
Figure 1Flow chart of patients included in the analysis.
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40095
Remdesivir is an antiviral medication originally intended to treat hepatitis C and respiratory syncytial virus infections [1]. Although unsuccessful for its original purpose, remdesivir proved effective against the Ebola virus disease and Marburg virus. Positive results on remdesivir's in vitro effectiveness against coronaviruses made it a promising treatment candidate for coronavirus disease 2019 (COVID-19) [2, 3]. Between May and July 2020, several regulatory agencies issued an emergency or conditional authorisation to treat patients hospitalised for COVID-19 with remdesivir [4]. Remdesivir also became the first fully licensed drug against COVID-19 by the United States Food and Drug Administration based on preliminary results from a randomised controlled trial (RCT) [5, 6]. However, the initial results were controversial: another RCT found no overall benefit [7], and there were concerns about the manufacturer’s role in the decision-making process [8].
In the context of the early RCT results, the World Health Organization (WHO) expert groups conducted a set of mortality trials (“Solidarity”) on four repurposed drugs. Remdesivir, hydroxychloroquine, lopinavir, and interferon beta-1a were included to determine whether any of them could reduce in-hospital mortality in patients hospitalised with COVID-19 [9]. Remdesivir was the only drug not discontinued after the interim report was published. The results showed a small benefit only among patients who were not ventilated during drug administration. The negative findings from the Solidarity trial were a major contributor to the WHO recommendation in November 2020 against using remdesivir to treat COVID-19 [10]. However, in April 2022, a weak conditional recommendation was issued to administer remdesivir for patients with non-severe COVID-19 but at the highest risk of hospitalisation [11].
These RCTs had small sample sizes and thus could not assess the potential modifying effect of characteristics such as age, sex, and comorbidities on the treatment outcome. Although the trials found no differences between remdesivir and the comparators overall, some results hinted that the effect of remdesivir may be heterogeneous across the population. Therefore, we analysed our large COVID-19 hospital database in Switzerland [12] to assess mortality among patients who received remdesivir and identify subpopulations that may benefit from remdesivir treatment.
The COVID-19 Hospital Surveillance System (CH-SUR) database is coordinated by the Federal Office of Public Health (FOPH) in collaboration with the University of Geneva [12]. It contains data on hospitalised patients diagnosed with COVID-19. To date, 21 university and cantonal hospitals across Switzerland participate in the system, covering most tertiary hospitals in Switzerland. Data collection was approved by the Ethics Committee of the Canton of Geneva, Switzerland (CCER, 2020–00827) and all local ethics committees. Study planning, conduct, and reporting were aligned with the Declaration of Helsinki (2013 revision). An up-to-date cohort description, protocol, codebook, questionnaire set, and further detail is available on the website https://www.unige.ch/medecine/hospital-covid/.
Two stays of the same patient were considered two episodes if the time between the discharge and new admission dates is more than 30 days or if the second hospitalisation was in a different hospital without data being shared. Various information was collected for each episode in a standardised manner, including demographics, admission details, complementary clinical details (comorbidities, complications, admission to an intermediate care or intensive care unit, and treatments), and follow-up status (death, discharge, or transfer).
We included adults (aged ≥18 years) with a laboratory-confirmed COVID-19 diagnosis who were hospitalised for more than 24 hours and recorded in CH-SUR between 1st February 2020 and 7th February 2022. The follow-up period was from the day of hospitalisation until the final discharge date of the first episode, death, or database closure. For subjects with more than one episode, only the first episode was included. We included patients who received either no treatment, remdesivir as the only (antiviral) treatment, or any treatment but not remdesivir. Patients who received remdesivir were split into those starting treatment within one week of the onset of symptoms (or hospitalisation, if hospitalised prior to symptom onset or data on symptoms were missing); and those starting later [13]. Patients who received remdesivir with other treatments were excluded since it would be challenging to differentiate between the effect of remdesivir and other drugs. Records were excluded if missing a patient’s outcome, outcome date, age, or sex or if records had inconsistencies in key dates. Treatment other than antivirals, interferon, and chloroquine (e.g. corticosteroids, antibodies, and immunomodulatory treatment) were not included in the analysis.
We split the patients into groups with similar treatment responses using model-based recursive partitioning (from this point forward, tree model) and the model4you R package (see "Technical description of the model-based recursive partitioning" and supplementary figure S1 in the appendix) [14–16]. In this method, a multivariable Cox proportional hazards regression analysis is performed on the entire dataset first. It adjusts for selected covariables, including the main variables of interest (treatment). Next, the patients are iteratively partitioned into groups by assessing the instabilities in the model parameters according to a pre-defined list of patient characteristics. A parameter is considered unstable if the partial derivative of its contribution to the partial likelihood correlates with at least one patient characteristic. The stability is assessed by splitting the dataset into two parts based on the values of each variable on the list using the Bonferroni-adjusted permutation test of statistics of a quadratic form [17]. If any parameter with significant instability (p <0.01) is found, we split the dataset into two parts according to the parameter with the lowest p-value. The same process is repeated for both groups until we find no parameter with instability.
In real-world settings, the decision to treat a patient with remdesivir or another drug usually depends on the patient’s characteristics, risk factors, comorbidities, and clinical signs and symptoms. To reduce the risk of confounding by indication, we considered a method called local centring [18–20]. The treatment variable T0 (which usually would be either 1 or 0, depending on whether the patient received the treatment or not) is replaced by T = T0 – P(X), where P(x) is the propensity of receiving treatment based on the covariables X. In other words, the higher the probability of receiving remdesivir, the higher the benefit needs to be to contribute to the regression with the same effect. We estimated the propensities of all three treatment options by random forests using the partykit package in R (see "Technical description of the model-based recursive partitioning" in the appendix) [21].
We performed four analyses using the tree model described above. First, we conducted two analyses where treatment, age, and sex were included in the initial Cox model as independent variables. The levels of treatment were: none; remdesivir within a week of symptom onset; remdesivir later; treatment with drugs other than remdesivir. Analysis I was without, and analysis II was with local centring.
Second, we conducted two analyses where treatment was the only independent variable in the Cox model, also without and with local centring (analyses III and IV, respectively). Remdesivir was included as a time-depending covariable by splitting the person-time into two records: from enrolment to treatment initiation and from treatment initiation to the end of follow-up. Other treatment was included as constant, ignoring the starting time of treatment.
We selected a set of metabolic markers and comorbidities for the partitioning that may be associated with the prognosis of COVID-19. The following variables, all measured at baseline, were included (table 1): body mass index (BMI) [22]; high blood urea nitrogen level (>19 mg/dl) [23]; high respiratory rate (>30/min) [24]; low blood pressure (diastolic <60 mmHg or systolic <90 mmHg) [23]; having chronic obstructive pulmonary disease (COPD) [23]; having a chronic cardiovascular disease [23]; having a chronic renal disease [25]; and having an oncological condition (cancer, tumour) [23]. We also included a composite variable based on a dichotomised CURB-65 score (≥2 vs <2). The CURB-65 score was defined as meeting at least two of the following conditions: confusion (abbreviated mental test score <9); high urea nitrogen level; high respiratory rate; low blood pressure; and age ≥65 years, (all cut-offs defined above) [26]. Age and sex were included as partitioning variables in analyses III and IV. Age and BMI were considered continuous, and all others as dichotomous variables. The determination of propensity scores was performed using all the variables mentioned above. Missing data were imputed using the R package mice and all included explanatory variables.
Table 1Variables included in the model. Analyses I–IV refer to the four analyses described in the main text. All variables were measured at baseline (hospital admission).
| Variable | Type | Definition/categorisation | Inclusion in analyses | |||
| I | II | III | IV | |||
| Treatment (crude) | Categorical | Remdesivir within 7 days of symptom onset; remdesivir later than 7 days after symptom onset; other treatment or their combination; or none | C | – | C | – |
| Treatment (locally centred)* | Categorical | Remdesivir within 7 days of symptom onset; remdesivir later than 7 days after symptom onset; other treatment or their combination; or none | – | C | – | C |
| Age | Continuous | Continuous | C | C | T | T |
| Sex | Dichotomous | Male vs female | C | C | T | T |
| Body mass index [22] | Continuous | Continuous: 104 * weight [kg] / (height [cm]2) | T | T | T | T |
| High blood urea nitrogen [23] | Dichotomous | >19 mg/dl blood urea nitrogen: yes or no | T | T | T | T |
| High respiratory rate [24] | Dichotomous | >30/minute: yes or no | T | T | T | T |
| Low blood pressure [23] | Dichotomous | Diastolic <60 mmHg or systolic <90 mmHg: yes or no | T | T | T | T |
| High CURB65 score [26] | Dichotomous | At least two (vs 1 or none) of the following indicators: age ≥65 years, high urea nitrogen, high respiratory rate, low blood pressure, abbreviated mental test score <9. | T | T | T | T |
| Respiratory disease [23] | Dichotomous | The patient has a chronic obstructive pulmonary disease: yes or no | T | T | T | T |
| Cardiovascular disease [23] | Dichotomous | The patient has a chronic cardiovascular disease: yes or no | T | T | T | T |
| Chronic renal disease [25] | Dichotomous | The patient has a chronic renal disease: yes or no | T | T | T | T |
| Oncological condition [23] | Dichotomous | The patient has been diagnosed with cancer, a tumour, or other oncological pathological finding: yes or no | T | T | T | T |
C, included in the initial Cox model as a covariable; T, included in the tree model as a partitioning variable.
* Local centring refers to an adjustment of the variable indicator, where it is defined as T = T0 – P(x) where T0 is the crude indicator (1 or 0), and P(x) is the probability that T0 = 1 given the covariables x.
We present the results of the Cox models as adjusted hazard ratios (aHR) with corresponding 95% confidence intervals (CI). The tree model results are presented graphically, showing the process from the full dataset into the final partitioning. For each leaf node (i.e., a subgroup defined according to the partitioning factors), the primary outcome is the regression coefficient of remdesivir use within seven days of symptom onset on mortality. The secondary outcomes were the regression coefficients for other covariables (late remdesivir use, use of another treatment, and age and sex if not included as partitioning variables). All analyses were conducted in R (version 4.2.1).
We conducted four sensitivity analyses. The first three were restricted to different time periods to account for the differences in variants, clinical practice, and other factors. In sensitivity analysis 1, we included the first wave (1st February 2020 until 31st October 2020). Sensitivity analysis 2 included the first two waves (until 28th February 2021). Sensitivity analysis 3 included the first to fourth waves (until 20th October 2021). Finally, we conducted sensitivity analysis 4, where the CURB-65 score (a composite of other variables included in the tree model) was excluded from the partitioning.
The funders had no role in the study design, the collection, analysis, or interpretation of data, the writing of the manuscript, or in the decision to submit the paper for publication.
The initial dataset included 30,653 records of 29,639 patients. Eighty-four records representing subsequent episodes with at least a 30-day gap were excluded. The records of patients returning to the hospital within 30 days were merged. As mentioned, records were excluded if there was a missing outcome or sex or had missing or inconsistent dates related to hospital admission or treatment initiation (n = 4176, 14.1%). They were also excluded for discharge on the day of entry (n = 143, 0.5%), receiving both remdesivir with other tratment (n = 198, 0.9%), and age <18 years (n = 921, 3.2%; supplementary figure S2). All 2411 patients (8.1%) from one hospital were excluded because of the disproportionately high risk of a missing outcome.
Ultimately, 21,790 patients were included (table 2): 1347 (6.2%) received remdesivir within the first seven days after symptom onset; 946 (4.3%) started remdesivir more than seven days after symptom onset; and 2517 (11.6%) received another treatment (chloroquine, ribavirin, interferon, and/or lopinavir combined with ritonavir). Patients who received remdesivir were younger than those who did not receive treatment, particularly those who started treatment more than seven days after symptom onset (median age 64 years [interquartile range, IQR 54–74]) compared to those who did not receive any treatment (median [IQR] age 70 years [56–81]). The proportion of males was higher among those who received remdesivir than those without treatment (65.0% vs 54.9%). The median (IQR) follow-up time was 11 (6–18) days. Total person-time was 261,988 person-days under no treatment, 18,408 under remdesivir treatment administered within seven days of symptoms, 11,439 under remdesivir treatment administered later, and 47,058 under another treatment.
Table 2Clinical characteristics of patients. For continuous variables, the values are given as median (interquartile range). For categorical variables, the percentages provided are calculated with respect to the total number of patients (by column).
| No treatment (n = 16,980) | Remdesivir early* (n = 1,347) | Remdesivir late* (n = 946) | Another treatment** (n = 2,517) | Total (n = 21,790) | ||
| Outcome | Discharged | 14,637 (86.2%) | 1,114 (82.7%) | 837 (88.5%) | 2,132 (84.7%) | 18,720 (85.9%) |
| Death | 2,343 (13.8%) | 233 (17.3%) | 109 (11.5%) | 385 (15.3%) | 3,070 (14.1%) | |
| Follow-up time | Median (IQR), days | 11 (6–17) | 11 (7–18) | 14 (9–21) | 12 (7–20) | 11 (6–18) |
| Age | Median (IQR), years | 70 (56–81) | 68 (57–77) | 64 (54–74) | 67 (55–79) | 69 (56–80) |
| Sex | Men | 9,320 (54.9%) | 879 (65.3%) | 612 (64.7%) | 1,561 (62.0%) | 12,372 (56.8%) |
| Women | 7,660 (45.1%) | 468 (34.7%) | 334 (35.3%) | 956 (3.0%) | 9,418 (43.2%) | |
| Body mass index | Median (IQR), kg/m2 | 26.6 (23.4–30.5) | 27.7 (24.5–31.3) | 27.6 (24.2–31.5) | 26.8 (23.7–30.5) | 26.8 (23.5–30.6) |
| Urea nitrogen level in blood | <19 mg/dl | 12,429 (73.2%) | 880 (65.3%) | 660 (69.8%) | 1,762 (70.0%) | 15,731 (72.2%) |
| ≥19 mg/dl | 4,551 (26.8%) | 467 (34.7%) | 286 (30.2%) | 755 (30.0%) | 6,059 (27.8%) | |
| Respiratory rate | <30/min | 15,031 (88.5%) | 1,082 (80.3%) | 768 (81.2%) | 1,880 (74.7%) | 18,761 (86.1%) |
| ≥30/min | 1,949 (11.5%) | 265 (19.7%) | 178 (18.8%) | 637 (25.3%) | 3,029 (13.9%) | |
| Blood pressure | Normal | 15,072 (88.8%) | 1,147 (85.2%) | 839 (88.7%) | 2,339 (92.9%) | 19,397 (89.0%) |
| Low*** | 1,908 (11.2%) | 200 (14.8%) | 107 (11.3%) | 178 (7.1%) | 2,393 (11.0%) | |
| CURB-65 score | <2 | 11,107 (65.4%) | 791 (58.7%) | 625 (66.1%) | 1,562 (62.1%) | 14,085 (64.6%) |
| ≥2 | 5,873 (34.6%) | 556 (41.3%) | 321 (33.9%) | 955 (37.9%) | 7,705 (36.3%) | |
| Respiratory disease | No | 14,025 (82.6%) | 1,081(80.3%) | 774 (81.8%) | 2,001 (79.5%) | 17,881 (82.1%) |
| Yes | 2,955 (17.4%) | 266 (19.7%) | 172 (18.2%) | 516 (20.5%) | 3,909 (17.9%) | |
| Cardiovascular disease | No | 10,310 (60.7%) | 800 (59.4%) | 630 (66.6%) | 1649 (65.5%) | 13,389 (61.4%) |
| Yes | 6,670 (39.3%) | 547 (40.6%) | 316 (33.4%) | 868 (34.5%) | 8,401 (38.6%) | |
| Renal disease | No | 13,270 (78.2%) | 1,063 (78.9%) | 795 (84.0%) | 2,142 (85.1%) | 17,270 (79.3%) |
| Yes | 3,710 (21.8%) | 284 (21.1%) | 151 (16.0%) | 375 (14.9%) | 4,520 (20.7%) | |
| Oncological condition | No | 14,550 (85.7%) | 1,117 (82.9%) | 786 (83.1%) | 2,194 (87.2%) | 18,647 (85.6%) |
| Yes | 2,430 (14.3%) | 230 (17.1%) | 160 (16.9%) | 323 (12.8%) | 3,143 (14.4%) | |
* Early and late treatment defined as starting remdesivir within 7 days of the onset of symptoms or later, respectively
** Includes the following drugs: chloroquine, ribavirin, interferon, lopinavir/ritonavir
*** Low blood pressure defined as diastolic <60 mmHg or systolic <90 mmHg
IQR: interquartile range. All values were measured at baseline (time of hospitalisation).
The initial Cox model without local centring (analysis I) was adjusted for treatment, age and sex. Remdesivir treatment both within the first week (aHR 1.54, 95% CI 1.35–1.77 vs no treatment) and later (aHR 1.27, 95% CI 1.05–1.55) was associated with elevated mortality (figure 1A). Older age (aHR 1.05, 95% CI 1.05–1.06 per incremental increase of one year) also increased mortality. Female sex (aHR 0.64, 95% CI 0.60–0.68 vs males) reduced the risk. The same was observed with local centring (analysis II; figure 1B; remdesivir treatment aHR 1.44, 95% CI 1.25–1.67 within the first week and aHR 1.23, 95% CI 1.00–1.51 after that; older age aHR 1.06, 95% CI 1.05–1.06; female sex aHR 0.64, 95% CI 0.59–0.69). Receiving treatment other than remdesivir was not associated with mortality.

Figure 1Flow chart of patients included in the analysis.
The partitioning did not differ between the models without or with local centring (figure 2; supplementary tables S1–S2). CURB-65 score caused the highest instability. Patients with high CURB-65 scores were next split by respiratory rate and those with low CURB-65 scores by oncological conditions. Ultimately, eight leaf nodes were identified. Almost half of the observations were in one leaf node (low CURB-65 score, no oncological conditions or renal disease, normal blood urea nitrogen). Early remdesivir use was not associated with lower mortality in any of the nodes. Late administration of remdesivir was beneficial in two cases: in patients with oncological conditions and a high CURB-65 score but a normal respiratory rate; and in patients with a low CURB-65 score and a high urea nitrogen level but no oncological or renal conditions.
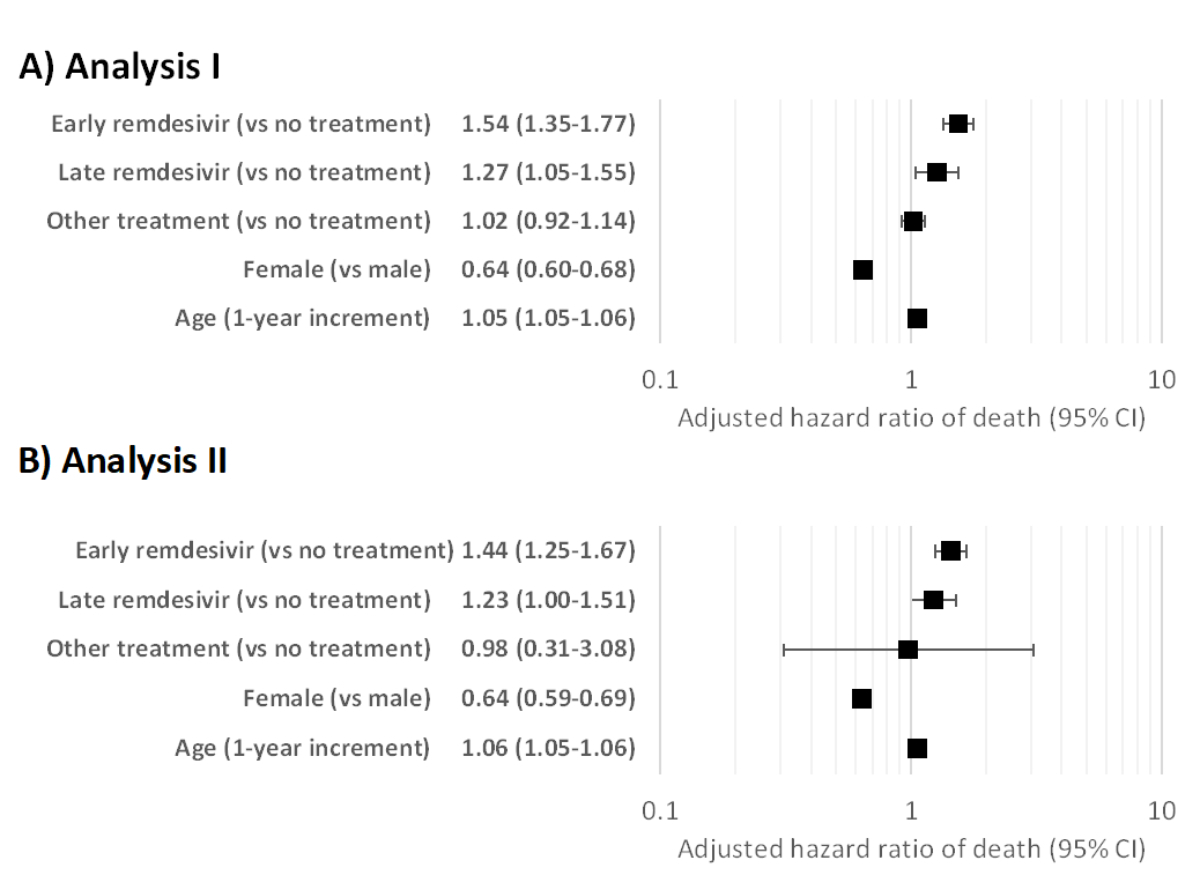
Figure 2Adjusted hazard ratios for mortality in the initial Cox regression model (full dataset) in analyses I and II. Panel A presents analysis I (without local centring), and panel B analysis II (with local centring).
The initial Cox model was adjusted only for treatment. Early treatment with remdesivir remained significantly associated with increased mortality in the overall population both without (analysis III; figure 3A; aHR = 1.29, 95% CI 1.13–1.48) and with (analysis IV; figure 3B; aHR = 1.28, 95% CI 1.11–1.47) local centring, although the effect size was smaller than in analyses I and II. Neither late remdesivir treatment nor treatment with other antivirals was associated with mortality.
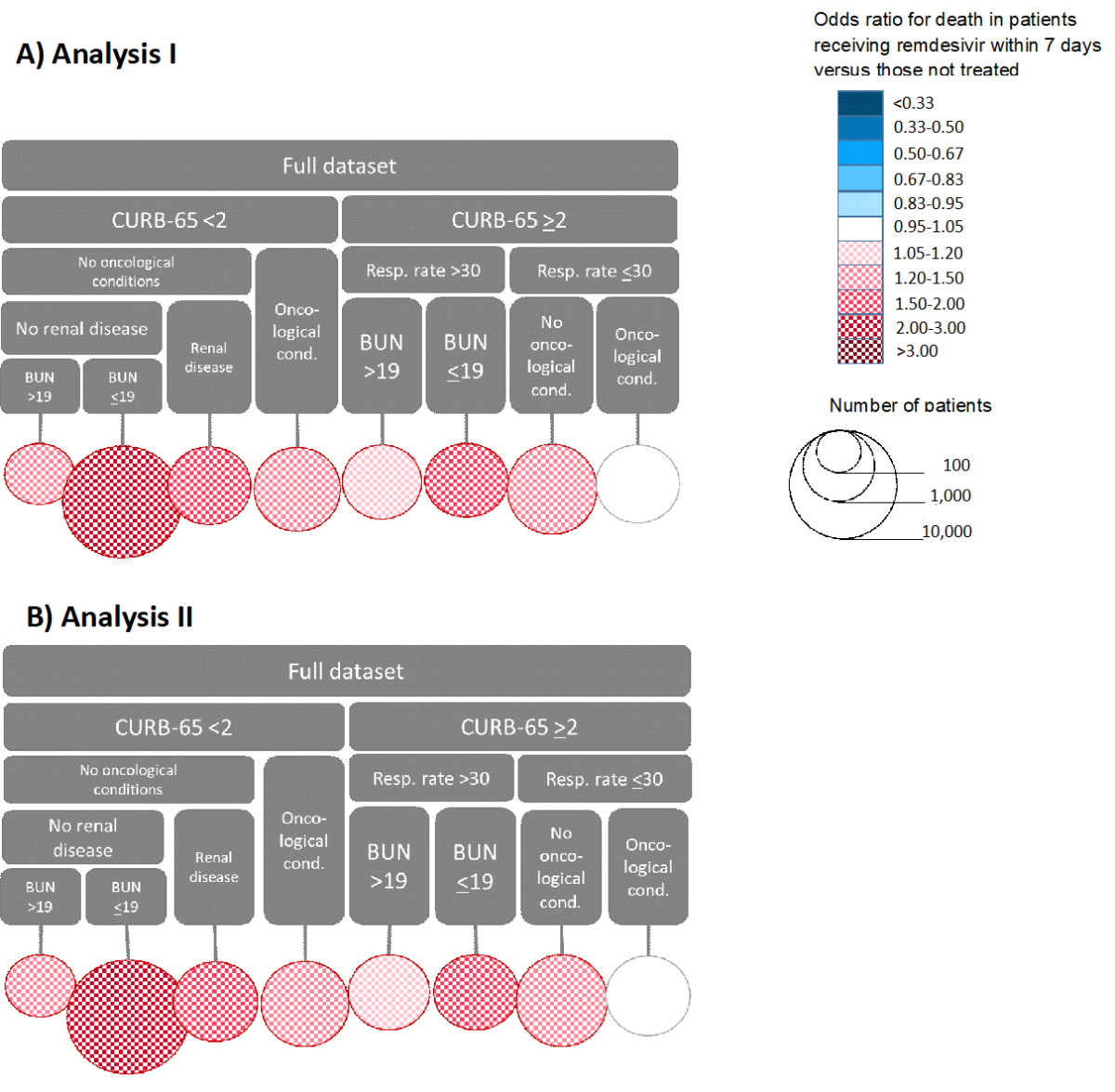
Figure 3Partitioning of the dataset in analyses I and II. The top part of both panels shows the variables that determine each branch of the tree, and the circles represent the final branches. For example, the leftmost branch of Panel A consists of patients with CURB-65 score above 2, respiratory rate >30 /min and BUN <19 mg/dl. The size of the circle represents the size of this population group, and the colour represents the effect of remdesivir treatment within 7 days of symptom onset on mortality. Panel A shows the results of analysis I without, and Panel B the results of analysis II with local centring. CURB-65: composite score for severe pneumonia depending on confusion, urea nitrogen, respiratory rate, blood pressure, and age; Resp. rate: respiratory rate per minute; BUN: blood urea nitrogen level (mg/dl).
CURB-65 score again had the highest instability (figure 4). Age had the next highest instability, split at 78 for those with high CURB-65 scores and 73 years for those with low scores (figure 5; supplementary tables S3–S4). The trees with and without local centring were almost identical. The number of leaf nodes was 20 in analysis III and 19 in analysis IV. One leaf node was clearly the largest, covering about a fourth of the observations. It included patients with a low CURB-65 score, age ≤59 years, no oncological conditions, and a normal blood urea nitrogen level. Early remdesivir use was beneficial in five nodes in both analyses. However, the sample sizes were relatively small, ranging from 192 to 663, and regression coefficients ranged between –0.26 and –0.91 without local centring and between –0.25 and –0.80 with local centring. The conditions determining the largest group were the presence of oncological comorbidity, a low CURB-65 score, and an age below 59 years. In addition, without local centring, patients with a high CURB-65 score and age ≤64 years had a lower risk of death when treated early with remdesivir.
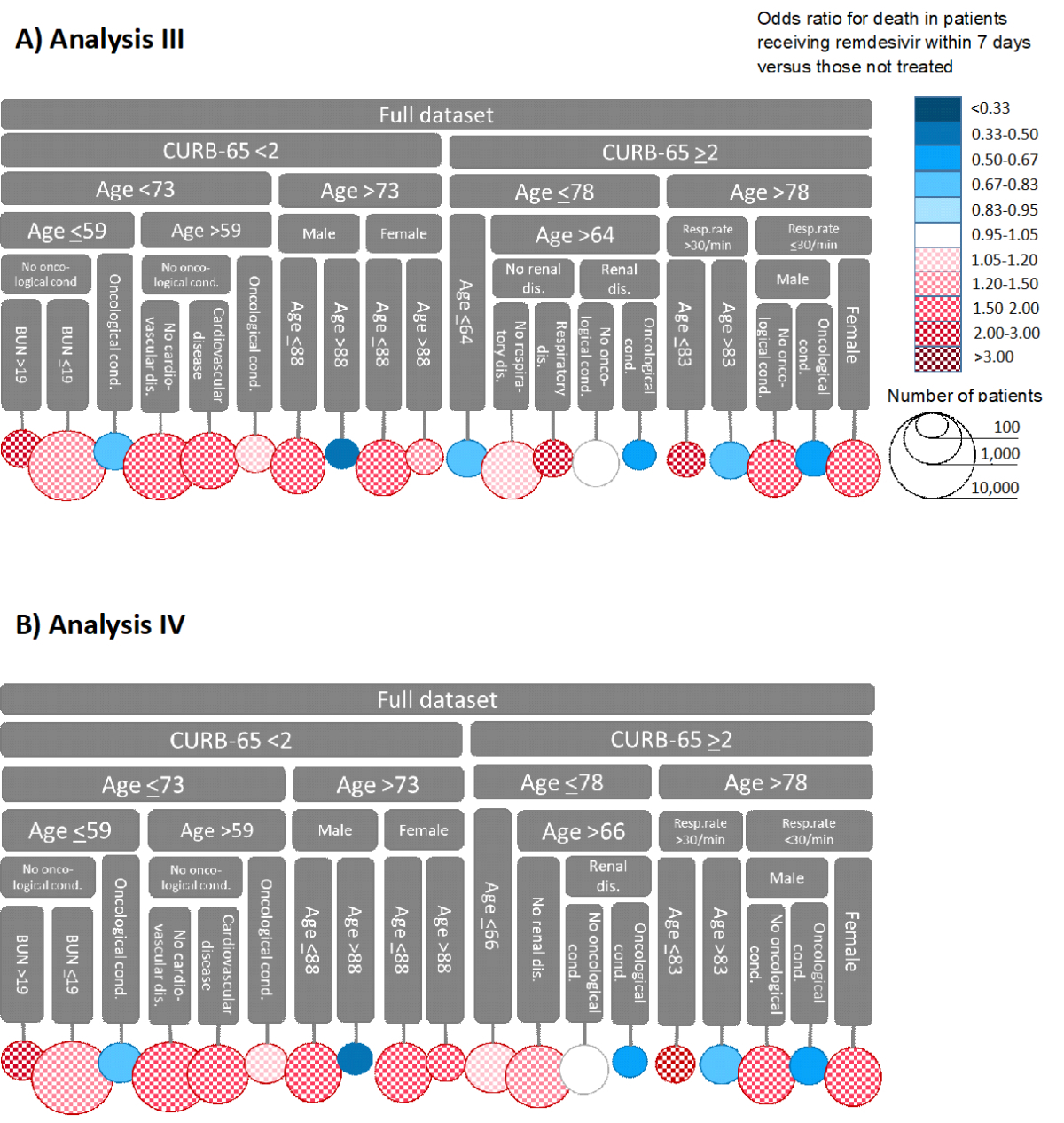
Figure 4Partitioning of the dataset in analyses III and IV. The top part of both panels shows the variables that determine each branch of the tree, and the circles represent the final branches. For example, the leftmost branch of Panel A consists of patients with CURB-65 scores above 2 and ages below 64. The size of the circle represents the size of this population group. The colour represents the effect of remdesivir treatment within 7 days of symptom onset on mortality. Panel A shows the results of analysis III without, and Panel B the results of analysis IV with local centring. CURB-65, the composite score for severe pneumonia depending on confusion, urea nitrogen, respiratory rate, blood pressure, and age; Resp. rate, respiratory rate per minute; BUN, blood urea nitrogen level (mg/dL); age, age in years.

Figure 5Adjusted hazard ratios for mortality in the initial Cox regression model (full dataset) in analyses III and IV. Panel A presents analysis III without local centring, and Panel B analysis IV with local centring.
In sensitivity analysis 1 (restricted to the first wave of the pandemic), early remdesivir use was associated with reduced mortality in the models without age or sex, although the association was significant only without local centring (aHR 0.66, 95% CI 0.44–0.97; supplementary table S5). In the model with age and sex, mortality was reduced moderately but not significantly. Early remdesivir use was particularly beneficial among those with a high CURB-65 score; patients with a low CURB-65 score and oncological conditions had higher mortality when remdesivir was administered early. When age and sex were used as splitting variables, a benefit of early remdesivir was seen, particularly among those over 78 years old.
When the second wave was included (sensitivity analysis 2), the protective effect of remdesivir disappeared: mortality was higher among those receiving remdesivir early (supplementary table S6). The same was seen in sensitivity analysis 3 when the period was extended to the end of the fourth wave. The trees were similar to those in the main analysis although not identical: the main components of the partitioning, and the branches where remdesivir was beneficial, were the same as in the main analysis (supplementary table S7).
In sensitivity analysis 4 (without CURB-65 score), blood urea nitrogen became the key partitioning variable. In the analyses with age and sex in the Cox model, all treatment levels were beneficial among patients who had high urea nitrogen and low blood pressure but a normal respiratory rate; however, the effect was minor among those treated early with remdesivir compared to other treatment options. In the analyses without age and sex in the Cox model, there were benefits of remdesivir use in two groups: older patients with a high urea nitrogen level and high respiratory rate; and older women with renal disease but normal urea nitrogen level and normal respiratory rate (supplementary table S8).
Hospitalised COVID-19 patients in Switzerland treated with remdesivir had higher mortality than those who received no antiviral treatment, but the association was not uniform between sub-populations. Overall, the CURB-65 score – a composite variable that combines older age (≥65 years) and the presence of selected clinical and laboratory findings – caused the most instability in the regression parameters. However, it was challenging to find patterns in the factors potentially modifying the treatment effect of remdesivir. The factors frequently appearing on the pathways to effective remdesivir included oncological comorbidities, male sex, and old age.
The CURB-65 score has been validated to predict mortality in community-acquired pneumonia [26], but its performance for COVID-19 outcomes is limited [27]. Nevertheless, CURB-65 was the most important variable in the partitioning process, possibly because it is a composite variable that combines several predictors. When CURB-65 was omitted from the analysis, urea nitrogen level and respiratory rate became the key components causing instability. Younger (≤59 years) individuals with oncological comorbidities were the largest group among those with low CURB-65 scores (≤1) to see benefits with remdesivir; their regression coefficients corresponded with at least 22% lower odds of death when they received early remdesivir than without treatment. Cancer patients usually have a weakened immune system because of cancer therapy and are thus more susceptible to SARS-CoV-2 infection. Studies from the early stages of the pandemic also suggested higher mortality among cancer patients than non-cancer patients [28, 29]. Nevertheless, the evidence related to antiviral treatment for COVID-19 in cancer patients is limited [30].
Despite the promising results in the early stages of the pandemic, evidence of the benefits of remdesivir is scarce. In April 2022, the WHO Living Guideline on Therapeutics and COVID-19 was updated with a weak conditional recommendation supporting remdesivir for patients with non-severe COVID-19 but at a high risk of being hospitalised [11]. An update is also expected for patients with severe COVID-19. The recommendation is based on up-to-date evidence from five trials with 2,710 patients. Although the differences in mortality and recovery speed are minimal, remdesivir clearly reduces the risk of hospitalisation; it prevented 73 hospital admissions among 1,000 patients, which is of the same magnitude as other treatments including nirmatrelvir, molnupiravir, sotrovimab, and casirivimab-imdevimab [11].
Another living systematic review has identified five RCTs assessing remdesivir treatment, two overlapping with the WHO review [31]. The conclusions were similar: no difference in mortality was observed, but there may be benefits in non-mortality outcomes. Nevertheless, to our knowledge, no RCT has shown any harmful outcomes associated with remdesivir. Remdesivir has also not been associated with any major adverse events [32]. Instead, a recent non-randomised study found a significant reduction in mortality in patients receiving remdesivir and dexamethasone versus dexamethasone alone [33]. Despite the lack of randomisation, the study arms were similar, at least regarding known patient characteristics.
Previous studies have suggested that remdesivir treatment is beneficial when given at an early stage [11, 13]. Our findings did not support this claim. The previous studies also had limitations that mitigate the strength of this finding. To our knowledge, the only RCT that assessed the role of treatment timing was conducted in China in the early days of the epidemic with less than 300 patients. The remdesivir arm had lower mortality than the placebo arm when treatment/placebo was started within 10 days of symptom onset, but if started later, remdesivir resulted in higher mortality than placebo [7]. However, these associations were not significant. A retrospective cohort study from the United Arab Emirates found lower mortality among patients receiving remdesivir within the first seven days than those treated later [34]. In another prospective study from Italy, receiving remdesivir within five days versus later also reduced mortality [35]. However, the reason for delayed treatment was the delayed admission to the hospital in most cases, so it is questionable to what extent these differences can be attributable to remdesivir. A large cohort study from Hong Kong compared the timing of remdesivir initiation relative to dexamethasone and found lower mortality if remdesivir was initiated before or at the same time as dexamethasone [36]. The overall mortality in our cohort was also comparable to that in other studies where remdesivir was much more common [37].
Our study has several limitations. First, the allocation of remdesivir was not random. Many of the variables we assessed probably influenced the decision to administer remdesivir. We attempted to mitigate the risk of the indication confounding the results through local centring; however, the results were similar with and without this correction method. Second, the variables for the partitioning were selected subjectively based on previous knowledge. Third, we excluded patients who received remdesivir together with other drugs. However, such patients were <10% of all patients treated with remdesivir, and excluding this group is unlikely to have a major impact. Fourth, we did not adjust for the centre effect; the 20 hospitals likely have different practices for administering different drugs. Fifth, we refrained from quantifying the uncertainty around the findings; although the mortality was lower in patients treated with remdesivir in some patient groups, this could be due to chance, especially in the smallest subgroups.
The results should be interpreted as a description of the CH-SUR data and not generalised to the overall population. Further, the tree model does not address associations between the partitioning variables and the outcome; it only attempts to divide the population into subgroups depending on the treatment response. The findings should thus not be misinterpreted as predictors of the effect of remdesivir. Finally, the selection of variables for the partitioning and regression model was made unsystematically.
In conclusion, in Swiss hospitals, COVID-19 patients treated with remdesivir had a higher risk of death than those without treatment. However, this rather controversial finding is likely a result of unmeasured confounders or artefacts related to the decisions on drug administration. While there is a growing consensus that the ability of remdesivir to reduce mortality is minimal [11, 31], some individuals may still benefit from it. Our results demonstrate that the association between remdesivir use and mortality varies substantially between patient groups. In particular, it would be worth examining the role of remdesivir in treating elderly males and patients with oncological comorbidities. Vaccines have become widely available to control the pandemic, and the evidence on COVID-19 is constantly growing and maturing. Therefore, the treatment and management of COVID-19 are moving from an emergency response towards a precision medicine approach[38] with the help of large collaborative studies.
The anonymised data can be accessed through a multi-stage process described elsewhere (https://www.unige.ch/medecine/hospital-covid/files/4015/9427/6937/CH_SUR_Multicentric_process_final.pdf). Applicants must complete a concept sheet and send it to the study team. An Executive Committee of experts and hospital participant representatives will review the concept. Depending on the goal of the analysis, additional ethics clearance might be needed. Data will be restricted to the request and shared through a secure platform.
We thank Camille Gaza Valera for editing the manuscript.
The analyses incorporated in this paper were supported by the Swiss Federal Office of Public Health under reference 333.0-20/1, the Swiss National Science Foundation (SNSF) under grant agreement No 163878, and the European Union’s Horizon Europe research and innovation programme under grant agreement No 101046314.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. HFG reports receiving grants/contracts from SNSF, National Institutes of Health, Gilead and Yvonne Jacob Foundation, travel grants from Gilead and board membership for Merck, Gilead Sciences, ViiV Healthcare, GlaxoSmithKline, Johnson & Johnson, Janssen and Novartis. UH reports consultancies for Sanofi-Pasteur, paid lectures for Infectopharm, Merck, Moderna, Pfizer, Roche, Sanofi Genzyme and Sanofi-Pasteur, board membership for Takeda, GlaxoSmithKline/Watermark, HilleVax, Coalition for Epidemic Preparedness Innovations, Seqirus, IQVIA and Merck, and unpaid activities for the Standing Committee on Vaccination, Paediatric Infectious Diseases Group (Switzerland) and Bündnis Kinder- und Jugendgesundheit (Germany). PWS reports speaker’s honorarium from Pfizer, travel grants from Pfizer and Gilead, and board membership for Pfizer and Gilead. DVG reports travel grants from A. Menarini GmbH and receiving equipment (for free) from Roche Diagnostics. AW reports board membership for Roche and board leadership role for the National Center for Infection Control (Swissnoso / Switzerland). All other authors declare no potential conflicts of interest.
1. Cihlar T, Mackman RL. Journey of remdesivir from the inhibition of hepatitis C virus to the treatment of COVID-19. Antivir Ther. 2022 Apr;27(2):13596535221082773. 10.1177/13596535221082773
2. Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016 Mar;531(7594):381–5. 10.1038/nature17180
3. Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017 Jun;9(396):eaal3653. 10.1126/scitranslmed.aal3653
4. Saint-Raymond A, Sato J, Kishioka Y, Teixeira T, Hasslboeck C, Kweder SL. Remdesivir emergency approvals: a comparison of the U.S., Japanese, and EU systems. Expert Rev Clin Pharmacol. 2020 Oct;13(10):1095–101. 10.1080/17512433.2020.1821650
5. U.S. Food & Drug Administration. FDA news release: FDA Approves First Treatment for COVID-19. October 22, 2020. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19
6. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al.; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020 Nov;383(19):1813–26. 10.1056/NEJMoa2007764
7. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 May;395(10236):1569–78. 10.1016/S0140-6736(20)31022-9
8. Moynihan R, Macdonald H, Bero L, Godlee F. Commercial influence and COVID-19. BMJ 2020 Jun 24;369:m2456. 10.1136/bmj.m2456
9. WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022 May;399(10339):1941–53. 10.1016/S0140-6736(22)00519-0
10. World Health Organization. WHO recommends against the use of remdesivir in COVID-19 patients. 20 November 2020, Geneva, Switzerland: World Health Organization. Available from: https://www.who.int/news-room/feature-stories/detail/who-recommends-against-the-use-of-remdesivir-in-covid-19-patients
11. World Health Organization. Therapeutics and COVID-19: living guideline. 14 July 2022, Geneva, Switzerland: World Health Organization. Available from: https://app.magicapp.org/#/guideline/nBkO1E
12. Thiabaud A, Iten A, Balmelli C, Senn L, Troillet N, Widmer A, et al. Cohort profile: SARS-CoV-2/COVID-19 hospitalised patients in Switzerland. Swiss Med Wkly. 2021 Feb;151(708):w20475. 10.4414/smw.2021.20475
13. Gil-Sierra MD, Briceño-Casado MP, Alegre-Del Rey EJ, Sánchez-Hidalgo M. Efficacy of early use of remdesivir: a systematic review of subgroup analysis. Rev Esp Quimioter. 2022 Jun;35(3):249–59. 10.37201/req/154.2021
14. Seibold H, Zeileis A, Hothorn T. Model-Based Recursive Partitioning for Subgroup Analyses. Int J Biostat. 2016 May;12(1):45–63.
15. Seibold H, Zeileis A, Hothorn T. Individual treatment effect prediction for amyotrophic lateral sclerosis patients. Stat Methods Med Res. 2018 Oct;27(10):3104–25.
16. Hothorn T, Hornik K, Zeileis A. Ctree: Conditional Inference Trees. Available from: https://cran.r-project.org/web/packages/partykit/vignettes/ctree.pdf
17. Hothorn T, Hornik K, Zeileis A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J Comput Graph Stat. 2006;15(3):651–74. 10.1198/106186006X133933
18. Athey S, Tibshirani J, Wager S. Generalized random forests. Ann Stat. 2019;47(2):1148–78. 10.1214/18-AOS1709
19. Robinson PM. Root-N-consistent semiparameteric regression. Econometrica. 1988;56(4):931–54. 10.2307/1912705
20. Gao Z, Hastie T. Estimating Heterogeneous Treatment Effects for General Responses [preprint]. 2022; arXiv:2103.04277.v4. https://doi.org/10.48550/arXiv.2103.04277
21. Hothorn T, Seibold H, Zeileis A. partykit: A Toolkit for Recursive Partytioning. Version 1.2-15. 2021; Available from: https://cran.r-project.org/web/packages/partykit/index.html
22. Mahamat-Saleh Y, Fiolet T, Rebeaud ME, Mulot M, Guihur A, El Fatouhi D, et al. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: a systematic review and meta-analysis of observational studies. BMJ Open. 2021 Oct;11(10):e052777. 10.1136/bmjopen-2021-052777
23. Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS One. 2020 Nov;15(11):e0241955. 10.1371/journal.pone.0241955
24. Mahendra M, Nuchin A, Kumar R, Shreedhar S, Mahesh PA. Predictors of mortality in patients with severe COVID-19 pneumonia - a retrospective study. Adv Respir Med. 2021;89(2):135–44. 10.5603/ARM.a2021.0036
25. Jdiaa SS, Mansour R, El Alayli A, Gautam A, Thomas P, Mustafa RA. COVID-19 and chronic kidney disease: an updated overview of reviews. J Nephrol. 2022 Jan;35(1):69–85. 10.1007/s40620-021-01206-8
26. Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003 May;58(5):377–82. 10.1136/thorax.58.5.377
27. Satici C, Demirkol MA, Sargin Altunok E, Gursoy B, Alkan M, Kamat S, et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis. 2020 Sep;98:84–9. 10.1016/j.ijid.2020.06.038
28. Han HJ, Nwagwu C, Anyim O, Ekweremadu C, Kim S. COVID-19 and cancer: from basic mechanisms to vaccine development using nanotechnology. Int Immunopharmacol. 2021 Jan;90:107247. 10.1016/j.intimp.2020.107247
29. Yeoh CB, Lee KJ, Rieth EF, Mapes R, Tchoudovskaia AV, Fischer GW, et al. COVID-19 in the Cancer Patient. Anesth Analg. 2020 Jul;131(1):16–23. 10.1213/ANE.0000000000004884
30. Belsky JA, Tullius BP, Lamb MG, Sayegh R, Stanek JR, Auletta JJ. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. 2021 Mar;82(3):329–38. 10.1016/j.jinf.2021.01.022
31. Kaka AS, MacDonald R, Linskens EJ, Langsetmo L, Vela K, Duan-Porter W, et al. Major Update 2: Remdesivir for Adults With COVID-19: A Living Systematic Review and Meta-analysis for the American College of Physicians Practice Points. Ann Intern Med. 2022 May;175(5):701–9. 10.7326/M21-4784
32. Humeniuk R, Mathias A, Cao H, Osinusi A, Shen G, Chng E, et al. Safety, Tolerability, and Pharmacokinetics of Remdesivir, An Antiviral for Treatment of COVID-19, in Healthy Subjects. Clin Transl Sci. 2020 Sep;13(5):896–906. 10.1111/cts.12840
33. Marrone A, Nevola R, Sellito A, et al. Remdesivir plus dexamethasone versus dexamethasone alone for the treatment of COVID-19 patients requiring supplemental O2 therapy: a prospective controlled non-randomized study. Clin Infect Dis. 2022 Aug 24;75(1):e403-9.
34. Hussain Alsayed HA, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Hussain AA, Hamid Q, Halwani R. Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: A retrospective cohort study. PLoS One. 2021 Oct;16(10):e0258643. 10.1371/journal.pone.0258643
35. Falcone M, Suardi LR, Tiseo G, Barbieri C, Giusti L, Galfo V, et al. Early Use of Remdesivir and Risk of Disease Progression in Hospitalized Patients With Mild to Moderate COVID-19. Clin Ther. 2022 Mar;44(3):364–73. 10.1016/j.clinthera.2022.01.007
36. Wong CK, Lau KT, Au IC, et al. Optimal Timing of Remdesivir Initiation in Hospitalized Patients With Coronavirus Disease 2019 (COVID-19) Administered With Dexamethasone. Clin Infect Dis. 2022 Aug 24;75(1):e499-508. https://doi.org/10.1093/cid/ciab728
37. ACTIV-3-Therapeutics for Inpatients with COVID-19 (TICO) Study Group. Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomized, double-blind, phase 3 trial. Lancet Respir Med. 2022 Oct;10(10):972-84. https://doi.org/10.1016/S2213-2600(22)00215-6
38. König IR, Fuchs O, Hansen G, von Mutius E, Kopp MV. What is precision medicine? Eur Respir J. 2017 Oct;50(4):1700391. 10.1183/13993003.00391-2017
The appendix is available in the pdf version of the article.