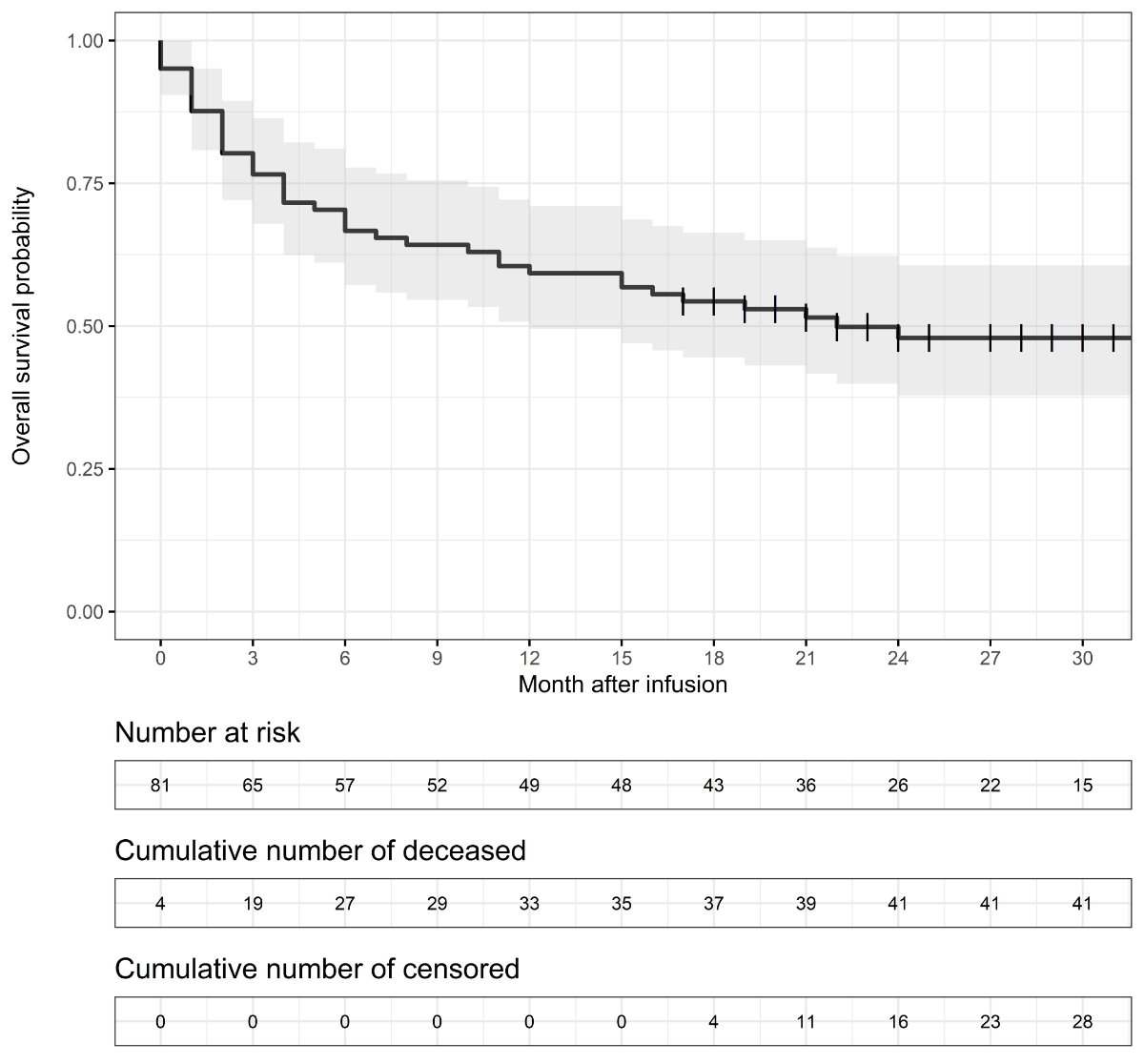
Figure 1Kaplan–Meier estimates of overall survival; vertical line = censoring mark; grey band = 95% confidence interval.
DOI: https://doi.org/https://doi.org/10.57187/s.3441
In October 2018, tisagenlecleucel (tisa-cel, Kymriah®) was the first chimeric antigen receptor T cell therapy (CAR-T) approved by Swissmedic, the Swiss Agency for Therapeutic Products. Axicabtagene ciloleucel (axi-cel, Yescarta®) was subsequently approved in April 2019. Based on the positive results of the pivotal trials (JULIET for tisa-cel [1], and ZUMA-1 for axi-cel [2]), these therapies promised substantial survival benefits and even cure for patients with diffuse large cell lymphoma (DLBCL) whose disease was relapsed or refractory (r/r) after two or more previous treatments. Tisa-cel was also approved to treat pediatric acute lymphoblastic leukaemia (p-ALL), which is outside the scope of this study. This patient group previously had a poor prognosis, with estimated 24-month overall survival rates of 17% (95% confidence interval = 13–22%) to 24% (95% CI = 18–30%), depending on disease history [3].
While the need for effective treatment options for r/r DLBCL is undisputed, reimbursement of CAR-T cell therapies poses two critical challenges to Swiss public payers. First, the established process to include new services into the reimbursement system for inpatient care has proven inadequate for highly-priced products with high unmet needs (see the Supplementary Material for details). Second, the high prices of CAR-T (average list price: CHF 373,000) raised questions about financial sustainability. Since CAR-T therapies are becoming more widespread (i.e. they are approved for new indications and earlier lines of treatment), their budget impact on public payers becomes even more relevant.
One hope was that cost offsets would reduce the budget impact of potentially curative CAR-T therapies because some subsequent treatments would be avoided [4]. The extent of these cost offsets remains highly uncertain. A pragmatic literature search on OVID found no real-world study analysing total healthcare expenditure (HCE) for patients over multiple years after CAR-T infusion. Several US studies analysed HCE during shorter post-infusion periods. Sahli et al. [5] identified 74 patients in a commercial insurance claims database and analysed the period between 30 days before and 56 days after CAR-T infusion. They estimated a mean HCE of $184,337 and a median HCE of $144,711, excluding CAR-T acquisition cost. Keating et al. [6] identified 191 patients in three US real-world databases. They estimated total HCE during the first three months after the CAR-T infusion to be $379,627 to $525,772 (including CAR-T acquisition cost). Total HCEs were generally higher among patients with cytokine release syndromes and neurological events; 27–29% of patients received subsequent salvage therapy after CAR-T infusion. Di et al. [7] analysed data for 305 patients with DLBCL in the Blue Cross Blue Shield Axis database between 41 days before and 154 days after CAR-T infusion and estimated a mean HCE of $611,900 and a median HCE of $573,300 (including CAR-T acquisition cost). Chihara et al. [8] found a median HCE of $352,572 (including CAR-T infusion costs) within 90 days after infusion among 445 Medicare patients aged ≥65; 106 patients who died or disenrolled during these 90 days were excluded from the HCE estimate. Chacim et al. [4] analysed HCE directly attributable to CAR-T cell therapy using data from 20 patients treated in a comprehensive cancer centre in Portugal. The mean HCE from referral to 150 days after infusion amounted to €358,809, including the CAR-T acquisition cost but excluding the cost of potential subsequent lines of treatment for relapsed patients and the cost of treatments for coexisting conditions.
We present a retrospective observational data study based on the administrative databases of several Swiss health insurers. This study’s primary objective is to assess pre-, peri-, and post-CAR-T infusion real-world healthcare expenditures from a public payers’ perspective. The estimates represent the total cost borne by mandatory health insurance and cantons in 2018–2022. This study’s secondary objective is to describe 24-month overall survival, the number of deaths 0–14 days after infusion, and the hospital stay length of patients who received CAR-T therapies.
This retrospective observational data analysis used anonymised patient data from the administrative databases of Concordia, CSS, Groupe Mutuel, Helsana, ÖKK, Sanitas, SWICA, Sympany, and Visana. Other insurers asked to participate could not contribute data due to a lack of technical or human resources. These health insurance companies or groups provided mandatory health insurance to approximately 78% of Swiss residents in 2021 [9]. Personal information was limited to age in five-year groups and sex. Patients were included if they received CAR-T infusion before 30 June 2021 (treatment identification cut-off). CAR-T therapies were identified using the relevant procedure codes in the supplemental catalogue of the SwissDRG system. The catalogues are available at www.swissdrg.org. This identification only included patients who received a CAR-T infusion. The patient was excluded if T-cells were collected for genetic modification but not infused. Only patients aged ≥30 years at the time of infusion were included because younger patients could be treated for pediatric acute lymphoblastic leukaemia, which is outside the scope of this study.
The primary outcome is direct healthcare expenditure (HCE) from a public payer’s perspective. Public payers of direct medical costs in Switzerland are mandatory health insurance and cantons. Cantons pay 55% of all hospital inpatient claims, while mandatory insurance funds all other benefits. It is beyond the scope of this study to analyse non-medical costs (such as home help), indirect costs (such as loss of working capacity), or intangible costs (such as loss of well-being due to pain and grief). Acquisition costs for CAR-T therapies were not included due to strict confidentiality.
HCE data were collected at 30-day intervals, starting 300 days before the patient was admitted to a hospital for a CAR-T infusion and ending 720 days after the infusion or with death or censoring, whichever occurred first. For simplification, a 30-day interval will be called a “month” in the remainder of this article. The “per patient per month” metric is frequently used in analyses of total HCE. Because insurance premiums are calculated per month, actuaries, regulators, and the general population are familiar with this metric. Claims were included if they had been processed until 31 October 2022 (data cut-off).
In the results table, expenditures are shown for three-month periods and categorised into pre-, peri-, and post-infusion. The peri-infusion period was defined as one month before to two months after the infusion. Since the peri-infusion period began one month before infusion, it might have been too short to include apheresis for all patients. The University Hospital of Zurich reported a median duration of 41 days (range = 31–62) between order placement and CAR-T cell delivery to the treatment site for patients treated until mid-2020 [10]. Nonetheless, this definition ensured a low chance of including prior lines of therapy in CAR-T treatment costs. In addition, we classified those patients as “low-cost survivors” who survived the respective three-month period and had mean monthly expenditures below CHF 500 (i.e. total three-month HCE below CHF 1,500). The CHF 1,500 threshold is high enough to cover outpatient visits, including diagnostic services, but not inpatient stays or specialised post-progression treatments.
Post-infusion healthcare expenditure can be incomplete at data cut-off because of the lag between the treatment date and claim processing and data censoring. Our primary strategy to avoid bias and a sensitivity analysis are described in the appendix.
For survival analysis, the start date was the date of the CAT-T infusion. For technical reasons, it was not possible to determine the exact date in 23% of the cases. For these cases, the hospital admission date was used instead, which might have caused a slight overestimation of the survival time after CAR-T therapy. For patients with an observed infusion date, 85% received infusion 0–5 days after hospital admission, so the overestimation of overall survival (OS) was likely to be limited. A sensitivity analysis was conducted by excluding these individuals (see supplementary materials in the appendix).
Ethical approval is not required for retrospective database studies on anonymised patient data. Potentially identifying variables such as the area of residence, treatment sites, or exact dates were not used in this study. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross sectional studies was followed.
Descriptive statistics were done using R version 4.2.1. OS was estimated applying the Kaplan-Meier method and using the R package “survival”.
This study identified 49 male and 32 female patients who underwent CAR-T therapy for LBCL between November 2018 and June 2021. For censored patients, the median time from treatment to data cut-off was 27 months, with a minimum of 17 months (interquartile range = 21–31). In our data set, the median age group was 70–74 years, with a minimum of 30–34 years and a maximum of 80–84. These estimates do not represent the entire treated population in Switzerland because patients aged <30 years were excluded. For comparison, table 1 also includes information on the population in a Swiss real-world data analysis by Stolz et al. [10] and the pivotal trials (JULIET for tisa-cel/Kymriah® [1, 11], and ZUMA-1 for axi-cel/Yescarta® [2, 12]). The 62 years reported by Stolz et al. is the best available estimate for the median age in the entire treated population in Switzerland.
Previous lines of therapy could not be analysed, but all patients must have had at least two lines of therapy to be eligible for reimbursement of CAR-T therapy [13]. Most patients were treated for r/r DLBCL, but data might include some individuals treated for r/r primary mediastinal LBCL. Diagnostic information is not routinely available in claims data, so it could not be used as a direct inclusion criterion. Among the 81 patients, 66.7% (54/81) were treated with tisa-cel (Kymriah®) and 33.3% (27/81) were treated with axi-cel (Yescarta®).
Table 1The patient population in the Swiss claims data study.
| Swiss claims data study | Stolz et al. (2022) | JULIET trial (tisa-cel) | ZUMA-1 trial (axi-cel) | |
| N (patients who received infusion) | 81 | 21 | 111 | 101 |
| Median age (range), 5 yr group or yr | 70–74 (30–34; 80–84)* | 62 (20; 76) | 56 (22; 76) | 58 (23; 76) |
| Age ≥65 yr, no. (%) | 54 (67%)* | nr | 25 (23%) | 24 (24%) |
| Male, no. (%) | 49 (60%) | 16 (76%) | nr | 68 (67%) |
| Previous treatments | ||||
| Number of prior treatment lines, mean (range) | nr (2-nr)** | 3.6 (2–7) | nr | nr |
| ≥3 prior lines of therapy | nr | nr | 57 (52%) | 70 (69%) |
* This number is not representative of the entire patient population treated in Switzerland because patients aged <30 years were excluded.
** the number of previous lines of therapy was not directly identifiable in claims data, but all patients must have had at least two lines of therapy to be eligible.
nr = not reported
The HCE per patient per month in the pre-, peri-, and post-CAR-T cell infusion periods are shown in table 2. Each row represents a three-month period. Patients who were deceased or censored during the period account for less than the full three months. During the peri-infusion period (one month before infusion and two months after), the mean expenditure per month amounted to CHF 38,490 and the median to CHF 25,765 (interquartile range = CHF 18,884–38,170). The high mean compared to the median indicates a right-skewed distribution, which is confirmed in figure S1 in the appendix. Eighty-six per cent of these expenditures were billed for hospital inpatient services (DRG payments and additional fees).
The mean monthly HCE during the first post-infusion year amounted to CHF 8,696–11,342 (table 2), and the median monthly HCE to CHF 3,446–4,733. The proportion of low-HCE survivors increased with time since infusion and amounted to approximately 15% in months 15–17 after treatment. Inpatient expenditures, including drugs administered during hospital stays, accounted for approximately 40–50% of total expenditure in the post-CAR-T infusion period. Many patients required inpatient treatment in the months and years after CAR-T infusion.
For comparison, expenditures in the 10 months before CAR-T treatment are shown in the first rows in table 2. These expenditures are likely to include the costs of second and/or later lines of therapy because they often follow quickly after each other. A recent review reported the median treatment duration for patients with DLBCL in the second and third treatment lines as 2.3 and 3.4 months, respectively [14]. For patients with rapid disease progression, even the first line of treatment might be included in this period. The mean expenditure in months 10 to 5 before CAR-T therapy was CHF 8,115–11,353 (median = CHF 4,524–8,044). The mean expenditure in months 4 to 2 before CAR-T infusion was CHF 22,564 (median = CHF 17,509).
Table 2HCE pre- and post-CAR-T infusion.
| HCE per month | Low-HCE survivors (%) | Share inpatients | Share drugs outpatients | |||||||
| Period | Pat. N | Pat. months | Mean | SE | 25th percentile | 50th percentile | 75th percentile | |||
| Pre: m 10–8 | 78 | 234 | 8,115 | 2,285 | 1,000 | 4,524 | 9,705 | 16 (21%) | 56% | 16% |
| Pre: m 7–5 | 78 | 234 | 11,353 | 3,007 | 2,130 | 8,044 | 15,207 | 4 (5%) | 59% | 12% |
| Pre: m 4–2 | 78 | 234 | 22,564 | 4,513 | 10,962 | 17,509 | 25,920 | 0 (0%) | 61% | 8% |
| Peri infusion* | 78 | 230 | 38,490 | 6,874 | 18,884 | 25,765 | 38,170 | 0 (0%) | 86% | 3% |
| Post: m 3–5 | 67 | 186 | 8,696 | 2,276 | 2,231 | 4,733 | 11,900 | 1 (2%) | 38% | 17% |
| Post: m 6–8 | 56 | 162 | 11,342 | 3,482 | 1,823 | 3,970 | 11,312 | 3 (5%) | 43% | 19% |
| Post: m 9–11 | 50 | 150 | 10,643 | 4,696 | 1,196 | 3,465 | 8,945 | 5 (10%) | 49% | 23% |
| Post: m 12–14 | 46 | 132 | 9,722 | 6,262 | 1,290 | 3,446 | 10,801 | 6 (13%) | 50% | 24% |
| Post: m 15–17 | 40 | 105 | 6,981 | 3,451 | 464 | 2,265 | 5,288 | 6 (15%) | 43% | 27% |
| Post: m 18–20 | 29 | 80 | 5,068 | 2,216 | 1,222 | 2,603 | 6,963 | 2 (7%) | 19% | 31% |
| Post: m 21–23 | 22 | 57 | 7,455 | 5,565 | 1,547 | 2,493 | 3,593 | 1 (5%) | 55% | 14% |
Pre/post = before or after CAR-T infusion; m = month; IQR = interquartile range; “Low-HCE survivors” are defined as patients who are alive and non-censored for the entire interval and have an average cost per month below CHF 500 during this interval.
* Expenditures for CAR-T therapies are not included (list price = CHF 373,000).
Table 3 shows the total HCE (excluding CAR-T acquisition costs) per patient during the entire observation period from 30 days before CAR-T cell infusion until death or censoring. The mean HCE, excluding CAR-T cell therapy, was CHF 215,737, with a median of CHF 151,539 and an interquartile range of 100,238 to 250,234.
In cost-effectiveness modelling, it is common to evaluate the costs for end-of-life care and curative treatments separately. A direct identification of end-of-life care was infeasible in our data. As an approximation, we calculated total healthcare expenditures in the last 30 days of life, excluding patients who died within 30 days of CAR-T infusion and patients who were admitted to the hospital more than 30 days before their death. The rationale behind this decision was a technical concern: Hospital inpatient services are billed per case in the DRG-based system. For patients who remained continuously hospitalised for more than 30 days before their eventual demise, the per-case hospital claim encompasses services rendered within and outside the specified 30-day window. Table 3 shows that mean expenditures in the last 30 days of life were CHF 29,193 (median = CHF 21,567). Most of these expenses were for hospital inpatient care.
Table 3Total HCE from treatment to the end of the observation period: HCE in the last 30 days of life.
| Period | Pat. | HCE peri- and post-infusion** | Share inpatients | Share drugs | ||||
| Mean | SE | 25th percentile | 50th percentile | 75th percentile | ||||
| Peri- and post-infusion* | 78 | 215,737 | 21,279 | 100,238 | 151,539 | 250,234 | 66% | 12% |
| Last 30 days of life | 34 | 29,193 | 4,947 | 12,189 | 21,567 | 39,682 | 83% | 7% |
* 24 months or until death/earlier censoring.
** HCE excluded the cost of CAR-T therapy (average list price = CHF 373,000).
Figure 1 shows the Kaplan-Meier curve of overall survival during the first 30 post-infusion months. The curve descends with observed deaths, while vertical lines represent data censorings. The complete Kaplan-Meier data and the cumulative hazard plot are provided in the appendix. Four per cent of patients (3/81) died within 0–2 days after CAR-T infusion, and another died within the first 14 days after CAR-T infusion. The estimated 12-month OS rate was 59% (95% CI 49–71%), and the estimated 24-month OS rate was 48% (38–61%). For the deceased patients, the mean survival time after CAR-T infusion was 7.4 months.

Figure 1Kaplan–Meier estimates of overall survival; vertical line = censoring mark; grey band = 95% confidence interval.
A few other patient-relevant outcomes could be observed in the claims data: 11% of patients (9/81) died during the hospital stay for CAR-T infusion, and 2% (2/81) had to stay in the hospital for at least two months after the CAR-T infusion. The median hospital stay duration after CAR-T infusion was 20 days, and the mean duration was 23 days (see figure S6 in the appendix for the distribution). It is important to note that providers in Switzerland are obliged to monitor patients daily within the first 10 days after CAR-T infusion to detect symptoms of cytokine release syndrome, neurological events, or other toxicities. Nine per cent of patients (7/81) were discharged 10–14 days after CAR-T infusion._Ref113865120
Our study described direct medical expenditures from a Swiss public payer’s perspective of patients with LBCL treated by CAR-T in 2019–2021. HCEs were categorised into pre-, peri-, and post-CAR-T infusion periods.
The observed post-infusion costs of CHF 5,068 to CHF 11,342 per month or CHF 215,000 over two years are higher than previous cost estimates for non-Hodgkin lymphoma in Switzerland. Wieser et al. [15] translated US estimates by Mariotto et al. [16] into Switzerland and estimated that patients with non-Hodgkin lymphoma incurred direct medical costs of CHF 33,429 to CHF 51,269 in the last treatment year. The high mean cost in a population treated with a potentially curative CAR-T therapy is remarkable. This patient population was expected to include many patients with durable responses whose low healthcare expenditures impact the grand mean. This point is best illustrated by an example. It is assumed that 20% of patients treated with CAR-T experience durable responses, and the 12-month overall survival is 60%. In this scenario, the patient population after 12 months can be divided into two groups: One-third of patients will be in a state of remission, while two-thirds will have experienced disease progression. The healthcare expenditures of the first group are expected to be low, significantly impacting the grand mean.
Two primary factors can explain the escalated cost per patient with disease progression. Firstly, many novel treatment alternatives have become accessible since the previous cost estimations. Secondly, patients with LBCL who received CAR-T therapy represent more severe cases than the average non-Hodgkin lymphoma case.
Our observed real-life expenditure over two years also exceeds the estimated lifetime expenditures of infused patients of CHF 139,000 in a recent cost-effectiveness study [17]. This cost-effectiveness study reported a total lifetime cost of CHF 403,470, but CHF 264,821 were attributable to CAR-T therapy costs, which were excluded in our study (see [18] and table 10 in the appendix). However, these costs are not directly comparable since our estimates include total healthcare expenditure, while cost-effectiveness studies focus on treating one specific disease. See appendix, section 6, for a discussion on the decomposition of healthcare expenditure.
Our findings of considerable total care costs relating to CAR-T infusion are consistent with several US real-world data analyses cited in the introduction. Jalbert et al. provided one possible explanation for high post-infusion expenditures [19]. They analysed 129 patients with DLBCL treated with CAR-T and estimated the risk of receiving radiation or systemic treatment as 36% (95% CI = 27–46%) after six months and 48% (95% CI = 44–71%) after 12 months.
Our study estimated overall survival rates as 59% (95% CI = 49–71%) after 12 months and 48% (95% CI = 38–61%) after 24 months. These estimates are comparable to other recent real-world data studies. Using data for 408 patients registered in the retrospective French DESCAR-T registry study, Bachy et al. [18] estimated 12-month overall survival at 64% (95% CI = 55–71%) with axi-cel and 49% (95% CI = 40–47%) with tisa-cel. In an analysis of cases enrolled in the German Registry for Stem Cell Transplantation, Bethge et al. [20] estimated 12-month survival at 52%, but their real-world population included patients with poor performance status who would not have been eligible for the ZUMA-1 trial. Using data for 149 patients in the Center for International Blood and Marrow Transplant Research (CIBMTR) database, Shadman et al. [21] estimated a 24-month OS rate of 47% (95% CI = 33–60%).
However, our estimates are less optimistic than a recent cost-effectiveness study that estimated an average life expectancy of 9.24 years after infusion [17]. In our real-world data, deceased patients had a mean survival time after CAR-T infusion of 7.4 months. In order to attain an average of 9.24 years in the entire dataset, the censored patients would have to attain a mean survival time of 17 years. This residual life expectancy is longer than the 16 years of 70-year-old male members of the Swiss general population [22], which seems implausible given the disease history and frailty of patients given CAR-T therapy.
An interesting question is how the survival rates of patients after CAR-T therapies compare to those of patients treated with other therapy options. It is beyond this study’s scope to indirectly compare our patient population with historical controls. An often-cited historical comparison is the SCHOLAR-1 study, which performed a patient-level pooled retrospective analysis of a population of patients with refractory DLBCL. For the subgroup refractory to second or greater line therapy, 24-month OS was 17% (95% CI = 13–22%) [3].
Maziarz et al. [23] indirectly compared tisagenlecleucel (tisa-cel; Kymriah®) and historical treatments for r/r DLBCL. Using standardised mortality ratio weights, they found an overall survival hazard ratio of 60% (95% CI = 44–77%) in favour of tisagenlecleucel in the intention-to-treat (ITT) population. In the same analysis, they found 24-month OS rates in the ITT population of 34% (95% CI = 26–42%) with tisa-cel compared to 16% (95% CI = 12–22%) with the other treatments, also favouring tisagenlecleucel. Salles et al. [24] conducted a matching-adjusted indirect comparison of another CAR-T therapy (lisocabtagene maraleucel) and salvage chemotherapy for r/r DLBCL. After matching and adjusting, they found an overall survival hazard ratio of 50% (95% CI = 40–60%) in favour of lisocabtagene maraleucel and a median overall survival of 20.5 months (95% CI = 12.1–unreached) with CAR-T therapy compared to 6.0 months (95% CI = unreported) with salvage chemotherapy, also favouring the CAR-T therapy (lisocabtagene maraleucel). Using real-world data from one clinical centre in the US, Sermer et al. [25] compared the outcomes of 69 patients treated with CAR-T therapy with a historical population of 146 patients treated with alternative therapies. Observed 12-month OS rates were 64% (95% CI = 54–77%) in the CAR-T group and 39% (95% CI = 31–48%) in the alternative treatment group. In a multivariate analysis adjusting for pre-treatment characteristics, the treatment type was not a significant predictor of OS.
Our analysis of HCE (primary outcome) had several limitations. First, some pre- and post-CAR-T treatments are not recorded in insurance claims data because they are part of clinical trials or early access programs. Those new treatments might be covered by mandatory health insurance in the future, leading to higher expenditure for public payers. Second, pre- and post-CAR-T treatment patterns are not standardised and are rapidly changing, further reducing the future generalizability of our results. Third, patient selection and optimal treatment timing remain under evaluation [10], and our study population might not represent future patients. Fourth, three patients had to be excluded from the sample because their insurers could not report complete data on expenditures. Fifth, our estimates are a lower bound for actual expenditures because of possible delayed claims and censoring. Based on the sensitivity analyses shown in the appendix, we expect our mean post-infusion HCE estimates to be underestimated by 3–4%.
The main limitation in measuring overall survival was the relatively short follow-up period. With less than 20% of patients still at risk after 30 months, long-term estimates are too uncertain. Two other limitations are common to most observational studies. The first is the absence of a control group. We could not assess how similar patients would have done after other treatment settings and were, therefore, unable to analyse cost-effectiveness. The second limitation is the presence of many post-infusion therapies, often also novel approaches, which make it difficult to assess the effectiveness of CAR-T therapies alone.
A general caveat of our study is its limited scope, which leaves unanswered many questions that are highly relevant to patients, physicians, and society. First, we could not describe the treated patient population in detail. Because Swiss health insurance data does not contain diagnostic information, neither the patient’s disease history nor relevant comorbidities could be analysed. Second, we could not analyse the duration of progression-free survival or the time to the next treatment. Data from registries or treatment sites should be used to analyse these highly relevant issues.
Two years after the introduction of CAR-T therapies for relapsed or refractory DLBCL, much uncertainty remains around the budget impact on public payers. We conducted a retrospective insurance claims data analysis and found high mean healthcare expenditure peri- and post-infusion (excluding CAR-T acquisition cost). Further research is needed to understand the drivers behind these expenditures. Data from registries and treatment sites should be used to analyse progression-free survival and post-infusion treatment patterns and relate them to prognostic factors.
The challenges of high and uncertain costs and uncertain long-term benefits are not limited to CAR-T therapies and apply to many newly approved therapies. For example, a recent study found that less than 40% of drugs approved through accelerated approval or conditional marketing authorisation pathways in Europe and the US from 2007 to 2021 were rated by official health technology assessment agencies in Germany, France, or Canada as providing at least moderate therapeutic value compared to existing therapies [26]. The authors concluded that post-approval studies should be given more importance in the decision-making process. Registries and administrative databases provide valuable sources of information for such studies. From a societal perspective, balancing fast access to therapies with sustainable public financing will be very challenging. Monitoring expenditures and benefits in the real world is an essential step towards creating transparency and finding new pathways for societal-decision making.
We thank Magdalena Bernzen (Helsana), Anne-Catherine Miranda Cruzado (Visana), Adrien De Marchi (Groupe Mutuel), Kazim Demir (Sympany), Heidi Rüttimann (CSS), and Thomas Sudler (Concordia) for excellent data preparation. We thank David Bumann (Groupe Mutuel), Claire Galesne (Groupe Mutuel), Dr. med. Beat Kipfer (KPT), Dr. Aurélien Sallin (SWICA), Dr. med. Ulrich Tanner (Concordia), Daniela Vögeli (SWICA), and Peter Welsch (Sympany) for providing valuable insights during the design and writing of this study.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Most authors and contributors work for health insurers providing mandatory health insurance in Switzerland. The companies didn’t directly influence the conceptualization or results presented, but the study was written from the perspective of a public payer. The academic experts Mark Pletscher and Niklaus Meier regularly advise different Swiss Health insurers in topics related health technology assessment and pricing.
1. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al.; JULIET Investigators. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019 Jan;380(1):45–56.
2. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017 Dec;377(26):2531–44.
3. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017 Oct;130(16):1800–8.
4. Chacim S, Monjardino T, Cunha JL, Medeiros P, Redondo P, Bento MJ, et al. Costs, effectiveness, and safety associated with Chimeric Antigen Receptor (CAR) T-cell therapy: results from a comprehensive cancer center. PLoS One. 2022 Dec;17(12):e0278950.
5. Sahli B, Eckwright D, Darling E, Gleason PP, Leach JW. Chimeric antigen receptor T-cell therapy real-world assessment of total cost of care and clinical events for the treatment of relapsed or refractory lymphoma. J Clin Oncol. 2021 May;39(15 _suppl):e19500–19500.
6. Keating SJ, Gu T, Jun MP, McBride A. Health Care Resource Utilization and Total Costs of Care Among Patients with Diffuse Large B Cell Lymphoma Treated with Chimeric Antigen Receptor T Cell Therapy in the United States. Transplant Cell Ther. 2022 Jul;28(7):404.e1–6.
7. M. Di et al., “Total Costs of Care during Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed/Refractory B Cell Non-Hodgkin Lymphoma: A Large Private Insurance Claim-Based Analysis,” Blood, vol. 140, no. Supplement 1, pp. 10818–10819, Nov. 2022, doi: .
8. D. Chihara et al., “Real-World Effectiveness and Economic Impact Associated with Chimeric Antigen Receptor T-Cell Therapy Among Older Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma in US,” Blood, vol. 140, no. Supplement 1, pp. 2421–2423, Nov. 2022, doi: .
9.Swiss Federal Office of Public Health (2022) Statistik der obligatorischen Krankenversicherung 2021. Tabelle T 5.05, access on 15.08.2022.
10. Stolz S, Roncador M, Rösler W, Zenz T, Manz MG, Müller AM, et al. Introducing innovative cellular therapies into the clinic: a 2-year retrospective experience of a chimeric antigen receptor T-cell programme at a single centre in Switzerland. Swiss Med Wkly. 2022 Jun;152(25–26):w30186. 10.4414/SMW.2022.w30186
11. Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021 Oct;22(10):1403–15.
12. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019 Jan;20(1):31–42.
13 Krankenpflege-Leistungsverordnung KLV, Anhang 1.
14. Morrison VA, Shou Y, Bell JA, Hamilton L, Ogbonnaya A, Raju A, et al. Evaluation of treatment patterns and survival among patients with diffuse large B-cell lymphoma in the USA. Future Oncol. 2019 Mar;15(9):1021–34.
15. Wieser S, et al. Die Kosten der nichtübertragbaren Krankheiten in der Schweiz. Winterthur: Zurich University of Applied Sciences, Winterthur Institute of Health Economics; 2014.
16. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011 Jan;103(2):117–28.
17. Moradi-Lakeh M, Yaghoubi M, Seitz P, Javanbakht M, Brock E. Cost-Effectiveness of Tisagenlecleucel in Paediatric Acute Lymphoblastic Leukaemia (pALL) and Adult Diffuse Large B-Cell Lymphoma (DLBCL) in Switzerland. Adv Ther. 2021 Jun;38(6):3427–43.
18. Jalbert JJ, Wu N, Chen CI, Ambati S, Ge W, Arnason JE. Real-World Treatment Patterns After CD19-Directed CAR T Cell Therapy Among Patients with Diffuse Large B Cell Lymphoma. Adv Ther. 2022 Jun;39(6):2630–40.
19. Bachy E, Le Gouill S, Di Blasi R, Sesques P, Manson G, Cartron G, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022 Oct;28(10):2145–54.
20. W. A. Bethge et al., “GLA/DRST real-world outcome analysis of CAR-T cell therapies for large B-cell lymphoma in Germany,” Blood, p. blood.2021015209, Mar. 2022, doi: .
21. Shadman M, Pasquini M, Ahn KW, Chen Y, Turtle CJ, Hematti P, et al. Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood. 2022 Mar;139(9):1330–9.
22 Swiss Federal Statistical Office (2022): Life expectancy.
23. Maziarz RT, Zhang J, Yang H, Chai X, Yuan C, Schwarz E, et al. Indirect comparison of tisagenlecleucel and historical treatments for relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2022 Apr;6(8):2536–47.
24. Salles G, Spin P, Liu FF, Garcia J, Kim Y, Hasskarl J. Indirect Treatment Comparison of Liso-Cel vs. Salvage Chemotherapy in Diffuse Large B-Cell Lymphoma: TRANSCEND vs. SCHOLAR-1. Adv Ther. 2021 Jun;38(6):3266–80.
25. Sermer D, Batlevi C, Palomba ML, Shah G, Lin RJ, Perales MA, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020 Oct;4(19):4669–78.
26. Vokinger KN, Kesselheim AS, Glaus CE, Hwang TJ. Therapeutic Value of Drugs Granted Accelerated Approval or Conditional Marketing Authorization in the US and Europe From 2007 to 2021. JAMA Health Forum. 2022 Aug;3(8):e222685.
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3441.