Association of chocolate consumption with neurological and cardiovascular outcomes
in atrial fibrillation: data from two Swiss atrial fibrillation cohort studies (Swiss-AF
and BEAT-AF)
DOI: https://doi.org/https://doi.org/10.57187/smw.40109
Annina Staubera,
Andreas Müllera,
Nikki Rommersbc,
Stefanie Aeschbacherbd,
Nicolas Rodondief,
Leo H. Bonatig,
Juerg H. Beerhi,
Raban V. Jegera,
David J. Kurza,
Claudia Liedtkea,
Peter Ammannj,
Marcello Di Valentinok,
Patricia Chocanoe,
Richard Kobzal,
Michael Kühnebd,
David Conenm,
Stefan Osswaldbd,
Alain M. Bernheima
a Department of Cardiology, Triemli
Hospital Zurich, Zurich, Switzerland
b Cardiovascular Research Institute
Basel, University Hospital Basel, Basel, Switzerland
c Department of Clinical Research, University
of Basel, Basel, Switzerland
d Cardiology Division, Department of
Medicine, University of Basel, Basel, Switzerland
e Institute of Primary Health Care
(BIHAM), University of Bern, Bern, Switzerland
f Department of General Internal
Medicine, Inselspital, Bern University Hospital, University of Bern, Bern,
Switzerland
g Neurology Division and Stroke
Centre, Department of Clinical Research, University Hospital Basel, Basel, Switzerland
h Department of Medicine, Cantonal
Hospital of Baden, Baden, Switzerland
i Molecular Cardiology, University
Hospital Zurich, Zurich, Switzerland
j Department of Cardiology,
Kantonsspital St Gallen, St. Gallen, Switzerland
k Department of Cardiology, Ospedale
San Giovanni, Bellinzona, Switzerland
l Department of Cardiology, Luzerner
Kantonsspital, Luzern, Switzerland
m Population Health Research
Institute, McMaster University, Hamilton, Canada
Summary
AIM: To
assess the associations of chocolate consumption with neurocognitive function, brain
lesions on magnetic resonance imaging (MRI), and cardiovascular outcome in
patients with atrial fibrillation (AF).
METHODS: We
analysed data from patients of two prospective multicentre Swiss atrial
fibrillation cohort studies (Swiss-AF) and (BEAT-AF). Assessments of MRI
findings and neurocognitive function were performed only in the Swiss-AF
population (in 1727 of 2415 patients [71.5%] with a complete data set), as
patients enrolled in BEAT-AF were not systematically evaluated for these
outcomes. Otherwise, the two cohorts had an equivalent set of clinical
assessments. Clinical outcome analysis was performed in 3931 patients of both
cohorts. Chocolate consumption was assessed by questionnaire. Patients were
categorised as no/low chocolate consumption (No/Low-Ch) ≤1 servings/week,
moderate chocolate consumption (Mod-Ch) >1–6 servings/week, and high chocolate
consumption (High-Ch) >6 servings/week, respectively. Brain lesions were
evaluated by MRI. Assessment of cognitive function was performed by
neurocognitive functional testing and included global cognition measurement
with a cognitive construct score. Cerebral MRI and cognition were evaluated at
baseline. Cross-sectional associations between chocolate consumption and MRI
findings were analysed by multivariate logistic regression models and associations
with neurocognitive function by multivariate linear regression models. Clinical
outcome events during follow-up were recorded and assessed by a clinical event
committee. The associations between chocolate consumption and clinical outcomes
were evaluated by Cox regression models. The median follow-up time was 6 years.
RESULTS:
Chocolate consumption was not associated with prevalence or volume of vascular
brain lesions on MRI, nor major adverse cardiac events (ischaemic stroke,
myocardial infarction, cardiovascular death). However, No/Low-Ch was
independently associated with a lower cognitive construct score compared to Mod-Ch
(No/Low-Ch vs. Mod-Ch: coeff. –0.05, 95% CI –0.10–0), whereas other
neurocognitive function tests were not independently associated with chocolate
consumption categories. In addition, there was a higher risk of heart failure
hospitalisation (No/Low-Ch vs. Mod-Ch: HR 1.24, 95% CI 1.01–1.52) and of
all-cause mortality (No/Low-Ch vs. Mod-Ch: HR 1.29, 95% CI 1.06–1.58) in No/Low-Ch
compared to Mod-Ch. No significant associations with the evaluated outcomes
were observed when High-Ch was compared to Mod-Ch.
CONCLUSION:
While chocolate consumption was not associated with MRI findings and major
adverse cardiac events in an atrial fibrillation population, No/Low-Ch was
associated with a lower cognitive construct score, higher risk of heart failure
hospitalisation and increased all-cause mortality compared to Mod-Ch.
ClinicalTrials.gov Identifier:
NCT02105844
Introduction
Atrial
fibrillation (AF) is the most common arrhythmia in European countries. In view
of an ageing population, its prevalence is expected to increase further [1]. Atrial
fibrillation has been associated with an increased risk of cardiovascular
morbidity and death [2]. According to previous investigations, chocolate
consumption is linked to a decreased risk of cardiovascular and cerebrovascular
disease [3–5] and may affect cognitive function [6–9].
The most
important components of
chocolate are cocoa flavonoids and methylxanthines [6]. A number of beneficial systemic
effects have been
attributed to flavonoids, including increased nitric oxide bioavailability [10],
antioxidant properties [10, 11], antithrombotic mechanisms and anti-inflammatory
effects [12]. Both flavonoids and methylxanthines may permeate the blood-brain
barrier. Whereas flavonoids may affect brain function via mechanisms such as
increased cerebral blood flow [8, 13], promotion of cerebral angiogenesis [9, 13],
or inhibition of neuronal death by apoptosis [9], methylxanthines may act as
mild central nervous system stimulants [6].
Chocolate
may have the most favourable effect when consumed in moderation [3, 4]. Moderate
chocolate intake may also be inversely related to the risk of clinically
apparent atrial fibrillation [14]. However, data regarding this association are
conflicting [15, 16]. To date, the effect of chocolate consumption on
neurocognitive function, cerebral and cardiovascular outcomes in patients
diagnosed with atrial fibrillation has not been studied. With a per capita
consumption of 9.9 kg/year, the Swiss population has the highest consumption of
this aliment in Europe [17]. The prevalence of atrial fibrillation in
Switzerland is 600–699/100,000 persons [18]. The widespread consumption of chocolate
in a
country with a high prevalence of atrial fibrillation gave rise to searching
for associations between chocolate intake and clinical outcomes in a Swiss AF
population.
Based on
the above-mentioned considerations, the aim of the present study was to
evaluate the association between chocolate consumption and 1) neurocognitive
function, 2) brain lesions on MRI, and 3) cardiovascular events in patients
with atrial fibrillation.
Methods
Study design, data
sources and participants
Data from two
ongoing prospective, observational multicentre cohort studies from Switzerland – the
Swiss Atrial Fibrillation
Cohort study (Swiss-AF) and the Basel Atrial Fibrillation Cohort study
(BEAT-AF) – were included in this study [19, 20]. Patients were eligible for
participation in Swiss-AF if they had a history of documented atrial fibrillation
and if they were aged 65 years or older. An additional 10–15% of patients between
45–65 years of age were aimed to be
enrolled to assess atrial fibrillation in individuals who are potentially in
the active workforce [19]. In BEAT-AF, patients with atrial fibrillation
documented on electrocardiogram were asked to participate [20]. In both cohort
studies, patients could be recruited from in- and outpatient clinics. Enrolment
of patients with acute illnesses was postponed until stabilisation. More
detailed information on the underlying cohorts is described elsewhere [19, 20]. Both
cohort studies use an equivalent set of clinical assessments, including
chocolate consumption, patient demographic characteristics, medical history,
and medication. During the course of the study, information on clinical events
is collected yearly.
For data
derived from brain magnetic resonance
imaging (MRI) as well as from cognitive functional testing, only data
from the Swiss-AF cohort were included. In BEAT-AF, these outcomes were not
systematically evaluated. For the analyses regarding brain lesions and
cognitive function testing, we used the baseline data (= data of the first
visit) from all enrolled Swiss-AF patients with a full data set, i.e., patients
that had a baseline MRI as well neurocognitive function testing performed at
the initiation visit. The association between chocolate consumption and
clinical events was assessed using the data of all Swiss-AF and BEAT-AF
patients with available information on baseline chocolate consumption.
The local Ethics Committees approved the study
protocols of both registries. All patients gave written informed consent.
Categorisation of chocolate consumption
Chocolate
consumption was reported by the patients on a yearly basis via a
multiple-choice question with the following nine answer options: never or less
than one bar (German: “Riegel”) per month, 1–3 bars per month, 1 bar per week, 2–4
bars per week, 5–6 bars per week, 1 bar per day, 2–3 bars per day, 4–5 bars per day,
and 6+ bars per day,
respectively.
One serving
is considered to correspond to approximately 30 g of chocolate [21]. As the size
and composition of a chocolate bar is variable and the chocolate content is therefore
difficult to determine, we chose to equate a bar of chocolate to one serving.
According to two meta-analyses, the quantity of chocolate intake potentially
associated with a reduction in the risk of cardiovascular disease ranges from
45g to 180 g/week, which is considered to represent moderate consumption [3, 4].
Consequently, we defined three groups of chocolate consumption. Patients were
stratified into the no/low chocolate consumption group (No/Low-Ch) if they
stated they ate ≤1 servings (corresponding to ≤30 g/week) of chocolate per week.
In the moderate chocolate consumption group (Mod-Ch), the weekly chocolate
intake ranged from >1 to 6 servings (>30 g to 180 g per week), and in the
high chocolate consumption group (High-Ch), chocolate consumption exceeded 6
servings per week (>180 g per week).
Clinical measures
Weight and
height were directly measured and body mass index (BMI) calculated. Patient
history was assessed at baseline. Educational status, smoking status, medical
history, and history of oral anticoagulation medication were obtained by
questionnaire. Atrial fibrillation type was classified as paroxysmal,
persistent, or permanent atrial fibrillation [18].
Outcome measures
The primary
interest of our study was to evaluate the association between chocolate
consumption and neurocognitive function in a real-world atrial fibrillation
population. Given the unique data set on cerebral MRI performed and
systematically analysed in a large number of Swiss-AF participants, we
additionally intended to search for associations between chocolate intake and
brain lesions. Moreover, the assembly of data from both the Swiss-AF and the
BEAT-AF cohorts enabled us to evaluate the association between chocolate
consumption and clinical outcome measures in a large population of patients
suffering from atrial fibrillation. The study was exploratory in nature and
aimed to be hypothesis-generating rather than confirmatory. Therefore, no
distinct primary endpoint was defined prior to analysis of data.
Cognitive testing
Centrally
trained study personnel performed standardised neurocognitive assessments. The
tests were provided in paper format in the main national languages of
Switzerland (i.e., German, French and Italian) [22]. Neurocognitive testing
included the Montreal Cognitive Assessment (MoCA)
which is a screening test to detect mild
cognitive impairment. Patients can obtain a score from 0 to 30 points [23]. The Trail
Making Test (TMT) parts A and B and the
Semantic Fluency Test (SF) were included for detection of dementia [24, 25]. In
the TMT, patients connect circled numbers in ascending order by drawing a
continuous line (trail) between them. Trails A and B are of different lengths. The
test score is defined as the time used divided by the total number of circles
correctly connected in that time [22, 24]. In the SF test, patients are asked to
name as many animals as possible within 60 seconds [25].
The Digit
Symbol Substitution Test (DSST) was used to evaluate psychomotor performance [26].
In this test, patients receive a key grid of numbers and matching symbols. The
score is the number of correct number-symbol matches achieved within 120
seconds [22, 26]. Additionally, we used the cognitive construct score, a factor
score developed for the Swiss-AF study that allows for quantification of
cognitive function. The test is composed of 17 differently weighted combined
items from all of the above-mentioned individual neurocognitive tests [22].
Brain MRI
Brain MRI
was performed on 1.5 or 3 Tesla MRI scanners. A standardised protocol was used
in all participating centres. The standard protocol did not demand the administration
of contrast agent. Brain lesions were assessed as small non-cortical infarcts
(SNCIs), large non-cortical or cortical infarcts (LNCCIs), microbleeds (Mb),
and white matter lesions (WML) [19]. As 99% of patients presented with WML, we
used the Fazekas score as a binary endpoint for WML in the analysis. At least
moderate disease was defined as a score ≥2 [27].
Main clinical
outcome measures
Main
clinical outcome measures were prespecified and included major adverse cardiac
events (combined end-point of ischaemic stroke, myocardial infarction and
cardiovascular death), stroke, major bleeding, myocardial infarction, hospitalisation
for heart failure, cardiovascular death, and all-cause death, respectively. If
a clinical outcome measure was reported or found in the medical records,
additional information was collected from involved hospitals and/or treating
physicians. All events were adjudicated by a blinded clinical event committee
[19].
Statistical
analysis
Baseline
patient characteristics by categories of chocolate consumption were described
by mean and standard deviation, or absolute and relative frequency, as
appropriate. Prevalence of lesions on baseline MRI were presented as absolute
and relative frequency, lesion volume and lesion count as median and
interquartile range. Results from neurocognitive tests were described by mean
and standard deviation. Group comparisons were performed by ANOVA tests (one-way,
three groups) for continuous variables and by
chi-squared tests for categorical variables.
In all
analyses, we considered available data from both the BEAT-AF and Swiss-AF
cohort until 13 May 2022.
In all the
analyses evaluating the association between chocolate consumption and outcomes,
Mod-Ch was used as the reference category and the two other categories were
compared to this reference category.
The
association between chocolate consumption and cerebral lesions on MRI was
examined using multivariable-adjusted mixed-effects logistic regression models,
including study centre as a random intercept. In this analysis, we included all
patients with a brain MRI reading available at baseline (n = 1727). In patients
that presented with cerebral lesions, we also analysed the association between
chocolate consumption and lesion volume using multivariable-adjusted
mixed-effects linear regression models with the lesion volumes (log-transformed
due to the skewed distribution and mean-centred) as the dependent variable.
LNCCIs, SNCI, WML and Mb were analysed. These models also included study centre
as a random intercept.
In all
patients with a brain MRI reading available at baseline, we also assessed the
cross-sectional association between chocolate consumption and cognitive
function using linear mixed-effects models with study centre as a random
intercept. The test scores of the performed neurocognitive tests as well as the
calculated cognitive construct factor score were used as continuous outcome
variables. Given the previous association between the neurocognitive test
scores and the presence as well as the volume of neurological lesions [28], we
additionally adjusted the models for neurocognitive outcomes for the presence
and volume of LNCCI, the presence of white matter lesions with Fazekas scale
≥2, as well as the volume of white matter lesions. The volumes were set to 0
for patients who did not present with the particular lesions. Given the very
low proportion of missing data at baseline, we performed an available case
analysis.
We
investigated the association between chocolate consumption and clinical events
using Cox proportional hazard models with study centre as a stratification
factor. All variables in the model were time-updated to account for variations
in chocolate consumption and other covariates over time (information updated
with each patient visit). In case of missing data occurring at follow-up visits
or of missed visits, we performed a simple imputation with the patient’s last
observation carried forward until the next visit or censoring. In potentially
recurring events, only the first event was considered.
For each
outcome, two models were constructed: (1) a model adjusted for age and gender,
and (2) a model adjusted for age, gender, and additional clinical covariates
(the lists of covariates are displayed in the corresponding tables). For each
model, the estimates (odds ratio for logistic regression models, coefficient
for linear models, and hazard ratios for Cox models) for the fixed effects of
the different levels of chocolate consumption along with their corresponding
95% confidence interval (CI) are reported.
As a
sensitivity analysis, we investigated the potential effect of the two different
cohorts by adding an interaction term of cohort with chocolate consumption.
This did not result in a better model fit and therefore it was not considered
in the analysis.
All
analyses were performed using the statistical software R version 4.2.2. The
analytical code is provided as supplementary material (appendix 1).
Ethics statement
The studies
involving human participants were reviewed and approved by the Ethics Committee
Nordwest- und Zentralschweiz, Switzerland and all local Ethics Committees at
the study sites. The project numbers were 2021-00701 for Swiss-AF and EK 331/09
for BEAT-AF (both numbers from the lead Ethics Committee).
Results
Brain MRI and
neurocognitive function analysis
Baseline
characteristics
Of the 2415
patients enrolled in the Swiss-AF cohort, 1727 (71.5%) patients were included
in the analysis for brain lesions on brain MRI and for neurocognitive function
testing. 672 (27.8%) patients did not undergo brain MRI; the main reason for
this was an implanted cardiac device (n = 461; [68.6%]). Other reasons were
contraindications for MRI and claustrophobia. 11 (0.5%) patients were excluded
from the analysis due to missing MoCA assessment during the baseline visit and
in 5 (0.2%) patients we did not have information on chocolate consumption at
baseline.
Baseline
characteristics of the 1727 included patients are displayed in table 1. 1127 (65.3%)
patients were in the No/Low Ch,
375 (21.7%) in the Mod-Ch, and 225 (13.0%) in the High-Ch group.
Table 1Baseline characteristics of patients in the
brain MRI and neurocognitive function analyses.
| Chocolate consumption
groups |
Overall |
No/Low-Ch |
Mod-Ch |
High-Ch |
p |
| n |
1727 |
1127 |
375 |
225 |
|
| Age (y), mean (SD) |
72.55 (8.39) |
72.49 (8.51) |
72.42 (8.21) |
73.09 (8.06) |
0.58 |
| Female, n (%) |
474 (27.4) |
292 (25.9) |
109 (29.1) |
73 (32.4) |
0.098 |
| BMI (kg/m2), mean (SD) |
27.66 (4.75) |
27.96 (4.83) |
27.39 (4.68) |
26.61 (4.32) |
<0.001 |
| Active smoker, n (%) |
130 (7.5) |
83 (7.4) |
26 (6.9) |
21 (9.3) |
0.53 |
| Arterial hypertension, n (%) |
1196 (69.3) |
793 (70.4) |
258 (68.8) |
145 (64.4) |
0.21 |
| Education level, n (%) |
|
|
|
|
0.44 |
| − basic |
203 (11.8) |
140 (12.4) |
34 (9.1) |
29 (12.9) |
|
| − middle |
843 (48.8) |
551 (48.9) |
184 (49.1) |
108 (48.0) |
|
| − advanced |
681 (39.4) |
436 (38.7) |
157 (41.9) |
88 (39.1) |
|
| AF-type (non-paroxysmal), n (%) |
936 (54.2) |
648 (57.5) |
175 (46.7) |
113 (50.2) |
0.001 |
| History of diabetes mellitus, n (%) |
270 (15.6) |
203 (18.0) |
48 (12.8) |
19 (8.4) |
<0.001 |
| History of stroke, n (%) |
228 (13.2) |
138 (12.2) |
57 (15.2) |
33 (14.7) |
0.27 |
| History of heart failure, n (%) |
373 (21.6) |
250 (22.2) |
76 (20.3) |
47 (20.9) |
0.7 |
| History of renal failure, n (%) |
312 (18.1) |
225 (20.0) |
60 (16.0) |
27 (12.0) |
0.009 |
| Oral anticoagulation, n (%) |
1555 (90.0) |
1015 (90.1) |
341 (90.9) |
199 (88.4) |
0.62 |
Prevalence and volume of brain lesions on MRI
Analysis of brain MRI showed LNCCI in 387
(22.4%) patients, SNCI in 367 (21.3 %), Mb in 371 (21.5 %), and WML in 1710
(99.0%) patients. Of the patients with WML, 926 (53.7%) presented with a
Fazekas score ≥2 (table 2).
Table 2Lesions
detected by brain MRI at baseline.
| Chocolate
consumption groups |
Overall |
No/Low-Ch |
Mod-Ch |
High-Ch |
|
n |
1727 |
1127 |
375 |
225 |
| LNCCI |
Prevalence |
387 (22.4) |
258 (22.9) |
78 (20.8) |
51 (22.7) |
| Volume |
1623 [255, 7314] |
1337 [229, 6824] |
2340 [399, 6107] |
1656 [227, 8805] |
| Count |
1.0 [1.0, 2.0] |
1.0 [1.0, 2.0] |
1.0 [1.0, 2.0] |
1.0 [1.0, 3.0] |
| SNCI |
Prevalence |
367 (21.3) |
238 (21.1) |
83 (22.1) |
46 (20.4) |
| Volume |
63 [30.0, 160.5] |
66 [30.0, 167.2] |
57 [30.0, 159.0] |
57 [33.0, 107.3] |
| Count |
1.0 [1.0, 3.0] |
2.0 [1.0, 3.0] |
1.0 [1.0, 2.0] |
1.0 [1.0, 2.0] |
| Microbleeds |
Prevalence |
371 (22.2) |
248 (22.8) |
71 (19.4) |
52 (24.0) |
| Number |
1.0 [1.0, 2.0] |
1.0 [1.0, 2.0] |
1.0 [1.0, 2.0] |
1.0 [1.0, 2.0] |
| WML |
Prevalence |
1710 (99.0) |
1121 (99.5) |
371 (98.9) |
218 (96.9) |
| Volume |
3921 [1446, 9786] |
3792 [1395, 9705] |
3738 [1476, 8954] |
5334 [1700, 11770] |
| Count |
23 [11.0, 41.0] |
22 [11.0, 40.0] |
23 [11.0, 41.0] |
25 [14.0, 44.8] |
| Fazekas scale ≥2 |
926 (53.7) |
591 (52.5) |
202 (53.9) |
133 (59.1) |
The
association between chocolate consumption and the prevalence of lesions detected
by brain MRI is presented in table 3. In the simple model adjusted for age and
gender, as well as in the full model adjusted for additional clinical
variables, there was no association found for prevalence of brain lesions on
brain MRI and the different groups of chocolate consumption (table 3).
Moreover, no significant associations were found between the volume of brain
lesions and chocolate consumption (table 3).
Table 3Association
between chocolate consumption and the prevalence of brain lesions.
|
Simple model |
Full model |
| No/Low-Ch vs Mod-Ch |
High-Ch vs Mod-Ch |
No/Low-Ch vs Mod-Ch |
High-Ch vs Mod-Ch |
| Outcome |
OR (95% CI) |
p |
OR (95% CI) |
p |
OR (95% CI) |
p |
OR (95% CI) |
p |
| LNCCI |
1.12 (0.84, 1.49) |
0.46 |
1.10 (0.73, 1.65) |
0.65 |
1.22 (0.88, 1.68) |
0.23 |
1.14 (0.72, 1.79) |
0.56 |
| SNCI |
0.93 (0.69, 1.24) |
0.61 |
0.87 (0.57, 1.33) |
0.52 |
0.96 (0.71, 1.29) |
0.79 |
0.87 (0.57, 1.34) |
0.53 |
| Microbleeds |
1.20 (0.89, 1.62) |
0.23 |
1.28 (0.85, 1.93) |
0.22 |
1.22 (0.90, 1.66) |
0.20 |
1.31 (0.86, 2.00) |
0.20 |
| Fazekas ≥2 |
0.93 (0.72, 1.21) |
0.59 |
1.20 (0.83, 1.73) |
0.334 |
0.91 (0.70, 1.18) |
0.48 |
1.17 (0.81, 1.70) |
0.40 |
| Outcome |
coeff (95% CI) |
p |
coeff (95% CI) |
p |
coeff (95% CI) |
p |
coeff (95% CI) |
p |
| LNCCI volume |
−0.29 (−0.81, 0.22) |
0.27 |
−0.06 (−0.78, 0.66) |
0.87 |
−0.24 (−0.71, 0.23) |
0.31 |
−0.01 (−0.67, 0.64) |
0.97 |
| SNCI volume |
0.07 (−0.23, 0.36) |
0.66 |
−0.09 (−0.52, 0.33) |
0.66 |
0.1 (−0.20, 0.40) |
0.50 |
−0.06 (−0.49, 0.37) |
0.80 |
| WML volume |
−0.02 (−0.16, 0.12) |
0.80 |
0.14 (−0.07, 0.34) |
0.19 |
−0.04 (−0.18, 0.10) |
0.59 |
0.13 (−0.07, 0.33) |
0.20 |
Neurocognitive
function tests
The
results of neurocognitive function tests are shown in table 4. Whereas MoCA and
cognitive construct varied between groups, the remaining neurocognitive test
results were similar. The association between chocolate consumption and
neurocognitive function in multivariate analysis is shown in table 5. In the
simple model adjusting for age and gender, Mod-Ch was associated with better
TMT-B, DSST, and cognitive construct results compared to No/Low-Ch. However, in
the full model of the multivariate analysis, only the association between
Mod-Ch and a better cognitive construct score remained significant. Evaluation
of neurocognitive test results in High-Ch versus Mod-Ch exhibited no
significant associations in the two statistical models (table 5).
Table 4Results of neurocognitive
function tests.
| Chocolate consumption
group |
Overall |
No/Low-C |
Mod-Ch |
High-Ch |
p |
| n |
1727 |
1127 |
375 |
225 |
|
| MoCA |
25.53 (3.03) |
25.38 (3.05) |
25.78 (2.98) |
25.81 (2.98) |
0.03 |
| TMT-A |
0.54 (0.22) |
0.54 (0.22) |
0.55 (0.20) |
0.53 (0.21) |
0.6 |
| TMT-B |
0.22 (0.11) |
0.21 (0.11) |
0.23 (0.11) |
0.21 (0.10) |
0.13 |
| DSST |
44.54 (14.19) |
44.02 (14.70) |
45.97 (13.03) |
44.72 (13.31) |
0.07 |
| SF |
19.04 (5.36) |
18.96 (5.46) |
19.35 (5.13) |
18.92 (5.18) |
0.45 |
| CoCo |
0.03 (0.53) |
0.01 (0.54) |
0.09 (0.49) |
0.03 (0.50) |
0.05 |
Table 5Association
between chocolate consumption and neurocognitive function.
|
Simple model |
Full model |
|
No/Low-Ch vs. Mod-Ch |
High-Ch vs. Mod-Ch |
No/Low-Ch vs. Mod-Ch |
High-Ch vs. Mod-Ch |
| Outcome |
coeff (95% CI) |
p |
coeff (95% CI) |
p |
coeff (95% CI) |
p |
coeff (95% CI) |
p |
| MoCA |
−0.41 (−0.74, −0.08) |
0.14 |
0.07 (−0.39, 0.54) |
0.76 |
−0.27 (−0.59, 0.06) |
0.11 |
0.07 (−0.38, 0.53) |
0.76 |
| TMT−A |
−0.01 (−0.03, 0.01) |
0.36 |
−0.01 (−0.04, 0.02) |
0.63 |
−0.004 (−0.03, 0.02) |
0.72 |
−0.01 (−0.04, 0.02) |
0.65 |
| TMT−B |
−0.01 (−0.02, −0.00) |
0.02 |
−0.01 (−0.02, 0.01) |
0.36 |
−0.01 (−0.02, 0.003) |
0.14 |
−0.01 (−0.02, 0.01) |
0.33 |
| DSST |
−1.81 (−3.26, −0.36) |
0.01 |
−0.60 (−2.65, 1.46) |
0.57 |
−1.09 (−2.46, 0.28) |
0.12 |
−0.53 (−2.46, 1.40) |
0.59 |
| SF |
−0.37 (−0.97, 0.22) |
0.22 |
−0.28 (−1.1, 0.56) |
0.51 |
−0.22 (−0.81, 0.37) |
0.47 |
−0.27 (−1.09, 0.56) |
0.53 |
| CoCo |
−0.08 (−0.13, −0.03) |
0.003 |
−0.03 (−0.10, 0.05) |
0.44 |
−0.05 (−0.10, 0) |
0.049 |
−0.03 (−0.10, 0.04) |
0.43 |
Clinical outcome
analysis
Baseline
characteristics of patients included in the clinical outcome analysis
Of the 4039
patients in the Swiss-AF and the BEAT-AF cohorts, 3931 (97.2%) were included in
the analysis. 108 patients were excluded due to missing data at baseline. The
median observation time was 6.05 years. The total person-years of follow-up
added up to 21,726 years. The baseline characteristics of the patients
stratified by the level of chocolate consumption are shown in table 6.
Table 6Baseline
characteristics of patients in the clinical outcome analysis.
| Chocolate consumption group |
Overall |
No/Low-Ch |
Mod-Ch |
High-Ch |
p |
| n |
3.931 |
2.548 |
836 |
547 |
|
| Age (y), mean (SD) |
71.42 (10.07) |
71.48 (9.79) |
71.38 (10.56) |
71.68 (10.35) |
0.86 |
| Female, n (%) |
1117 (28.2) |
678 (26.6) |
249 (29.8) |
178 (32.5) |
0.9 |
| BMI (kg/m2),
mean (SD) |
27.46 (4.76) |
27.74 (4.80) |
27.18 (4.64) |
26.54 (4.51) |
<0.001 |
| Active smoker, n (%) |
313 (7.9) |
212 (8.3) |
59 (7.1) |
38 (6.9) |
0.35 |
| Arterial hypertension, n
(%) |
2736 (69.1) |
1790 (70.3) |
571 (68.3) |
356 (65.1) |
0.49 |
| Highest education level,
n (%) |
|
|
|
|
0.4 |
| − basic |
483 (12.3) |
324 (12.8) |
77 (9.2) |
75 (13.7) |
|
| − middle |
1936 (49.1) |
1256 (49.4) |
421 (50.5) |
254 (46.5) |
|
| − advanced |
1520 (38.6) |
961 (37.8) |
336 (40.3) |
217 (39.7) |
|
| AF type non-paroxysmal, n
(%) |
2020 (51.0) |
1355 (53.2) |
397 (47.5) |
253 (46.3) |
0.1 |
| History of diabetes, n
(%) |
635 (16.0) |
469 (18.4) |
106 (12.7) |
54 (9.9) 106 (19.4) |
<0.001 |
| History of stroke / TIA,
n (%) |
676 (17.1) |
418 (16.4) |
151 (18.1) |
|
0.18 |
| History of heart failure,
n (%) |
942 (23.8) |
621 (24.4) |
187 (22.4) |
126 (23.0) |
0.44 |
| History of renal failure,
n (%) |
741 (18.7) |
521 (20.5) |
144 (17.3) |
75 (13.7) |
0.1 |
| History of MI, n (%) |
582 (14.7) |
398 (15.6) |
110 (13.2) |
71 (13.0) |
0.1 |
| History of CAD, n (%) |
1059 (26.7) |
730 (28.7) |
206 (24.6) |
117 (21.4) |
0.1 |
| History of major
bleeding, n (%) |
152 (3.8) |
95 (3.7) |
30 (3.6) |
27 (4.9) |
0.37 |
| Oral anticoagulation, n
(%) |
3333 (84.2) |
2141 (84.1) |
703 (84.1) |
464 (85.0) |
0.87 |
| Antithrombotic
medication, n (%) |
189 (4.8) |
133 (5.3) |
33 (4.0) |
22 (4.0) |
0.21 |
Association between
chocolate consumption and clinical outcomes
During follow-up, a total of 1358 (34.5%)
patients experienced one or more clinical events. Major adverse cardiac events
occurred in 560, stroke in 210, major bleeding in 392, hospitalisation for
heart failure in 632, myocardial infarction in 150, cardiovascular death in
446, and all-cause death in 726 patients, respectively. The association between
chocolate consumption and clinical outcomes in multivariate analysis is shown
in Table 7. No independent association of chocolate consumption with major
adverse cardiac events was found. In the simple model adjusting for age and gender
as well as following adjustment for various additional clinical parameters, the
risk of hospitalisation for heart failure and of all-cause death was higher in No/Low-Ch
compared to Mod-Ch (table 7). For the comparison between High-Ch vs. Mod-Ch no
corresponding associations were seen. The Kaplan-Meier curves for the
end-points hospitalisation for heart failure and all-cause death in the three
groups of chocolate consumption are presented in figure 1.
Table 7Association
between chocolate consumption and clinical outcomes.
| |
Simple
model |
Full
model |
| HR |
95% CI |
HR |
95% CI |
| MACE |
No/Low-Ch vs. Mod-Ch |
1.21 |
[0.98, 1.50] |
1.12 |
[0.90, 1.39] |
| High-Ch vs Mod-Ch |
1.02 |
[0.76, 1.38] |
0.98 |
[0.72, 1.32] |
| Stroke |
No/Low-Ch vs. Mod-Ch |
0.99 |
[0.71, 1.39] |
0.99 |
[0.70, 1.38] |
| High-Ch vs Mod-Ch |
0.94 |
[0.59, 1.51] |
0.88 |
[0.54, 1.42] |
| Major bleeding |
No/Low-Ch vs. Mod-Ch |
1.25 |
[0.97, 1.61] |
1.23 |
[0.95, 1.59] |
| High-Ch vs. Mod-Ch |
1.06 |
[0.74, 1.51] |
1.08 |
[0.75, 1.54] |
| Hospitalisation for acute heart failure |
No/Low-Ch vs. Mod-Ch |
1.36 |
[1.11, 1.67] |
1.24 |
[1.01, 1.52] |
| High-Ch vs Mod-Ch |
0.82 |
[0.60, 1.11] |
0.84 |
[0.62, 1.15] |
| Myocardial infarction |
No/Low-Ch vs. Mod-Ch |
1.36 |
[0.89, 2.07] |
1.26 |
[0.82, 1.92] |
| High-Ch vs Mod-Ch |
1.10 |
[0.61, 2.00] |
1.14 |
[0.63, 2.07] |
| Cardiac death |
No/Low-Ch vs. Mod-Ch |
1.19 |
[0.94, 1.52] |
1.08 |
[0.85, 1.39] |
| High-Ch vs Mod-Ch |
1.04 |
[0.75, 1.45] |
0.92 |
[0.66, 1.30] |
| All-cause death |
No/Low-Ch vs. Mod-Ch |
1.42 |
[1.16, 1.73] |
1.29 |
[1.06, 1.58] |
| High-Ch vs Mod-Ch |
1.24 |
[0.95, 1.62] |
1.15 |
[0.88, 1.51] |
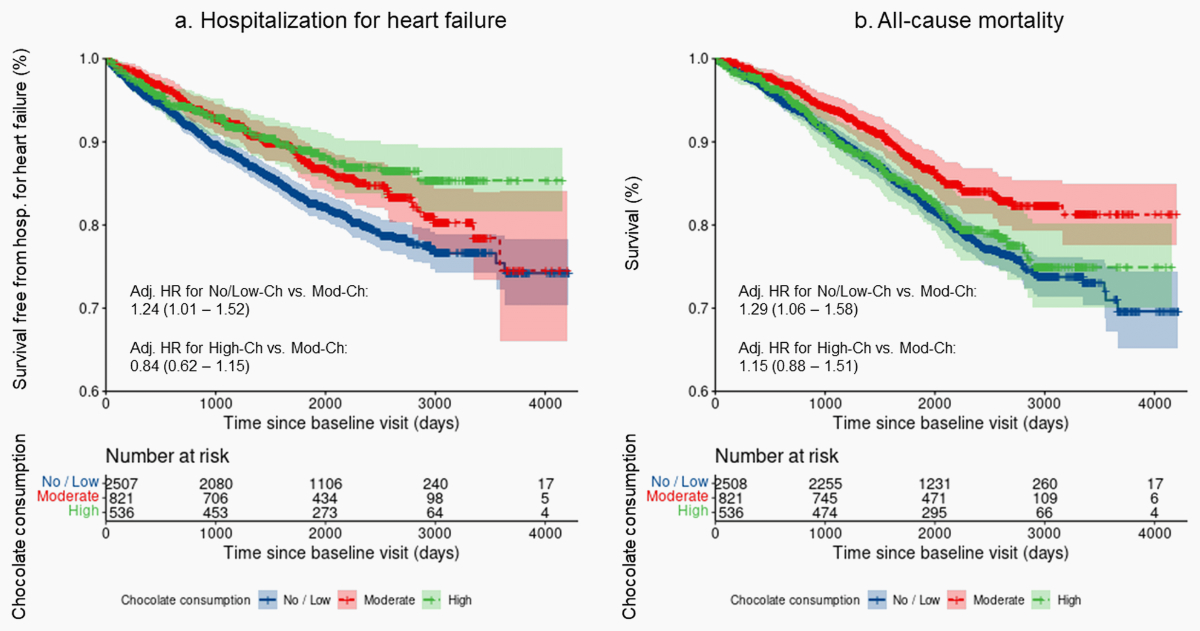
Figure 1Kaplan-Meier
curves with confidence bands showing: (a) the probability of survival free from
hospitalisation for heart failure and (b) the survival probability for
all-cause mortality for each group of chocolate consumption. Hosp.:
hospitalisation; adj.: adjusted; HR: hazard ratio; No/Low-Ch: no or low chocolate
consumption; Mod-Ch: moderate chocolate consumption; High-Ch: high chocolate
consumption
Discussion
In this
large cohort study of patients with atrial fibrillation, we observed no
association between chocolate consumption and the prevalence or volume of
vascular brain lesions on brain MRI. On the other hand, No/Low-Ch, when
compared to Mod-Ch, was independently associated with lower neurocognitive
function as assessed by the cognitive construct score. Moreover, whereas major
adverse cardiac events, comprising ischaemic stroke, myocardial infarction and
cardiovascular death, appeared to be unrelated to chocolate consumption, No/Low-Ch
was associated with an increased risk of hospitalisation for heart failure and
all-cause mortality when compared to Mod-Ch.
It is
generally accepted that a healthy lifestyle has a positive effect on health in
patients with atrial fibrillation and lifestyle modification is considered an
important therapeutic intervention in these patients according to current atrial
fibrillation guidelines [18]. Chocolate, when consumed in moderation, may
potentially represent a nutritional contribution to the well-being of atrial
fibrillation patients.
Of note, in
our study, BMI was lower in patients with higher chocolate consumption, a
finding that has previously been observed by others [14]. This association is
somewhat counter-intuitive. Potentially, it might indicate a generally
healthier lifestyle in patients consuming more chocolate. Alternatively, the
finding may have been influenced by disease, as obese patients, particularly
those with diabetes mellitus, are generally advised to reduce their consumption
of high-sugar foods, including chocolate. Moreover, as chocolate intakes were self-reported
by patients, underreporting by obese subjects due to social desirability bias could
have added to this finding. Interestingly, according to a previous study in a population
with no history of
cardiovascular disease, frequent chocolate consumption may independently be
linked to lower BMI. The observed association was not explained by calorie intake,
activity, or other potential confounders [29]. These results are intriguing,
but in line with preclinical data in animals [30]. To account for the
differences in baseline characteristics observed among the three groups of
chocolate consumption, we corrected for these potential confounders in
multivariate analyses.
The
beneficial systemic effects of chocolate are thought to be primarily mediated
by cocoa flavonoids, a group of polyphenols that may improve endothelial
function, decrease platelet reactivity, decrease sympathetic tone and lower
blood pressure [10, 31]. Additionally, oxidative stress reduction and
anti-inflammatory effects have been attributed to chocolate intake [11, 12, 14].
Cardiovascular
health is closely linked to cognitive performance [8]. Moreover, components of
chocolate such as flavonoids and methylxanthines may cross the blood-brain
barrier and therefore exhibit direct cerebral effects [6, 9]. Flavonoids may
increase central blood flow, promote angiogenesis, inhibit neuronal cell
destruction by neurotoxicants, and interact with cellular and molecular signalling
cascades in regions involved in learning and memory [8, 9, 13]. Methylxanthines
may act as mild central nervous system stimulants and lead to expression of
neurotrophins that influence neurocognitive function [6].
Published
data indicate that moderate consumption of chocolate may have a positive effect
on cardiovascular health [3, 4]. However, the link between chocolate consumption
and atrial fibrillation is less well-established. To date, research in the
field has mainly focused on the risk of atrial fibrillation and results have
been inconsistent. Although in the Danish Diet, Cancer and Health Study, an
inverse association between moderate chocolate intake and the incidence of atrial
fibrillation was observed [14], other studies did not find a similar link
between chocolate consumption and the risk of atrial fibrillation occurrence [15,
16].
To the best
of our knowledge, our study is the first to evaluate the potential associations
between chocolate consumption and neurocognitive function, vascular brain
lesions, and clinical outcome in patients with an established diagnosis of atrial
fibrillation.
Whereas
findings on brain MRI appeared to be unrelated to chocolate intake, better
neurocognitive function as assessed by the cognitive construct score was
independently associated with moderate chocolate consumption. The cognitive
construct score, as a summary measure of the common aspects of the four
neurocognitive tests used in our study, has previously been shown to reveal
good psychometric properties and to increase measurement sensitivity when
applied to the Swiss-AF population [22]. This may explain the fact that a
significant independent association between neurocognitive function and
chocolate consumption was only found when the cognitive construct score was
used. However, the absence of independent associations between chocolate intake
and any of the four tests performed with the patients may call into question
the clinical relevance of our finding.
In line
with the observed link between the cognitive construct score and moderate
chocolate consumption, a positive association between cognitive performance and
improved memory has previously been attributed to habitual chocolate intake in
patients without atrial fibrillation [6–9]. Interestingly, it has also been
recognised that the highest number of Nobel Prize winners can be found in
countries with the highest chocolate consumption [32]. However, this potential
link is rather speculative and has not been elaborated in detail. It cannot be
excluded that the association might simply be due to the fact that in highly
developed countries, where the luxury good chocolate is more frequently
consumed, more research can be afforded and conducted and with this, the
likelihood of citizens receiving Nobel Prizes may rise.
In our
study, a decreased risk of hospitalisation for heart failure could be seen for
Mod-Ch compared to No/Low-Ch, when assessed in multivariate analysis. This
observation is in accordance with a previously found association between
moderate chocolate consumption and a lower risk of heart failure [5]. Improvement
of endothelial function with activation of nitric oxide, reduction in
sympathetic tone, lowering of blood pressure and anti-inflammatory properties
are potential effects of flavonoids that may contribute to a positive influence
on heart failure [10, 12, 31, 33].
Unlike for
heart failure hospitalisation, we did not find a link between chocolate
consumption and the risk of myocardial infarction or cardiovascular death. This
contrasts with previously published data implying a beneficial effect of
chocolate consumption on the risk of cardiac events, including myocardial
infarction and cardiac death [3, 4, 34, 35]. The discrepancy in findings regarding
cardiovascular outcomes is difficult to explain. A potential factor might be a
variability in the consumed concentration of plant-derived flavonoids. Whereas
protective effects on the cardiovascular system have been attributed to high
amounts of flavonoids ingested either directly via daily cocoa consumption or
by intake of dark chocolate [10, 33, 34], we had no information on the type of
chocolate consumed in our study.
In contrast
to the lack of an association with cardiovascular mortality, moderate chocolate
consumption was independently related to a decreased risk of all-cause mortality
in our study. Given the large difference in event rates of the two endpoints, this
disparity in findings, at least in part, may have been related to a statistical
power issue. A true dissociation between cardiovascular and all-cause mortality
would imply the presence of relevant effects of chocolate consumption on
extra-cardiovascular systems. For example, this might be mediated by
anti-inflammatory actions or decreased genotoxicity due to antioxidant properties
[11, 12, 36, 37].
Limitations
In the
present study, we did not have information on the type of chocolate or the
cocoa concentration consumed. However, it cannot be assumed that patients only
ever eat the same type of chocolate and our data may represent a real-life
setting in this regard. In Switzerland, the most frequently consumed chocolate
is milk chocolate. Therefore, the amount of flavonoids consumed in our study
may have been lower than if patients had eaten only dark chocolate. Despite this limitation,
we observed significant associations between
chocolate consumption and important clinical endpoints in multivariate
analyses. Whether the associations would have been more pronounced if only dark
chocolate was used remains speculative.
Our study
was exploratory in nature. Therefore, a high number of tests on associations
between chocolate consumption and outcome measures were performed, no clear
primary endpoint was defined, and no adjustment for multiple comparisons was done.
Consequently, given an alpha level of 5%, we cannot exclude that some of the
findings may have resulted by chance and therefore, the data should be
interpreted with caution. However, as discussed above, similar associations were
found in other studies investigating different populations.
Another
limitation of our study is adherent to its non-randomised, observational
design. Although we performed multivariate analyses adjusting for multiple
co-variables, the possibility of residual confounding due to selection bias,
including Berkson’s bias, or due to unmeasured potentially influencing factors,
as well as the possibility of reverse causality, cannot be excluded. This must
be taken into account when interpreting the results. Due to its exploratory
nature, our work is hypothesis-generating rather than confirmatory.
Conclusion
Based on
our findings, Mod-Ch consumption may potentially be beneficial in patients with
atrial fibrillation. No/Low-Ch, when compared to Mod-Ch, was associated with lower
cognitive function as assessed by the cognitive construct factor score, higher
risk of heart failure hospitalisation and increased all-cause mortality, while
there were no associations of chocolate consumption with MRI findings and major
adverse cardiac events in a real-world atrial fibrillation population.
Data availability
statement
Due to
restrictions by the Ethical Committee, data is not publicly available. Requests
to access the datasets should be directed to the corresponding author.
Alain M.
Bernheim, MD
Department
of Cardiology
Triemli Hospital
Zurich
Birmensdorferstrasse 497
CH-8063 Zurich
Alain.Bernheim[at]stadtspital.ch
References
1 Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, et al. Projections
on the number of individuals with atrial fibrillation in the European Union, from
2000 to 2060. Eur Heart J. 2013 Sep;34(35):2746–51. 10.1093/eurheartj/eht280
2 Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation
and risks of cardiovascular disease, renal disease, and death: systematic review and
meta-analysis. BMJ. 2016 Sep;354:i4482. 10.1136/bmj.i4482
3 Yuan S, Li X, Jin Y, Lu J. Chocolate Consumption and Risk of Coronary Heart Disease,
Stroke, and Diabetes: A Meta-Analysis of Prospective Studies. Nutrients. 2017 Jul;9(7):688.
10.3390/nu9070688
4 Ren Y, Liu Y, Sun XZ, Wang BY, Zhao Y, Liu DC, et al. Chocolate consumption and risk
of cardiovascular diseases: a meta-analysis of prospective studies. Heart. 2019 Jan;105(1):49–55.
10.1136/heartjnl-2018-313131
5 Petrone AB, Gaziano JM, Djoussé L. Chocolate consumption and risk of heart failure
in the Physicians’ Health Study. Eur J Heart Fail. 2014 Dec;16(12):1372–6. 10.1002/ejhf.180
6 Sumiyoshi E, Matsuzaki K, Sugimoto N, Tanabe Y, Hara T, Katakura M, et al. Sub-Chronic
Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth
Factors: A Parallel-Group Randomized Trial. Nutrients. 2019 Nov;11(11):2800. 10.3390/nu11112800
7 Orozco Arbelaez E, Banegas JR, Rodríguez Artalejo F, López García E. Consumo habitual
de chocolate y estado cognitivo en los adultos mayores españoles [Influence of habitual
chocolate consumption over the Mini-Mental State Examination in Spanish older adults].
Nutr Hosp. 2017 Jul;34(4):841–6. 10.20960/nh.630
8 Crichton GE, Elias MF, Alkerwi A. Chocolate intake is associated with better cognitive
function: The Maine-Syracuse Longitudinal Study. Appetite. 2016 May;100:126–32. 10.1016/j.appet.2016.02.010
9 Nehlig A. The neuroprotective effects of cocoa flavanol and its influence on cognitive
performance. Br J Clin Pharmacol. 2013 Mar;75(3):716–27. 10.1111/j.1365-2125.2012.04378.x
10 Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, et al. Dark chocolate
improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007 Nov;116(21):2376–82.
10.1161/CIRCULATIONAHA.107.713867
11 Serafini M, Bugianesi R, Maiani G, Valtuena S, De Santis S, Crozier A. Plasma antioxidants
from chocolate. Nature. 2003 Aug;424(6952):1013. 10.1038/4241013a
12 Goya L, Martín MÁ, Sarriá B, Ramos S, Mateos R, Bravo L. Effect of cocoa and its flavonoids
on biomarkers of inflammation: studies of cell culture, animals and humans. Nutrients.
2016 Apr;8(4):212. 10.3390/nu8040212
13 Sokolov AN, Pavlova MA, Klosterhalfen S, Enck P. Chocolate and the brain: neurobiological
impact of cocoa flavanols on cognition and behavior. Neurosci Biobehav Rev. 2013 Dec;37(10
Pt 2):2445–53. 10.1016/j.neubiorev.2013.06.013
14 Mostofsky E, Berg Johansen M, Tjønneland A, Chahal HS, Mittleman MA, Overvad K. Chocolate
intake and risk of clinically apparent atrial fibrillation: the Danish Diet, Cancer,
and Health Study. Heart. 2017 Aug;103(15):1163–7. 10.1136/heartjnl-2016-310357
15 Khawaja O, Petrone AB, Kanjwal Y, Gaziano JM, Djoussé L. Chocolate Consumption and
Risk of Atrial Fibrillation (from the Physicians’ Health Study). Am J Cardiol. 2015 Aug;116(4):563–6.
10.1016/j.amjcard.2015.05.009
16 Larsson SC, Drca N, Jensen-Urstad M, Wolk A. Chocolate consumption and risk of atrial
fibrillation: two cohort studies and a meta-analysis. Am Heart J. 2018 Jan;195:86–90.
10.1016/j.ahj.2017.09.013
17 CHOCOSUISSE & CAOBISCO, statistical bulletin. Available from: https://www.chocosuisse.ch/services/facts-figures
18 Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al.;
ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management
of atrial fibrillation developed in collaboration with the European Association for
Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of
atrial fibrillation of the European Society of Cardiology (ESC) Developed with the
special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur
Heart J. 2021 Feb;42(5):373–498. 10.1093/eurheartj/ehaa612
19 Conen D, Rodondi N, Mueller A, Beer J, Auricchio A, Ammann P, et al. Design of the
Swiss Atrial Fibrillation Cohort Study (Swiss-AF): structural brain damage and cognitive
decline among patients with atrial fibrillation. Swiss Med Wkly. 2017 Jul;147:w14467.
20 Ruperti Repilado FJ, Doerig L, Blum S, Aeschbacher S, Krisai P, Ammann P, et al. Prevalence
and predictors of atrial fibrillation type among individuals with recent onset of
atrial fibrillation. Swiss Med Wkly. 2018 Sep;148:w14652. 10.4414/smw.2018.14652
21 Larsson SC, Åkesson A, Gigante B, Wolk A. Chocolate consumption and risk of myocardial
infarction: a prospective study and meta-analysis. Heart. 2016 Jul;102(13):1017–22.
10.1136/heartjnl-2015-309203
22 Springer A, Monsch AU, Dutilh G, Coslovsky M, Kievit RA, Bonati LH, et al.; Swiss-AF
Study Investigators. A factor score reflecting cognitive functioning in patients from
the Swiss Atrial Fibrillation Cohort Study (Swiss-AF). PLoS One. 2020 Oct;15(10):e0240167.
10.1371/journal.pone.0240167
23 Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The
Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment.
J Am Geriatr Soc. 2005 Apr;53(4):695–9. 10.1111/j.1532-5415.2005.53221.x
24 Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat
Protoc. 2006;1(5):2277–81. 10.1038/nprot.2006.390
25 Lopes M, Brucki SM, Giampaoli V, Mansur LL. Semantic Verbal Fluency test in dementia:
preliminary retrospective analysis. Dement Neuropsychol. 2009;3(4):315–20. 10.1590/S1980-57642009DN30400009
26 Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. New York: Oxford
University Press; 2004. pp. 368–70. ISBN: 978-0-19-511121-7.
27 Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at
1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987 Aug;149(2):351–6.
10.2214/ajr.149.2.351
28 Conen D, Rodondi N, Müller A, Beer JH, Ammann P, Moschovitis G, et al.; Swiss-AF Study
Investigators. Relationships of Overt and Silent Brain Lesions With Cognitive Function
in Patients With Atrial Fibrillation. J Am Coll Cardiol. 2019 Mar;73(9):989–99. 10.1016/j.jacc.2018.12.039
29 Golomb BA, Koperski S, White HL. Association between more frequent chocolate consumption
and lower body mass index. Arch Intern Med. 2012 Mar;172(6):519–21. 10.1001/archinternmed.2011.2100
30 Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, et al. (-)-Epicatechin
enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol. 2011 Sep;589(Pt
18):4615–31. 10.1113/jphysiol.2011.209924
31 Bruno RM, Ghiadoni L. Polyphenols, antioxidants and the sympathetic nervous system.
Curr Pharm Des. 2018;24(2):130–9. 10.2174/1381612823666171114170642
32 Messerli FH. Chocolate consumption, cognitive function, and Nobel laureates. N Engl
J Med. 2012 Oct;367(16):1562–4. 10.1056/NEJMon1211064
33 Corti R, Flammer AJ, Hollenberg NK, Lüscher TF. Cocoa and cardiovascular health. Circulation.
2009 Mar;119(10):1433–41. 10.1161/CIRCULATIONAHA.108.827022
34 Bayard V, Chamorro F, Motta J, Hollenberg NK. Does flavanol intake influence mortality
from nitric oxide-dependent processes? Ischemic heart disease, stroke, diabetes mellitus,
and cancer in Panama. Int J Med Sci. 2007 Jan;4(1):53–8. 10.7150/ijms.4.53
35 Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, et al. Flavonoid
intake and cardiovascular disease mortality: a prospective study in postmenopausal
women. Am J Clin Nutr. 2007 Mar;85(3):895–909. 10.1093/ajcn/85.3.895
36 Garcia JP, Santana A, Baruqui DL, Suraci N. The Cardiovascular effects of chocolate.
Rev Cardiovasc Med. 2018 Dec;19(4):123–7.
37 Leyva-Soto A, Chavez-Santoscoy RA, Lara-Jacobo LR, Chavez-Santoscoy AV, Gonzalez-Cobian LN.
Daily Consumption of Chocolate Rich in Flavonoids Decreases Cellular Genotoxicity
and Improves Biochemical Parameters of Lipid and Glucose Metabolism. Molecules. 2018 Sep;23(9):2220.
10.3390/molecules23092220
Appendix: analytical code
The appendix is available in the pdf version of the article.
