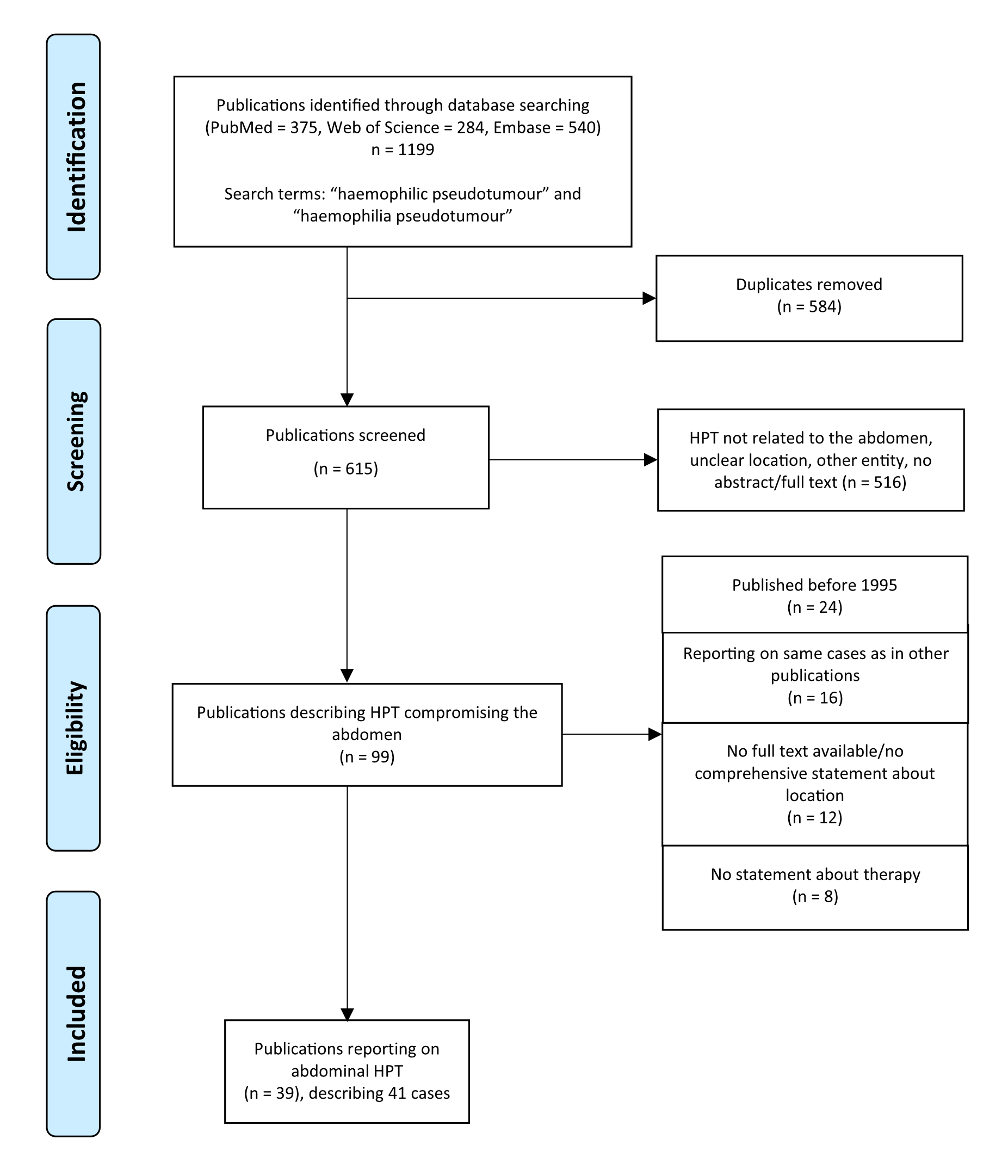
Figure 1PRISMA flow diagram.
HPT: haemophilic pseudotumour
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40094
Haemophilia is a rare, inherited disease resulting from the deficiency or dysfunction of coagulation protein factor VIII in haemophilia A and factor IX in haemophilia B. Since the mutations causing the disease are inherited X-linked recessively, men are nearly exclusively affected. The severity of both types of haemophilia is defined by the residual clotting factor activity in the plasma. A factor activity of over 50% is considered normal; clotting factor activity of 5–50% defines mild and 1–5% moderate haemophilia. Factor activity of less than 1% corresponds to severe haemophilia, which is characterised by spontaneous bleeds, whereas bleeding in mild and moderate haemophilia usually occurs after trauma [1–3]. Factor replacement therapy is recommended as the standard of care for patients with severe haemophilia to prevent bleeding [4, 5].
Haemophilic pseudotumours are rare complications. The prevalence in patients with severe haemophilia is around 1–2%, persisting despite the use of clotting factor replacement therapy over the last decades [6]. Haemophilic pseudotumours result from repetitive spontaneous bleeding over years and correspond histologically to encapsulated chronic hematomas in different states of organisation. They mainly occur in the musculoskeletal system, where the rigidity of the tissue prevents major bleeding. Abdominal haemophilic pseudotumours share the same entity but are extremely rare and may cause severe bleeding despite factor replacement and due to the dilatability of the abdominal cavity. Additionally, they may reach a size that compromises the function of abdominal organs [7–11]. However, the rarity of abdominal haemophilic pseudotumours prevents a clear treatment consensus. The purpose of this systematic review is to evaluate the therapy strategies for patients with symptomatic abdominal haemophilic pseudotumours, focusing on clinical outcomes.
An electronic search for relevant publications between 1995 and February 2023 was performed in three international databases (Medline [PubMed], Web of Science Core Collection and EMBASE). The search terms were “haemophilic pseudotumour” and “haemophilia pseudotumour” (fig. 1) [12]. Studies were included in the analysis if they met the following criteria: a) reports on patients with haemophilic pseudotumour directly or indirectly related to the abdomen and b) reports on therapy strategies of the abdominal haemophilic pseudotumour. The “relation to the abdomen” was defined as adherence to and/or displacement, infiltration or obstruction of intraabdominal or retroperitoneal organs or vessels. Studies were excluded from the analysis if a) they were published before 1995, because the recommendation for haemophilia prophylaxis was implemented by the World Federation for Haemophilia and World Health Organization in 1995 [13] or b) the full text was missing. The references of incorporated studies were inspected, and relevant publications were included.

Figure 1PRISMA flow diagram.
HPT: haemophilic pseudotumour
All articles were eligible when they reported cases of abdominal or abdomen-related haemophilic pseudotumours and described an acute treatment strategy. Two reviewers independently performed the eligibility assessment and data extraction. To avoid errors in data extraction, a double data-entry method was applied, and discrepancies were compared and discussed by two authors to achieve consensus. Information from each case was extracted, including the year of publication, haemophilia type and severity, localisation and tumour size, invasion or displacements including complications, and therapeutic management and follow-up.
The abdominal haemophilic pseudotumour’s localisation was divided into “intraperitoneal” or “extraperitoneal” causing abdominal symptoms. Symptoms were defined as displacement of or adherence to abdominal organs without compromising organ function or as compression with a consecutive impact on organ function, fistulation, infiltration, abscess formation or bleeding (table 1). Treatment was divided into conservative or interventional alone, interventional treatment with step-up to surgery or primary surgical. Interventional therapy strategies were embolisation or percutaneous drainage of the haemophilic pseudotumour or local radiotherapy. Surgery was divided into “surgical resection”, including all surgical attempts to completely resect the haemophilic pseudotumour; “surgical management”, including surgical procedures to resolve complications due to the haemophilic pseudotumour without resecting the tumour; and “surgical drainage” (table 1). The outcome was considered “favourable” for patients with an improvement in symptoms and no early recurrence of haemophilic pseudotumour.
Table 1Overview of all included haemophilic pseudotumours.
Haemophilia type: 1 mild; 2 moderate; 3 severe. Location: (presumed) origin stated first, size if stated.
| Case | Ref. | Age (y) | HT | Location(size in cm) | Symptoms | 1° management (timing) | Complications (management) | Outcome (follow-up duration) |
| 1 | [16] | 15 | A1 | EP; R, P (8 × 8 × 11) | Displacing bladder, intestines | I à S | – | Favourable (10 months) |
| 2 | [17] | 20 | A | EP; R | Organ displacement | C/I | BX (S, Em) | NS |
| 3 | [18] | 34 | A3 | EP; R (35 × 25 × 20) | Displacing kidney, renal and iliac vessels | S (El) | – | Favourable (17 days) |
| 4 | [19] | 17 | A2 | IP (8 × 11 × 6) | Intestinal obstruction | S (Em) | – | Favourable (1 year) |
| 5 | [20] | 65 | A3 | EP; R (22 × 22 × 25) | Displacing kidney, bowel | C/I | – | Favourable (3 years) |
| 6 | [21] | 72 | A3 | EP; P (8 × 3.5) | Compressing ureter | C/I | Skin fistula (C/I) | Size stable (8 years) |
| 7 | [22] | 11 | A1 | EP; R (4 × 8 × 8) | Infiltrating kidney, haematuria | C/I | – | Favourable (4 weeks) |
| 8 | [23] | 66 | A3 | IP (30) | Colocutaneous fistula, adherence (bowel, aorta, vena cava, ureter), sepsis | S (Em) | Bowel fistula, sepsis (S, Em) | Favourable (8 months) |
| 9 | [24] | 34 | A3 | EP; P (7) | Displacing bladder, bleeding | S (Em) | – | Favourable (14 days) |
| 10 | [25] | 42 | NS3 | IP | Stomach fistula, melaena | I à S | – | Favourable (1 week) |
| 11 | [26] | 57 | B1 | EP; Pleura | Displacing liver, lungs | S (El) | – | Favourable (6 months) |
| 12 | [27] | 30 | A3 | EP; R, P | Compressing kidney, haematuria | C/I | Sepsis (S, Em) | NS |
| 13 | [28] | 30 | B3 | EP; R (21) | Duodenal fistula, abscess, haematuria, sepsis | I (Em) à S (El) | – | Favourable (1 month) |
| 14 | [29] | 75 | A3 | EP; R, P (15 litres) | Compressing ureters, sepsis | C/I | Bowel fistula (C/I) | Death (sepsis, 5 years) |
| 15 | [29] | 39 | A3 | EP; R (8) | Abscess | C/I | Colonic fistula (S, El) | Favourable (1 year) |
| 16 | [30] | 52 | B | IP (21 litres) | Compressing intestines, satiety | S | – | Death (sepsis, timing NS) |
| 17 | [31] | 22 | A3 | EP; R (12 × 13 × 20 and 4 × 5 × 6) | Displacing kidney, bowel | S (El) | – | Favourable (3 months) |
| 18 | [32] | 45 | A3 | EP; R, P (20) | Displacing ureter, iliac vessels | C/I | – | Size stable (1 year) |
| 19 | [33] | 61 | A1 | EP; R (8 × 5 × 5) | Abscess, sepsis | C/I | Colonic fistula (S, El), BX (2×S, Em) | Favourable (3 years) |
| 20 | [34] | 53 | B3 | EP; R | Adherence (ureter, iliac/mesenteric vessels) | S (El) | Bowel fistula, abscess (C/I) | Death (sepsis, 8 months) |
| 21 | [35] | 64 | A3 | EP; R | Compressing ureter, haematuria | C/I | – | Death (BX, 10 days) |
| 22 | [6] | 38 | A1 | EP; R, P | Obstructive nephropathy, dialysis | S | Bladder fistula (C/I), coiling of EIA (S) | Death (sepsis, 1 year) |
| 23 | [36] | 23 | A | EP; R (30 × 25) | Compression, hydronephrosis | S (El) | – | Favourable (1 year) |
| 24 | [37] | 37 | A2 | EP; R (20 × 15) | Compressing ureter, hydronephrosis | S (El) | – | Favourable (1 year) |
| 25 | [37] | 51 | A2 | EP; R (10 × 6) | Displacing adjacent organs | S (El) | – | Favourable (5 years) |
| 26 | [38] | 31 | A3 | EP; P (40 × 30) | Compressing bowel and bladder, Ileus | S (El) | – | NS |
| 27 | [39] | 35 | B3 | EP; P (30 × 25) | Compressing ureter, colon | S | – | Favourable(9 months) |
| 28 | [40] | 48 | A3 | EP; P, R (23 × 21 × 14) | Displacing kidney, bowel, iliac vessels, skin fistula | C/I | Infection (S, C/I) | Recurrent infections (8 years) |
| 29 | [41] | 26 | A3 | EP; lumbar, R (20 × 9 × 6) | Compressing dural sac, nerve roots, ureter, large vessels | I à S (El) | – | Favourable (2 years) |
| 30 | [42] | 47 | B | EP; R (27 × 18) | Displacing diaphragm and bowel, compressing vena cava | I à S | – | Favourable (3 months) |
| 31 | [43] | 35 | A | EP; P | Compressing colon, ileus | S | – | NS |
| 32 | [44] | 37 | A3 | EP; P, R (75, 60) | Compressing pelvic/liver vessels, lung | I à S (El) | Migration of Kirschner wire (S, El) | Favourable (1 year) |
| 33 | [45] | 22 | A2 | EP; P (27 × 13 × 10) | Organ displacement | S (El) | – | Favourable (3 months) |
| 34 | [46] | 70 | A3 | EP; R (22 × 14 × 8, 21 × 16 × 7, 20 × 17 × 11) | Adjacent to right side of liver, compressing kidney | C/I | – | Favourable (2 years) |
| 35 | [47] | 51 | A3 | EP; R (6.8 kg) | Compressing kidney, spleen, stomach, bowel | S (El) | – | Favourable (1 month) |
| 36 | [48] | 47 | A1 | EP; R (34 × 30) | Displacing colon, ureter | S (El) | Skin fistula (S, El) | Favourable (2 years) |
| 37 | [49] | 30 | A3 | EP; R | Skin and bowel fistula, hematemesis, melaena | S (El) | – | Favourable (10 years) |
| 38 | [50] | 56 | B2 | EP; P | Colocutaneous fistula | I à S (El) | – | Favourable (8 months) |
| 39 | [51] | 43 | A3 | EP; P, R (21 × 18 × 8) | Displacing bowel, ureter, iliac vessels | S (El) | – | Favourable (2.7 years) |
| 40 | [52] | 34 | A1 | EP; R, P (10 × 10 × 15) | Displacing pelvic organs | I à S (El) | – | Favourable (5 years) |
| 41 | [53] | 22 | A3 | EP; P, R (21 × 25 × 28) | Displacing organs, iliac vessels, rupture of HPT: bleeding | S (Em) | – | Favourable (8 years) |
A: haemophilia A; B: haemophilia B; BX: bleeding; C/I: conservative or interventional therapy alone; EIA: external iliac artery; El: elective; Em: emergency; EP: extraperitoneal; HPT: haemophilic pseudotumour; HT: haemophilia type; I à S: interventional treatment with step-up to surgery; IP: intraperitoneal; NS: not stated; P: pelvis; R: retroperitoneum; Ref.: reference; S: surgical therapy alone
All included articles underwent a quality assessment according to the JBI critical appraisal checklist for case reports by two appraisers [14]. A maximum of 8 points could be reached (table 2).
Table 2Quality assessment according to the JBI critical appraisal checklist.
| Points | 1/8 | 2/8 | 3/8 | 4/8 | 5/8 | 6/8 | 7/8 | 8/8 |
| References | – | [42, 43, 53] | [6, 25, 30, 35, 36, 39, 45] | [20, 38, 40] | [19, 22, 29, 32, 34, 41, 49, 50] | [17, 21, 27, 28, 31, 37, 46, 47] | [26, 33, 44, 48, 52] | [16, 18, 23, 24, 51] |
Data analysis was performed with Prism 9.1.2 [12]. Categorical variables are expressed as frequency and percentage. Because of the heterogeneity of the review (a collection of case reports) and the small number of included cases, further statistical analyses were not conducted.
Considering the design of our study, ethical approval and consent were not required.
The data search in the three electronic databases using the defined terms extracted 1199 potential articles. After removing 584 duplicates, the titles and abstracts were screened for eligibility. Consequently, 516 articles were excluded due to an unclear or extraabdominal location of the reported haemophilic pseudotumour or for other blood cyst entities than haemophilia. The remaining 99 articles documented abdominal haemophilic pseudotumour with intraperitoneal or extraperitoneal location, the latter with contact to the abdomen by compressing or displacing abdominal organs. Twelve were excluded because of missing full texts or incomplete information regarding location. Eight articles were removed due to missing statements about therapy management. Another 16 articles reported the same cases as other already included publications and were therefore removed. Twenty-four articles were excluded due to publication before 1995. Eventually, 39 relevant articles published between 1995 and 2023 involving 41 cases were included in the final analysis (fig. 1).
The results of the quality assessment analysis are displayed in table 2. Of the articles, 25.6% (n = 10) reached eight or seven of eight points on the JBI critical appraisal checklist for case reports, whereas 41% (n = 16) fulfilled five or six points of the maximum of eight, and only 7.7% (n = 3) received one or two points in total.
All patients were male, with a mean age of 41.1 (SD ±16.5) years; the youngest patient was 11 and the oldest 75 years old. The type of haemophilia was specified in 40 (97.6%) cases. The majority were diagnosed with haemophilia A (n = 33; 80.5%). The severity of the bleeding disorder was mentioned in 36 (87.8%) cases: 24 (58.5%) were severe, five (12.2%) moderate and seven (17%) mild. In 37 (90.2%) cases, the reported haemophilic pseudotumours were extraperitoneal, and four (9.8%) haemophilic pseudotumours originated primarily intraperitoneally. All patients with acute bleeding had a baseline treatment with factor replacement therapy.
Twenty-three (56.1%) haemophilic pseudotumours caused organ impairment by compression (n = 16), fistulas (n = 6) or infiltration (n = 1). Eighteen (43.9%) haemophilic pseudotumours provoked no organ impairment, but two led to bleeding, and another two caused local abscesses. The follow-up was stated in 37 cases (90.2%).
Of all documented haemophilic pseudotumours, 12 cases (29.3%) underwent conservative or interventional treatment alone (fig. 2).
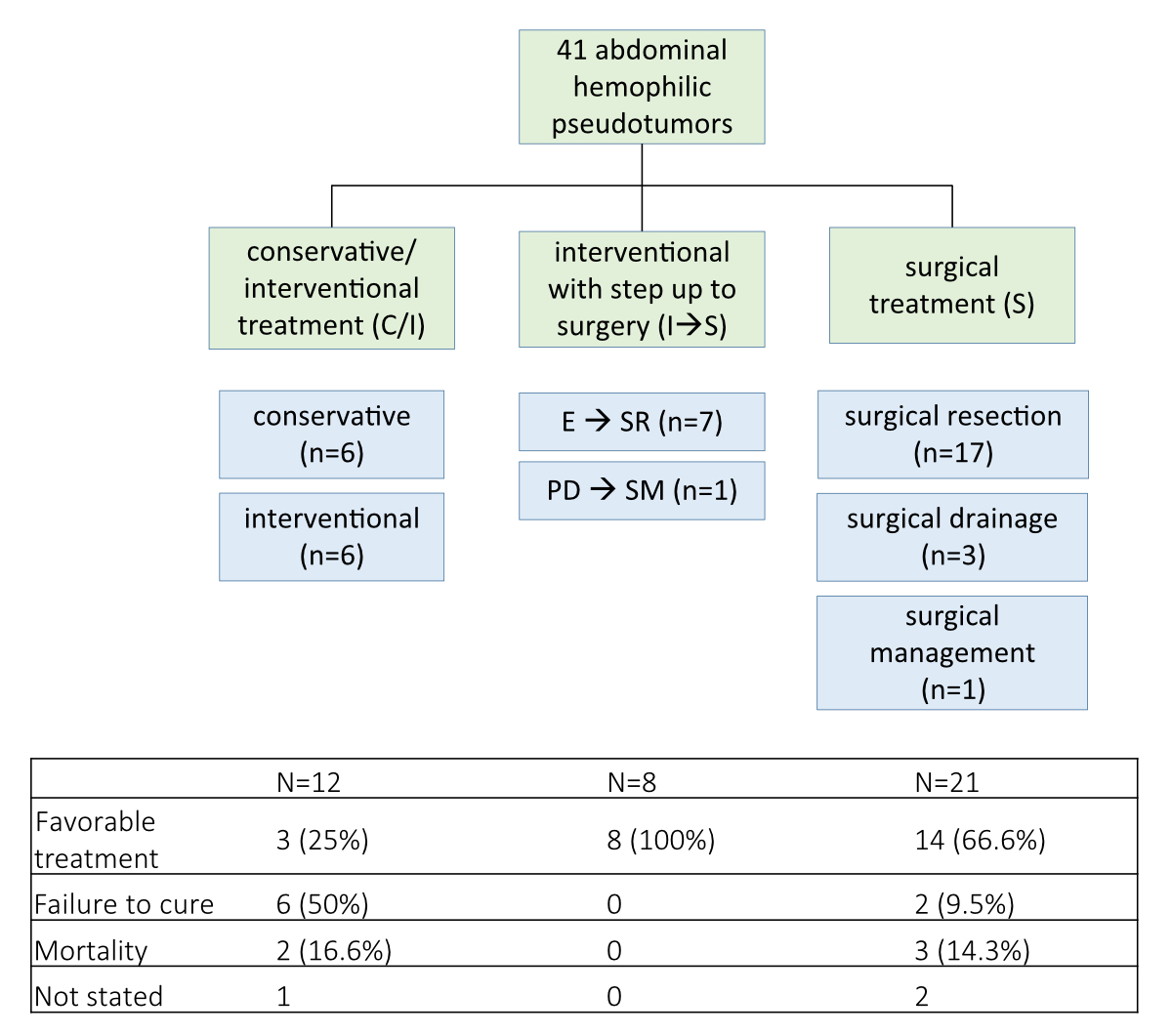
Figure 2Overview of treatments of haemophilic pseudotumours.
C/I: conservative or interventional therapy alone; I à S: interventional treatment with step-up to surgery; E à SR: Embolisation first, then surgical resection (complete resection); PD à SM: percutaneous drainage first, then surgical management (no resection: e.g. colostomy alone); S: surgical therapy alone
Six cases (50%) were treated only conservatively (cases 5, 6, 7, 21, 28 and 34). The age was either very young (11 years old) or mainly over 60 years. The outcome was reported in all cases and could be considered “favourable” in three (table 1).
Interventional therapy management alone was described in six cases (cases 2, 12, 14, 15, 18 and 19). Case two was only biopsied. Embolisation alone was the definite treatment in patient 18. The other three patients obtained percutaneous drainage alone as primary therapy (including one nephrostomy with previous radiotherapy 14 years ago in patient 14).
Interventional therapy followed by a planned step-up to surgical therapy was applied in eight cases (19.5%) (fig. 2). In seven patients, embolisation preceded surgical resection. Percutaneous drainage was followed by surgical management in case 13 (bypassing a duodenal fistula). The outcome after interventional management with step-up to surgery was described in all patients and could be considered “favourable” in all (table 1).
Primary surgical therapy was performed in 21 cases (51.2%) (fig. 2). Primary surgical resection of the haemophilic pseudotumours was performed in 17 cases (cases 3, 8, 9, 11, 16, 17, 20, 23, 24, 25, 26, 27, 33, 35, 36, 39 and 41); in case 20, only partial resection was performed. Surgical management alone was applied in case 31 as a colostomy due to compression of the colon by the haemophilic pseudotumour. Surgical drainage was the initial therapeutic concept in three cases (cases 4, 22 and 37). Case 37 was followed by surgical management (temporary colostomy). The outcome after primarily performed surgery was described in 19 of 21 patients and could be considered “favourable”in 16 patients (table 1).
After conservative therapy, one out of six patients (case 21) died due to unmanageable gastrointestinal bleeding 10 days after the therapy onset. Case 6 developed a skin fistula under conservative treatment. Case 28 received surgical drainage and radiotherapy after recurrent infections while being treated conservatively.
Interventional therapy alone led to complications in five out of six cases. Case 2 required surgical drainage after a biopsy complicated by bleeding. Three cases required surgical resection after percutaneous drainage: case 12 needed left nephrectomy and splenectomy due to sepsis, and cases 15 and 19 required surgery due to bowel fistulas (case 19 needed multiple surgeries due to postoperative bleeding). Case 14 died from sepsis five years after interventional treatment due to the development of a bowel fistula that was treated conservatively.
One out of eight patients (case 32) received interventional management followed by surgical management one year after initial surgery due to the migration of a Kirschner wire.
Four of 21 primarily surgically treated haemophilic pseudotumours required re-operation or intervention to treat complications: case 8 needed surgery because of a bowel fistula followed by sepsis and case 36 because of a skin fistula. Case 20 developed an intraabdominal abscess six months after partial surgical resection that needed percutaneous drainage and was followed by a large bowel fistula; he died 14 months after initial surgery due to sepsis. Case 22 developed a bladder fistula with haematuria following surgery and was scheduled for embolisation of the internal iliac artery. An accidental coiling of the external iliac artery occurred, which required surgical management, followed by an inguinal hematoma with neurological compromise; the patient declined further interventions and died one year later due to sepsis. Case 16 died due to sepsis after the initial surgery.
As an example, we include here a case description from our institution, which has not been published and is not included in this systematic review.
A 25-year old male patient with moderate hemophilia B and progressive abdominal pain was admitted to our emergency department. The initial hemoglobin was 65 g/l. The abdominal CT scan detected ubiquitous intraabdominal free fluid and a tumor in the right hemiabdomen (figure 3). The exploratory laparotomy presented non-coagulated blood in all four quadrants and a coagulum-like tumor in the omentum majus attached to the right colon. Complete resection followed. Histology revealed a hemophilic pseudotumor (13 × 8 × 6 cm). The postoperative course and 3 months follow-up was uneventful.
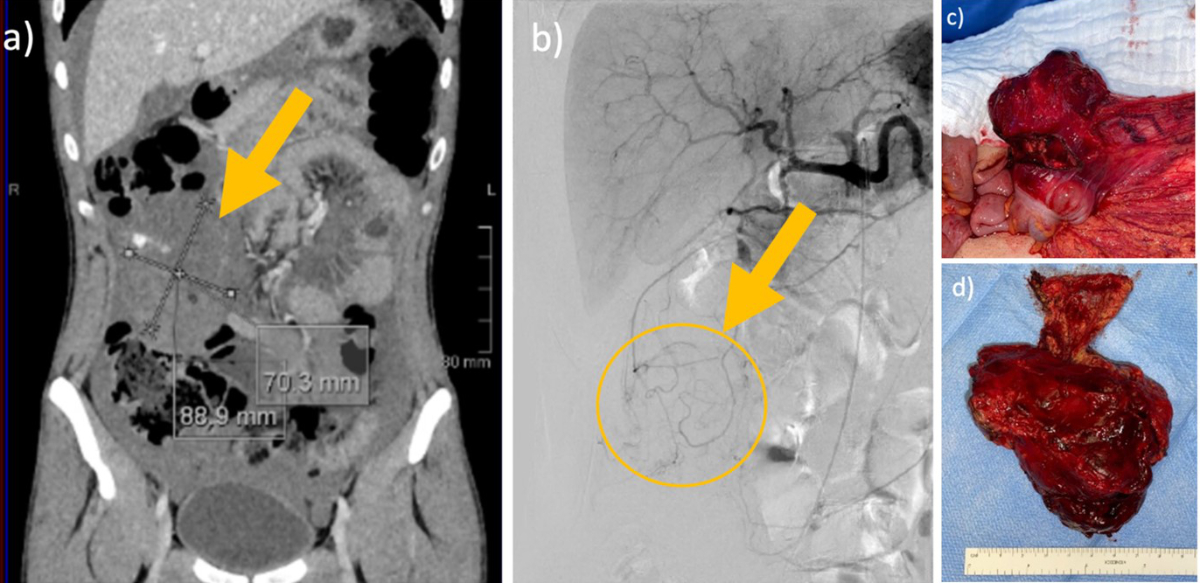
Figure 3Intraabdominal pseudotumour in a 25-year-old male patient.
25-year-old male patient with moderate haemophilia B with progressive abdominal pain and a haemoglobin of 65 g/l. Abdominal CT scan detected ubiquitous intraabdominal free fluid and a tumour in the right hemiabdomen. Exploratory laparotomy presented non-coagulated blood in all four quadrants and a coagulum-like tumour in the omentum majus attached to the right colon. Complete resection followed. Histology revealed a haemophilic pseudotumour (13 × 8 × 6 cm).Postoperative course and three-month follow-up were uneventful.
(a) Contrast CT venous phase with tumour 6.6 × 7.4 × 9.6 cm, (b) Angiography with no sign of active bleeding, (c) Intraoperative finding, (d) Resected specimen
This systematic review summarises the current publications on therapy strategies for symptomatic, abdominal haemophilic pseudotumours. Due to the rarity of this haemophilic complication, only 39 case reports were included, describing 41 cases with abdominal or abdominal-related haemophilic pseudotumours. The quality of the included reports was mostly good (table 2). Overall, the following main findings can be recapitulated: first, optimal therapy for symptomatic, abdominal haemophilic pseudotumour seems to be surgical resection. Second, preoperative embolisation can be an effective bridging option to stabilise patients and perform surgery in a second step. Third, diagnostic biopsy and percutaneous drainage tended to cause severe bleeding complications in the included reports. This could lead to the suggestion of avoiding biopsies or punctures in abdominal haemophilic pseudotumours.
The treatment goal for haemophilic patients is to not experience any bleeds by regularly administrating therapeutic products aimed at maintaining haemostasis. For patients with severe haemophilia, the World Federation of Hemophilia strongly recommends prophylaxis, which should be individualised, considering for example the patient’s bleeding phenotype and the availability of medication. Prophylaxis with clotting factor concentrates is referred to as regular replacement therapy, in contrast to episodic replacement therapy (on-demand), which is administrated only at the time of the bleed. Episodic therapy, regardless of the doses used, does not alter the bleeding profile significantly and, hence, does not change the natural history of haemophilia leading to complications due to bleeding. The cases included in this systematic review were, whenever information was available, mainly under on-demand administration of clotting factors. In the emergency settings, clotting factors were replaced in all cases and supported interventional or surgical treatment.
However, the current literature confirms the exceptionally rare incidence of abdominal haemophilic pseudotumour. The overall incidence of all haemophilic pseudotumours is already low at 1–2%, depending on the prophylactic regimen [9]. In general, the higher factor levels at all times, the less the spontaneous bleeding. In countries with healthcare constraints and for patients with limited access to clotting factor concentrates, less intensive prophylaxis may be used, with the possible price of more bleeds [4]. The reviewed publications came mainly from Asia, especially Turkey and India, both countries with wide rural areas and healthcare constraints. A lack of regular and adequate prophylactic therapy in severe haemophilia may promote the formation of haemophilic pseudotumours.
A recent literary review from 1983–2015 reported 134 patients with ubiquitous haemophilic pseudotumour [7]. Similar to our findings, 82% of the cases were associated with haemophilia A, and in only 16.5% of the cases, haemophilia B was present; in 1.5%, no information on the type of haemophilia was available. Of the patients, 51.9% had severe haemophilia, 25.6% moderate and 10.5% mild, visualising the importance of the phenotype. Of all included haemophilic pseudotumour, only seven cases (5.2%) of abdominal or retroperitoneal (not clearly stated) localisations were reported. Again, this illustrates the low prevalence of abdominal haemophilic pseudotumours. Surgical intervention was needed in 79 of these cases (59%), of which 56 (71%) reported complete resection of the tumour.
In this systematic review, the treatment management of the 41 analysed cases was divided into three comprehensible and clinically relevant categories: conservative alone, including interventional treatment; interventional treatment with step-up to surgery according to a two-stage procedure; and surgical approach “alone”. All symptomatic cases received a baseline treatment with clotting factor concentrates, which was the treatment alone in conservative management and supportive in all other treatments. However, the first group, including abdominal haemophilic pseudotumours treated conservatively or interventionally, had a high complication rate of over 50%. This could suggest that conservative management of symptomatic abdominal haemophilic pseudotumour is not optimal and the dynamic of the pseudotumours may lead to further complications. However, the numbers are too low to draw conclusions.
All cases in the second group with the step-up treatment documented no complication or mortality. From the limited information of the case reports, we hypothesise that the initial interventional treatment (the first step) was undertaken to control present bleeding or reduce the risk of intraoperative haemorrhage in terms of preoperative conditioning. Eventually, surgery was undertaken in a more controlled and stable situation. Hence, preoperative embolisation may be an effective bridging therapy to surgery.
The majority of all cases were treated surgically with resection of the abdominal haemophilic pseudotumours and had mainly favourable outcomes. The mean follow-up in this group was 1.9 years (SD ±2.7 years), which is clearly not representative to make a statement about the recurrence rate of abdominal haemophilic pseudotumours after surgical resection.
This systematic review has clear limitations. Due to the nature of this rare disease, mainly case series and case reports over 27 years could be included. Missing data parameters due to the heterogeneity of all case reports make the comparability difficult, and the results must be interpreted with caution. Additionally, the study has a relatively small sample size, limiting the conclusion from the presented data.
Our review confirms that haemophilic pseudotumours with abdominal contact are uncommon, and intraperitoneal pseudotumours are even more exceptionally rare. Complete surgical resection of symptomatic, abdominal haemophilic pseudotumours should be evaluated as the treatment of choice, and preoperative embolisation may be used as a bridging therapy before surgery. Diagnostic biopsy and percutaneous drainage should be avoided because they can lead to bleeding and the development of bowel fistulation and cutaneous fistulation [15]. However, regular and individualised prophylactic treatment in severe haemophilia may prevent haemophilic pseudotumours.
FvS and JW collected and analysed the data, wrote the paper, and created the tables and figures. IH, RS and KL analysed the data, contributed essential information to the conception, provided a factual review and helped edit the manuscript.
This research received no specific grant from any funding agency.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. van den Berg HM, De Groot PH, Fischer K. Phenotypic heterogeneity in severe hemophilia. J Thromb Haemost. 2007 Jul;5 Suppl 1:151–6. 10.1111/j.1538-7836.2007.02503.x
2. Hermans C, Dolan G, Jennings I, Windyga J, Lobet S, Rodríguez-Merchán EC, et al. Managing Haemophilia for Life: 5th Haemophilia Global Summit. Eur J Haematol. 2015 Oct;95 Suppl 78:1–25. 10.1111/ejh.12617
3. Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica. 2019 Sep;104(9):1702–9. 10.3324/haematol.2019.221093
4. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia, 2020. 26 Suppl 6: p. 1-158.
5. Olasupo OO, Lowe MS, Krishan A, Collins P, Iorio A, Matino D. Clotting factor concentrates for preventing bleeding and bleeding-related complications in previously treated individuals with haemophilia A or B. Cochrane Database Syst Rev. 2021 Aug;8(8):CD014201.
6. Lim MY, Nielsen B, Ma A, Key NS. Clinical features and management of haemophilic pseudotumours: a single US centre experience over a 30-year period. Haemophilia. 2014 Jan;20(1):e58–62. 10.1111/hae.12295
7. Doyle AJ, Back DL, Austin S. Characteristics and management of the haemophilia-associated pseudotumours. Haemophilia. 2020 Jan;26(1):33–40. 10.1111/hae.13870
8. Gavrel M, Rafowicz A, d’Oiron R, Franchi-Abella S, Lambert T, Adamsbaum C. Imaging features of atypical bleeds in young patients with hemophilia. Diagn Interv Imaging. 2019 Mar;100(3):135–45. 10.1016/j.diii.2018.11.010
9. Lin S, Tong K, Wang G, Zhong Z, Cao S, Feng Z. Clinical characteristics and surgical treatment of haemophilic pseudotumor: A retrospective analysis of thirty-four patients. Haemophilia. 2020 Sep;26(5):873–81. 10.1111/hae.14109
10. Rodriguez-Merchan EC. Haemophilic cysts (pseudotumours). Haemophilia. 2002 May;8(3):393–401. 10.1046/j.1365-2516.2002.00609.x
11. Rodriguez-Merchan EC. Hemophilic Pseudotumors: diagnosis and Management. Arch Bone Jt Surg. 2020 Mar;8(2):121–30.
12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009 Oct;62(10):e1–34. 10.1016/j.jclinepi.2009.06.006
13. Fischer K, Berntorp E. Targeting factor replacement therapy in severe hemophilia: which level is important? Semin Thromb Hemost. 2015 Nov;41(8):860–3. 10.1055/s-0035-1552562
14. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020.
15. Magallón M, Monteagudo J, Altisent C, Ibáñez A, Rodríguez-Pérez A, Riba J, et al. Hemophilic pseudotumor: multicenter experience over a 25-year period. Am J Hematol. 1994 Feb;45(2):103–8. 10.1002/ajh.2830450202
16. Ahuja SP, Sidonio R Jr, Raj AB, Bertolone SJ, Silverman C, Antekeier DP, et al. Successful combination therapy of a proximal haemophilic pseudotumour with surgery, radiation and embolization in a child with mild haemophilia A. Haemophilia. 2007 Mar;13(2):209–12. 10.1111/j.1365-2516.2006.01425.x
17. Al Saadi AS, Al Wadan AH, El Hamarneh SA, Emad ME. Life-threatening biopsy of an iliopsoas pseudotumour in a patient with haemophilia: a case report. J Med Case Rep. 2008 Apr;2(1):135. 10.1186/1752-1947-2-135
18. Bian YY, Wu H, Huang Z, Zhai J, Liu Y, Weng XS. Surgical treatment of a giant iliopsoas haemophilic pseudotumour with adjacent structure compressions: A case report. Haemophilia. 2017 Nov;23(6):e507–12. 10.1111/hae.13299
19. Chatterjee S, Mukhopadhyay R. Intra-Abdominal Mesenteric Haemophilic Pseudotumour in an Undiagnosed Case of Haemophilia: a Rare Cause of Intestinal Obstruction. Indian J Surg. 2020;82(6):1284–6. 10.1007/s12262-020-02274-z
20. Dupont MV, Coche EE. CT and MRI Aspects of an Abdominal Hemophilic Pseudotumor. J Belg Soc Radiol. 2015 Dec;99(2):50–2. 10.5334/jbr-btr.887
21. Ferreira de Matos C, Claeyssens-Donadel S, Debard A, Piel-Julian ML. A pelvic mass. Rev Med Interne. 2022;43(5):323–4. 10.1016/j.revmed.2022.02.005
22. Fouda AE, Mansour AK, Al-Tonbary YA. Not a true tumour, but a renal pseudotumour: a case report of an 11.5 year old mild haemophilic child. Haemophilia. 2010 Nov;16(6):956–8. 10.1111/j.1365-2516.2010.02334.x
23. Frezin J, Marique L, Coubeau L, Hubert C, Lambert C, Hermans C, et al. Successful emergency resection of a massive intra-abdominal hemophilic pseudotumor. World J Gastrointest Surg. 2015 Mar;7(3):43–6. 10.4240/wjgs.v7.i3.43
24. García-Pérez R, Torres-Salmerón G, Sánchez-Bueno F, García-López A, Parrilla-Paricio P. Intraabdominal hemophilic pseudotumor: case report. Rev Esp Enferm Dig. 2010 Apr;102(4):275–80. 10.4321/S1130-01082010000400009
25. Garge S, Keshava SN, Moses V, Mammen S, Ahmed M, Chiramel GK, et al. Role of endovascular embolization in treatment of acute bleeding complications in haemophilia patients. Br J Radiol. 2016 Aug;89(1064):20151064. 10.1259/bjr.20151064
26. Goel MK, Juneja D, Jain SK, Chaudhuri SK, Kumar A. An unusual case of pleural-based tumor with life-threatening post-operative complication. Indian J Crit Care Med. 2012 Jan;16(1):48–51. 10.4103/0972-5229.94436
27. Gürkan E, Oçal F. Renal haemophilic pseudotumour. Haemophilia. 2005 Sep;11(5):559–60. 10.1111/j.1365-2516.2005.01126.x
28. Hahn SY, Hahn SM, Jin SL, Kim HS, Lyu CJ, Lee JG, et al. Huge retroperitoneal complicated pseudotumour in haemophilia B with inhibitor. Haemophilia. 2016 Jan;22(1):e45–7. 10.1111/hae.12799
29. Heaton DC, Robertson RW, Rothwell AG. Iliopsoas haemophiliac pseudotumours with bowel fistulation. Haemophilia. 2000 Jan;6(1):41–3. 10.1046/j.1365-2516.2000.00349.x
30. Iqbal M, Comp PC, Wu DH. Progression of an untreated pseudotumor. Haemophilia. 2017 Sep;23(5):e464–6. 10.1111/hae.13284
31. Kamal AF, Pradana AS, Prabowo Y. Bilateral iliopsoas haemophilic “soft tissue pseudotumours”: A case report. Int J Surg Case Rep. 2015;13:19–23. 10.1016/j.ijscr.2015.05.018
32. Keller A, Terrier F, Schneider PA, Bianchi S, Howarth N, De Moerloose P. Pelvic haemophilic pseudotumour: management of a patient with high level of inhibitors. Skeletal Radiol. 2002 Sep;31(9):550–3. 10.1007/s00256-002-0518-8
33. Kilic YA, Dundar SV, Onat D, Akhan O. Iliopsoas hemophilic pseudotumor with bowel fistulization. Bratisl Lek Listy. 2009;110(11):729–31.
34. Kumar L, Varghese R, Menon RN, Siddharthan N. Perioperative management of a patient with severe Haemophilia B for abdominal pseudotumour Surgery. Indian J Anaesth. 2015 Jul;59(7):461–2. 10.4103/0019-5049.160978
35. Kuo CC, Huang CC, Chu TS. Renal haemophilic pseudotumour. Acta Clin Belg. 2009;64(6):555–6. 10.1179/acb.2009.095
36. Liu S, Zhou X, Song A, Huo Z, Wang Y, Liu Y. Successful treatment of giant retroperitoneal haemophilic pseudotumour. Postgrad Med J. 2019 Aug;95(1126):457. 10.1136/postgradmedj-2019-136719
37. Liu S, Zhou X, Song A, Huo Z, Wang Y, Liu Y. Successful resection of giant abdominal hemophilic pseudotumor: surgical treatment and follow-up outcomes in one single center. Medicine (Baltimore). 2019 Nov;98(46):e17998. 10.1097/MD.0000000000017998
38. López-Gómez J, Contreras JS, Figueroa-Ruiz M, Servín-Torres E, Velázquez-García J, Bevia-Pérez F, et al. Management of the hemophilic pseudotumor of the abdomen: A rare pathological entity. Int J Surg Case Rep. 2014;5(11):789–92. 10.1016/j.ijscr.2014.08.022
39. Luther A, Mahajan AV, Pandey S. Hemophilic pseudotumor of abdomen: A rare case report. Indian Journal of Vascular and Endovascular Surgery. 2020;7(1):91–3. 10.4103/ijves.ijves_21_19
40. Malkan UY, Gunes G, Eliacik E, et al. Giant Hemophilic Pseudotumor of the Iliopsoas: case Report. UHOD Uluslar Hematol Onkol Derg. 2014;24(4):286–8.
41. Nachimuthu G, Arockiaraj J, Krishnan V, Sundararaj GD. Hemophilic pseudotumor of the first lumbar vertebra. Indian J Orthop. 2014 Nov;48(6):617–20. 10.4103/0019-5413.144238
42. Nguyen H, Nakakura E, Karp S, et al. Management of an enormous hemophilic pseudotumor with arterial embolization followed by surgical evacuation. Haemophilia. 2008;14:61–61.
43. O’Dowd M, Geoghegan T, Munk PL, McAuley G, Torreggiani WC. Haemophilic pseudotumour presenting with large bowel obstruction. Australas Radiol. 2006 Aug;50(4):386–8. 10.1111/j.1440-1673.2006.01607.x
44. Pennekamp PH, Strauss AC, Klein C, Marx A, Goldmann G, Friedrich M, et al. Giant haemophilic pseudotumour of the pelvis: case report and literature review. Haemophilia. 2015 Nov;21(6):e484–6. 10.1111/hae.12752
45. Poudyal BS, Shrestha GS. Giant hemophilic pseudotumor eroding the iliac bone. Oxf Med Case Rep. 2021 Mar;2021(3):omab005. 10.1093/omcr/omab005
46. Sagheer S, Atkins A, McRae S. Successful use of tranexamic acid in the management of haemophilic pseudotumour. Haemophilia. 2016 Jul;22(4):e306–9. 10.1111/hae.12911
47. Salaj P, Gurlich R, Svorcová V, Marková M, Cetkovský P. Prophylactic preparation and surgical extirpation of a very large abdominal blood cyst in a severe haemophilia A patient with inhibitors managed by rFVIIa. Haemophilia. 2009 Jan;15(1):380–2. 10.1111/j.1365-2516.2008.01929.x
48. Şenol K, Tütüncü T, Yüksek YN, Dağlar Özdemir G, Güney Y, Kama NA. Management of a recurrent massive abdominal haemophilic pseudotumour with adjuvant radiotherapy. Haemophilia. 2015 Jul;21(4):e333–6. 10.1111/hae.12707
49. Serban M, Mihailov D, Savescu D, et al., Long-term outcome of an unusual haemophilic pseudotumour. Hamostaseologie, 2012. 32 Suppl 1(Suppl 1): p. S43-4.
50. Sevilla J, Alvarez MT, Hernández D, Canales M, De Bustos JG, Magallón M, et al. Therapeutic embolization and surgical excision of haemophilic pseudotumour. Haemophilia. 1999 Sep;5(5):360–3. 10.1046/j.1365-2516.1999.00330.x
51. Valentino LA, Martinowitz U, Doolas A, Murali P. Surgical excision of a giant pelvic pseudotumour in a patient with haemophilia A. Haemophilia. 2006 Sep;12(5):541–4. 10.1111/j.1365-2516.2006.01318.x
52. Ying SH, Chen WM, Wu PK, Chen CF, Liu CL, Chen TH. Pelvic hemophilic pseudotumor presenting as severe sciatic pain in a patient with no history of hemophilic symptoms. J Orthop Sci. 2012 Jul;17(4):490–4. 10.1007/s00776-011-0094-7
53. Zheng J, Chen K, Liu F, Deng Y, Mao Z, Lv J, et al. Treatment of pelvic haemophilic pseudotumour: A retrospective study. Haemophilia. 2020 Nov;26(6):e308–14. 10.1111/hae.14148
Table S1The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports (last amended in 2017).
Website: https://joannabriggs.org/critical_appraisal_tools; https://wiki.joannabriggs.org/display/MANUAL/Appendix+7.4+Critical+appraisal+checklist+for+case+reports
| Major components | Response options | |||
| 1. Were the patient’s demographic characteristics clearly described? | Yes | No | Unclear | Not applicable |
| 2. Was the patient’s history clearly described and presented as a timeline? | Yes | No | Unclear | Not applicable |
| 3. Was the current clinical condition of the patient on presentation clearly described? | Yes | No | Unclear | Not applicable |
| 4. Were diagnostic tests or assessment methods and the results clearly described? | Yes | No | Unclear | Not applicable |
| 5. Was the intervention(s) or treatment procedure(s) clearly described? | Yes | No | Unclear | Not applicable |
| 6. Was the post-intervention clinical condition clearly described? | Yes | No | Unclear | Not applicable |
| 7. Were adverse events (harms) or unanticipated events identified and described? | Yes | No | Unclear | Not applicable |
| 8. Does the case report provide takeaway lessons? | Yes | No | Unclear | Not applicable |
| Overall appraisal: Include □ Exclude □ Seek further info □ | ||||