoelkerungszahlen/
17. Federal Office of Public Health FOPH. Communicable Diseases Legislation – Epidemics
Act, (EpidA) [Internet]. Bern: Federal Office of Public Health; 2020 [cited 13 December
2021]. Available from: https://www.bag.admin.ch/bag/en/home/gesetze-und-bewilligungen/gesetzgebung/gesetzgebung-mensch-gesundheit/epidemiengesetz.html
18. Federal Office of Public Health FOPH. COVID-19 Switzerland [Internet]. Swiss Confederation;
2022 [cited 21 October 2022]. Available from: https://www.covid19.admin.ch/en/epidemiologic/
19. World Health Organization. Tracking SARS-CoV-2 variants [Internet]. 2022 [cited 12th
August 2022]. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants/
20. CumulusPro. Straatos. CumulusPro; 2022.
21. GmbH SP. Menschen. Prozesse. Digitalisierung. . Lenzburg: Strub & Partner GmbH; 2022.
22. Resolve to Save Lives. Covid-19 Contact Tracing Playbook [Internet]. 2022 [cited 11
August 2022]. Available from: https://contacttracingplaybook.resolvetosavelives.org/checklists/metrics
23. Federal Office of Public Health FOPH. Coronavirus: SwissCovid app [Internet]. 2023.
Available from: https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/swisscovid-app-und-contact-tracing.html
24. Nadeau SA, Vaughan TG, Beckmann C, Topolsky I, Chen C, Hodcroft E, et al. Swiss public
health measures associated with reduced SARS-CoV-2 transmission using genome data.
Sci Transl Med. 2023;15(680):eabn7979. 10.1126/scitranslmed.abn7979
25. Borah BF, Pringle J, Flaherty M, Oeltmann JE, Moonan PK, Kelso P. High Community Transmission
of SARS-CoV-2 Associated with Decreased Contact Tracing Effectiveness for Identifying
Persons at Elevated Risk of Infection – Vermont. Clin Infect Dis. 2022;75(S2):S334-7.
10.1093/cid/ciac518
26. Herrero M, Ciruela P, Mallafré-Larrosa M, Mendoza S, Patsi-Bosch G, Martínez-Solanas È,
et al.; Epidemiological Surveillance Network of Catalonia. SARS-CoV-2 Catalonia contact
tracing program: evaluation of key performance indicators. BMC Public Health. 2022 Jul;22(1):1397.
10.1186/s12889-022-13695-8
27. Pei S, Kandula S, Cascante Vega J, Yang W, Foerster S, Thompson C, et al. Contact
tracing reveals community transmission of COVID-19 in New York City. Nat Commun. 2022 Oct;13(1):6307.
10.1038/s41467-022-34130-x
28. Walker A, Houwaart T, Finzer P, Ehlkes L, Tyshaieva A, Damagnez M, et al.; German
COVID-19 OMICS Initiative (DeCOI). Characterization of Severe Acute Respiratory Syndrome
Coronavirus 2 (SARS-CoV-2) Infection Clusters Based on Integrated Genomic Surveillance,
Outbreak Analysis and Contact Tracing in an Urban Setting. Clin Infect Dis. 2022 Mar;74(6):1039–46.
10.1093/cid/ciab588
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/smw.2023.40112.



