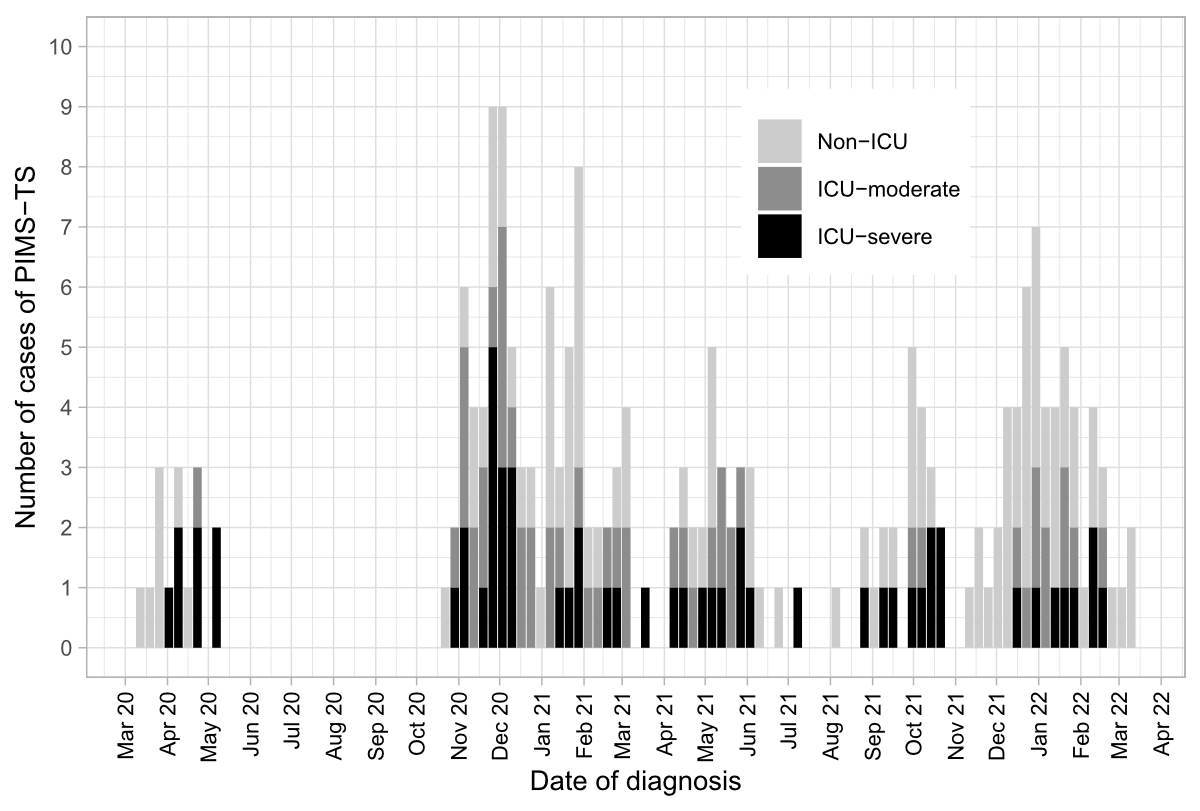
Figure 1Number of children with PIMS-TS according to ICU admission over time.
ICU: intensive care unit; PIMS-TS: paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2.
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40092
Since the description of the first cases of paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in April 2020, this disease has affected thousands of children and adolescents worldwide. In the United States of America alone, the Centers for Disease Control and Prevention (CDC) reported more than 9000 cases on the COVID Data Tracker as of 3 October 2022. PIMS-TS usually occurs 4 to 8 weeks after SARS-CoV-2 infection [1, 2]. Clinical features include fever and multiple organ dysfunction, including cardiac involvement often requiring intensive care unit (ICU) admission [3, 4]. The pandemic has generated several successive waves of various intensities and SARS-CoV-2 will continue to circulate, with an ongoing risk of PIMS-TS. Due to the potential severe course of the disease, efforts must be maintained to understand how PIMS-TS affects the heart and what long-term consequences are to be expected. The acute presentation of PIMS-TS has been described exhaustively, but the short-, medium- and long-term manifestations are still poorly understood and rely on reports from a small number of children [5, 6]. This study provides a detailed description of cardiac involvement according to disease severity in a large cohort of children and adolescents with PIMS-TS. It focuses on the detailed short-term evolution during the hospital stay and in the first two months thereafter.
The prospective national observational cohort study included children and adolescents below 18 years of age who were hospitalised with PIMS-TS in Switzerland from 1 March 2020 to 31 March 2022.
PIMS-TS was defined according to national guidelines [7]:
Data was collected as part of a larger study on SARS-COV-2 infections in children through the Swiss Paediatric Surveillance Unit (SPSU, https://www.spsu.ch) [8, 9]. All 29 paediatric hospitals in Switzerland were asked to submit cases monthly using an electronic clinical report form through REDCap (Research Electronic Data CAPture) [10] (see supplementary data for the questionnaire). For each patient, detailed epidemiological and clinical data, laboratory values, transthoracic echocardiographic findings, treatment and outcome were recorded during hospitalisation and four to six weeks after hospital discharge. Normal values for troponin T and N-terminal pro B-type natriuretic peptide (NT-proBNP) were defined according to international normal ranges for age [11]. Coronary artery dimensions and left ventricular ejection fraction (LVEF) were taken from the echocardiography report, which was completed by a paediatric cardiologist. Coronary artery involvement was classified according to the AHA guidelines [12] and using the z-score system by Dallaire and Dahdah [13], which has the following categories: no involvement: z-score <2; dilation: z-score ≥2 to <2.5; small aneurysm: z-score ≥2.5 to <5; medium aneurysm: z-score ≥5 to <10; giant aneurysm: z-score ≥10. A normal LVEF was defined as an LVEF of 55% or higher. Mild systolic dysfunction was defined as LVEF ≥45% to <55%; moderate systolic dysfunction as LVEF >35% to <45%; and severe systolic dysfunction as LVEF ≤35%. Right ventricular systolic function and right or left ventricular diastolic function were deemed abnormal or not on the basis of the echocardiography report. For the analysis, children were stratified into three groups according to disease severity: “non-ICU”: children not admitted to the ICU; “ICU-moderate”: children admitted to the ICU but not requiring invasive ventilation or inotropic support; “ICU-severe”: children admitted to the ICU and requiring invasive ventilation and/or inotropic support.
The study was approved by the Ethikkommission Nordwest- und Zentralschweiz (EKNZ 2020-01130).
Descriptive statistics were used to summarise the epidemiological and clinical data (median and interquartile range [IQR] for continuous data and proportions for categorical data). Categorical data were compared using the Chi-square test and comparisons between the three groups were assessed using the Kruskal-Wallis rank-sum test for non-normally distributed data. The Pearson and Spearman correlation coefficients were used for normally and non-normally distributed data, respectively, to assess the association between inflammatory biomarker values and coronary artery abnormalities or left ventricular systolic dysfunction. Missing data is mentioned in the tables summarising echocardiographic findings. To aid readability of tables, all other missing data are mentioned only in the supplementary data section. All analyses were performed with R software (version 2022.07.1).
Detailed data were collected for 223 children; 220 fulfilled the diagnostic criteria of PIMS-TS. Of these, 16 children were excluded from the analysis because of duplicate entry (patients referred to other hospitals). Of the 204 children included in the final analysis, 194 (95.1%) had follow-up data available at a median of 41.0 (IQR 28.0–50.0) days. Figure 1 illustrates the number of PIMS-TS cases reported over time. Median age at presentation was 9.0 (IQR 6.0–11.5) years and 142 (69.6%) children were male (table 1).

Figure 1Number of children with PIMS-TS according to ICU admission over time.
ICU: intensive care unit; PIMS-TS: paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2.
Most children had a positive SARS-CoV-2 serology (175 [85.8%]). A positive nasopharyngeal polymerase chain reaction (PCR) was reported in 54 (26.5%) children. One (0.5%) child had a positive SARS-CoV-2 antigen test. Eight (3.9%) children had a negative PCR and/or serology but had a history of SARS-CoV-2 exposure. One (0.5%) child had no laboratory evidence of SARS-CoV-2 infection or exposure to an index case but fulfilled all other clinical criteria. None of the children had been vaccinated against SARS-CoV-2. Baseline characteristics are summarised in table 1. More than half of the children (105 [51.5%]) required ICU admission; 55 (52.4%) were classified into the ICU-severe group as they needed inotropic support, with 14 of them (13.3%) also needing mechanical ventilation.
Table 1Baseline characteristics of children with PIMS-TS, by disease severity group. (Non-ICU: child not admitted to the ICU; ICU-moderate: child admitted to the ICU without need for mechanical ventilation and/or inotropic support; ICU-severe: child admitted to the ICU with need for mechanical ventilation and/or inotropic support.)
| Total | Non-ICU | ICU-moderate | ICU-severe | p-value | ||
| n (%) | n (%) | n (%) | n (%) | |||
| 204 (100) | 99 (48.5) | 50 (24.5) | 55 (26.9) | |||
| Age category (years) | 0.135 | |||||
| 0–2 | 9 (4.4) | 6 (6.1) | 3 (6.0) | – | ||
| 2–5 | 30 (14.7) | 18 (18.2) | 4 (8.0) | 8 (14.5) | ||
| 5–10 | 80 (39.2) | 39 (39.4) | 23 (46.0) | 18 (32.7) | ||
| 10–15 | 78 (38.2) | 33 (33.3) | 20 (40.0) | 25 (45.5) | ||
| 15–18 | 7 (3.4) | 3 (3.0) | – | 4 (7.3) | ||
| Female | 62 (30.4) | 33 (33.3) | 12 (24.0) | 17 (30.9) | 0.502 | |
| Ethnicity | 0.466 | |||||
| Caucasian | 128 (62.7) | 68 (68.7) | 30 (60.0) | 30 (54.5) | ||
| Black | 22 (10.8) | 5 (5.1) | 8 (16.0) | 9 (16.4) | ||
| Asian | 5 (2.5) | 3 (3.0) | 1 (2.0) | 1 (1.8) | ||
| Hispanic | 5 (2.5) | 3 (3.0) | 1 (2.0) | 1 (1.8) | ||
| Other or Unknown | 39 (19.1) | 17 (17.2) | 10 (20.0) | 12 (21.8) | ||
| Pre-existing medical conditions | ||||||
| Respiratory disease* | 9 (4.4) | 5 (5.1) | 1 (2.0) | 3 (5.5) | 0.629 | |
| Cardiovascular disease** | 6 (2.9) | 2 (2.0) | 2 (4.0) | 2 (3.6) | 0.747 | |
| Other disease*** | 23 (11.3) | 12 (12.1) | 7 (14.0) | 4 (7.3) | 0.516 | |
| SARS-CoV-2 PCR**** | 0.367 | |||||
| Positive | 54 (26.5) | 31 (31.3) | 9 (18.0) | 14 (25.5) | ||
| Negative | 134 (65.7) | 61 (61.6) | 38 (76.0) | 35 (63.6) | ||
| Not done | 16 (7.8) | 7 (7.1) | 3 (6.0) | 6 (10.9) | ||
| SARS-CoV-2 serology | 0.373 | |||||
| Positive | 175 (85.8) | 80 (80.8) | 45 (90.0) | 50 (90.9) | ||
| Negative | 6 (2.9) | 4 (4.0) | 2 (4.0) | – | ||
| Not done | 21 (10.3) | 14 (14.1) | 3 (6.0) | 4 (7.3) | ||
* Asthma 8; obstructive sleep apnoea 1.
** Atrial septal defect 3; right aortic arch 1; left heart hypoplasia after Glenn surgery 1; partial anomalous pulmonary venous connection 1.
*** Grade V vesicoureteral reflux 1; PFAPA (periodic fever, aphthous stomatitis, pharyngitis, adenitis) syndrome 1; PCDH-19 encephalopathy 1; polymalformative syndrome 1; varicella infection one week prior 1; obesity 7; Osgood Schlatter disease 3; neurofibromatosis type 1 1; unilateral multicystic dysplastic kidney 1; mild developmental delay 1; glucose-6-phosphate dehydrogenase deficiency 1; attention deficit hyperactivity disorder 3; congenital anomalies of the kidney and urinary tract 1.
**** Result of first nasopharyngeal PCR test.
ICU: intensive care unit; PCR: polymerase chain reaction; PIMS-TS: paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2.
Echocardiography was done in 201 (98.5%) children. Of these, 132 (65.7%) had a novel cardiac abnormality recorded during hospitalisation or follow-up. Left ventricular systolic dysfunction was present in 73 (35.8%) children and was more common in those admitted to the ICU (21.5% in the non-ICU group compared to 42.9% and 58.2% in the ICU-moderate and ICU-severe group, respectively [p <0.001]). Among the 73 children with left ventricular systolic dysfunction, 43 (58.9%) had mild, 13 (17.8%) moderate and 7 (9.6%) severe dysfunction (table 2). The LVEF was not documented for ten children, therefore severity of left ventricular systolic dysfunction could not be assessed. Left ventricular systolic dysfunction was present at admission in 62 (31.5%) children and developed during hospitalisation in 11 (5.4%) children. Left ventricular systolic dysfunction resolved during hospitalisation in 67 (91.8%) children. In children not admitted to the ICU, rapid resolution occurred whereas in children admitted to the ICU left ventricular systolic dysfunction initially deteriorated in some cases. Full recovery at discharge or follow-up was observed in all children (figure 2).

Figure 2Evolution of left ventricular systolic dysfunction over time, by disease severity. To make trends visible, we assigned a value of 55% (= normal) when a patient with left ventricular systolic dysfunction had recovered at the next echocardiography. (Non-ICU: child not admitted to the ICU; ICU-moderate: child admitted to the ICU without need for mechanical ventilation and/or inotropic support; ICU-severe: child admitted to the ICU with need for mechanical ventilation and/or inotropic support.) For discharged the closest echocardiography before discharge was used.
Right ventricular systolic dysfunction was reported at admission in 14 (6.9%) children and resolved in all, except one (0.5%), before discharge. No child had persistent left or right ventricular systolic dysfunction at follow-up (table 3).
Left ventricular diastolic dysfunction was reported in 23 (11.9%) children at admission and developed in eight (3.9%) children during hospitalisation. Five (2.5%) children had left ventricular diastolic dysfunction at follow-up: two (1.0%) children from the ICU-severe group had persistent diastolic dysfunction and three (1.5%) children were newly diagnosed with diastolic dysfunction, one patient in each severity group.
Coronary artery abnormalities were reported in 45 (22.1%) children (20 [20.2%], 11 [22.0%] and 14 [25.5%] in the non-ICU, ICU-moderate and ICU-severe group, respectively) (detailed in table 2). The abnormalities were diagnosed in 29 (64.4%) children at admission, in 9 (20.0%) during hospitalisation, in 4 (8.9%) at discharge and in 3 (6.7%) at follow-up. The degree of coronary artery dilation was mild in 9 (9.4%), 4 (8.0%) and 3 (5.5%) children in the non-ICU, ICU-moderate and ICU-severe group, respectively. Among the 29 children who had a coronary artery abnormality at admission, 17 (58.6%) still had some dilation during hospitalisation, and 5 (17.2%) and 4 (13.8%) had persistent coronary artery dilation at discharge and at follow-up, respectively.
Table 2Echocardiographic findings in children with PIMS-TS at admission. (Non-ICU: child not admitted to the ICU; ICU-moderate: child admitted to the ICU without need for mechanical ventilation and/or inotropic support; ICU-severe: child admitted to the ICU with need for mechanical ventilation and/or inotropic support.)
| Total | Non-ICU | ICU-moderate | ICU-severe | p-value | |
| n (%) | n (%) | n (%) | n (%) | ||
| 204 (100) | 99 (48.5) | 50 (24.5) | 55 (26.9) | ||
| Coronary involvement | 0.469 | ||||
| Normal (z-score <2) | 156 (76.5) | 76 (76.8) | 39 (78.0) | 42 (76.4) | |
| Dilation (2≤ to <2.5) | 16 (7.8) | 9 (9.1) | 4 (8.0) | 2 (3.6) | |
| Small aneurysm (2.5≤ to <5) | 27 (13.2) | 11 (11.1) | 6 (12.0) | 10 (18.2) | |
| Medium aneurysm (5≤ to <10) | 2 (1.0) | – | 1 (2.0) | 1 (1.8) | |
| Missing data | 3 (1.5) | 3 (3.0) | – | – | |
| Pericardial effusion | 50 (24.5) | 16 (16.2) | 15 (30.0) | 19 (34.5) | 0.026 |
| Missing data | 4 (2.0) | 4 (4.0) | – | – | |
| Right ventricular systolic dysfunction | 18 (8.8) | 6 (6.1) | 3 (6.0) | 9 (16.4) | 0.079 |
| Missing data | 3 (1.5) | 3 (3.0) | – | – | |
| Left or right ventricular diastolic dysfunction | 34 (16.7) | 11 (11.1) | 12 (24.0) | 11 (20.0) | 0.115 |
| Missing data | 3 (1.5) | 3 (3.0) | – | – | |
| Left ventricular systolic dysfunction | <0.001 | ||||
| Normal (LVEF ≥55%) | 124 (60.8) | 73 (73.7) | 28 (56.0) | 23 (41.8) | |
| Mild (LVEF ≥45% to <55%) | 43 (21.1) | 13 (13.1) | 13 (26.0) | 17 (30.9) | |
| Moderate (LVEF <35% to <45%) | 13 (6.4) | 4 (4.0) | 3 (6.0) | 6 (10.9) | |
| Severe (LVEF ≤35%) | 7 (3.4) | - | 2 (2.0) | 5 (9.1) | |
| LVEF not specified | 10 (4.9) | 3 (3.0) | 3 (6.0) | 4 (7.3) | |
| Missing data | 7 (3.4) | 6 (6.1) | 1 (2.0) | – | |
| Mitral valve regurgitation | 0.149 | ||||
| None | 135 (66.2) | 70 (70.7) | 33 (66.0) | 32 (58.2) | |
| Mild | 56 (27.5) | 22 (22.2) | 15 (30.0) | 19 (34.5) | |
| Moderate | 3 (1.5) | – | 1 (2.0) | 2 (3.6) | |
| Severe | 1 (0.5) | – | – | 1 (1.8) | |
| Missing data | 9 (4.4) | 7 (7.1) | 1 (2.0) | 1 (1.8) |
LVEF: left ventricular ejection fraction
At follow-up, coronary artery abnormalities were reported in 12 (5.9%) children. Details on coronary artery involvement and treatment during hospitalisation are summarised in the supplementary data (table S6).
Pericardial effusion was present in 50 (25.0%) children, occurring more frequently in children admitted to the ICU (p = 0.035). Three (1.7%) children with pericardial effusion during hospitalisation had persistent pericardial effusion at follow-up (table 3). No child had pericardial effusion that required an intervention.
Table 3Echocardiography findings during hospitalisation and follow-up (n represents an abnormal echocardiographic finding at each timepoint, not necessarily a newly diagnosed abnormality).
| Echocardiography findings | At admission | During hospitalisation | At discharge | At follow-up |
| n/total (%) | n/total (%) | n/total (%) | n/total (%) | |
| Pericardial effusion | 31/195 (15.9) | 26/138 (18.8) | 18/113 (15.9) | 3/181 (1.7) |
| Right ventricular systolic dysfunction | 14/194 (7.2) | 9/137 (6.6) | 1/112 (0.9) | 0/183 (–) |
| Left ventricular systolic dysfunction | 62/197 (31.5) | 32/139 (23.0) | 6/113 (5.3) | 0/183 (–) |
| Diastolic dysfunction | 23/193 (11.9) | 10/137 (7.3) | 5/113 (4.4) | 5/183 (2.7) |
| Coronary involvement | 29/197 (14.7) | 27/139 (19.4) | 13/113 (11.5) | 12/183 (6.6) |
n/total: abnormal results/total; %: percentage of abnormal results
Mitral valve regurgitation was reported in 60 children (29.4%) and was classified as mild in most (93.3%) cases (table 3).
An abnormal NT-proBNP peak value was reported in 182 (97.8%) children and an abnormal troponin T value in 183 (69.4%) (table S5). The median troponin T and NT-proBNP values were higher in the ICU-severe group than in the ICU-moderate and non-ICU groups (table S5). There was no correlation between coronary artery involvement and inflammatory markers (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR] or ferritin) (Pearson r coefficient of 0.0 for CRP; Spearman r coefficient of -0.2 and -0.1 for ESR and ferritin, respectively). However, a trend of increased CRP values and left ventricular systolic dysfunction was found (Pearson r coefficient of 0.22). No correlation was found between ESR or ferritin values and left ventricular systolic dysfunction (Spearman r coefficient of 0.1 and 0.04, respectively). Figure 3 summarises the key echocardiographic and laboratory abnormalities documented during hospitalisation and follow-up according to disease severity.
Electrocardiogram (ECG) abnormalities were noted in 37 (18.1%) children (table S2). Ten (4.9%) children had atrioventricular (AV) block (1st degree n = 7; 2nd degree n = 1; 3rd degree n = 2), which was significantly more frequent in children admitted to the ICU (1 [1.0%], 2 [4.0%] and 7 [12.7%] in the non-ICU, ICU-moderate and ICU-severe group, respectively) (p = 0.005). At follow-up, three (1.5%) children had persistent ECG abnormalities (right axis deviation n = 2; abnormal ST- and T-wave segment n = 1).
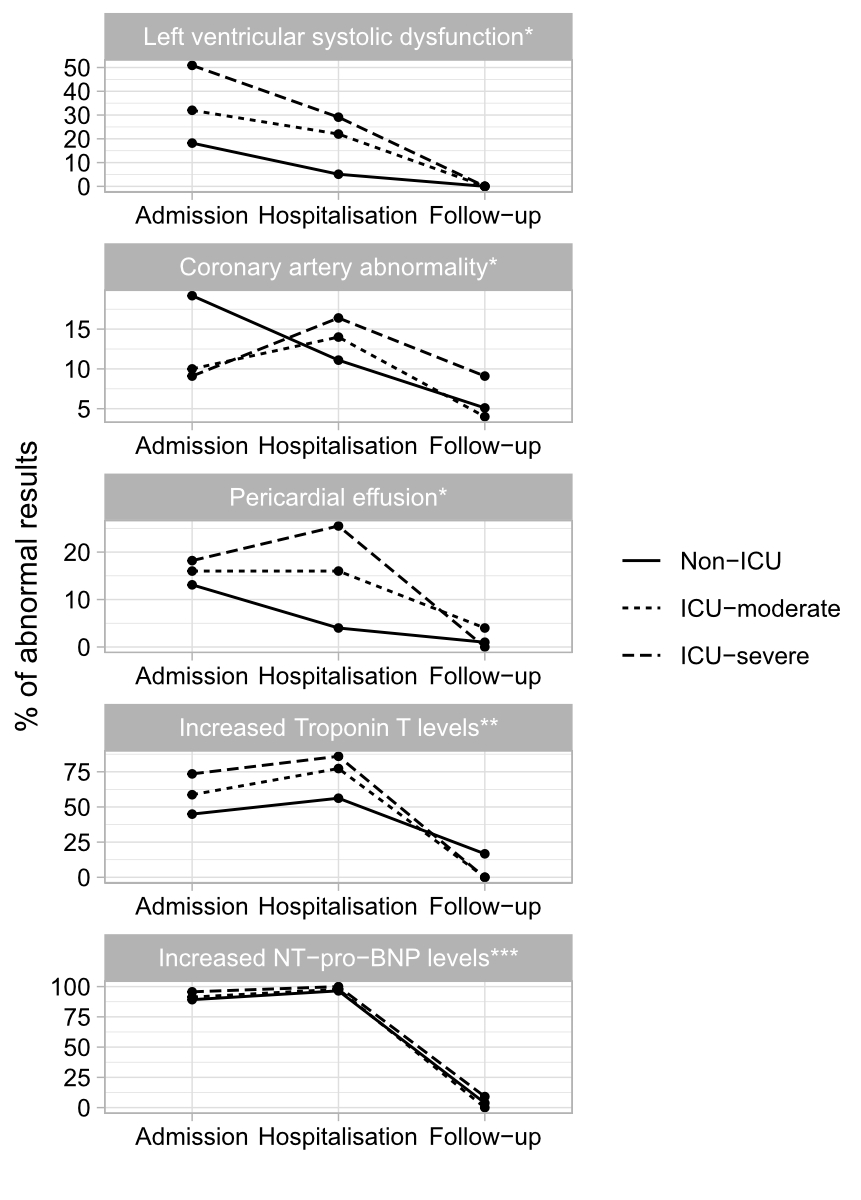
Figure 3Summary of echocardiographic and laboratory findings during admission, hospitalisation and follow-up according to disease severity. (Non-ICU: child not admitted to the ICU; ICU-moderate: child admitted to the ICU without need for mechanical ventilation and/or inotropic support; ICU-severe: child admitted to the ICU with need for mechanical ventilation and/or inotropic support.)
* Non-ICU n = 99; ICU-moderate n = 50; ICU-severe n =55.
** Troponin T reference value <14 ug/l; percentage calculated using n abnormal/total.
*** NT-proBNP reference value according to age [11]; percentage calculated using n abnormal/total.
Among the six children who had a pre-existing cardiac comorbidity, four were admitted to the ICU. Two children, one with left heart hypoplasia and one with atrial septal defect, presented with ventricular systolic dysfunction and one required inotropic support.
One child with partial abnormal pulmonary venous return presented with hypotensive shock requiring inotropic support. One child with right aortic arch presented with coronary artery dilation and mild mitral valve regurgitation.
Acetylsalicylic acid and intravenous immunoglobulin (IVIG) were the most frequently used treatments (161 [82.1%] and 155 [77.9%], respectively). The combination of IVIG and corticosteroids was used in 124 (60.8%) children. In total, 31 (15.2%) and 30 (14.7%) children, respectively, received either IVIG or corticosteroids as single treatment. Children admitted to the ICU were more likely to receive corticosteroids, anti-coagulation treatment and anakinra (p <0.001) (table S1). Thirty-two children (15.7%) had been recruited into the Swissped-Recovery Trial for randomised treatment with methylprednisolone or immunoglobulin (non-ICU n = 16; ICU-moderate n = 6; ICU-severe n = 10) [14].
The median length of hospitalisation was 6.0 (IQR 5.0–8.0) days and the duration of ICU admission was 2.0 (1.5–5.0) days. No child required treatment with extracorporeal membrane oxygenation and no deaths occurred. Follow-up data were available from 194 (95.1%) children (table 4). Limitation in daily life activities because of fatigue was reported in 15 (7.9%) children without differences according to initial disease severity (p = 0.189). School or kindergarten attendance was disrupted in 16 (8.9%) children: 4 (2.1%) complained of difficulty concentrating, 7 (3.6%) were attending school only part-time and 5 (2.6%) had not been able to return to school. Children admitted to the ICU were more likely to experience limitations in school attendance (p = 0.011). Physical activity was restricted in most children (106 [58.6%]) according to local recommendations. Few children complained of persisting symptoms at 6-week follow-up, with headache, abdominal pain and rash being the most frequently reported (10 [5.3%], 8 [4.2%] and 6 [3.1%], respectively). The frequency of these symptoms was reported regardless of initial disease severity (p >0.05).
Table 4General activity and symptoms at follow-up. Missing data is not included in this table; for further details on missing data, see table S4.
(Non-ICU: child not admitted to the ICU; ICU-moderate: child admitted to the ICU without need for mechanical ventilation and/or inotropic support; ICU-severe: child admitted to the ICU with need for mechanical ventilation and/or inotropic support.)
| Total | Non-ICU | ICU-moderate | ICU-severe | p-value | |
| n (%) | n (%) | n (%) | n (%) | ||
| 194 (100) | 92 (47.4) | 49 (25.3) | 53 (27.3) | ||
| Daily life limitation | |||||
| General activity | 15 (7.9) | 8 (9.0) | 1 (2.0) | 6 (11.3) | 0.189 |
| School/kindergarten | 16 (8.9) | 2 (2.5) | 5 (10.4) | 9 (17.6) | 0.011 |
| Sport | 106 (58.6) | 44 (52.4) | 30 (63.8) | 32 (64.0) | 0.291 |
| Persisting symptoms | |||||
| Headache | 10 (5.3) | 8 (9.1) | – | 2 (3.8) | 0.068 |
| Abdominal pain | 8 (4.2) | 6 (6.7) | – | 2 (3.8) | 0.176 |
| Rash | 6 (3.1) | 1 (1.1) | 1 (2.0) | 4 (7.7) | 0.084 |
| Fever | 3 (1.6) | 1 (1.1) | – | 2 (3.8) | 0.279 |
| Respiratory distress | 3 (1.6) | – | 1 (2.1) | 2 (3.8) | 0.204 |
| Cough | 3 (1.6) | 2 (2.2) | 1 (2.1) | – | 0.556 |
| Hypertension | 2 (1.1) | – | 1 (2.1) | 1 (2.0) | 0.402 |
| Hepato-/splenomegaly | 1 (0.5) | – | 1 (2.0) | – | 0.238 |
ICU: intensive care unit.
This large multicentre national study presents detailed cardiological data and follow-up from active surveillance of children diagnosed with PIMS-TS during the first two years of the COVID pandemic in Switzerland. The overall epidemiological data are comparable with that of other European and North American studies, including the rate of cardiac involvement [15–19]. Although the number of children of Black ethnicity is not available in Switzerland, they were likely overrepresented in our cohort, a finding that is in line with data from a previous meta-analysis [20].
Left ventricular systolic dysfunction was one of the key cardiac findings in this cohort of children with PIMS-TS, reported in approximately one in three children. This finding is also frequently reported in other studies ranging from 13% to 80% [15, 16, 21–24]. Most commonly, detection of left ventricular systolic dysfunction has been described on admission with rapid resolution before discharge [25], as documented in most of our patients. One novel finding in our cohort is a decrease in left ventricular systolic dysfunction several days after admission, which has rarely been seen previously [24]. This deterioration of cardiac function after admission emphasises the need for regular echocardiographic assessment during hospitalisation in patients presenting with PIMS-TS as left ventricular systolic dysfunction, particularly in children with more severe disease.
In our cohort, coronary artery involvement occurred regardless of the severity of illness. Interestingly, there was no correlation between coronary artery involvement and anti-inflammatory markers like CRP, ferritin and ESR. However, children with coronary artery aneurysm were more likely to be admitted to the ICU. One novel finding in our study is that coronary artery involvement can occur several days after admission. This has only rarely been reported previously: one study described a series of three children who presented with coronary artery dilation after initial presentation and treatment with IVIG [26].
We found that few children with coronary artery aneurysms had persistent aneurysms at follow-up. Most studies with short-term follow-up of coronary artery abnormalities in PIMS-TS patients describe a rapid regression to normal size of the coronary arteries — as was seen in our cohort [27–32]. Only a few coronary aneurysms persisted in our short-term follow-up; most regressed to normal size. However, regression to normal size does not equate to resolution and absence of long-term coronary artery sequelae. The pathophysiology of coronary artery aneurysms in PIMS-TS is not well understood. The occurrence may be caused by vasodilation in a highly proinflammatory milieu as described in febrile children [33]. Alternatively it may occur as a result of the destruction of the arterial wall by inflammatory cells as is known to occur in Kawasaki disease [34]. It is supposed that coronary artery dilation, as it is mostly seen in children with PIMS-TS, resolves without consequences whereas coronary artery aneurysms caused by the destruction of the arterial wall are known to increase the long-term risk of thrombosis, coronary artery stenosis due to vascular remodelling or endothelial dysfunction even in case of regression to normal diameter [12].
In our cohort, pericardial effusion was present in one in four children but was not haemodynamically relevant and did not require intervention. In the literature, pericardial effusion is generally reported as mild and has been described in 23% to 31% of patients with PIMS-TS with rapid resolution during hospitalisation [15, 16, 35]. In contrast, in our cohort the pericardial effusion was still present at discharge in some cases, with resolution at follow-up echocardiography in most cases. Mitral valve regurgitation was frequently reported but most commonly classified as mild, which was also seen in another study [15]. Moderate or severe mitral valve regurgitation was rare and only seen in patients admitted to the ICU. One study demonstrated that the resolution of mitral valve regurgitation, which follows the development of the myocardial dysfunction, lags the resolution of the left ventricular dysfunction. But almost all patients showed a complete resolution of the mitral valve regurgitation within six months [36].
We found cardiac markers including troponin T and NT-proBNP to be higher in children admitted to the ICU. Cardiac biomarkers are frequently elevated in children with PIMS-TS [37]. One study reported that NT-proBNP values were higher in children with cardiac involvement compared to those without [38]. Similarly, further studies found an association between the severity of left ventricular systolic dysfunction and higher values of troponin T and NT-proBNP [39, 40]. The rapid resolution of the left ventricular systolic dysfunction and the cardiac biomarker levels documented in our study lends weight to the hypothesis that the myocardial injury results from a temporary myocardial oedema caused by severe inflammation rather than from direct virus-mediated myocardial damage [41].
ECG abnormalities were less commonly reported in our cohort with one in five children having an abnormal ECG. In the literature, abnormal ST- and T-wave segments are more frequently noted, up to 46%, as is atrioventricular block, up to 25% [15, 24, 42, 43]. One study found that an atrioventricular block was more frequent in children admitted to the ICU [43]. In our cohort, children with atrioventricular block were also more frequently admitted to the ICU but the reason for ICU admission was most likely related to left ventricular systolic dysfunction and/or shock requiring inotropic support. Moreover, the atrioventricular conduction abnormalities were usually present at admission or prior to corticosteroid treatment, except for two children who developed atrioventricular block two days after initiation of treatment with corticosteroids. The proposed mechanism of such atrioventricular conduction abnormalities is that the hyperinflammatory response leads to widespread myocardial injury as well as oedema of the conduction tissue [44].
The diversity of treatment regimens observed in our study reflects the absence of evidence-based recommendations regarding PIMS-TS treatment. Observational study and expert consensus recommendation had guided treatment strategy [45, 46]. The results of a recent randomised controlled trial comparing methylprednisolone and IVIG treatment are expected soon and will potentially influence the future management of children with PIMS-TS [14].
The high rate of sport limitation in our study was mainly due to physical activity being advised against for three to six months if cardiac involvement was documented, as suggested by national and international guidelines [45, 47]. Children without a cardiac abnormality were recommended to stop physical activity for two weeks only [7]. Interestingly, persisting clinical symptoms on follow-up did not correlate with initial disease severity.
The strengths of this study are a relatively large number of children included with detailed clinical data available. Moreover, almost all children included had complete follow-up data available. Despite the active and established surveillance system, some cases might not have been captured in our study. Our classification of disease severity may not be applicable to other countries as the decision to admit apatient to the ICU instead of a standard ward might be determined by local practices and therefore differ between centres. However, the definition of severe cases is likely comparable as ventilation and inotropic support are clear definitions. ECG abnormalities were reported according to medical record and not all ECG were reviewed by a cardiologist, therefore we might have underestimated the rate of abnormalities. Coronary artery involvement in children is mostly assessed by transthoracic echocardiography; cardiac MRI was rarely done, therefore this data was not included for analysis.
Our study shows that cardiac involvement occurs in the majority of children and adolescents with PIMS-TS, the extent of which is central to the course of the disease. In the acute stage, ventricular dysfunction is most prominent, which increases the risk for ICU admission. For the longer-term consequences, coronary artery aneurysms are more important as they carry an increased risk of coronary artery stenosis and thrombosis. With a timely diagnosis and adequate therapy of PIMS-TS, recovery was documented in a few weeks. Close monitoring and regular follow-ups of children with PIMS-TS particularly those with coronary artery changes is required due to the possibility of long-term cardiac consequences.
Data collected for the study and the study protocol will be made available to others on request.
We thank the representatives of Swiss paediatric units: M. Albisetti; V. Bernet; F. Cachat; B. Deubzer; S. Fluri; M. Gebauer; M. Gehri; E. Giannoni; S. Grupe; S. Gysi; M. Horn; T. Karen; U. Kerr; G. Laube; B. Laubscher; J. Llor; H. Madlon; A. Malzacher; A. Merglen; S. Minocchieri; V. Muehlethaler; J. Mc Dougall; T. Riedel; M. Russo; P. Schillinger; F. Stollar; A. Woerner; A. Zemmouri. We would also like to acknowledge the administrative support by Daniela Beeli, Mirjam Mäusezahl and Fabian Tschagellar, from the Federal Office of Public Health Switzerland.
Author contributions: The study was designed by PZ and NR. Data analysis and interpretation were done by AU, PZ and NR. DW and SB critically reviewed cardiological data. Regular online meetings were conducted with all authors for critical review of the analysis. The first draft was written by AU; all authors have read and approved the final manuscript.
The study is funded by grants from the Paediatric infectious Diseases Group (PIGS) and the Federal Office of Public Health (FOPH).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
1. Roarty C, Waterfield T. Review and future directions for PIMS-TS (MIS-C). Arch Dis Child 2022;0:archdischild-2021-323143. doi:
2. Jiang L, Tang · Kun, Irfan O, et al. Epidemiology, Clinical Features, and Outcomes of Multisystem Inflammatory Syndrome in Children (MIS-C) and Adolescents-a Live Systematic Review and Meta-analysis. Curr Pediatr Rep. 2022;1:3.
3. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MB, et al.; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020 Jul;383(4):334–46. 10.1056/NEJMoa2021680
4. Radia T, Williams N, Agrawal P, Harman K, Weale J, Cook J, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr Respir Rev. 2021 Jun;38:51–7.
5 Flood J, Shingleton J, Bennett E, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS): Prospective, national surveillance, United Kingdom and Ireland, 2020. Lancet Reg Heal - Eur 2021;3:100075. doi:
6. Santos MO, Gonçalves LC, Silva PA, et al. Multisystem inflammatory syndrome (MIS-C): a systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J Pediatr (Rio J). 2021;:
7. Schlapbach LJ, Andre MC, Grazioli S, Schöbi N, Ritz N, Aebi C, et al.; PIMS-TS working group of the Interest Group for Pediatric Neonatal Intensive Care (IGPNI) of the Swiss Society of Intensive Care and the Pediatric Infectious Diseases Group Switzerland (PIGS). Best Practice Recommendations for the Diagnosis and Management of Children With Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2 (PIMS-TS; Multisystem Inflammatory Syndrome in Children, MIS-C) in Switzerland. Front Pediatr. 2021 May;9:667507.
8. Uka A, Buettcher M, Bernhard-Stirnemann S, et al. Factors Associated with Hospital and Intensive Care Admission in Paediatric SARS-CoV-2 Infection: A Prospective Nationwide Observational Cohort Study. Eur J Pediatr Published Online First; 2021.
9. Zimmermann P, Uka A, Buettcher M, et al. Neonates with SARS-CoV-2 infection: spectrum of disease from a prospective nationwide observational cohort study. 2022. doi: 10.4414/SMW.2022.w30185
10. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. doi:
11. Nir A, Lindinger A, Rauh M, Bar-Oz B, Laer S, Schwachtgen L, et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol. 2009 Jan;30(1):3–8.
12. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al.; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation. 2017 Apr;135(17):e927–99. 10.1161/CIR.0000000000000484
13. Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011 Jan;24(1):60–74.
14. Welzel T, Schöbi N, André MC, Bailey DG, Blanchard-Rohner G, Buettcher M, et al.; Swissped Recovery Trial. Multicenter Randomized Trial of Methylprednisolone vs. Intravenous Immunoglobulins to Treat the Pediatric Inflammatory Multisystem Syndrome-Temporally Associated With SARS-CoV-2 (PIMS-TS): protocol of the Swissped RECOVERY Trial. Front Pediatr. 2022 May;10:905046.
15. Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al.; AEPC COVID-19 Rapid Response Team*. Acute Cardiovascular Manifestations in 286 Children With Multisystem Inflammatory Syndrome Associated With COVID-19 Infection in Europe. Circulation. 2021 Jan;143(1):21–32.
16. Belay ED, Abrams J, Oster ME, et al. Trends in Geographic and Temporal Distribution of US Children With Multisystem Inflammatory Syndrome During the COVID-19 Pandemic Editorial Supplemental content. Published Online First: 2021. doi:
17. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al.; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020 Jul;324(3):259–69.
18. Mannarino S, Raso I, Garbin M, Ghidoni E, Corti C, Goletto S, et al. Cardiac dysfunction in Multisystem Inflammatory Syndrome in Children: an Italian single-center study. Ital J Pediatr. 2022 Feb;48(1):25.
19. Hejazi OI, Loke YH, Harahsheh AS. Short-term Cardiovascular Complications of Multi-system Inflammatory Syndrome in Children (MIS-C) in Adolescents and Children. Curr Pediatr Rep. 2021;9(4):93–103.
20. Yasuhara J, Watanabe K, Takagi H, Sumitomo N, Kuno T. COVID-19 and multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Pediatr Pulmonol. 2021 May;56(5):837–48.
21. Caro-Patón GL, de Azagra-Garde AM, García-Salido A, Cabrero-Hernández M, Tamariz A, Nieto-Moro M. Shock and Myocardial Injury in Children With Multisystem Inflammatory Syndrome Associated With SARS-CoV-2 Infection: What We Know. Case Series and Review of the Literature. J Intensive Care Med. 2021 Apr;36(4):392–403.
22. Minocha PK, Phoon CK, Verma S, Singh RK. Cardiac Findings in Pediatric Patients With Multisystem Inflammatory Syndrome in Children Associated With COVID-19. Clin Pediatr (Phila). 2021 Feb;60(2):119–26.
23. Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2021 Jul;22(8):896–903.
24 Ramcharan T, Nolan O, Chui ·, et al. Paediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS): Cardiac Features, Management and Short-Term Outcomes at a UK Tertiary Paediatric Hospital. 2020;41:1391–401. doi:
25. Das N, Hill R, Trivedi M, et al. Longitudinal Assessment of Cardiac Function Following Multisystem Inflammatory Syndrome in Children Associated with COVID-19. Pediatr Cardiol Published Online First. 2022 Jul;21: 10.1007/s00246-022-02972-3
26. Nelson MC, Mrosak J, Hashemi S, Manos C, Prahalad S, Varghese S, et al. Delayed Coronary Dilation with Multisystem Inflammatory Syndrome in Children. CASE (Phila). 2021 Sep;6(1):31–5.
27 Capone CA, Misra N, Ganigara M, et al. Six Month Follow-up of Patients With Multi-System Inflammatory Syndrome in Children. doi:
28. Sanil Y, Misra A, Safa R, Blake JM, Eddine AC, Balakrishnan P, et al. Echocardiographic Indicators Associated with Adverse Clinical Course and Cardiac Sequelae in Multisystem Inflammatory Syndrome in Children with Coronavirus Disease 2019. J Am Soc Echocardiogr. 2021 Aug;34(8):862–76.
29. Aziz OA, Sadiq M, Qureshi AU, et al. Short to midterm follow-up of multi-system inflammatory syndrome in children with special reference to cardiac involvement. Cardiol Young Published Online First; 2022.
30. Stasiak A, Kędziora P, Kierzkowska B, Niewiadomska-Jarosik K, Perdas E, Smolewska E. Changes in the cardiovascular system in children with pediatric multisystem inflammatory syndrome temporally associated with COVID-19 - A single center experience. Int J Cardiol. 2022 Aug;361:126–33.
31. Yildirim Arslan S, Sahbudak Bal Z, Bayraktaroglu S, et al. Cardiac Assessment in Children with MIS-C: Late Magnetic Resonance Imaging Features. Pediatr Cardiol. •••;1:3.
32. Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al.; Overcoming COVID-19 Investigators. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA. 2021 Mar;325(11):1074–87. 10.1001/jama.2021.2091
33. Muniz JC, Dummer K, Gauvreau K, Colan SD, Fulton DR, Newburger JW. Coronary artery dimensions in febrile children without Kawasaki disease. Circ Cardiovasc Imaging. 2013 Mar;6(2):239–44. 10.1161/CIRCIMAGING.112.000159
34 Noval Rivas M, Arditi M. Kawasaki disease: pathophysiology and insights from mouse models. doi:
35. Wong J, Theocharis P, Regan W, Pushparajah K, Stephenson N, Pascall E, et al. Medium-Term Cardiac Outcomes in Young People with Multi-system Inflammatory Syndrome: the Era of COVID-19. Pediatr Cardiol. 2022 Dec;43(8):1728–36.
36 Chakraborty A, Johnson JN, Spagnoli J, et al. Long-Term Cardiovascular Outcomes of Multisystem Inflammatory Syndrome in Children Associated with COVID-19 Using an Institution Based Algorithm. ;1:3. doi:
37. Alsaied T, Tremoulet AH, Burns JC, Saidi A, Dionne A, Lang SM, et al. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation. 2021 Jan;143(1):78–88.
38. Harahsheh AS, Krishnan A, Debiasi RL, et al. Cardiac echocardiogram findings of severe acute respiratory syndrome coronavirus-2-associated multi-system inflammatory syndrome in children. Cardiol Young Published Online First; 2021.
39 Sirico D, Basso A, Sabatino J, et al. Evolution of echocardiographic and cardiac magnetic resonance imaging abnormalities during follow-up in patients with multisystem inflammatory syndrome in children. Eur Hear J - Cardiovasc Imaging Published Online First: 21 July 2022. doi:
40. Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, Campbell AP, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021 May;5(5):323–31.
41. Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation. 2020 Aug;142(5):429–36.
42. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al.; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020 Jul;324(3):259–69.
43. Dionne A, Mah DY, Son MB, Lee PY, Henderson L, Baker AL, et al. Atrioventricular Block in Children With Multisystem Inflammatory Syndrome. Pediatrics. 2020 Nov;146(5):e2020009704.
44. Carmona CA, Levent F, Lee K, et al. Case Report Atrioventricular Conduction Abnormalities in Multisystem Inflammatory Syndrome in Children. Published Online First; 2021.
45. Schlapbach LJ, Andre MC, Grazioli S, Schöbi N, Ritz N, Aebi C, et al.; PIMS-TS working group of the Interest Group for Pediatric Neonatal Intensive Care (IGPNI) of the Swiss Society of Intensive Care and the Pediatric Infectious Diseases Group Switzerland (PIGS). Best Practice Recommendations for the Diagnosis and Management of Children With Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2 (PIMS-TS; Multisystem Inflammatory Syndrome in Children, MIS-C) in Switzerland. Front Pediatr. 2021 May;9:667507.
46. Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, et al.; French Covid-19 Paediatric Inflammation Consortium. Association of Intravenous Immunoglobulins Plus Methylprednisolone vs Immunoglobulins Alone With Course of Fever in Multisystem Inflammatory Syndrome in Children. JAMA. 2021 Mar;325(9):855–64.
47. Chowdhury D, Fremed MA, Dean P, Glickstein JS, Robinson J, Rellosa N, et al. Return to Activity After SARS-CoV-2 Infection: Cardiac Clearance for Children and Adolescents. Sports Health. 2022;14(4):460–5.
Table S1Treatment according to disease severity.
| Total | Non-ICU | ICU-moderate | ICU-severe | p-value | |
| n (%) | n (%) | n (%) | n (%) | ||
| 204 (100) | 99 (48.5) | 50 (24.5) | 55 (26.9) | ||
| IVIG | 155 (76.0) | 69 (69.7) | 42 (84.0) | 44 (80.0) | 0.233 |
| Missing data | 5 (2.5) | 2 (2.0) | 1 (2.0) | 2 (3.6) | |
| Methylprednisolone | 122 (59.8) | 42 (42.4) | 33 (66.0) | 47 (85.5) | <0.001 |
| Missing data | 6 (2.9) | 4 (4.0) | 1 (2.0) | 1 (1.8) | |
| Prednisolone | 122 (59.8) | 46 (46.5) | 36 (72.0) | 40 (72.7) | 0.001 |
| Missing data | 9 (4.4) | 5 (5.1) | – | 4 (7.3) | |
| ASA (high dose)* | 42 (20.6) | 26 (26.3) | 9 (18.0) | 7 (12.7) | 0.287 |
| Missing data | 12 (5.9) | 4 (4.0) | 4 (8.0) | 4 (7.3) | |
| ASA (low dose)** | 161 (78.9) | 74 (74.7) | 43 (86.0) | 44 (80.0) | 0.428 |
| Missing data | 8 (3.9) | 6 (6.1) | 1 (2.0) | 1 (1.8) | |
| Anakinra | 20 (9.8) | 3 (3.0) | 2 (4.0) | 15 (27.3) | <0.001 |
| Missing data | 7 (3.4) | 3 (3.0) | 1 (2.0) | 3 (5.5) | |
| Tocilizumab | 4 (2.0) | 1 (1.0) | – | 3 (5.5) | 0.207 |
| Missing data | 8 (3.9) | 4 (4.0) | 1 (2.0) | 3 (5.5) | |
| Unfractionated heparin | 57 (27.9) | 2 (2.0) | 18 (36.0) | 37 (67.3) | <0.001 |
| Missing data | 9 (4.4) | 4 (4.0) | 1 (2.0) | 4 (7.3) | |
| LMWH | 49 (24.0) | 11 (11.1) | 12 (24.0) | 26 (47.3) | <0.001 |
| Missing data | 9 (4.4) | 4 (4.0) | 1 (2.0) | 4 (7.3) | |
| Oxygen support | 60 (29.4) | 9 (9.1) | 20 (40.0) | 31 (56.4) | <0.001 |
| Missing data | – | – | – | – |
* 50 to 80 mg/kg/day
** 2 to 5 mg/kg/day
ASA: acetylsalicylic acid; ICU: intensive care unit; IVIG: intravenous immunoglobulin; LMWH: low-molecular-weight heparin
Table S2ECG findings during hospitalisation and follow-up.
| Total | Non-ICU | ICU-moderate | ICU-severe | p-value | |
| n (%) | n (%) | n (%) | n (%) | ||
| During hospitalisation | 204 (100) | 99 (48.5) | 50 (24.5) | 55 (26.9) | |
| ECG normal | 142 (69.6) | 74 (74.7) | 35 (70.0) | 33 (60.0) | 0.162 |
| ECG not done | 25 (12.3) | 15 (15.2) | 1 (2.0) | 9 (16.4) | 0.554 |
| ECG AV block | 10 (4.9) | 1 (1.0) | 2 (4.0) | 7 (12.7) | 0.005 |
| ECG Prolonged QT interval | 6 (2.9) | 3 (3.0) | 3 (6.0) | – | 0.191 |
| ECG Tachyarrhythmia | 1 (0.5) | 1 (1.0) | – | – | 0.587 |
| ECG Other abnormality * | 20 (9.8) | 5 (5.1) | 9 (18.0) | 6 (10.9) | 0.041 |
| During follow-up | 194 (100) | 92 (47.4) | 49 (25.3) | 53 (27.3) | |
| ECG normal | 164 (84.5) | 78 (84.8) | 43 (86.0) | 43 (78.2) | 0.514 |
| ECG not done | 27 (13.9) | 13 (14.1) | 6 (12.0) | 8 (14.5) | 0.896 |
| ECG abnormality ** | 3 (1.5) | 1 (1.1) | – | 2 (3.6) | 0.263 |
* abnormal ST-and T wave segment 9, new bundle branch block 4, supraventricular extrasystole 2, junctional rhythm 2, biphasic P wave in V1-V2 1, sinus bradycardia 1, low voltage 1
** axis deviation 2, abnormal ST- and T wave segment 1
AV: atrioventricular; ECG: electrocardiography; ICU: intensive care unit
Table S3Treatment on-going at follow-up.
| Total | Non-ICU | ICU-moderate | ICU-severe | p-value | |
| n (%) | n (%) | n (%) | n (%) | ||
| 194 (100) | 92 (47.4) | 49 (25.3) | 53 (27.3) | ||
| Low-dose ASA (2 to 5 mg/kg/day) | 150 (77.7) | 68 (74.7) | 36 (73.5) | 46 (86.8) | 0.174 |
| Missing data | – | – | – | – | |
| Prednisone | 26 (13.5) | 13 (14.3) | 6 (12.2) | 7 (13.2) | 0.906 |
| Missing data | 2 (1.0) | 1 (1.1) | – | 1 (1.9) | |
| LMWH | 1 (0.5) | 1 (1.1) | – | – | 0.887 |
| Missing data | 4 (2.1) | 2 (2.2) | 1 (2.0) | 1 (1.9) |
ICU: intensive care unit; ASA: acetylsalicylic acid; LMWH: Low-molecular-weight heparin
Table S4General activity and symptoms at follow-up.
| Total | Non-ICU | ICU-moderate | ICU-severe | p-value | |
| n (%) | n (%) | n (%) | n (%) | ||
| 194 (100) | 92 (47.4) | 49 (25.3) | 53 (27.3) | ||
| Daily life limitation | |||||
| General activity | 15 (7.8) | 8 (8.8) | 1 (2.0) | 6 (11.3) | 0.229 |
| Missing data | 2 (1.0) | 2 (2.2) | – | – | |
| School/kindergarten | 16 (8.3) | 2 (2.2) | 5 (10.2) | 9 (17.0) | 0.007 |
| Missing data | 13 (6.7) | 10 (11.0) | 1 (2.0) | 2 (3.8) | |
| Sport | 106 (54.9) | 44 (48.4) | 30 (61.2) | 32 (60.4) | 0.522 |
| Missing data | 12 (6.2) | 7 (7.7) | 2 (4.1) | 3 (5.7) | |
| Persisting symptoms | |||||
| Headache | 10 (5.2) | 8 (8.8) | – | 2 (3.8) | 0.119 |
| Missing data | 5 (2.6) | 3 (3.3) | 2 (4.1) | – | |
| Abdominal pain | 8 (4.1) | 6 (6.6) | – | 2 (3.8) | 0.447 |
| Missing data | 3 (1.6) | 1 (1.1) | 1 (2.0) | 1 (1.9) | |
| Rash | 6 (3.1) | 1 (1.1) | 1 (2.0) | 4 (7.5) | 0.213 |
| Missing data | 2 (1.0) | 1 (1.1) | – | 1 (1.9) | |
| Fever | 3 (1.6) | 1 (1.1) | – | 2 (3.8) | 0.462 |
| Missing data | 2 (1.0) | 1 (1.1) | 1 (2.0) | – | |
| Respiratory distress | 3 (1.6) | – | 1 (2.0) | 2 (3.8) | 0.375 |
| Missing data | 2 (1.0) | 1 (1.1) | 1 (2.0) | – | |
| Cough | 3 (1.6) | 2 (2.2) | 1 (2.0) | – | 0.695 |
| Missing data | 2 (1.0) | 1 (1.1) | 1 (2.0) | – | |
| Hypertension | 2 (1.0) | – | 1 (2.0) | 1 (1.9) | 0.757 |
| Missing data | 7 (3.6) | 3 (3.3) | 2 (4.1) | 2 (3.8) | |
| Hepato-/splenomegaly | 1 (0.5) | – | 1 (2.0) | – | 0.327 |
| Missing data | 4 (2.1) | 3 (3.3) | – | 1 (1.9) | |
ICU: intensive care unit
Table S5Cardiac biomarkers values during hospitalisation and at follow-up according to disease severity
| Non-ICU | ICU-moderate | ICU-severe | p-value | ||||
| n abnormal/total (%) | median (IQR) | n abnormal/total (%) | median (IQR) | n abnormal/total (%) | median (IQR) | ||
| Troponin T in μg/l (ref <14) | |||||||
| Admission | 40/89 (44.9) | 11.0 (5.0, 35.0) | 27/46 (58.7) | 23.0 (11.5, 39.0) | 36/49 (73.5) | 31.0 (13.8, 130.3) | <0.001 |
| Peak value | 50/89 (56.2) | 19.5 (7.0, 40.0) | 34/44 (77.3) | 32.0 (17.0, 65.0) | 43/50 (86.0) | 55.0 (28.5, 168.8) | <0.001 |
| At discharge | 31/86 (36.0) | 10.0 (5.0, 19.0) | 15/43 (34.9) | 8.5 (5.8, 21.5) | 29/49 (59.2) | 17.0 (8.0, 41.0) | 0.002 |
| At follow-up | 3/18 (16.7) | 4.0 (3.0, 8.3) | 0/7 (0.0) | 3.0 (3.0, 3.5) | 0/15 (0.0) | 3.0 (3.0, 3.5) | 0.271 |
| NT-pro-BNP in pg/ml* | |||||||
| Admission | 74/83 (89.2) | 1630 (528, 4505) | 42/46 (91.3) | 1871 (1142, 5693) | 45/47 (95.7) | 6035 (2431, 11224) | <0.001 |
| Peak value | 83/86 (96.5) | 3435 (1772, 5930) | 45/46 (97.8) | 5711 (2521, 11990) | 50/50 (100) | 10925 (7217, 23998) | <0.001 |
| At discharge | 71/82 (86.6) | 1213 (398, 2652) | 37/45 (82.2) | 804 (274, 1808) | 41/50 (82.0) | 1025 (367, 2430) | 0.252 |
| At follow-up | 1/26 (3.8) | 42.2 (26.8, 59.8) | 0/13 (0.0) | 50.0 (31.6, 60.0) | 2/22 (9.1) | 40.0 (23.1, 50.0) | 0.437 |
* NT-pro-BNP reference value according to age[11]
ICU: intensive care unit; IQR: interquartile range; PIMS-TS: Paediatric inflammatory multisystem syndrome-temporally related to severe acute respiratory syndrome coronavirus 2
Table S6Details of coronary artery involvement during hospitalisation and follow-up.
| Patient characteristics | Coronary artery involvement (z-score) | ||||||
| Age* | Sex | Severity group | Treatment | At admission | During hospitalisation | Before discharge | At follow-up |
| 4/3 | M | Non-ICU | IVIG, Pred, high**- and low-dose*** ASA | LAD (3.8) | LAD (3.8) | LAD (2.5) | LAD (4.1) |
| 8/0 | F | Non-ICU | IVIG, low-dose ASA | LMCA (3.7), LAD (2.7) | LMCA (2.8) | None | LMCA (2.2) |
| 11/3 | F | Non-ICU | IVIG, MP, Pred, low-dose ASA | LAD (2.5) | Not done | Not done | LMCA (2.3) |
| 5/6 | M | Non-ICU | IVIG, high- and low-dose ASA | RCA (2.2), LMCA (4.7), LAD (2.7), LCX (2.3) | Not done | Not done | RCA (2.1) |
| 0/5 | F | Non-ICU | IVIG, high- and low-dose ASA | None | Not done | None | LMCA (2.5) |
| 10/8 | M | ICU-moderate | IVIG, Pred, high- and low-dose ASA, clopidogrel | RCA (5.3) | RCA (5.3) | RCA (5.3) | RCA (3.5) |
| 7/5 | M | ICU-severe | IVIG, MP, Pred, low-dose ASA | None | Not done | Not done | LMCA (2.3), LAD (2.5) |
| 8/6 | M | ICU-severe | IVIG, MP, Pred, low-dose ASA | None | None | LMCA (2.8) | LMCA (2.7) |
| 2/11 | M | ICU-severe | IVIG, MP, Pred, high- and low-dose ASA | None | None | LAD (3.4) | LAD (2.0) |
| 12/11 | M | ICU-severe | IVIG, MP, Pred, low-dose ASA | None | None | RCA (5.5) | LAD (2.7), LCX (2.4) |
| 10/3 | M | ICU-severe | IVIG, PM, anakinra, low-dose ASA | None | RCA (3.3), LAD (4.5) | RCA (4.0), LAD (4.5) | LAD (2.3), LCX (2.5) |
| 4/10 | M | ICU-severe | MP, high-dose ASA | None | None | Not done | RCA (2.4) |
* years/months
** 50 to 80 mg/kg/day
*** 2 to 5 mg/kg/day
ASA: acetylsalicylic acid; IVIG: intravenous immunoglobulin; MP: methylprednisolone; Pred: prednisolone