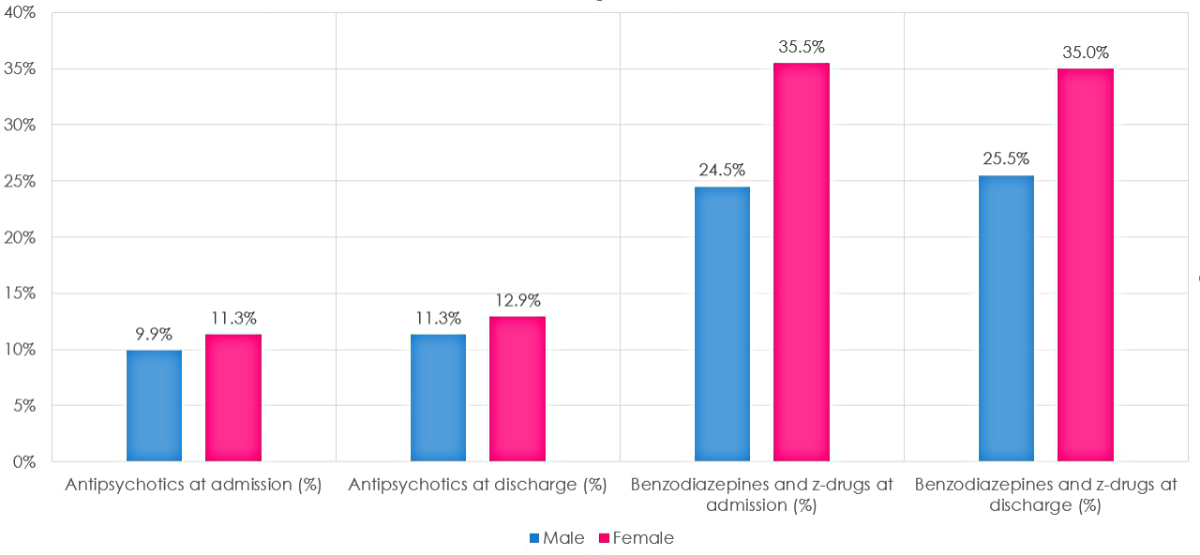
Figure 1 Percentage of antipsychotics, benzodiazepines and Z-drugs at admission and at discharge. Comparisons between males and females.
DOI: https://doi.org/https://doi.org/10.57187/s.3409
Antipsychotic medications, formerly known as neuroleptics, are a class of drugs employed in the treatment of many mental health illnesses. They are classified into first generation (typical), acting as dopamine receptor antagonists, and second generation (atypical) antipsychotics, which are able to block both dopamine and serotonin receptors [1]. In current medical practice, the modern second-generation antipsychotics are more frequently prescribed due to their higher efficacy and reduced incidence of side effects, particularly extrapyramidal symptoms [1] .
The US Food and Drug Administration (FDA) has approved the use of atypical antipsychotics for the treatment of schizophrenia, bipolar affective disorder, major depressive disorder (adjunctive therapy), agitation associated with schizophrenia or bipolar mania, autism, Tourette’s syndrome and psychosis [2, 3]. Almost the same indications have been approved by the European Medicines Agency (EMA) and Swissmedic [4].
In recent years, under the promotion of clinical trials by drug makers, off-label prescribing of such drugs has increased in popularity. In fact, owing to their sedative-hypnotic properties, atypical antipsychotics are often the agents of choice for mood disorders, insomnia and agitation, obsessive-compulsive disorders, post-traumatic stress disorders and anxiety disorders, among others [5–7]. Therefore, in the hospital setting, antipsychotics have found wide application for the management of behavioural, neuropsychiatric and sleeping disturbances [8], mainly in dementia [9, 10] and delirium [11, 12]. In 2005, however, the FDA released an alert to notify clinicians about the increased mortality risk in elderly patients treated with atypical antipsychotics for dementia-related psychosis (DRP), extending the warning in 2008 to typical antipsychotics [13]. According to the latest literature data on the efficacy, safety and acceptability of atypical antipsychotics, these drugs have no (or insignificant) benefits in terms of DRP improvement compared to placebo; instead, they are associated with an increased risk of mortality, cerebrovascular events and discontinuations due to adverse events [14]. A recent systematic review also demonstrated the presence of a correlation between the off-label use of these medications and a greater risk of metabolic complications, namely weight gain [15]. In addition, atypical antipsychotics may cause sedation/somnolence, extrapyramidal symptoms, memory and cognitive impairment, falls, injuries and fractures as common side effects [16].
The recommendation from the most recent NICE guidelines is to treat patients with dementia with antipsychotics only if they are at risk of harming themselves or others, or if they are severely distressed [17]. The drug with the best evidence supporting its use in dementia is risperidone, a medication licensed for short-term (up to six weeks) treatments. However, the focus should remain on non-pharmacological therapies, which are to be preferred and tried before prescribing antipsychotics [18].
Taking into account the potential complications of the treatment, as well as the difficulties related to deprescription in some patients [15, 16, 19], a careful risk-benefit assessment should be performed when prescribing atypical antipsychotics as a new or first-line treatment [17, 20].
According to an international survey, antipsychotic use progressively increased from 2005 to 2014 [21]. A New Zealand analysis also demonstrated a rise in antipsychotic use in the elderly between the years 2005 and 2019, while a parallel decrease in the use of hypnotics, sedatives and anxiolytics was registered [22]. In Irish nursing homes, in 2019, the prevalence of antipsychotic medications was also high (48%), especially among patients with dementia (67%) [23]. More recently, a retrospective Australian study recognised that 73% of hospitalised patients with dementia or delirium were prescribed a new antipsychotic during their hospital stay, 48% received non-pharmacological interventions as first-line therapy and 48% were discharged with antipsychotics [24].
In Switzerland, patients’ exposure to sedative prescriptions has been high as well, as documented in a survey conducted by Helsana [25], one of the main Swiss health insurance agencies: 13% of the people insured and 30% of those aged over 65 years were exposed to benzodiazepines, Z-drugs and antipsychotics in 2017. The same report conducted in 2020 report [26] stated that 13% of patients with home care were users of multiple antipsychotics. Furthermore, almost a quarter (24%) of all long-term home-care patients received a prescription of benzodiazepines (or analogues) more than three times in 2019Q2, hinting at the magnitude of long-term use.
As part of a Choosing Wisely campaign called smarter medicine, promoted by the Swiss Society of General Internal Medicine (SSGIM), a recommendation intended to curb the use of benzodiazepines and other sedative-hypnotics in older people with insomnia, agitation or delirium was published in 2016 [27]. The recommendation, released during the SSGIM’s annual congress as part of a Top 5 list of practices to be avoided, and accompanied by a media campaign, was intended to increase clinicians’ awareness regarding the risks of sedative-hypnotic prescriptions.
We joined the campaign with our network of public teaching hospitals belonging to the Ente Ospedaliero Cantonale (EOC), in the Italian-speaking part of Switzerland, on the one hand by monitoring the prescribing of benzodiazepines/Z-drugs and antipsychotics, and on the other hand with a multimodal intervention designed to raise awareness and generate peer pressure to change prescribing habits [28–31]. The campaign was successful, resulting in a 2.5% absolute reduction in new benzodiazepine prescriptions at discharge in an 18-month period between 2016 and 2017. The concomitant monitoring of new antipsychotic prescriptions showed an upward trend that was not however statistically significant [29].
At the end of 2017, the intervention and continuous inter-hospital benchmarking of new benzodiazepine prescriptions was interrupted in favour of other projects targeting Choosing Wisely recommendations [32, 33]. Considering the fact that the other projects were themselves about medication and diagnostic test overuse, we were confident that the awareness of potentially negative effects of prescribing sedative drugs would remain and mark a cultural change.
In the domain of prescribing, however, clinicians’ inertia is very difficult to avoid and, considering the six years that have passed, the potential impact of pandemic-related stress [34], and the importance of the topic in terms of public health, we decided to reactivate the monitoring of benzodiazepine, Z-drug and antipsychotic prescriptions and to analyse whether the evolution in prescribing these medications showed interdependent changes.
The present study aimed (a) to retrospectively explore the time trend of antipsychotic, benzodiazepine and Z-drug prescriptions and deprescriptions in internal medicine wards (primary outcome), as well as (b) to follow up the long-term outcomes of the Choosing Wisely awareness campaign conducted to improve benzodiazepine and Z-drug prescription habits and of the COVID-19 pandemic (secondary outcome).
This is a longitudinal study conducted from the fourth quarter of 2014 (2014Q4) to the second quarter of 2023 (2023Q2). We made available again a previously used dashboard designed to collect data about new benzodiazepine/Z-drug and antipsychotic prescriptions in the internal medicine wards of a network of four public teaching hospitals (H1-H4) belonging to the Ente Ospedaliero Cantonale (EOC), in the Italian-speaking part of Switzerland. Although the internal medicine wards of the four hospitals are gathered in a single department, local service heads act independently both in terms of patients’ care and education of attending physicians. The original investigation was aimed at analysing the impact of continuous monitoring and benchmarking of new benzodiazepine/Z-drug and antipsychotic prescriptions (meaning medications not previously used by the patients, introduced during the hospital stay or prescribed at discharge), supported by education and peer-endorsement interventions [28–31]. Each Internal Medicine Department in the network of hospitals had access to a dedicated dashboard such that it was possible, using electronic medical records, to continuously detect the differences between the prescription of benzodiazepines/Z-drugs and antipsychotics at admission and at discharge for patients admitted to the department. Each case was extracted with an anonymous identification number.
We analysed all medications commercially available in Switzerland. Regarding benzodiazepines and Z-drugs, we considered alprazolam (Xanax®), bromazepam (Lexotanil®), clorazepate (Tranxilium®), clobazam (Urbanyl®), chlordiazepoxide (Librax®), diazepam (Paceum®, Psychopax®, Stesolid®, Valium®), flurazepam (Dalmadorm®), flunitrazepam (Rohypnol®), ketazolam (Solatran®), lorazepam (Lorasifar®, Sedazin®, Temesta®, Somnium®), lormetazepam (Loramet®, Noctamid®), oxazepam (Seresta®, Anxiolit®), prazepam (Demetrin®), temazepam (Normison®), triazolam (Halcion®), zolpidem (Zoldorm®, Stilnox®) and zoplicone (Imovane®); for antipsychotics we considered amisulpride (Solian®, Amisulprid®), aripripazole (Abilify®, Aripiprazol®), asenapine (Sycrest®), clotiapine (Entumine®), clozapine (Leponex®, Clopin®), lurasidone (Latuda®), olanzapine (Olanza®, Olanpax®, Olanzapin®), paliperidone (Invega®, Xeplion®), quetiapine (Quetiapin®, Seroquel®, Sequase®) and risperidone (Risperdal®, Zanirisp®).
Original data (medication prescriptions at hospital admission and discharge, sex, age and case mix) were collected as part of standard hospital quality monitoring and did not contain information allowing identification of the patients.
Without a predefined population size target, we considered all the hospitalisations in the department of internal medicine of the four hospitals, from 2014Q4 to 2023Q2, without any exclusion criteria. We only had access to data that had already been collected and anonymised, and each data collection refers to a single case, not to a specific patient. We did not have access to daily prescriptions; we only knew whether a specific medication was present or not in domiciliary therapy and/or in therapy at discharge. A treatment present at admission but not at discharge was considered a deprescription; while a treatment absent at admission but present at discharge was considered a new prescription.
To obtain the % of new prescriptions of a given medication (indicated with M), we used the formula:
% new prescriptions of M = [(no of cases with M at discharge) – (no of cases with M both at admission and at discharge)] / [total cases – (no of cases with M at admission)]
To obtain the % of deprescriptions of a given medication (indicated with M), we used the formula:
% deprescriptions of M = [(no of cases with M at admission) – (no of cases with M both at admission and at discharge)] / no of cases with M at admission
The study was compliant with the “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement” guidelines [35]. According to Swiss law, studies based solely on anonymous secondary data do not require approval from an ethics review board [36]. The protocol was not registered as it was a retrospective study.
We used pivot tables to group the results. Descriptive statistics are expressed as relative frequencies and percentages for categorical/dichotomous variables, while means and standard deviations (SD) were used for quantitative variables after checking the normal distribution with the Shapiro-Wilk test. Comparisons between males and females were performed using the t-test for independent samples. A univariate analysis was also conducted to assess differences in antipsychotic and benzodiazepine/Z-drug use at admission and at discharge between males and females. Statistically significant changes attributable to the Choosing Wisely campaign or the COVID-19 pandemic were analysed using the interrupted time-series approach (ITSA), figure S1 in the appendix. The model used was the ordinary least-squares model with Neway-West standard errors (STATA command: itsa for single group). A serial correlation analysis was performed according to the test proposed by Cumby and Huizinga [37] (STATA command: actest). After checking for possible serial correlation, each ITSA model was rerun with the correct lags according to the serial correlation test. A graphical analysis of the time trends was also performed to determine whether a change occurred in the time period of interest (Choosing Wisely campaign or COVID-19 pandemic). If a change was suspected, the presence of a concomitant change in ITSA was assessed. To assess the temporal trends independently of the COVID-19 pandemic and the influence of hospital H4 (whose internal medicine head was involved in drafting the smarter medicine – Choosing Wisely recommendation on antipsychotic prescription released by the SSGIM in 2023 [38]), we ran OLS regression models for the time-series analysis excluding data related to the COVID-19 pandemic and to H4. In this way, we calculated the overall trend of outcomes of interest as if the COVID-19 pandemic had not occurred and hospital H4 had managed psychotropic drugs like the other hospitals of the network.
Statistical significance was set at 0.05. All statistical analyses were performed using STATA18 (StataCorp., College Station, TX, USA).
The final dataset consisted of 140 observations (35 for each hospital), representing the aggregated data for the period of interest. Overall, the total number of patient-events was 74,659, of which 14,645 were in hospital H1, 16,083 in hospital H2, 24,285 in hospital H3 and 19,646 in hospital H4, with a mean (±SD) case mix (metric that reflects the diversity, complexity and severity of the treated patients) for the hospitals in the quarter of 1.08 ± 0.14 (1.09 ± 0.17 for the other Swiss hospitals of the same category; years 2021–2022). The mean age was 73 ± 2 years, without significant differences between the four hospitals.
With regard to antipsychotic therapy, 10.6% of patients were receiving it prior to admission and 12.1% were discharged with it (new prescriptions 3.3 ± 0.7%; deprescriptions 13.3 ± 5.2%). With regard to benzodiazepines or Z-drugs, 30.1% of patients already had a prescription prior to admission and 30.3% were discharged with one (new prescriptions 5.6 ± 0.9%; deprescriptions 12.4 ± 1.6%). Full results are shown in table 1.
Table 1 Descriptive statistics related to aggregated data. Period from October 2014 to June 2023. Results are reported as either count (percentage) or mean (standard deviation).
| Hospital | Total patients | Total of antipsychotics at admission | Total of antipsychotics at discharge | Total of benzodiazepines and Z-drugs at admission | Total of benzodiazepines and Z-drugs at discharge | % of new antipsychotic prescriptions (SD) | % of new benzodiazepine and Z-drug prescriptions (SD) | % of antipsychotic deprescriptions (SD) | % of benzodiazepine and Z-drug deprescriptions (SD) | |
| Overall | H1 | 14,645 | 1719 (11.7%) | 1902 (13.0%) | 4743 (32.4%) | 4746 (32.4%) | 3.0 (1.6) | 5.4 (2.0) | 12.2 (6.4) | 11.3 (3.1) |
| H2 | 16,083 | 1397 (8.7%) | 1533 (9.5%) | 4644 (28.9%) | 4603 (28.6%) | 2.4 (1.1) | 4.6 (1.4) | 16.3 (7.1) | 12.4 (3.6) | |
| H3 | 24,285 | 2798 (11.5%) | 3405 (14.0%) | 6998 (28.8%) | 6985 (28.8%) | 2.4 (1.1) | 6.8 (2.4) | 13.0 (4.5) | 13.9 (2.7) | |
| H4 | 19,646 | 2005 (10.2%) | 2184 (11.1%) | 6067 (30.9%) | 6272 (31.9%) | 4.8 (1.5) | 5.5 (1.2) | 12.1 (3.7) | 11.8 (3.4) | |
| Total | 74,659 | 7919 (10.6%) | 9024 (12.1%) | 22,452 (30.1%) | 22,606 (30.3%) | 3.3 (0.7) | 5.6 (0.9) | 13.3 (5.2) | 12.4 (1.6) | |
| Male | H1 | 7110 | 795 (11.2%) | 881 (12.4%) | 1873 (26.3%) | 1917 (27.0%) | 2.9 (2.0) | 5.5 (2.4) | 12.8 (9.2) | 13.0 (4.2) |
| H2 | 8175 | 714 (8.7%) | 764 (9.3%) | 1895 (23.2%) | 1939 (23.7%) | 2.3 (1.3) | 4.7 (1.3) | 17.4 (9.8) | 13.3 (5.3) | |
| H3 | 11,597 | 1250 (10.8%) | 1523 (13.1%) | 2765 (23.8%) | 2849 (24.6%) | 2.3 (1.2) | 6.7 (2.5) | 12.4 (6.0) | 15.0 (3.9) | |
| H4 | 10,157 | 891 (8.8%) | 1001 (9.9%) | 2548 (25.1%) | 2745 (27.0%) | 4.5 (1.8) | 5.5 (1.4) | 11.8 (5.3) | 12.3 (4.8) | |
| Total | 37,039 | 3650 (9.9%) | 4169 (11.3%) | 9081 (24.5%) | 9450 (25.5%) | 3.0 (0.9) | 5.7 (1.0) | 13.1 (3.2) | 13.4 (2.1) | |
| Female | H1 | 7535 | 924 (12.3%) | 1021 (13.6%) | 2870 (38.1%) | 2829 (37.5%) | 3.0 (2.0) | 5.4 (2.2) | 11.7 (8.1) | 10.3 (3.7) |
| H2 | 7908 | 683 (8.6%) | 769 (9.7%) | 2749 (34.8%) | 2664 (33.7%) | 2.6 (1.3) | 4.5 (2.5) | 15.3 (6.7) | 11.9 (5.0) | |
| H3 | 12,688 | 1548 (12.2%) | 1882 (14.8%) | 4233 (33.4%) | 4136 (32.6%) | 2.5 (1.5) | 6.9 (2.9) | 13.7 (5.9) | 13.2 (3.2) | |
| H4 | 9489 | 1114 (11.7%) | 1183 (12.5%) | 3519 (37.1%) | 3527 (37.2%) | 5.0 (1.6) | 5.4 (1.8) | 12.1 (5.7) | 11.3 (3.7) | |
| Total | 37,620 | 4269 (11.3%) | 4855 (12.9%) | 13,371 (35.5%) | 13,156 (35.0%) | 3.5 (0.8) | 5.6 (1.1) | 12.9 (2.9) | 11.7 (1.8) |
In the subsequent analysis, females showed a higher percentage of antipsychotic and benzodiazepine/Z-drug therapy at admission and at discharge (p <0.001 in all comparisons) (figure 1).

Figure 1 Percentage of antipsychotics, benzodiazepines and Z-drugs at admission and at discharge. Comparisons between males and females.
There were also differences in the mean percentage values of new antipsychotic prescriptions (males: 3.0 ± 0.9%; females: 3.5 ± 0.8%; p = 0.01) and in benzodiazepine/Z-drug deprescriptions (males: 13.4 ± 2.1%; females: 11.7 ± 1.8%; p <0.001). However, there were no significant differences between males and females in terms of temporal trends (figure 2).
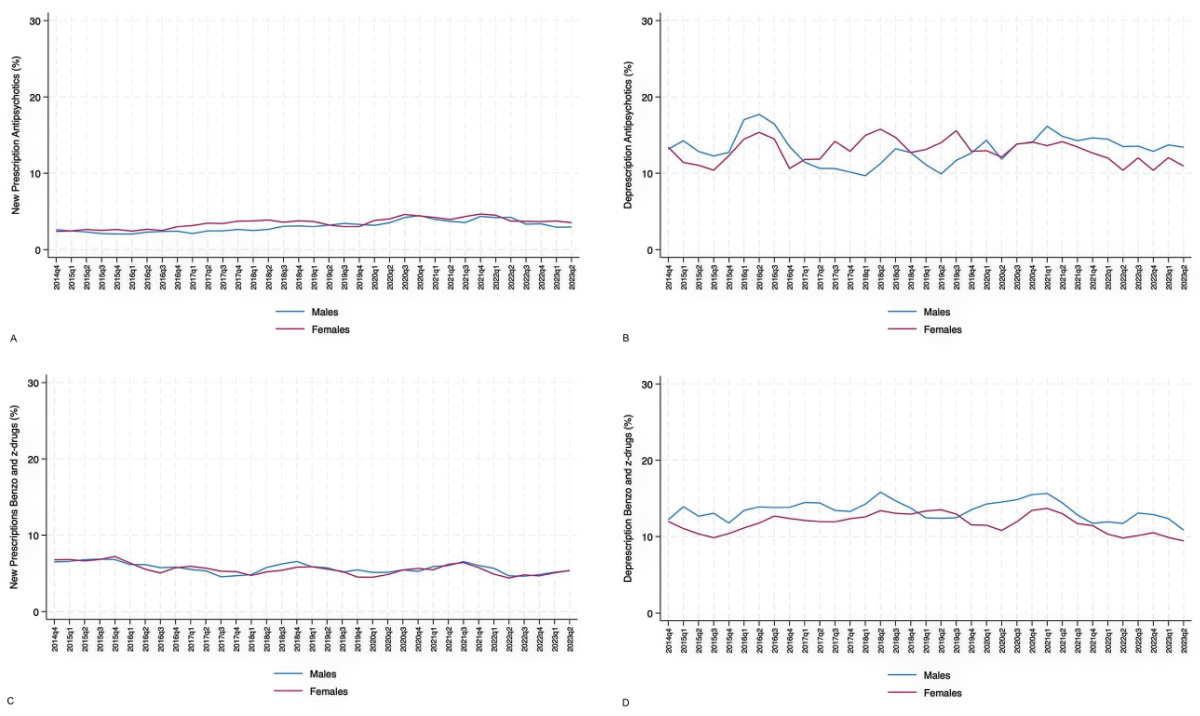
Figure 2 Temporal trend comparisons between males and females for (A) new antipsychotic prescriptions, (B) antipsychotic deprescriptions, (C) new benzodiazepine and Z-drug prescriptions and (D) benzodiazepine and Z-drug deprescriptions in the internal medicine departments of the four teaching hospitals involved in the analysis (H1-H4); trend from October 2014 to June 2023 (moving average calculated with 1-quarter intervals). The Choosing Wisely campaign began in January 2016 and ended in June 2017.
The percentage of new antipsychotic prescriptions showed an upward trend over time, albeit with some fluctuations, some of which reached statistical significance. A first significant fluctuation was observed in the third quarter of 2020, when the percentage of prescribed antipsychotics during hospitalisation decreased by 0.11% (95% CI: −0.16 – −0.05%, p <0.01). The percentage then increased again until the fourth quarter of 2021, when it decreased by 0.28% (95% CI: −0.39 – −0.16%, p <0.001) (figure 3). New antipsychotic prescriptions showed important differences between hospitals, with H3 always above the average and H2 almost always below. Hospital H3 had the highest percentage of new antipsychotic prescriptions during the COVID-19 period, with a peak of 7.8% in the third quarter of 2020.
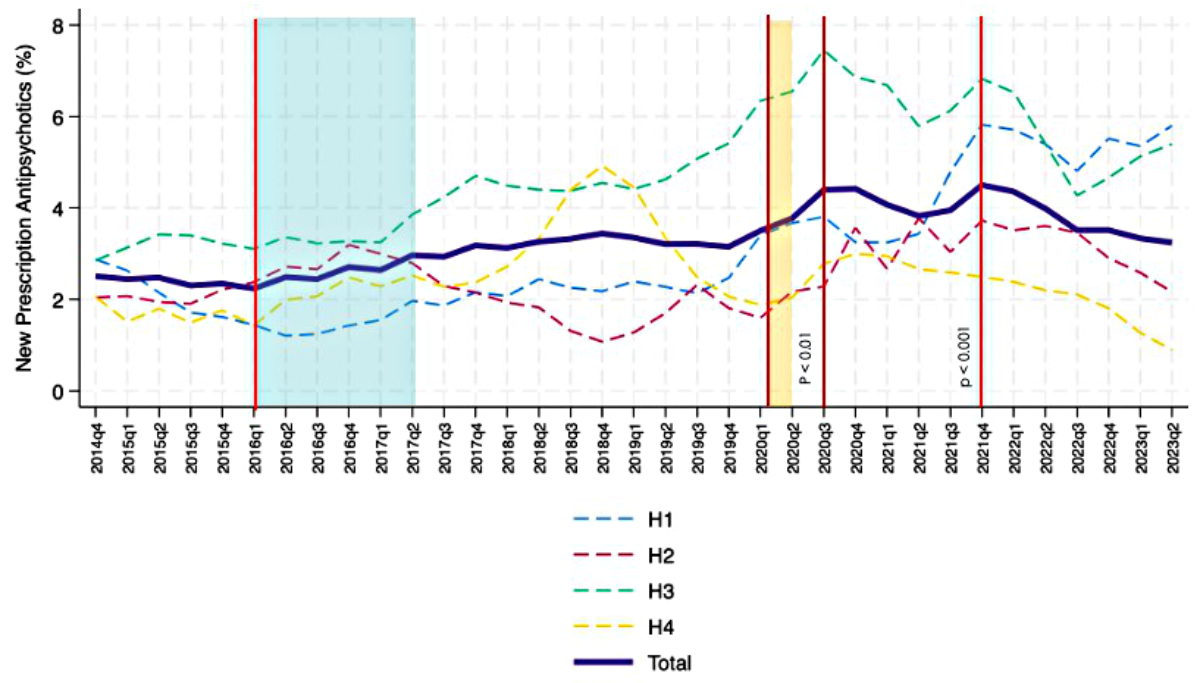
Figure 3 Temporal trends in new antipsychotic prescriptions (bold purple line) in the internal medicine wards of the four teaching hospitals (H1-H4) involved in the analysis; trend from October 2014 to June 2023 (moving average calculated with 1-quarter intervals). The Choosing Wisely campaign started in January 2016 and finished in June 2017 (blue box). In our region, the pandemic started in March 2020 (vertical orange line). Interrupted time-series analyses (vertical red lines) were performed to assess trend changes in quarters 2016Q1 (start of Choosing Wisely campaign) and 2020Q1 (start of COVID-19 pandemic). Furthermore, given changes in the overall trend, interrupted time-series analyses were also performed for quarters 2020Q3 and 2021Q4.
The overall trend analysis, which was carried out excluding the influence of the COVID-19 pandemic and hospital H4 (involved in the planning of the national campaign aimed at limiting the prescription of antipsychotics), showed a significant annual change of +0.20% (95% CI: 0.08–0.30%, p <0.001) in new antipsychotic prescriptions (figure 7A).
The percentage of antipsychotic deprescriptions during hospitalisation showed a non-significant decrease although some differences among the four hospitals were noted (figure 4). Analysis of the temporal trend revealed two short-term non-significant changes.
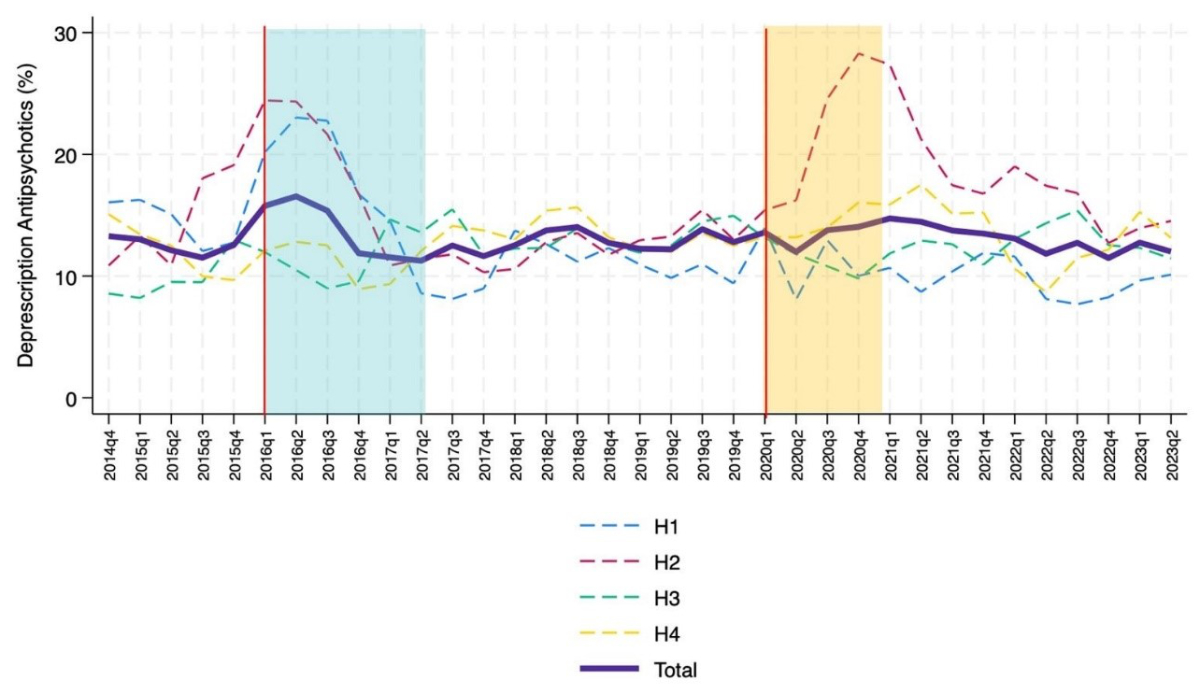
Figure 4 Temporal trends in antipsychotic deprescriptions (bold purple line) in the internal medicine wards of the four teaching hospitals (H1-H4) involved in the analysis; trend going from October 2014 to June 2023 (moving average calculated with 1-quarter intervals). The Choosing Wisely campaign started in January 2016 and finished in June 2017 (blue box). In our region, the pandemic started in March 2020 (vertical orange line). Interrupted time-series analyses (vertical red lines) were performed to assess trend changes in quarters 2016Q1 (start of Choosing Wisely campaign) and 2020Q1 (start of COVID-19 pandemic).
The percentage of patients already treated with antipsychotics at hospital admission showed an annual increase of 0.36% (p <0.001).
The percentage of new benzodiazepine and Z-drug prescriptions showed a significant change of −0.20% annually (95% CI: −0.36 – −0.02%, p = 0.013) (figure 7C), with a significant change in the first quarter of 2016, when the Choosing Wisely campaign started (p <0.005) (figure 5); other variations occurred over the period but none reached statistical significance.
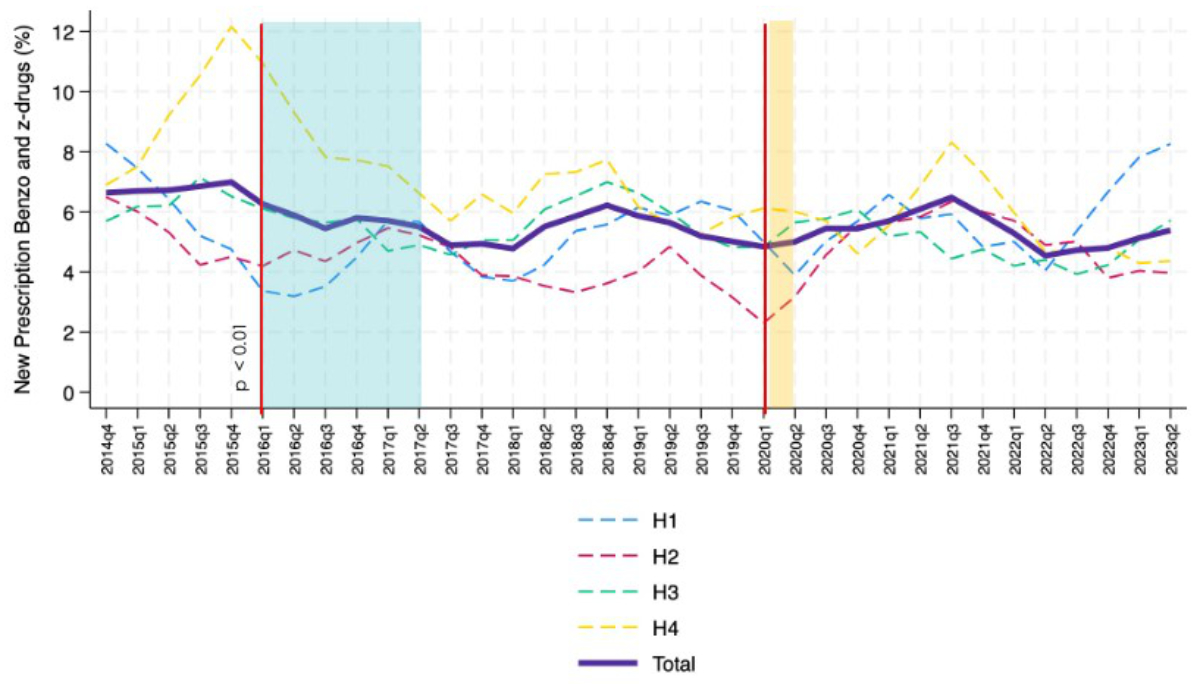
Figure 5 Temporal trends in new benzodiazepine and Z-drug prescriptions (bold purple line) in the internal medicine wards of the four teaching hospitals (H1-H4) involved in the analysis; trend going from October 2014 to June 2023 (moving average calculated with 1-quarter intervals). Vertical red lines indicate the trend changes. The Choosing Wisely campaign started in January 2016 and finished in June 2017 (blue box). In our region, the pandemic started in March 2020 (vertical orange line). Interrupted time-series analyses (vertical red lines) were performed to assess trend changes in quarters 2016Q1 (start of Choosing Wisely campaign) and 2020Q1 (start of COVID-19 pandemic).
The overall trend in the percentage of deprescriptions for benzodiazepines and Z-drugs fell significantly in the period under review: −0.28% (95% CI: −0.44–−0.12%, p <0.001) (figure 7D). This decline showed a relevant downward trend from the second quarter of 2021, when a quarterly change of −0.22 (95% CI: −0.41 – −0.03, p = 0.027) was observed (figure 6 and figure S2 in the appendix).

Figure 6 Temporal trends in benzodiazepine and Z-drug deprescriptions (bold purple line) in the internal medicine wards of the four teaching hospitals (H1-H4) involved in the analysis; trend going from October 2014 to June 2023 (moving average calculated with 1-quarter intervals). The Choosing Wisely campaign started in January 2016 and ended in June 2017 (blue box). In our region, the pandemic started in March 2020 (vertical orange line). Interrupted time-series analyses (vertical red lines) were performed to assess trend changes in quarters 2016Q1 (start of Choosing Wisely campaign) and 2020Q1 (start of COVID-19 pandemic). Furthermore, given changes in the overall trend, interrupted time-series analyses were also performed for quarter 2021Q2.
The percentage of patients already treated with benzodiazepines/Z-drugs at hospital admission showed an annual change of −0.32% (p <0.001).
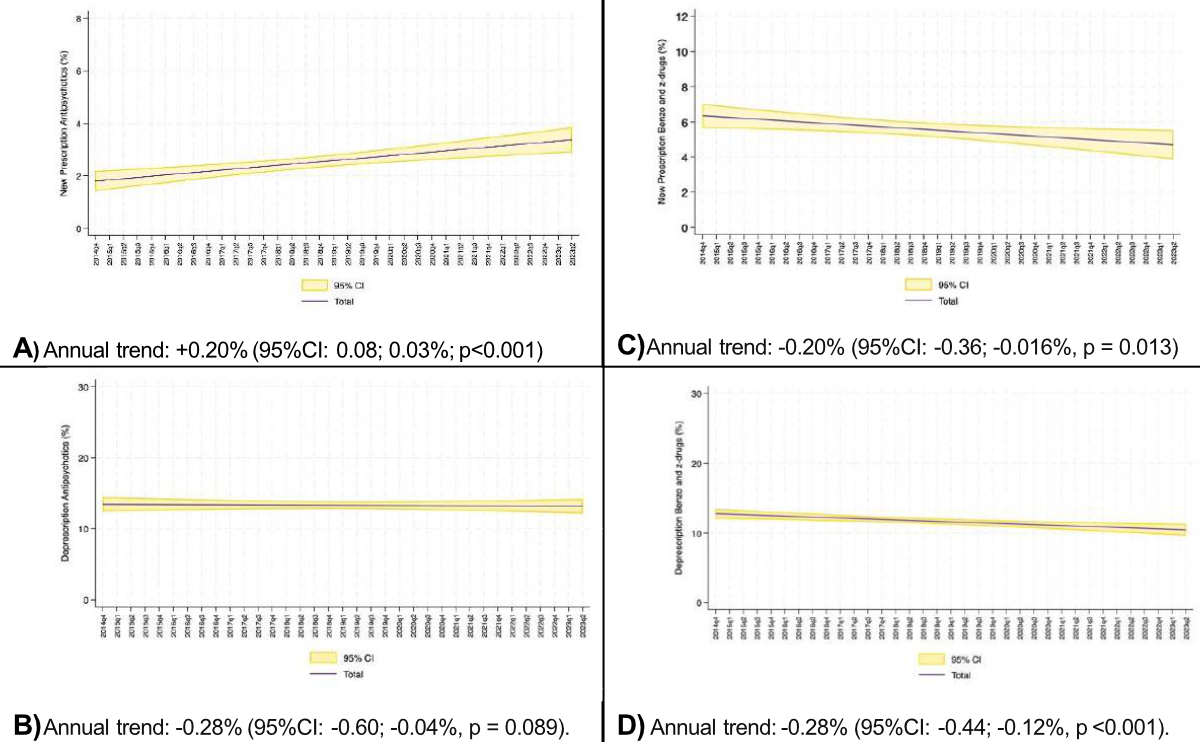
Figure 7 Overall trend in the percentage of (A) New antipsychotic prescriptions, (B) Antipsychotic deprescriptions, (C) New benzodiazepine and Z-drug prescriptions, and (D) Benzodiazepine and Z-drug deprescriptions. Annual trend determined excluding the impact of the COVID-19 pandemic and hospital H4.
In the present study we analysed whether the evolution of new in-hospital antipsychotic prescriptions, as well as benzodiazepines and Z-drugs, had seen any changes in the years following an internal Choosing Wisely campaign and if the pandemic had significantly influenced the trend.
Even allowing for the important differences between hospitals, our results showed that new intra-hospital prescriptions of antipsychotics in internal medicine wards increased linearly between 2014 and 2023. Considering the linearity of the time-related increase, it can be assumed that the long-term trend was not influenced by identifiable individual events, even if the COVID-19 pandemic contributed to a transient increase in consumption (see figure 3). With regard to the trend changes detected with interrupted time-series analyses, we interpreted the decrease in new antipsychotic prescriptions in 2020Q3 and 2021Q4 as consequences of the epidemiologically favourable phases of the pandemic (both quarters were at the maximum distance from the previous COVID-19 incidence peak), while the downward trend in benzodiazepine and Z-drug deprescriptions in 2021Q2 could be explained by the second wave of COVID-19, which in our region was in 2020Q4 and 2021Q1 [39].
The prescription strategy for both antipsychotics and benzodiazepines/Z-drugs, as seen in figures 3 and 5, seems to be a hospital characteristic with a reproducible trend: some hospitals were prescribing more antipsychotics and less benzodiazepines and Z-drugs (see H3), while others were doing the opposite (see H2). The internal 2016–2017 Choosing Wisely campaign reduced new benzodiazepine and Z-drug prescriptions and inter-hospital variation without affecting that of antipsychotics in a measurable way [29].
Our data show an upward trend in new intra-hospital antipsychotic prescriptions (+0.20% year) that is quantitatively comparable to the downward trend in new intra-hospital benzodiazepine/Z-drug prescriptions (−0.20% year). The same trend was visible in the ongoing prescriptions at admission, suggesting a similar out-of-hospital approach (−0.32% year of benzodiazepines/Z-drugs and +0.36% year of antipsychotics). This suggests a change of sedative prescription strategy rather than the choice of alternative, non-pharmacological approaches.
Regarding differences between the sexes (subsequent analysis), the percentage of females with benzodiazepines/Z-drugs at hospital admission was higher than that of males (35.5% vs 24.5%); the same applies, albeit to a lesser degree, to antipsychotics (11.3% vs 9.9%). The temporal trend was however comparable between the two sexes. The fact that benzodiazepine/Z-drug deprescriptions were significantly higher in females while new prescriptions were comparable, was probably influenced by numerosity, even if a difference in the original prescription appropriateness, as a potential co-factor, is acknowledged in the literature [40, 41].
The intra-hospital variation of drug prescriptions and medical procedures is a universally recognised challenge [42–48] and our network was not an exception to this phenomenon [30]. Over the years analysed, the internal medicine departments of the four hospitals, H3 in particular, displayed different trends in new prescriptions of antipsychotics and other sedative drugs. This circumstance was detected within the network despite dealing with a similar patient population in terms of age (mostly older people) and disease severity. We hypothesise that these differences are related to differences in the medical background and in the prescription thresholds at the individual physician and team levels and also involve the predisposition to treat off-label indications such as insomnia and states of psychomotor agitation. Considering the fact that the use of antipsychotics could be deleterious in terms of side effects (such as drowsiness, QT prolongation, falls, pneumonia and cerebrovascular events), the prescription of these drugs should not be significantly influenced by individual treatment strategies but rather be the expression of a proper risk-benefit assessment. Therefore, systematically monitoring and benchmarking prescription trends among hospitals and raising awareness of potentially inappropriate prescriptions remains essential.
As limitations and potential sources of bias, we emphasise that the study allowed only a quantitative analysis of deprescriptions/prescriptions and that we did not have information about underlying diagnoses and daily medication dosage. This did not allow us to analyse the appropriateness of prescriptions and the reasons behind the observed patterns. We were also unable to detail the correlation between the increase in antipsychotic prescriptions and the decrease of benzodiazepines/Z-drugs. Additionally, having studied only the Italian-speaking part of Switzerland, we cannot be sure that similar trends are present in the rest of the country and that results can be generalised.
Furthermore, a potential confounding factor is the fact that the therapeutic reconciliation process was limited to the data given by GPs and patients themselves, without the systematic involvement of the pharmacy.
Last but not least, the reasons behind the differences in antipsychotic and benzodiazepine/Z-drug prescriptions among the network hospitals were not analysed.
With more than 10% and 30% of patients admitted to the internal medicine wards already treated with antipsychotics and benzodiazepines/Z-drugs, respectively, this study confirmed the very high prevalence of sedative use in Switzerland. In addition, the percentage of patients with an ongoing antipsychotic treatment at admission and new in-hospital prescriptions rose linearly between 2014 and 2023. In parallel, we observed a linear reduction of new in-hospital benzodiazepine/Z-drug prescriptions and of the percentage of patients already treated with them at hospital admission. This change, suggesting a shift in sedative prescription strategy is worth further investigation, both in hospital and family medicine settings. Another important point is the difference between sexes, confirming that females have higher percentages of chronic use, and deprescriptions/prescriptions. Significant inter-hospital variations in prescribing, common in other healthcare aspects, were once again highlighted.
The previous successful local campaign to curb benzodiazepine/Z-drug prescriptions did not affect short-term antipsychotic use but was probably one of the determinants of the progressive shift toward antipsychotics. The COVID-19 pandemic seems, on the contrary, to only have had an impact in the epidemic phase of the disease.
Continuously monitoring new prescriptions of antipsychotics and other sedatives, and ensuring benchmarking, is a necessary prerequisite for detecting potentially inappropriate drifts, creating awareness and taking action in a targeted way.
Study data are obtainable from the authors upon reasonable request.
Author contributions: Conceptualisation: LG, VG, AC, AG, NG – Methodology: LG, VG – Validation: LG, VG, AC, NG – Formal analysis: LG, VG, AC, NG – Investigation: VG, GS, AG, AM, VFB – Resources: LG – Data curation: VG, LC, MZ – Original draft preparation: LG, VG, AC, AG, NG, GS, VFB – Review and editing: LG, VG, AC, AG, NG, GS, AM, VFB, JG, MZ – Supervision: LG – Funding acquisition: LG.
All authors have read and agreed to the published version of the manuscript.
This research was funded by the Fondo di Ricerca del Dipartimento di Medicina dell’Ospedale San Giovanni di Bellinzona, Bellinzona, Switzerland.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005 Jun;172(13):1703–11.
2. FDA. (2016). Atypical Antipsychotic Drugs Information.
3. Krutika Chokhawala LS. (2022). Antipsychotic Medications.
4. Swissmedicinfo. (2021). Quietapin-Mepha. https://www.swissmedicinfo.ch/ViewMonographie?isNewUpdate=1
5. Gaertner J, Eychmueller S, Leyhe T, Bueche D, Savaskan E, Schlögl M. Benzodiazepines and/or neuroleptics for the treatment of delirium in palliative care?-a critical appraisal of recent randomized controlled trials. Ann Palliat Med. 2019 Sep;8(4):504–15.
6. Højlund M, Andersen JH, Andersen K, Correll CU, Hallas J. Use of antipsychotics in Denmark 1997-2018: a nation-wide drug utilisation study with focus on off-label use and associated diagnoses. Epidemiol Psychiatr Sci. 2021 Apr;30:e28.
7. Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011 Feb;20(2):177–84.
8. Walsh JK. Drugs used to treat insomnia in 2002: regulatory-based rather than evidence-based medicine. Sleep. 2004 Dec;27(8):1441–2.
9. Reus VI, Fochtmann LJ, Eyler AE, Hilty DM, Horvitz-Lennon M, Jibson MD, et al. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia. Am J Psychiatry. 2016 May;173(5):543–6.
10. Gareri P, Segura-García C, Manfredi VG, Bruni A, Ciambrone P, Cerminara G, et al. Use of atypical antipsychotics in the elderly: a clinical review. Clin Interv Aging. 2014 Aug;9:1363–73.
11. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in Older Persons: Advances in Diagnosis and Treatment. JAMA. 2017 Sep;318(12):1161–74.
12. Nikooie R, Neufeld KJ, Oh ES, Wilson LM, Zhang A, Robinson KA, et al. Antipsychotics for Treating Delirium in Hospitalized Adults: A Systematic Review. Ann Intern Med. 2019 Oct;171(7):485–95.
13. FDA. (2023). Drug Alerts and Statements.
14. Yunusa I, Rashid N, Demos GN, Mahadik BS, Abler VC, Rajagopalan K. Comparative Outcomes of Commonly Used Off-Label Atypical Antipsychotics in the Treatment of Dementia-Related Psychosis: A Network Meta-analysis. Adv Ther. 2022 May;39(5):1993–2008.
15. Stogios N, Smith E, Bowden S, Tran V, Asgariroozbehani R, McIntyre WB, et al. Metabolic adverse effects of off-label use of second-generation antipsychotics in the adult population: a systematic review and meta-analysis. Neuropsychopharmacology. 2022 Feb;47(3):664–72.
16. Yunusa I, Alsumali A, Garba AE, Regestein QR, Eguale T. Assessment of Reported Comparative Effectiveness and Safety of Atypical Antipsychotics in the Treatment of Behavioral and Psychological Symptoms of Dementia: A Network Meta-analysis. JAMA Netw Open. 2019 Mar;2(3):e190828.
17. Dementia: Assessment, management and support for people living with dementia and their carers. London: National Institute for Health and Care Excellence (NICE); 2018 Jun.
18. England LCNN. Appropriate prescribing of antipsychotic medication in dementia. 2022.
19. Jahnsen JA, Widnes SF, Schjøtt J. Quetiapine, Misuse and Dependency: A Case-Series of Questions to a Norwegian Network of Drug Information Centers. Drug Healthc Patient Saf. 2021 Jul;13:151–7.
20. Corbett A, Burns A and Ballard C. Don't use antipsychotics routinely to treat agitation and aggression in people with dementia. Bmj. 2014; 349(nov03 1):g6420-g6420.
21. Hálfdánarson Ó, Zoëga H, Aagaard L, Bernardo M, Brandt L, Fusté AC, et al. International trends in antipsychotic use: A study in 16 countries, 2005-2014. Eur Neuropsychopharmacol. 2017 Oct;27(10):1064–76.
22. Nishtala PS, Chyou TY. An Updated Analysis of Psychotropic Medicine Utilisation in Older People in New Zealand from 2005 to 2019. Drugs Aging. 2022 Aug;39(8):657–69.
23. Kelleher JE, Weedle P, Donovan MD. The Prevalence of and Documented Indications for Antipsychotic Prescribing in Irish Nursing Homes. Pharmacy (Basel). 2021 Sep;9(4):160.
24. Tumusiime WA, Hardman CJ, Breen JL. Antipsychotic prescribing in people admitted to hospital with dementia or delirium. Australas J Ageing. 2022 Jun;41(2):258–64.
25. Assurance H. (2018). Helsana-Arzneimittelreport. Available at: file:///C:/Users/susan/Downloads/arzneimittelreport-2018.pdf
26. Assurance H. Helsana-Arzneimittelreport. 2020. Available at: https://www.helsana.ch/de/helsana-gruppe/medien-publikationen/mitteilungen/arzneimittelreport-2020.html
27. Smarter Medicine CwS. SmarterMedicine_Flyer_ENG_web300. 2016. Available at https://www.smartermedicine.ch/fileadmin/user_upload/Adaptionen/smartermedicine/Dokumente/Listen_Flyer_neu/SmarterMedicine_Flyer_ENG_web300.pdf
28. Del Giorno R CA, Gabutti L. Benzodiazépines chez les patients âgés : le côté sombre d’une pilule magique [Benzodiazepines in elderly patients : the dark side of a magic pill]. 2017;13(547):282-84.
29. Del Giorno R, Greco A, Zasa A, Clivio L, Pironi M, Ceschi A, et al. Combining prescription monitoring, benchmarking, and educational interventions to reduce benzodiazepine prescriptions among internal medicine inpatients; a multicenter before and after study in a network of Swiss Public Hospitals. Postgrad Med. 2018 Sep;130(7):627–36.
30. Del Giorno R, Ottini A, Greco A, Stefanelli K, Kola F, Clivio L, et al. Peer-pressure and overuse: the effect of a multimodal approach on variation in benzodiazepine prescriptions in a network of public hospitals. Int J Clin Pract. 2020 Mar;74(3):e13448.
31. Del Giorno R, Schneiders C, Stefanelli K, Ceschi A, Gyoerik-Lora S, Aletto I, et al. Unexpected Increase in Benzodiazepine Prescriptions Related to the Introduction of an Electronic Prescribing Tool: Evidence from Multicenter Hospital Data. Diagnostics (Basel). 2019 Nov;9(4):190.
32. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: A longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018 Apr;50:52–9.
33. Erard Y, Del Giorno R, Zasa A, De Gottardi S, Della Bruna R, Keller F, et al. A multi-level strategy for a long lasting reduction in unnecessary laboratory testing: A multicenter before and after study in a teaching hospital network. Int J Clin Pract. 2018 Oct;73(3):e13286.
34. Moreno C, Wykes T, Galderisi S, Nordentoft M, Crossley N, Jones N, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020 Sep;7(9):813–24.
35. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007 Oct;335(7624):806–8.
36. Kofam. (2023). Koordinationsstelle Forschung am Menschen. Available at: https://kofam.ch/de
37. Cumby RE, Huizinga J. Testing the Autocorrelation Structure of Disturbances in Ordinary Least-Squares and Instrumental Variables Regressions. Econometrica. 1992;60(1):185–95.
38. Smarter Medicine CwS. (2023). Liste «Top 5» Médecine Interne Générale hospitalière. Available at : https://www.smartermedicine.ch/fr/liste-top-5/medecine-interne-generale-hospitaliere-2023
39. cantonale T-Udm. Situazione epidemiologica in Ticino. 2023. Available at: https://www4.ti.ch/dss/dsp/covid19/popolazione/situazione-epidemiologica
40. Morgan SG, Weymann D, Pratt B, Smolina K, Gladstone EJ, Raymond C, et al. Sex differences in the risk of receiving potentially inappropriate prescriptions among older adults. Age Ageing. 2016 Jul;45(4):535–42.
41. Resnick B, Boltz M, Galik E, Kuzmik A, McPherson R, Drazich B, et al. Differences in Medication Use by Gender and Race in Hospitalized Persons Living with Dementia. J Racial Ethn Health Disparities. 2024 Oct;11(5):2839–47.
42. Waring JJ. A qualitative study of the intra-hospital variations in incident reporting. Int J Qual Health Care. 2004 Oct;16(5):347–52.
43. Sandora TJ, Fung M, Melvin P, Graham DA, Rangel SJ. National Variability and Appropriateness of Surgical Antibiotic Prophylaxis in US Children’s Hospitals. JAMA Pediatr. 2016 Jun;170(6):570–6.
44. Sanchez Traun KB, Schauberger CW, Ramirez LD, Jones CW, Lindberg AF, Molero Bravo RA, et al. Opioid prescribing trends in postpartum women: a multicenter study. Am J Obstet Gynecol MFM. 2019 Nov;1(4):100055.
45. Rogers MA, Blumberg N, Saint S, Langa KM, Nallamothu BK. Hospital variation in transfusion and infection after cardiac surgery: a cohort study. BMC Med. 2009 Jul;7(1):37.
46. McLeod M, Ahmed Z, Barber N, Franklin BD. A national survey of inpatient medication systems in English NHS hospitals. BMC Health Serv Res. 2014 Feb;14(1):93.
47. Lelong A, Léger S, Vendittelli F, Blanquet M, Thuong CT, Belgacem B, et al. A quality indicator can be biased by intra-hospital heterogeneity: the case for quality of patient record keeping in France. Eur J Public Health. 2015 Oct;25(5):787–91.
48. Hudelson P, Vilpert S. Overcoming language barriers with foreign-language speaking patients: a survey to investigate intra-hospital variation in attitudes and practices. BMC Health Serv Res. 2009 Oct;9(1):187.
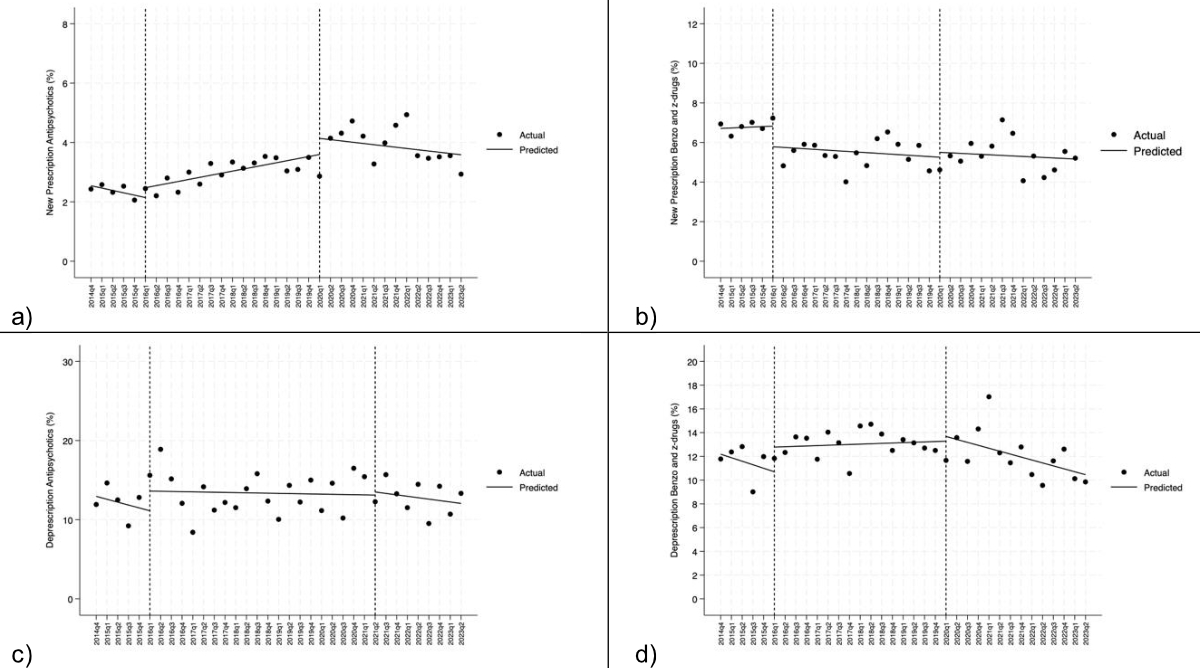
Figure S1 Interrupted time-series analysis performed in 2016Q1 (start of Choosing Wisely campaign) and 2020Q1 (start of COVID-19 pandemic): (A) new prescription of antipsychotics (%); (B) new prescription of benzodiazepines and Z-drugs (%); (C) Deprescription of antipsychotics (%); (D) Deprescription of benzodiazepines and Z-drugs (%).
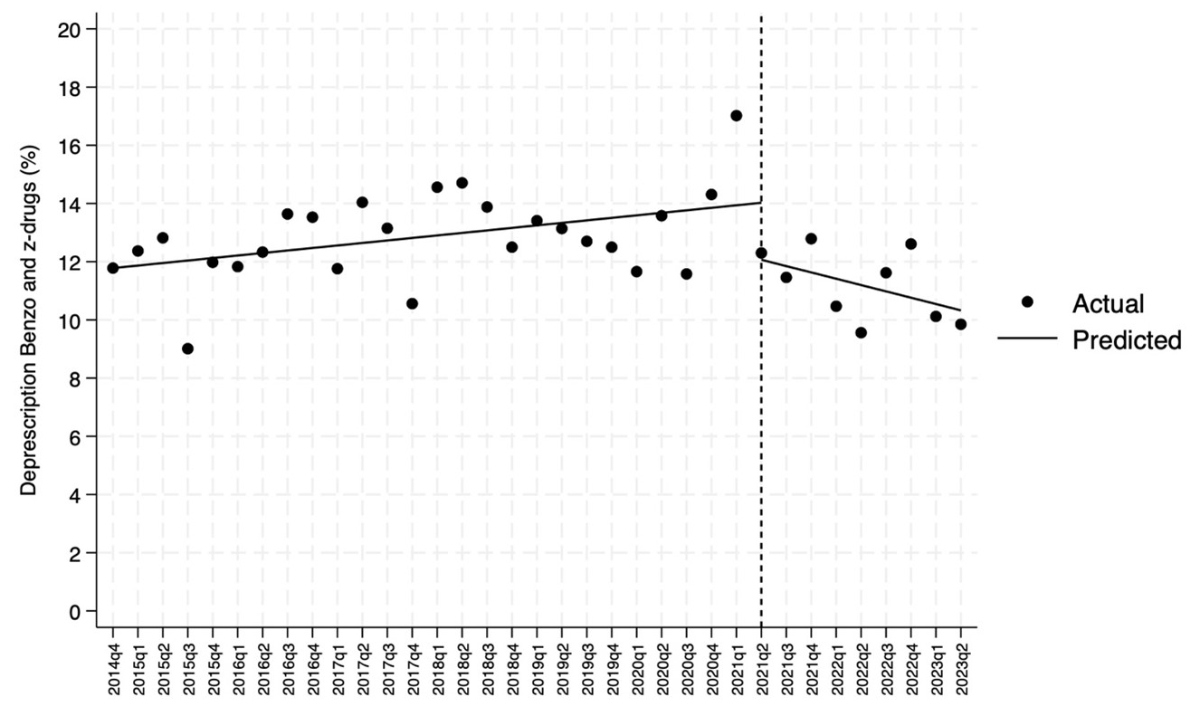
Figure S2 Interrupted time-series analysis. Focus on the second quarter of 2021 (2021Q2). The Choosing Wisely campaign started in January 2016 and finished in June 2017. In our region, the pandemic started in March 2020.