Patients with refractory musculoskeletal pain syndromes undergoing a multimodal assessment
and therapy programme: a cross-sectional study
DOI: https://doi.org/https://doi.org/10.57187/s.3466
Tiffany Prétata,
Thomas Hüglea,
Johanna Mettlera,
Marc Suterb,
Sandy Jean Scherbb,
Reine-Laure Tailya,
Charlotte Hansa,
Marielle Hoarauc,
Laurent Monodd,
Pierre Frossarda,
Sonia Turchie,
Guillaume Marillierf,
Nastasya Delavignettef,
Marc Blancharda,
Antonio Le Thanhf,
Pedro Ming Azevedoa
a Department of Rheumatology, Lausanne
University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
b Pain Center, Department of Anesthesiology,
Lausanne University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
c Musculoskeletal department, Chiropractic
Unit, University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
d School of Health Sciences Fribourg (HEdS-FR),
HES-SO University of Applied Sciences and Arts Western, Fribourg, Switzerland
e Musculoskeletal department, Occupational
therapy Unit, University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
f Department of Psychiatry, Lausanne University
Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
Summary
BACKGROUND: Chronic musculoskeletal pain syndromes,
including fibromyalgia, are heterogeneous entities with a major socioeconomic burden.
Multimodal treatment programmes have shown greater efficacy than conventional approaches
for these patients, at least in the short term. A profound understanding of
chronic musculoskeletal pain syndrome patients treated in multimodal treatment
programmes is important for their development and to provide insight into these
conditions.
AIM: To provide a comprehensive and objective
description of medical, psychosocial and sleep characteristics of the treatment-refractory
chronic musculoskeletal pain syndrome patients treated at the multimodal
treatment programmes provided by our tertiary service in Switzerland.
METHODS: This was a cross-sectional analysis
of 202 refractory chronic musculoskeletal pain syndrome patients with or without
a concomitant autoimmune disorder hospitalised between 2018 and 2022 in a 12-day
Swiss multimodal treatment programme. They underwent a comprehensive self-assessment
with eight different questionnaires and assessments by a psychiatrist, rheumatologist,
pain specialist, occupational therapist and physiotherapist. Sleep assessment was
performed via actigraphy. Clinical and demographic variables were selected by consensus
of three experienced rheumatologists and chronic pain specialists. The Fibromyalgia
Rapid Screening Test (FiRST), American College of Rheumatology (ACR)-2010 criteria
(ACR2010) and Toronto Alexithymia Scale-20 (TAS-20) were also applied.
RESULTS: The mean age of the patients was 47
years (SD = 10), 73% were female, and 30% were obese. Half (50%) were not from Switzerland,
and 12% came from conflict zones. Almost half (40%) lived alone. Back pain was the
principal site (90%). Of the patients, 78% fulfilled the ACR2010 criteria for
fibromyalgia, and 17% were diagnosed with an underlying immune-mediated disorder,
mostly spondylarthritis. Pain since childhood occurred in 45% of the patients, and
68% had pain since adolescence. Disability financial aid had been pursued by
69%, and 46% were still awaiting a response. Psychiatric comorbidities were highly
prevalent (73%), of which 56% consisted of depression. Of all patients, 15% were
diagnosed with enduring personality changes after a catastrophic experience (EPCACE),
and 10% had post-traumatic stress disorder. Alexithymia affected 34% of patients.
Objective sleep disorder was observed in 78% of patients, and 41% were under opioid
therapy.
CONCLUSION: This analysis reveals the complex
psychosomatic and socioeconomic patterns of the patients treated in Switzerland
with refractory chronic musculoskeletal pain syndromes, often originating in childhood
and adolescence. Obesity, immigration, social isolation, psychiatric comorbidities,
sleep deprivation and opiate use, among others, stood out as target characteristics
for further research.
Introduction
Chronic pain is the leading cause of years lived
with disability in the world [1] and the leading reason for seeking medical help
in the United States [2]. It can have a profound impact on individuals’ quality
of life, leading to depression, anxiety, social isolation and reduced function.
Chronic pain can also impose a significant economic burden. Studies in the United
States have estimated the annual cost of chronic pain to be in the range of $560
to $635 billion, with a substantial portion attributed to indirect costs such as
lost productivity and disability [3]. Additionally, chronic pain is associated with
higher healthcare costs, including increased use of medications, hospitalisation
and outpatient consultations [4]. It is a major problem in Switzerland as it is
worldwide. In 2006, a European telephonic survey found that 16% of Swiss participants
had chronic musculoskeletal pain syndromes (CMPS), of which 32% were reported as
severe. Most participants (54%) thought that their pain was inadequately controlled,
with direct consequences on their quality of life, work and social life [5].
The impact of chronic pain highlights the need
for effective prevention and management strategies to reduce the burden on individuals
and society. The effectiveness of interventions may vary depending on the underlying
cause and characteristics of the pain, making personalised treatment plans essential
[6]. Despite uncertainties about optimal treatment, the need for a personalised
and multidisciplinary approach is a consensus. In this context, multimodal
treatment programmes (MMPs) emerge as the most effective intervention, at least
in the short term [7–9]. Significant benefits of multicomponent treatment have
been documented in a meta-analysis [9], and multimodal programmes are proposed in
most guidelines for resistant cases [10, 11].
Since 2018, the department of rheumatology of
our institution (Centre Hospitalier Universitaire [CHUV], Lausanne, Switzerland)
has provided a multimodal treatment programme for patients with chronic pain and
fatigue syndromes. This 2-week inpatient programme includes patient evaluations
by several health professionals, including rheumatologists, anaesthesiologists,
physiotherapists, occupational therapists, chiropractors, osteopaths and psychiatrists.
It aims to rediscuss the diagnoses, build an overall action plan for the patient
and find solutions concerning health insurance-related issues.
Multimodal programmes are implemented globally,
yet no agreed best approach exists for individual patients. Understanding the typical
profiles of patients who attend these programmes is crucial to effectively customising
care, optimising both the costs and benefits of these interventions.
The present work is part of a larger research
project aimed at optimising the effectiveness and cost-effectiveness of the
multimodal treatment programme provided by our institution for chronic pain syndromes.
The priorities of the project are defining and investigating (a) the most relevant
endpoints to be studied prospectively and (b) the clinical, social and psycho-behavioural
characteristics that seem to influence such endpoints and can be used to separate
patients in clusters.
Study aims
The study goal was to provide a comprehensive
and objective description of medical, psychosocial and sleep characteristics of
the treatment-refractory chronic musculoskeletal pain syndrome patients treated
at the multimodal programme provided by our tertiary service in Switzerland.
Methods
This was a cross-sectional study involving 207
patients who completed the 2-week inpatient multimodal treatment programme for chronic
musculoskeletal pain syndromes provided by CHUV from its beginning in March 2018
up to November 2022. The exclusion criteria for the study were refusing to sign
the informed consent, failing to complete the multimodal treatment programme and
missing data. The population targeted by the MMP consists of adult chronic
musculoskeletal pain syndrome and chronic fatigue syndrome patients who have failed
ambulatory treatment and are therefore sent to this tertiary university MMP by their
physicians in secondary or primary care. The inclusion criteria are age between
18 and 70 years and the ability to fluently speak French. The exclusion criteria for
the MMP are the existence of cognitive,
physical, cultural or psychiatric difficulties that prevent patients from understanding
or adhering to MMP procedures. We estimate that one in every five patients sent
to a pre-MMP consultation is excluded because of these criteria.
Clinical questionnaires
The questionnaires used were chosen beforehand
by a group of physician specialists in the treatment of chronic musculoskeletal
pain syndromes when designing the multimodal treatment programme. The
questionnaires are the validated and traditionally used French versions for patients
with chronic pain. They assess various aspects of these conditions, including cognitive-behavioural
factors (kinesiophobia, catastrophism, avoidance beliefs regarding work and physical
activity, and patterns of activities), emotional distress (anxiety and depression),
physical functioning and pain severity.
Patients were asked to complete the clinical
questionnaires at the start of hospitalisation and before discharge. The questionnaires
were completed online using secure software (REDCap®), and patients’ identities
were replaced by a security code. Access to the questionnaires was given to patients
3 days before hospitalisation. Patients who did not complete the electronic forms
alone did so with assistance at entry and discharge.
Evaluation of cognitive-behavioural factors
Kinesiophobia
The Tampa Scale for Kinesiophobia (TSK) is a psychological assessment
tool used to measure kinesiophobia, or fear of movement, through 17 items that gauge
participants’ feelings about pain and movement. Commonly used in clinical settings,
the TSK helps determine how fear influences a patient’s avoidance of physical activity,
guiding treatment strategies and interventions [12].
Catastrophism
The Pain Catastrophizing Scale (PCS) is a psychological tool used to
measure an individual’s tendency to catastrophise pain, a process where pain is
anticipated or experienced with exaggerated negative mental responses. The PCS evaluates
thoughts and feelings about pain across three dimensions: rumination, magnification
and helplessness. Widely used in both clinical and research settings, the PCS helps
assess the impact of pain catastrophising on pain experiences and outcomes, especially
in managing chronic pain [13].
Avoidance beliefs regarding work and physical
activity
The Fear-Avoidance Beliefs Questionnaire (FABQ)
is a tool designed to evaluate individuals’ beliefs about how physical activity
and work might cause or worsen pain. It is frequently used to understand how these
beliefs contribute to the avoidance of physical activity and the resulting disability
in chronic pain conditions [14].
Patterns of activities
The Patterns of Activity Measure (POAM) questionnaire is designed to
evaluate daily activity behaviours in individuals with chronic pain. It identifies
three main behavioural strategies: pacing (POAM-P), which is adaptive, and avoidance
(POAM-A) and overdoing (POAM-O), which are often maladaptive and linked to the development
and persistence of chronic pain. The POAM-A measures the extent to which individuals
avoid activities due to fear of pain or re-injury. The POAM-O assesses how individuals
exceed their physical limits during activities, often ignoring pain signals, which
may lead to increased pain and a cycle of overactivity followed by enforced rest
[15].
Evaluation of emotional distress
Anxiety and depression
The Hospital Anxiety and Depression Scale (HADS) is a common screening
tool used to detect potential anxiety disorders and depression. It is especially
valuable for assessing patients with ongoing physical discomfort, such as
chronic musculoskeletal pain syndromes [16].
Evaluation of pain severity, physical functioning
and quality of life
The Brief Pain Inventory (BPI)
is a standardised tool often used to measure pain severity and its effects on daily
activities in individuals with chronic pain. The BPI-Severity (BPI-S) section has
patients rate their pain from 0 (no pain) to 10 (worst pain imaginable), and the
BPI-Impact (BPI-I) section assesses how pain affects aspects of daily life such
as general activity, mood, mobility, work, social interactions, sleep and enjoyment
of life. In the study’s longitudinal component, not discussed in this paper, BPI-Impact
variation was selected as the primary outcome for multimodal treatment programmes,
with other questionnaires serving as secondary measures [17].
Clinical and demographic variables
Three experienced rheumatologists and chronic
pain specialists selected clinical and demographic variables to understand
chronic musculoskeletal pain syndromes and patient treatment responses. These included
demographics (age, sex, origin, family status, disability insurance), BMI, clinical
and psychiatric diagnoses, comorbidities, pain types (nociceptive, nociplastic,
neuropathic), sleep patterns (assessed over 5 nights with actigraphy), medication
use and lab results. Details on the variables chosen and their definitions are shown
in tables S1 to S4 (see appendix).
“Fibromyalginess”, the extent of central fibromyalgia
features, was evaluated using the ACR 2010 and FiRST criteria. Alexithymia was assessed
with the TAS-20 scale and two psychiatric evaluations.
Data analysis
Measures of central tendency and dispersion
were assessed for all numerical data. The mean and standard deviation (SD) are shown
for variables with approximately normal distribution. The median, mode and range
are shown where the distribution was not normal. Normality was evaluated with normal
probability plots. To account for missing data, we used proportions to make the
different clinical variables comparable, while systematically specifying the total
number of non-missing data.
The data were analysed with Microsoft Office
Excel 2019 and Stata/MP 13.
Ethics approval
Ethics approval was granted by the Commission
Cantonale d'Éthique de la Recherche sur l’Être Humain (CER-VD) on 06 December 2021
(Project ID 2021-00853). Some of the patients signed a specific informed consent
form for this research, and all signed the hospital’s general consent form, allowing
the use of their clinical and epidemiological data as long as their identity was
kept secret. The study was performed without a preregistered protocol.
Results
Demographic data
Two hundred and seven patients initially participated
in the study, but 5 were excluded – 3 for not completing the multimodal
treatment programme and two for not completing the questionnaires – leaving a final
sample of 202 patients. The sample comprised 73% women and 27% men, with an average
age of 47 years, ranging from 23 to 74 years (figure 1). Demographic details are
available in table S1.
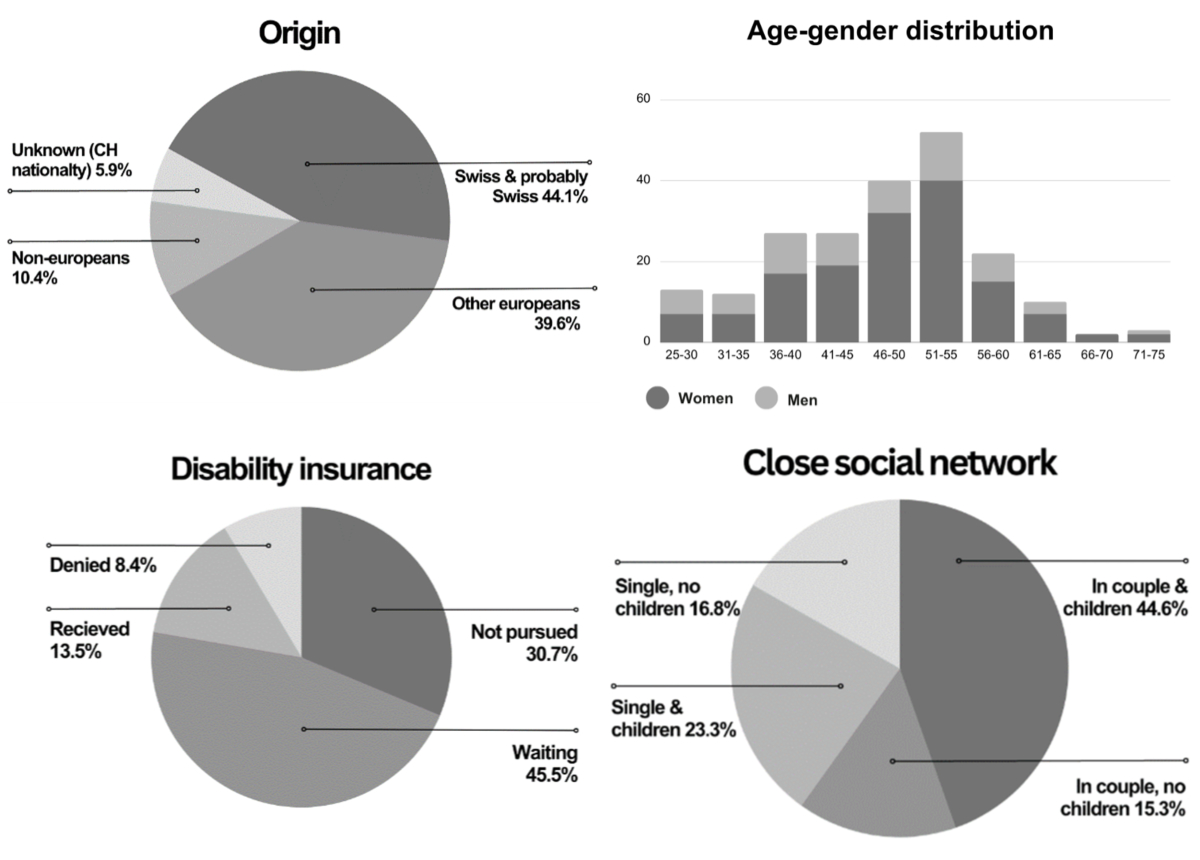
Figure 1Main demographic
features (age and sex distribution, patients’ origin and disability insurance
status). Origin of patients was recorded for 190/202 (94%) individuals, with 12
missing data. “Probably Swiss“ refers to patients whose origin was not formally
investigated but were francophone. “Unknown origin but Swiss nationality“ refers
to patients whose origin was not formally investigated but who possessed Swiss
citizenship and were not originally francophone. (A) Origin (n = 190; 12
data missing); (B) Age – sex distribution (n = 202; no data missing); (C)
Disability insurance status (n = 198; data on 4 patients missing); (D)
Close social network (relationship status n = 202; no data missing).
The country of origin was identified for 94%
(190/202) of the patients, with distributions depicted in figure 1. Regarding living
arrangements, around 60% of patients lived with a partner (57% women, 67% men),
and the majority (68%) had children. Disability insurance status, reported by 98%
of patients, showed that 69% had applied for it, 13% had been granted, 8%
were denied and 45% were awaiting for a decision (figure 1).
Clinical characteristics
The clinical characteristics of patients are
represented in tables 1 and 2.
Table 1Patients’
body mass index (BMI) and main clinical comorbidities. Missing data is indicated
where the total number is different from 202.
| Clinical characteristic |
All patients |
Women |
Men |
| Weight |
BMI |
Median = 27, mode = 25, range: 16.3–50 |
Median = 27.5, mode = 25, range: 16.3–46.9 |
Median = 26.2, mode = 28.4, range: 16.5–50 |
| Underweight: n/total (%) |
7/158 (4%) |
5/115 (4%) |
2/43 (4%) |
| Healthy weight: n/total (%) |
49/158 (31%) |
35/115 (30%) |
14/43 (33%) |
| Overweight: n/total (%) |
53/158 (33%) |
35/115 (30%) |
18/43 (423%) |
| Obese: n/total (%) |
48/158 (30%) |
39/115 (34%) |
9/43 (21%) |
| Main comorbidities n/total (%) |
Obstructive sleep apnoea: n, total (%) |
19/202 (9%) |
11/148 (7%) |
8/54 (15%) |
| COPD: n, total
(%) |
6/202 (3%) |
2/148 (1%) |
4/54 (7%) |
| Asthma: n,
total (%) |
18, 202 (9%) |
12, 148 (8%) |
6, 54 (11%) |
| Diabetes: n,
total (%) |
12, 202 (6%) |
9, 148 (6%) |
3, 54 (6%) |
Table 2Pain
characterisation and diagnosis. Missing data is indicated where the total number
is different from 202.
| Clinical characteristic |
All patients |
Women |
Men |
| n/total (%) |
n/total (%) |
n/total (%) |
| Pain site |
Back pain |
Any back pain |
182/202 (90%) |
135/148 (91%) |
47/54 (87%) |
| Buttock |
65/202 (32%) |
50/148 (34%) |
15/54 (28%) |
| Lumbar |
166/202 (82%) |
128/148 (86%) |
38/54 (70%) |
| Dorsal |
63/202 (31%) |
51/148 (3%) |
12/54 (22%) |
| Cervical pain |
136/202 (67%) |
105/148 (71%) |
31/54 (57%) |
| Shoulder pain |
92/202 (45%) |
69/148 (47%) |
23/54 (43%) |
| Pain type |
Peripheral |
Nociplastic |
152/202 (75%) |
122/148 (83%) |
30/54 (56%) |
| Nociceptive |
Mechanical |
124/202 (61%) |
91/148 (61%) |
33/54 (61%) |
| Inflammatory |
12/202 (6%) |
7/148 (5%) |
5/54 (9%) |
| Neuropathic |
40/202 (20%) |
29/148 (20%) |
11/54 (20%) |
| Axial |
Nociplastic (functional) component |
Clinical diagnosis |
131/202 (65%) |
100/148 (68%) |
31/54 (57%) |
| Waddell score |
n = 84 (42%), mode = 1, SD = 1.5 |
n = 65 (43%), mode = 1.5, SD = 2 |
n = 19
(35%), mode = 1, SD = 1.6 |
| Nociceptive |
Mechanical |
139/202 (69%) |
108/148 (73%) |
31/54 (57%) |
| Inflammatory |
8/202 (4%) |
4/148 (3%) |
4/54 (7%) |
| Neuropathic (radiculopathy) |
34/202 (17%) |
19/148 (13%) |
15/54 (28%) |
| Pain history |
Pain in childhood |
39/86 (45%) |
32/70 (46%) |
7/16 (44%) |
| Pain in adolescence |
58/85 (68%) |
52/71 (73%) |
6/14 (43%) |
| Pain in adolescence but not childhood |
23/58 (40%) |
21/52 (40%) |
2/5 (40%) |
| Biomechanical disorders |
68/202 (34%) |
54/148 (36%) |
14/54 (26%) |
| Spine surgery >1 |
14/100 (14%) |
9/75 (12%) |
5/25 (20%) |
| Hypermobility |
Clinical diagnosis |
50/181 (28%) |
43/130 (33%) |
7/51 (14%) |
| Abnormal Beighton score |
n = 40, median = 6, mode = 7, range: 0–9 |
n = 33, median = 6, mode = 7, range: 1–9 |
n = 7, median = 4, mode = 4, range: 0–7 |
| Clinical diagnosis associated with peripheral pain |
Autoimmune rheumatic diseases |
34/202 (17%) |
25/148 (17%) |
9/54 (17%) |
| Significant osteoarthritis |
57/202 (28%) |
48/148 (32%) |
9/54 (17%) |
| Bursitis |
12/202 (6%) |
11/148 (7%) |
1/54 (2%) |
| Enthesopathy |
53/202 (26%) |
37/148 (25%) |
16/54 (30%) |
| Tendinopathy |
62/202 (37%) |
46/148 (31%) |
16/54 (30%) |
| Microcrystalline disease |
7/202 (3%) |
7/148 (5%) |
0/54 (0%) |
| Fibromyalgia |
FM ACR 2010 fulfilled |
36/46 (78%) |
29/36 (81%) |
7/10 (70%) |
| FM FiRST criteria fulfilled |
47/58 (81%) |
38/47 (81%) |
9/11 (82%) |
Body Mass Index and main clinical comorbidities
The overweight and obese categories accounted
for 33% and 30% of the patients, respectively. Four and a half per cent of the patients
were underweight. The main comorbidities observed included sleep obstructive apnoea
(9.4%), chronic obstructive pulmonary disease (3%), asthma (9%), diabetes (6%) and
microcrystalline disease (3%).
Pain characterisation and diagnosis
The vast majority of patients reported back
pain. The lumbar region was painful for 82% of all patients. Cervical pain affected
67% of patients. Axial pain was considered to have a nociplastic component in 65%
(pain characteristics could not be attributed
to a neuropathic or nociceptive process). Nevertheless, the Waddell score (proposed
as a marker of “non-organic“ pain) showed a median of 1 for all patients.
The characterisation of peripheral pain sites
led to too many variables of questionable importance and was abandoned. Shoulder
pain was retained because it was a frequent complaint (reported by 45% of all patients)
that can be associated with neck pain and biomechanical processes. Nociplastic pain
was the most represented type of peripheral pain, diagnosed in 75% of all patients
(82% of women and 56% of men). Fibromyalgia, based on the 2010 ACR and FiRST criteria,
was present in 78% and 81% of patients, respectively. Peripheral nociceptive pain
was prevalent among 61% of all patients. Peripheral osteoarthritis was observed
in 28% of patients (32% of women and 17% of men). Tendinopathy was present in 37%
of patients. Peripheral neuropathy was reported by 20% of all patients. Inflammatory
pain was rare.
A detailed personal pain history was collected
from 2021 onwards. In 45% of the investigated cases (n = 86, representing 43% of
the total sample), persistent or recurrent pain was reported in childhood, and in
68.2%, from adolescence. All patients experiencing pain in childhood also reported
pain in adolescence, and 40% of the latter did not recall pain in childhood.
Among the entire sample, 34% of patients received
a diagnosis implicating an intrinsic biomechanical mechanism (36% of women and 26%
of men).
Clinically diagnosed hypermobility was present
in 27.6% of patients for whom data were available (33% of women and 14% of men).
Beighton scores were measured in 101 patients, with a median of 2. When patients
were diagnosed with hypermobility, the median score was 6 for women and 4 for men.
Autoimmune rheumatic diseases at entry to the
programme were reported by 22% of the total sample, but this diagnosis was not confirmed
by our investigation in 23% of cases. Active peripheral autoimmune inflammation
was present in 6% of all patients.
Psychiatric comorbidities
The psychiatric comorbidities of patients are
detailed in table 3.
Table 3Psychiatric
comorbidities.
| Psychiatric comorbidity |
All patients |
Women |
Men |
| n/total (%) |
n/total (%) |
n/total (%) |
| At least one psychiatric disorder |
147/202 (73%) |
108/148 (73%) |
41/54 (76%) |
| Depression |
Clinically diagnosed |
114/202 (56%) |
84/148 (57%) |
30/54 (56%) |
| HDS 10 |
75/194 (39%) |
55/145 (38%) |
20/49 (41%) |
| Anxiety |
Clinically diagnosed |
62/202 (31%) |
45/148 (30%) |
17/54 (31%) |
| HAS >10 |
111/193 (57%) |
78/144 (54%) |
33/49 (67%) |
| Post-traumatic stress disorder |
20/202 (10%) |
17/148 (11%) |
3/54 (6%) |
| Enduring personality change after disaster
experience |
31/202 (15%) |
26/148 (18%) |
5/54 (9%) |
| Alexithymia |
Clinical diagnosis |
69/202 (34%) |
47/148 (32%) |
22/54 (41%) |
| TAS-20 |
TAS-20 total: |
n = 30 (15%), mean = 53.5, SD = 16, range: 31–82 |
n = 24 (16%), mean = 53.7, SD = 16.9, range: 31–82 |
n = 6 (11%), mean = 52.5, SD = 13.4, range: 38–72 |
| TAS-20 ≥52 |
17/30 (57%) |
14/24 (58%) |
3/6 (50%) |
| TAS-20 >60 |
9/30 (30%) |
8/24 (33%) |
1/6 (17%) |
| Personality disorder |
54/202 (27%) |
42/148 (28%) |
12/54 (22%) |
| Bipolar disorder |
7/202 (3%) |
4/148 (3%) |
3/54 (6%) |
Among all patients, 73% had at least one psychiatric
disorder.
Clinically diagnosed depression was observed
in 56% of all patients, with a comparable prevalence between genders. According
to the HDS, depression affected 39% of patients (38% of women and 41% of men).
Clinically diagnosed anxiety was found in 31%
of all patients, evenly distributed between genders. Additionally, 57.5% of all
patients had a high anxiety score, with a HAS of >10 (54% of women and 67% of
men).
Post-traumatic stress disorder (PTSD) was found
in 10% of patients (11% of women and 6% of men), with enduring personality changes
after a catastrophic experience (EPCACE) in 15% (18% of women and 9% of men). Most
(85%) post-traumatic stress disorder patients had concomitant enduring
personality changes after a catastrophic experience, and 85% were Europeans, including
60% Swiss patients. Twenty per cent of post-traumatic stress disorder patients came
from conflict zones. Conversely, 8% of patients coming from a conflict zone demonstrated
post-traumatic stress disorder. Eighty-four per cent of patients with enduring
personality changes after a catastrophic experience were Europeans, including 45%
Swiss patients. Approximately one third originated from a conflict zone. Conversely,
21% of patients coming from a conflict zone demonstrated enduring personality
changes after a catastrophic experience.
Clinical alexithymia was present in 34% of all
patients (32% of women and 41% of men). Alexithymia can be assessed through the
TAS-20 score; 15% of the sample completed this, of whom 30% had a score of ≥61 (probable
alexithymia).
Medications
Medication details are provided in table 4.
| Medication |
All patients |
Women |
Men |
| n/total (%) |
n/total (%) |
n/total (%) |
| Opiates |
Total |
83/202 (41%) |
62/148 (42%) |
21/54 (39%) |
| Strong |
28/202 (14%) |
22/148 (15%) |
6/54 (11%) |
| Weak |
55/202 (27%) |
40/148 (27%) |
15/54 (28%) |
| Antidepressants (AD) |
Total |
95/202 (47%) |
77/148 (52%) |
18/54 (33%) |
| Tricyclic or tetracyclic |
22/202 (11%) |
15/148 (10%) |
7/54 (13%) |
| Dual |
31/202 (15%) |
27/148 (18%) |
4/54 (7%) |
| Vilazodone |
5/202 (2%) |
4/148 (3%) |
1/54 (2%) |
| Mirtazapine |
2/202 (1%) |
2/148 (1%) |
0/54 (0%) |
| Trazodone |
23/202 (11%) |
20/148 (13%) |
3/54 (6%) |
| Gabapentinoid drugs |
37/202 (18%) |
25/148 (17%) |
12/54 (22%) |
| Anti-seizure medication (ASM) |
8/202 (4%) |
7/148 (5%) |
1/54 (2%) |
| Neuroleptics |
12/202 (6%) |
11/148 (7%) |
1/54 (2%) |
| Benzodiazepines |
59/202 (29%) |
46/148 (31%) |
13/54 (24%) |
| Nonbenzodiazepines (Z-drugs) |
26/202 (13%) |
20/148 (13%) |
6/54 (11%) |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) |
113/202 (56%) |
82/148 (55%) |
31/54 (57%) |
| Paracetamol/metamizole |
130/202 (64%) |
90/148 (61%) |
40 54 (74%) |
| Myorelaxants |
28/202 (14%) |
21/148 (14%) |
7/54 (13%) |
| Cannabidiol (CBD) |
3/202 (1%) |
0/70 (0%) |
3/25 (12%) |
| Prednisone |
9/202 (4%) |
8/148 (5%) |
1/54 (2%) |
| Biologics |
10/202 (5%) |
8/148 (5%) |
2/54 (4%) |
The most used medications were non-opioid analgesics
such as paracetamol and metamizole (64%), followed by NSAIDs (56%). A total of 41%
were taking opiates, with 4% taking strong opiates.
Laboratory results
Details of laboratory results can be found in
supplementary table S3.
The median C-reactive protein (CRP) level was
1 mmol/l (2 mmol/l women, 1 mmol/l men) with a range of 0 to 37. It was tested in
approximately 69% of our sample, of which 14% showed higher than 5 mmol/l (the laboratory
normality cutoff). The median sedimentation rate was 7 mm/h (11 mm/h in women, 4
mm/h in men) with a range from 1 to 63.
Sleep analysis
Details of the sleep analysis can be found in
supplementary table S4.
The median total sleep time was 7 hours and
44 minutes (07:44), with a range of 04:39–11:14. When considering sleep efficacy
(percentage of time spent asleep), 47% of the patients had an efficacy below the
cutoff of 85%.
Regarding the sleep fragmentation index, 78%
of patients showed a value greater than the cutoff of 20, indicating regular sleep
interruptions. The overall percentage of individuals with a delayed sleep phase
(defined as turning off the lights after
midnight) was 21%.
Discussion
We present here a detailed panel of the clinical,
psychiatric and epidemiological characteristics of patients with refractory
chronic musculoskeletal pain syndromes seen in tertiary centres in French Switzerland.
This characterisation is important because it provides insights allowing for possible
improvements in the treatment and prevention of such cases.
In 2019, a study measured the effects of a Swiss
multimodal treatment programme aimed at improving chronic pain among Italian- or
German-speaking patients [18]. Despite similar average ages and sex distributions
across their cohort and our French-speaking population, social networks varied.
Specifically, 17% of our patients were single and without children, compared to
22–27% of German speakers and 3–12% of Italian speakers. Additionally, 36–66% of
German-speaking patients were unemployed, and 28–43% were working part-time. For
Italian-speaking patients, 68–80% were unemployed, and 14–23% worked part-time.
We did not directly measure the employment rate, but we were interested in the need
to resort to disability insurance, which indirectly indicates difficulties in maintaining
full-time employment. This concerned 69% of our population, which is then closer
to the Italian cohort in this matter. The authors of that study only reported the
number of comorbidities of their patients, ranging from 0 to >6, and did not
characterise the pain types and mechanisms, nor patient behavioural, psychiatric
or sleep characteristics. We believe that a need exists to specify and list these
aspects as objectively and exhaustively as possible to better understand these complex
patients.
Socioeconomic characteristics
We highlight the relatively young age of our
patients, the predominance of women, the higher proportion of patients living alone,
and the high proportion of immigrants and obesity.
The young age of our patients is partially related
to the programme’s age limits (18 to 70 years). Nevertheless, the distribution curve
was close to normal, with no tendency to deviate towards older ages, which suggests
a low influence of selection bias on this result. The peak incidence was concentrated
between the ages of 45 and 55 years. The reasons for this are unclear. Menopause
and perimenopause are probably factors associated with this phenomenon, but the
same distribution was observed in men. Interestingly, 33% (n = 67) of our patients
showed a predominant “overdoing” behavioural response profile (POAM-O). It could
be argued that this adaptive strategy fails around the age of 45 years, when physical
endurance begins to diminish. The need for performance, however, continues to be
demanded by society and the patients themselves, which leads to crisis.
The female predominance is not a surprise. Around
half of chronic pain conditions are more common in women, including fibromyalgia,
with only 20% having a higher prevalence in men [19]. This reinforces that women
are more exposed and should be a priority target for prevention.
The sample demonstrated a high proportion of
patients living alone. Based on 2018 data from the SFSO [20],approximately
17% of women and 16% of men aged 34–54 years in Switzerland are single. In comparison,
36% of patients for the same age group in our sample were single. Nevertheless,
our patients had children more often than the paired population in Switzerland (88%
versus 75% in the 50–59 years age group) [21]. Higher singlehood levels coupled with
more children might
suggest a higher rate of divorce or single parenthood. Furthermore, a lower education
level is linked to having
more children, especially for women [22].
Social isolation in chronic pain is multifactorial. Mobility restrictions
limit social interactions, as do the disbelief and stigmatisation of chronic pain
patients [23]. High rates of fatigue, depression and other psychiatric conditions
likewise play a role. If chronic pain triggers isolation, isolation might also be
a risk factor for chronic pain [24, 25]. One benefit of the in-hospital
multimodal treatment programme is that it enables patients to bond over shared experiences
and offer each other support. Additionally, the inpatient setting isolates patients
from their normal environments, which often contribute to their conditions, facilitating
behavioural and social change. Furthermore, this setting generally allows for extended
screening time, giving healthcare providers better opportunities to monitor progress,
conduct diagnostic tests and tailor treatments accordingly.
At least 56% of patients were not originally
from Switzerland, a higher proportion than that found in the population of Lausanne
(estimated at 43% in 2019). The excess could be attributed to immigration stress,
adaptation difficulties and economic factors. The living conditions before immigration
are an issue, of course. Accordingly, 12% of the sample came from conflict areas.
Nevertheless, post-traumatic stress disorder and enduring personality changes
after a catastrophic experience were not more prevalent among war refugees and immigrants
than among Swiss patients.
We observed a higher prevalence of obesity compared
to the general Swiss population. According to a 2017 report by the Swiss Federal
Statistical Office (SFSO) [26], 11%
of women and 13% of men in the
35–54 years age range in Switzerland are obese, against29% of women and 21% of men
with
this age in our sample. The
link between socioeconomic status, obesity and chronic pain is well established
[27]: a low socioeconomic status is associated with a higher risk of developing
chronic pain, and it is also associated with a higher risk of obesity [28]. This
seems to be especially true for women living in high-income countries [29].
Psychiatric features
Approximately 73% of patients had at least one
psychiatric disorder, and a notably high proportion was affected by
post-traumatic stress disorder and enduring personality changes after a
catastrophic experience. The latter diagnoses were more prevalent among women, which
could be explained by the common presence of domestic violence and sexual abuse
[30]. Notably, patients from territories in conflict did not show higher proportions
of post-traumatic stress disorder or enduring personality changes after a
catastrophic experience, and at least half of the patients with these diagnoses
were from Switzerland.
Irrespective of sex, 56% of patients presented
clinically diagnosed depression. However, when assessed using the HDS, depression
was reported in 38% of women and 41% of men. A potential gender bias in the HDS
assessment has been recognised, with several studies tackling this topic [31, 32].
Similarly, anxiety disorders were clinically
diagnosed in 31% of the sample and evenly distributed between genders. Nonetheless,
54% of women and 67% of men were considered anxious according to the HAS. We conclude
that, as for the HDS, the HAS seems to be more useful as a monitoring tool than
a diagnostic one. Further research is needed to assess the ideal HAS cutoff, as
well as gendered tendencies for the chronic musculoskeletal pain syndrome population.
Alexithymia, defined by difficulties in identifying
and expressing emotions, was also a prevalent disorder, with a higher prevalence
among men. Meta-analysis findings have indicated that chronic pain samples had significantly
higher mean alexithymia scores compared with controls. In chronic
musculoskeletal pain syndromes, alexithymia was significantly positively associated
with anxiety, depression, pain intensity and interference, although the latter relationships
may be accounted for by negative affect [33]. Only 30 patients answered the TAS-20
that was introduced later in the programme. All nine patients with TAS-20 scores
of >60 were diagnosed with alexithymia, but 15 alexithymic patients had a TAS-20
of ≤60. In conclusion, the positive predictive value for this test seems to be good
for this cutoff but at the expense of a poor negative predictive value.
Clinical characteristics
Among the clinical characteristics presented
by this population, some stand out for their high incidence or for suggesting pathophysiological
mechanisms, including the presence of peripheral arthritis, pain since childhood
or adolescence, the use of opioids, the presence of fibromyalgia, the presence of
sleep disorders and the coexistence of autoimmune rheumatic diseases.
Twenty-eight per cent of our sample had clinically
significant peripheral osteoarthritis. Age, weight, hypermobility and biomechanical
disorders are among the known causes of osteoarthritis. The mean age of patients
diagnosed with osteoarthritis was 51 years, 29% were overweight, 38.2% were obese,
32% were diagnosed with hypermobility and 42% were classified as having a biomechanical
disorder. In comparison, the entire sample was younger on average and displayed
lower rates of each of these factors.
Remarkably, almost half of the patients had
experienced pain since childhood or adolescence. Evidence suggests that chronic
pain in childhood is likely to continue in adulthood, with more risk of depression,
anxiety or opioid misuse [34, 35]. In our sample, the proportion of opiate usage
between all patients (41%) and patients with pain since childhood or adolescence
was similar (36%). A chi-square test did not reveal any significant association
between the two conditions, nor with depression or anxiety. Nevertheless, we found
a significant association between pain impact (according to the BPI) and the presence
of pain since childhood or adolescence (p = 0.034) but not with pain severity (p
= 0.097). Musculoskeletal pain in children and adolescents may be a highly underestimated
problem,and questions remain about its causes and
whether early intervention could help prevent the development of chronic pain [36].
Evidence suggests that long-term opiates do
not improve the quality of life for patients with chronic musculoskeletal pain
syndromes, while posing risks of addiction, opiate-induced hyperalgesia, myocardial
infarction and fractures [37, 38]. Despite recommendations, 40.9% of our patients
reported the use of these medications. Opioid consumption is increasing worldwide,
and Switzerland ranked second in 2019 in terms of opioid use per habitant [39].
Fibromyalgia was formally tested in only 29%
of the patients. Nevertheless, it was diagnosed in 78% of patients tested with the
ACR 2010 criteria and in 81% of patients tested with the FiRST criteria. Most patients
tested by both (92%) fulfilled both tests. Studies directly comparing the 2010 ACR
criteria and FiRST are scarce, but sensitivities of 83% and 74% were found when
using the modified 2010 ACR criteria and FiRST compared to the 1990 criteria, respectively
[40, 41]. Fibromyalgia as a diagnosis has been criticised as artificial and not
anchored by pathophysiology. The more recent concept of nociplasty is now largely
accepted and better represents the increasingly understood pathophysiological mechanisms
behind non-nociceptive and non-neurological pain [42]. By definition,
fibromyalgia always implies peripheric nociplastic pain, but not all peripheral
nociplastic pain is fibromyalgia. In our sample, 81% of patients considered to have
peripheral nociplastic pain fulfilled the ACR 2010 criteria, and 88% fulfilled the
FiRST criteria. Pure nociplastic pain was rare (only 7% of patients).
Many patients experienced fragmented sleep (78%),
decreased sleep efficacy (47%) and sleep delay (20%). These results align with a
recent systematic review and meta-analysis revealing that between 73% and 75% of
patients with chronic pain experienced sleep disturbances [43]. The relationship
between pain and sleep disorders is bidirectional, with each exacerbating the other
[44]. This puts sleep as a priority target for future research and care management.
Medications generally only offer partial relief and are not without side effects,
especially with prolonged use. Non-pharmacological interventions exist, such as
mindfulness, relaxation techniques, exercise and cognitive-behavioural therapy (CBT).
More specifically, CBT approaches for insomnia, pain or both have shown good efficacy
[45].
In 23% of cases, the pre-multimodal
treatment programme diagnosis of autoimmune rheumatic diseases was abandoned upon
evaluation. This was primarily due to the confusion between fibromyalgia-induced
allodynia and polyenthesopathy associated with spondylarthritis (SpA) or hypermobility,
especially in post-menopause women. In total, 40% of hypermobile patients fulfilled
either the 2010 ACR criteria or the FiRST criteria. Conversely, 25% of patients
fulfilling the 2010 ACR criteria were hypermobile, compared to 31% for the FiRST.
Limitations
This study has several limitations. Firstly,
data were missing for many variables collected later in the multimodal
treatment programme history. The MMP protocol was improved over time, which explains
why the first patients were not fully assessed. However, data were collected consecutively
and consistently, so the partial results can be extrapolated to the entire sample.
Secondly, this cross-sectional study is subject
to bias and imprecision in measurement. For instance, assessing pain experienced
during childhood relies on patients’ recall and interpretation of early events in
life. A huge effort was made to limit the impact of subjectivity by precisely defining
each variable (table S2) and by addressing many variables through various means.
Thirdly, the population described here does
not correspond to chronic musculoskeletal pain syndrome patients seen in outpatient
clinics, which raises questions about how applicable our conclusions are to the
majority of these patients.
Lastly, while this study acknowledges the presence
of promising variables, it does not explicitly explore their impact on the efficacy
of the multimodal treatment programme and pain outcomes. This limitation leaves
room for future investigations.
Future research and improvements
This study is part of a larger effort to optimise
multimodal programmes by prospectively identifying patients who best respond to
the programme and its elements. Defining what “response“ means is crucial
because chronic musculoskeletal pain syndromes are complex and highly subjective.
Although reducing pain intensity is important, this is not always achievable or
the sole objective. Independently of pain, physical dysfunction, fatigue and mood,
for instance, are important impediments to a normal life. Therefore, the characterisation
of endpoints is fundamental. Additionally, the separation of patients in clusters
is necessary to define the profiles prone to endpoint improvements. The definition
of clusters begins with the definition of clinically significant variables, and
the present study allows several insights.
Firstly, our findings highlight several key
variables for future investigation, including obesity, social isolation, psychiatric
comorbidities (depression, anxiety, post-traumatic stress disorder and enduring
personality changes after a catastrophic experience, alexithymia), pain history,
immigration, hypermobility and sleep quality. These may serve as both markers of
progress and potential endpoints, alongside subjective patient evaluations. Objective
measures such as mobility levels tracked via actigraphy should also be used in future
research.
Secondly, subjective variables should be defined
as objectively as possible and measured by more than one mean where possible. The
present work shows that this is possible, if imperfect. Thirdly, the new classification
of pain (nociplastic, nociceptive, neurologic) appears useful for research.
Our team acknowledges that the assessment of
other important dimensions is lacking and should be developed in future analysis
(e.g. intellectual and introspection capacity, autonomic nervous system status).
Conclusion
Obesity, living alone, psychiatric comorbidities,
sleep deprivation and opiate misuse stood out as common characteristics. Half of
the sample did not originate from Switzerland, and 12% originated from unstable
conflictual regions, yet they were not more prone to post-traumatic stress
disorder than Swiss patients. Additionally, many patients had experienced pain since
childhood or adolescence, which suggests that chronic musculoskeletal pain
syndromes are often rooted in early life experiences, with a significant effect
on pain impact. Multimodal treatment programmes are recognised as an efficacious
strategy to address chronic musculoskeletal pain syndromes, yet relevant endpoints
still need to be defined and long-term outcomes must still be assessed. Moreover,
a consensus on the optimal structure for multimodal treatment programmes is lacking.
Dr. Pedro Ming
Azevedo
Department of
Rheumatology
University Hospital
Lausanne (CHUV)
CH-1011
Lausanne
pedro.ming-azevedo[at]chuv.ch
References
1. Stanaway, Jeffrey D et al. Global, regional, and national comparative risk assessment
of 84 behavioural, environmental and occupational, and metabolic risks or clusters
of risks for 195 countries and territories, 1990-2017: a systematic analysis for the
Global. Lancet. 2018 Nov 10;392(10159):1923-1994.
2. St Sauver JL, Warner DO, Yawn BP, Jacobson DJ, McGree ME, Pankratz JJ, et al. Why
patients visit their doctors: assessing the most prevalent conditions in a defined
American population. Mayo Clin Proc. 2013 Jan;88(1):56–67. doi: https://doi.org/10.1016/j.mayocp.2012.08.020
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012 Aug;13(8):715–24.
doi: https://doi.org/10.1016/j.jpain.2012.03.009
4. Schopflocher D, Taenzer P, Jovey R. The prevalence of chronic pain in Canada. Pain
Res Manag. 2011;16(6):445–50. doi: https://doi.org/10.1155/2011/876306
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain
in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006 May;10(4):287–333.
doi: https://doi.org/10.1016/j.ejpain.2005.06.009
6. Mansfield KE, Sim J, Jordan JL, Jordan KP, Bedson J, Pacey V. A systematic review
and meta-analysis of the prevalence of chronic widespread pain in the general population.
Pain. 2020;161(9):2023–37.
7. Carville SF, Arendt-Nielsen L, Bliddal H, Blotman F, Branco JC, Buskila D, et al.;
EULAR. EULAR evidence-based recommendations for the management of fibromyalgia syndrome.
Ann Rheum Dis. 2008 Apr;67(4):536–41. doi: https://doi.org/10.1136/ard.2007.071522
8. Häuser W, Bernardy K, Arnold B, Offenbächer M, Schiltenwolf M. Efficacy of multicomponent
treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical
trials. Arthritis Rheum. 2009 Feb;61(2):216–24. doi: https://doi.org/10.1002/art.24276
9. Kaiser U, Treede RD, Sabatowski R. Multimodal pain therapy in chronic noncancer pain-gold
standard or need for further clarification? Pain. 2017 Oct;158(10):1853–9. doi: https://doi.org/10.1097/j.pain.0000000000000902
10. Häuser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome
- a systematic review. Eur J Pain. 2010 Jan;14(1):5–10. doi: https://doi.org/10.1016/j.ejpain.2009.01.006
11. Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Fluß E, et al. EULAR revised
recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017 Feb;76(2):318–28.
doi: https://doi.org/10.1136/annrheumdis-2016-209724
12. Nour Y, Abdelmoneem Y, Sameh G, Mohamed HE. Translation and validation of the Tampa
Scale of Kinesiophobia Arabic version in chronic low back pain. Ann Phys Rehabil Med.
2017;60 S13:e20. doi: https://doi.org/10.1016/j.rehab.2017.07.145
13. French DJ, Noël M, Vigneau F, French JA, Cyr CP, Evans RT. PCS-CF: A French-language,
French-Canadian adaptation of the Pain Catastrophizing Scale. Can J Behav Sci. 2005;37:181–92.
doi: https://doi.org/10.1037/h0087255
14. Chaory, K., Fayad, F., Rannou, F., Lefèvre-Colau M. M., Fermanian, J., Revel, M.,
Poiraudeau, S. (2004). Validation of the French version of the fear avoidance belief
questionnaire. Spine (Phila Pa 1976) 15;29(8), 908-13. doi: https://doi.org/10.1097/00007632-200404150-00018
15. Benaim, Charles, Léger, Bertrand, Vuistiner, Philippe, Luthi, François, Validation
of the French Version of the “Patterns of Activity Measure” in Patients with Chronic
Musculoskeletal Pain, Pain Research and Management, 2017, 6570394, 7 pages, 2017.
16. Bocéréan C, Dupret E. A validation study of the Hospital Anxiety and Depression Scale
(HADS) in a large sample of French employees. BMC Psychiatry. 2014 Dec;14(1):354.
doi: https://doi.org/10.1186/s12888-014-0354-0
17. Poundja, J. B. A., Fikretoglu, D., Guay, F., Brunet, A. (2007 Validation of the French
Version of the Brief Pain Inventory in Canadian Veterans Suffering from Traumatic
Stress. Journal of Pain and Symptom Management, 33 (6), Issue 6, 720-726. doi: https://doi.org/10.1016/j.jpainsymman.2006.09.031
18. Benz T, Lehmann S, Brioschi R, Elfering A, Aeschlimann A, Angst F. Comparison of short-
and mid-term outcomes of Italian- and German-speaking patients after an interdisciplinary
pain management programme in Switzerland: A prospective cohort study. J Rehabil Med.
2019 Feb;51(2):127–35. doi: https://doi.org/10.2340/16501977-2514
19. Osborne NR, Davis KD. Sex and gender differences in pain. In: Moro E, Arabia G, Tartaglia
MC, Ferretti MT, editors. International Review of Neurobiology. Vol. 164. Academic
Press; 2022. p. 277-307. doi: https://doi.org/10.1016/bs.irn.2022.06.013
20. Le couple [The couple] (2018). Swiss Federal Statistical Office. Available from https://www.bfs.admin.ch/bfs/fr/home/statistiques/population/familles/couple.html
21. Berrut S, Mosimann A, Nicolet-dit-Félix M. (2018) Enquête sur les familles et les
générations 2018 [Survey on families and generations 2018]. Swiss Federal Statistical
Office. Available from https://www.bfs.admin.ch/bfs/fr/home/statistiques/population/enquetes/efg.assetdetail.10467789.html
22. Souhait d'enfants, parentalité [desire for children, parenthood] (2018) Swiss Federal
Statistical Office. Available from https://www.bfs.admin.ch/bfs/fr/home/statistiques/population/familles/souhait-enfants-parentalite.html
23. Newton, B. J., Southall, J. L., Raphael, J. H., Ashford, R. L., & LeMarchand, K. (2013).
A narrative review of the impact of disbelief in chronic pain. Pain management nursing:
official journal of the American Society of Pain Management Nurses, 14(3), 161–171.
doi: https://doi.org/10.1016/j.pmn.2010.09.001
24. Gonder ME, Orr WN, Khan TW. The Impact of Isolation During COVID-19 on Chronic Musculoskeletal
Pain in the Geriatric Population: A Narrative Review. Pain Physician. 2022 Mar;25(2):E185–91.
25. Oliveira VC, Ferreira ML, Morso L, Albert HB, Refshauge KM, Ferreira PH. Patients’
perceived level of social isolation affects the prognosis of low back pain. Eur J
Pain. 2015 Apr;19(4):538–45. doi: https://doi.org/10.1002/ejp.578
26. Galati M, Kaeser M, Semaani C, Storni M. (2017) Excès de poids et obésité. [Overweight
and obesity]. Swiss Federal Statistical Office. Available from https://www.bfs.admin.ch/bfs/fr/home/statistiques/sante/determinants/exces-poids.html
27. Santé des migrants [Migrants and health] (2019). Swiss Federal Statistical Office.
Available from https://www.bfs.admin.ch/bfs/fr/home/statistiques/sante/etat-sante/migrants.html
28. Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res.
2015 Jul;8:399–408. doi: https://doi.org/10.2147/JPR.S55598
29. Prego-Domínguez J, Khazaeipour Z, Mallah N, Takkouche B. Socioeconomic status and
occurrence of chronic pain: a meta-analysis. Rheumatology (Oxford). 2021 Mar;60(3):1091–105.
doi: https://doi.org/10.1093/rheumatology/keaa758
30. Häuser W, Galek A, Erbslöh-Möller B, Köllner V, Kühn-Becker H, Langhorst J, et al. Posttraumatic
stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between
posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain.
2013 Aug;154(8):1216–23. doi: https://doi.org/10.1016/j.pain.2013.03.034
31. Langvik E, Hjemdal O, Nordahl HM. Personality traits, gender differences and symptoms
of anhedonia: what does the Hospital Anxiety and Depression Scale (HADS) measure in
nonclinical settings? Scand J Psychol. 2016 Apr;57(2):144–51. doi: https://doi.org/10.1111/sjop.12272
32. Nortvedt MW, Riise T, Sanne B. Are men more depressed than women in Norway? Validity
of the Hospital Anxiety and Depression Scale. J Psychosom Res. 2006 Feb;60(2):195–8.
doi: https://doi.org/10.1016/j.jpsychores.2005.07.002
33. Aaron RV, Fisher EA, de la Vega R, Lumley MA, Palermo TM. Alexithymia in individuals
with chronic pain and its relation to pain intensity, physical interference, depression,
and anxiety: a systematic review and meta-analysis. Pain. 2019 May;160(5):994–1006.
doi: https://doi.org/10.1097/j.pain.0000000000001487
34. Palermo T. M. (2020). Pain prevention and management must begin in childhood: the
key role of psychological interventions. Pain, 161 Suppl 1(Suppl), S114–S121. doi: https://doi.org/10.1097/j.pain.0000000000001862
35. Groenewald CB, Law EF, Fisher E, Beals-Erickson SE, Palermo TM. Associations Between
Adolescent Chronic Pain and Prescription Opioid Misuse in Adulthood. J Pain. 2019 Jan;20(1):28–37.
doi: https://doi.org/10.1016/j.jpain.2018.07.007
36. Hatakeyama BA, Camargo BI, Santos VS, Leite MN, Espirito Santo CM, Kamper SJ, et al. Prevalence
of disabling musculoskeletal pain in children and adolescents in Brazil: A cross-sectional
study. Braz J Phys Ther. 2024;28(1):100593. doi: https://doi.org/10.1016/j.bjpt.2024.100593
37. Murray M, Stone A, Pearson V, Treisman G. Clinical solutions to chronic pain and the
opiate epidemic. Prev Med. 2019 Jan;118:171–5. doi: https://doi.org/10.1016/j.ypmed.2018.10.004
38. Chou R, Hartung D, Turner J, et al. (2020). Opioid Treatments for Chronic Pain [Internet].
Rockville (MD): Agency for Healthcare Research and Quality (US); (Comparative Effectiveness
Review, No. 229.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK556253/
39. Ju C, Wei L, Man KK, Wang Z, Ma TT, Chan AY, et al. Global, regional, and national
trends in opioid analgesic consumption from 2015 to 2019: a longitudinal study. Lancet
Public Health. 2022 Apr;7(4):e335–46. doi: https://doi.org/10.1016/S2468-2667(22)00013-5
40. Bennett RM, Friend R, Marcus D, Bernstein C, Han BK, Yachoui R, et al. Criteria for
the diagnosis of fibromyalgia: validation of the modified 2010 preliminary American
College of Rheumatology criteria and the development of alternative criteria. Arthritis
Care Res (Hoboken). 2014 Sep;66(9):1364–73. doi: https://doi.org/10.1002/acr.22301
41. Fan A, Tournadre A, Pereira B, Tatar Z, Couderc M, Malochet-Guinamand S, et al. Performance
of Fibromyalgia Rapid Screening Tool (FiRST) to detect fibromyalgia syndrome in rheumatic
diseases. Rheumatology (Oxford). 2016 Oct;55(10):1746–50. doi: https://doi.org/10.1093/rheumatology/kew244
42. Gilhus NE, Deuschl G. Neuroinflammation - a common thread in neurological disorders.
Nat Rev Neurol. 2019 Aug;15(8):429–30. doi: https://doi.org/10.1038/s41582-019-0227-8
43. Sun Y, Laksono I, Selvanathan J, Saripella A, Nagappa M, Pham C, et al. Prevalence
of sleep disturbances in patients with chronic non-cancer pain: A systematic review
and meta-analysis. Sleep Med Rev. 2021 Jun;57:101467. doi: https://doi.org/10.1016/j.smrv.2021.101467
44. Whale K, Gooberman-Hill R. The Importance of Sleep for People With Chronic Pain: Current
Insights and Evidence. JBMR Plus. 2022a Jun;6(7):e10658. doi: https://doi.org/10.1002/jbm4.10658
45. Whale K, Dennis J, Wylde V, Beswick A, Gooberman-Hill R. The effectiveness of non-pharmacological
sleep interventions for people with chronic pain: a systematic review and meta-analysis.
BMC Musculoskelet Disord. 2022b May;23(1):440. doi: https://doi.org/10.1186/s12891-022-05318-5
Appendix: supplementary tables
Table S1Patients’ socioeconomic variables.
| Variable |
All patients |
Women |
Men |
| Sex: n (%) |
202 (100%) |
148 (73%) |
54 (27%) |
| Age |
Mean = 47, SD = 10, mode = 48, range: 23–74 |
Mean = 48, SD = 9.1, 48, [27–73 |
Mean = 44.8, SD = 11.3, mode = 36, range: 22–73 |
| Origin |
Swiss (n/total, %) |
89/202 (44%) |
71/148 (48%) |
18; 54 (33%) |
| Unknown origin with Swiss nationality (n/total,
%) |
12/202 (6%) |
7/148 (5%) |
5/54 (9%) |
| Other European countries (n/total, %) |
80/202 (40%) |
53/202 (26%) |
27/202 (13%) |
| Non-Europeans (n/total, %) |
21/202 (10%) |
17/148 (11%) |
4/54 (7%) |
| Conflict zones (n/total, %) |
25/202 (12%) |
15/148 (7%) |
10/54 (18%) |
| Reported traumatic war experience (n/total,
%) |
6/27 (22%) |
3/17 (18%) |
3/10 (30%) |
| Living in couple (n/total, %) |
121/202 (60%) |
85/148 (57%) |
36/54 (67%) |
| Having children (n/total, %) |
137/202 (68%) |
107/148 (72%) |
30/54 (56%) |
| Disability insurance status (n = 198) |
Not pursued (n/total, %) |
62/198 (31%) |
46/144 (32%) |
16/54 (30%) |
| Pursued (n/total, %) |
136/198 (69%) |
98/144 (68%) |
38/54 (70%) |
| |
Under consideration (n/total, %) |
92/198 (45%) |
64/144 (44%) |
28/54 (52%) |
| Received (n/total, %) |
27/198 (13%) |
22/144 (15%) |
5/54 (9%) |
| Denied (n/total, %) |
17/198 (8%) |
12/144 (8%) |
5/54 (9%) |
| Time off work in years: median, mode,
[range] |
Median = 1.5, mode = 0, range: 0–30 |
Median = 1.1, mode = 0, range: 0–30 |
Median = 2, mode = 0, range: 0–13 |
Table S2Clinical variables
studied and definitions.
| Domain |
Variable |
Definition and measurement |
| Demographics |
Age |
Age in years at multimodal treatment programme admission. |
| Sex |
Only birth sex was computed (women and men). |
| Relationship status |
Choice between “in couple” or “not in couple”. |
| Parenthood status |
Choice between “having children“ or “not having children”. |
| Disability status (DI) insurance |
Choice between DI “received“, “denied“ or “under consideration“.
“Received“ DI being rediscussed was considered “under consideration“. |
| Time off work |
Time in years from the moment patient lost or quit
their job to the date of multimodal treatment programme admission. No distinction
was made between whether the patient lost the job because of pain or not (impossible
to differentiate in most cases). |
| Physical characteristics |
BMI |
This was tested as a continuous variable and as a
categorical variable according to the World Health Organization (<18.5 = underweight,
18.5 to <25 = healthy, 25.0 to <30 = overweight, 30.0 or higher = obesity).
|
| Menopausal status |
Women >55 years old were automatically considered
menopaused, and those <40 years old were considered non-menopaused. FSH and
oestrogen levels were tested between 40 and 55 years of age. |
| Hypermobility |
The Beighton score was tested as a continuous variable.
However, the Beighton score highly disagreed with past diagnoses of hypermobility.
Thus, patients were considered hypermobile if this diagnosis was previously made
by a medical doctor or if they had a history of sprains and subluxations in childhood
or adolescence and referred to themselves as more flexible than their peers then. |
| Biomechanical disorders |
Musculoskeletal disorders due to persistent
biomechanical overload. This variable was considered present when the final diagnosis
appointed this mechanism. |
| Comorbidities |
Autoimmune rheumatism |
Any previous diagnosis of autoimmune rheumatic condition,
regardless of potential effect on pain. This included rheumatoid arthritis, Sjögren’s
syndrome, lupus, undifferentiated connective tissue disease, and axial and peripheric
spondylarthritis. At the entry, these conditions were considered “suspected“ and
reclassified as “confirmed“ or “not confirmed“ after clinical investigation during
hospitalisation. |
| Peripheral significant osteoarthritis (OA) |
Patients were considered to have significant peripheral
OA when complaints of pain or physical limitation were attributed to non-spinal
OA. |
| Airway comorbidities |
This included any previous diagnosis of COPD, asthma
or emphysema (other diseases were absent in our sample). |
| Metabolic disease |
Including previous diagnosis of diabetes, asymptomatic
high uric acid levels, gout, calcium pyrophosphate deposition disease, dyslipidaemia. |
| Depression or anxiety |
Hospital Anxiety Scale and Hospital Depression Scale
were tested as a continuous or categorical variable (according to the official
cutoff of 10 for these scales). The diagnosis for both conditions was determined
by the MMP’s psychiatrists according to the DSM-5 definitions. |
| Post-traumatic stress disorder and enduring personality
changes after a catastrophic experience |
The diagnosis for post-traumatic stress disorder and
enduring personality changes after a catastrophic experience was determined by
the MMP’s psychiatrists according to the DSM-5 and ICD-10 definitions (respectively). |
| Other psychiatric conditions |
Relevant disorders in this category included bipolar
and personality disorders (DSM-5 definitions) and alexithymia. The diagnosis of
these conditions was determined by the MMP’s psychiatrists. Alexithymia was also
tested by the Toronto Alexithymia Scale-20 (TAS-20), which was tested as a continuous
and categorical variable (according to the author’s guidelines). Whenever discordance
existed between the TAS-20 diagnosis and the psychiatric diagnosis, the latter
was considered correct. |
| Pain characterisation |
Localisation |
Back pain |
Back pain corresponded to dorsal pain or lumbar pain.
Cervical pain alone was not considered back pain. |
| Peripheral pain |
Pain was called “peripheral“ when it was not spinal,
visceral or in the head. An attempt was made to pinpoint the source of pain (e.g.
neuropathies, arthrosis, enthesopathies, bursitis, tendinopathies, arthritis). |
| Types of pain |
An effort was made to classify each referred pain
into three categories: neuropathic (either radicular or peripheral), nociceptive
and nociplastic pain. The latter was diagnosed when the pain itself, its characteristics
or its intensity could not be attributed to a neuropathic or nociceptive process.
“Functional“ pain was considered equal to nociplastic pain. These categories were
not mutually exclusive. |
| Pain in childhood and adolescence |
Patients were questioned about recurrent or persistent
pain in childhood and adolescence. All answers were noted, but only pain with
significant functional impact (from the patient’s viewpoint) was counted for statistical
analysis. |
| Medications |
Opiates |
Opiates were initially classified as “weak“ (tramadol,
codeine and tapentadol) and “strong“ (all others) for future testing in outcome
association studies. However, this subdivision is polemic and led to small N sizes
in each group so all opioids were analysed together in this study. |
| Antidepressants |
Antidepressants were further classified as tricyclics,
tetracyclics, duals, selective serotonin reuptake inhibitors, atypical and vilazodone.
Mirtazapine was classified among the tetracyclic antidepressants. Trazodone is
rarely used as an antidepressant and thus was analysed separately. Due to their
similar profiles and the scarcity of data, tricyclics and tetracyclics were analysed
together. |
| Anticonvulsants and gabapentinoids |
No patient used anticonvulsants for seizure control.
In all cases, they were being used to control neuropathic pain. Gabapentinoids
were also used in the control of nociplastic pain. Because of the small sample
size, both were analysed together. |
| Z-drugs and benzodiazepines (BZD) |
Despite their action on benzodiazepine receptors,
the “Z-drugs“ (zopiclone, eszopiclone, zaleplon and zolpidem) have different profiles
and often different uses so the two were first analysed separately. Due to the
small sample, these medications were also tested together. |
| Non-opioid analgesics |
This class included paracetamol and metamizole. |
| Immunosuppressants |
Included classical and selective synthetic disease-modifying
antirheumatic drugs and biologics of any kind. Prednisone was considered separate. |
| Myorelaxants |
Tizanidine and tolperisone were the only specimens
of this class present in our sample. |
| Others |
The use of non-steroidal anti-inflammatory drugs (NSAIDs)
and neuroleptics was also analysed. |
| Laboratory work |
Acute phase reactants |
C-reactive protein (CRP) and erythrocyte sedimentation
rate (ESR) were systematically analysed and helped clinical judgment on whether
an active inflammatory condition was directly (“inflammatory pain“) or indirectly
(“secondary fibromyalgia“) causing pain. |
| Markers of metabolic diseases |
Markers of metabolic diseases such as cholesterol,
triglycerides, glycosylated haemoglobin, thyroid-stimulating hormone and uric
acid levels were not systematically performed but were used when available. In
practice, these results never led to a new musculoskeletal disorder diagnosis
in our sample. |
| Rheumatic disease markers |
All rheumatic disease markers were tested when clinically
appropriated to define or refine the diagnosis. |
| Virtual reality (VR) response |
Response to VR (Visual Analogue Scale of pain and
anxiety before and after VR treatment) was analysed. |
Table S3Laboratory results.
| Laboratory marker |
All patients |
Women |
Men |
| C-reactive protein |
Total tested |
n/total = 187/202 (93%), median = 1, mode = 0, range: 0–37 |
n/total = 139/148 (94%), median = 2, mode = 0, range: 0–37 |
n/total = 48/54 (89%), median = 1, mode = 0, range: 0–8 |
| Abnormal (n/total, %) |
129/187 (69%) |
96/139 (69%) |
33/8 (69%) |
| >5 mmol/l (n/total, %) |
27/187 (14%) |
22/139 (23%) |
5/48 (10%) |
| >10 mmol/l (n/total, %) |
11/187 (6%) |
11/139 (11%) |
0/48 (0%) |
| Sedimentation rate |
n/total = 177/202 (88%), median = 7, mode = 6, range: 1–63 |
n/total = 132/148 (89%), median = 11, mode = 6, range: 1–63 |
n/total = 45/54 (83%), median = 4, mode = 1, range: 1–33 |
| Anti-nuclear antibodies: n/total (%) |
Total tested |
136/202 (67%) |
107, 148 (72%) |
29, 54 (54%) |
| Present |
34/136 (25%) |
28, 107 (26%) |
6, 29 (21%) |
| Rheumatoid factor: n/total (%) |
Total tested |
134/202 (66%) |
104/148 (70%) |
30/54 (56%) |
| Present |
1/134 (1%) |
1/104 (1%) |
0/30 (0%) |
| Anti-cyclic citrullinated peptide: n/total
(%) |
Total tested |
103/202 (51%) |
77/148 (52%) |
26/54 (48%) |
| Present |
2/103 (2%) |
1/77 (1%) |
1/26 (4%) |
| Human leucocyte antigen B27 n/total (%) |
Total tested |
71/202 (35%) |
45/148 (30%) |
26/54 (48%) |
| Present |
10/71 (14%) |
8/45 (18%) |
2/26 (8%) |
Table S4Sleep analysis according to actigraphy.
| Sleep analysis |
All patients |
Women |
Men |
| Sleep time (hours) |
n/total, median, mode,
[range] |
173/202, 08h10”, 07h27” [04:40–12:04] |
134/148, 08h16”, 08h09”, [04:41–12:04] |
39, 07h44”, 07h27”, [04:40–11:11] |
| <6 h: n/total (%) |
6/173 (3%) |
2/134 (1%) |
4/39 (10%) |
| Efficacy <85%: n/total (%) |
82/173 (47%) |
63/134 (47%) |
19/39 (49%) |
| Fragmentation index >20: n/total (%) |
135/173 (78%) |
103/134 (77%) |
32/39 (82%) |
| Delayed sleep phase: n/total (%) |
35, 173 (20%) |
24, 134 (18%) |
11, 39 (28%) |
