Assessment of focal liver lesions in non-cirrhotic liver – expert opinion statement
by the Swiss
Association for the Study of the Liver and the Swiss
Society of Gastroenterology
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40099
Mikael
Sawatzkiab,
Daniela B. Husarikc,
David Semelaa
a Department of Gastroenterology and Hepatology, Kantonsspital St. Gallen, St. Gallen,
Switzerland
b Praxis für Gastroenterologie und Hepatologie, St. Gallen, Switzerland
c Institute of Radiology and Nuclear Medicine, Kantonsspital St. Gallen, St. Gallen,
Switzerland
Summary
Focal liver
lesions are common, with a prevalence up to 20%. The lesions must be evaluated
in context of risk factors associated with malignancy. Risk factors include age
>40 years, known current or past malignancy, presence of liver cirrhosis or
chronic liver disease (i.e. suspected by elevated liver elastography
measurement ≥8 kPa or FIB-4 score ≥1.3), unintentional weight loss, fever or
night sweats, newly detected focal liver lesions, documented growth of focal
liver lesions, current or past use of androgens (e.g. testosterone,
oxymetholone, danazol), increased serum tumour markers (i.e. alpha-fetoprotein, carbohydrate
antigen 19-9 [CA19-9], carcinoembryonic antigen [CEA]) and family history of
malignancy.
In patients without risk factors of malignancy, regional (non-)fatty
changes, simple liver cysts and typical haemangiomas can be diagnosed by
conventional ultrasound (without contrast). Conventional ultrasound Doppler is
recommended to rule out vascular malformations such as portosystemic shunts.
In
all other cases of focal liver lesions, contrast-enhanced imaging is indicated
for differentiation in benign and malignant dignity.
Contrast-enhanced
ultrasound (CEUS) as a first diagnostic step and contrast-enhanced magnetic resonance
imaging (MRI) are accurate tests to diagnose haemangioma and focal nodular
hyperplasia.
Hepatocellular adenoma is diagnosed by contrast-enhanced MRI
and/or histology.
“Wash out” on CEUS is highly
suspicious for a malignant focal liver lesion. Additional investigations aimed
at identifying the primary tumour, as well as staging-computed tomography, MRI
and/or histology may be necessary and should be decided on a case-by-case
basis.
A biopsy of focal liver lesions is indicated in cases of unclear
dignity, malignant aspect and focal liver lesions of unclear origin as well as for
guiding surgical and oncological management.
Introduction
This
document represents the first version of the Swiss Association for the Study of
the Liver (SASL) Expert Opinion Statement for the assessment of focal liver
lesions in non-cirrhotic liver. Recommendations are based on the results of original
studies and selected reviews, the European Association for the Study of the
Liver (EASL) Clinical Practice Guidelines on the management of benign liver tumours
and cystic liver diseases (www.easl.eu) [1, 2], the European Federation of
Societies for Ultrasound in Medicine and Biology (EFSUMB)
(http://www.efsumb.org) and the World Federation For Ultrasound in Medicine and
Biology (WFUMB) (https://wfumb.info/guidelines) guidelines and good clinical
practice recommendations for contrast enhanced ultrasound (CEUS) in the liver [3,
4], as well as the American College of Radiology (ACR) appropriateness criteria
for initial liver lesion characterisation [5]. Sonographic evaluation of the
liver is an integral part of the training of Swiss gastroenterologists and
hepatologists; therefore, this expert opinion statement will emphasise the
application of conventional and CEUS in focal liver
lesions.
Focal liver
lesions are often diagnosed in asymptomatic and symptomatic patients as incidental
lesions (incidentaloma) or as suspected liver tumours. Widespread imaging of
the liver is performed by ultrasound (US), CEUS,
contrast-enhanced computer tomography (CT) and magnetic resonance imaging (MRI).
In general, the use of diagnostic imaging has increased significantly and has contributed
to rising healthcare costs [6]. These recommendations for the workup of focal
liver lesions aim at state-of-the-art and cost-efficient diagnostic work up, as
well as reducing non-indicated imaging with ionising radiation and futile
biopsies. This document focusses on the evaluation of focal liver lesions in the
non-cirrhotic liver. The management of focal liver lesions in cirrhotic liver is
not discussed in this document and is covered partially in the SASL expert
opinion statement on hepatocellular carcinoma [7].
Background
Risk
classification of patients presenting with focal liver lesions
Focal liver
lesions are common, with a prevalence of 5–18% in imaging series [8, 9] and 20%
in autopsy series [10]. Most focal liver lesions are of a benign nature (i.e.
liver cysts, hepatic haemangioma, focal nodular hyperplasia, focal
(non-)steatosis) and do not require biopsy, treatment or follow-up. However, it
is crucial to identify pre-malignant as well as malignant focal liver lesions
early and reliably to offer appropriate treatment, such as curative resection
or ablation. The a priori probability of malignant nature of focal liver lesions
is dependent on the clinical context (i.e. presence of chronic liver disease
with increased risk of hepatocellular carcinoma and cholangiocarcinoma) and the
patient’s medical history (i.e. current or previous malignancy with increased
risk of liver metastasis). After obtaining a detailed patient history and a physical
examination, patients with focal liver lesions should be assessed for the
presence of risk factors associated with malignancy (table 1) [11]. In
addition, certain drugs are associated with lesions such as hepatocellular
adenoma (e.g. oral contraceptives, androgens) and hepatocellular carcinoma (e.g.
androgens). Depending on the clinical context, assessment of serum tumour
markers, such as alpha-fetoprotein (AFP) in hepatocellular carcinoma, carcinoembryonic
antigen (CEA) in liver metastasis and carbohydrate antigen (CA) 19-9 with CEA in
cholangiocarcinoma, can be helpful (i.e.
chronic hepatitis B, also non-alcoholic steatohepatitis [MAFLD] / non-alcoholic
steatohepatitis [NASH] without cirrhosis or patients with a history of a
previous malignancy such as germ-cell tumour).
Table 1Risk factors for potential malignancy of focal liver
lesions (modified according to [11]).
| Age >40
years |
| Known current
or past malignancy |
| Presence of
liver cirrhosis or chronic liver disease (especially
in advanced fibrosis (pathological elastography (≥8 kPa) or FIB-4 score ≥1.3),
chronic hepatitis B virus infection, MAFLD/NASH, haemochromatosis) |
| Unintentional
weight loss, fever or night sweats |
| Newly (especially
if not documented on previous imaging) detected focal liver lesion(s) |
| Documented
growth of focal liver lesion(s) |
| Current or
past use of androgens (e.g. testosterone, oxymetholone, danazol) |
| Increased
serum tumour markers (e.g. alpha-fetoprotein, CA19-9, CEA) |
| Family history
of malignancy |
Bacterial
and parasitic infections also should be considered when evaluating focal liver
lesions. The presence of fever, night sweats, leukocytosis or elevated C-reactive
protein can be suggestive of a liver abscess. Complex cystic lesions and
calcifications can be findings related to echinococcosis, which require serological
testing.
In
combination with the following imaging characteristics and/or histology,
accurate diagnosis is possible in almost all focal liver lesions.
Imaging modalities
Focal liver
lesions should be documented regarding their number, size, shape, localisation
and probable mass effect on the surrounding liver parenchyma, vasculature and
bile ducts. Depending on the imaging modality and the use of contrast agents,
further characteristics of a lesion, such as echogenicity, homogeneous or
heterogeneous aspect and perfusion patterns in relation to the surrounding
liver parenchyma (i.e. arterial enhancement and venous washout), are crucial
for the differential diagnosis and must be documented.
Conventional
ultrasound
Conventional ultrasound is the most frequently used first imaging modality of the
liver. In one
of the largest studies performed, the prevalence of benign focal liver lesions in
45,319 hospitalised patients was 15.1%, with 6.3% accounting for focal fatty
sparing, 5.8% cysts, 3.3% haemangioma, 0.2% focal nodular hyperplasia and 0.04%
hepatocellular adenoma [12]. In patients with focal liver lesions of unclear
dignity, particularly in patients with risk factors (table 1), further
contrast-enhanced imaging is needed. Conventional ultrasound should always
include a Doppler to rule out an intrahepatic or extrahepatic congenital portosystemic
shunt. Nodules in an otherwise healthy liver are known to be a common mode of
presentation of these vascular malformations [13].
Contrast-enhanced ultrasound (CEUS)
Contrast-enhanced ultrasound (CEUS) is a cost-effective imaging modality that avoids
ionising radiation and
takes advantage of a non-nephrotoxic contrast agent with an excellent safety
profile (table 2).
Table 2Comparison of contrast-enhanced imaging modalities.
| |
CEUS |
CT |
MRI |
| Severe anaphylactoid reactions |
0.01% |
0.04% |
0.01% |
| Paravasation |
No complication |
Complication |
Complication |
| Thyroid affection |
No |
Rare |
No |
| Kidney affection |
No |
Possible |
No |
| Ionising radiation |
No |
Yes |
No |
| Costs |
<300 CHF |
700 CHF |
900 CHF |
| Accuracy |
CEUS = MRI |
CEUS, MRI > CT |
MRI = CEUS |
| Diagnostic delay after ultrasound |
No |
Yes |
Yes |
| Examination time (netto) |
5 min. |
4 min. |
35 min. |
| Contrast volume application |
1−2.5 ml |
100−120 ml |
10−20 ml |
| Examination conditions |
Variable |
Excellent |
Excellent |
| Interobserver variation |
Strong |
Low |
Low |
A contrast agent is intravenously injected (i.e.
SonoVue®), and the focal liver lesion is studied for contrast enhancement
and/or washout in comparison to the surrounding liver parenchyma (figure 1 and 2,
table 3). A detailed description of the technical aspects and clinical
performance of CEUS has been published [14]. In comparison
to CT and MRI the CEUS contrast agent is a pure blood pool agent which remains
strictly intravascular whereas the majority of contrast agents for CT and MRI show
a late distribution of the contrast agent from the blood pool into the
extravascular space. CEUS can evaluate focal liver
lesions in real time and in higher temporal resolution than other imaging
modalities [3, 15–17]. Appropriate experience of the operator is required for CEUS
performance and interpretation.

Figure 1Proposed
diagnostic algorithm for work-up of focal liver lesions.
cCT:
contrast-enhanced computed tomography; cMRI: contrast-enhanced magnetic
resonance imaging; FLL: focal liver lesion; FNH: focal nodular hyperplasia;
HCA: hepatocellular adenoma; HCC: hepatocellular carcinoma; CCC:
cholangiocellular carcinoma.
1 Including
also pathological liver elastography ≥8 kPa or FIB-4 score ≥1.3.
2 CEUS with
unclear finding and with risk factors or multiple lesions → cMRI; CEUS with unclear
finding and
no risk factors → Follow up 3 and/or 6 months or cMRI.
3 cMRI
preferred; cCT for patients with claustrophobia, inability to hold breath and
MRI contraindications.
4 Abscess (due
to inflammation/hyperaemia) and acute hematoma (by compressing portal veins)
show peripheral contrast enhancement.
5 Necrotic
metastases often have a peripheral contrast enhancement due to vascularisation
and can be detected more accurately by CEUS.

Figure 2aSchematic CEUS enhancement patterns of selected benign focal liver lesions.
1. CEUS in regional fatty sparing and focal steatosis of the liver: Homogenous contrast
enhancement in all phases similar to the surrounding liver tissue.
2. CEUS in liver cyst: lack of contrast enhancement in all phases.
3. CEUS in echinoccocal cyst: lack of contrast enhancement in all phases and possible
additional calcifications.
4. CEUS in hepatic abscess: arterial phase with well delineated avascular/necrotic
area with surrounding hyperenhancement (hyperaemia).
5. CEUS in hemangioma: Peripheal nodular arterial contrast enhancement with (slow
or fast) centripetal contrast enhancement. Complete or incomplete filing in the portal-venous
or late venous phase. Hyper- or isoenhanced in the late venous phase.
6. CEUS in focal nodular hyperplasia: centrifugal rapid arterial contrast enhancement
(also called “spoke wheel pattern”) or decentral arterial contrast enhancement. Portal-venous
phase and late-venous phase with hyper- or isoenhancement, occasionally with visible
central (non-perfused) scar.
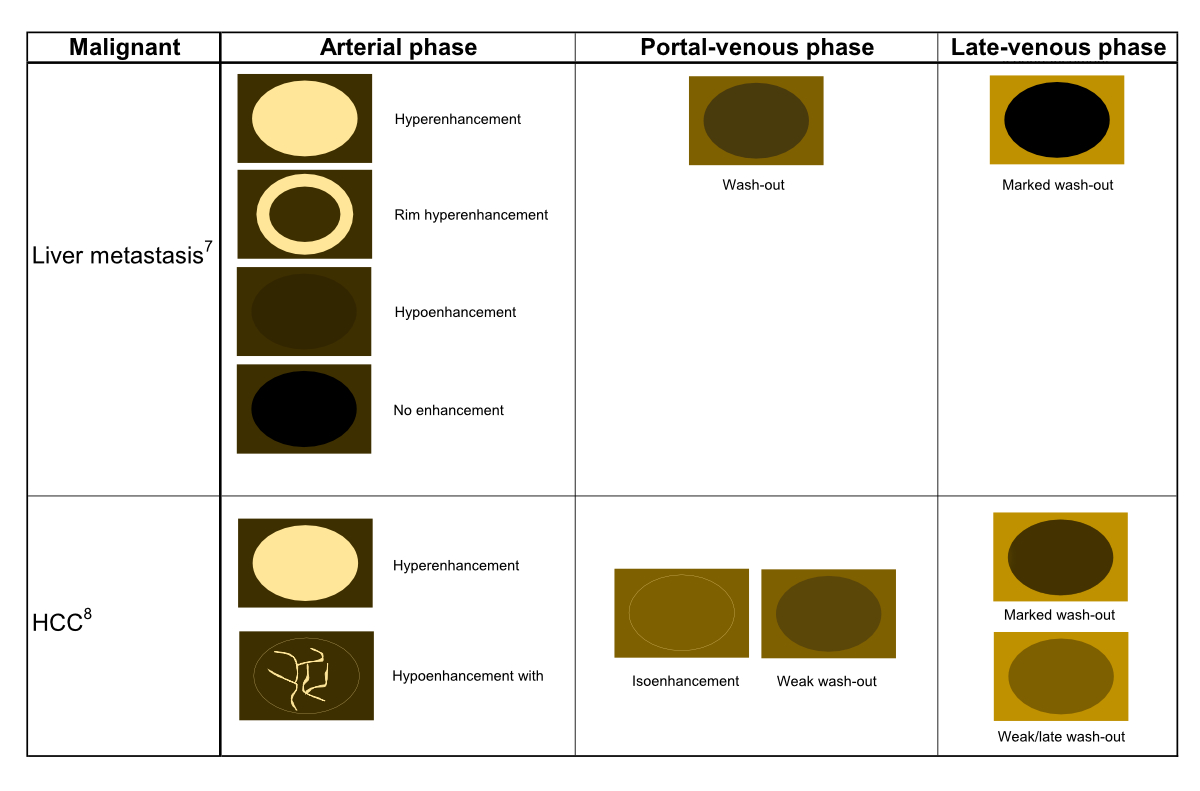
Figure 2bSchematic CEUS enhancement patterns of selected malignant focal liver lesions.
7. CEUS in hypervascular, hypovascular and avascular in liver metastases with early
wash-out.
8. CEUS in hypervascular or hypovascular hepatocellular carcinoma with late wash-out.
Table 3Contrast enhanced ultrasound: contrast phases after intravenous injection of Sonovue®.
| Contrast phases |
Start (seconds p.i.) |
End (seconds p.i.) |
| Arterial phase(AP) |
10−20 |
30−40 |
| Portal venous phase (PVP) |
30−45 |
120 |
| Late venous phase (LVP) |
>120 |
Up to 360−480 |
Obesity,
meteorism and subdiaphragmal localisation of focal liver lesions can significantly
limit CEUS performance. As an imaging modality in pregnant patients, CEUS has been
studied in case control studies [18, 19]. However, the contrast agent
Sonovue® is not currently approved in pregnant patients (off label use also in children);
thus, MRI without a contrast agent is the preferred imaging modality.
Computed
tomography
Utilising
X-rays, CT is the most commonly used cross-sectional imaging tool. Organs can be
depicted without superpositions on multidetector spiral-CT scanners by
capturing entire volumes during a single breath hold. When performed for the
evaluation of liver lesions, a CT protocol must include at least two phases for
assessing the dynamic enhancement pattern. These phases include the hepatic
arterial phase (30–40 s post injection) and the portal venous phase (50–90 s post
injectionem). In addition, an initial unenhanced scan and a delayed phase (3–10
min post injectionem) can be acquired. The use of iodinated intravenous
contrast increases soft tissue contrast and is an essential component of
detection and characterisation of focal liver lesions. A disadvantage of CT is the
limitation for the use of intravenous
contrast in patients with reduced renal function or prior allergic reaction to
the contrast agent.
Magnetic resonance
imaging
Due to its superior soft-tissue contrast, MRI offers
some advantages compared to CT for detection and characterisation of focal
liver lesions in particular in patients with liver cirrhosis [20]. Multiphasic
dynamic imaging using non-specific (extracellular) or liver-specific
(hepatobiliary) gadolinium-based contrast agents allows for a definitive
diagnosis in most cases avoiding invasive procedures such as liver biopsy. The
liver-specific hepatobiliary contrast agents (e.g. gadobenate dimeglumine, gadolinum-BOPTA,
gadoxetic acid, gadolinum-EOB-DTPA), are eliminated through both renal and
hepatic excretion pathways and therefore provide both early perfusion
information and later hepatocyte-selective information [21]. The major
advantage of gadolinum-EOB-DTPA over gadolinum-BOPTA is the earlier time point
for imaging the hepatobiliary phase (20 min after injection vs. 90 min to 120
min). However, with gadolinum-BOPTA there is the possibility to analyze delayed
phase equilibrium images, while this is not possible with gadolinum-EOB-DTPA
due to the rapid uptake by the hepatocytes as early as 2 minutes after
injection (transitional phase). Thanks to advances to shorten scan time,
reduced breath-holding capacity is becoming less of an issue for MRI. Patients
with cardiac pacemakers, neurostimulators or metallic foreign bodies still have
limited access to MRI.
Types and imaging characteristics
of focal liver lesions
Focal steatosis and
focal fatty sparing
The most
common focal liver lesions are either areas with increased (focal steatosis) or
decreased steatosis (focal non-steatosis). Focal fatty-sparing areas are
typically located in the gallbladder fossa, the periportal region and the
segment of the ligamentum falciforme (figure 3A). Similar locations
are observed for focal infiltrations of steatosis (figure 3D–F) [22, 23].
These lesions have typically a landscape-shaped appearance but can also mimic
solid tumours (so-called pseudotumour). The lesions can be diffuse, focal or multifocal
and are often located either in the perivascular or subcapsular regions [24]. Conventional
ultrasound imaging with typical landscape-shaped findings in typical above-mentioned
localisation without mass effect on vessels can easily diagnose these
pseudolesions (hypoechoic in fatty-sparing and hyperechoic in the focal
infiltration of fat) [23, 24]. In cases of atypical appearance and localisation
(figures 3E, 3F), particularly in patients with risk factors (table 1),
CEUS is accurate in most cases [3]. Perfusion of such
lesions is isoechoic (figure 3C) in comparison to the surrounding
liver tissue and neither hyperenhancement nor washout can be documented on CEUS imaging.
In unclear situations contrast-enhanced CT or MRI should be
used as next non-invasive imaging modality [22–25].
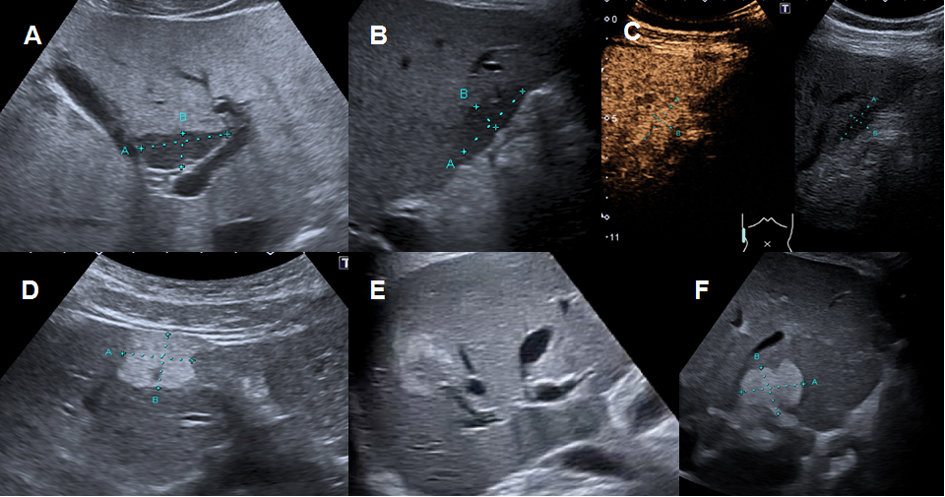
Figure 3Regional fatty sparing and focal steatosis on ultrasound and CEUS.
A Focal sparing periportal in a fatty liver on US. B Focal sparing subcapsular on US C without wash-out (= isoenhancing) in the late venous phase. D Focal steatosis in a female patient. E Focal inhomogeneous steatosis in a young patient with cystic fibrosis. F Focal steatosis in a young female patient.
On CT, steatosis will result in decreased (hypodense) attenuation on
non-contrast scans, with normal liver attenuation of 50–57 Hounsfield unit (HU)
remaining. The attenuation will remain hypodense compared to normal liver and
the spleen during the portal venous phase at about 70 seconds. However, MRI
should be used as the next non-invasive imaging modality in unclear cases due
to its superior ability to detect fat after conventional ultrasound / CEUS (figure
4). MRI can demonstrate microscopic fat
content resulting in signal intensity drop from in-phase to opposed-phase
imaging. MRI even allows to quantify the fat content [26].
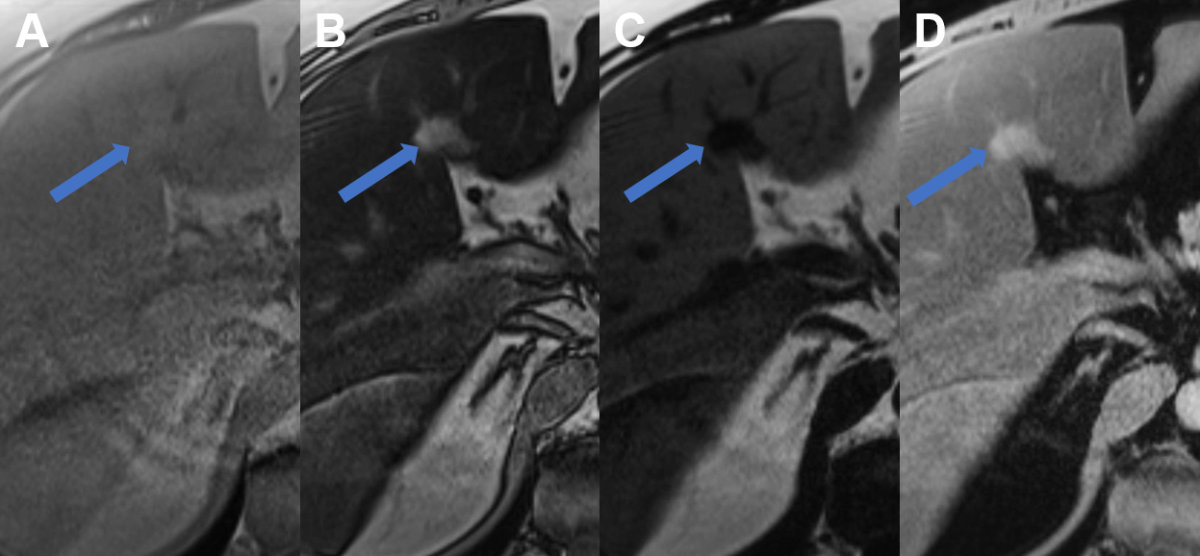
Figure 4Regional fatty sparing and focal steatosis on MRI.
A MRI T1 weighted image of a case with focal sparing (arrow) of steatosis in the porta
hepatis with B signal drop in the remaining liver on opposed phase images and persisting high signal
in the spared area. C On fat only images, the fatty liver shows signal with the spared area appearing dark,
while on D the water only image, the spared area is brighter than the steatotic surrounding
liver.
Liver cysts
The
prevalence of liver cysts ranges from 2.5% to 18% with a diameter from <1 cm
up to 30 cm. Liver cysts should be differentiated in simple and complex cysts as
well as infectious and non-infectious cysts [9, 27].
Conventional
ultrasound is the first imaging modality to demonstrate fluid-containing lesion
with smooth thin wall with a sensitivity and specificity of 90% [2, 9]. Simple
liver cysts are non-enhancing on CEUS [3]. Septation,
mural irregularity/mural nodularity or echoic internal material define a
complex liver cyst needing further investigation. Vascular perfusion with septa
or solid enhancing noduli of the liver cyst can be demonstrate or excluded by CEUS
(figure 5). Alternatively, CT or MRI are very sensitive
imaging modalities in this scenario.
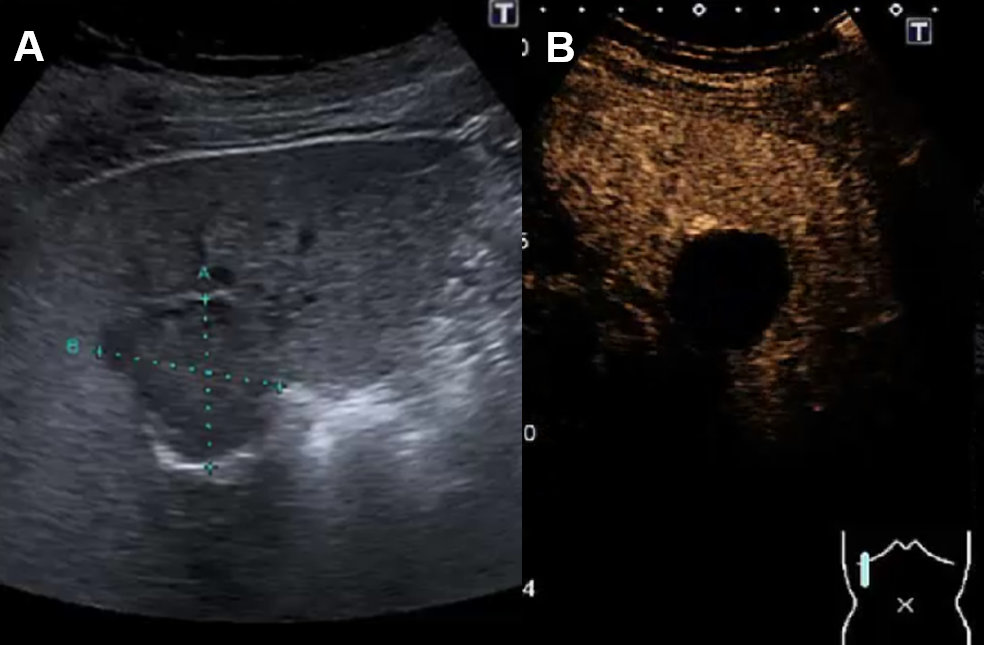
Figure 5Liver cyst on ultrasound and CEUS.
A Ultrasound with cystic lesion with echogenic content. B CEUS without contrast-enhancement demasking a complex hepatic cyst.
Hepatic cysts, particularly larger than 1 cm, can generally be characterised
on CT by their homogeneous low attenuation (–10 to +20 HU), sharp margination
and lack of enhancement. In small lesions, attenuation measurements can be
inaccurate. On MRI imaging (figure 6), a hepatic cyst follows the
signal intensity of water on all sequences with homogeneous low signal
intensity on T1-weighed T(1w) images, increased signal intensity, greater than
other T2 hyperintense liver lesions (e.g. haemangiomas) on T2-weighed (T2w)
images, and a lack of enhancement after the administration of contrast agents. MRI
is superior in detecting and characterising complex cysts. Complex cysts in the
liver should be characterized by MRI, particularly when suspected to be haemorrhagic,
to differentiate complex liver cysts from mucinous cystic neoplasms. A
difficult issue remains to differentiate complex cysts from biliary mucinous
cystic neoplasms and cystic metastases (e.g. in ovarian cancer and
gastrointestinal stromal tumours). Therefore, a low density on CT is not
definitive for a simple cyst in certain patients with underlying malignancy [28].
Rupture, bleeding and superinfection represent rare complications in liver
cysts. Definitive diagnosis of simple cysts and complex liver cysts needing
interventions can be performed by conventional ultrasound.
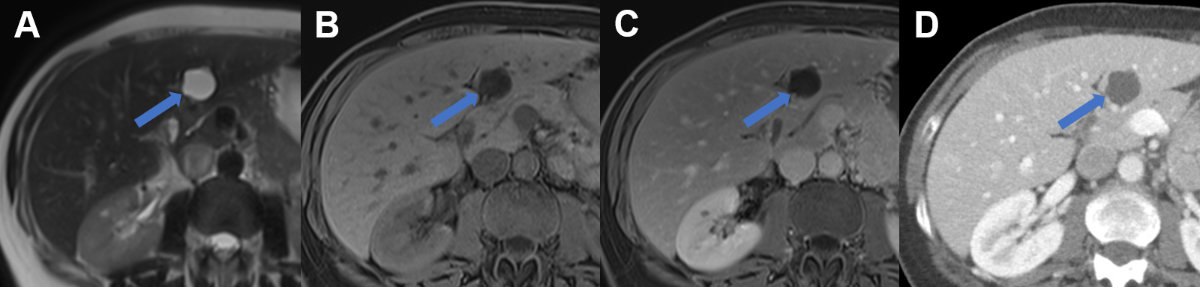
Figure 6Liver cyst on MRI and CT.
Typical liver cyst in segment III with A hyperintense signal on T2 weighted MR image, B hypointense signal on T1 weighted MR image, and C lack of enhancement on portal venous MR. D On CT the cyst appears sharply demarcated with fluid attenuation and lack of enhancement.
Echinococcosis of the liver
There are
two main types of echinococcosis: cystic echinococcosis (CE) caused by Echinococcus
granulosus (also known as hydatid disease) and alveolar echinococcosis
caused by Echinococcus multilocularis. Echinococcosis
of the liver is diagnosed by grey-scale ultrasound as the screening method of
choice and in combination with serology. Ultrasound classification for cystic echinococcosis
was elaborated by the World Health Organisation-Informed Working Group in
Echinococcus (WHO-IWGE) [29, 30]. These cystic lesions vary from a simple
anechoic cyst (CE 1) to vesicular multiseptated cysts with a “wheel-like”, “rosette-like”
or “honeycomb-like” structure (CE 2) to anechoic content with a detachment of the
laminated membrane from the cyst wall visible as a floating membrane or “water-lily”
sign (CE 3). Heterogenous hypoechoic or inhomogenous degenerative contents
without daughter cysts are seen on conventional ultrasound in type CE 4. A thick,
calcified wall is typical in CE 5 and highly suggestive of cystic
echinococcosis (figure 7) [31].
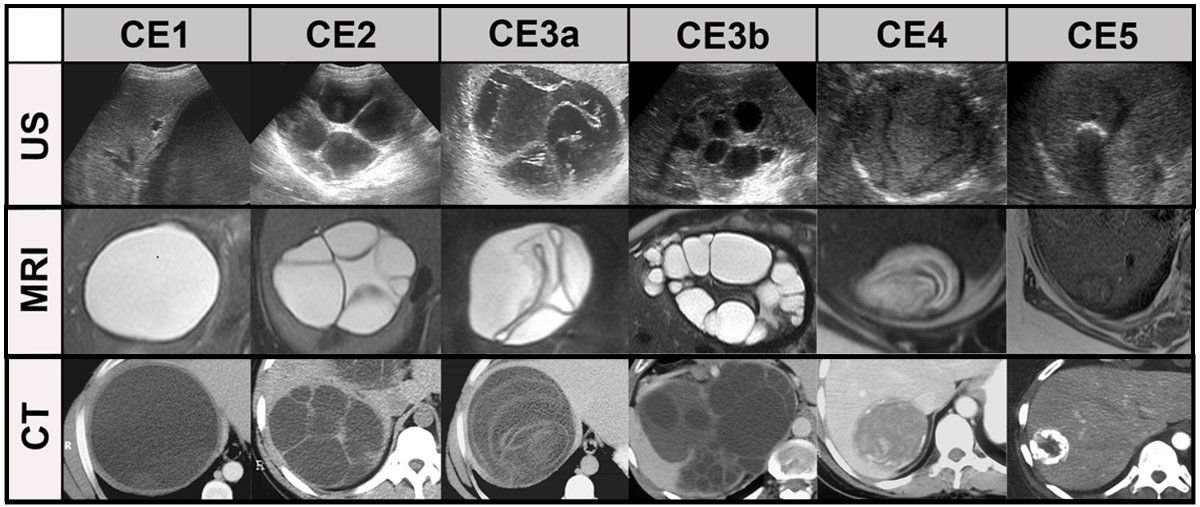
Figure 7Systemic comparison of all stages in cystic echinococcosis (CE) by ultrasound, CT
and MRI (modified from [29]).
CE1: unilocular, simple cysts with liquid content and often with the CE1-specific
‘‘double line sign’’, CE2: multivesicular, multiseptated cysts, CE3a: cysts with liquid
content and the CE3a-specific detached endocyst, CE3b: unilocular cysts with daughter
cysts inside a mucinous or solid cyst matrix, CE4: heterogenous solid cysts with degenerative
CE4-specific canalicular structure of the cyst content and CE5: cysts with degenerative
content and heavily calcified wall.
Alveolar echinococcosis is endemic in
Switzerland. Most conventional ultrasound findings (70%) are hyper- and hypoechoic
areas mimicking a tumour with irregular margins (figure 8) and
central necrosis (pseudocyst) surrounded by an irregular hyperechogenic ring [32].
Haemangioma-like hyperechogenic nodules as the initial lesion and small
calcified lesions can be found on conventional ultrasound in 30% of cases. In
both cases, CEUS can easily demask this pseudotumour as
a “simple” cystic lesion without contrast enhancement (figure 8) and
demonstrate biliary or vascular infiltration.
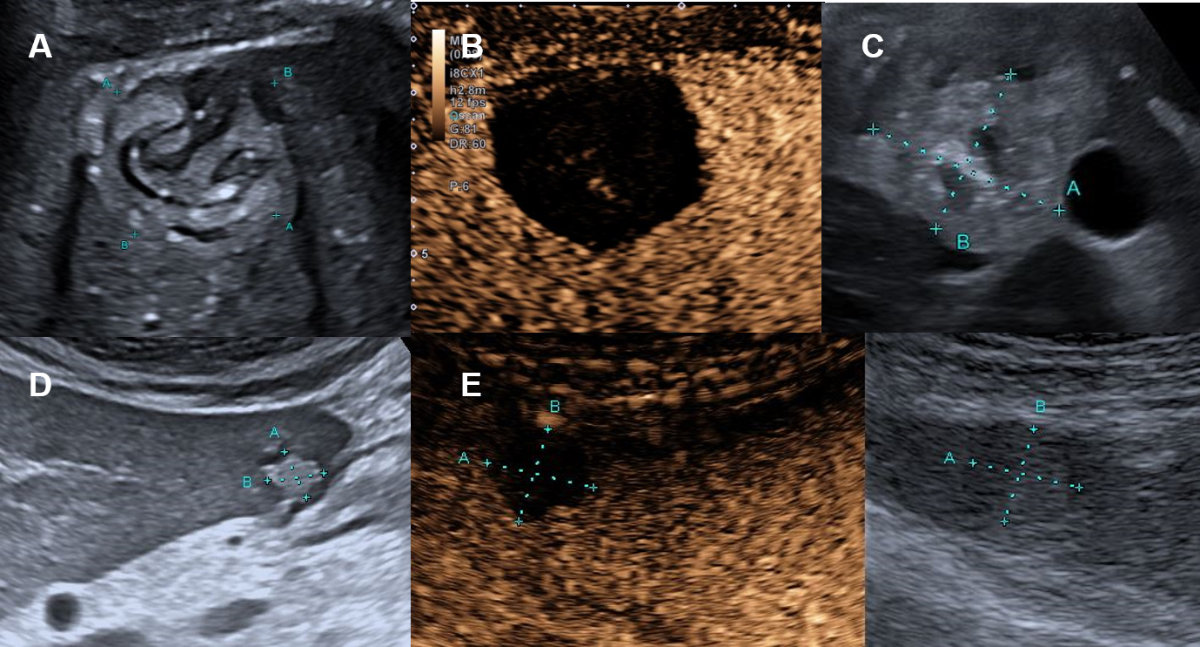
Figure 8Hepatic echinococcosis on conventional ultrasound and CEUS.
A Echogenic 4 cm hepatic lesion in the right liver lobe in a cystic echinococcosis
(E. granulosus) with floating membrane / “water-lily” sign (CE3) in a 39-yea-old female patient
from Kosovo. B CEUS without contrast-enhancement. C Echogenic hepatic lesion in the right liver lobe in an alveolar echinococcosis (E. multilocularis) in a 57 year old Swiss patient. D Echogenic hepatic lesion in the left liver lobe in an alveolar echinococcosis (E. multilocularis) in a 50-year-old Swiss male with alveolar echinococcosis. E CEUS without contrast-enhancement.
With conventional
ultrasound being the recommended main imaging modality for echinococcosis, CT
and MRI are used in cases where conventional ultrasound cannot clearly assess
the extent of the disease (e.g. in obese patients, in subdiaphragmatic or
extra-abdominal location). These techniques are used to perform staging in
newly discovered echinococcosis, assess complications such as cysto-biliary
fistulas and for pre-surgical evaluation.
The
WHO-IWGE classification for cystic echinococcosis can be applied for CT and
MRI, with MRI being superior to CT in reproducing the conventional ultrasound-stages
of cystic echinococcosis, and CT being superior to conventional ultrasound and
MRI in demonstrating minute calcifications [33]. Cystic echinococcosis fluid on
CT demonstrates fluid attenuation (approximately 0 HU). Calcifications are seen
as hyperdensities, with faint calcifications potentially being missed after the
administration of an intravenous contrast agent, which is the reason why
unenhanced images should be acquired. On MRI, theses cysts are hyperintense on
T2w images, and daughter cysts or membranes are more easily visible than on CT
(figure 9).
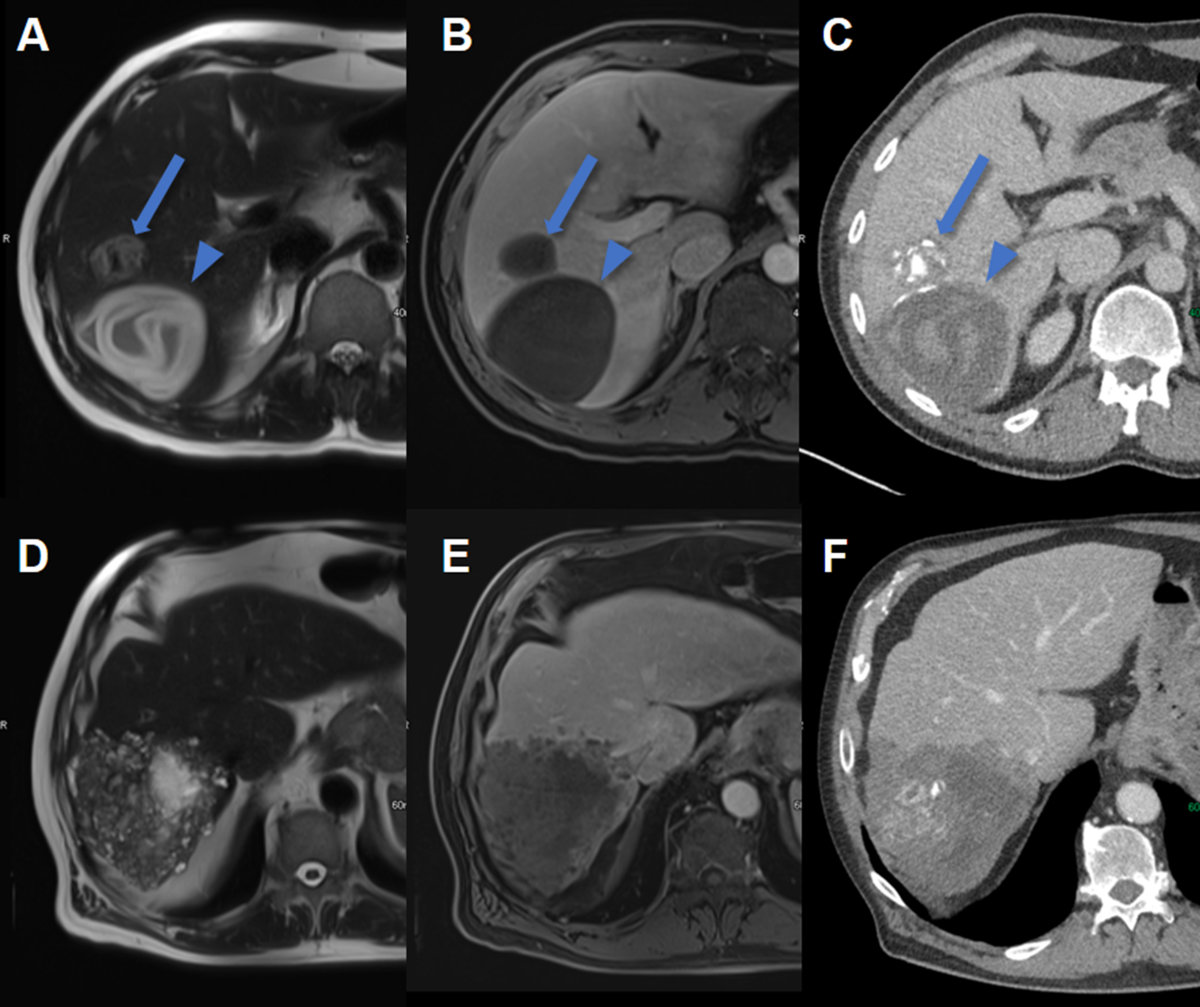
Figure 9Hepatic echinococcosis on CT and MRI.
A T2w axial MRI, B T1w fs axial image after gadolinium based i.v. contrast and C CT image after iodine based i.v. contrast in a 37-year-old male patient with cystic
echinococcosis (E. granulosus) demonstrating two lesions: the anterior with heterogenous signal on T2w, lack of
enhancement and coarse calcifications visualized on CT (arrow, CE5). The posterior
lesion with hyperintense detached membrane on T2w, lack of enhancement and faint visibility
of the membrane on CT (arrowhead, CE3).
D T2w axial MRI, E T1w fs axial image after gadolinium based i.v. contrast and F CT image after iodine based i.v. contrast in a 74-year-old male patient with alveolar
echinococcosis (E. mulitlocularis) with pathognomonic microcystic features on T2w image and partial necrosis, infiltrative
aspect and coarse calcifications seen on CT.
MRI is also
the second imaging modality of choice for alveolar echinococcosis after conventional
ultrasound. On MRI, microcystic, alveolar structures are a pathognomonic
feature of alveolar echinococcosis (figure 9). However, many lesions
are atypical and of an infiltrative character. For detection of calcifications
and in patients incompatible with MRI, CT usually has a role. On CT images, alveolar
echinococcosis presents as mixed hyperdense-hypodense lesions with possible
necroses.
MRI with
hepatobiliary contrast can increase the detection of cysto-biliary fistulas by
adding a contrast-enhanced magnetic resonance cholangiography (MRC) to
conventional T2w MRC [34].
Although
18F-fluorodeoxyglucose-positron emission tomography computed tomography
(18F-FDG-PET-CT) does not play a diagnostic role in hepatic alveolar echinococcosis,
it is the imaging modality of choice for assessing the inflammation surrounding
the lesions and is helpful for patient management (i.e. when deciding to stop
long term medical treatment).
Pyogenic liver
abscess
Abscesses
can be diagnosed relatively easy by grey-scale ultrasound, particularly in the
context of typical clinical manifestations such as fever, chills, leukocytosis
and increased C-reactive protein. However, the demarcation and extension of
abscesses with liquid necrosis (anechoic or hypoechoic) in the liver can be
underestimated by grey-scale conventional ultrasound. Abscesses in early stages
with inflammation but without necrosis can be missed (figure 10). CEUS is helpful
in diagnosing pyogenic liver abscesses, including
demarcation and extension (figure 10). Regarding the pathogenesis of
liver abscess formation with bacterial infection, inflammation, thrombosis of
small vessels and ischemia provoking necrosis (inducing vicious circle with
bacterial infection), a classification of pyogenic liver abscesses by CEUS has been
proposed [35]. With CEUS,
necrotic liver (anechoic) tissue can easily be distinguished from ischemic
liver tissue, which is crucial information guiding further diagnostic and
therapeutic management.
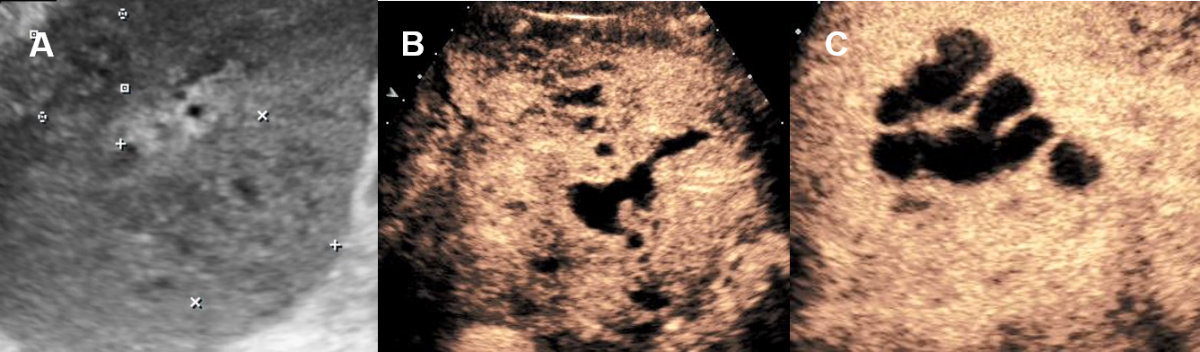
Figure 10Pyogenic liver abscess on conventional ultrasound and CEUS.
A 60-year-old male patient with clinical signs of infection with hardly delimitable
hepatic lesions on ultrasound. B, C CEUS with confluent well delimitable liver abscess with anechoic (avascular/necrotic)
lined by with surrounding hyperenhancement (hyperaemia).
The
appearance of liver abscesses on CT is variable, however they generally
demonstrate peripheral enhancement and central hypoattenuation due to necrosis
and only rarely contain central gas [36]. In early contrast-enhanced CT images,
segmental, wedge-shaped or circumferential increased perfusion can be seen. A
double target sign is a characteristic finding on contrast-enhanced CT with
central low attenuation (fluid) surrounded by a higher attenuation inner rim
(abscess membrane) and low attenuation outer ring (oedema of the liver
parenchyma) [37].
On MRI,
typical imaging features include hypointense signal on T1w, hyperintense signal
on T2w, similar enhancement characteristics as seen on CT. Abscesses show
restricted diffusion with high signal on diffusion weighted images and low
signal on apparent diffusion coefficient maps in the abscess cavity as well as
a lack of diffusion restriction in the periphery (figure 11). These imaging feature
help to differentiate between abscess and
cystic or necrotic tumour with low signal on DWI and high signal on apparent
diffusion coefficient [38].
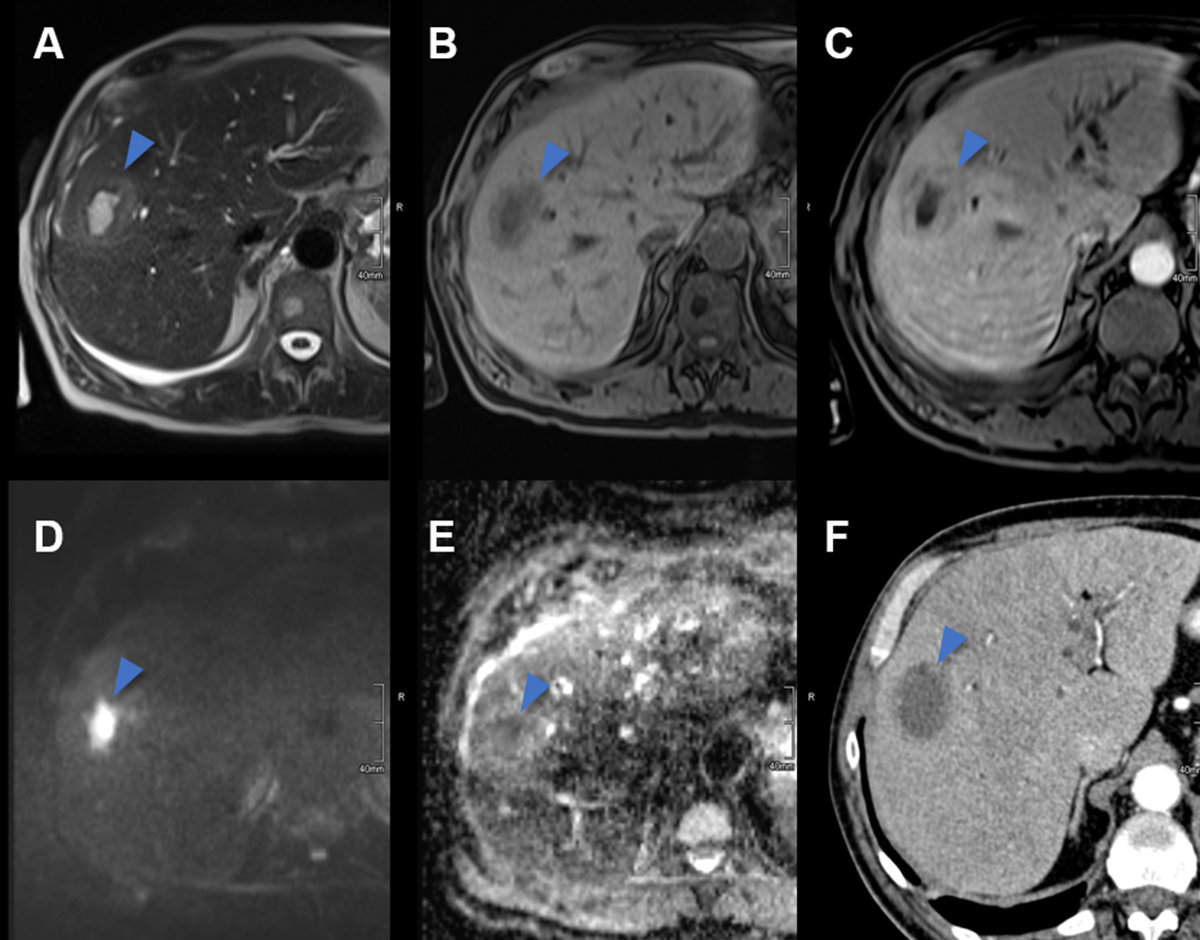
Figure 11Pyogenic liver abscess on CT and MRI.
A Axial T2w MRI image with a central hyperintense abscess (arrowhead) in the right
liver lobe of a 62-year-old male patient with clinical signs of infection. B On T1w unenhanced MR image the abscess is hypointense. C T1w contrast enhanced MRI revealing the double target sign with a contrast enhancing
abscess wall and a narrow peripheral hypointense edematous rim as well as segmental
adjacent hyperenhancement. D On diffusion weighted images the abscess is centrally hyperintense due to restricted
diffusion with E correlating low signal on the ADC map. F On contrast enhanced CT, the abscess is centrally hypodense with a slightly hyperdense
rim and adjacent hyperperfusion.
Note that
depending on the infectious origin, patients with liver abscesses may have
different clinical presentation and/or imaging patterns, that is, Klebsiella
species or fungal infections may not produce liquefactive necrosis [39]. In
addition, amoebic liver abscesses caused by Entamoebia histolytica have
unique characteristics in terms of risk factors, origin, symptoms and treatment
and have recently been reviewed in detail [40].
Hepatic haemangioma
Hepatic haemangioma
is the most common benign liver tumour, with a prevalence up to 20% in autopsy
series [1, 41]. Haemangiomas are often solitary and small (<4 cm), though they
can reach 20 cm in diameter. Additionally, most haemangiomas are asymptomatic
incidental findings. No relationship is seen between the size of haemangiomas
and rare complications (for instance, discomfort in the case of large or giant haemangioma,
bleeding after trauma) and little relationship is shown between symptoms and size.
This benign liver tumour may change in size during long-term follow-up (i.e.
reported annual growth rates of 0.3 to 3.4 mm) [1, 41].
Conventional
ultrasound is the first imaging modality to detect hepatic haemangioma. Most haemangiomas
can be demonstrated as hyperechoic (78%) (figure 12A) but also as
hypoechoic (15%) (figure 12D) or isoechoic lesion (7%) [42].
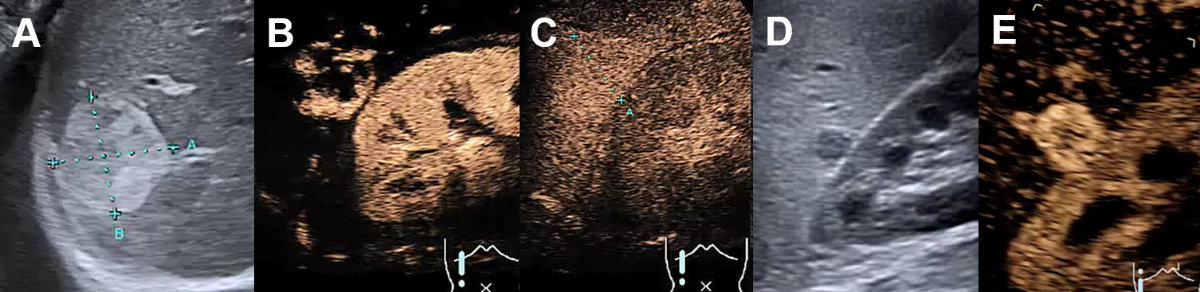
Figure 12Haemangioma on conventional ultrasound and CEUS.
A Hyperechogenic haemangioma on ultrasound, B with centripetal contrast enhancement on CEUS in the arterial phase, C with complete contrast enhancement and without wash-out in the late venous phase.
D Hypoechogenic hemangioma next to the kidney on ultrasound. E Complete contrast enhancement on CEUS in the arterial phase.
In the
presence of risk factors (table 1) a contrast-enhanced imaging modality is
mandatory to exclude malignant focal liver lesions (e.g., hyperechoic liver
metastasis, figure 20A). CEUS is accurate for
the diagnosis of haemangiomas in about 95% of the cases [3]. The peripheral nodular
enhancement with gradual centripetal filling (= iris-diaphragm sign) without washout
is a highly specific finding for a typical haemangioma [1, 42]. CEUS can classify
these lesions as incomplete (22%) or complete (78%)
centripetal filling haemangioma [3, 17]. Atypical features include “shunt haemangiomas”
with abundant arterio- (porto-)venous shunts (also called high-flow or flash-filling
haemangiomas) mostly with a hypoechoic appearance on conventional ultrasound (figure
13A), sclerosing haemangiomas and haemangiomas with regressive changes
such as calcifications, thrombosis and phleboliths [42–44]. The findings of CEUS are
characterised with similar but faster centripetal
contrast-enhancement in high-flow haemangiomas (figure 13).
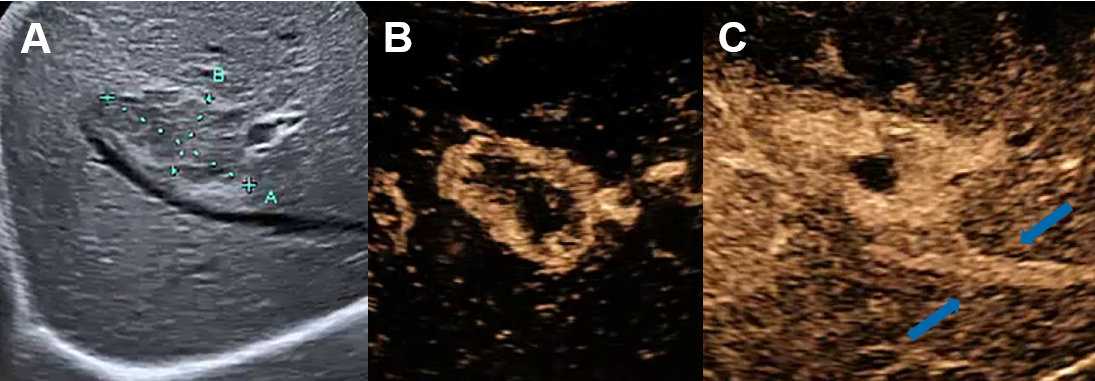
Figure 13Shunt haemangioma on conventional ultrasound and CEUS.
A Hypoechoenic high flow shunt-hemangioma on ultrasound, B with rapid arterial centripetal filling in few seconds on CEUS with C arterioportal shunt (arrows) in the arterial phase.
In the case
of slow-filling haemangiomas, the reinjection of contrast agents without
scanning the patient during the first minute may better show contrast
accumulation within the haemangioma. The diagnostic challenge of some atypical haemangiomas
is washout due to the shunts mimicking malignant focal liver lesions such as
metastasis or hepatocellular carcinoma. In unclear CEUS
findings or the unavailability of CEUS, MRI is the most accurate imaging modality
with a sensitivity and specificity of 91–100% [1, 41, 44]. Note that CT has a
sensitivity of 98.3% and a specificity of 55% [41].
Hepatic haemangiomas appear isodense to the blood pool on unenhanced CT
images. After the administration of contrast material, they demonstrate
peripheral, nodular enhancement with progressive centripetal fill-in in later contrast
phases [45]. In late
phases, hepatic haemangiomas can
be iso- to hyperdense to the normal liver. Large haemangiomas may have central
cystic degeneration, thrombosis, or fibrosis with a lack of enhancement. Small haemangiomas
will uniformly enhance in the arterial phase – described as “flash-filling”. Therefore,
it can be challenging to differentiate small haemangiomas from hypervascular
neoplasms (e.g. metastases from neuroendocrine tumours or hepatocellular
carcinoma) on arterial phase imaging. In contrast to haemangiomas, malignant
neoplasms usually become hypodense relative to the normal liver on portal
venous and/or delayed phase images [46].
Enhancement
characteristics of haemangiomas in MRI (figure 14) are analogous to
those on CT. On T2w images, haemangiomas are typically very bright /
hyperintense with internal fibrotic areas appearing dark. For patients with
incidental liver lesions, multiphase contrast-enhanced CT has a sensitivity of 75.6%
to 86.7% (accuracy of 91% to 95%), and MRI has a sensitivity of 86.7% to 97.8%
(accuracy of 95% to 99%) for diagnosis of haemangiomas [47].
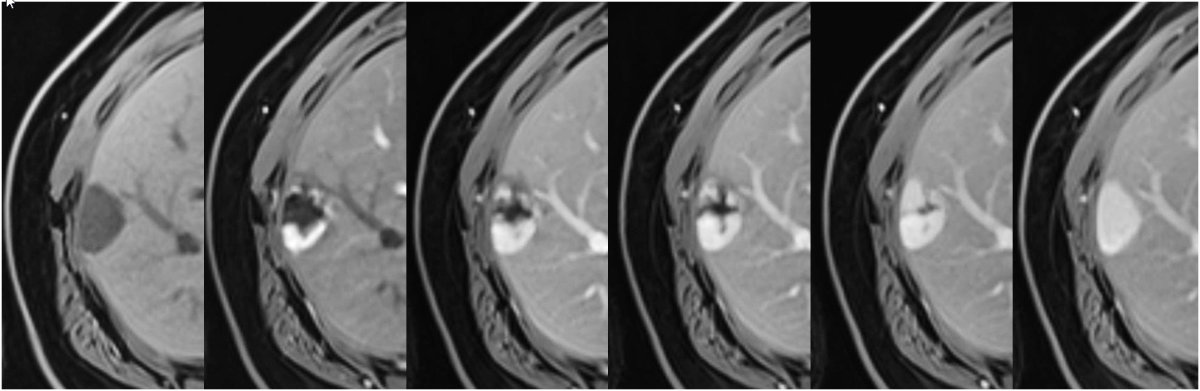
Figure 14Haemangioma on MRI.
Subcapsular haemangioma with hypointense signal on the unenhanced T1w image (left)
and after contrast administration peripheral nodular enhancement with filling in over
time and hyperintense signal matching the blood pool on delayed phase (right).
For the
majority of patients with typical haemangioma without washout on CEUS (see above),
a conservative approach is appropriate. Pregnancy and
the use of oral contraceptive pills are not contraindicated in the presence of
stable asymptomatic haemangioma. Follow-up imaging is unnecessary in typical
cases with a low risk profile [1]. A multidisciplinary approach is recommended
for growing and/or symptomatic haemangiomas by compression and in the case of
Kasabach-Merritt
syndrome.
Focal nodular
hyperplasia
Focal nodular hyperplasia is the second-most common benign hepatic tumour
with a prevalence
of 0.03% (0.4 to 3% in autopsy series) in predominantly middle-aged (35 to 50
years) female patients (10:1 female ratio) with mostly solitary manifestations
smaller than 5 cm (multiple focal nodular hyperplasia in 20–30% of cases) [1].
In most cases, the focal nodular hyperplasia size remains stable over time [48].
On a conventional ultrasound, focal nodular hyperplasia usually appears
slightly hypo- or isoechoic (figure 15A), sometimes with a lobulated
contour and a pseudocapsule, which is caused by compression of the surrounding
liver tissue or vessels and sometimes with a central scar (figure 15C).
Central feeding arteries can be demonstrated on colour Doppler with spoke-wheel
pattern (figure 15B) with typical arterial flow in the pw-Doppler. However,
malignant tumours can also present the spoke-wheel sign, for instance. in
fibrolamellar hepatocellular carcinoma [17, 49]. Therefore, contrast-enhanced
imaging is mandatory in suspected focal nodular hyperplasia.
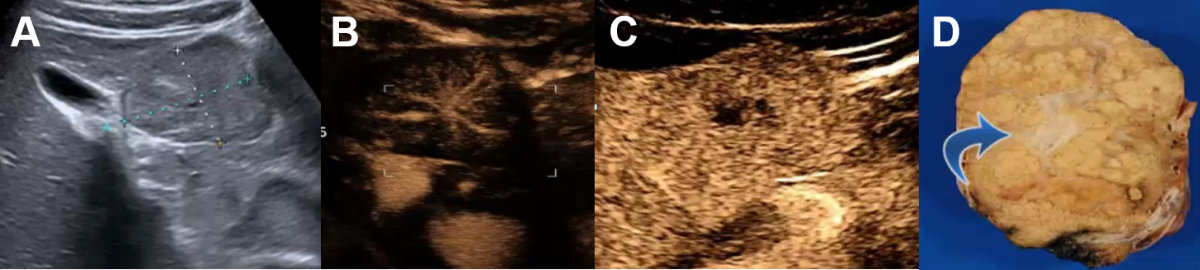
Figure 15Focal nodular hyperplasia on conventional ultrasound and CEUS.
A Symptomatic focal nodular hyperplasia on ultrasound next to the gallbladder. B CEUS with centrifugal arterial contrast enhancement (“spoke wheel sign”). C Late phase with central scar (hypoenhancing) of the dystrophic central artery (D, blue arrow) shown on the resection sample.
CEUS is an excellent imaging modality to accurately diagnose focal
nodular hyperplasia, particularly if the diameter is below 3 cm [1]. The
typical finding shows a fast arterial centrifugal uptake of the contrast agent,
which becomes hyperechoic in seconds. This fast, dynamic process can be missed
by CT or MRI. Hyperenhancing focal liver lesions can be demonstrated in the
arterial, portal-venous phase up to the late venous phase (sometime iso-enhancing
in the late venous phase), but in most cases without washout (figure 15C)
[3, 17]. This vascular malformation can be divided into different groups by CEUS according
to the vascular patterns. Atypical variants of focal
nodular hyperplasia, that is, without a central scar and/or with decentral
contrast enhancement, are reported in about 20% of cases [48, 50]. In general, CEUS
is more accurate than MRI in focal nodular hyperplasia smaller than 3 cm,
whereas the opposite is true in larger focal nodular hyperplasia lesions [1].
On CT, focal
nodular hyperplasia is usually hypo- or isodense relative to the normal liver
on unenhanced images, with a hypodense scar seen in one-third of the cases. Focal
nodular hyperplasia are avidly enhanced in the arterial phase, becoming
isodense in the portal venous phase and later phases. If present, the central
scar enhances gradually and can appear hyperdense on delayed-phase images. For
patients with incidental liver lesions, multiphase contrast-enhanced CT has an
accuracy of 85% to 93% for the diagnosis of focal nodular hyperplasia [51].
Enhancement
characteristics of focal nodular hyperplasia on MRI (figure 16) are
again similar to those on CT with avid arterial enhancement of the lesion and becoming
isointense to the surrounding liver during the portal venous phase. On
unenhanced T1w images, focal nodular hyperplasia is isointense relative to the
normal liver and on T2w images isointense to slightly hyperintense [45, 46].
The central scar is typically dark on T1w images and bright on T2w images. For
patients with incidental liver lesions, multiphase contrast-enhanced MRI has an
accuracy of 88% to 99% for the diagnosis of focal nodular hyperplasia [51].
High specificity (close to 100%) of CEUS and MRI in focal nodular hyperplasia
avoids biopsy and allows conservative treatment. Follow-up imaging in the vast
majority of patients is not necessary.
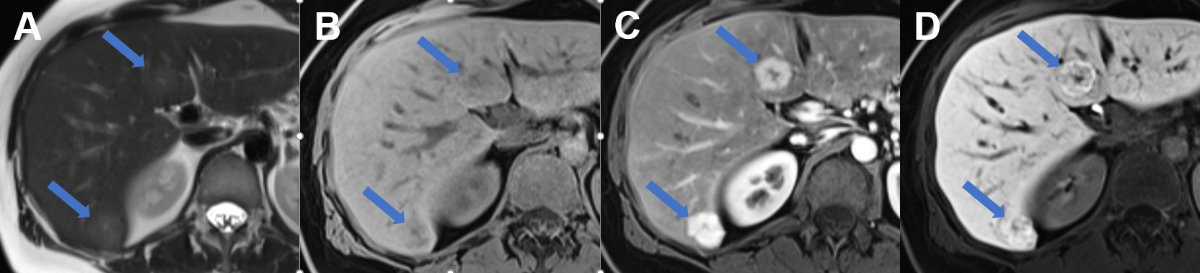
Figure 16Focal nodular hyperplasia on MRI.
MRI of focal nodular hyperplasia in liver segment VI und IVb (arrows) with A slight hyperintense signal on T2w image with hyperintense central scar, B slight hypointense signal on T1w image with hypointense central scar, C avid arterial enhancement and D increased metabolisation of the hepatobiliary contrast agent during the hepatobiliary
phase.
To
distinguish focal nodular hyperplasia from hepatocellular adenomas,
hepatobiliary agents such as gadoxetate disodium are helpful with focal nodular
hyperplasias, which unlike hepatocellular adenomas demonstrate high uptake on
hepatobiliary phase images [52]. A modified EASL flowchart for the management
of focal nodular hyperplasia is shown in figure 17. In asymptomatic patients, no
follow-up is needed even during pregnancy, and oral contraceptives do not have
to be stopped [1]. However, follow-up is indicated in the case of underlying
vascular liver disease (e.g. chronic Budd-Chiari syndrome, Fontan-associated
liver disease) as focal nodular hyperplasia and focal nodular hyperplasia-like
lesions as well as hepatocellular carcinoma are seen more frequently in
vascular liver diseases. The imaging features of these lesions can be less
typical [53–55]. Therefore, liver biopsy is advisable in case of uncertain
diagnosis or when focal nodular hyperplasia is diagnosed outside of the
classical clinical context.

Figure 17Recommended
management of a focal nodular hyperplasia (modified flowchart for the
management of focal nodular hyperplasia by EASL [1]); imaging modalities may include
ultrasound, CEUS and MRI
with a hepatobiliary contrast agent. In suspected focal nodular hyperplasia on ultrasound,
size of the focal
liver lesion is important for choosing contrast-enhanced image modality. For
large lesions >3 cm, MRI sensitivity is excellent. CEUS or MRI are
recommended for lesions <3 cm. If doubt remains after CEUS and MRI, patients
should be referred to a specialist centre where percutaneous biopsy (or
resection) may be considered. CEUS:
Contrast-enhanced ultrasound; EASL: European Association for the Study of the
Liver; FNH: focal nodular hyperplasia; MRI: magnetic resonance imaging; US:
conventional ultrasound
Symptomatic
focal nodular hyperplasias due to relevant size should be presented at a multidisciplinary
board to discuss exceptional resection or transarterial embolisation.
Hepatocellular
adenoma
Hepatocellular
adenomas are rare, with a prevalence of 0.001–0.004%, and are most commonly
found in middle-aged women (10:1 female to male, aged 35 to 40 years) [1]. Hepatocellular
adenomas are usually solitary, sometimes pedunculated and of various sizes ranging
from several millimetres to 30 cm. Oral contraceptive use increases the
incidence of this hormone-sensitive focal liver lesions 30–40-fold. Hepatocellular
adenomas are also associated with obesity and metabolic syndrome. In males,
androgenic steroids are associated with hepatocellular adenomas. In particular,
hepatocellular adenomas ≥5 cm have higher risk of haemorrhage and malignant
transformation (particularly β-catenin activated hepatocellular adenomas). The
molecular classification of hepatocellular adenomas with associated risk
factors, bleeding and malignant transformation has been described in detail [56].
As a result of the sensitivity to hormones, hepatocellular adenomas can also
grow in size with an increased risk of bleeding during pregnancy, especially in
the last trimester, but also after childbirth (rapid drop in oestrogen levels
with a possible massive hepatocellular adenoma regression). On CEUS, hyperenhancement
in the arterial phase can be seen in the periphery
and in the centre of the lesion, with chaotic, and usually centripetal,
behaviour. Washout is usually absent.
On
unenhanced CT, the attenuation of hepatocellular adenomas varies depending on recent
haemorrhage, which can be hyperdense, or fat content, which will appear
hypodense. Generally, hepatocellular adenomas are well marginated and
demonstrate homogenous enhancement on arterial phase images, returning to isodensity
on portal venous and delayed-phase images.
MRI is
superior to all other imaging modalities for the characterisation of hepatocellular
adenomas (figure 18). For diagnosing HNF-1a inactivated hepatocellular
adenomas, MRI with extracellular contrast agents ranges from 87% to 91% sensitivity
and 89% to 100% specificity, and for diagnosing inflammatory hepatocellular
adenomas from 85% to 88% sensitivity and 88% to 100% specificity [1]. Meanwhile,
the identification of β-catenin-activated hepatocellular adenoma and its
distinction with unclassified hepatocellular adenoma and hepatocellular
carcinoma is not possible by any imaging technique. Biopsy may be considered in
these cases to exclude malignancy (particularly for all adenomas that are not
steatotic to inform management decisions; unless in males or >5 cm for which
resection/ablation can be recommended, see below). In the case of histologically
proven β‑catenin-activated hepatocellular adenoma, curative intervention is
advised irrespective of size. Hepatocellular adenomas <5 cm of the HNF-1α
subtype, or those that are either inflammatory or β‑catenin non-activated on
biopsy, can be managed conservatively. Lifestyle
changes such as discontinuation of oral contraceptives as well as weight loss
should be recommended. The current management of hepatocellular adenoma relies
ever more on the molecular classification. Therefore, the role of
biopsy is increasingly important for diagnostic and prognostic purposes. We
recommend using a recently published algorithm for guidance [57].

Figure 18Hepatocellular adenoma in MRI.
MRI of a HNF-1a-activated hepatocellular
adenoma in liver segment II (arrow) with A hyperintense signal on the T2w image, signal drop from B T1 weighted in- to C opposed-phase, D arterial enhancement persisting in E the portal venous phase and F due to lack of contrast metabolisation hypointense signal on hepatobiliary phase
images.
On MRI
images, inflammatory hepatocellular adenomas are hyperintense on T2w images and
isointense or mildly hyperintense on T1w images with minimal or no signal
drop-off on opposed-phase images. After the administration of gadolinium-based
contrast material, inflammatory hepatocellular adenomas usually demonstrate
avid arterial enhancement, which persists in the portal venous and delayed
phases [58]. HNF-1α-inactivated hepatocellular adenomas are hyper- or
isointense on T1w images, with typical diffuse signal drop-off on opposed phase
due to intracellular fat [58].
For the
differentiation between adenoma and focal nodular hyperplasia, low signal on
hepatobiliary phase images is 100% specific, 92% sensitive and 97% accurate for
hepatocellular adenoma [59].
For all presumed hepatocellular adenomas, a
reassessment with MRI is advised after 6 months. Hepatocellular adenomas persistently
greater than 5 cm or increasing in size (>20% diameter – as per
RECIST criteria for solid malignant tumours) should be considered for resection
or curative treatment irrespective of their molecular or histological subtype
because of the risk of haemorrhage. In women, lesions less than 5 cm should be
reassessed at 1 year, and annual imaging adopted thereafter. For lesions stable
or reducing in size after 5 years, biannual imaging can be proposed. In men,
all hepatocellular adenomas should be resected. In the case of haemodynamic-relevant
bleeding, hepatocellular adenomas should be embolised. The management of hepatocellular
adenomas recommended
by the European
Association for the Study of the Liver is shown in figure 19.
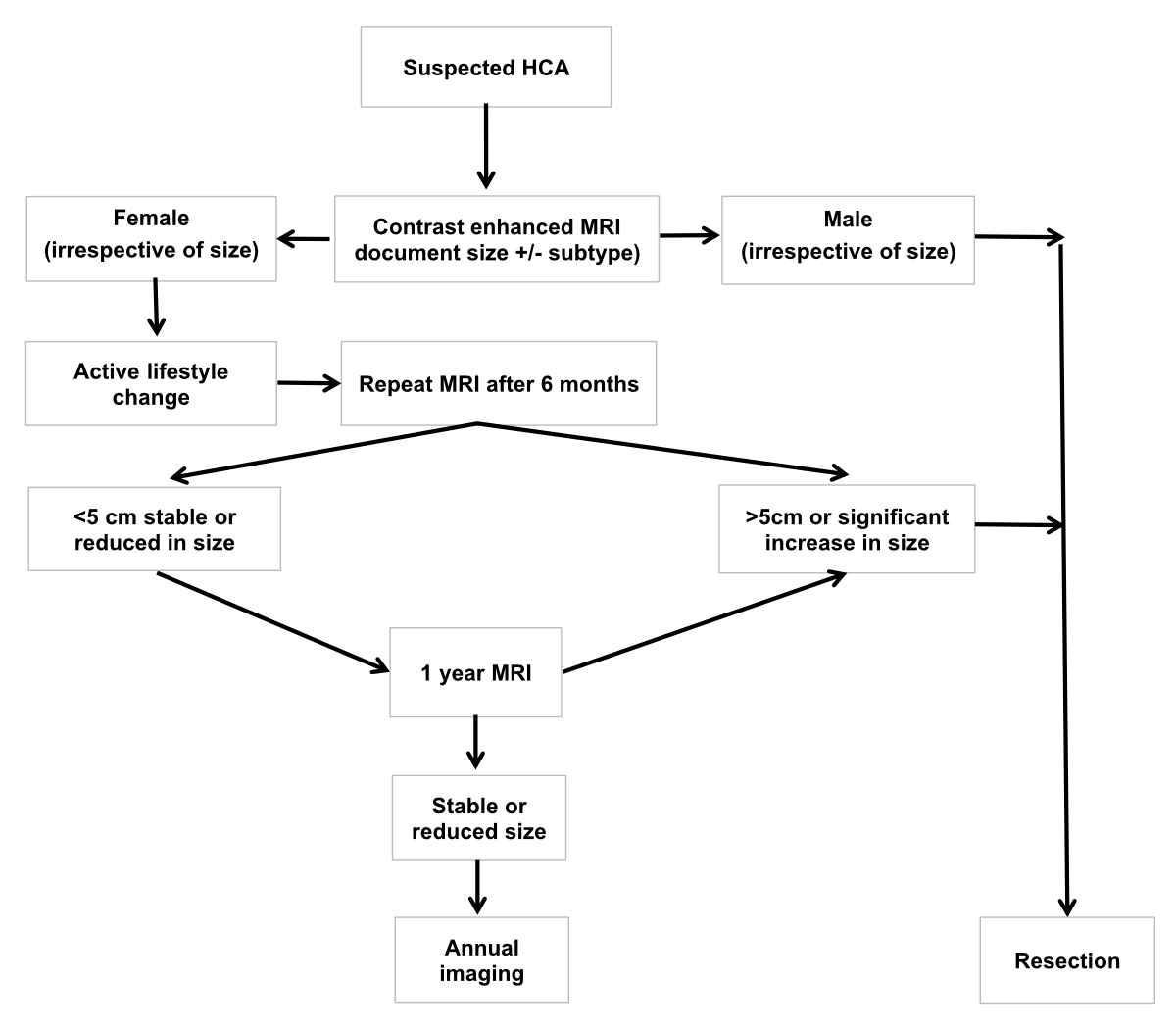
Figure 19Recommended
management of a presumed hepatocellular adenoma according to the European
Association for the Study of the Liver [1]: Baseline
MRI is necessary to help to confirm a diagnosis of hepatocellular adenoma and characterise
it. In
men, resection (or ablation) of hepatocellular adenomas of any size is recommended.
In women, an
observation period of 6 months after lifestyle changes is appropriate.
Resection is indicated in lesions persistently greater than 5 cm, or in case of
increasing size on follow-up. In smaller lesions, a conservative approach with
interval imaging can be adopted. In specialist centres practising MRI subtyping
of hepatocellular adenoma, longer intervals between scans may be preferred for H-HCA.
Biopsy is
reserved for those cases where the diagnosis of HCA is uncertain on imaging and
malignancy must be ruled out. EASL: European
Association for the Study of the Liver; HCA: hepatocellular adenoma; MRI:
magnetic resonance imaging
Malignant focal
liver lesions and liver metastases
Liver metastases
are the most common malignant focal liver lesions in a non-cirrhotic
liver. The correct diagnosis is crucial for determining the next
diagnostic and therapeutic steps or the appropriate follow-up interval. On conventional
ultrasound hepatic metastases vary in echogenicity and can be hypoechoic,
isoechoic or hyperechoic or cystic. Particularly patients with risk factors and
newly documented or increasing focal liver lesions need further contrast-enhanced
imaging (because e.g., a hyperechoic focal liver lesions on conventional
ultrasound could be a haemangioma or hyperechoic liver metastasis) (figures 12 A and
20 A).
When
detecting these focal liver lesions on conventional ultrasound, CEUS could be immediately
performed, with appropriate expertise, with an
excellent accuracy to differentiate benign focal liver lesions from malignant focal
liver lesions. According to the degree of vascularisation in the arterial phase
the focal liver lesion can be categorised by CEUS as
hyper-, hypo- or avascular (corresponding to necrosis) metastases. Ten to 15%
of liver metastases are hypervascular [17] (figure 20, B and E). A
common and highly specific feature of metastatic lesions or other malignant focal
liver lesions (as hepatocellular carcinoma and cholangiocarcinoma) is the
washout of the contrast agent in the portal venous or late phase (figure
20, C, E and G) after initial contrast-enhancement [4, 60].
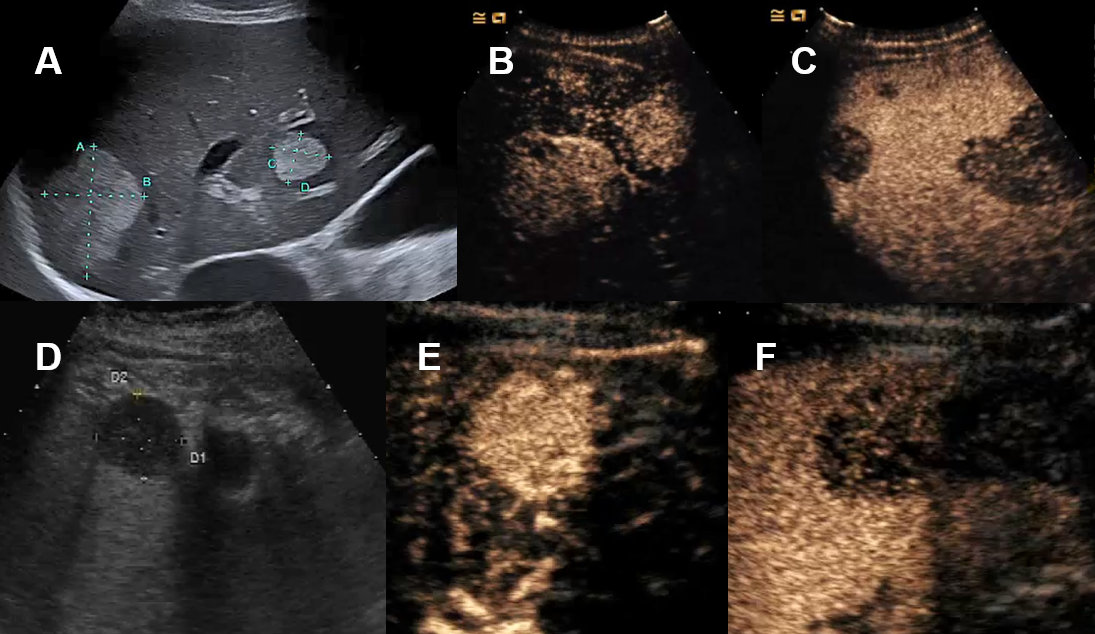
Figure 20Liver metastasis on conventional ultrasound and CEUS.
A Hyperechogenic liver metastases on ultrasound. B Hyperenhancement of biopsy proven neuroendocrine tumor-metastasis on CEUS. C Wash-out on CEUS. D Patient with abdominal wall abscess with cystic lesion of unclear dignity on MRI
with features of a cystic hepatic lesion on ultrasound. E Complete arterial contrast enhancement of this solid mass on CEUS. F Wash-out CEUS of the biopsy proven primary lymphoma. G CEUS with arterial contrast enhancement of a melanoma metastasis demonstrating necrosis
on the non-enhancing areas (which is an important information when planning ultrasound-guided
biopsy avoiding biopsy of the necrotic area).
In the
largest prospective multicentre trials sensitivity and specificity of CEUS in the
differentiation of benign and malignant focal liver lesions was
not inferior to CT and MRI even for small focal liver lesions [61–63].
Meta-analyses
involving hepatocellular carcinomas, metastatic cancers, cholangiocarcinomas
and other malignant focal liver lesions found a comparable sensitivity and
specificity of >90% for CEUS, CT and MRI regardless of whether the standard
of reference included histology or the studies were blinded or unblinded [64,
65] but with a lower cost for CEUS [15].
In
Switzerland, even in clinical practice, CEUS is reported
with a sensitivity of 96.0%–97.2% for malignant focal liver lesions and a
specificity of 84.2%–90.6% for benign focal liver lesions [66]. CEUS is useful as
a first and immediate diagnostic imaging tool after conventional
ultrasound to accurately diagnose or exclude malignant focal liver lesions.
Unnecessary further imaging or biopsies can be avoided. Nevertheless, CEUS is also
helpful in the detection of missed colorectal liver
metastases after staging-CT. Moreover, CEUS is
particularly useful in colorectal cancer with colorectal tumour stage T3/T4 and
in cases with focal liver lesions of uncertain dignity after staging CT with an
accuracy of 98.4% for CEUS in determining dignity [67].
However,
CEUS cannot replace CT or MRI in these oncological patients. CT is mandatory
for tumour staging and MRI is more accurate to evaluate the exact number and
localisation of liver metastases, especially in limited conventional ultrasound/CEUS-conditions
mentioned above.
Liver metastases are typically hypodense on unenhanced CT, enhancing less
than surrounding liver following contrast administration (except metastases
from neuroendocrine tumour or renal cell carcinoma). If there is concomitant
hepatic steatosis, it can be more difficult to detect metastases due to their
isodensity in a steatotic liver. Enhancement of metastases is typically
peripheral with washout, helping distinguish them from haemangiomas. The
resolution of CT does not allow for a definitive characterisation of lesions
<1 cm. Moreover, small hypervascular metastases, for instance from renal
cell carcinoma, thyroid carcinoma, and neuroendocrine tumours, may be difficult
to distinguish from flash-filling haemangiomas [46]. Wall thickening, peripheral
enhancement, mural nodules as well as multiplicity and lesion growth raise the
likelihood of malignancy.
The appearance of hepatic metastases on MRI is variable depending on the
primary tumour and the size of the metastases. On MRI, hepatic metastases often
demonstrate hypointensity on T1w images, hyperintensity on T2w images and
restricted diffusion (figure 21). Occasionally, hepatic metastases are
difficult to detect on unenhanced images without diffusion-weighted images. In
the hepatobiliary phase, metastases appear hypointense due to the lack of
metabolisation of the contrast agent.
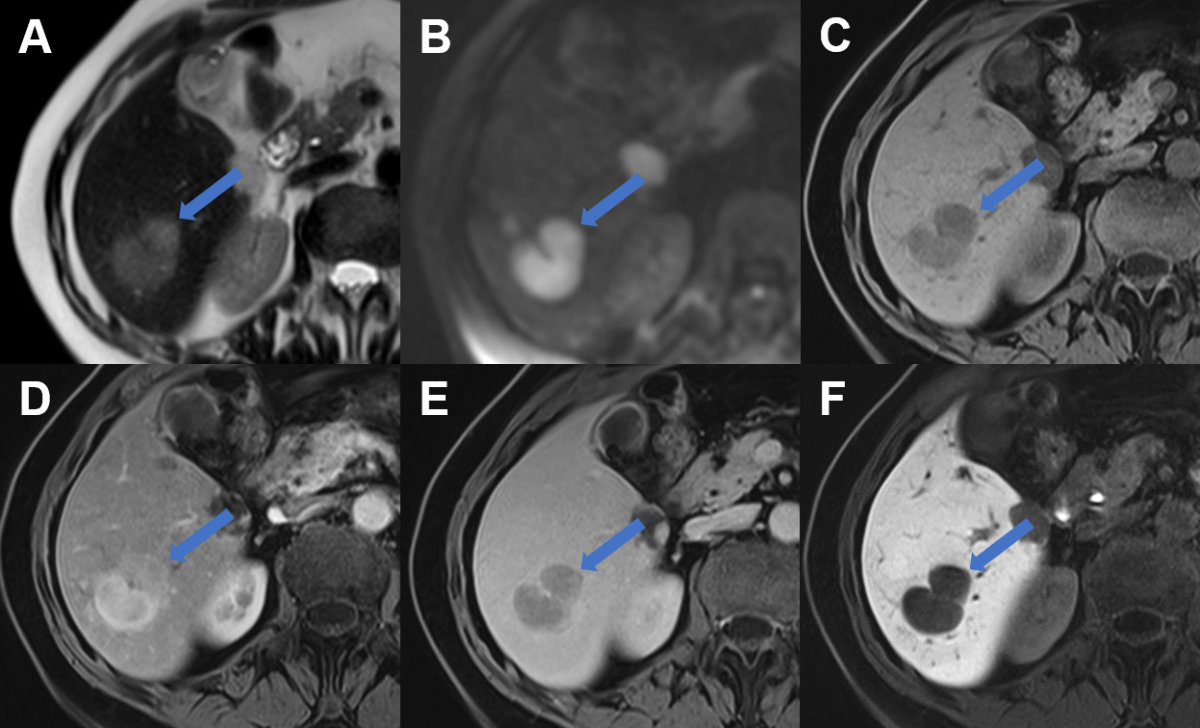
Figure 21Liver metastasis on MRI.
Liver metastasis in segment VI laterally (arrow) with A slight hyperintense signal on T2w image, B restricted diffusion with high signal on b800 diffusion weighted image, C hypointense signal on T1w image, D arterial enhancement, E portal venous wash-out and F hypointense signal during the hepatobiliary phase due to lack of contrast metabolisation.
In patients with a history of extrahepatic malignancy, contrast-enhanced
CT can differentiate between metastases and benign lesions with an accuracy of
74% and MRI with an accuracy of 83% and 91%, increasing to 94% with the
addition of dynamic the hepatobiliary phase [68].
The accuracy of CT, however, strongly depends on the size of liver
metastases. The overall accuracy at preoperative CT was 81% but only 55% for
detecting colorectal liver metastases measuring 6–10 mm, and only 8% for colorectal
liver metastases measuring 1–5 mm in
patients undergoing liver resection [69].
Hepatocellular carcinoma can occur in non-cirrhotic liver disease (particularly
in chronic hepatitis B and non-alcoholic steatohepatitis with advanced fibrosis).
Imaging characteristics of hepatocellular carcinoma in non-cirrhotic and
cirrhotic patients are similar except hepatocellular carcinomas in
non-cirrhotic livers frequently present as a solitary mass with or without
satellite lesions and are much larger in tumour size and often seen with a
central scar [70]. In contrast to hepatocellular carcinoma in liver cirrhosis,
which can be diagnosed non-invasively based on typical contrast-enhanced MRI
and CT features, suspected hepatocellular carcinoma in non-cirrhotic liver
requires a biopsy of the focal liver lesion [7].
Biopsy of solitary
liver lesions in non-cirrhotic liver
Biopsy and
histological analysis of a focal liver lesion should be performed if the
clinical evaluation, tumour markers, serological testing and state-of-the-art
imaging do not allow for characterizing the lesion and/or if suspicion of malignancy
remains high. Biopsy of a suspected liver metastasis is often helpful in
establishing diagnosis by identifying the primary tumour as well as for tumour
staging purposes. In addition, hepatocellular adenoma can sometimes be difficult to
differentiate from well-differentiated hepatocellular
carcinoma. In this scenario, the indication for liver biopsy should be
generously made, particularly in the event of ambiguous imaging [71].
A biopsy of
focal liver lesions should be performed by experienced physicians to avoid potential
tumour cell seeding and post-interventional bleeding [72, 73]. When performing a
biopsy of a focal liver lesion, it is mandatory to acquire a second biopsy from
the surrounding liver to rule out chronic liver disease, advanced fibrosis or cirrhosis.
The biopsy of the adjacent liver is important in diagnosing a focal liver
lesion, particularly hepatocellular lesions. Detection of cirrhosis will change
the patient’s focal liver lesion management. Imaging of the correct location
for sonography-guided biopsy can be enabled by performing CEUS (i.e. for focal liver
lesions with insufficient demarcation in conventional
ultrasound and for avoiding biopsy of avascular/necrotic tumour area).
Conclusion
In clinical
routines, conventional ultrasound is the first imaging modality in patients
with focal liver lesions in non-cirrhotic liver. Patient history, physical
examination, tumour markers and imaging findings together with risk factors for
malignancy or infection determine the need of further investigation.
Contrast-based imaging studies such as CEUS, CT or MRI allow for the accurate differentiation
of focal liver lesions in most cases. In case CEUS is unavailable, inconclusive
or if there is inadequate experience by the operator, MRI is recommended. If a focal
liver lesion remains unclear after imaging, a biopsy of the lesion and the
surrounding liver should be considered.
Acknowledgements
Reviewed and approved by:
Christine Bernsmeier, Annalisa Berzigotti, Philip Bruggmann, Andreas Cerny, Andrea
De Gottardi,
Montserrat Fraga, Nicolas Goossens, Beat Helbling, Andreas E. Kremer, Anja
Lachenmayer, Valérie McLin, Joachim C. Mertens, Darius Moradpour and Achim
Weber as council members of the Swiss Association for the Study of the Liver
(SASL) and by Bruno Balsiger, Jan Borovicka, Stephan Brand, Lukas Degen, Tobias
Ehmann, Florian Riniker, Kaspar Truninger and Alain Vonlaufen as council members
of the Swiss Society of Gastroenterology (SSG) as well as, Pietro Majno, Beat
Müllhaupt, Christine Sempoux and Daniel Weiss.
Mikael
Sawatzki, MD
Kantonsspital
Sankt Gallen
Rorschacher
Strasse 95
CH-9007 St.
Gallen
mikael.sawatzki[at]kssg.ch
References
1. European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines
on the management of benign liver tumours. J Hepatol. 2016 Aug;65(2):386–98. 10.1016/j.jhep.2016.04.001
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines
on the management of cystic liver diseases. J Hepatol. 2022 Oct;77(4):1083–108. 10.1016/j.jhep.2022.06.002
3. Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al. Guidelines
and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)
in the liver — update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives
of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013 Feb;34(1):11–29.
4. Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, et al. Guidelines
and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS)
in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS.
Ultrasound Med Biol. 2020 Oct;46(10):2579–604. 10.1016/j.ultrasmedbio.2020.04.030
5. Chernyak V, Horowitz JM, Kamel IR, Arif-Tiwari H, Bashir MR, Cash BD, et al.; Expert
Panel on Gastrointestinal Imaging. ACR Appropriateness Criteria® Liver Lesion-Initial
Characterization. J Am Coll Radiol. 2020 Nov;17(11S 11s):S429–46. 10.1016/j.jacr.2020.09.005
6. Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, et al. Use
of diagnostic imaging studies and associated radiation exposure for patients enrolled
in large integrated health care systems, 1996-2010. JAMA. 2012 Jun;307(22):2400–9.
10.1001/jama.2012.5960
7. Goossens N, Toso C, Heim MH. Management of hepatocellular carcinoma: SASL expert opinion
statement. Swiss Med Wkly. 2020 Jul;150(3132):w20296. 10.4414/smw.2020.20296
8. Horta G, López M, Dotte A, Cordero J, Chesta C, Castro A, et al. [Benign focal liver
lesions detected by computed tomography: review of 1,184 examinations]. Rev Med Chil.
2015 Feb;143(2):197–202. 10.4067/S0034-98872015000200007
9. Rawla P, Sunkara T, Muralidharan P, Raj JP. An updated review of cystic hepatic lesions.
Clin Exp Hepatol. 2019 Mar;5(1):22–9. 10.5114/ceh.2019.83153
10. Bahirwani R, Reddy KR. Review article: the evaluation of solitary liver masses. Aliment
Pharmacol Ther. 2008 Oct;28(8):953–65. 10.1111/j.1365-2036.2008.03805.x
11. Gore RM, Pickhardt PJ, Mortele KJ, Fishman EK, Horowitz JM, Fimmel CJ, et al. Management
of Incidental Liver Lesions on CT: A White Paper of the ACR Incidental Findings Committee.
J Am Coll Radiol. 2017 Nov;14(11):1429–37. 10.1016/j.jacr.2017.07.018
12. Kaltenbach TE, Engler P, Kratzer W, Oeztuerk S, Seufferlein T, Haenle MM, et al. Prevalence
of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients.
Abdom Radiol (NY). 2016 Jan;41(1):25–32. 10.1007/s00261-015-0605-7
13. McLin VA, Franchi Abella S, Debray D, Guérin F, Beghetti M, Savale L, et al.; Members
of the International Registry of Congenital Porto-Systemic Shunts. Congenital Portosystemic
Shunts: Current Diagnosis and Management. J Pediatr Gastroenterol Nutr. 2019 May;68(5):615–22.
10.1097/MPG.0000000000002263
14. Dietrich CF, Averkiou M, Nielsen MB, Barr RG, Burns PN, Calliada F, et al. How to
perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int Open. 2018 Jan;4(1):E2–15.
10.1055/s-0043-123931
15. Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, Lee K, et al. Contrast-enhanced
ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced
computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation
of focal liver lesions and detection of liver metastases: a systematic review and
cost-effectiveness analysis. Health Technol Assess. 2013 Apr;17(16):1–243. 10.3310/hta17090
16. Piscaglia F, Bolondi L; Italian Society for Ultrasound in Medicine and Biology (SIUMB)
Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications:
retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006 Sep;32(9):1369–75.
10.1016/j.ultrasmedbio.2006.05.031
17. Jang JY, Kim MY, Jeong SW, Kim TY, Kim SU, Lee SH, et al. Current consensus and guidelines
of contrast enhanced ultrasound for the characterization of focal liver lesions. Clin
Mol Hepatol. 2013 Mar;19(1):1–16. 10.3350/cmh.2013.19.1.1
18. Schwarze V, Marschner C, Negrão de Figueiredo G, Rübenthaler J, Clevert DA. Single-Center
Study: Evaluating the Diagnostic Performance and Safety of Contrast-Enhanced Ultrasound
(CEUS) in Pregnant Women to Assess Hepatic Lesions. Ultraschall Med. 2020 Feb;41(1):29–35.
10.1055/a-0973-8517
19. Schwarze V, Froelich MF, Marschner C, Knösel T, Rübenthaler J, Clevert DA. Safe and
pivotal approaches using contrast-enhanced ultrasound for the diagnostic workup of
non-obstetric conditions during pregnancy, a single-center experience. Arch Gynecol
Obstet. 2021 Jan;303(1):103–12. 10.1007/s00404-020-05735-8
20. Purysko AS, Remer EM, Veniero JC. Focal liver lesion detection and characterization
with GD-EOB-DTPA. Clin Radiol. 2011 Jul;66(7):673–84. 10.1016/j.crad.2011.01.014
21. Neri E, Bali MA, Ba-Ssalamah A, Boraschi P, Brancatelli G, Alves FC, et al. ESGAR
consensus statement on liver MR imaging and clinical use of liver-specific contrast
agents. Eur Radiol. 2016 Apr;26(4):921–31. 10.1007/s00330-015-3900-3
22. Karcaaltincaba M, Akhan O. Imaging of hepatic steatosis and fatty sparing. Eur J Radiol.
2007 Jan;61(1):33–43. 10.1016/j.ejrad.2006.11.005
23. Venkatesh SK, Hennedige T, Johnson GB, Hough DM, Fletcher JG. Imaging patterns and
focal lesions in fatty liver: a pictorial review. Abdom Radiol (NY). 2017 May;42(5):1374–92.
10.1007/s00261-016-1002-6
24. Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging
patterns and pitfalls. Radiographics. 2006;26(6):1637–53. 10.1148/rg.266065004
25. Dioguardi Burgio M, Bruno O, Agnello F, Torrisi C, Vernuccio F, Cabibbo G, et al. The
cheating liver: imaging of focal steatosis and fatty sparing. Expert Rev Gastroenterol
Hepatol. 2016 Jun;10(6):671–8. 10.1586/17474124.2016.1169919
26. Lupsor M, Badea R. Imaging diagnosis and quantification of hepatic steatosis: is it
an accepted alternative to needle biopsy?. Rom J Gastroenterol. 2005 Dec;14(4):419–25.
27. Vachha B, Sun MR, Siewert B, Eisenberg RL. Cystic lesions of the liver. AJR Am J Roentgenol.
2011 Apr;196(4):W355-66. 10.2214/AJR.10.5292
28. Labib PL, Aroori S, Bowles M, Stell D, Briggs C. Differentiating Simple Hepatic Cysts
from Mucinous Cystic Neoplasms: Radiological Features, Cyst Fluid Tumour Marker Analysis
and Multidisciplinary Team Outcomes. Dig Surg. 2017;34(1):36–42. 10.1159/000447308
29. Working Group WH; WHO Informal Working Group. International classification of ultrasound
images in cystic echinococcosis for application in clinical and field epidemiological
settings. Acta Trop. 2003 Feb;85(2):253–61. 10.1016/S0001-706X(02)00223-1
30. Pakala T, Molina M, Wu GY. Hepatic Echinococcal Cysts: A Review. J Clin Transl Hepatol.
2016 Mar;4(1):39–46. 10.14218/JCTH.2015.00036
31. Stojkovic M, Rosenberger K, Kauczor HU, Junghanss T, Hosch W. Diagnosing and staging
of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound?.
PLoS Negl Trop Dis. 2012;6(10):e1880. 10.1371/journal.pntd.0001880
32. Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for
the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta
Trop. 2010 Apr;114(1):1–16. 10.1016/j.actatropica.2009.11.001
33. Taourel P, Marty-Ane B, Charasset S, Mattei M, Devred P, Bruel JM. Hydatid cyst of
the liver: comparison of CT and MRI. J Comput Assist Tomogr. 1993;17(1):80–5. 10.1097/00004728-199301000-00014
34. Kantarci M, Pirimoglu B, Ogul H, Bayraktutan U, Eren S, Aydinli B, et al. Can biliary-cyst
communication be predicted by Gd-EOB-DTPA-enhanced MR cholangiography before treatment
for hepatic hydatid disease?. Clin Radiol. 2014 Jan;69(1):52–8. 10.1016/j.crad.2013.08.005
35. Kunze G, Staritz M, Köhler M. Contrast-enhanced ultrasound in different stages of
pyogenic liver abscess. Ultrasound Med Biol. 2015 Apr;41(4):952–9. 10.1016/j.ultrasmedbio.2014.12.001
36. Lee TY, Wan YL, Tsai CC. Gas-containing liver abscess: radiological findings and clinical
significance. Abdom Imaging. 1994;19(1):47–52. 10.1007/BF02165861
37. Bächler P, Baladron MJ, Menias C, Beddings I, Loch R, Zalaquett E, et al. Multimodality
Imaging of Liver Infections: Differential Diagnosis and Potential Pitfalls. Radiographics.
2016;36(4):1001–23. 10.1148/rg.2016150196
38. Chan JH, Tsui EY, Luk SH, Fung AS, Yuen MK, Szeto ML, et al. Diffusion-weighted MR
imaging of the liver: distinguishing hepatic abscess from cystic or necrotic tumor.
Abdom Imaging. 2001;26(2):161–5. 10.1007/s002610000122
39. Rossi B, Gasperini ML, Leflon-Guibout V, Gioanni A, de Lastours V, Rossi G, et al. Hypervirulent
Klebsiella pneumoniae in Cryptogenic Liver Abscesses, Paris, France. Emerg Infect
Dis. 2018 Feb;24(2):221–9. 10.3201/eid2402.170957
40. Roediger R, Lisker-Melman M. Pyogenic and Amebic Infections of the Liver. Gastroenterol
Clin North Am. 2020 Jun;49(2):361–77. 10.1016/j.gtc.2020.01.013
41. Leon M, Chavez L, Surani S. Hepatic hemangioma: what internists need to know. World
J Gastroenterol. 2020 Jan;26(1):11–20. 10.3748/wjg.v26.i1.11
42. Dietrich CF, Mertens JC, Braden B, Schuessler G, Ott M, Ignee A. Contrast-enhanced
ultrasound of histologically proven liver hemangiomas. Hepatology. 2007 May;45(5):1139–45.
10.1002/hep.21615
43. Kim KW, Kim AY, Kim TK, Kim SY, Kim MJ, Park MS, et al. Hepatic hemangiomas with arterioportal
shunt: sonographic appearances with CT and MRI correlation. AJR Am J Roentgenol. 2006 Oct;187(4):W406-14.
10.2214/AJR.05.0611
44. Mamone G, Di Piazza A, Carollo V, Cannataci C, Cortis K, Bartolotta TV, et al. Imaging
of hepatic hemangioma: from A to Z. Abdom Radiol (NY). 2020 Mar;45(3):672–91. 10.1007/s00261-019-02294-8
45. Cogley JR, Miller FH. MR imaging of benign focal liver lesions. Radiol Clin North
Am. 2014 Jul;52(4):657–82. 10.1016/j.rcl.2014.02.005
46. Kamaya A, Maturen KE, Tye GA, Liu YI, Parti NN, Desser TS. Hypervascular liver lesions.
Semin Ultrasound CT MR. 2009 Oct;30(5):387–407. 10.1053/j.sult.2009.06.001
47. Chung YE, Kim MJ, Kim YE, Park MS, Choi JY, Kim KW. Characterization of incidental
liver lesions: comparison of multidetector CT versus Gd-EOB-DTPA-enhanced MR imaging.
PLoS One. 2013 Jun;8(6):e66141. 10.1371/journal.pone.0066141
48. Bauditz JF, Wermke W. Sonomorphologie, Vaskularisation und Wachstumsverhalten von
Fokal Nodulären Hyperplasien der Leber im Langzeitverlauf. Z Gastroenterol. 2007;45(8):45.
10.1055/s-2007-988117
49. Dong Y, Wang WP, Mao F, Zhang Q, Yang D, Tannapfel A, et al. Imaging Features of Fibrolamellar
Hepatocellular Carcinoma with Contrast-Enhanced Ultrasound . Ultraschall Med. 2020
.
50. Zarzour JG, Porter KK, Tchelepi H, Robbin ML. Contrast-enhanced ultrasound of benign
liver lesions. Abdom Radiol (NY). 2018 Apr;43(4):848–60. 10.1007/s00261-017-1402-2
51. Zech CJ, Grazioli L, Breuer J, Reiser MF, Schoenberg SO. Diagnostic performance and
description of morphological features of focal nodular hyperplasia in Gd-EOB-DTPA-enhanced
liver magnetic resonance imaging: results of a multicenter trial. Invest Radiol. 2008 Jul;43(7):504–11.
10.1097/RLI.0b013e3181705cd1
52. McInnes MD, Hibbert RM, Inácio JR, Schieda N. Focal Nodular Hyperplasia and Hepatocellular
Adenoma: Accuracy of Gadoxetic Acid-enhanced MR Imaging—A Systematic Review. Radiology.
2015 Nov;277(2):413–23. 10.1148/radiol.2015142986
53. Sempoux C, Balabaud C, Paradis V, Bioulac-Sage P. Hepatocellular nodules in vascular
liver diseases. Virchows Arch. 2018 Jul;473(1):33–44. 10.1007/s00428-018-2373-6
54. Mamone G, Carollo V, Di Piazza A, Cortis K, Degiorgio S, Miraglia R. Budd-Chiari Syndrome
and hepatic regenerative nodules: magnetic resonance findings with emphasis of hepatobiliary
phase. Eur J Radiol. 2019 Aug;117:15–25. 10.1016/j.ejrad.2019.05.015
55. Kogiso T, Tokushige K. Fontan-associated liver disease and hepatocellular carcinoma
in adults. Sci Rep. 2020 ;10(1):21742. PubMed PMID: 33303924. PMCID: PMC7728791.
10.1038/s41598-020-78840-y
56. Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, et al.; GENTHEP Investigators.
Molecular Classification of Hepatocellular Adenoma Associates With Risk Factors, Bleeding,
and Malignant Transformation. Gastroenterology. 2017 Mar;152(4):880–894.e6. 10.1053/j.gastro.2016.11.042
57. Romailler É, Schmidt Kobbe S, Moradpour D, Sempoux C. [Hepatocellular adenoma: update
2020]. Rev Med Suisse. 2020 Sep 2;16(704):1554-9. PubMed PMID: 32880111. Epub 2020/09/04.
Adénomes hépatocellulaires : update 2020. fre.
58. Katabathina VS, Menias CO, Shanbhogue AK, Jagirdar J, Paspulati RM, Prasad SR. Genetics
and imaging of hepatocellular adenomas: 2011 update. Radiographics. 2011 Oct;31(6):1529–43.
10.1148/rg.316115527
59. Purysko AS, Remer EM, Coppa CP, Obuchowski NA, Schneider E, Veniero JC. Characteristics
and distinguishing features of hepatocellular adenoma and focal nodular hyperplasia
on gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol. 2012 Jan;198(1):115–23.
10.2214/AJR.11.6836
60. Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al.; World Federation
for Ultrasound in Medicine; European Federation of Societies for Ultrasound. Guidelines
and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS)
in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives
of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013 Feb;39(2):187–210.
10.1016/j.ultrasmedbio.2012.09.002
61. Seitz K, Bernatik T, Strobel D, Blank W, Friedrich-Rust M, Strunk H, et al. Contrast-enhanced
ultrasound (CEUS) for the characterization of focal liver lesions in clinical practice
(DEGUM Multicenter Trial): CEUS vs. MRI—a prospective comparison in 269 patients.
Ultraschall Med. 2010 Oct;31(5):492–9. 10.1055/s-0029-1245591
62. Seitz K, Strobel D, Bernatik T, Blank W, Friedrich-Rust M, Herbay A, et al. Contrast-Enhanced
Ultrasound (CEUS) for the characterization of focal liver lesions - prospective comparison
in clinical practice: CEUS vs. CT (DEGUM multicenter trial). Parts of this manuscript
were presented at the Ultrasound Dreiländertreffen 2008, Davos. Ultraschall Med. 2009 Aug;30(4):383–9.
10.1055/s-0028-1109673
63. Strobel D, Bernatik T, Blank W, Schuler A, Greis C, Dietrich CF, et al. Diagnostic
accuracy of CEUS in the differential diagnosis of small (≤ 20 mm) and subcentimetric
(≤ 10 mm) focal liver lesions in comparison with histology. Results of the DEGUM multicenter
trial. Ultraschall Med. 2011 Dec;32(6):593–7. 10.1055/s-0031-1271114
64. Friedrich-Rust M, Klopffleisch T, Nierhoff J, Herrmann E, Vermehren J, Schneider MD,
et al. Contrast-Enhanced Ultrasound for the differentiation of benign and malignant
focal liver lesions: a meta-analysis. Liver Int. 2013 May;33(5):739–55. 10.1111/liv.12115
65. Zhang L, Zhang L, Wang H, Chen L, Sui G. Diagnostic performance of contrast-enhanced
ultrasound and magnetic resonance imaging for detecting colorectal liver metastases:
A systematic review and meta-analysis. Dig Liver Dis. 2019 Sep;51(9):1241–8. 10.1016/j.dld.2019.06.004
66. Sawatzki M, Meyenberger C, Brand S, Semela D. Contrast-enhanced ultrasound (CEUS)
has excellent diagnostic accuracy in differentiating focal liver lesions: results
from a Swiss tertiary gastroenterological centre. Swiss Med Wkly. 2019 Jun;149:w20087.
10.4414/smw.2019.20087
67. Sawatzki M, Güller U, Güsewell S, Husarik DB, Semela D, Brand S. Contrast-enhanced
ultrasound can guide the therapeutic strategy by improving the detection of colorectal
liver metastases. J Hepatol. 2021 Feb;74(2):419–27. 10.1016/j.jhep.2020.09.036
68. Haimerl M, Wächtler M, Platzek I, Müller-Wille R, Niessen C, Hoffstetter P, et al. Added
value of Gd-EOB-DTPA-enhanced Hepatobiliary phase MR imaging in evaluation of focal
solid hepatic lesions. BMC Med Imaging. 2013 Dec;13(1):41. 10.1186/1471-2342-13-41
69. Ko Y, Kim J, Park JK, Kim H, Cho JY, Kang SB, et al. Limited detection of small (≤
10 mm) colorectal liver metastasis at preoperative CT in patients undergoing liver
resection. PLoS One. 2017 Dec;12(12):e0189797. 10.1371/journal.pone.0189797
70. Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver:
A comprehensive review. World J Hepatol. 2019 Jan;11(1):1–18. 10.4254/wjh.v11.i1.1
71. Cho SW, Marsh JW, Steel J, Holloway SE, Heckman JT, Ochoa ER, et al. Surgical management
of hepatocellular adenoma: take it or leave it?. Ann Surg Oncol. 2008 Oct;15(10):2795–803.
10.1245/s10434-008-0090-0
72. Neuberger J, Patel J, Caldwell H, Davies S, Hebditch V, Hollywood C, et al. Guidelines
on the use of liver biopsy in clinical practice from the British Society of Gastroenterology,
the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020 Aug;69(8):1382–403.
10.1136/gutjnl-2020-321299
73. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for
the Study of Liver Diseases. Liver biopsy. Hepatology. 2009 Mar;49(3):1017–44. 10.1002/hep.22742





















