Interdisciplinary Swiss consensus recommendations on staging and treatment of advanced
prostate cancer
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40108
Arnoud J. Templetonab,
Aurelius Omlincd,
Dominik Bertholde,
Jörg Beyerf,
Irene A. Burgerg,
Daniel Eberlih,
Daniel Engeleri,
Christian Fankhauserj,
Stefanie Fischerk,
Silke Gillessenl,
Guillaume Nicolasm,
Stephanie Kroezen,
Anja Lorcho,
Michael Müntenerp,
Alexandros Papachristofilouq,
Niklaus Schaeferr,
Daniel Seilers,
Frank Stennert,
Petros Tsantoulisu,
Tatjana Vlajnicvw,
Thomas Zillix,
Daniel Zwahleny,
Richard Cathomasz
a Medical Oncology, St. Claraspital, Basel,
Switzerland / St. Clara Research Ltd., Basel, Switzerland
b Faculty of
Medicine, University of Basel, Basel, Switzerland
c Onkozentrum Zurich, University of Zurich
d Tumorzentrum Hirslanden Zurich,
Switzerland
e Medical Oncology, CHUV Lausanne, Vaud, Switzerland
f Medical Oncology, Inselspital, Universitätsspital,
Bern, Switzerland
g Department of Nuclear Medicine, Kantonsspital
Baden, Baden, Switzerland
h Department of Urology, Universitätsspital Zürich,
Zurich, Switzerland
i Department of Urology, Kantonsspital St. Gallen,
St. Gallen, Switzerland
j Department of Urology, Luzerner Kantonsspital,
Luzern, Switzerland
k Department of Medical Oncology and Hematology,
Kantonsspital St. Gallen, St. Gallen, Switzerland
l Medical Oncology, Ente Ospedaliero Cantonale
(EOC), Bellinzona, Switzerland
m Department of Nuclear Medicine, Universitätsspital
Basel, Basel, Switzerland
n Department of Radio-Oncology, Kantonsspital Aarau,
Aarau, Switzerland
o Medical Oncology and Hematology,
Universitätsspital Zürich, Zurich, Switzerland
p Department of Urology, Stadtspital Zürich Triemli,
Zurich, Switzerland
q Department of Radio-Oncology, Universitätsspital
Basel, Basel, Switzerland
r Department of Visceral Surgery, CHUV, Lausanne,
Vaud, Switzerland
s Department of Urology, Rotes Schloss Zürich,
Zurich, Switzerland
t Medical Oncology and Hematology,
Universitätsspital Basel, Basel, Switzerland
u Medical Oncology and Hematology, Université de
Genève (HUG), Geneva, Switzerland
v Institute of Pathology, Kantonsspital Graubünden,
Chur, Switzerland
w Institute of Medical Genetics and Pathology,
Universitätsspital Basel, Basel, Switzerland
x Department of Radio-Oncology, Ente Ospedaliero
Cantonale (EOC), Bellinzona, Switzerland
y Department of Radio-Oncology, Kantonsspital
Winterthur, Winterthur, Switzerland
z Division of Oncology/Hematology, Kantonsspital
Graubünden, Chur, Switzerland
Summary
The management of prostate
cancer is undergoing rapid changes in all disease settings. Novel imaging tools
for diagnosis have been introduced, and the treatment of high-risk localized,
locally advanced and metastatic disease has changed considerably in recent
years. From clinical and health-economic perspectives, a rational and optimal use
of the available options is of the utmost importance. While international
guidelines list relevant pivotal trials and give recommendations for a variety
of clinical scenarios, there is much room for interpretation, and several
important questions remain highly debated. The goal of developing a national
consensus on the use of these novel diagnostic and therapeutic strategies in
order to improve disease management and eventually patient outcomes has
prompted a Swiss consensus meeting. Experts from several specialties, including
urology, medical oncology, radiation oncology, pathology and nuclear medicine,
discussed and voted on questions of the current most important areas of
uncertainty, including the staging and treatment of high-risk localized
disease, treatment of metastatic hormone-sensitive prostate cancer (mHSPC) and use
of new options to treat metastatic castration-resistant prostate cancer
(mCRPC).
Introduction
Prostate
cancer remains the most common cancer diagnosed in men and a major cause of
cancer deaths in Switzerland, with around 6,500 men newly diagnosed and 1,400
men dying from prostate cancer
every year (www.krebs.bfs.admin.ch). In recent years, several new diagnostic
options and treatment strategies have emerged (figure 1). These are broadly
summarized in various international guidelines, but there is much room
for interpretation, and several important questions remain debated. To enhance
patient management and outcomes, a Swiss consensus meeting was held to promote
the coordinated use of innovative diagnostic and therapeutic approaches on a
national scale. The consensus discussion focused on the current most
important areas of uncertainty, including the staging and treatment of
high-risk localized disease, treatment of metastatic hormone-sensitive prostate
cancer (mHSPC) and use of new options to treat metastatic castration-resistant
prostate cancer (mCRPC).
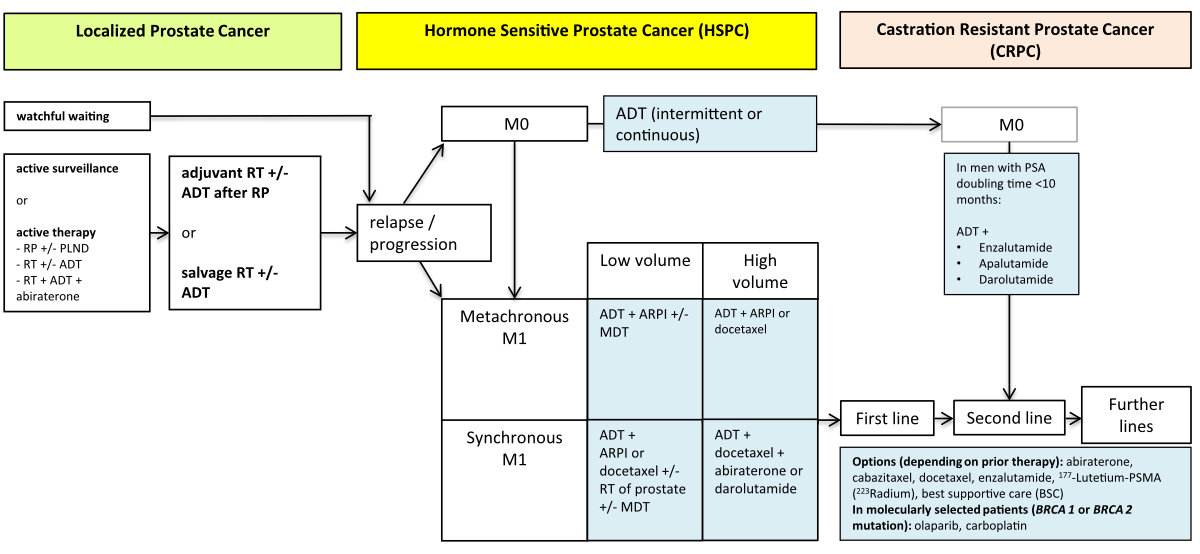
Figure 1The
therapy landscape for prostate cancer, 2023. ADT, androgen deprivation (with LHRH
agonist or
antagonist or bilateral
orchiectomy); ARPI, androgen receptor pathway inhibitor (i.e. abiraterone,
apalutamide, enzalutamide); PLND, pelvic lymph node dissection; M0, no evidence
of distant metastases; M1 evidence of distant metastases; MDT, metastasis
directed therapy. Please refer to https://www.swissmedicinfo.ch for approved indications.
The meeting took place in Bern on November 24, 2022. Most questions were selected
from the 2022 international Advanced Prostate Cancer Consensus Conference (APCCC)
[1], but some questions were reformulated to facilitate discussion, and some new questions
were formulated by the corresponding authors. All questions were circulated to all
experts before the meeting to allow participants to prepare. All questions were discussed
and subsequently voted on. All votes were submitted electronically and anonymously.
Consensus was defined as at least 80% of votes favouring a specific answer.
This article summarizes the discussion and voting results and is intended to serve
as guidance for the formulation of recommendations by institutional multidisciplinary
tumour boards and as a basis for discussion with individual patients. However, these
recommendations are not compulsory regulations and cannot replace careful and interdisciplinary
shared decision making with patients while considering important individual-specific
factors (figure 2).

Figure 2Considerations for individual decisions regarding the treatment of metastatic
hormone-sensitive prostate cancer.
Composition of the
panel
The Swiss Group
for Clinical Cancer Research (SAKK) invited a total of 22 Swiss prostate cancer
experts from different specialties, including urology, radiation oncology,
nuclear medicine, pathology and medical oncology, to join the panel. Experts were
identified using the network of the SAKK project group for urogenital tumours.
Fifteen experts were able to participate in person at the meeting. Participants
were permitted to vote on all questions presented, regardless of possible
conflicts of interest (e.g., authorship of scientific work discussed).
The co-authors Irene A. Burger, Daniel Eberli, Stefanie Fischer, Silke Gillessen,
Guillaume Nicolas, Stephanie Kroeze, Niklaus Schaefer, Thomas Zilli, and Daniel Zwahlen
could not attend the
consensus meeting in person.
Staging of
localized prostate cancer
In the
first part of the consensus meeting, questions involving modern imaging (i.e.,
PSMA [prostate-specific membrane antigen] PET/CT and whole-body MRI) as staging modalities
in localized prostate cancer were
addressed. The possibility of staging at diagnosis using PSMA PET/CT was the centre
of the discussion. There was consensus (86%) that staging with PSMA PET/CT is
indicated in cases of very high-risk or high-risk localized disease, according
to NCCN (National Comprehensive Cancer Network)
definitions (table 1), and one-third of panellists also recommended this method
of staging in cases of unfavourable intermediate risk.
Table 1 Risk stratification according to the National Comprehensive Cancer Network (NCCN).
| Risk group |
Clinical/pathological features |
| Very low |
Has all of the following: |
cT1c |
| Gleason 6 (ISUP Grade Group 1) |
| PSA <10 ng/ml |
| <3 prostate
biopsy fragments/cores positive, ≤50% cancer in each fragment/core |
| PSA density <0.15 ng/ml/g |
| Low |
Has all of the
following but does not qualify for very low risk: |
cT1–cT2a |
| Gleason 6 (ISUP
Grade Group 1) |
| PSA <10 ng/ml |
| Intermediate |
Has all of the following: |
No high-risk group features |
| No very high-risk group
features |
| Has one or more intermediate
risk factors: |
cT2b–cT2c |
| Gleason 7 (7a = 3 + 4 or 7b
= 4 + 3) (ISUP Grade Group 2 or 3) |
| PSA 10–20 ng/ml |
| Favourable intermediate |
Has all of the following: |
1 intermediate-risk feature |
| Gleason 6 or 7a (ISUP Grade
Group 1 or 2) |
| <50% biopsy cores
positive (e.g., <6 of 12 cores) |
| Unfavourable intermediate |
Has one or more of the
following: |
2 or 3 intermediate-risk
features |
| Gleason 7b (ISUP
Grade Group 3) |
| ≥50% biopsy cores
positive (e.g., ≥6 of 12 cores) |
| High |
Has no very high-risk
features and has exactly one high-risk feature: |
cT3a OR |
| Gleason 8–10 (ISUP Grade
Group 4 or 5) OR |
| PSA >20 ng/ml |
| Very high |
Has at least one of the
following: |
cT3b–cT4 |
| Primary Gleason
pattern 5 |
| 2 or 3 high-risk features |
| >4 cores with Gleason 8–10
(ISUP Grade Group 4 or 5) |
The
discussion highlighted that the problem of balancing false positive or
ambiguous findings on one hand and sensitivity for locoregional lymph node
metastasis (cN1) and/or distant metastases (cM1) on the other hand is difficult
given that these findings prompt the determination of a curative versus
palliative treatment strategy. Based on the available literature, the
sensitivity and specificity of 18F-PSMA-1007 PET/CT (the tracer most
often used in Swiss institutions) for detection of locoregional lymph node
metastases are 54% and 97%, respectively [2].
A comparative study of conventional staging, both whole-body MRI and 18F-PSMA-1007
PET/CT showed a higher sensitivity and better inter-reader agreement for staging prostate cancer, despite the known
limitation of unspecific bone uptake (UBU) [3,
4]. There are very limited data on the comparison of 18F-PSMA-1007
and 68Ga-PSMA-11, and both radiotracers are used in routine clinical
practice.
The panel
discussed how to proceed in patients with high-risk prostate cancer for whom
radical local treatment (radical prostatectomy or radiotherapy) of the primary
tumour is planned and who have up to three lesions in the bone with low
uptake (as defined by the institution) evident on an upfront 18F-PSMA
PET/CT without a correlate on the CT component. The majority of experts (71%)
felt that, in general, no further investigations are needed in this case. The rationale
is to avoid undertreatment in the case of false positive findings (i.e.,
inadequate local treatment in the case of wrongly assuming distant metastatic
disease). In fact, a recent PSMA PET/CT–guided biopsy study confirmed that
the majority of such lesions are caused by false positive uptake [5]. In contrast,
in the case of intense
uptake (as defined by the institution), only 13% of experts considered this approach
appropriate, whereas two-thirds recommended correlative imaging (usually targeted
MRI), and 20% recommended a biopsy. There was consensus (93%) not to use whole-body
MRI instead of PSMA PET/CT for initial staging.
Initial treatment
of very high-risk localized prostate cancer
Active
treatment with curative intent encompasses radical prostatectomy or external
beam radiation in combination with androgen deprivation therapy. A recent
report from the STAMPEDE platform protocol [6]
found that the addition of abiraterone/prednisone (for 2 years) and androgen
deprivation therapy (for 3 years) to local radiotherapy led to a 9% increase in the
6-year absolute survival
rate in men with locally advanced disease (definition according to STAMPEDE
protocol: cN1 cM0 disease; or cN0 cM0 disease with at least two of the following:
clinical stage T3 or T4, Gleason score
8–10 and prostate-specific antigen [PSA] concentration ≥40 ng/ml). When asked
about the relative preference of surgery versus radiotherapy plus androgen deprivation
therapy and abiraterone in men with cN0 disease with at least two high-risk
criteria, experts showed no preference (40% for both options). If these
patients are treated surgically, experts recommended that this be considered a
first step of a multimodal approach with a high likelihood that postoperative
radiation therapy will be required. In the case of radiotherapy, there was consensus
(87%) to include pelvic lymph nodes in the radiation volume. For men with cN1
cM0 disease detectable by modern imaging, a majority of experts
(60%) favoured radiotherapy with androgen deprivation therapy and abiraterone, whereas
40% opted for surgery with or without
radiotherapy. Notably, three out of four urologists present preferred the
combination of radiotherapy, androgen deprivation therapy and abiraterone in
this situation, possibly reflecting the perceptions that this treatment is best
supported by data and that cN1 likely reflects systemic disease that requires
both local and systemic treatment.
In the
prostate cancer community, there is some controversy regarding further
management in the case of proven lymph node involvement (pN1) following radical
prostatectomy and undetectable PSA. In the absence of high-risk features
(i.e., Gleason 8–10, positive margins [R1] or pT3), there was consensus (92%) to monitor
PSA
and only initiate salvage radiotherapy to the prostate bed and pelvic lymph
node drainage area in cases of PSA increase. In the case of the presence of two
or three of these high-risk features, however, only 43% of experts opted for
monitoring and salvage radiotherapy in cases of PSA increase, whereas 57% were
in favour of adjuvant radiotherapy, and a minority (21%) of those experts suggested
radiotherapy in combination with androgen deprivation therapy. In the case of
three or more positive lymph nodes, the latter approach was favoured by 50% of
experts in the absence and 67% in the presence of high-risk features. When
asked specifically about management of patients following radical prostatectomy (R0)
and extended pelvic
lymph node dissection without lymph node involvement (pN0) and an undetectable
postoperative PSA with a high risk of relapse (i.e., both Gleason 8–10 and pT3b/4
but negative
margins [R0]), the majority of experts (72%) opted
for monitoring and early salvage radiotherapy with or without androgen
deprivation therapy in cases of rising PSA. In a similar scenario but with R1,
53% of the participants opted for adjuvant radiotherapy with or without androgen
deprivation therapy as soon as the patient has regained continence after
surgery. Generally, in cases of monitoring, restaging should be performed with
PSMA PET/CT early, i.e., before PSA rises >0.5ng/ml. For patients treated
with radiotherapy after surgery (adjuvant/additive or salvage), 47% of experts
advocated the use of androgen deprivation therapy for 6 months, 33% for 12–24 months
and 20% not at all.
This result reflects a pragmatic interpretation of somewhat conflicting results
from the RADICALS-HD trial, which found that, in men receiving postoperative
radiotherapy after radical prostatectomy, 24 months of androgen deprivation
therapy improved metastasis-free survival (MFS) compared to 6 months of
androgen deprivation therapy, while 6 months of androgen deprivation therapy did
not improve MFS compared to no androgen deprivation therapy [7]. Treatment recommendations
should therefore
be individualized based on patient-specific risk factors. The results of the
clinically most relevant votes are summarized in figure 3.
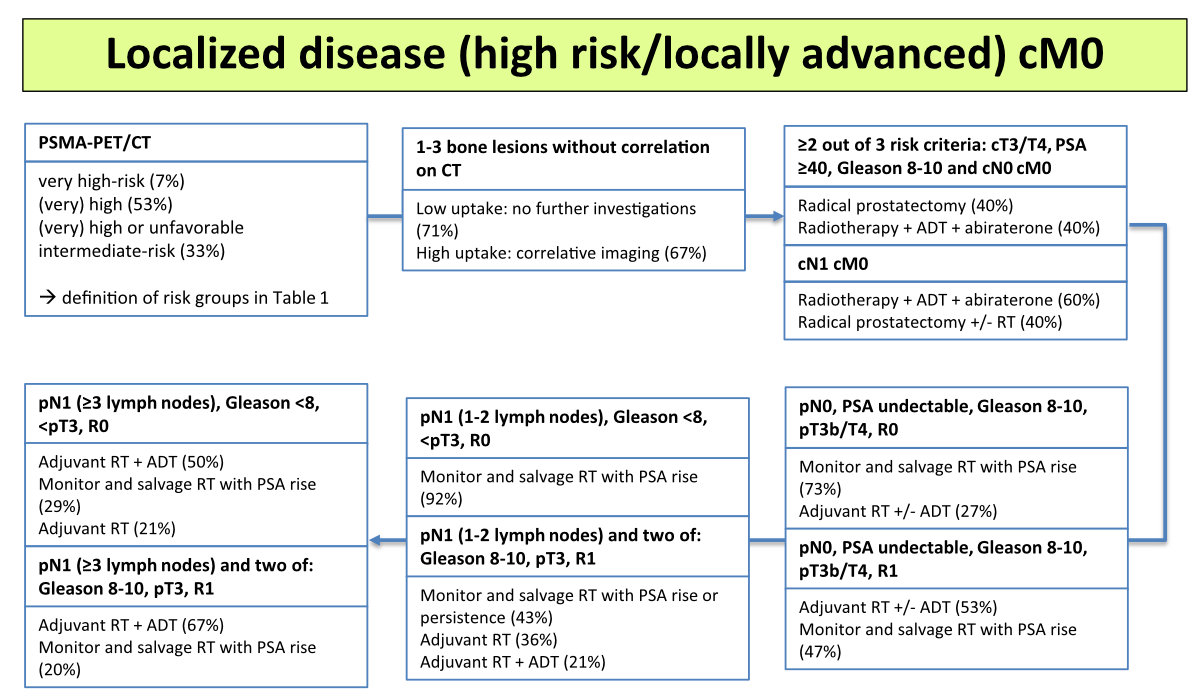
Figure 3Staging and treatment of (very) high-risk localized and locally advanced
disease (no distant metastasis), with percentages indicating the voting
results. ADT, androgen deprivation therapy; cM0, no distant metastases;
cN0, no locoregional lymph node metastases; CT, computed tomography; PSA,
prostate-specific antigen; PET, positron emission tomography; PSMA, prostate-specific
membrane antigen; RT,
radiotherapy. Please refer to https://www.swissmedicinfo.ch for approved indications.
Treatment of metastatic
hormone-sensitive prostate cancer (mHSPC)
Around 5–10% of men with prostate cancer are
found to have metastatic disease at first presentation, i.e., have synchronous
or de novo metastatic (M1) disease [8].
This initial presentation with M1 disease contrasts with metastatic disease recurring
after prior local therapy of the prostate, i.e., metachronous metastatic
disease. The latter presents a more favourable prognosis than synchronous
metastatic disease [9]. Furthermore, the extent
of metastatic disease, namely the presence of visceral disease (e.g.,
metastases in the liver), and the number and localization of bone metastases
are prognostic factors [10]. As a result,
in 2015 high volume disease was defined as the presence of visceral
metastatic disease and/or the presence of at least four bone metastases, of
which at least one was not in the spine or pelvis (with low volume
disease representing all situations in which this high volume definition is not
met) [10]. However, it should be noted
that volume definitions are based on conventional imaging and may be adjusted
in the future with the use of novel imaging (such as PSMA-PET or whole-body
MRI). Median overall survival in cases of synchronous high volume mHSPC is
around 3 years, while median overall survival in cases of metachronous low
volume disease is around 8 years [9, 11].
In recent years, early treatment intensification, with the addition of any of
the novel androgen receptor pathway inhibitors (ARPI: abiraterone, apalutamide
or enzalutamide) or of docetaxel to androgen deprivation therapy, has become the
standard of care for most men with mHSPC, depending on the timing of diagnosis
and extent of disease [12]. In 2022, two
randomized phase 3 studies investigated a triple combination of androgen
deprivation therapy, docetaxel and an ARPI (abiraterone in the PEACE-1 study [13]
and darolutamide in the ARASENS study [14]). Most men in these studies had de novo
mHSPC (all in PEACE-1 and 86% in ARASENS). The addition of either abiraterone or
darolutamide to androgen deprivation therapy and docetaxel was associated with
longer survival; in the PEACE-1 study this result was restricted to men with
high volume disease, for whom median overall survival was prolonged by approximately
1.6 years [13]. A post hoc
analysis of the ARASENS study also demonstrated an increased benefit for high volume
patients [15]. The panel voted on the
question of treatment recommendations for men with synchronous high volume
mHSPC: 60% were in favour of a triplet therapy, and 33% recommended doublet
therapy (i.e., androgen deprivation therapy plus docetaxel or ARPI). In cases
of synchronous low volume disease, there was consensus (80%) for the use
of androgen deprivation therapy plus ARPI (while 20% of experts recommended androgen
deprivation therapy plus docetaxel or ARPI). For men with metachronous high
volume mHSPC, 47% of experts recommended triplet therapy, 27% androgen
deprivation therapy plus ARPI and 27% androgen deprivation therapy plus
docetaxel or ARPI. For men with metachronous low volume mHSPC, experts
reached consensus (93%) for the use of androgen deprivation therapy plus ARPI.
In the latter situation, 50% were in favour of treatment until progression (as
in the pivotal studies), whereas 29% opted for holding both androgen
deprivation therapy and ARPI in the case of a favourable response (i.e., PSA
<0.2 ng/ml) after 2 years with rechallenge upon progression (21% recommended
discontinuing the ARPI only with rechallenge at progression). In the case of
triplet therapy, 40% and 20% of experts were in favour of using darolutamide and
abiraterone, respectively, while the remaining 40% had no preference.
Low volume
mHSPC (defined as up to four bone metastases) has been shown to be predictive of
benefits from local radiotherapy of the prostate, with an 8% gain in absolute overall
survival after 3 years [16]. However,
none of the participants in this trial had received an ARPI, so it remains
uncertain whether a combination of both modalities is needed. Of all Swiss panellists,
47% recommended radiation therapy of the primary tumour in addition to ARPI for
the majority of patients, while 47% recommended this approach only in select
patients (e.g., younger patients), and 7% did not recommend radiation of the
prostate in addition to ARPI.
Metastasis-directed
therapy (MDT)
A majority of
experts (73%) agreed that treatment recommendations for MDT should not be based
on conventional imaging (i.e., CT plus a bone scan) only. In cases of
synchronous low volume mHSPC with one to three bone lesions on PSMA PET/CT, 57%
of panellists favoured systemic therapy plus local treatment of the primary tumour
plus MDT, while 36% voted for systemic therapy plus local treatment of the
primary tumour only. In a similar scenario with metachronous disease, 53% of
experts recommended systemic therapy plus MDT, while 27% favoured MDT without
systemic treatment, and 20% favoured systemic treatment alone. Clear consensus
(93%) was reached regarding the type of MDT, namely radiotherapy. For men with
synchronous low volume mHSPC and one to three PSMA PET/CT–positive retroperitoneal
lymph nodes,
there was no preference (47% of the votes each) for systemic therapy plus local
treatment of the primary tumour or systemic therapy plus local treatment of the
primary tumour plus MDT. The
results of relevant votes are summarized in figure 4. Importantly, the evidence
for the effectiveness of MDT in these patients is currently based on small
phase 2 trials and is not supported by large trials showing improvement of
relevant oncological outcomes.
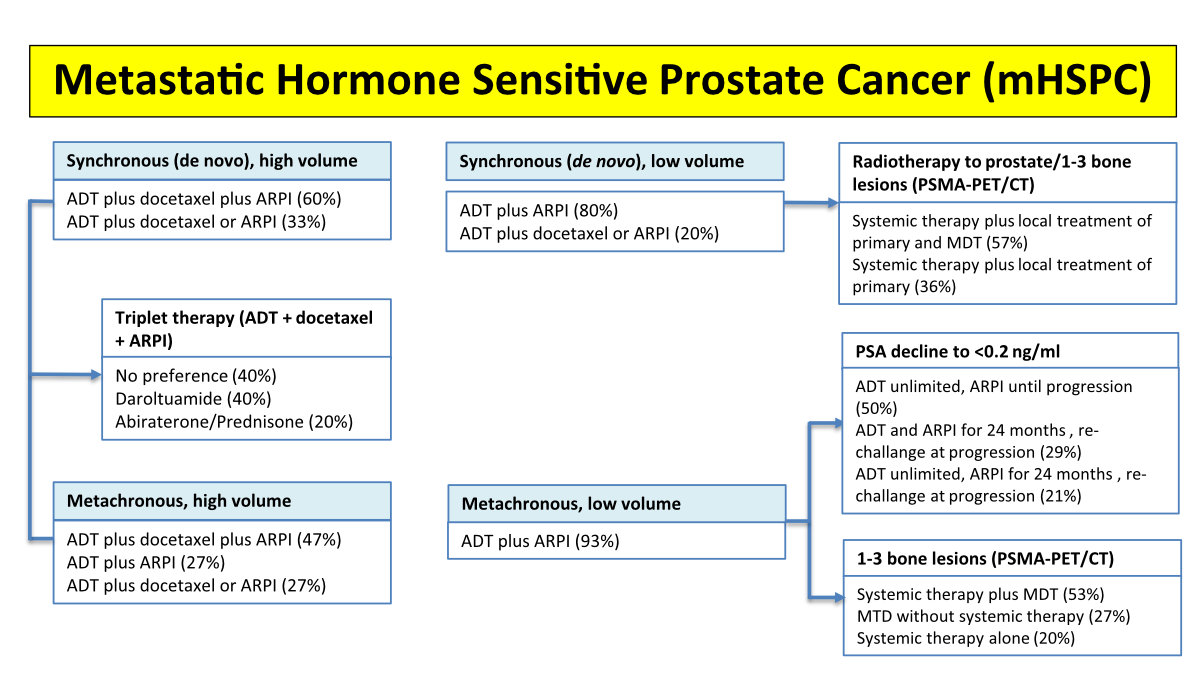
Figure 4Treatment of metastatic hormone-sensitive prostate cancer (mHSPC), with
percentages indicating the voting results. High volume: visceral
disease and/or at least 4 bone metastasis of which at least one outside the
pelvis and vertebral column. Low volume: high volume criteria not met. ADT,
androgendeprivation therapy; ARPI, androgen receptor pathway inhibitor (abiraterone,
enzalutamide, apalutamide, darolutamide); CT, computed tomography; MTD,
metastasis directed therapy; PET, positron emission tomography; PSA,
prostate-specific antigen; PSMA, prostate-specific membrane antigen; RT,
radiotherapy. Please refer to https://www.swissmedicinfo.ch for approved indications.
Additional investigations
and follow-up in mHSPC patients
There was
consensus (87%) that molecular tests (i.e., next-generation sequencing) would
not influence the decision of the first-line treatment for mHSPC, even if
available without restrictions. Given the lack of high-quality data on
follow-up modalities and intervals during the treatment of mHSPC, the following
questions were discussed. In the absence of symptoms, 47% of experts recommended
regular imaging, e.g., every 6–12 months, regardless of PSA, while 33% recommended
imaging after about
6–12 months and
then no more imaging until confirmed PSA progression, and 20% recommended imaging
prompted only by rising PSA. As for imaging modality, 53% of panellists opted
for conventional imaging (CT with or without a bone scan), whereas 27% and 13% favoured
PET/CT and whole-body MRI, respectively. Again, there is very limited evidence for
how to interpret, e.g., PSMA PET/CT in patients responding to systemic therapy,
and, in fact, in Switzerland PSMA PET/CT is not approved or reimbursed in this situation.
Metastatic
castration-resistant prostate cancer (mCRPC)
Selection of first-line
therapy and treatment sequence
In the
absence of alterations of DNA damage response and repair (DDR) genes, all
experts (100%) recommended an ARPI as a first-line therapy for mCRPC in men who
received androgen deprivation therapy as monotherapy for mHSPC. In cases of
time to castration resistance of less than 6 months (i.e., progression within 6
months of the start of androgen deprivation therapy), the use of ARPI or
chemotherapy was considered adequate (both options were recommended by 47% of
experts). For men treated with an ARPI in the case of mHSPC, all panellists
(100%) recommend a switch to chemotherapy, irrespective of time to castration resistance.
Most
experts (64%) did not recommend a switch to another ARPI therapy in the
majority of patients who have received one line of ARPI and then have progressed.
By contrast, 29% deemed a switch appropriate in select patients who had a prior
response to abiraterone and subsequently progressed. The basis for the latter
recommendation is a study showing a PSA response rate of 19% in this situation [17],
while, e.g., abiraterone after
enzalutamide was associated with a very low PSA response rate of around 1% [18].
PARP inhibition
In around 10%
of mCRPC cases, tumours harbour a pathogenic BRCA1/2 alteration (around
half of which is germ line) [19, 20] that
is predictive of benefits from PARP inhibition. Recently, studies combining new
endocrine therapies (e.g., abiraterone or enzalutamide) with PARP inhibitors (e.g.,
olaparib, niraparib or talazoparib) have reported longer radiographic
progression-free survival with the combination, irrespective of DDR status, at
the cost of increased toxicity and no improvement in overall survival for
unselected populations [21–23]. In
all these studies, in most cases castration resistance had occurred in patients
receiving androgen deprivation therapy as monotherapy. There was consensus
(93%) not to combine ARPI with a PARP inhibitor as first-line therapy for mCRPC,
irrespective of DDR status. However, for men with mCRPC with a pathogenic BRCA1/2
alteration who developed castration resistance during androgen deprivation
therapy and an ARPI (with or without docetaxel), 38% of experts recommended a switch
to PARP inhibitor monotherapy, whereas others favoured a switch to chemotherapy
(31%) or the addition of a PARP inhibitor to continued ARPI (31%). In cases of
other (i.e., not BRCA1/2) pathogenic DNA repair gene alterations, there
was consensus (86%) to switch to chemotherapy.
Radionuclide
therapy
The use of 177Lu-PSMA
has led to an overall survival benefit in patients with mCRPC and pretreatment
with ARPI and docetaxel if PSMA avidity has been demonstrated on a staging PSMA
PET/CT [24]. In men with symptomatic
mCRPC who met criteria for treatment with both 223Ra and 177Lu-PSMA,
there was consensus (80%) in favour of using 177Lu-PSMA, while 13%
of experts had no preference. Furthermore, there was consensus (93%) to
recommend 177Lu-PSMA after prior treatment with docetaxel and an ARPI.
Imaging and
follow-up for mCRPC patients
The majority
of the panel (73%) recommended imaging every 3–6 months for men being treated for
mCRPC, regardless of PSA and in the absence of new symptoms. In terms of
imaging modality, 47% of panellists favoured CT scans (with or without a bone
scan), while 29%, 14% and 7% opted for a CT scan plus a bone scan, whole-body
MRI and PET/CT, respectively.
The results
of relevant votes are summarized in figure 5.
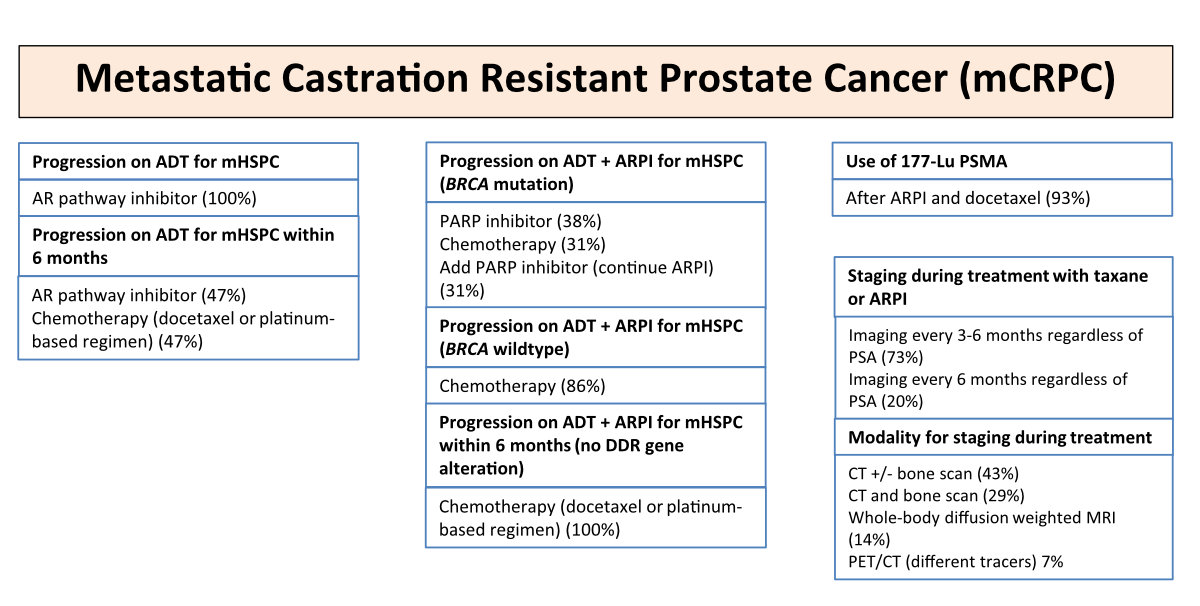
Figure 5Treatment and staging of metastatic castration-resistant prostate cancer
(mCRPC), with percentages indicating the voting results. ADT,
androgendeprivation therapy; AR, androgen receptor; ARPI, androgen receptor
pathway inhibitor; BRCA, breast cancer gene; DDR, DNA Damage Response and Repair;
Lu, Lutetium; mHSPC,
metastatic hormone sensitive prostate cancer; CT, computed tomography; MRI,
magnet resonance imaging; PARP, Poly ADP-Ribose Polymerase; PET, positron
emission tomography; PSA, prostate-specific antigen; PSMA,
prostate-specific membrane antigen. Please refer to https://www.swissmedicinfo.ch for approved indications.
Health-economic
aspects
Concerns
about the availability and costs of modern therapies were prevalent among the
participants in the consensus meeting. When asked whether financial cost to the
health care system should be considered when making treatment decisions or recommendations,
89% of experts responded “yes, absolutely” and 11% “no, not at all”. It remains
to be determined how this can be achieved in daily practice while ensuring
optimal treatment for all our patients. First steps might be using generic
drugs, if available; de-escalation strategies; and strict adherence to the
principle that diagnostic procedures must have a therapeutic consequence.
Acknowledgements
The authors
would like to thank Fabienne Trattner from Medtoday Switzerland and Nadja Burri
from the Swiss Group of Clinical Cancer Research (SAKK) in Bern for their great
administrative support in organizing the meeting.
PD Dr. Arnoud Templeton
Medical Oncology
Claraspital Basel
CH-4058 Basel
arnoud.templeton[at]claraspital.ch
PD Dr. Richard Cathomas
Division of Oncology/Hematology
Kantonsspital
Graubünden
CH-7000
Chur
richard.cathomas[at]ksgr.ch
References
1. Gillessen S, Bossi A, Davis ID, de Bono J, Fizazi K, James ND, et al. Management of
Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-risk and Locally
Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report
of the Advanced Prostate Cancer Consensus Conference 2022. Eur Urol. 2022.
2. Ingvar J, Hvittfeldt E, Trägårdh E, Simoulis A, Bjartell A. Assessing the accuracy
of [18F]PSMA-1007 PET/CT for primary staging of lymph node metastases in intermediate- and
high-risk prostate cancer patients. EJNMMI Res. 2022 Aug;12(1):48. 10.1186/s13550-022-00918-7
3. Anttinen M, Ettala O, Malaspina S, Jambor I, Sandell M, Kajander S, et al. A Prospective
Comparison of 18F-prostate-specific Membrane Antigen-1007 Positron Emission Tomography Computed Tomography,
Whole-body 1.5 T Magnetic Resonance Imaging with Diffusion-weighted Imaging, and Single-photon
Emission Computed Tomography/Computed Tomography with Traditional Imaging in Primary
Distant Metastasis Staging of Prostate Cancer (PROSTAGE). Eur Urol Oncol. 2021 Aug;4(4):635–44.
10.1016/j.euo.2020.06.012
4. Grünig H, Maurer A, Thali Y, Kovacs Z, Strobel K, Burger IA, et al. Focal unspecific
bone uptake on [18F]-PSMA-1007 PET: a multicenter retrospective evaluation of the distribution, frequency,
and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur
J Nucl Med Mol Imaging. 2021 Dec;48(13):4483–94. 10.1007/s00259-021-05424-x
5. Vollnberg B, Alberts I, Genitsch V, Rominger A, Afshar-Oromieh A. Assessment of malignancy
and PSMA expression of uncertain bone foci in [18F]PSMA-1007 PET/CT for prostate cancer-a single-centre experience of PET-guided biopsies.
Eur J Nucl Med Mol Imaging. 2022 Sep;49(11):3910–6. 10.1007/s00259-022-05745-5
6. Attard G, Murphy L, Clarke NW, Cross W, Jones RJ, Parker CC, et al.; Systemic Therapy
in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE)
investigators. Abiraterone acetate and prednisolone with or without enzalutamide for
high-risk non-metastatic prostate cancer: a meta-analysis of primary results from
two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet.
2022 Jan;399(10323):447–60. 10.1016/S0140-6736(21)02437-5
7. Parker CC, Clarke N, Cook A, Catton C, Cross WR, Kynaston H, et al. Duration of androgen
deprivation therapy (ADT) with post-operative radiotherapy (RT) for prostate cancer:
First results of the RADICALS-HD trial (ISRCTN40814031). Annals of Oncology 2022;33
(suppl_7):S808-S69. 10.1016/annonc/annonc89 (LBA 9).
8. Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, et al. Prostate cancer.
Nat Rev Dis Primers. 2021 Feb;7(1):9. 10.1038/s41572-020-00243-0
9. Francini E, Gray KP, Xie W, Shaw GK, Valença L, Bernard B, et al. Time of metastatic
disease presentation and volume of disease are prognostic for metastatic hormone sensitive
prostate cancer (mHSPC). Prostate. 2018 Sep;78(12):889–95. 10.1002/pros.23645
10. Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal
Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015 Aug;373(8):737–46.
10.1056/NEJMoa1503747
11. Gravis G, Boher JM, Chen YH, Liu G, Fizazi K, Carducci MA, et al. Burden of Metastatic
Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from
Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur Urol. 2018 Jun;73(6):847–55.
10.1016/j.eururo.2018.02.001
12. Cornford P, van den Bergh RC, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M,
et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update:
Treatment of Relapsing and Metastatic Prostate Cancer. Eur Urol. 2021 Feb;79(2):263–82.
10.1016/j.eururo.2020.09.046
13. Fizazi K, Foulon S, Carles J, Roubaud G, McDermott R, Fléchon A, et al.; PEACE-1 investigators.
Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in
de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre,
open-label, randomised, phase 3 study with a 2×2 factorial design. Lancet. 2022 Apr;399(10336):1695–707.
10.1016/S0140-6736(22)00367-1
14. Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al.; ARASENS
Trial Investigators. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate
Cancer. N Engl J Med. 2022 Mar;386(12):1132–42. 10.1056/NEJMoa2119115
15. Hussain M, Tombal B, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide
Plus Androgen-Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate
Cancer by Disease Volume and Risk Subgroups in the Phase III ARASENS Trial. J Clin
Oncol. 2023 Jul;41(20):3595–607. 10.1200/JCO.23.00041
16. Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al.; Systemic Therapy
for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE)
investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic
prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018 Dec;392(10162):2353–66.
10.1016/S0140-6736(18)32486-3
17. de Bono JS, Chowdhury S, Feyerabend S, Elliott T, Grande E, Melhem-Bertrandt A, et
al. Antitumour Activity and Safety of Enzalutamide in Patients with Metastatic Castration-resistant
Prostate Cancer Previously Treated with Abiraterone Acetate Plus Prednisone for ≥24
weeks in Europe. Eur Urol. 2018 Jul;74(1):37–45. 10.1016/j.eururo.2017.07.035
18. Attard G, Borre M, Gurney H, Loriot Y, Andresen-Daniil C, Kalleda R, et al.; PLATO
collaborators. Abiraterone Alone or in Combination With Enzalutamide in Metastatic
Castration-Resistant Prostate Cancer With Rising Prostate-Specific Antigen During
Enzalutamide Treatment. J Clin Oncol. 2018 Sep;36(25):2639–46. 10.1200/JCO.2018.77.9827
19. Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative
clinical genomics of advanced prostate cancer. Cell. 2015 May;161(5):1215–28. 10.1016/j.cell.2015.05.001
20. de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for Metastatic
Castration-Resistant Prostate Cancer. N Engl J Med. 2020 May;382(22):2091–102. 10.1056/NEJMoa1911440
21. Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore N, Loredo E, et al. Abiraterone
and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid. 2022;2022(9):1.
10.1056/EVIDoa2200043
22. Chi KN, Rathkopf DE, Smith MR, Efstathiou E, Attard G, Olmos D, et al. Phase 3 MAGNITUDE
study: First results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP)
as first-line therapy in patients (pts) with metastatic castration-resistant prostate
cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations.
Journal/ASCO GU abstract #12 2022;
23. Pfizer. Pfizer Announces Positive Topline Results from Phase 3 TALAPRO-2 Trial 2022
(Press release on Oct 4);
24. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al.; VISION Investigators.
Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl
J Med. 2021 Sep;385(12):1091–103. 10.1056/NEJMoa2107322




