Vulnerability to
heat-related mortality and the effect of prevention measures: a time-stratified
case-crossover study in Switzerland
DOI: https://doi.org/https://doi.org/10.57187/s.3418
Martina S.
Ragettlia,b,
Benjamin
Flückigera,b,
Danielle
Vienneaua,b,
Silvia
Domingo-Irigoyenc,
Markus
Koschenzc,
Martin
Rööslia,b
a Swiss Tropical and Public Health
Institute (Swiss TPH), Allschwil, Switzerland
b University of Basel, Basel,
Switzerland
c Lucerne University of Applied
Sciences and Arts. School of Engineering and Architecture, Horw, Switzerland
Summary
BACKGROUND: Swiss climate scenarios predict increases
in the frequency and intensity of extreme heat episodes in the future. For the effective
prevention of heat-related mortality, several aspects of the population’s
vulnerability to heat must be understood on a local level.
METHODS: A nationwide analysis of individual death
records was conducted, enabling a more comprehensive understanding than typical
heat studies based on aggregated data. A total of 320,306 individual death records
from the Swiss National Cohort with precise address information during the warm season
(May to September) from 2003–2016 were linked to indoor and outdoor high-resolution
daily temperature estimates. A time-stratified case-crossover study combined
with distributed lag non-linear models was then performed to assess the temperature-mortality
associations for various causes of death and to estimate the potential effect
modification of individual characteristics. Additionally, it was explored whether
the effect of extreme heat changed over time in regions with and without
cantonal heat-health action plans (HHAPs).
RESULTS: Using the temperature with the lowest cause-specific
mortality risk (minimum mortality temperature) as the reference temperature, extreme
heat (defined as ambient daily maximum temperature reaching 33 °C) was
associated with a strong increase in all-cause mortality (odds ratio (OR):
1.21, 95% CI: 1.17–1.25) and disease-specific mortality from Alzheimer’s disease and
dementia (OR: 1.67, 95% CI: 1.48–1.88), COPD (OR: 1.37, 95% CI: 1.12–1.67), diabetes
(OR: 1.34, 95% CI: 1.06–1.70), and myocardial infarction (OR: 1.26, 95% CI:
1.10–1.44). Indoor temperatures above 24 °C were found to be critical for
mortality. The population most vulnerable to heat included older adults (≥75
years), unmarried individuals, people with a low education level, older women
with low neighbourhood socioeconomic position, and men under 75 years old with
low socioeconomic position. Overall, the risk of heat-related all-cause
mortality in 2009–2016 was lower than that in 2003–2008. The decrease was
significantly stronger in the region where cantonal HHAPs were implemented.
CONCLUSIONS: This study provides
important information for planning targeted and effective measures to reduce heat-related
health risks in Switzerland. It demonstrates that HHAPs contribute to reducing
heat-related mortality, although they may not reach the high-risk population of
individuals with low socioeconomic position. Future prevention efforts should also
target the less privileged population, including people younger than 75 years.
Introduction
Increasing heat stress is one of Switzerland’s most critical climate-related risks
[1]. Currently, Switzerland is experiencing long-term warming that is approximately
twice that of the global average. According to the CH2018 Swiss Climate Scenarios,
the frequency and intensity of heatwaves are expected to increase, presenting a substantial
public health risk [2]. Exposure to high ambient temperatures is associated with various
morbidity outcomes and an increased risk of premature death [3–7]. In Switzerland,
recent heatwaves have caused substantial excess mortality of several hundred deaths
[8–11]. Although heatwaves are part of naturally varying weather conditions, estimates
indicate that approximately one-third of the heat-related deaths from 1991 to 2018
were attributable to human-induced climate change [12]. Given the continuing warming
trend, preventative measures to reduce the heat-related mortality burden are crucial.
To protect the health of the population from heat, European public health authorities
have introduced intervention measures. Many of these were implemented after the heatwave
of 2003, which contributed to 70,000 excess deaths across the region [13], including
nearly 1000 in Switzerland [14]. Health authorities in some cantons of Switzerland
have implemented heat-health action plans (HHAPs). Such plans are recommended by the
Word Health Organization (WHO) Europe and support a systematic and comprehensive public
health response that consists of a portfolio of measures at different levels [15].
The canton of Ticino in the south of Switzerland and cantons in the Lake Geneva region
implemented HHAPs between 2004 and 2008. These cantons followed the example of neighbouring
countries France and Italy, which were among the first to adopt national HHAPs [10,
16]. Cantonal HHAPs are coordinated by the cantonal health departments and include
various public health measures involving different partners from the health and social
care system. Measures are targeted to inform the population and health professionals
about heat-related health risks, protect the most vulnerable groups during heat events,
and support long-term adaptation to increasing heat stress. In cantons without HHAPs,
public health measures are lacking or have only been partly implemented; partial implementations
are mostly limited to single actions to raise awareness about heat-related health
risks during heat episodes [8, 17, 18].
To effectively prevent heat-related health effects, several questions must be answered
at the local level. The identification of the most vulnerable population groups is
essential for planning effective interventions. A better understanding of the causes
of death that are associated with heat might elucidate possible underlying vulnerabilities.
Although it is important to determine the critical outdoor temperatures for heat-related
mortality, an understanding of the role of indoor temperatures in buildings is also
needed [19–21]. People spend most of their time indoors [22, 23]. However, to date,
most studies have only reported the exposure-response relationships for outdoor temperature,
and thus critical indoor temperatures remain unclear.
To better understand these important aspects of the prevention of heat-related mortality
in Switzerland, a large, nationwide analysis of individual death records was carried
out, providing a deeper understanding than typical heat studies that rely on aggregated
mortality data. Using a case-crossover study design and a high spatial resolution
model of daily temperature exposure, we aimed to systematically identify heat-related
causes of death, investigate critical indoor temperatures for heat-related mortality,
and define the most vulnerable populations by assessing various individual risk factors.
In addition, we aimed to determine whether the prevention measures implemented in
Switzerland changed the effect of extreme temperatures on mortality between 2003 and
2016.
Materials and methods
Study population
Individual death records from the Swiss National Cohort (SNC) from 2003 to 2016 were
used [24]. The SNC is a long-term cohort based on the linkage of mortality and national
census records for all people residing in Switzerland. It encompasses individual data
on sex, age, marital status, education, accommodation, and other factors. The SNC
also contains a Swiss-specific indicator for neighbourhood-based socioeconomic position,
which allows the assessment of health effects by socioeconomic profiles [25, 26].
For the present 14-year analysis, only deaths in the permanent resident population
that occurred during the warm season (May to September) were included. Observations
with missing information on the residential building were excluded (n = 309), which
left 320,306 deaths during the study period.
The main outcome was all-cause mortality (International Classification of Diseases,
10th version: A00–Z99). In addition, specific primary causes of death indicated by
previous studies [6, 27] to have a plausible association with heat exposure were investigated.
These included neoplasms (C00-D48), endocrine diseases (E00-E90), mental and behavioural
disorders (F00-F99), diseases of the nervous system (G00-G99), cardiovascular diseases
(CVDs; I00-I99), respiratory diseases (J00-J99), digestive diseases (K00-K93), renal
diseases (N00-N39), and external-cause mortality (V01-Y98). CVDs were divided into
cerebrovascular diseases (I60-I69), stroke (I60-I64), ischaemic heart diseases (I20-I25),
myocardial infarction (I21-I22), and hypertensive diseases (I10-I15). Additionally,
we considered the following primary death causes: diabetes (E10-E14), Alzheimer’s
disease and dementia (F00-F03, G30-G31.1, G31.8-G31.9), chronic obstructive pulmonary
disease (COPD; J41-J44), suicide (X60-X84), and accidents (V01-X59).
Outdoor and indoor temperature exposure
The exposure to ambient temperature at the residential address of each deceased person
was assessed using the temperature model by Flückiger et al. [28]. The model predicts
the daily maximum temperature (Tmax), daily mean temperature (Tmean), and daily minimum
temperature (Tmin) 2 m above ground level with a fine spatial resolution of 100 ×
100 m across Switzerland. It was developed to capture small-scale temperature variability
within cities and in complex topographic terrains using satellite data, station-based
temperature measurements, atmospheric re-analysis data, and land-use information.
Tmax was used for the analysis of the effect of heat on mortality to assess the effect
of the most extreme warm season temperatures. Previous studies in Switzerland have
shown that Tmean, Tmax, and Tmin are highly correlated and result in similar exposure-response
relationships with mortality [10, 29]. However, to assess whether warm nights affected
cause-specific mortality differently, the cause-specific exposure-response curves
for outdoor Tmin are presented in the appendix.
Exposure to daily maximum indoor temperature (indoor Tmax) was estimated for each
death using a simplified thermal building model for three typical Swiss building types
[30]. Indoor temperature levels were simulated using predefined physical building
parameters for single-family houses, apartment houses built in or before 1990, and
apartment houses built after 1990 (table S1 in the appendix). The individual-level
information on building type and building period was provided by the SNC. It was assumed
that the windows were open (not tilted) during the night for natural cooling. The
indoor temperature model considered individual outdoor Tmax levels and daily mean
global radiation on the day of death and in the 14 days prior to account for the influence
of the weather conditions on the thermal mass of the building. Outdoor temperature
at the residential address was estimated with the fine-grained temperature model by
Flückiger et al. [28]. The data on global radiation was collected from the MeteoSwiss
monitoring network from the station closest to the building. A detailed description
of the indoor temperature simulation and its comparison to a more complex thermal
building simulation program is provided in the appendix A.
Statistical analysis
A time-stratified case-crossover design was used to investigate the acute effects
of short-term temperature increases on mortality. In this approach, the temperature
exposure on and before the day of death (at the address of the deceased person) was
compared with exposure during non-event (control) days [31]. Because each participant
served as his or her own control, potentially confounding effects of individual time-invariant
characteristics, such as age or sex, were controlled for. Control days were matched
to the same day of the week within the month that the death occurred to avoid overlap
bias [32]. Thus, a maximum of four control days per death was possible. In the time-stratified
case-crossover design, acute heat-related health effects were assessed on the basis
of individual temperature exposures. In contrast to classical time series studies
relying on central monitoring stations, small-scale spatial contrasts in temperature
exposure were explicitly considered in this study design.
Conditional logistic regression models with distributed lag non-linear models (DLNMs)
were run to estimate associations between cause-specific mortality risks and outdoor
and indoor temperatures, accounting for the nonlinear form of exposure-response functions
[33]. The DLNM method relies on the definition of a cross-basis function. For outdoor
temperature, the exposure dimension of the cross-basis term was modelled with a quadratic
B-spline with two internal knots placed at the 50th and 90th percentile (P) of the warm season temperature distribution. The lag dimension was
specified as a natural cubic spline with two internal knots placed at equally spaced
values on the log scale, similar to the methods of previous studies investigating
temperature-mortality associations during the warm season [29, 34, 35]. We considered
lagged effects up to a week before death to capture potential short-term harvesting.
An alternative number of lags (10) and the positions of the knots at P75 and P90,
as well as two knots (P10/P90 and P75/P95), were tested for all-cause mortality, and
the best model was selected according to the lowest Akaike information criterion (AIC).
Cumulative temperature-mortality associations were evaluated across the whole lag
period for each death cause. These associations were centred at the cause-specific
minimum mortality temperature (MMT), following a common approach of studies applying
DLNM. This defines the temperature with the least mortality risk, corresponding to
an odds ratio (OR) of 1. Similar to previous work [11, 36], the MMT was limited to
values between P25 and P90 of warm season temperature distributions in this study.
To characterise the effect of extreme heat exposure on cause-specific mortality, cumulative
(over lags 0–7) odds ratios (ORs) of mortality are presented as the change in mortality
risk from the cause-specific MMT to the P98 of the warm season Tmax (33 °C).
The indoor temperature-mortality associations on the day of death were modelled on
the basis of the exposure dimension of the DLNM method only (one-basis function; quadratic
B-spline, 2 degrees of freedom) because indoor temperature levels were simulated using
the temperature levels up to 14 days before death. Preliminary analyses, which also
considered potential lagged effects of indoor temperature, revealed that these effects
were negligible. The model parameters (degrees of freedom and alternative spline functions)
were selected during preliminary analyses and validated using AIC. To evaluate the
robustness of the method of defining the MMT of the temperature-mortality association
for indoor Tmax, a sensitivity analysis was performed by widening the range from P25-P90
to P2-P90 of the warm season temperature distribution. All outdoor and indoor temperature-mortality
models included an indicator variable for national public holidays.
To study effect modification by individual characteristics, stratified analyses for
outdoor Tmax and all-cause mortality were conducted by age (<75 years, 75–84 years,
>85 years), sex, marital status (married, single, divorced, and widowed adults), nationality
(Swiss, European, and other nationalities), education (compulsory or less, upper secondary,
and tertiary), neighbuorhood-based socioeconomic position (low, medium, and high),
building period (before 1970, 1970–1990, and after 1990), and floor of residence (ground
floor to 2nd floor, which includes single-family homes, 3rd to 5th floor, and >5th
floor).
To assess whether public health interventions and public awareness contributed to
a reduction in heat-related mortality in Switzerland, we compared the ORs of all-cause
mortality during two time periods (2003–2008 versus 2009–2016) in three regions (all
of Switzerland, cantons that had implemented HHAPs, and cantons without HHAPs). The
cantonal HHAPs had all been implemented between 2004 and 2008. Thus, the more recent
period marks the time when HHAPs were in place [10]. The models were run separately
for each period and region to estimate the effect of extreme temperatures (the P98
of period- and region-specific warm season Tmax values) using the period and region-specific
MMT as a reference.
All analyses were conducted with R software (version 4.1.3). The DLNM models were
fitted in R using the dlnm package [33] following the exposure history approach described
by Gasparrini [37].
Protocol and registration
No protocol has been registered for this observational study.
Ethical consent
The SNC was approved by the ethics committees of the canton of Bern (No KeK 153/2014,
PB_2020-00050).
Results
Table 1 describes the characteristics of the study population and daily temperature
levels for Switzerland and two regions of cantons (defined according to whether they
had implemented HHAPs before 2009). Most of the participants (68%) were aged 75 or
older. The age and sex distributions were similar in the two regions. However, the
percentage of people with low socioeconomic position was higher in the region with
HHAPs (32%) than in the region without HHAPs (18%). Outdoor Tmax was highly correlated
with Tmin (0.80, Pearson correlation coefficient) and indoor Tmax (0.88). In both
periods (2003–2008 and 2009–2016), the median exposure to outdoor Tmax was higher
in cantons that had implemented HHAPs compared to those without such prevention measures.
Annual means of outdoor Tmax on the day of death increased in both regions during
the warm summers of 2003 and 2015 when major heatwaves occurred [9, 14] (figure S2
in the appendix). Mortality from CVD (34.3%) and neoplasms (29%) accounted for the
largest fraction of deaths (table S2 in the appendix). Alzheimer’s disease and dementia,
external-cause mortality, mental disorders, respiratory diseases, digestive diseases,
diabetes, and renal diseases accounted for 6.8%, 6.3%, 5.7%, 5.4%, 4.1%, 2.1%, and
1.3% of mortality, respectively.
Figure 1 shows the cause-specific cumulative temperature-mortality associations for
outdoor Tmax with the corresponding MMT. The risk of mortality generally increased
with temperature, except for the risk of mortality from digestive diseases, which
was not significantly associated with warm season temperature. For most diseases,
temperature had a significant effect even at moderately warm temperatures. However,
for various cardiovascular diseases and neoplasms, the risk increased most markedly
above 30 °C. Using the MMT as a reference, extreme heat (33 °C) was associated with
a significant increase in all-cause mortality across the lag period of 0–7 days (OR:
1.21, 95% CI: 1.17–1.25), and the mortality of multiple disease groups, including
mental disorders (OR: 1.64, 95% CI: 1.44–1.86), respiratory diseases (OR: 1.62, 95%
CI: 1.42–1.86), nervous disorders (OR: 1.43, 95% CI: 1.42–1.86), endocrine diseases
(OR: 1.33, 95% CI: 1.10–1.62), CVD (OR: 1.21, 95% CI: 1.14–1.29), external causes
(OR: 1.21, 95% CI: 1.07–1.38), and digestive diseases (OR: 1.11, 95% CI: 1.01–1.22)
(table S2). Strong associations were found for the specific diseases Alzheimer’s and
dementia (OR: 1.67, 95% CI: 1.48–1.88), COPD (OR: 1.37, 95% CI: 1.12–1.67), diabetes
(OR: 1.34, 95% CI: 1.06–1.70), and myocardial infarction (OR: 1.26, 95% CI: 1.10–1.44).
Exposure-response curves for outdoor Tmin were similar (figure S3, table S2 in the
appendix).
Table 1Descriptive statistics of individual mortality and temperature data in Switzerland
by time period (2003–2008; 2009–2016) and region (with and without heat-health action
plans, HHAPs between 2003 and 2016).
|
Time
period and region |
| 2003–2016 |
2003–2008 |
2009–2016 |
|
|
| Switzerland |
With HHAPs |
Without HHAPs |
With HHAPs |
Without HHAPs |
| Population, count (%) |
320,306 |
100.0% |
37,908 |
100.0% |
99,637 |
100.0% |
50,929 |
100.0% |
131,832 |
100.0% |
| Number of cantons |
26 |
|
6a |
|
20 |
|
6a |
|
20 |
|
| Age (years) |
<75 |
101,763 |
31.8% |
12,784 |
33.7% |
31,806 |
31.9% |
16,532 |
32.5% |
40,641 |
30.8% |
| 75–84 |
94,049 |
29.4% |
11,292 |
29.8% |
30,567 |
30.7% |
14,075 |
27.6% |
38,115 |
28.9% |
| 85+ |
124,494 |
38.9% |
13,832 |
36.5% |
37,264 |
37.4% |
20,322 |
39.9% |
53,076 |
40.3% |
| Sex |
Female |
161,850 |
50.5% |
19,142 |
50.5% |
50,930 |
51.1% |
25,465 |
50.0% |
66,313 |
50.3% |
| Male |
158,456 |
49.5% |
18,766 |
49.5% |
48,707 |
48.9% |
25,464 |
50.0% |
65,519 |
49.7% |
| Socioeconomic positionb |
Low 1st
quintile |
70,797 |
22.1% |
12,143 |
32.0% |
18,644 |
18.7% |
15,995 |
31.4% |
24,015 |
18.2% |
| Medium 2nd–4th
quintile |
179,534 |
56.1% |
19,460 |
51.3% |
57,473 |
57.7% |
26,383 |
51.8% |
76,218 |
57.8% |
| High 5th
quintile |
49,214 |
15.4% |
3,438 |
9.1% |
16,785 |
16.8% |
4,968 |
9.8% |
24,023 |
18.2% |
| Unknown |
20,761 |
6.5% |
2,867 |
7.6% |
6,735 |
6.8% |
3,583 |
7.0% |
7,576 |
5.7% |
| Education |
School-age or younger |
1,733 |
0.5% |
187 |
0.5% |
451 |
0.5% |
298 |
0.6% |
797 |
0.6% |
| Compulsory or less |
119,845 |
37.4% |
17,519 |
46.2% |
39,672 |
39.8% |
19,593 |
38.5% |
43,061 |
32.7% |
| Upper secondary |
144,958 |
45.3% |
14,504 |
38.3% |
45,978 |
46.1% |
20,544 |
40.3% |
63,932 |
48.5% |
| Tertiary level |
43,078 |
13.4% |
4,932 |
13.0% |
11,746 |
11.8% |
7,788 |
15.3% |
18,612 |
14.1% |
| Unknown |
10,692 |
3.3% |
766 |
2.0% |
1,790 |
1.8% |
2,706 |
5.3% |
5,430 |
4.1% |
| Urbanisation |
Urban |
211,763 |
66.1% |
25,223 |
66.5% |
65,546 |
65.8% |
33,784 |
66.3% |
87,210 |
66.2% |
| Peri-urban |
59,510 |
18.6% |
7,072 |
18.7% |
18,143 |
18.2% |
9,754 |
19.2% |
24,541 |
18.6% |
| Rural |
49,033 |
15.3% |
5,613 |
14.8% |
15,948 |
16.0% |
7,391 |
14.5% |
20,081 |
15.2% |
| Outdoor Tmax, median (P5,
P98) |
22.4 |
13.8, 32.5 |
23.2 |
14.8, 32.6 |
22.2 |
13.5, 32.6 |
23.1 |
14.6, 32.4 |
22.0 |
13.6, 32.5 |
| Outdoor Tmin, median (P5,
P98) |
12.3 |
6.0, 18.4 |
12.8 |
6.3, 19.1 |
12.3 |
5.6, 18.0 |
12.7 |
6.5, 19.1 |
12.1 |
6.1, 18.2 |
| Indoor Tmax, median (P5,
P98) |
23.0 |
20.0, 28.0 |
23.0 |
20.1, 28.0 |
22.7 |
20.0, 28.0 |
23.4 |
20.3, 29.0 |
23.0 |
20.1, 28.0 |
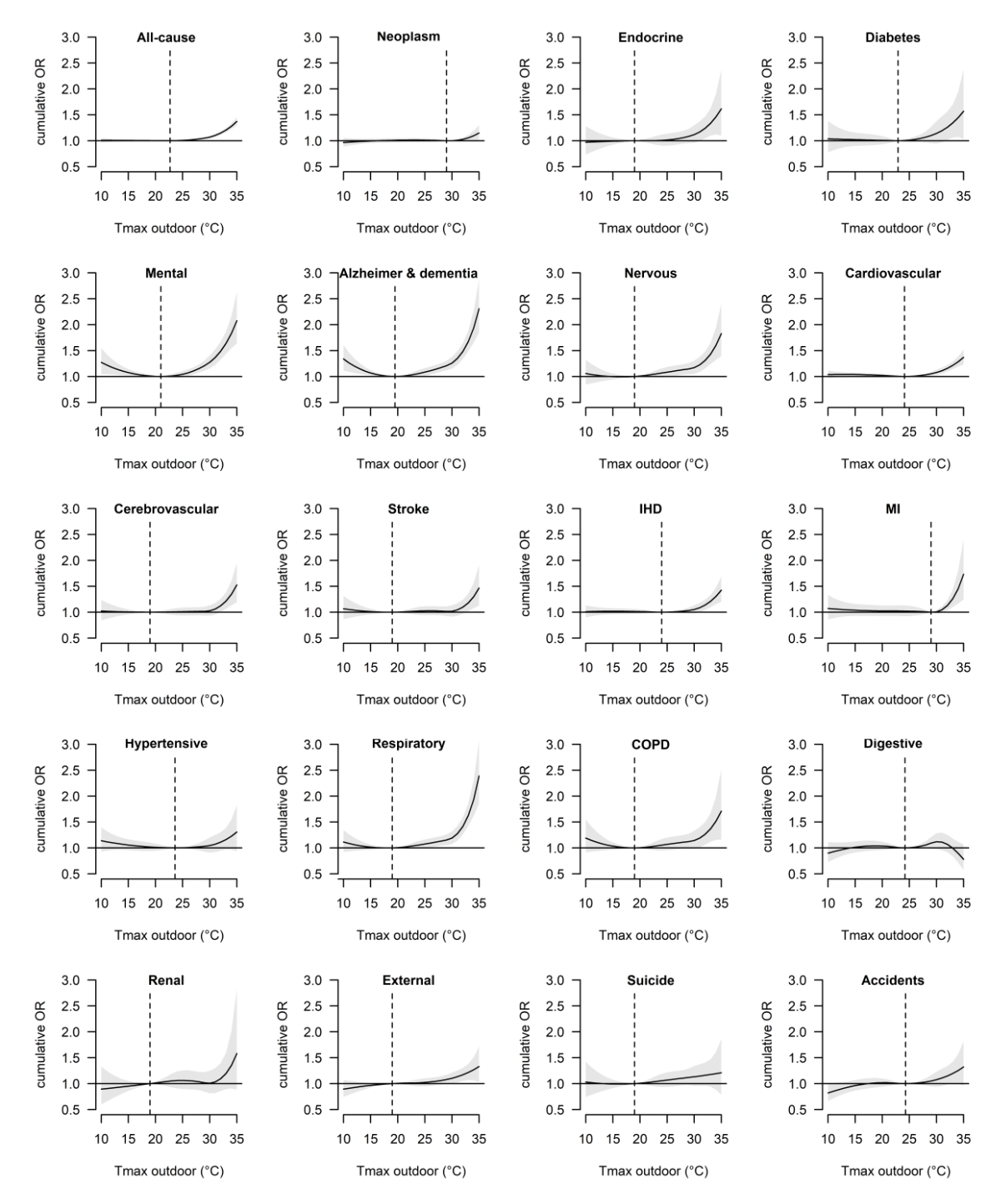
Figure 1
Overall cumulative exposure-response associations (with 95% confidence intervals)
between outdoor daily maximum temperature (Tmax) and cause-specific mortality along
7 days of lag in Switzerland during the warm seasons (May to September) of 2003–2016.
The dashed vertical lines indicate the cause-specific minimum mortality temperature.
OR: odds ratio; IHD: ischaemic heart disease; MI: myocardial infarction; COPD: chronic
obstructive pulmonary disease.
Figure 2 compares the risk of all-cause mortality and cause-specific mortality as
a function of indoor and outdoor Tmax. The curves for indoor Tmax follow a similar
pattern as those for outdoor Tmax. As indicated by the dotted vertical lines in figure
2, the ORs of mortality at the P98 of the warm season temperature distribution, which
was 28 °C for indoor Tmax and 33 °C for outdoor Tmax, were comparable. Slightly higher
ORs at the P98 were found for outdoor Tmax than for indoor Tmax for Alzheimer’s disease
and respiratory diseases (table S2). A statistically significant effect of indoor
Tmax on all-cause mortality with the 95% confidence interval of the OR >1.0 was observed
when indoor Tmax reached 23–24 °C. The indoor temperature-mortality associations for
all investigated death causes are provided in the appendix (figure S4 in the appendix).
Sensitivity analysis revealed that the exposure-response associations for indoor Tmax
did not differ with alternative search strategies to define the MMT (figure S5 in
the appendix).
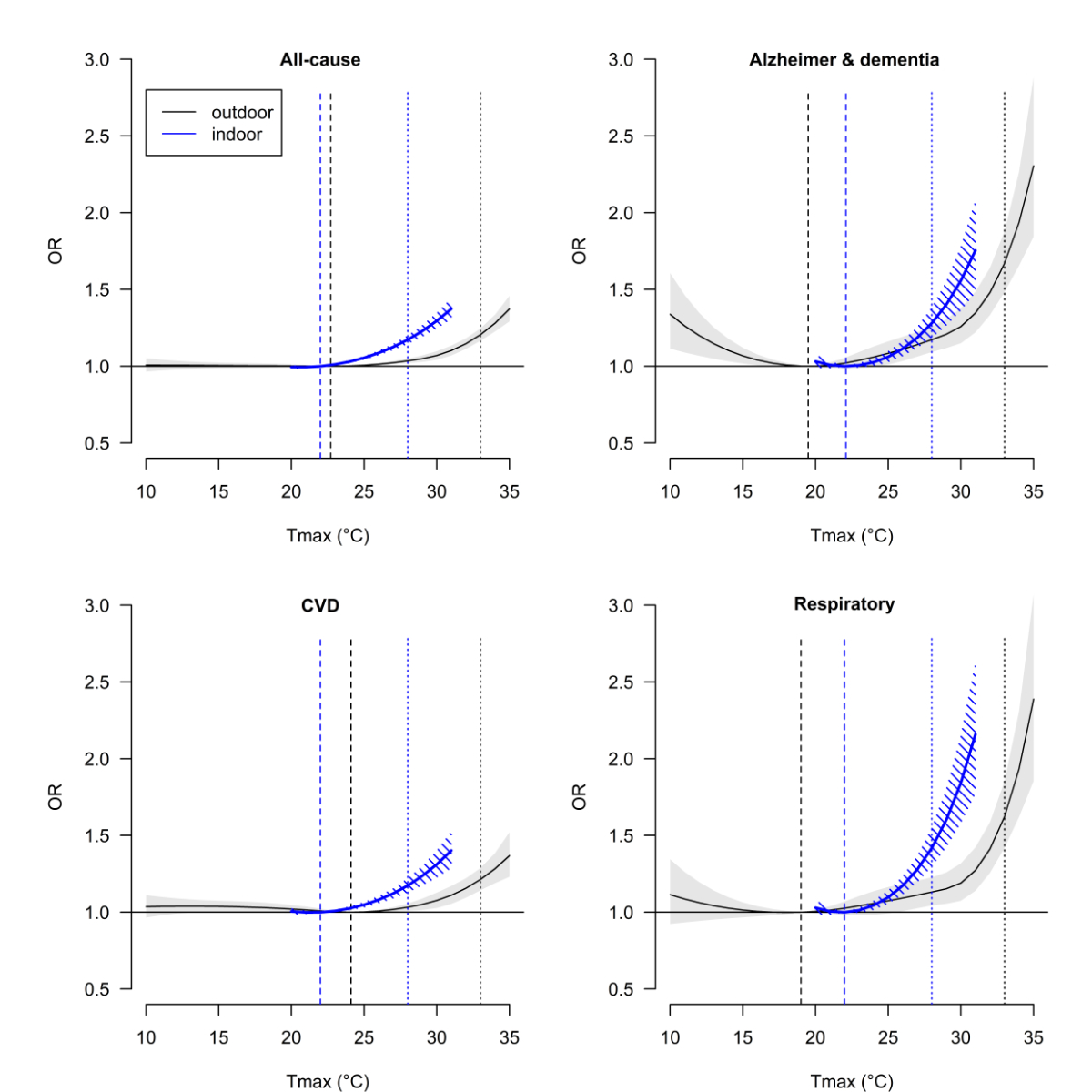
Figure 2
Comparison of outdoor (black curve) and indoor (blue curve) temperature-mortality
associations (with 95% confidence intervals) for specific mortality causes in Switzerland
during the warm seasons of 2003–2016. Odds ratios (ORs) represent the change in mortality
risk associated with outdoor and indoor daily maximum temperature (Tmax) versus the
cause-specific minimum mortality temperature (dashed vertical lines). Dotted vertical
lines mark the 98th percentile of the warm season Tmax.
In addition, we observed significant effect modification by neighbuorhood-based socioeconomic
position and education. The heat-related effects were significantly stronger in people
with low education levels than in those with high education levels (Chi-squared test
p = 0.035). The effects of extreme heat on all-cause mortality were stronger in older
women and men aged under 75 years with low socioeconomic position than in participants
of the same age and sex with an intermediate or high socioeconomic position (figure
S6 in the appendix). However, in men aged ≥75 years, the heat-related effects tended
to be stronger among those with a high socioeconomic position. Overall, building characteristics
and floor were not effect modifiers; however, in the population with a low socioeconomic
position, risks increased in persons who lived in a building constructed from 1970
to 1990, lived on the 3rd to 5th floor, or experienced high indoor temperatures (≥24 °C) (figure S6 in the appendix).
By contrast, for the population with a high socioeconomic position, living in an apartment
building on the 3rd floor or higher was not a risk factor for heat-related mortality.
In Switzerland, the risk for heat-related all-cause mortality was significantly lower
in 2009–2016 than in 2003–2008 (figure 3, figure S7 in the appendix). Although the
decrease in mortality risk for outdoor temperatures reaching the P98 of period- and
region-specific warm season Tmax values was statistically significant in the region
of cantons that have implemented HHAPs, a smaller and nonsignificant difference was
found across cantons where no such measures had been taken (table S3 in the appendix).
When comparing the period-specific ORs of vulnerable population groups in the region
that had introduced HHAPs, we observed a stronger reduction in risk in those aged
75 and older than in those with low socioeconomic position (table S3). For the latter,
no statistically significant difference in effect was found between the two time periods.
However, when 2003 was excluded from the analysis, the observed decrease in risk associated
with extreme heat between the two periods became smaller (and nonsignificant) in the
region that had introduced HHAPs (figure S8 in the appendix). In the region without
HHAPs, no risk reduction was observed.
Table 2Cumulative odds ratios (ORs) with 95%
confidence intervals of heat-related all-cause mortality at outdoor daily
maximum temperatures of 33 °C in relation to the minimum mortality temperature of
22.7 °C, stratified by individual characteristics. Significant differences (p <0.05,
assessed by Chi-squared tests) across categories in the total population (Ptotal)
and between males and females (Pmale/Pfemale) are marked in bold.
| |
Total |
Male |
Female |
|
| Population |
Deaths |
OR |
95%
CI |
Ptotal |
Deaths |
OR |
95%
CI |
Deaths |
OR |
95%
CI |
Pmale/Pfemale |
| Total |
320,306 |
1.21 |
1.17–1.25 |
|
158,456 |
1.17 |
1.11–1.23 |
161,850 |
1.24 |
1.18–1.30 |
0.074 |
| Age (3 groups) |
Under 75 |
101,763 |
1.11 |
1.05–1.18 |
0.006 |
64,001 |
1.14 |
1.06–1.23 |
37,762 |
1.07 |
0.97–1.18 |
0.291 |
| 75–84 |
94,049 |
1.24 |
1.16–1.32 |
|
49,711 |
1.21 |
1.11–1.32 |
44,338 |
1.27 |
1.16–1.38 |
0.469 |
| 85+ |
124,494 |
1.26 |
1.19–1.33 |
|
44,744 |
1.16 |
1.06–1.27 |
79,750 |
1.31 |
1.23–1.40 |
0.033 |
| Age (2 groups) |
Under 75 |
101,763 |
1.11 |
1.05–1.18 |
0.002* |
64,001 |
1.14 |
1.06–1.23 |
37,762 |
1.07 |
0.97–1.18 |
0.291 |
| 75+ |
218,543 |
1.25 |
1.20–1.30 |
|
94,455 |
1.19 |
1.11–1.26 |
124,088 |
1.30 |
1.23–1.37 |
0.033 |
| Marital status |
Married |
129,561 |
1.12 |
1.06–1.18 |
0.002 |
91,853 |
1.13 |
1.06–1.20 |
37,708 |
1.10 |
1.00–1.22 |
0.728 |
| Single |
37,211 |
1.38 |
1.25–1.52 |
|
18,309 |
1.39 |
1.21–1.60 |
18,902 |
1.37 |
1.19–1.57 |
0.878 |
| Widowed |
116,291 |
1.23 |
1.16–1.30 |
|
29,514 |
1.14 |
1.02–1.27 |
86,777 |
1.26 |
1.18–1.34 |
0.134 |
| Divorced |
30,488 |
1.22 |
1.10–1.35 |
|
15,280 |
1.16 |
0.99–1.35 |
15,208 |
1.28 |
1.10–1.48 |
0.371 |
| Education |
Compulsory or less |
119,845 |
1.26 |
1.19–1.33 |
0.084 |
39,036 |
1.21 |
1.09–1.34 |
80,809 |
1.28 |
1.19–1.36 |
0.376 |
| Upper secondary |
144,958 |
1.19 |
1.13–1.25 |
|
79,519 |
1.17 |
1.09–1.25 |
65,439 |
1.21 |
1.12–1.30 |
0.540 |
| Tertiary |
43,078 |
1.12 |
1.02–1.23 |
|
33,485 |
1.11 |
1.00–1.23 |
9,593 |
1.16 |
0.96–1.40 |
0.671 |
| Socioeconomic positionb |
Low |
70,797 |
1.25 |
1.16–1.35 |
0.465* |
36,546 |
1.20 |
1.08–1.33 |
34,251 |
1.31 |
1.17–1.46 |
0.266 |
| Medium |
179,534 |
1.19 |
1.13–1.24 |
|
88,629 |
1.13 |
1.06–1.20 |
90,905 |
1.24 |
1.17–1.32 |
0.033 |
| High |
49,214 |
1.18 |
1.08–1.28 |
|
24,555 |
1.28 |
1.14–1.44 |
24,659 |
1.08 |
0.96–1.21 |
0.036 |
| Nationality |
Swiss |
246,820 |
1.20 |
1.16–1.25 |
0.493 |
118,864 |
1.15 |
1.09–1.22 |
127,956 |
1.25 |
1.19–1.32 |
0.032 |
| European |
18,757 |
1.30 |
1.14–1.49 |
|
11,768 |
1.28 |
1.08–1.52 |
6,989 |
1.34 |
1.08–1.67 |
0.716 |
| Other nationality |
54,729 |
1.19 |
1.10–1.28 |
|
27,824 |
1.19 |
1.07–1.33 |
26,905 |
1.19 |
1.07–1.32 |
0.951 |
| Building construction
period |
Before 1970 |
217,172 |
1.18 |
1.14–1.23 |
0.253 |
107,614 |
1.16 |
1.09–1.23 |
109,558 |
1.21 |
1.14–1.28 |
0.329 |
| 1970–1990 |
98,781 |
1.26 |
1.18–1.34 |
|
48,691 |
1.19 |
1.09–1.30 |
50,090 |
1.32 |
1.21–1.44 |
0.102 |
| After 1990 |
1,320 |
1.26 |
0.76–2.12 |
|
615 |
1.18 |
0.56–2.51 |
705 |
1.17 |
0.57–2.43 |
0.990 |
| Floor |
Floor 0–2 |
151,282 |
1.16 |
1.10–1.22 |
0.872 |
73,198 |
1.17 |
1.08–1.26 |
78,084 |
1.15 |
1.07–1.23 |
0.727 |
| Floor 3–5 |
45,420 |
1.19 |
1.07–1.32 |
|
20,181 |
1.19 |
1.02–1.39 |
25,239 |
1.19 |
1.03–1.37 |
0.979 |
| Floor 6+ |
23,047 |
1.13 |
0.91–1.40 |
|
9,582 |
1.01 |
0.75–1.38 |
13,465 |
1.26 |
0.93–1.72 |
0.322 |
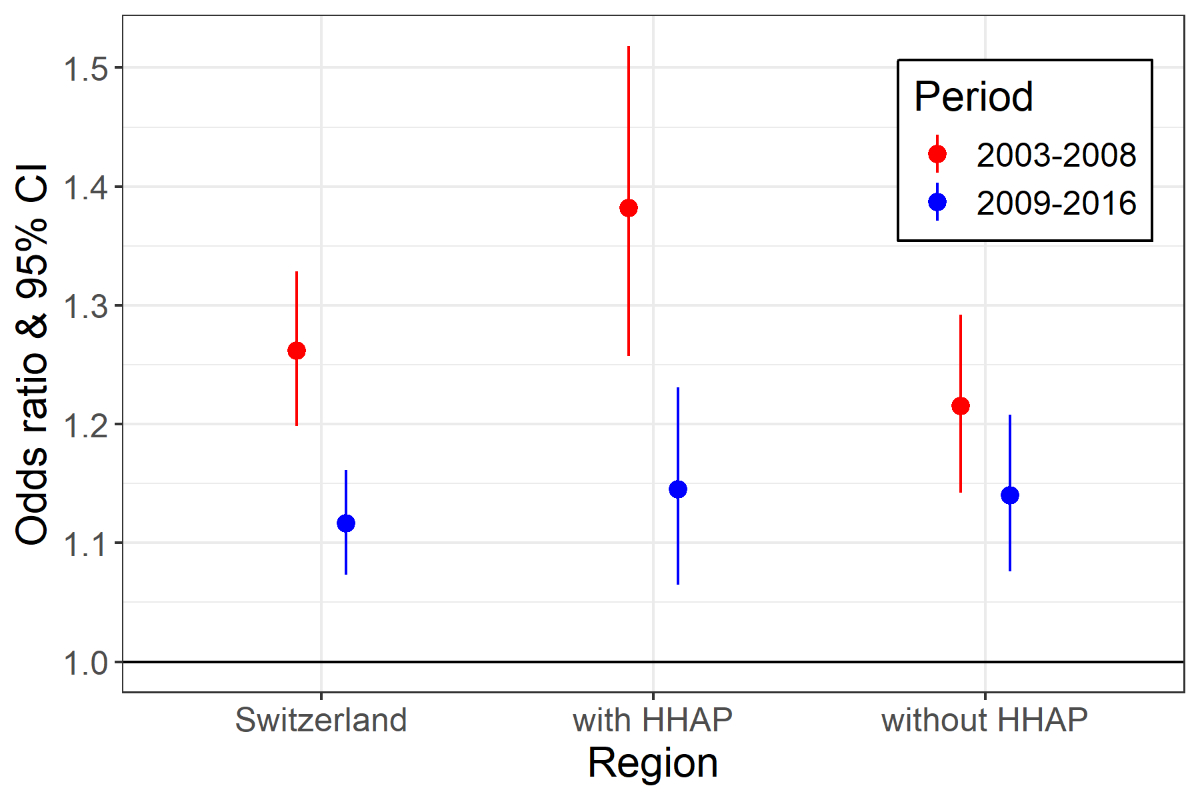
Figure 3
Cumulative odds ratios (ORs) with 95% confidence intervals (CIs) of heat-related all-cause
mortality at region- and period-specific (2003–2008 versus 2009–2016) extreme outdoor
daily maximum temperatures (Tmax) in Switzerland. Extreme temperatures are defined
as the 98th percentile of the region- and period-specific Tmax. The region with heat-health action
plans (HHAPs) covers cantons that implemented HHAPs between 2003 and 2008.
Discussion
Using individual mortality data from all of Switzerland, we assessed various crucial
aspects of the prevention of heat-related mortality. In addition to all-cause mortality,
extreme heat, defined as a Tmax of 33 °C, was strongly associated with specific diseases,
including Alzheimer’s disease and dementia, COPD, diabetes, and myocardial infarction.
Our analysis also suggests that maximum indoor temperatures above 24 °C have a significant
effect on mortality. Older adults (≥75 years old) were at an increased risk of heat-related
mortality, along with individuals who were not married and those with a low education
level. In addition, a low socioeconomic position increased the risk for heat-related
mortality in older women and men under 75 years old.
Our study provides further evidence that HHAPs help reduce heat-related mortality
risk in regions with heat stress. In the cantons that implemented HHAPs between 2004
and 2008, the ORs of mortality associated with extreme heat were significantly lower
in the more recent period (2009–2016). In the region without HHAPs, the risk reduction
was considerably smaller and not statistically significant. In this region, public
health measures to protect the population from the dangers of hot weather are lacking
at the cantonal or city level or have been only partially introduced. Assessments
of HHAPs at the city or country level in Europe and North America have also found
that HHAPs lower the health burden of heatwaves, although a causal effect remains
to be clearly established [38, 39]. Overall, the region without HHAPs was less affected
by heat exposure than the Lake Geneva region and the canton of Ticino in the south
of Switzerland. Therefore, Switzerland provides an example that in regions particularly
affected by heat exposure and its associated mortality risks, joint action from different
stakeholders helps protect the population from heat. Nevertheless, this ecological
comparison of regions is limited in terms of causal inference. Our sensitivity analysis
excluded the year 2003 and showed a less pronounced risk reduction between the two
periods in the region where cantonal HHAPs were introduced. However, the analysis
revealed no risk reduction in the region without HHAPs when the year 2003 was excluded.
Therefore, it is likely that HHAPs have contributed to reducing heat-related mortality
in the region with such measures in the more recent period.
In addition, we found that the less socioeconomically privileged population is at
a high risk of heat-related mortality. Our effect modification analysis revealed that
a low education level and a low neighbuorhood-based socioeconomic position are risk
factors for heat-related mortality. The latter risk factor was most evident among
older women and young men. Older women are generally considered more adversely affected
by hot weather than men in the same age group. The reasons for the higher heat-related
mortality risk among women are multifaceted; potential explanations include women’s
higher life expectancy, their lower thermoregulation capacity, the increased risk
of cardiovascular events in postmenopausal women, and other gender-related sociocultural
factors [40]. Our results indicate that a low socioeconomic position can further increase
the vulnerability of older women. Reducing the physical and social isolation of older
women during heat events may minimise their susceptibility to high temperatures. In
men under 75 years old, a lower socioeconomic position might increase the heat-related
mortality risk for several reasons, including increased heat exposure at work and
at home, more comorbidities, and personal behavioural factors, such as underestimating
the health risks of extreme heat and not adhering to recommendations on how to behave
during heat episodes [34]. Prevention strategies in recent years have targeted older
adults. However, the prevention measures did not seem to effectively reach people
with low socioeconomic positions. We found no significant difference in effect among
the population with low socioeconomic position when comparing the two periods in the
region with cantonal HHAPs. Therefore, our results suggest that future prevention
efforts are needed to specifically target the less socioeconomically privileged population,
including men under 75 years old. To reach this group, participatory approaches to
developing targeted information and alert systems are recommended [15].
Surprisingly, similar to a recent study on heat-related cardiovascular mortality in
the Zurich region, the odds of heat-related all-cause mortality were elevated among
older men with a high neighbourhood-based socioeconomic position [34]. Although we
cannot fully explain this finding, the potentially longer life expectancy of more
socioeconomically privileged men could play a role. In our study population, however,
the mean age among men aged 75 and older with low (85 years) and high socioeconomic
position (86 years) was similar.
Our results illustrate that several disease systems are sensitive to heat. These findings
show that it is crucial to intensify the care of patients with chronic respiratory
diseases such as COPD, Alzheimer’s disease and dementia, diabetes and certain cardiovascular
health problems during heat episodes. Different mechanisms may be at play. For cardiovascular
diseases, we found significant associations with stroke, ischaemic heart disease,
and myocardial infarction, similar to previous investigations on heat-related cardiovascular
mortality [27, 34, 41, 42]. Biological mechanisms to regulate the body temperature
cause a redistribution of the blood flow away from core organs, an increased heart
rate, and organ oxygen demand. This elevates cardiovascular strain and may explain
the elevated likelihood of acute coronary events during heat episodes [6]. The increased
mortality risk for hypertensive diseases during hot weather seems counterintuitive,
as exposure to heat leads to reduced blood pressure and peripheral vasodilatation.
It is plausible that the elevated heat-related mortality risk in patients with chronic
hypertension is attributable to an inadequate dose reduction of anti-hypertensive
medication [43].
We found a particularly strong association between high temperatures and mortality
from Alzheimer’s disease and dementia, especially at ambient temperatures above 30
°C. People with dementia, specifically Alzheimer’s disease, should thus be considered
a high-risk group during hot weather. Patients with Alzheimer’s disease and dementia
may have a higher risk of heat-related mortality because of their limited ability
to recognise and alleviate heat stress. In addition, some of their medications reduce
thermoregulation capacity (e.g. neuroleptics). The high heat-related mortality risk
associated with Alzheimer’s disease and dementia may also be related to the age of
the patients. In our study population, the average age of individuals with this main
death cause was 87 years. In addition, previous studies reported that a low income
is an additional risk factor for heat-related mortality in patients with Alzheimer’s
disease and dementia [44, 45].
A growing number of national and international studies have reported that heat can
affect external causes of death, such as accidents and suicides [27, 46, 47]. It remains
unclear whether these causes of death are causally related to heat or rather to good
weather conditions favouring risky activities. Strikingly, heat-mortality associations
tend to be more linear for external death causes compared with other outcomes, which
suggests that causal explanations other than heat may be involved.
Ensuring comfortable indoor temperatures is essential for preventing the exacerbation
of symptoms of at-risk groups, which often spend most of their time indoors. The associations
found between indoor temperature and mortality in our study reflected the association
with outdoor temperature. The high correlation between indoor Tmax and outdoor Tmax
and the rarity of air conditioning in residential buildings in Switzerland likely
explain this finding. Nonetheless, periods of prolonged heat and certain building
characteristics can considerably reduce thermal comfort in indoor environments [19,
20]. For Alzheimer’s disease and dementia as well as respiratory diseases, we found
a strong increase in mortality risk at high indoor temperatures. The exacerbation
of respiratory symptoms with increasing indoor temperatures has been reported in case
studies; one study reported increased respiratory emergency distress calls at indoor
temperatures above 26 °C in New York during summer 2013 [21]. Others described adverse
effects of high indoor temperatures on diabetes management, schizophrenia, and dementia
symptoms without reporting exposure-response functions [20]. Considering climate change,
the public health sector must work with other relevant stakeholders (e.g. architects
and city planners) to prevent indoor overheating. Our analysis of heat-related mortality
suggests that indoor temperatures above 24 °C are associated with a significantly
increased mortality risk. The temperature threshold for health-supporting indoor environments
may be higher for the young and healthy population. Further studies assessing the
indoor temperature threshold above which adverse effects become stronger and more
frequent are warranted.
This is the first case-crossover study assessing cause-specific heat-related mortality
risk and individual effect modifiers of extreme heat using individual death records
from all of Switzerland. The strength of this study is that we assigned the temperature
exposure at the address of each deceased person using daily temperature data with
a high spatial resolution. Therefore, our study likely provides less biased and more
precise effect estimates than most time-series studies using aggregated numbers of
daily deaths and exposure data from central monitoring stations or temperature models
with coarser spatial resolutions. Moreover, to our knowledge, this is the first study
to examine both outdoor and indoor temperature relationships with various causes of
mortality.
Several limitations must be acknowledged. Some exposure misclassification of outdoor
and indoor temperature exposure is expected, as we only assigned daily temperature
levels at the residential address and ignored exposure away from home. Given that
most of the study population was ≥75 years old and we considered 7 days of exposure
before death, it is likely that the study population spent most of their time at home.
Additionally, indoor temperature estimates have some uncertainty because of assumptions
about the input variables and the simplified thermal building model used. Further
analyses exploring the association between mortality and indoor temperature with more
detailed information on the residential building, occupancy/ventilation behaviour,
and time spent indoors are warranted. Another limitation is that we did not consider
other potential environmental risk factors, such as air pollution. During periods
of hot weather, ozone levels tend to be high and may increase the health risks of
heat. However, previous studies have found that the acute effect of ozone on mortality
is relatively small [48, 49]. Moreover, because temperature is a contributory cause
of the formation of ozone with no reverse causal effect, we consider it legitimate
to ignore the effects of temperature on mortality mediated by ozone.
Conclusions
This study provides important information for planning targeted and effective measures
to reduce the health risks of heat in Switzerland. It adds to the growing literature
showing a positive effect of coordinated measures such as HHAPs in reducing the health
burden of heat, especially in regions with heat stress. However, our results suggest
that HHAPs must better address the less socioeconomically privileged population, particularly
men under 75 years old. Additionally, future prevention efforts should focus specifically
on patients with Alzheimer’s and dementia, diabetes, and COPD. Due to rising temperatures, coordinated measures between stakeholders to reduce vulnerability to heat are becoming increasingly important, and such measures should be extended to other regions in Switzerland.
Data sharing statement
The SNC data cannot be shared by the authors. The data
are the responsibility of the Federal Statistical Office, and may be ordered
here: https://www.bfs.admin.ch/bfs/en/home/statistics/population/surveys/snc.html. The
R code is available upon request from the corresponding author.
Acknowledgments
We thank the Swiss Federal Statistical Office for providing mortality and census data
and the support that made the Swiss National Cohort and this study possible. The members
of the Swiss National Cohort Scientific Board are M. Zwahlen (University of Berne),
M. Egger (University of Berne), V. von Wyl (University of Zurich), O. Hämmig (University
of Zurich), M. Bochud (University of Lausanne), M. Röösli (University of Basel), and
M. Schwyn (Federal Statistical Office). We thank Christian Schindler for the statistical
support.
Dr. Martina S. Ragettli
Swiss Tropical and Public Health
Institute (Swiss TPH)
Kreuzstrasse
2
CH-4123
Allschwil
Martina.Ragettli[at]swisstph.ch
References
1. Federal Office for the Environment FOEN. Climate-related risks and opportunities.
A synthesis for Switzerland – short version. Extended summary of the publication "Klimabedingte
Risiken und Chancen. Eine schweizweite Synthese" www.bafu.admin.ch/uw-1706-d. Bern: Federal Office for the Environment FOEN, 2018.
2. NCCS. CH2018 - Climate Scenarios for Switzerland. Zurich: National Centre for Climate
Services (NCCS), 2018.
3. Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. Mortality
risk attributable to high and low ambient temperature: a multicountry observational
study. Lancet. 2015 Jul;386(9991):369–75. 10.1016/S0140-6736(14)62114-0
4. Song X, Wang S, Hu Y, Yue M, Zhang T, Liu Y, et al. Impact of ambient temperature
on morbidity and mortality: an overview of reviews. Sci Total Environ. 2017 May;586:241–54.
10.1016/j.scitotenv.2017.01.212
5. Ragettli MS, Vicedo-Cabrera AM, Flückiger B, Röösli M. Impact of the warm summer 2015
on emergency hospital admissions in Switzerland. Environ Health. 2019 Aug;18(1):66.
10.1186/s12940-019-0507-1
6. Ebi KL, Capon A, Berry P, Broderick C, de Dear R, Havenith G, et al. Hot weather and
heat extremes: health risks. Lancet. 2021 Aug;398(10301):698–708. 10.1016/S0140-6736(21)01208-3
7. Romanello M, Di Napoli C, Drummond P, Green C, Kennard H, Lampard P, et al. The 2022
report of the Lancet Countdown on health and climate change: health at the mercy of
fossil fuels. Lancet. 2022 Nov;400(10363):1619–54. 10.1016/s0140-6736(22)01540-9 doi: https://doi.org/10.1016/S0140-6736(22)01540-9
8. Ragettli MS, Röösli M. Die Bedeutung von Präventionsmassnahmen. Hitzebedingte Sterblichkeit
im Sommer 2019. Prim Hosp Care. 2021;21(03):90–5. 10.4414/phc-d.2021.10296
9. Vicedo-Cabrera AM, Ragettli MS, Schindler C, Röösli M. Excess mortality during the
warm summer of 2015 in Switzerland. Swiss Med Wkly. 2016;146:w14379-w. doi: 10.4414/smw.2016.14379.
10. Ragettli MS, Saucy A, Flückiger B, Vienneau D, de Hoogh K, Vicedo-Cabrera AM, et al. Explorative
Assessment of the Temperature-Mortality Association to Support Health-Based Heat-Warning
Thresholds: A National Case-Crossover Study in Switzerland. Int J Environ Res Public
Health. 2023 Mar;20(6):4958. 10.3390/ijerph20064958
11. Vicedo-Cabrera AM, de Schrijver E, Schumacher DL, Ragettli MS, Fischer EM, Seneviratne SI.
The footprint of human-induced climate change on heat-related deaths in the summer
of 2022 in Switzerland. Environ Res Lett. 2023 Jul;18(7):074037. 10.1088/1748-9326/ace0d0
12. Vicedo-Cabrera AM, Scovronick N, Sera F, Royé D, Schneider R, Tobias A, et al. The
burden of heat-related mortality attributable to recent human-induced climate change.
Nat Clim Chang. 2021 Jun;11(6):492–500. 10.1038/s41558-021-01058-x
13. Robine JM, Cheung SL, Le Roy S, Van Oyen H, Griffiths C, Michel JP, et al. Death toll
exceeded 70,000 in Europe during the summer of 2003. C R Biol. 2008 Feb;331(2):171–8.
10.1016/j.crvi.2007.12.001
14. Grize L, Huss A, Thommen O, Schindler C, Braun-Fahrländer C. Heat wave 2003 and mortality
in Switzerland. Swiss Med Wkly. 2005 Apr;135(13-14):200–5. 10.4414/smw.2005.11009
15. Martinez GS, Kendrovski V, Salazar MA, de’Donato F, Boeckmann M. Heat-health action
planning in the WHO European Region: status and policy implications. Environ Res.
2022 Nov;214(Pt 1):113709. 10.1016/j.envres.2022.113709
16. Kotharkar R, Ghosh A. Progress in extreme heat management and warning systems: A systematic
review of heat-health action plans (1995-2020). Sustain Cities Soc. 2022;76:103487.
10.1016/j.scs.2021.103487
17. Ragettli MS, Röösli M. [Heat-health action plans to prevent heat-related deaths-experiences
from Switzerland]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz.
2019 May;62(5):605–11. 10.1007/s00103-019-02928-8
18. Federal Office for the Environment FOEN. (2019). Hitze und Trockenheit im Sommer 2018.
Auswirkungen auf Mensch und Umwelt: Umwelt-Zustand Nr. 1909. Bern, Federal Office
for the Environment FOEN (FOEN) 1-91. [cited July 10, 2024]. Available from: https://www.nccs.admin.ch/dam/nccs/de/dokumente/website/sektoren/gesundheit/bafu-hitze-und-trockenheit-im-sommer-2018.pdf.download.pdf/Hitze%20und%20Trockenheit%20im%20Sommer%202018%20(BAFU,%202019)%20.pdf
19. Murage P, Kovats S, Sarran C, Taylor J, McInnes R, Hajat S. What individual and neighbourhood-level
factors increase the risk of heat-related mortality? A case-crossover study of over
185,000 deaths in London using high-resolution climate datasets. Environ Int. 2020 Jan;134:105292.
10.1016/j.envint.2019.105292
20. Tham S, Thompson R, Landeg O, Murray KA, Waite T. Indoor temperature and health: a
global systematic review. Public Health. 2020 Feb;179:9–17. 10.1016/j.puhe.2019.09.005
21. Uejio CK, Tamerius JD, Vredenburg J, Asaeda G, Isaacs DA, Braun J, et al. Summer indoor
heat exposure and respiratory and cardiovascular distress calls in New York City,
NY, U.S. Indoor Air. 2016 Aug;26(4):594–604. 10.1111/ina.12227
22. WHO Regional Office for Europe. Heat and health in the WHO European Region: updated
evidence for effective prevention. Copenhagen: Word Health Organization (WHO). Europe.
2021.
23. Hasan F, Marsia S, Patel K, Agrawal P, Razzak JA. Effective community-based interventions
for the prevention and management of heat-related illnesses: a scoping review. Int
J Environ Res Public Health. 2021 Aug;18(16):8362. 10.3390/ijerph18168362
24. Spoerri A, Zwahlen M, Egger M, Bopp M. The Swiss National Cohort: a unique database
for national and international researchers. Int J Public Health. 2010 Aug;55(4):239–42.
10.1007/s00038-010-0160-5
25. Bopp M, Spoerri A, Zwahlen M, Gutzwiller F, Paccaud F, Braun-Fahrländer C, et al. Cohort
Profile: the Swiss National Cohort—a longitudinal study of 6.8 million people. Int
J Epidemiol. 2009 Apr;38(2):379–84. 10.1093/ije/dyn042
26. Panczak R, Galobardes B, Voorpostel M, Spoerri A, Zwahlen M, Egger M; Swiss National
Cohort and Swiss Household Panel. A Swiss neighbourhood index of socioeconomic position:
development and association with mortality. J Epidemiol Community Health. 2012 Dec;66(12):1129–36.
10.1136/jech-2011-200699
27. Ma Y, Zhou L, Chen K. Burden of cause-specific mortality attributable to heat and
cold: A multicity time-series study in Jiangsu Province, China. Environ Int. 2020 Nov;144:105994.
10.1016/j.envint.2020.105994
28. Flückiger B, Kloog I, Ragettli MS, Eeftens M, Röösli M, de Hoogh K. Modelling daily
air temperature at a fine spatial resolution dealing with challenging meteorological
phenomena and topography in Switzerland. Int J Climatol. 2022;42(12):1–16. 10.1002/joc.7597
29. Ragettli MS, Vicedo-Cabrera AM, Schindler C, Röösli M. Exploring the association between
heat and mortality in Switzerland between 1995 and 2013. Environ Res. 2017 Oct;158:703–9.
10.1016/j.envres.2017.07.021
30. Koschenz M, Domingo-Irigoyen S, Niffeler M, Ragettli MS, Flückiger B, Kafadar M, et
al. ResCool: Klimaanpassung von Neu-, Um-und bestehenden Wohnbauten – effiziente Kühlkonzepte.
2021 https://www.aramis.admin.ch/Default?DocumentID=68310&Load=true
31. Maclure M. The case-crossover design: a method for studying transient effects on the
risk of acute events. Am J Epidemiol. 1991 Jan;133(2):144–53. 10.1093/oxfordjournals.aje.a115853
32. Janes H, Sheppard L, Lumley T. Case-crossover analyses of air pollution exposure data:
referent selection strategies and their implications for bias. Epidemiology. 2005 Nov;16(6):717–26.
10.1097/01.ede.0000181315.18836.9d
33. Gasparrini A. Distributed lag linear and non-linear models in R: the package dlnm.
J Stat Softw. 2011 Jul;43(8):1–20. doi: https://doi.org/10.18637/jss.v043.i08
34. Saucy A, Ragettli MS, Vienneau D, de Hoogh K, Tangermann L, Schäffer B, et al. The
role of extreme temperature in cause-specific acute cardiovascular mortality in Switzerland:
A case-crossover study. Sci Total Environ. 2021 Oct;790(147958):147958. 10.1016/j.scitotenv.2021.147958
35. Gasparrini A, Guo Y, Hashizume M, Kinney PL, Petkova EP, Lavigne E, et al. Temporal
variation in heat–mortality associations: a multicountry study. Environ Health Perspect.
2015 Nov;123(11):1200–7. 10.1289/ehp.1409070
36. Tobías A, Hashizume M, Honda Y, Sera F, Ng CF, Kim Y, et al. Geographical variations
of the minimum mortality temperature at a global scale: A multicountry study. Environ
Epidemiol. 2021 Sep;5(5):e169. 10.1097/ee9.0000000000000169 doi: https://doi.org/10.1097/EE9.0000000000000169
37. Gasparrini A. Extensions of the dlnm package: The Comprehensive R Archive Network;
2021 [cited 20 June]. Available from: https://cran.r-project.org/web/packages/dlnm/vignettes/dlnmExtended.pdf
38. Martinez GS, Linares C, Ayuso A, Kendrovski V, Boeckmann M, Diaz J. Heat-health action
plans in Europe: challenges ahead and how to tackle them. Environ Res. 2019 Sep;176:108548.
10.1016/j.envres.2019.108548
39. Benmarhnia T, Bailey Z, Kaiser D, Auger N, King N, Kaufman JS. A difference-in-differences
approach to assess the effect of a heat action plan on heat-related mortality, and
differences in effectiveness according to sex, age, and socioeconomic status (Montreal,
Quebec). Environ Health Perspect. 2016 Nov;124(11):1694–9. 10.1289/EHP203
40. van Steen Y, Ntarladima AM, Grobbee R, Karssenberg D, Vaartjes I. Sex differences
in mortality after heat waves: are elderly women at higher risk? Int Arch Occup Environ
Health. 2019 Jan;92(1):37–48. 10.1007/s00420-018-1360-1
41. Khraishah H, Alahmad B, Ostergard RL Jr, AlAshqar A, Albaghdadi M, Vellanki N, et
al. Climate change and cardiovascular disease: implications for global health. Nat
Rev Cardiol. 2022 Dec;19(12):798–812. 10.1038/s41569-022-00720-x
42. Alahmad B, Khraishah H, Royé D, Vicedo-Cabrera AM, Guo Y, Papatheodorou SI, et al. Associations
Between Extreme Temperatures and Cardiovascular Cause-Specific Mortality: Results
From 27 Countries. Circulation. 2023 Jan;147(1):35–46. 10.1161/circulationaha.122.061832 doi: https://doi.org/10.1161/CIRCULATIONAHA.122.061832
43. Schulte F, Röösli M, Ragettli MS. Heat-related cardiovascular morbidity and mortality
in Switzerland: a clinical perspective. Swiss Med Wkly. 2021 Sep;151(3738):w30013.
10.4414/SMW.2021.w30013
44. Xu Z, Tong S, Cheng J, Zhang Y, Wang N, Zhang Y, et al. Heatwaves, hospitalizations
for Alzheimer’s disease, and postdischarge deaths: A population-based cohort study.
Environ Res. 2019 Nov;178:108714. 10.1016/j.envres.2019.108714
45. Gong J, Part C, Hajat S. Current and future burdens of heat-related dementia hospital
admissions in England. Environ Int. 2022 Jan;159:107027. 10.1016/j.envint.2021.107027
46. Bär S, Bundo M, de Schrijver E, Müller TJ, Vicedo-Cabrera AM. Suicides and ambient
temperature in Switzerland: A nationwide time-series analysis. Swiss Med Wkly. 2022 Mar;152(910 w30115):w30115.
10.4414/smw.2022.w30115 doi: https://doi.org/10.4414/SMW.2022.w30115
47. Kim Y, Kim H, Gasparrini A, Armstrong B, Honda Y, Chung Y, et al. Suicide and ambient
temperature: a multi-country multi-city study. Environ Health Perspect. 2019 Nov;127(11):117007.
10.1289/EHP4898
48. Vicedo-Cabrera AM, Sera F, Liu C, Armstrong B, Milojevic A, Guo Y, et al. Short term
association between ozone and mortality: global two stage time series study in 406
locations in 20 countries. BMJ. 2020 Feb;368:m108. 10.1136/bmj.m108
49. Buckley JP, Samet JM, Richardson DB. Commentary: does air pollution confound studies
of temperature? Epidemiology. 2014 Mar;25(2):242–5. doi: https://doi.org/10.1097/EDE.0000000000000051
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3418.


