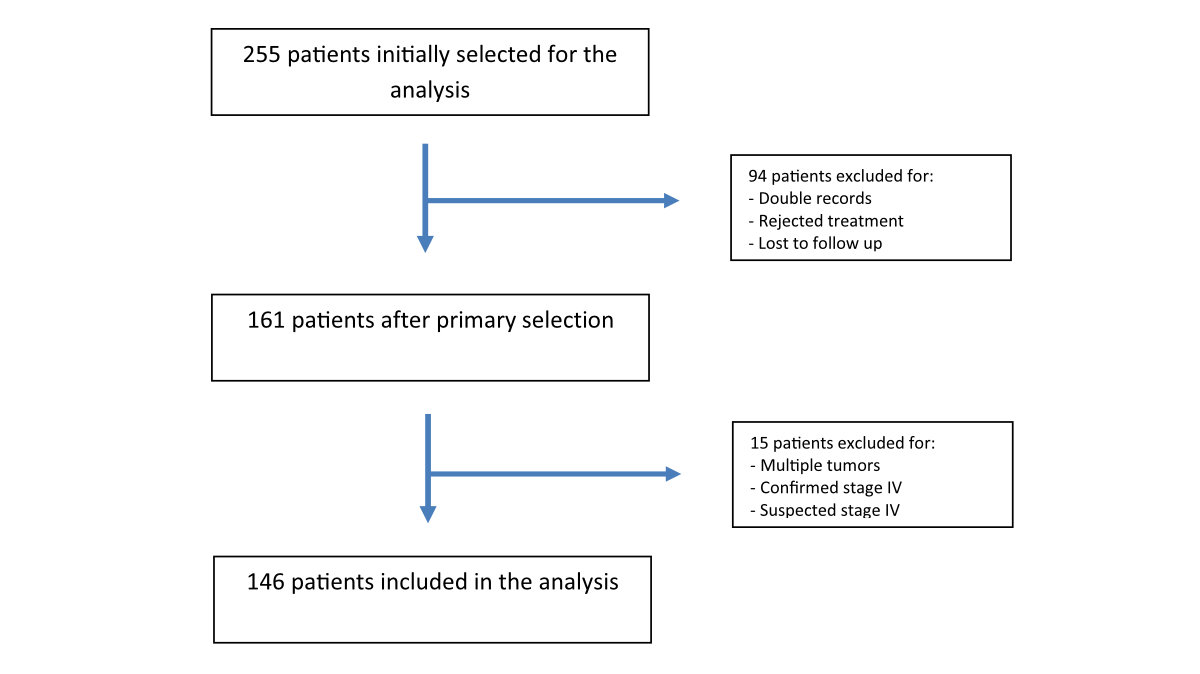
Figure 1Study flow chart.
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40110
Immune-checkpoint inhibitors, such as anti-programmed death-1 (anti-PD-1) and anti-programmed death-ligand 1 (anti-PD-L1) monoclonal antibodies, either alone or in combination with chemotherapy, are the backbone of modern treatment of metastatic non-small cell lung cancer that does not carry actionable mutations (such as EGFR, ALK, ROS1, BRAF or MET). These treatments have led to response and survival rates that were unattainable just a few years ago [1, 2]. Patients whose tumours express PD-L1 in at least 50% of cells are more likely to respond to compounds such as pembrolizumab, nivolumab or atezolizumab and to survive longer [3, 4]. Therefore, the prognostic value of PD-L1 in advanced disease depends on the availability of immunotherapy.
While the prognostic implication of PD-L1 tumour expression is well established in the metastatic setting, its significance remains unclear for stage I to III non-small cell lung cancer treated with curative intent [5–18]. In fact, the use of PD-1 or PD-L1 inhibitors in a neoadjuvant or adjuvant setting has been restricted to studies that are still in the late research phase or have only just begun publishing very early results [19, 20].
Thus, the prognostic and predictive significance of PD-L1 expression in the early-stage setting is currently receiving more attention, but the available information is limited [21].
Studies from the past decade have reported contradictory results, likely due to methodological flaws, such as lack of reproducibility of the PD-L1 expression assays, heterogeneity of disease stages, timing of specimen collection, inclusion of cases with actionable genetic alterations and heterogenous therapeutic interventions [14, 22, 23].
Several studies conducted in the pre-immunotherapy era, primarily in Asian countries, reported a negative correlation between increasing PD-L1 expression levels and survival in the early-stage setting [7–9, 24–27]. Contradictory results were observed among Caucasian patients, as some studies reported a positive correlation between increasing PD-L1 expression levels and survival [12–14].
The aim of this retrospective study was to investigate the prognostic significance of PD-L1 tumour expression in a homogeneous Swiss population of patients with non-small cell lung cancer in the early stages (I to III according to the TNM lung cancer staging system, 8th edition). PD-L1 was assessed using a standardised assay, and all cases were treated with curative intent, either with surgery, radiation therapy (including stereotactic body radiation therapy (SBRT) or conventional radiotherapy), chemoradiation or a combination of these modalities, in accordance with current standards [28, 29].
This information could be very useful in helping clinicians decide whether it is appropriate to include in therapeutic standards adjuvant treatment with atezolizumab or neoadjuvant treatment with nivolumab, based on the results of the IMpower010 and CheckMate 816 studies, respectively. Both studies showed clear benefits, in terms of disease-free survival in the first case and event-free survival in the second, especially for patients whose tumours had PD-L1 expression ≥50% [19, 20]. Nevertheless, these results need to be explored further to establish the effect of these two immune-oncology strategies on overall survival.
This study was conducted according to the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) Statement guidelines for observational studies [30].
Patients had to fulfil the following inclusion criteria: (a) diagnosis of adenocarcinoma, squamous cell carcinoma or undifferentiated carcinoma subtypes of the lung (neuroendocrine tumours, pure or mixed, were excluded) and (b) resectable disease, stage I to III (TNM lung cancer staging system, 8th edition), as per a thoracic surgeon’s assessment. In addition, patients had to have initiated a treatment with curative intent, with the main treatment method considered to be surgery, ablative radiotherapy or a multimodal approach. Completion of an adjuvant or neoadjuvant chemotherapy, according to current international standards, was not an inclusion criterion, but this information was thoroughly recorded to allow for its inclusion in the analysis. Tumour samples were assessed for PD-L1 expression and genetic aberrations.
We retrieved data on 255 patients with localised, technically resectable non-small cell lung cancer who were treated with curative intent at the Oncology Institute of Southern Switzerland (IOSI) and/or at the Thoracic Surgery Department of Ente Ospedaliero Cantonale (EOC) in Canton Ticino, Switzerland. Among them, 146 patients, who were treated between 2016 and 2019, met all inclusion criteria and were included in the present analysis (figure 1). Histological or cytological diagnosis of non-small cell lung cancer was obtained for all patients either preoperatively, by transbronchial or transthoracic biopsy, or intraoperatively, with wedge resection and frozen section. PD-L1 expression and molecular characterisation were determined before any systemic treatment began.

Figure 1Study flow chart.
Clinical and pathological data were obtained from institutional medical records. Follow-up visits were scheduled according to our local policy, which is based on current international guidelines [31]. Participants were censored at the time of study completion.
We analysed the following variables: PD-L1 status at different levels of protein expression, gender, smoking status, completeness of the therapeutic plan, presence of driver mutations, histopathological subtypes, grade of differentiation, disease stage at diagnosis, systemic treatment at relapse and type of treatment (i.e., tyrosine-kinase inhibitors, immunotherapy or chemotherapy). In addition, we evaluated other clinic-pathological prognostic factors, such as actionable alterations (EGFR, KRAS, BRAF, MET or HER-2 mutations or ALK, ROS1, NTRK or RET gene fusions), completeness of the treatment plan and the main modality of treatment received.
The immunohistochemical evaluation of PD-L1 was performed at the Institute of Pathology, EOC in Locarno, Switzerland, using the SP263 monoclonal rabbit anti-human antibody (Ventana/Roche, Ventana Medical Systems) on an automated instrument (Benchmark GX, Ventana/Roche) according to the standard protocol. The tumour proportion score (TPS) was used [32].
The presence of druggable genomic alterations was assessed in tumour biopsies from 107 of 109 cases with non-squamous histology, while no molecular analysis was performed on squamous cell tumours. Genomic DNA extraction was performed at the Institute of Pathology, EOC, Locarno, Switzerland, using the QIAamp DNA FFPE Tissue Kit (Qiagen, Chatsworth, CA, USA) and starting from three 8-µm-thick serial sections of the selected FFPE tissue block. The extracted DNA was molecularly characterised using a next-generation sequencing approach. We employed the S5XL Ion Torrent (IOT) platform and used the Ion AmpliSeq Colon and Lung Cancer Panel v2 (Thermo Fisher Scientific, Waltham, MA, USA), which determines the mutational status of 22 genes, including those most relevant and most frequently mutated in non-small cell lung cancer (i.e., EGFR, KRAS, BRAF and HER-2).
IOT results were considered usable only when the target regions were covered by ≥300 reads, the depth values were >2000X and the uniformity was >90%. The limit of detection of the variant allele frequencies was set between 2% and 5%. All the mutations demonstrated in the literature to be polymorphic and all the variants in intronic regions were excluded.
The presence of chromosomal alterations (gene fusions) in the ALK gene was determined using a fluorescence in situ hybridisation (FISH) assay on 4-μm FFPE tissue sections treated with a Paraffin Pretreatment Kit II (Pretreatment Reagent VP2000, Abbott Molecular AG, Baar, Switzerland) and processed with the VP2000 automatic processor (Abbott Molecular AG) according to the manufacturer’s instructions, using the ALK Break Apart FISH Probe Kit (Abbott Molecular Vysis, North Chicago, IL, USA). We evaluated FISH results following published criteria [33–35]. For FISH analyses, a minimum of 100 morphologically clear, non-overlapping nuclei from at least 8–10 areas were scored for each patient. Only experiments with ≥90% hybridisation efficiency were considered. In order to avoid false positive/negative results, a cut-off of 15% was applied for considering a tissue positive for ALK gene fusion.
We related predefined outcome measures, overall survival and disease-free survival, to different levels of PD-L1 expression. Overall survival was defined as the time between the beginning of treatment and death from any cause, and disease-free survival was defined as the time between the beginning of treatment and disease relapse or death from any cause. The sample size was not predefined. However, with the currently expected 5-year overall survival rate of early or locally advanced non-small cell lung cancer ranging from 35–63% [36], our sample size of 146 patients would have a power of 88.95% to estimate a survival change from 50–63% with alpha set at 0.05 (two-sided test). Survival probabilities were calculated using the life table method, and survival curves were estimated using the Kaplan-Meier method; differences between curves were analysed using the log-rank test. Follow-up was calculated as median time to censoring using the reverse Kaplan-Meier estimator (i.e., by flipping the meaning of event (i.e., death) and censor of the standard Kaplan-Meier survival curve) [37]. Binomial exact 95% confidence intervals (95% CIs) were calculated for incidence rates. Continuous variables were expressed as median and interquartile range, and differences between groups were compared using the Mann-Whitney U test. Fisher's exact test was used to test for differences between frequencies of categorical data, as appropriate. Hazard ratios (HRs) and their 95% CIs in both univariable and multivariable analyses of prognostic factors were estimated using a Cox proportional hazards model. Multivariable analysis of clinical prognostic factors affecting either overall survival or disease-free survival was performed by a backward stepwise Cox regression starting from the variables with a significant impact on outcomes in the univariable analyses. The validity of the proportional hazard assumption was confirmed using Schoenfeld residuals, and the overall statistical significance of the Cox model was assessed using a likelihood-ratio test. P-values ≤0.05 (two-sided test) were considered to indicate statistical significance. Statistical analyses were conducted using STATA 16.1 statistical software (Stata Corp., College Station, TX, USA).
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Canton Ticino (ProjectID 2021-01392).
A total of 146 patients treated between 2016 and 2019 met all the inclusion criteria and were included in the analysis.
Patient demographic and clinical characteristics are summarised in table 1. Cases were distributed according to the pathologic disease stage (or the clinical staging in cases of no surgery) as follows: stage I, 56.2%; stage II, 25.3% and resectable stage III, 18.5%. The percentages of male and female patients were 54.8% and 45.2%, respectively. Median age at diagnosis was 70 years (range 47–84). In terms of histology subtype, 23.3% of patients had a squamous cell variant, 74.7% were classified as adenocarcinoma and 2% had undifferentiated histology. Data on tobacco use were available for 140 patients: 47.1% of patients were current smokers, 44.3% were former smokers and the remaining 8.6% of patients had never smoked. Patients received surgery (90%) or radiotherapy (10%) as the main upfront treatment. Treatments were classified as complete or incomplete, according to following standards: stage I, radical surgery; stage II, radical surgery and adjuvant chemotherapy; stage III, either radical surgery and adjuvant chemotherapy ± mediastinal radiation or neoadjuvant chemoradiation followed by radical resection or definitive chemoradiation. According to this rule, 72% of patients completed their treatment, and 28% did not, including 3 patients (2%) allocated to neoadjuvant chemotherapy who could not proceed to surgery. Information on presence or absence of driver mutations was available in 73% of cases.
Table 1Baseline clinical characteristics.
| Feature | n (%) | |
| Age | <65 years | 44 (30%) |
| 65–75 years | 66 (45%) | |
| >75 years | 36 (25%) | |
| Sex | Male | 80 (55%) |
| Female | 66 (45%) | |
| Histologic type | Squamous | 34 (23%) |
| Non-squamous (adenocarcinoma) | 109 (75%) | |
| Undifferentiated | 3 (2%) | |
| T stage | 0 (minimally invasive adenocarcinoma) | 2 (1%) |
| 1 | 63 (43%) | |
| 2 | 47 (32%) | |
| 3 | 24 (16%) | |
| 4 | 10 (8%) | |
| N stage | X | 1 (0.7%) |
| 0 | 118 (81%) | |
| 1 | 14 (10%) | |
| 2 | 13 (9%) | |
| TNM VIII stage | IA1 | 20 (14%) |
| IA2 | 24 (16%) | |
| IA3 | 13 (9%) | |
| IB | 25 (17%) | |
| IIA | 10 (7%) | |
| IIB | 27 (19%) | |
| IIIA | 25 (17%) | |
| IIIB | 2 (1%) | |
| Smoking status | Current smoker | 66 (47%) |
| Former smoker | 62 (44%) | |
| Never a smoker | 12 (9%) | |
| Main curative strategy | Chemotherapy | 3 (2%) |
| Surgery | 126 (86%) | |
| Radiotherapy | 13 (9%) | |
| Multimodal | 4 (3%) | |
| Immunotherapy at relapse | Yes | 22 (15%) |
| No | 124 (85%) | |
| Target therapy at relapse | Yes | 3 (2%) |
| No | 143 (98%) | |
Treatments for stages I and II were registered as standard (surgery) in 90.9% of the cases and non-standard (ablative radiation) in 9.1% of cases. PD-L1 expression status was available for 129 patients and was reported as following ranges: TPS <1% (44.2%), TPS 1–24% (27.9%), TPS 25–49% (7%), TPS 50–74% (13.2%) and TPS ≥75% (7.4%). In terms of cumulative frequencies, we found positive PD-L1 expression (>1%) in 55.8% of patients and strong positive PD-L1 expression (≥50%) in 20.9% (table 2). In terms of histology subtypes, positive PD-L1 expression was observed in 75% of squamous cell carcinomas and 47.8% of adenocarcinomas.
Table 2Main histological and biological features.
| Feature | (%) | |
| Histology | MiA | 2 (1%) |
| Lepidic | 13 (8%) | |
| Acinary | 62 (43%) | |
| Mucinous | 11 (8%) | |
| Micropapillary | 5 (3%) | |
| Papillary | 24 (17%) | |
| Cribriform | 1 (1%) | |
| Tubular | 2 (1%) | |
| Solid | 24 (17%) | |
| Unknown | 2 (1%) | |
| PD-L1 expression | <1 | 57 (39%) |
| 1–24 | 36 (25%) | |
| 25–49 | 9 (6%) | |
| 50–74 | 17 (12%) | |
| 75–100 | 10 (6%) | |
| Unknown | 17 (12%) | |
| Druggable molecular alteration | Yes | 22 (15%) |
| No | 85 (58%) | |
| Unknown | 39 (27%) | |
| KRAS mutation | Yes | 41 (28%) |
| No | 65 (44%) | |
| Unknown | 40 (27%) | |
| EGFR mutation | Yes | 13 (9%) |
| No | 94 (64%) | |
| Unknown | 39 (27%) | |
| PT53 mutation/deletion | Yes | 38 (26%) |
| No | 69 (47%) | |
| Unknown | 39 (27%) | |
The number of patients with druggable genetic alterations was small; therefore, we could not perform a meaningful analysis. Classical epidermal growth factor receptor (EGFR) alterations, either exon 19 deletions or exon 21 point mutations (p.L858R), were present in 12.2% of patients; BRAF-V600 mutations in 1.87% and ALK rearrangements in 1.9%. KRAS mutations (including the G12C subtype, considered non-druggable at the time of data collection) were present in 38.7% of cases. TP53 inactivation was documented in 35.8% of patients. Other, less frequent genetic alterations are detailed in figure 2.

Figure 2Distribution of molecular alterations in 107 cases with adenocarcinomas of the lung.
At a median follow-up time of 20 months (interquartile range 11–32), 32 patients (22%) experienced disease progression: 19 of them (61%) had a local progression, 7 had a distant relapse (23%) and 5 had both local and systemic disease progression. The median disease-free survival in the entire cohort of 146 patients was 4.6 years (interquartile range 1.8−4.9), with 1-year and 2-year disease-free survival rates of 85% (95% CI 77–90) and 72% (95% CI 62–80), respectively.
Fifteen patients (10%) died, all due to lung cancer progression. The median overall survival was not reached, and 1-year and 2-year overall survival rates were 94% (95% CI 89–97) and 88% (95% CI 80–93), respectively.
Univariable analyses of disease-free survival and overall survival are summarised in tables 3 and 4, respectively.
Table 3Univariable analyses of factors that may affect disease-free survival.
| Variable (n) | 1-yr disease-free survival: % (95% CI) | 2-yr disease-free survival: % (95% CI) | HR (95% CI) | p-value (Cox) | |
| All patients (146) | 94 (89–97) | 88 (80–93) | |||
| PD-L1 >25% | No (93) | 87 (77–93) | 74 (60–84) | 1.0 | 0.049 |
| Yes (36) | 73 (54–85) | 56 (36–72) | 1.9 (1.0–3.9) | ||
| PD-L1 >50% | No (102) | 79 (57–91) | 62 (37–79) | 1.0 | 0.604 |
| Yes (27) | 83 (74–90) | 70 (57–80) | 1.2 (0.6–2.6) | ||
| PD-L1 >50% or druggable mutation | No (59) | 85 (69–93) | 74 (55–86) | 1.0 | 0.516 |
| Yes (45) | 84 (71–92) | 66 (49–79) | 0.8 (0.3–1.7) | ||
| Druggable mutation | No (85) | 95 (67–99) | 95 (67–99) | 1.0 | 0.326 |
| Yes (22) | 85 (75–92) | 69 (55–79) | 0.6 (0.2–1.7) | ||
| KRAS mutation | No (65) | 80 (63–90) | 65 (44–80) | 1.0 | 0.300 |
| Yes (41) | 91 (80–96) | 79 (63–88) | 1.5 (0.7–3.4) | ||
| Ever a smoker | No (12) | 84 (75–89) | 69 (58–78) | 1.0 | 0.312 |
| Yes (128) | 100 (NA–NA) | 100 (NA–NA) | 2.8 (0.4–20.6) | ||
| Smoking status | Never (12) | 100 (NA–NA) | 100 (NA–NA) | 1.0 | 0.358 |
| Former (62) | 84 (71–91) | 70 (55–82) | 2.6 (0.3–19.6) | ||
| 0.281 | |||||
| Current (66) | 83 (70–91) | 68 (51–80) | 3.0 (0.4–23.1) | ||
| Standard treatment completed | No (41) | 90 (81–94) | 75 (63–84) | 1.0 | 0.012 |
| Yes (105) | 73 (55–84) | 64 (44–78) | 0.4 (0.2–0.8) | ||
| Main local treatment | Surgery (130) | 86 (78–92) | 76 (65–84) | 1.0 | 0.003 |
| Radiotherapy (13) | 67 (34–86) | 36 (10–63) | 3.2 (1.5–6.9) | ||
| Stage | I (82) | 94 (85–98) | 81 (65–90) | 1.0 | 0.003 |
| >I (64) | 74 (61–86) | 62 (47–74) | 3.0 (1.4–6.2) | ||
| Sex | Female (66) | 98 (88–99) | 88 (72–96) | 1.0 | 0.177 |
| Male (80) | 85 (74–92) | 82 (70–90) | 1.6 (0.8–3.2) | ||
NA: not available.
Table 4Univariable analyses of factors that may affect overall survival.
| Variable () | 1-yr overall survival: % (95% CI) | 2-yr overall survival: % (95% CI) | HR (95% CI) | p-value (Cox) | |
| All patients (146) | 94 (89–97) | 88 (80–93) | |||
| PD-L1 >25% | No (93) | 96 (88–99) | 87 (75–94) | 1.0 | 0.087 |
| Yes (36) | 88 (71–95) | 84 (65–93) | 2.4 (0.9–6.7) | ||
| PD-L1 >50% | No (102) | 94 (87–97) | 85 (73–92) | 1.0 | 0.874 |
| Yes (27) | 92 (71–98) | 92 (71–98) | 1.1 (0.3–3.4) | ||
| PD-L1 >50% or druggable mutation | No (59) | 98 (87–99) | 89 (73–96) | 1.0 | 0.799 |
| Yes (45) | 92 (78–97) | 92 (78–97) | 1.2 (0.3–4.1) | ||
| Druggable mutation | No (85) | 96 (88–99) | 90 (78–95) | 1.0 | 0.478 |
| Yes (22) | 95 (67–99) | 95 (67–99) | 0.5 (0.06–3.8) | ||
| KRAS mutation | No (65) | 96 (87–99) | 91 (78–97) | 1.0 | 0.518 |
| Yes (41) | 94 (79–98) | 89 (70–97) | 1.5 (0.4–5.8) | ||
| Ever a smoker | No (12) | 100 (NA–NA) | 100 (NA–NA) | NA* | 0.263 (Log-rank test)* |
| Yes (128) | 94 (87–97) | 86 (77–92) | |||
| Smoking status | Never (12) | 100 (NA–NA) | 100 (NA–NA) | 0.191 | |
| Former (62) | 94 (84–98) | 92 (79–97) | 1.0* | ||
| Current (66) | 93 (82–97) | 80 (64–90) | 2.0 (0.7–5.6) | ||
| Standard treatment completed | No (41) | 92 (80–99) | 86 (66–95) | 1.0 | 0.327 |
| Yes (105) | 94 (87–98) | 89 (78–94) | 0.6 (0.2–1.7) | ||
| Main local treatment | Surgery (130) | 95 (89–98) | 91 (82–96) | 1.0 | 0.009 |
| Radiotherapy (13) | 83 (48–96) | 62 (28–84) | 4.2 (1.4–12.2) | ||
| Stage | I (82) | 97 (88–99) | 92 (78–97) | 1.0 | 0.079 |
| >I (64) | 91 (81–96) | 84 (71–92) | 2.8 (0.9–8.8) | ||
| Sex | Female (66) | 100 (NA–NA) | 97 (82–100) | 1.0 | 0.096 |
| Male (80) | 89 (79–94) | 80 (67–89) | 2.6 (0.8–8.3) | ||
NA: not available.
* There are no deaths among non-smokers; hence, hazard for that group is zero, which means that the hazard ratio for any group that does have an event will tend to infinity. For this reason, the only meaningful HR that we could calculate is the one for the current smoker group vs the pooled groups of former and never smokers.
The sole clinical factor affecting overall survival was the type of main local treatment. Patients with inoperable tumours who had radiotherapy only had significantly poorer outcomes (HR 4.2, 95% CI 1.4–12.2) (figure 3).

Figure 3Kaplan-Meier survival curves (DFS and OS) by main treatment modality and treatment completion. Kaplan-Meier curves for DFS (A) and OS (B) in the subgroup of patients treated with surgery (medically operable) vs radiotherapy (medically inoperable) in univariable analyses. Kaplan-Meier curves for DFS (C) and OS (D) for patients who completed treatment vs patients who did not complete treatment in univariable analyses. A positive significancy on OS for the subgroup of patients who completed treatment was not reached.DFS: disease-free survival; OS: overall survival.
Expression of PD-L1 in >25% of tumour cells was associated with lower disease-free survival (HR 1.9, 95% CI 1.0–3.9) (figure 4).

Figure 4Kaplan-Meier survival curves (DFS and OS) for PD-L1 expression levels. In univariable analyses, PDL1 expression of ≥25% was a significant poor prognostic factor for DFS but was not a significant predictor of OS. Kaplan-Meier curves for DFS (A) and OS (B) for PD-L1 expression for all cut-offs chosen for the analysis of our non-small cell lung cancer patient population and Kaplan-Meier curves for DFS (C) and OS (D) in patients with a PD-L1 tumour proportion score of ≥25%.DFS: disease-free survival; OS: overall survival.
Other factors significantly affecting disease-free survival were the completion of standard treatment (HR 0.4, 95% CI 0.2–0.8), presentation with inoperable disease (HR 3.2, 95% CI 1.5–6.9) and stage >I (HR 3.0, 95% CI 1.4–6.2) (figure 3).
The frequency of cases with PD-L1 expression >25% was similar in patients with stage I disease and those with higher stage disease (30% vs 26%, p = 0.695). Inoperability was higher in patients with disease stage >I (69% vs 31%), but the difference was not statistically significant (p = 0.074). The rate of treatment completion was significantly lower in patients with disease stage >I (41% vs 96%, p <0.001).
In the multivariable analysis (stepwise Cox regression starting from the aforementioned variables that were significantly associated with outcomes in univariable analyses), having an inoperable tumour and stage >I remained significantly associated with lower disease-free survival (table 5).
Table 5Stepwise Cox regression of prognostic factors associated with disease-free survival.
| Variable* | HR | 95% CI | p-value (Wald test) |
| Stage >I | 2.7 | 1.2–6.0 | 0.012 |
| Inoperable tumour | 3.2 | 1.4–7.4 | 0.005 |
N subjects = 126; n failures = 34; likelihood ratio test p = 0.0003.
* starting variables: completed treatment, stage >I, inoperable disease, PD-L1 >25%
PD-1 belongs to the CD28 superfamily and is an inhibitory surface receptor that is expressed on activated T, B and natural killer lymphocytes and regulates their activation and expansion [38]. PD-L1, which belongs to the B7 family, is the main ligand of PD-1 and is frequently upregulated in several human malignancies, including lung cancer [12, 39]. The activation of the PD-1/PD-L1 signalling pathway can inhibit other pathways, such as RAS/MEK/ERK and PI3K/AKT, to suppress T cell proliferation [40]. In the tumour microenvironment, PD-L1 expression can induce depletion of infiltrating T lymphocytes, leading to a reduction in immune surveillance [41]. PD-L1 favours the upregulation of regulatory T cells and their negative regulatory functions; in addition, it inhibits the activity of effector T cells [42].Consequently, PD-L1 expression helps cancer cells to avoid the antitumour immune response [43], while blocking PD-1/PD-L1 signalling restores this immune response against multiple tumour types, as demonstrated in recent clinical trials [44, 45].
These physiological mechanisms suggest that the expression of PD-L1 by tumour cells may lead to immune evasion and favour tumour growth.
Efforts have been made to determine the prognostic significance of PD-L1 expression in early-stage non-small cell lung cancer patients, but the results are highly heterogeneous.
First, several case series from the pre- and post-immunotherapy era, which assessed Asian and Caucasian patients with early-stage non-small cell lung cancer, were unable to demonstrate a consistent correlation between PD-L1 expression and overall survival or disease-free survival [21, 46, 47]. Second, a few other studies of Caucasian patients concluded that the expression of PD-L1 in tumour tissue correlated with a favourable prognosis [12–14, 48]. Finally, several studies of Asian patients suggested that PD-L1 expression was associated with a poor prognosis in terms of overall survival and/or disease-free survival [7–9, 24, 26, 27, 43]. There are many potential explanations for these discrepancies; for example, during the development of various commercially available immune checkpoint inhibitors (ICIs), different antibodies and platforms were used, along with different methodologies, target tumour material, scoring methods and thresholds of expression levels. Consensus could not be reached in these early phases, and harmonisation and quality assessment efforts were conducted later. The Blueprint PD-L1 Immunohistochemistry Assay Comparison Project, an industrial-academic collaborative partnership to provide information on the analytical and clinical comparability of four PD-L1 immunohistochemistry assays used in clinical trials (22C3 and 28-8 manufactured by Dako, SP142 and SP263 manufactured by Ventana/Roche) investigated this issue [49]. This study indicated that, despite similar analytical performance of three (28-8, 22C3 and SP263) of four assays of PD-L1 expression, interchanging assays and cut-offs leads to “misclassification” of PD-L1 status of the same tumour sample. In an additional study by Huijuan Li and colleagues, no association was found between PD-L1 expression and survival when SP263, 22C3 and ab58810 were used, but a poor prognosis was evident if PD-L1 was assessed by SP142, E1L3N or 28-8 antibodies [47].
The most relevant finding of our analysis was that PD-L1 expression >25% was correlated with lower disease-free survival in a univariable analysis. Although this effect was not confirmed in the multivariable analysis, it may suggest the need for additional therapeutic interventions, especially for patients with tumours with PD-L1 ≥25%, in order to equalise survival outcomes with those of the more favourable group. In January 2022, atezolizumab became the first cancer immunotherapy available in Switzerland for adjuvant treatment of patients with early-stage non-small cell lung cancer. Its approval was based on the IMpower010 phase III study, which showed that adjuvant atezolizumab improved disease-free survival and reduced the risk of recurrence by 57% in the subpopulation of patients with PD-L1 ≥50% and stage II–IIIA non-small cell lung cancer, compared with the best supportive care[19]. Data needed to assess overall survival in this study are not yet available; however, the Swiss regulatory authority Swissmedic, and, recently, the European Medicines Agency, considered this disease-free survival difference enough to deserve the aforementioned label. The Food and Drug Administration (FDA), in contrast, extended approval to patients with PD-L1 expression ≥1%.
The recently published CheckMate 816 randomised study assessing neoadjuvant nivolumab plus chemotherapy versus neoadjuvant chemotherapy alone reported similar results in favour of immunotherapy [20]. The median event-free survival was 31.6 months (95% CI 30.2 to not reached) with nivolumab plus chemotherapy and 20.8 months (95% CI 14.0–26.7) with chemotherapy alone (HR 0.63, 97.38% CI 0.43–0.91, p = 0.005). This greater event-free survival with chemo-immunotherapy compared to chemotherapy alone seemed to be due mainly to the subpopulation of patients with high PD-L1 expression (≥50%). In addition, this study found that, at the first prespecified interim analysis, the hazard ratio for death was 0.57 (99.67% CI 0.30–1.07) and was not significant. Upon the report of these event-free survival results, the FDA approved neoadjuvant nivolumab for stage II and III patients regardless the tumour’s PD-L1 expression.
From the perspective of a clinical oncologist, it could be challenging to recommend adjuvant or neoadjuvant treatments based on a disease-free survival advantage alone, without a confirmed overall survival advantage. At present, due to the predominance of patients treated with ICIs for metastatic tumours, the potential long-term or permanent harm caused by these drugs may be underestimated. This possibility should be seriously considered before embracing perioperative treatments with anti-PD-1 or anti-PD-L1 agents as a standard of care. Jain and colleagues conducted a large retrospective pharmacovigilance study on cardiovascular adverse events associated with ICIs in US patients. They reported a high incidence of subacute or chronic cardiovascular toxicity, with rates of stroke 4.6%, heart failure 3.5%, atrial fibrillation 2.1%, conduction disorders 1.5%, myocardial infarction 0.9%, myocarditis 0.05%, vasculitis 0.05% and pericarditis 0.2% [50]. In addition, among patients who developed nephritis, use of ICIs was associated with a twofold to threefold higher risk of myocardial infarction (HR 2.03, 95% CI 1.25–3.31, p = 0.004), heart failure (HR 2.37, 95% CI 1.86–3.03, p <0.001), conduction disorders (HR 3.06, 95% CI 2.17–4.3, p ≤0.001, atrial fibrillation (HR 3.29, 95% CI 2.46–4.4, p <0.001) and stroke (HR 1.75, 95% CI 1.39–2.2, p <0.001). Additionally, the development of pneumonitis while on ICIs was associated with heart failure (HR 2.61, 95% CI 1.23–5.52), and the development of encephalitis was associated with conduction disorders (HR 4.35; 95% CI 1.6–11.87). Effects like these could theoretically counterbalance event-free survival or disease-free survival advantages, precluding the desired overall survival benefit. This possibility is even more relevant for subgroups of patients with a biologically better prognosis, as may be the case for those with PD-L1 expression <25%. By contrast, immediate adoption of perioperative immunotherapy could be justified for patients whose tumours express high levels of PD-L1 (≥50%), given that both aforementioned randomised trials showed the greatest event-free survival and disease-free survival benefit, respectively, in this category.
Our patient population was relatively small but homogeneous in terms of diagnostic work-up and treatment allocation criteria and delivery. In addition, unlike in prior studies, PD-L1 was measured according to modern diagnostic and therapeutic standards.
Our results confirmed many of the principles determined by many years of research on lung cancer treatment. For example, both the impossibility of completing the treatment strategy and a disease stage >I were associated with lower disease-free survival in univariable analyses. Undergoing radiotherapy as the main treatment modality was associated with lower disease-free survival and overall survival in univariable analyses, probably mostly due to a selection bias, since, at our institute, stereotactic body radiation therapy is offered to frail patients deemed unable to tolerate surgery. In the multivariable analysis, radiotherapy as the main treatment and a disease stage >I correlated with lower disease-free survival. As mentioned above, analysing the treatment modality as a variable in a retrospective study may suffer from several biases, such as selection of patients by operability or resectability, omission of pathological nodal evaluation in the case of stereotactic body radiation therapy and underrepresentation of the SBRT arm, among other characteristics [51–54]. Findings from single institutions have always showed mixed results, ranging from surgery being the best option [55] to similar outcomes for surgery and radiotherapy [51–53, 56]. Two studies on SEER-Medicare linked data found superior long-term outcomes of lobectomy compared with stereotactic body radiation therapy [57, 58]. A study of US veterans observed that patients who underwent surgery had a higher cancer-specific mortality rate than those who underwent SBRT [54]. In contrast, a meta-analysis by Zheng et al. [59] concluded that survival outcomes were not significantly different between the two groups after adjusting for age and operability parameters. In conclusion, based on our results and the aforementioned studies, we recommend, for very early stages, a surgical resection over SBRT whenever possible. Weaknesses of the present analysis include its retrospective nature, the relatively small number of patients and the rather short median follow-up period for a population with predominantly stage I or II disease (for which a longer follow-up period is needed to record a substantial number of events).
In our cohort of early-stage non-small cell lung cancer patients treated with curative intent, the population of patients with PD-L1 expression ≥25% who were treated during the pre-immunotherapy era had a worse prognosis. This finding supports the use of adjuvant immunotherapy based on current evidence derived from disease-free survival outcomes. For the group of patients with PD-L1 expression <25%, it may be reasonable to wait for the overall survival data to be available before adopting perioperative immunotherapy as the standard of care.
We thank our patients and their families. Special thanks go to Prof. Emanuele Zucca for critical inputs in the study design, data analysis and manuscript writing as well to Dr. Luciano Cascione for the fundamental help in the statistical analysis.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.
1.Califano R, Kerr K, Morgan RD, Lo Russo G, Garassino M, Morgillo F, et al. Immune Checkpoint Blockade: A New Era for Non-Small Cell Lung Cancer. Curr Oncol Rep. 2016 Sep;18(9):59.
2.Marrone KA, Brahmer JR. Immune Checkpoint Therapy in Non-Small Cell Lung Cancer. Cancer J. 2016;22(2):81–91.
3.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al.; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016 Nov;375(19):1823–33.
4.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016 Apr;387(10027):1540–50.
5. Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107-16. Epub 2013/11/13. doi: . PubMed PMID: 24217091; PubMed Central PMCID: PMCPMC6125250.
6.Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014 May;50(7):1361–9.
7. Wu S, Shi X, Sun J, Liu Y, Luo Y, Liang Z, et al. The significance of programmed cell death ligand 1 expression in resected lung adenocarcinoma. Oncotarget. 2017;8(10):16421-9. Epub 2017/02/02. doi: . PubMed PMID: 28145884; PubMed Central PMCID: PMCPMC5369973.
8.Okita R, Maeda A, Shimizu K, Nojima Y, Saisho S, Nakata M. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother. 2017 Jul;66(7):865–76.
9.Takada K, Okamoto T, Toyokawa G, Kozuma Y, Matsubara T, Haratake N, et al. The expression of PD-L1 protein as a prognostic factor in lung squamous cell carcinoma. Lung Cancer. 2017 Feb;104:7–15.
10.Guo Q, Sun Y, Yu S, Bai H, Zhao J, Zhuo M, et al. Programmed cell death-ligand 1 (PD-L1) expression and fibroblast growth factor receptor 1 (FGFR1) amplification in stage III/IV lung squamous cell carcinoma (SQC). Thorac Cancer. 2017;8(2):73-9. Epub 2016/12/23. doi: . PubMed PMID: 28008744; PubMed Central PMCID: PMCPMC5334288.
11.Sterlacci W, Fiegl M, Droeser RA, Tzankov A. Expression of PD-L1 Identifies a Subgroup of More Aggressive Non-Small Cell Carcinomas of the Lung. Pathobiology. 2016;83(5):267–75.
12.Ameratunga M, Asadi K, Lin X, Walkiewicz M, Murone C, Knight S, et al. PD-L1 and Tumor Infiltrating Lymphocytes as Prognostic Markers in Resected NSCLC. PLoS One. 2016;11(4):e0153954. Epub 2016/04/23. doi: . PubMed PMID: 27104612; PubMed Central PMCID: PMCPMC4841565.
13.Sorensen SF, Zhou W, Dolled-Filhart M, Georgsen JB, Wang Z, Emancipator K, et al. PD-L1 Expression and Survival among Patients with Advanced Non-Small Cell Lung Cancer Treated with Chemotherapy. Transl Oncol. 2016;9(1):64-9. Epub 2016/03/08. doi: . PubMed PMID: 26947883; PubMed Central PMCID: PMCPMC4800057.
14.Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015 Aug;89(2):181–8.
15.Zhang Y, Wang L, Li Y, Pan Y, Wang R, Hu H, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014;7:567-73. Epub 2014/04/22. doi: . PubMed PMID: 24748806; PubMed Central PMCID: PMCPMC3990506.
16.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014 Oct;25(10):1935–40.
17.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012 Nov;98(6):751–5. 10.1177/030089161209800612
18.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011 Sep;28(3):682–8.
19.Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al.; IMpower010 Investigators. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021 Oct;398(10308):1344–57.
20.Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al.; CheckMate 816 Investigators. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 2022 May;386(21):1973–85.
21.D'Arcangelo M, D'Incecco A, Ligorio C, Damiani S, Puccetti M, Bravaccini S, et al. Programmed death ligand 1 expression in early stage, resectable non-small cell lung cancer. Oncotarget. 2019;10(5):561-72. Epub 2019/02/08. doi: . PubMed PMID: 30728907; PubMed Central PMCID: PMCPMC6355175.
22.Ma J, Chi D, Wang Y, Yan Y, Zhao S, Liu H, et al. Prognostic value of PD-L1 expression in resected lung adenocarcinoma and potential molecular mechanisms. J Cancer. 2018;9(19):3489-99. Epub 2018/10/13. doi: . PubMed PMID: 30310505; PubMed Central PMCID: PMCPMC6171018.
23.Chen YY, Wang LB, Zhu HL, Li XY, Zhu YP, Yin YL, et al. Relationship between programmed death-ligand 1 and clinicopathological characteristics in non-small cell lung cancer patients. Chin Med Sci J. 2013 Sep;28(3):147–51. 10.1016/S1001-9294(13)60040-1
24.Zhang M, Wang D, Sun Q, Pu H, Wang Y, Zhao S, et al. Prognostic significance of PD-L1 expression and (18)F-FDG PET/CT in surgical pulmonary squamous cell carcinoma. Oncotarget. 2017;8(31):51630-40. Epub 2017/09/09. doi: . PubMed PMID: 28881674; PubMed Central PMCID: PMCPMC5584275.
25.Inamura K, Yokouchi Y, Sakakibara R, Kobayashi M, Subat S, Ninomiya H, et al. Relationship of tumor PD-L1 expression with EGFR wild-type status and poor prognosis in lung adenocarcinoma. Jpn J Clin Oncol. 2016 Oct;46(10):935–41.
26.Sun JM, Zhou W, Choi YL, Choi SJ, Kim SE, Wang Z, et al. Prognostic Significance of PD-L1 in Patients with Non-Small Cell Lung Cancer: A Large Cohort Study of Surgically Resected Cases. J Thorac Oncol. 2016 Jul;11(7):1003–11.
27.Tang Y, Fang W, Zhang Y, Hong S, Kang S, Yan Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6(16):14209-19. Epub 2015/04/22. doi: . PubMed PMID: 25895031; PubMed Central PMCID: PMCPMC4546461.
28.Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017 Jul;28 suppl_4:iv1–21.
29.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw. 2021 Mar;19(3):254–66.
30.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-7. doi: Doi . PubMed PMID: WOS:000250386000022.
31.Vansteenkiste J, Crinò L, Dooms C, Douillard JY, Faivre-Finn C, Lim E, et al.; Panel Members. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol. 2014 Aug;25(8):1462–74.
32.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al.; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015 May;372(21):2018–28.
33.Thunnissen E, Bubendorf L, Dietel M, Elmberger G, Kerr K, Lopez-Rios F, et al. EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch. 2012;461(3):245-57. Epub 2012/07/25. doi: . PubMed PMID: 22825000; PubMed Central PMCID: PMCPMC3432214.
34.Tsuta K, Kawago M, Yoshida A, Sekine S, Asamura H, Furuta K, et al. Primary lung adenocarcinoma with morule-like components: a unique histologic hallmark of aggressive behavior and EGFR mutation. Lung Cancer. 2014 Jul;85(1):12–8.
35.Bubendorf L, Buttner R, Al-Dayel F, Dietel M, Elmberger G, Kerr K, et al. Testing for ROS1 in non-small cell lung cancer: a review with recommendations. Virchows Archiv. 2016;469(5):489-503. doi: . PubMed PMID: WOS:000387226000002.
36.Fatti e cifre sul cancro 2023 2023. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html
37.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996 Aug;17(4):343–6. 10.1016/0197-2456(96)00075-X
38.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. Epub 2016/03/05. doi: . PubMed PMID: 26936508; PubMed Central PMCID: PMCPMC4859220.
39.Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014 Dec;23(12):2965–70.
40.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5(230):ra46. Epub 2012/06/29. doi: . PubMed PMID: 22740686; PubMed Central PMCID: PMCPMC5498435.
41.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73(23):6900-12. Epub 2013/08/27. doi: . PubMed PMID: 23975756; PubMed Central PMCID: PMCPMC3851914.
42.Park HJ, Kusnadi A, Lee EJ, Kim WW, Cho BC, Lee IJ, et al. Tumor-infiltrating regulatory T cells delineated by upregulation of PD-1 and inhibitory receptors. Cell Immunol. 2012;278(1-2):76–83.
43.Shimoji M, Shimizu S, Sato K, Suda K, Kobayashi Y, Tomizawa K, et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer. 2016 Aug;98:69–75.
44.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275-87. Epub 2016/04/16. doi: . PubMed PMID: 27079802; PubMed Central PMCID: PMCPMC5381938.
45.Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9(1):47. Epub 2016/05/29. doi: . PubMed PMID: 27234522; PubMed Central PMCID: PMCPMC4884396.
46.Zhang M, Li G, Wang Y, Wang Y, Zhao S, Haihong P, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017;7(1):10255. Epub 2017/09/02. doi: . PubMed PMID: 28860576; PubMed Central PMCID: PMCPMC5578960.
47.Li H, Xu Y, Wan B, Song Y, Zhan P, Hu Y, et al. The clinicopathological and prognostic significance of PD-L1 expression assessed by immunohistochemistry in lung cancer: a meta-analysis of 50 studies with 11,383 patients. Transl Lung Cancer Res. 2019;8(4):429-49. Epub 2019/09/27. doi: . PubMed PMID: 31555517; PubMed Central PMCID: PMCPMC6749117.
48.Schmidt LH, Kummel A, Gorlich D, Mohr M, Brockling S, Mikesch JH, et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One. 2015;10(8):e0136023. Epub 2015/08/28. doi: . PubMed PMID: 26313362; PubMed Central PMCID: PMCPMC4552388.
49.Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017 Feb;12(2):208–22.
50. Jain P, Gutierrez Bugarin J, Guha A, Jain C, Patil N, Shen T, et al. Cardiovascular adverse events are associated with usage of immune checkpoint inhibitors in real-world clinical data across the United States. ESMO Open. 2021;6(5):100252. Epub 2021/08/31. doi: . PubMed PMID: 34461483; PubMed Central PMCID: PMCPMC8403739.
51.Matsuo Y, Chen F, Hamaji M, Kawaguchi A, Ueki N, Nagata Y, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. Eur J Cancer. 2014 Nov;50(17):2932–8.
52.Varlotto J, Fakiris A, Flickinger J, Medford-Davis L, Liss A, Shelkey J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer. 2013 Aug;119(15):2683–91.
53Verstegen NE, Oosterhuis JW, Palma DA, Rodrigues G, Lagerwaard FJ, van der Elst A, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol. 2013 Jun;24(6):1543–8.
54Bryant AK, Mundt RC, Sandhu AP, Urbanic JJ, Sharabi AB, Gupta S, et al. Stereotactic Body Radiation Therapy Versus Surgery for Early Lung Cancer Among US Veterans. Ann Thorac Surg. 2018 Feb;105(2):425–31.
55Crabtree TD, Denlinger CE, Meyers BF, El Naqa I, Zoole J, Krupnick AS, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010 Aug;140(2):377–86.
56Grills IS, Mangona VS, Welsh R, Chmielewski G, McInerney E, Martin S, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010 Feb;28(6):928–35.
57Shirvani SM, Jiang J, Chang JY, Welsh JW, Gomez DR, Swisher S, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84(5):1060-70. Epub 2012/09/15. doi: . PubMed PMID: 22975611; PubMed Central PMCID: PMCPMC3776428.
58Yu JB, Soulos PR, Cramer LD, Decker RH, Kim AW, Gross CP. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer. 2015;121(14):2341-9. Epub 2015/04/08. doi: . PubMed PMID: 25847699; PubMed Central PMCID: PMCPMC4490059.
59Zheng X, Schipper M, Kidwell K, Lin J, Reddy R, Ren Y, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2014 Nov;90(3):603–11.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest was disclosed.