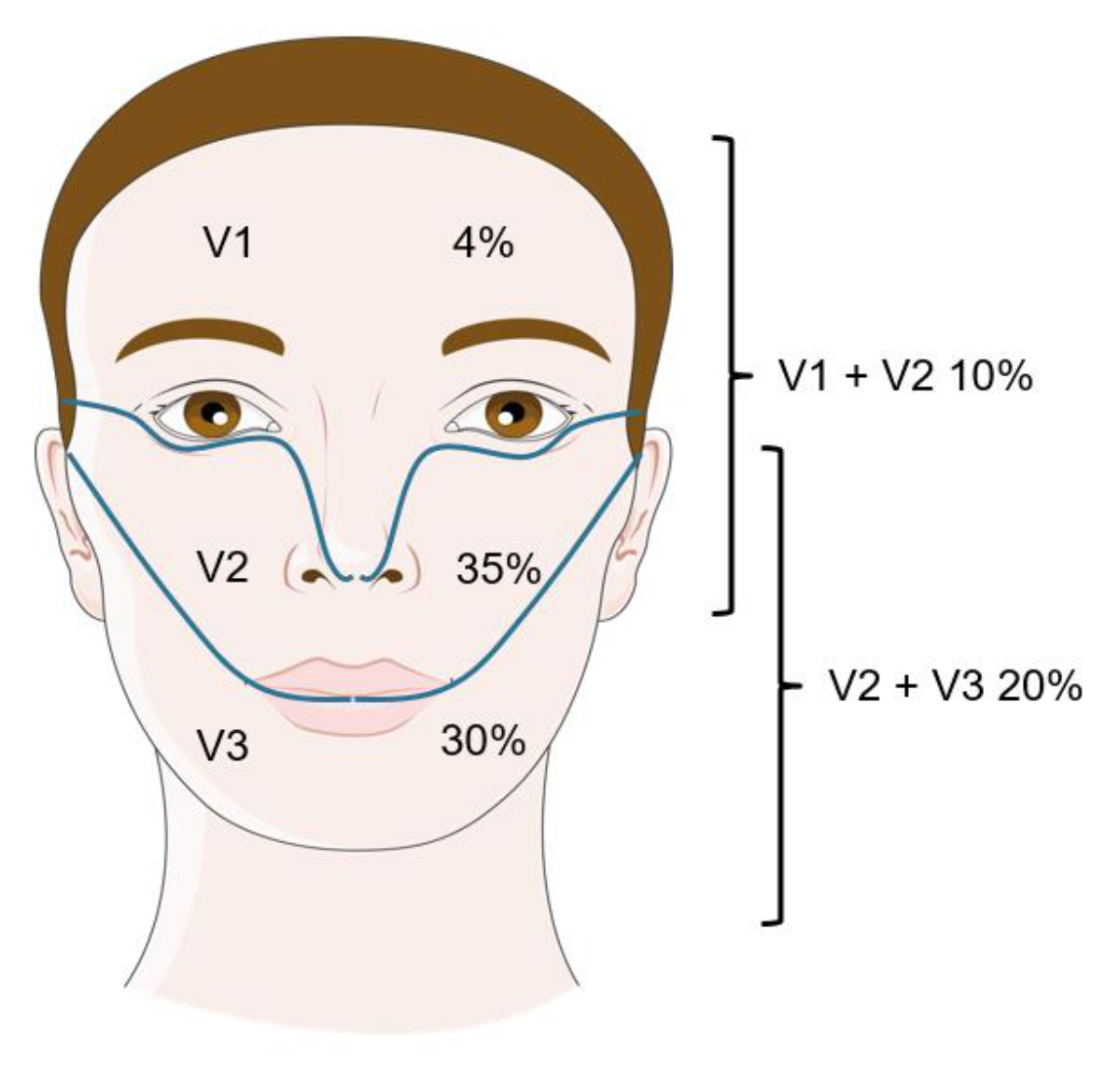
Figure 1Distribution of trigeminal neuralgia. Figure modified with text, markings and annotation after adaptation of “Feeling” from Servier Medical Art by Servier, licensed under a CC BY 4.0 license.
DOI: https://doi.org/https://doi.org/10.57187/s.3460
Trigeminal neuralgia is a recurring, paroxysmal and unilateral facial pain in an area innervated by one or more branches of the trigeminal nerve.
The annual incidence of trigeminal neuralgia is about 4.3–27 per 100,000, with a lifetime prevalence of 160–300 per 100,000. Women are twice as likely to be affected as men (F:M 2:1) and the average age of onset for classic trigeminal neuralgia is 53 years [1, 2]. Onset before age 50 suggests a secondary aetiology. Although some isolated cases within families imply a hereditary form of this condition, the genetic basis is still not well understood.
The “ignition hypothesis” stipulates that trigeminal neuralgia is due to a lesion of myelinated afferent trigeminal neurons in the trigeminal root or ganglion, leading to hyperexcitability and synchronised after-discharge activity that result in pain paroxysms [3].
In aetiological terms, classic, symptomatic and idiopathic trigeminal neuralgia are defined. Classic trigeminal neuralgia (approximately 85% of all cases) is characterised by pathological neurovascular contact, with compression of the trigeminal nerve by the superior cerebellar artery accounting for the majority of cases (75–80%). Compression by a vein is less common [4].
Symptomatic trigeminal neuralgia (10–15% of cases) may be caused by multiple sclerosis (MS), brainstem ischaemia and space-occupying lesions (tumours in the cerebellopontine angle, including schwannomas and metastases). MS patients have a 20-fold increased risk of developing trigeminal neuralgia, with a prevalence of 4% in this group [5]. The MS-associated form often occurs at a younger age, is frequently associated with sensory deficits and is bilateral in 18% of cases [6].
When a comprehensive diagnostic work-up fails to identify an underlying cause, the condition is classified as idiopathic.
The main symptom of trigeminal neuralgia is a sudden, shooting facial pain in the distribution of one or more trigeminal nerve branches. The second branch (V2, maxillary nerve) is most commonly affected (35%), followed by the third branch (V3, mandibular nerve; 30%), with the right side being somewhat more frequently affected (about 60%, the reason for this laterality remains unknown). Simultaneous occurrence in V2 and V3 is not uncommon (20%), even in classic trigeminal neuralgia. Isolated trigeminal neuralgia in the V1 area (ophthalmic nerve) is found in only 1–5% of patients and is particularly frequent in symptomatic trigeminal neuralgia [7, 8] (figure 1). Side switching does not occur in classic trigeminal neuralgia and is only observed in secondary trigeminal neuralgia.

Figure 1Distribution of trigeminal neuralgia. Figure modified with text, markings and annotation after adaptation of “Feeling” from Servier Medical Art by Servier, licensed under a CC BY 4.0 license.
The sudden electric shock-like pain lasts from a fraction of a second to a maximum of two minutes (usually only a few seconds) and is among the most painful conditions reported [2]. It can occur as a single attack or in a series of up to 100 times a day and can be triggered by innocuous stimuli such as chewing, speaking or touch (e.g. while washing one’s face or brushing one’s teeth). Usually, the pain is not present between attacks occurring in a series (refractory period). Spontaneous onset without any existing trigger factors is also possible. In addition, approximately 14–50% of affected individuals report developing moderately intense persistent pain during the disease. Other neurological deficits such as hearing loss, absence of corneal reflex or weakness of facial muscles are suggestive of a symptomatic aetiology, necessitating a comprehensive multidisciplinary work-up [2, 8, 9] (table 1). Trigeminal-autonomic symptoms, such as tearing, are also not uncommon and can be observed in one in three patients [2].
Table 1“Red flags” for the presence of secondary trigeminal neuralgia [2, 9].
| Known diagnosis such as multiple sclerosis or tumour |
| Onset before the age of 50 |
| Symptoms in the area of the first branch (V1) |
| Bilateral symptoms or symptoms that change sides |
| Significant sensory disturbances in the affected area |
| Other neurological symptoms |
In clinical practice, the diagnosis is made based on the clinical presentation and the diagnostic criteria outlined in ICHD-3 (International Classification of Headache Disorders, 3rd edition) [10, 11] (table 2).
Table 2Diagnostic ICHD-3 criteria (the international classification of headache disorders 3rd edition) for trigeminal neuralgia (ICHD-3 13.1.1) [11].
| Recurrent paroxysms of unilateral facial pain in the distribution(s) of one or more divisions of the trigeminal nerve, with no radiation beyond*, and fulfilling criteria B and C. |
| A. Pain has all of the following characteristics: |
| 1. Lasting from a fraction of a second to 2 minutes** |
| 2. Severe intensity*** |
| 3. Electric shock-like, shooting, stabbing or sharp in quality |
| B. Precipitated by innocuous stimuli within the affected trigeminal distribution**** |
| C. Not better accounted for by another ICHD-3 diagnosis. |
* In a few patients, pain may radiate to another division, but it remains within the trigeminal dermatomes.
** Duration can change over time, with paroxysms becoming more prolonged. A minority of patients will report attacks predominantly lasting for >2 minutes.
*** Pain may become more severe over time.
**** Some attacks may be, or appear to be, spontaneous, but there must be a history or finding of pain provoked by innocuous stimuli to meet this criterion. Ideally, the examining clinician should attempt to confirm the history by replicating the triggering phenomenon. However, this may not always be possible because of the patient’s refusal, awkward anatomical location of the trigger and/or other factors.
Every patient with suspected trigeminal neuralgia requires imaging to distinguish between classic trigeminal neuralgia and secondary causes. The current gold standard is magnetic resonance imaging (MRI), including high-resolution constructive interference in steady-state (CISS) sequences to exclude neurovascular compression, T2 sequences to detect possible inflammatory processes and angiography [9, 10] (figures 2, 3, 4). When assessing a possible pathological neurovascular contact, it is important (for planning potential surgical procedures) to distinguish between nerve displacement and atrophy [12].
Studies show that 25–85% of the population have neurovascular contact, which in most cases does not lead to trigeminal neuralgia [13]. To avoid bias, some authors have recommended not disclosing to the radiologist which side is affected [14].
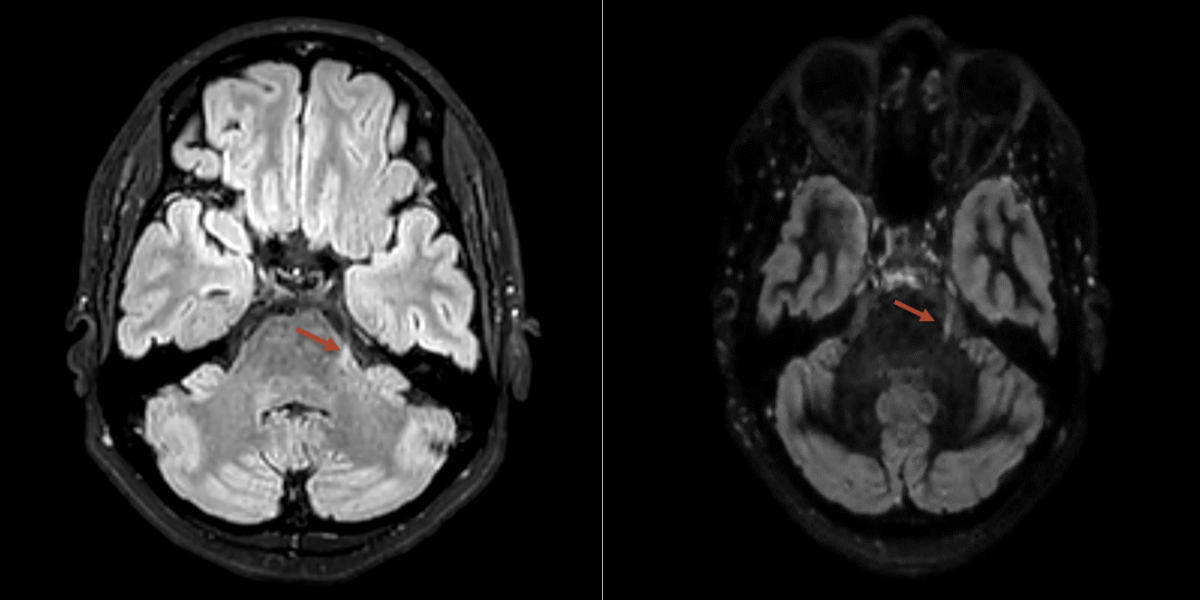
Figure 2MRI showing an multiple sclerosis lesion at the exit of the left trigeminal nerve in the area of the lateral pons (red arrow).
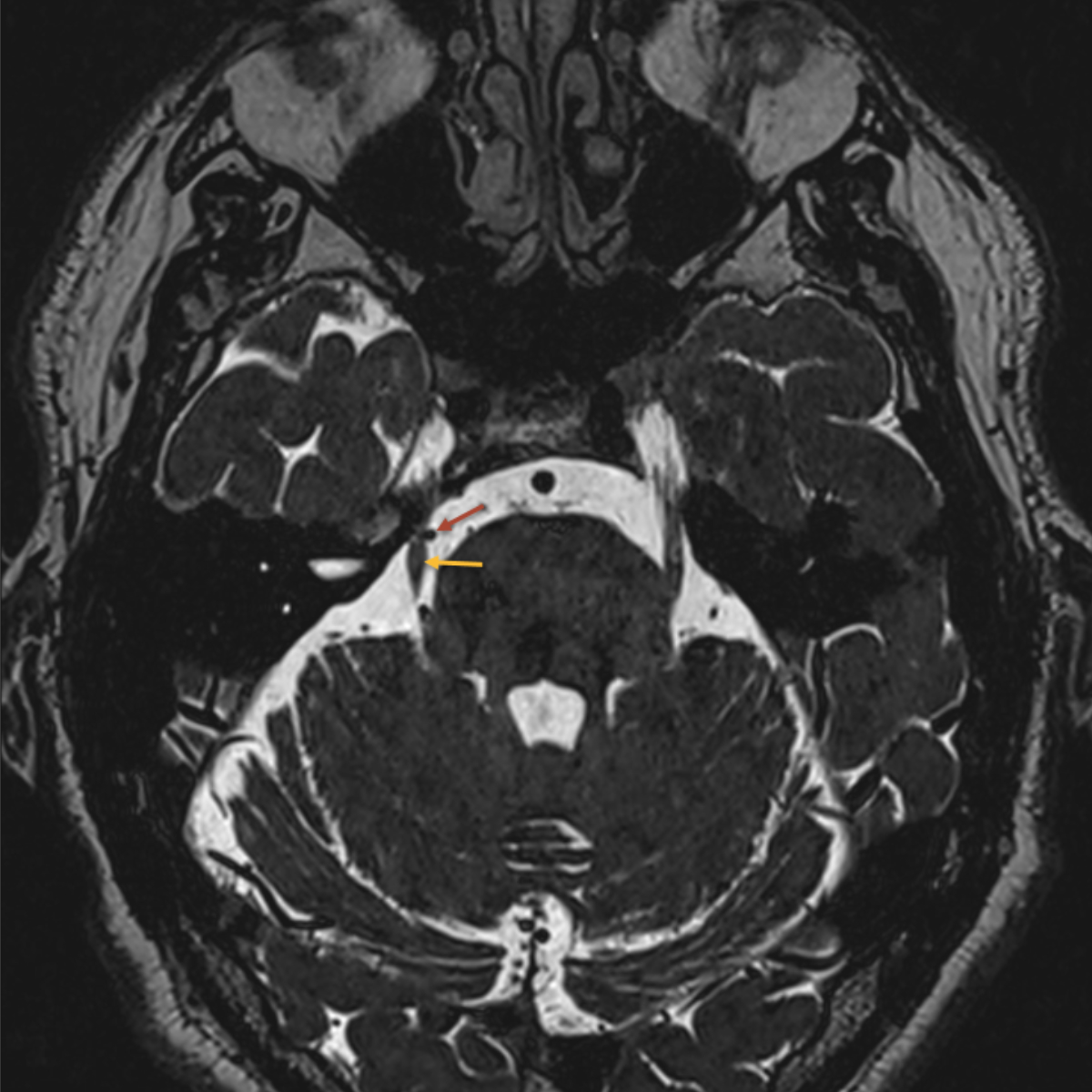
Figure 3MRI showing pathological neurovascular contact between the right superior cerebellar artery (red arrow) and the trigeminal nerve (yellow arrow).
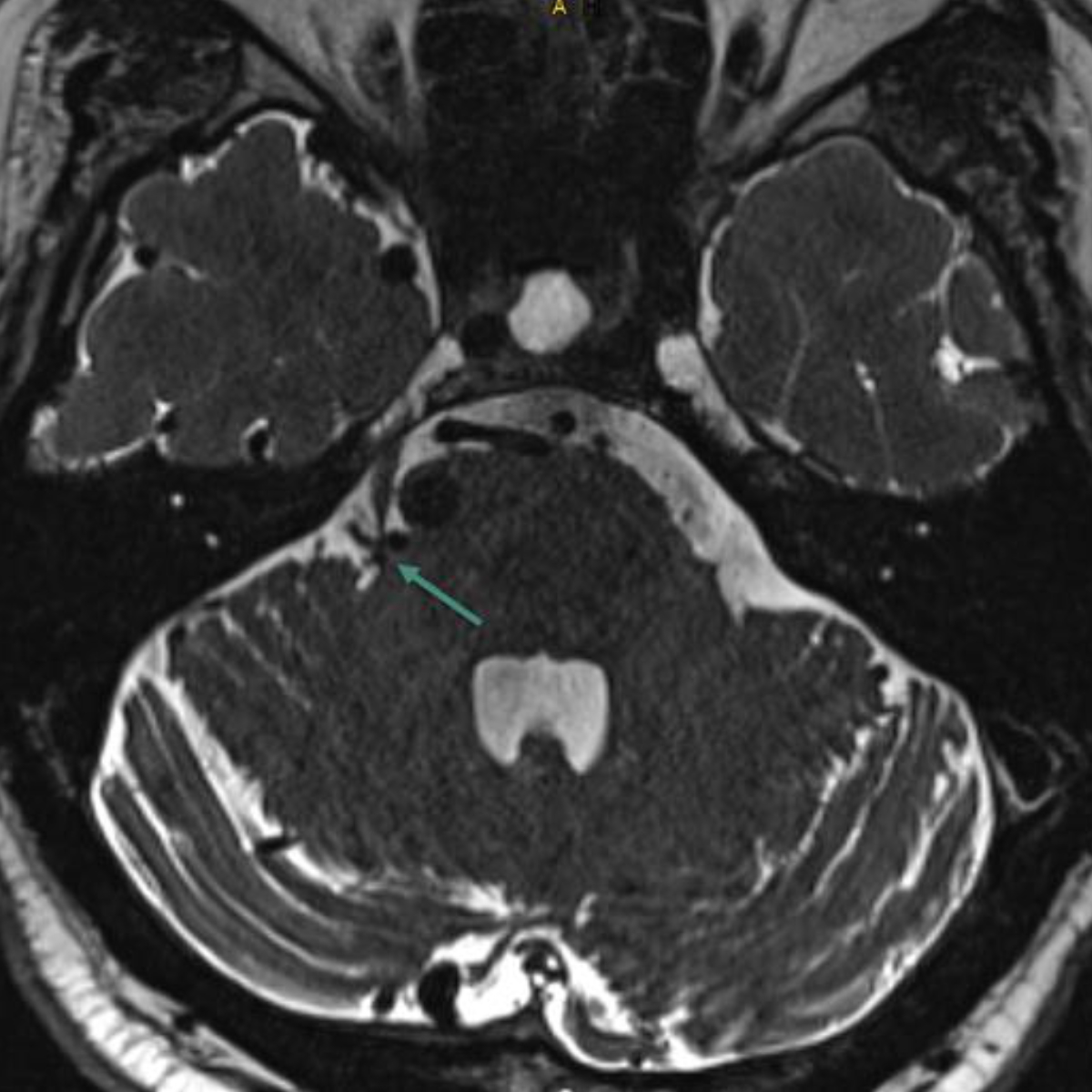
Figure 4MRI showing pathological neurovascular contact of the right trigeminal nerve at the root entry zone due to a dilated vein associated with an arteriovenous malformation (green arrow).
If an inflammatory cause is suspected, a diagnostic lumbar puncture should be performed, along with tests for immunological markers of lupus erythematosus, Sjögren's syndrome and panarteritis nodosa. In addition, tests for borrelia, toxoplasmosis and HIV titers are indicated, as well as measurement of ACE, sIL-2R and vitamin B12 levels.
In most cases, the combination of clinical findings and MRI imaging is considered sufficient for achieving an accurate diagnosis. However, in cases of diagnostic uncertainty or when MRI is not possible, neurophysiological diagnostics can be performed to demonstrate an affection of the trigeminal branch (trigeminal somatosensory evoked potential [SEP] to assess V2 and V3; blink reflex to assess V1) (figure 5).
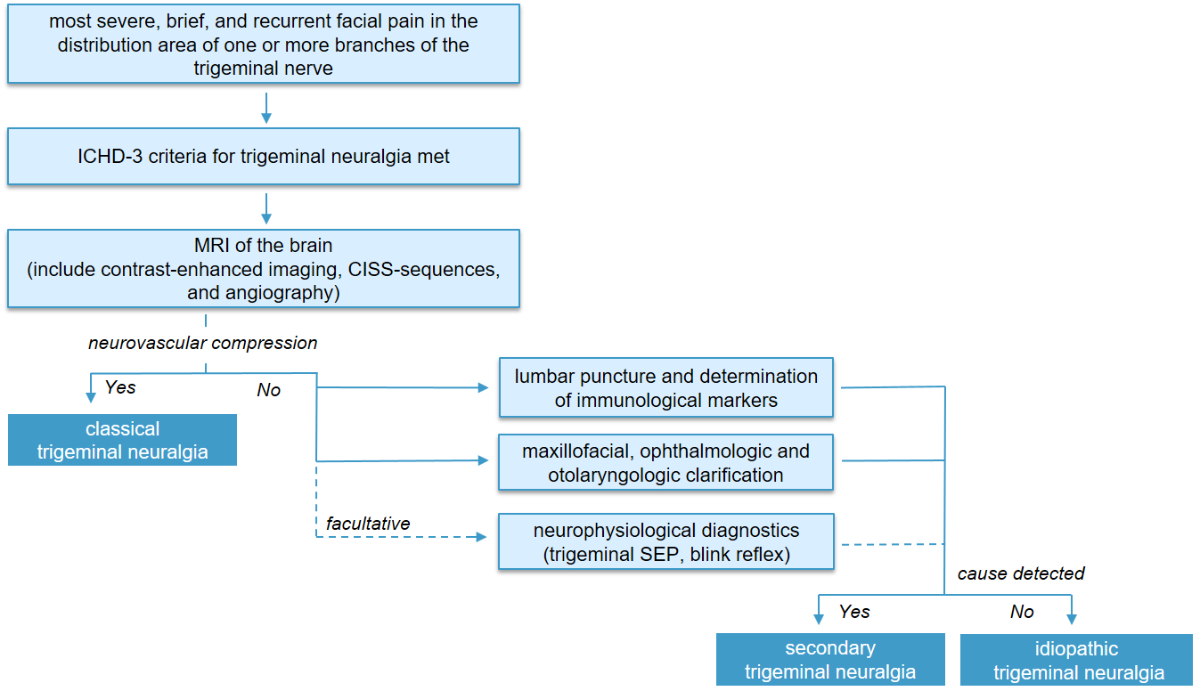
Figure 5Diagnostic work-up for suspected trigeminal neuralgia.
CISS: constructive interference in steady-state; ICHD-3: International Classification of Headache Disorders, 3rd edition; MRI: magnetic resonance imaging; SEP: somatosensory evoked potential.
Although the clinical presentation of trigeminal neuralgia is very distinctive, differential diagnoses must be considered. The most important ones include persistent idiopathic facial pain, paroxysmal hemicrania, Short-lasting Unilateral Neuralgiform headache with Conjunctival injection and Tearing (SUNCT syndrome), trigeminal neuropathy and cluster headache (table 3). Trigeminal neuropathy, characterised by a burning and persistent pain of moderate intensity in the trigeminal area caused by neural damage, is discussed in more detail below [10, 11].
Table 3Main differential diagnoses of trigeminal neuralgia [10, 11].
| Diagnosis | ICHD-3 | Brief description |
| Paroxysmal hemicrania | 3.2 | Unilateral orbital, supraorbital and/or temporal pain attacks lasting 2 to 30 minutes, occurring multiple times a day with accompanying symptoms of lacrimation, nasal congestion, rhinorrhoea, sweating in the forehead and face, miosis, ptosis and/or lid oedema. Good response to indomethacin. |
| SUNCT syndrome | 3.3 | Spontaneous or touch-triggered stabbing orbital, supraorbital and/or temporal pain accompanied by ipsilateral autonomic symptoms. Unlike trigeminal neuralgia, the pain can switch sides. |
| Cluster headache | 3.1 | A very severe, episodic, strictly unilateral pain in the orbital and/or temporal region lasting from 15 to 180 minutes, accompanied by ipsilateral autonomic symptoms. |
| Persistent idiopathic facial pain | 13.12 | Ongoing facial pain occurring for more than 2 hours per day over more than 3 months; no focal neurological deficits. |
| Painful trigeminal neuropathy | 13.1.2 | A dull, burning, persistent pain of moderate intensity in the trigeminal area; additional brief paroxysmal pain attacks are possible. Sensory disturbances (from anaesthesia to allodynia) are typical and usually much more pronounced than in trigeminal neuralgia. |
ICHD-3: International Classification of Headache Disorders, 3rd edition; SUNCT: Short-lasting Unilateral Neuralgiform headache with Conjunctival injection and Tearing.
When headaches and facial pain occur together, differential diagnoses in the otorhinolaryngology (ORL) field should be considered, especially in cases of atypical presentation. The most common cause is sinusitis. Acute rhinosinusitis mostly causes throbbing, sometimes positional and often unilateral pain, although it can also radiate to the areas of trigeminal branches V1 and V2. Sinus pain is generally associated with at least one other nasal symptom, such as nasal obstruction or rhinorrhoea. However, these symptoms can also occur secondarily with non-sinus-related pain. Less commonly, pain in the trigeminal supply area can be caused by infiltrating malignancies or compressive space-occupying lesions close to the nerves, such as adenoid cystic carcinomas or extensive tumours in the pterygopalatine fossa. However, in most cases, imaging can rule out an ORL cause as a differential diagnosis. A dental or maxillofacial surgical examination should be performed, as acute or chronic inflammatory conditions of the teeth or jaw can be confused with trigeminal neuralgia symptoms. Therefore, good communication between treating disciplines is necessary [15]. Many patients with facial pain initially seek dental care and only after unsuccessful dental treatment with persistent symptoms are referred to a neurologist or neurosurgeon. For this reason, it is crucial to know whether the pain in the trigeminal region was already present before the tooth surgery or whether it occurred afterwards.
Trigeminal neuralgia may also be caused by rheumatological conditions. For example, the incidence of trigeminal neuralgia in systemic sclerosis (SSc) is 5.8 per 1000 person-years, which is considerably higher than that reported in the general population [16, 17]. Inflammatory myositis and inflammatory arthritis are also significantly associated with trigeminal neuralgia, and there are trends suggesting associations with fibrotic manifestations of the disease, including diffuse cutaneous involvement and interstitial lung disease (ILD) [18]. Cases of trigeminal neuralgia and carpal tunnel syndrome preceding the diagnosis of SSc have also been reported [19–21].
Furthermore, trigeminal neuralgia is the most common neurological manifestation of mixed connective tissue disease (MCTD) and may be the only symptom. Trigeminal neuralgia may be the presenting symptom of systemic autoimmune diseases, thus early recognition is important to avoid significant delays in diagnosis and treatment [18].
Due to the specific nature of the pain, commonly used pain scales such as the numerical rating scale (NRS) or visual analogue scale (VAS) are not optimal for objectifying trigeminal neuralgia. In particular, they do not consider the specificity of the symptoms (such as triggers and daily life impairments) and do not provide a complete overview of the disease course.
The relatively new and not widely used Penn Facial Pain Scale-Revised (Penn-FPS-R) scale, specific to trigeminal neuralgia, provides a better method for assessing the quality of life of affected patients and monitoring treatment success [22]. The scale takes into account nine areas of daily life in which people with trigeminal neuralgia are often restricted, including “speaking”, “self-care”, “eating”, “eating hard foods/chewing food”, “daily activities”, “activities with temperature changes”, “touching”, “mood” and “relationships”. It is characterised by disease-specific questions, practical application in oral or written form and comprehensive evaluation. Regular assessments using Penn-FPS-R yield detailed information about the disease course and treatment effectiveness.
Another option is the Brief Pain Inventory-Facial (BPI-Facial) scale [23], which although not specific to trigeminal neuralgia provides a good overview of facial pain. BPI-Facial consists of 18 questions divided into three groups (pain intensity; interference with general activities; interference with facial-specific activities) and uses the classic NRS scale for quantification. The BPI-Facial scale is very simple, easy to evaluate and quick to administer.
Emotional factors can have a significant impact on the intensity and frequency of trigeminal neuralgia. Severe stress can exacerbate symptoms, leading to increased intensity and frequency of pain attacks, as well as shorter intervals between them [24]. For this reason, adopting a holistic approach is essential. The emotional and psychological wellbeing of patients and their overall quality of life can be significantly improved by integrating psychological support, including pain-coping techniques, stress management, and education on avoiding triggers and preventing secondary complications due to non-physical behaviours such as chewing or talking. In addition, targeted physiotherapeutic interventions, such as isometric neck exercises, transcutaneous electrical stimulation (TENS) and interferential therapy (IFT), can play an important supportive role alongside pharmacological therapy (figure 6).
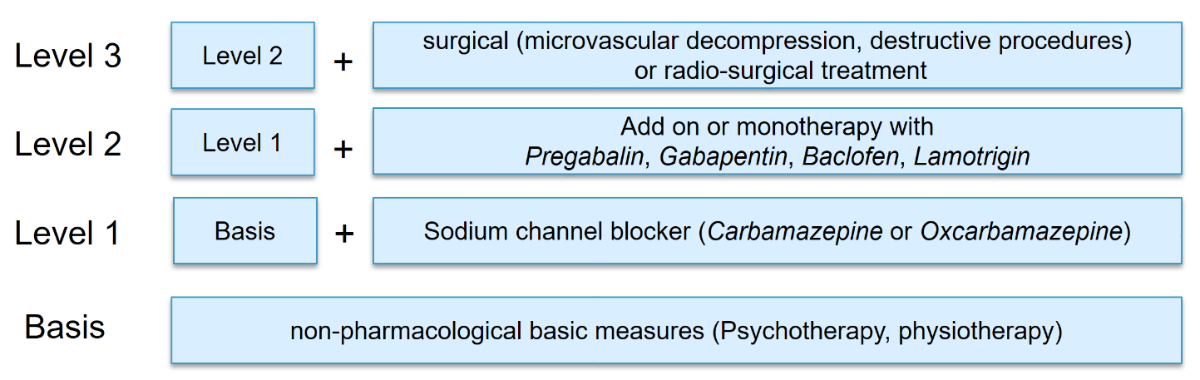
Figure 6Stepwise treatment of trigeminal neuralgia.
In view of the high pain burden, it is necessary to initiate prophylactic treatment to reduce the frequency and severity of attacks.
Sodium-channel blockers such as carbamazepine and (off-label) oxcarbazepine are considered first-line therapy in the pharmacological treatment of trigeminal neuralgia [14]. The initial response rate is approximately 90% and adequate pain relief often occurs within a few weeks of starting treatment. However, long-term efficacy drops to around 50%, meaning higher doses are required to achieve the same clinical effect.
Switching from carbamazepine to oxcarbazepine is recommended, particularly in cases of good efficacy but pronounced side effects or interactions (treatment can be switched directly to the equipotent dose of carbamazepine: the recommended carbamazepine-to-oxcarbazepine conversion ratio is 1:1.5) [10].
In order to minimise the risk of unwanted side effects, treatment with both medications should be started at a low dose and slowly increased. The daily dose should be divided into at least two doses (table 4).
Table 4First-line medicinal long-term treatment for trigeminal neuralgia.
| Medication | Dose |
| Carbamazepine | Start with 2 × 100 mg/day, increase by 100 mg/week, maximum dose of 1200 mg/day. If using high doses, divide into multiple doses throughout the day. Dose reduction may be necessary for older patients. |
| Oxcarbamazepine off-label use | Start with 2 × 300 mg/day, increase by 300 mg/week, maximum dose of 1800 mg/day. If using high doses, divide into multiple doses throughout the day. Dose reduction may be necessary for older patients. |
The most common side effects of both medications are dizziness, fatigue and nausea. Hyponatraemia and leukopenia are frequent. For this reason, serum level monitoring (for carbamazepine: therapeutic range is 4 to 12 µg/ml; toxic range >15 µg/ml) and laboratory monitoring of sodium and liver values are necessary during treatment (weekly at the beginning of treatment, then every six months after complete titration). During the first weeks of treatment, the possibility of severe side effects such as Stevens-Johnson syndrome and toxic epidermal necrolysis should also be considered. Stevens-Johnson syndrome is a life-threatening condition that manifests as painful ulcerations, high fever, body aches and possibly toxic epidermal necrolysis with epidermolysis. Specific genetic variants increase the risk of severe side effects. For example, the HLA allele B1502, which is particularly prevalent in Han Chinese populations (8/1,000,000 or 3–4 times more common than in the European population), can lead to carbamazepine-induced Stevens-Johnson syndrome [25]. Moreover, in individuals with the known HLA allele A3101 (2–5% of the North European population), the risk of severe side effects increases from 5.0% to 26.0% [26]. In our institution, genetic testing is only recommended if there is a specific suspicion, for example, due to ethnicity or a clinical history of similar skin reactions.
If treatment with first-line medications is not successful, combination therapy (or monotherapy) with second-line drugs such as pregabalin, gabapentin, lamotrigine and baclofen should be considered [14] (table 5). If one add-on therapy proves ineffective, another can be evaluated. In our experience, a combination of three or more medications is generally not advisable, but may be considered in exceptional cases of therapy-resistant forms of trigeminal neuralgia.
Table 5Second-line medicinal long-term treatment for trigeminal neuralgia.
| Medication | Dose |
| Baclofen | Starting with 5 mg 0-0-1, target dose 20–60 mg/day. |
| Gabapentin | Starting with 100 mg 1-1-1, target dose 2400–3600 mg/day. |
| Pregabalin | Starting with 25–75 mg 1-0-1, target dose 300–600 mg/day. |
| Lamotrigine | Starting with 25 mg 0-0-1, target dose 200 mg/day. |
The latest guidelines of the European Academy of Neurology also recommend botulinum toxin as an effective and safe second-line treatment. In published clinical trial data, 25–75 IU of onabotulinumtoxin A were injected mainly subcutaneously – with additional submucosal injections for intraoral pain – and distributed over up to 15 points in the painful area (2.5–5 IU per point) [27–29]. The treatment response was first observed after about 1–2 weeks. Secondary trigeminal neuralgia showed a comparable response to treatment compared with classic and idiopathic forms, regardless of patient age [30, 31].
Third-line pharmacological prophylaxis includes levetiracetam, lacosamide, valproate and the use of local anaesthetics such as capsaicin or ambroxol cream, but experience with their use is still limited and their effectiveness appears to be moderate.
According to a recent randomised trial, the calcitonin gene-related peptide antagonist erenumab is ineffective in treating trigeminal neuralgia [32].
There is currently a new sodium-channel blocker in phase 3 clinical trials for the treatment of trigeminal neuralgia: vixotrigine. The drug selectively targets the voltage-gated sodium channel Nav1.7 and, importantly, does not require titration in clinical practice. In previous studies, the rate of side effects was also significantly lower compared to carbamazepine and oxcarbazepine [9, 33].
There are no randomised controlled trials evaluating the acute medical treatment of exacerbated trigeminal neuralgia. While common analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and opiates, are usually ineffective, some medications have shown efficacy in managing acute pain and are often used off-label for this purpose [10].
One of the most commonly used drugs in the treatment of acute symptoms of trigeminal neuralgia is intravenous phenytoin [10]. During this therapy, it is essential to be aware of the increased risk of asystole, especially in patients with pre-existing heart rhythm disorders. Therefore, phenytoin is contraindicated in patients who have had a myocardial infarction within the previous 3 months or have decreased left ventricular function, or second- or third-degree AV block. It should be administered at a low infusion rate (max. 25 ml/min) and under blood pressure and ECG monitoring through a separate i.v. line. Alternatively, lidocaine or lacosamide can be used in case of treatment-resistant pain. According to some recent studies, lacosamide may be better tolerated and lead to sustained pain relief: 77.8% of cases with pain reduction compared to 72.8% with phenytoin [34, 35]. For mild-to-moderate exacerbation, a carbamazepine suspension (solution) can be used as an emergency measure, but the onset of action is not as rapid as with intravenous therapy and the effect is often insufficient. Other, less common treatments for acute exacerbations of trigeminal neuralgia include the use of pimozide, clonazepam, levetiracetam or the subcutaneous application of sumatriptan or local anaesthetics in the trigeminal area or on the occipital nerve (table 6).
Table 6Medicinal acute treatment for trigeminal neuralgia.
| Medication | Dose |
| Phenytoin | 250 mg intravenously over 15 minutes, or 15 mg/kg body weight, slow infusion (maximum rate of 25 mg/min) under blood pressure and ECG monitoring. |
| Lacosamide | Maximum 400 mg intravenously, slow infusion over 15–40 minutes (depending on doses) under blood pressure and ECG monitoring. |
| Lidocaine | 5 mg/kg intravenously over 60 minutes under blood pressure and ECG monitoring, or as an intranasal / intraoral spray. |
Surgical treatment of classic trigeminal neuralgia is primarily indicated in cases of inadequate response or significant side effects to medical therapy. However, there is currently no definitive study determining the optimal number of drugs to be tried before considering surgery. From the clinical point of view, it is recommended to initially test carbamazepine and oxcarbazepine and subsequently combine the most effective drug with either gabapentin, pregabalin or lamotrigine, before considering surgical intervention [10].
The gold standard in treating classic trigeminal neuralgia is microvascular decompression, also known as the Jannetta procedure [10]. This operation involves releasing the contact between the trigeminal nerve and (usually) the superior cerebellar artery. To this end, a Teflon sponge is typically placed between the vessel and the nerve to prevent the recurrence from recurring. About 84% of patients report freedom from symptoms in the first year after surgery without additional medication; after 5 years, this figure is still 72–85% [10]. Other invasive procedures include destructive methods such as percutaneous balloon compression (pressure damage to pain fibres), glycerol rhizotomy and radiofrequency thermocoagulation (thermal damage to pain fibres). These are associated with a lower risk of intra- and post-operative complications but with an increased risk of permanent sensory disturbances in the area of the trigeminal nerve. As a result, destructive procedures are more frequently recommended for older patients and good efficacy has also been demonstrated in patients with multiple sclerosis [9, 36]. It has become standard clinical practice to maintain drug therapy unchanged until surgery and then taper it gradually post-operatively.
Radiosurgery is a non-invasive, ablative technique. It involves stereotactic radiation of the trigeminal nerve, typically using a gamma knife (GK), cyberknife or linear accelerator with a single dose between 70 and 90 Gy, which is delivered in close proximity to the brainstem. This method is mainly used to treat treatment-refractory classic and symptomatic trigeminal neuralgia in patients with multiple sclerosis. The short-term pain relief rate for GK is between 75% and 90% at 1 year, but the long-term effectiveness after 5 years falls to between 44% and 65% – lower than that of microvascular decompression. Furthermore, the recurrence rate is over 20% – higher than in other methods [36, 37]. Dysaesthesia in the area of the trigeminal nerve occurs more frequently after radiosurgical procedures; however, the risk of anaesthesia dolorosa is very low (0.2%) [36]. As expected, post-operative complications such as haemorrhage, nerve injury, infection or cerebrospinal fluid leakage are less frequently observed than with surgical procedures [37].
Radiosurgery may be considered a safer alternative if the patient is not an appropriate candidate for surgery due to co-morbidities, age or other factors.
While showing efficacy in about 70% of patients, invasive neuromodulatory procedures such as deep brain stimulation (DBS) and motor cortex stimulation (MCS) are usually reserved as last-resort treatments due to their invasive nature [38]. Another new and potentially promising approach for patients with refractory trigeminal neuralgia may be MRgFUS (Magnetic Resonance-guided Focused UltraSound) targeting the thalamus, but experience is still limited and more studies are needed [39, 40].
Due to the different causes and therapeutic strategies, it is essential for clinical practice to distinguish between trigeminal neuralgia and painful trigeminal neuropathy (table 7).
Neuropathic trigeminal pain results from neural damage caused by injuries, surgeries, inflammation or space-occupying lesions in the face and jaw. It occurs in the distribution area of one or more branches of the trigeminal nerve, especially unilaterally and infraorbitally in the area of the nasolabial fold, and tends to spread to other facial and neck regions. Compared with trigeminal neuralgia, the pain is permanent and has a pressing or burning character. Short paroxysmal pain attacks may occur but are not the dominant type of pain. Sensory disorders (from anaesthesia to allodynia) are typically more pronounced than in trigeminal neuralgia [41]. An MRI scan with contrast agent to identify the cause of neuropathy is essential. In addition, a trigeminal SEP can be carried out to corroborate the diagnosis (it reveals nerve damage, which is characteristic of trigeminal neuropathy) (figure 7). If an inflammatory cause is suspected, a diagnostic lumbar puncture should be performed, along with tests for immunological markers of lupus erythematosus, Sjögren's syndrome and panarteritis nodosa. In addition, tests for borrelia, toxoplasmosis and HIV titers are indicated, as well as measurement of ACE, sIL-2R and vitamin B12 levels.
The therapy relies on a medication-based approach to pain relief (table 8), which has demonstrated high efficacy but typically does not result in complete pain remission [42].
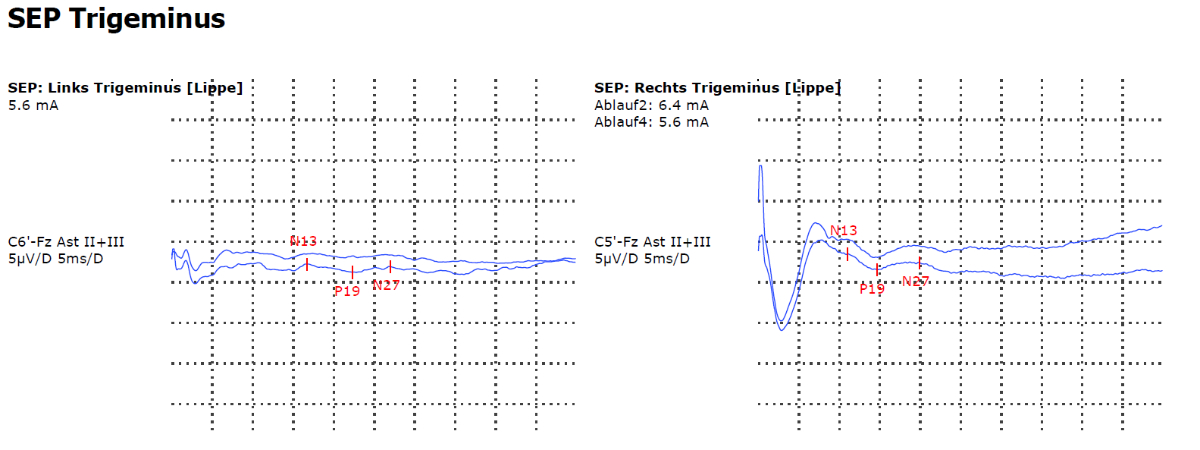
Figure 7Somatosensory evoked potentials (SEPs) of the trigeminal nerve demonstrating evidence of left-sided trigeminal neuropathy.
Table 7Comparison of trigeminal neuralgia and trigeminal neuropathy.
| Trigeminal neuralgia (TN) | Painful trigeminal neuropathy | |
| Aetiology | Neurovascular compression (classic TN), multiple sclerosis, brainstem ischaemia, space-occupying lesions (secondary TN), or unknown (idiopathic TN) | Neural damage (caused by injuries, surgeries, inflammations or tumours in the face and jaw) |
| Location | One or more trigeminal nerve branches (usually V2 and/or V3) | Most commonly the periorbital region (V2) with a tendency to spread to other facial and neck regions |
| Character | Sudden, shooting pain (possible additional constant pain) | Burning, pressing (additional brief paroxysmal pain attacks may occur) |
| Intensity | Very strong | Moderately strong |
| Duration of episodes | Fraction of second to two minutes | Constant |
| Triggers | Touch, such as brushing teeth or eating | Constant |
| Frequency of episodes. | Single attack or in series (up to 100/day) | Constant |
| Neurological examination | Usually normal | May reveal sensory loss or other abnormalities |
| First-line therapy | Carbamazepin, oxcarbamazepin | Amitriptyline, duloxetin, pregabalin, gabapentin |
Combination therapy is possible but is only recommended if various monotherapies are unsuccessful [43]. The efficacy of surgical procedures is questionable and they are only recommended for specific indications. In cases of therapy-refractory pain (about 5% of cases), the implantation of a deep brain stimulator (the ventral posteromedial nucleus of the thalamus [VPM] and the periaqueductal grey [PAG]) can be evaluated. Although this method is not a standard procedure, its high efficacy in the treatment of neuropathic pain has been demonstrated in numerous studies [44, 45].
Table 8Medicinal long-term treatment of trigeminal neuropathy.
| Medication | Dose |
| Gabapentin | Starting with 100 mg 1-1-1, target dose 2400–3600 mg/day |
| Pregabalin | Starting with 25–75 mg 1-0-1, target dose 300–600 mg/day |
| Amitriptyline | Starting with 10–25 mg 0-0-1, target dose 75–150 mg/day |
| Duloxetin | Starting with 30 mg 1-0-0, target dose 60–120 mg/day |
Despite numerous studies and advances in research on trigeminal neuralgia in recent years, many important questions and issues remain unresolved. In clinical practice, there is a particularly urgent need to develop effective and well-tolerated drugs for long-term prophylaxis and to develop effective mechanisms for the treatment of pain exacerbation. More research is necessary to better understand the mechanism of pain generation and the possible genetic inheritance of trigeminal neuralgia.
Given the specialised nature of its treatment, it is crucial to increase awareness about symptoms and diagnosis of trigeminal neuralgia and establish treatment guidelines, such as those of the Swiss Headache Society (www.headache.ch). Furthermore, effectively distinguishing it from other trigeminal nerve and headache disorders is enhanced by an interdisciplinary approach. A board setting offers numerous advantages for the diagnosis and treatment of trigeminal neuralgia. It enables more comprehensive patient care by ensuring a coordinated and concerted treatment approach that addresses all relevant aspects of the condition.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Araya EI, Claudino RF, Piovesan EJ, Chichorro JG. Trigeminal Neuralgia: Basic and Clinical Aspects. Curr Neuropharmacol. 2020;18(2):109–19. 10.2174/1570159X17666191010094350
2. Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Trigeminal neuralgia—a prospective systematic study of clinical characteristics in 158 patients. Headache. 2014;54(10):1574–82. 10.1111/head.12441
3. Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18(1):4–13. 10.1097/00002508-200201000-00002
4. Barker FG 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996 Apr;334(17):1077–83. 10.1056/NEJM199604253341701
5. Martinelli Boneschi F, Colombo B, Annovazzi P, Martinelli V, Bernasconi L, Solaro C, et al. Lifetime and actual prevalence of pain and headache in multiple sclerosis. Mult Scler. 2008 May;14(4):514–21. 10.1177/1352458507085551
6. Di Stefano G, Maarbjerg S, Truini A. Trigeminal neuralgia secondary to multiple sclerosis: from the clinical picture to the treatment options. J Headache Pain. 2019 Feb;20(1):20. 10.1186/s10194-019-0969-0
7. Bennetto L, Patel NK, Fuller G. Trigeminal neuralgia and its management. BMJ. 2007 Jan;334(7586):201–5. 10.1136/bmj.39085.614792.BE
8. Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945-1984. Ann Neurol. 1990 Jan;27(1):89–95. 10.1002/ana.410270114
9. Ruscheweyh R, Lutz J, Mehrkens JH. Trigeminusneuralgie : Moderne Diagnostik und Therapie [Trigeminal neuralgia : Modern diagnostic workup and treatment]. Schmerz. 2020 Dec;34(6):486–94. 10.1007/s00482-020-00496-4
10. Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Leal PR, Nurmikko T, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020 Sep;19(9):784–96. 10.1016/S1474-4422(20)30233-7
11. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. 3rd edition. Cephalalgia. 2018;38(1):1-211. doi:
12. Masur H, Papke K, Bongartz G, Vollbrecht K. The significance of three-dimensional MR-defined neurovascular compression for the pathogenesis of trigeminal neuralgia. J Neurol. 1995 Jan;242(2):93–8. 10.1007/BF00887823
13. Peker S, Dinçer A, Necmettin Pamir M. Vascular compression of the trigeminal nerve is a frequent finding in asymptomatic individuals: 3-T MR imaging of 200 trigeminal nerves using 3D CISS sequences. Acta Neurochir (Wien). 2009 Sep;151(9):1081–8. 10.1007/s00701-009-0329-y
14. Bendtsen L, Zakrzewska JM, Abbott J, Braschinsky M, Di Stefano G, Donnet A, et al. European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol. 2019 Jun;26(6):831–49. 10.1111/ene.13950
15. von Eckardstein KL, Keil M, Rohde V. Unnecessary dental procedures as a consequence of trigeminal neuralgia. Neurosurg Rev. 2015 Apr;38(2):355–60. 10.1007/s10143-014-0591-1
16. Maltez N, Choi MY, Troyanov Y, Wang M, Jantz M, Fritzler MJ, et al.; Canadian Scleroderma Research Group. Trigeminal neuralgia in systemic sclerosis. Semin Arthritis Rheum. 2021 Feb;51(1):318–23. 10.1016/j.semarthrit.2021.01.001
17. MacDonald BK, Cockerell OC, Sander JW, Shorvon SD. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain. 2000 Apr;123(Pt 4):665–76. 10.1093/brain/123.4.665
18. Maikap D, Padhan P. Trigeminal Neuralgia as an Initial Presentation of Systemic Autoimmune Diseases: A Case Series. Mediterr J Rheumatol. 2022;33(3):333-338. Published 2022 Sep 30. doi:
19. Nascimento IS, Bonfá E, de Carvalho JF, Saad CG, Vendramini MB, Teixeira MJ, et al. Clues for previously undiagnosed connective tissue disease in patients with trigeminal neuralgia. J Clin Rheumatol. 2010 Aug;16(5):205–8. 10.1097/RHU.0b013e3181e928e6
20. Scardina GA, Mazzullo M, Messina P. La diagnosi precoce della sclerosi sistemica progressiva. Il ruolo delle manifestazioni oro-facciali [Early diagnosis of progressive systemic sclerosis: the role of oro-facial phenomena]. Minerva Stomatol. 2002;51(7-8):311–7.
21. Machet L, Vaillant L, Machet MC, Esteve E, Muller C, Khallouf R, et al. Carpal tunnel syndrome and systemic sclerosis. Dermatology. 1992;185(2):101–3.
22. Symonds T, Randall JA, Hoffman DL, Zakrzewska JM, Gehringer W, Lee JY. Measuring the impact of trigeminal neuralgia pain: the Penn Facial Pain Scale-Revised. J Pain Res. 2018 Jun;11:1067–73. 10.2147/JPR.S152958
23. Sandhu SK, Halpern CH, Vakhshori V, Mirsaeedi-Farahani K, Farrar JT, Lee JY. Brief pain inventory—facial minimum clinically important difference. J Neurosurg. 2015 Jan;122(1):180–90. 10.3171/2014.8.JNS132547
24. Lee KH. Facial pain: trigeminal neuralgia. Ann Acad Med Singap. 1993 Mar;22(2):193–6.
25. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004 Apr;428(6982):486. 10.1038/428486a
26. McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperavičiūtė D, Carrington M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011 Mar;364(12):1134–43. 10.1056/NEJMoa1013297
27. Wu CJ, Lian YJ, Zheng YK, Zhang HF, Chen Y, Xie NC, et al. Botulinum toxin type A for the treatment of trigeminal neuralgia: results from a randomized, double-blind, placebo-controlled trial. Cephalalgia. 2012 Apr;32(6):443–50. 10.1177/0333102412441721
28. Zúñiga C, Piedimonte F, Díaz S, Micheli F. Acute treatment of trigeminal neuralgia with onabotulinum toxin A. Clin Neuropharmacol. 2013;36(5):146–50. 10.1097/WNF.0b013e31829cb60e
29. Morra ME, Elgebaly A, Elmaraezy A, Khalil AM, Altibi AM, Vu TL, et al. Therapeutic efficacy and safety of Botulinum Toxin A Therapy in Trigeminal Neuralgia: a systematic review and meta-analysis of randomized controlled trials. J Headache Pain. 2016 Dec;17(1):63. 10.1186/s10194-016-0651-8
30. Liu J, Xu YY, Zhang QL, Luo WF. Efficacy and Safety of Botulinum Toxin Type A in Treating Patients of Advanced Age with Idiopathic Trigeminal Neuralgia. Pain Res Manag. 2018;2018:7365148. Published 2018 Apr 5. 10.1155/2018/7365148
31. Asan F, Gündüz A, Tütüncü M, Uygunoğlu U, Savrun FK, Saip S, et al. Treatment of multiple sclerosis-related trigeminal neuralgia with onabotulinumtoxinA. Headache. 2022 Nov;62(10):1322–8. 10.1111/head.14414
32. Schott Andersen AS, Maarbjerg S, Noory N, Heinskou TB, Forman JL, Cruccu G, et al. Safety and efficacy of erenumab in patients with trigeminal neuralgia in Denmark: a double-blind, randomised, placebo-controlled, proof-of-concept study. Lancet Neurol. 2022 Nov;21(11):994–1003. 10.1016/S1474-4422(22)00294-0
33. Gambeta E, Chichorro JG, Zamponi GW. Trigeminal neuralgia: an overview from pathophysiology to pharmacological treatments. Mol Pain. 2020;16:1744806920901890. 10.1177/1744806920901890
34. Masrour S. Lacosamide for refractory trigeminal neuralgia and other facial pain-Case report. Headache. 2022 Oct;62(9):1227–30. 10.1111/head.14367
35. Muñoz-Vendrell A, Teixidor S, Sala-Padró J, Campoy S, Huerta-Villanueva M. Intravenous lacosamide and phenytoin for the treatment of acute exacerbations of trigeminal neuralgia: A retrospective analysis of 144 cases. Cephalalgia. 2022 Sep;42(10):1031–8. 10.1177/03331024221092435
36. Bick SK, Eskandar EN. Surgical Treatment of Trigeminal Neuralgia. Neurosurg Clin N Am. 2017 Jul;28(3):429–38. 10.1016/j.nec.2017.02.009
37. Lee S, Lee JI. Gamma Knife Radiosurgery for Trigeminal Neuralgia : review and Update. J Korean Neurosurg Soc. 2022 Sep;65(5):633–9. 10.3340/jkns.2021.0303
38. Rasche D, Tronnier VM. Clinical Significance of Invasive Motor Cortex Stimulation for Trigeminal Facial Neuropathic Pain Syndromes. Neurosurgery. 2016 Nov;79(5):655–66. 10.1227/NEU.0000000000001353
39. Ishaque M, Moosa S, Urban L, Kundu B, Qureshi Z, Spears T, et al. Bilateral focused ultrasound medial thalamotomies for trigeminal neuropathic pain: a randomized controlled study. J Neurosurg. 2023 Dec:1–11. 10.3171/2023.10.JNS23661
40. Taranta V, Saporito G, Ornello R, Splendiani A, Bruno F, Sucapane P, et al. Magnetic Resonance-guided Focused Ultrasound thalamotomy for refractory neuropathic pain: a systematic review and critical appraisal of current knowledge. Ther Adv Neurol Disord. 2023 Jun;16:17562864231180729. 10.1177/17562864231180729
41. Peñarrocha M, Cervelló MA, Martí E, Bagán JV. Trigeminal neuropathy. Oral Dis. 2007 Mar;13(2):141–50. 10.1111/j.1601-0825.2006.01356.x
42. Gierthmuehlen M, Gierthmuehlen P, Hugger A, Gierthmuehlen J, Türp JC. Neuropathische Gesichtsschmerzen – eine interdisziplinäre Herausforderung. Swiss Dent J. 2020 Apr;130(4):321–7. 10.61872/sdj-2020-04-03
43. Tesfaye S, Sloan G, Petrie J, White D, Bradburn M, Julious S, et al.; OPTION-DM trial group. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial [Erratum in: Lancet. 2022 Sep 10;400] [10355]. Lancet. 2022 Aug;400(10353):680–90. 10.1016/S0140-6736(22)01472-6
44. Owen SL, Green AL, Nandi DD, Bittar RG, Wang S, Aziz TZ. Deep brain stimulation for neuropathic pain. Acta Neurochir Suppl (Wien). 2007;97(Pt 2):111–6.
45. Pereira EA, Aziz TZ. Neuropathic pain and deep brain stimulation. Neurotherapeutics. 2014 Jul;11(3):496–507. 10.1007/s13311-014-0278-x