Outcome of kidney transplantation from senior deceased donors: a single centre study
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40098
Kris Magerla,
Matthias Diebolda,
Caroline Wehmeiera,
Patrizia Amicoab,
Michael Dickenmanna,
Jürg Steigera,
Stefan Schaubab,
Patricia Hirt-Minkowskia
a Clinic for Transplantation Immunology and Nephrology, University
Hospital Basel, Basel, Switzerland
b HLA-Diagnostic and lmmunogenetics, Department of Laboratory Medicine,
University Hospital Basel, Basel, Switzerland
c KM and DM contributed
equally to this work as co-first authors
Summary
BACKGROUND: Addressing the current demographic
development, the efficacy and safety of kidney transplantations from very
senior donors needs to be carefully evaluated. The aim of this study was to
analyse patient and graft outcomes of kidney allograft recipients stratified by
donor age.
METHODS: We retrospectively investigated n = 491 patients
from a prospective, observational renal transplant cohort. Patients with
kidneys from very old donors (n = 75, aged >70 years), elderly donors (n = 158,
between 60–70 years), and regular donors (n = 258, aged <60 years) were
investigated. The primary outcome was death-censored graft survival within the
predefined donor age groups.
RESULTS: Overall, n = 57 death-censored graft
losses occurred. Graft loss was proportionally highest in the very old donor
group (n = 11/75), but this did not reach statistical significance when compared
to the elderly (14/158) and regular donor groups (32/258); (p = 0.37).
Kaplan-Meier analysis demonstrated that 3-year/5-year
death-censored graft survival in the very old
donor group was 96%/86% and did not differ from the other age groups (p = 0.44).
Median estimated glomerular filtration rate (eGFR), calculated by the Chronic
Kidney Disease Epidemiology Collaboration
(CKD-EPI) formula (in ml/min/1.73 m2 of body surface) 12 months
post-transplant did not differ between the elderly donor and very old donor
groups (p = 0.53). However, patients who received regular donor kidneys had
higher median eGFR compared to recipients in both the elderly and very old
donor groups (p <0.0001). During follow-up, 31% of patients developed at
least one acute rejection episode. Time-to-event analysis demonstrated no
difference in occurrence of any acute rejection event across all three groups
(p = 0.11).
CONCLUSIONS: This
study demonstrates that kidney transplantation from carefully selected very old
donors seems a valid option with reasonable short- and mid-term outcomes.
Introduction
Kidney
transplantation is the therapy of choice for many patients with end stage renal
disease and has evolved rapidly during the past few decades. However, due to
the progressively increasing gap between the number of available organs and the
number of patients on kidney transplantation wait lists (in Switzerland as well
as in other countries) strategies to expand the donor pool – such as using living
donors, ABO-incompatible
transplantation, donation after cardiac death, and extending the age limit for
deceased kidney donors – have been developed [1, 2]. In Switzerland, in 2022, there
were
1041 patients with end stage renal disease registered on the waiting list for a
kidney transplantation: 231 kidney transplantations were performed from
deceased donors, of which 91 organs were from donation after cardiac death; and
111 kidney transplantations were performed from living kidney donors (data
received from https://www.swisstransplant.org). In 2002, the United
Network for Organ Sharing (UNOS) proposed increasing the kidney donor pool by
considering kidneys from expanded criteria donors [3], even though previous studies
addressing the use of expanded
criteria donors (in general defined as aged ≥60 years or 50–59 years old with
comorbidities) indicated that the discard rate of these kidneys is high [4]. During
the past decade in the United
States, >40% of expanded criteria donor kidneys were not transplanted [4]. A single
centre study performed in a
transplant centre in Europe investigated the outcomes of performed
transplantations and kidney discard rate (KDR) of potential expanded criteria
donors, stratified by age groups (50–59 years old, 60–69 years old, 70–79 years
old, and ≥80 years old) between 2003 and 2013 [5].
The KDRs of the three younger age groupings were similar and ranged between
15.4% and 20.1%, whereas the KDR of potential octogenarian donors was 48.2% [5]. In
a Swiss national cohort study, Kuhn et
al. recently reported that the KDR at all Swiss transplant centres was about
20% [6]. They further pointed out that
donor candidates who were refused were older, had a higher prevalence of cardiac
death, heart disease, hypertension, diabetes mellitus, acute kidney injury, and
preexisting kidney disease compared to donor candidates whose kidneys were
transplanted [6]. Furthermore, the long-term
survival of expanded criteria donor kidneys is about 10–40% lower than those of
younger donors [7, 8]. Nevertheless,
large studies have shown that transplanting allografts from marginal donors is preferable
to remaining on renal replacement therapy [7, 9].
To date, reports of kidney transplantations from donors aged 70 years and older,
with long-term follow-up, are limited [6, 10–15].
Taking the changing
donor epidemiology and current demographic development into account, the
efficacy and safety of kidney transplantations from very senior donors needs to
be carefully evaluated. To address these questions, this study aimed to
retrospectively analyse patient and graft outcomes of kidney allograft
recipients from very old donors (aged >70 years old) compared to elderly (between
60–70 years old) and younger donors (<60 years old).
Materials
and methods
Patient
population
This retrospective
study was performed with the approval of the ethics committee of Northwestern
and Central Switzerland (www.eknz.ch;
project-ID 2021-01475). Patients from a prospective, observational renal
transplant cohort, who were transplanted at our centre between March 2005 and June
2020 were selected for the study. We took advantage of a cohort that had
prospective risk stratification, with an adaptation of the induction regimen,
and received contemporary maintenance immunosuppression consisting of
tacrolimus (Tac) and mycophenolate acid (MPA). Inclusion criteria were a kidney
transplantation from a deceased adult donor and a minimum follow-up period of
1-year posttransplant. Exclusion criteria were living donor
transplantations, combined transplantations, and donor/recipients aged <18
years at the time of transplantation. Briefly, between March 2005 and June 2020, 577
patients received a kidney
allograft from a deceased donor at our centre. Of those, n = 60 were from paediatric
donors (aged less than 5 years), n = 23 were from child donors (aged between 5
and 17 years), and n = 3 recipients were under 18 years of age at the time of
transplantation, and were therefore excluded. The final study population
consisted of n = 491 adult recipients of deceased donor kidney transplantations.
Outcomes were evaluated until September 13, 2021 (figure 1).

Figure 1Study
flowchart. For exclusion criteria, paediatric donors are classified as donors aged
less than 5 years and
child donors are classified as donors aged between 5 and 17 years. Young
recipients are aged less than 18 years at the time of transplantation. Causes of death-censored
graft loss as well as causes of
patient death are indicated. Low graft function at 12 months posttransplant was
defined as chronic kidney disease stage 4 or higher (eGFR CKD-EPI <30
ml/min/1.73 m2). eGFR:
estimated glomerular filtration rate (calculated by the CKD-EPI formula; in
ml/min/1.73 m2 of body surface); CKD-EPI: Chronic Kidney Disease
Epidemiology Collaboration
Posttransplant
management
Initial
immunosuppression was selected based on the pretransplant risk stratification policy
used at our centre; i.e., the presence or absence of pretransplant donor-specific
human leukocyte antigen (HLA)-antibodies determined by single-antigen flow bead
(SAFB) technology on a Luminex platform (LABScreen single antigen, OneLambda,
West Hills CA, USA) as described previously [16–19].
Briefly, recipients of an allograft without pretransplant donor-specific
HLA-antibodies received induction therapy with basiliximab (Simulect, Novartis)
and triple therapy with Tac-MPA-prednisone. In the case of a rejection-free
clinical course, immunosuppression was modified and reduced within the first
six months to establish a dual Tac-MPA therapy in the long-term. Target trough
levels of tacrolimus were 10–12 ng/ml
for the first month, 8–10 ng/ml for months two to three, and around 6 ng/ml
thereafter. Recipients
with pretransplant donor-specific HLA-antibodies received an induction therapy
with polyclonal anti T-cell globulin (Grafalon) prior to reperfusion of the
allograft and on day 1–4, or anti-thymocyte globulin (Thymoglobuline,
Sanofi-Aventis) for 4 days, as well as intravenous immunoglobulins [16, 17]. Maintenance
immunosuppression and target
trough levels were the same as for renal allograft recipients without
pretransplant donor-specific HLA-antibodies. Steroids were started at 0.5 mg/kg
of bodyweight and tapered biweekly to 0.1 mg/kg body weight by month three
posttransplant, and maintained at this level. All biopsy-proven acute rejection
episodes were treated according to the histological phenotype and severity,
including borderline rejection, as described in earlier studies [17, 18].
Investigated
parameters and outcomes
Recipient
characteristics and outcomes were collected from patient charts and stored in a
research database. Donor derived factors were retrieved from the SOAS database
(Swiss Organ Allocating System). Baseline values such as: recipient age, sex,
history of kidney transplantation, dialysis status, time of renal replacement
therapy before transplantation, cold ischemia time, serum creatinine and eGFR
(CKD-EPI), as well as number of rejection episodes and rejection phenotypes,
were extracted from the research database. Kidney Donor Risk Index (KDRI)
combines a variety of donor factors to summarise the risk of graft failure
after kidney transplantation into a single number, including donor age (https://optn.transplant.hrsa.gov).
Thus, from the SOAS database, donor age, sex, and further values to calculate the
Kidney Donor Risk Index and cause of donor death, were extracted. Donor age was
categorically grouped into regular donors (age <60 years), elderly donors
(age between 60 and 70 years), and very old donors (age >70 years).
The primary
outcome was to investigate death-censored graft survival within the predefined
age groups. Secondary outcomes were patient survival and graft outcome (i.e., evaluation
of graft function at one and five years posttransplant). Furthermore, we analysed
the incidence of
(sub)clinical allograft rejection for the predefined donor age groups during
follow-up. In addition, we investigated independent predictors of graft loss.
Renal
allograft biopsies
From 2005
until September 2017, surveillance biopsies were routinely performed at 3 and 6
months posttransplant. Due to a change of policy at our centre, from September
2017 onwards no surveillance biopsies were performed anymore at 3/6 months
posttransplant. Clinically indicated allograft biopsies were performed when
serum creatinine increased by more than 20% from baseline, or in cases of
increasing proteinuria or glomerular haematuria. Histology
work-up followed standard procedures (of 2 biopsy cores obtained with a
16-gauge needle) including evaluation by light microscopy, immunofluorescence (staining
for immunoglobulins, complement including C4d, and HLA-DR), and
immunohistochemistry (staining for SV40 large T-antigen). All biopsies were scored
and classified according to the Banff 2013/2015 classification conventions [20, 21].
Statistical
analysis
We used JMP
software version 16.0 (SAS Institute Inc.,
Cary, NC) for statistical analysis. Categorical
data are presented as counts and percentages and
were analysed by Fisher's exact test or Pearson's chi-square test as
appropriate. Continuous data are summarised as median and interquartile ranges
(IQR) unless stated otherwise and analysed by the Wilcoxon or Kruskal-Wallis rank
sum tests. No sample size calculation was performed since this represents a
retrospective analysis. Recipient and donor baseline characteristics are shown
within table 1 for the entire study population and stratified by donor age. Furthermore,
donor age was compared across different transplant periods during the
observation time and represented with violin plots. Patient, graft, and
death-censored graft survival was analysed by the Kaplan-Meier method and
groups were compared using the log-rank test. Allograft function at one and
five years posttransplant was compared across the donor age groups and represented
with violin plots. Kidney Donor Risk Index was calculated according to the
guidelines for calculating and interpreting KDRI (available from https://optn.transplant.hrsa.gov)
using R version 4.0.2 and the package transplant. Mainly due to missing creatinine values of the donors, sufficient donor
factors to calculate KDRI were only available for 431 patients. Multivariable Cox
proportional
hazards regression analysis was performed to adjust for potential confounders. Potential
confounders were selected based on pre-existing knowledge about worse graft
outcome and graft loss. No p-value threshold or automated variable selection
was used. For all models, transplantations with primary
non-function organs (n = 6) were excluded. The final model was chosen based on the
number of events and the consensus of requiring 10 events per independent variable.
The variables chosen for the final model were delayed graft function, cold
ischemia time, donor-specific HLA-antibodies, HLA-mismatches, donation after
cardiac death, and the variable of interest: donor age group. The proportional hazard
assumption was tested using Schoenfeld
residuals. A two-tailed p-value <0.05 was considered to indicate statistical significance.
No imputation was used to address missing values.
Table 1Recipient and donor baseline characteristics stratified by donor age, n
= 491.
| |
Entire
study population (n = 491) |
Donors aged
<60 years (n = 258) |
Donors aged between 60 and 70 years (n = 158) |
Donors aged
>70 years (n = 75) |
p-value* |
| Recipient |
| Age (years) |
58 (49–65) |
55 (44–62) |
61 (54–66) |
64 (57–69) |
<0.0001 |
| Female,
n (%) |
165 (34) |
88 (34) |
51 (32) |
26 (35) |
0.91 |
| Primary disease, n (%) |
Adult polycystic kidney disease |
79 (16) |
32 (13) |
30 (19) |
17 (23) |
0.01 |
| Diabetic |
59 (12) |
24 (9) |
24 (15) |
11 (15) |
|
| Vascular |
54 (11) |
24 (9) |
24 (15) |
6 (8) |
|
| Glomerulopathy |
163 (33) |
93 (36) |
45 (29) |
25 (33) |
| Other |
87 (18) |
57 (22) |
23 (14) |
7 (9) |
|
| Unknown |
49 (10) |
28 (11) |
12 (8) |
9 (12) |
|
| Immunological parameters |
Number of transplant, % 1/2/≥3 |
79/18/3 |
74/23/3 |
83/13/4 |
85/14/1 |
0.04 |
| Pretransplant DSA, % 0/1/2/≥3 |
81/10/5/4 |
75/12/6/7 |
83/9/6/2 |
93/3/4/0 |
0.01 |
| DSA
Class, % I/II/I+II |
40/35/25 |
35/35/30 |
50/35/15 |
60/40/0 |
0.03 |
| Cumulative MFI |
3183 (1393–7717) |
3766 (1362–10947) |
2357 (1311–5433) |
4152 (1372–4303) |
0.50 |
| HLA-A/B/DR/DQ
MM |
5 (4–7) |
5 (4–6) |
5 (4–6) |
5 (4–7) |
0.59 |
| Induction therapy |
Basiliximab, n (%) |
374 (76) |
180 (70) |
128 (81) |
66 (88) |
0.0001 |
| ATG +/- IvIg, n (%) |
109 (22) |
76 (29) |
24 (15) |
9 (12) |
|
| None, n (%) |
8 (2) |
2 (1) |
6 (4) |
0 |
|
| Baseline IS |
Tac-MMF/Myf-P, n (%) |
447 (91) |
232 (90) |
146 (92) |
69 (92) |
0.66 |
| Tac-MMF/Myf-mTOR,
n (%) |
44 (9) |
26 (10) |
12 (8) |
6 (8) |
|
| Dialysis, n (%) |
479 (98) |
254 (98) |
155 (98) |
70 (93) |
0.04 |
| Dialysis time, months |
39.3 (23.4–56.7) |
40.4 (25.4–59.3) |
39.7 (22.6–58.3) |
31.9 (20.4–63.5) |
0.07 |
| Deceased donor |
| Age, y |
59 (47–67) |
48 (39–54) |
65 (62–68) |
75 (72–79) |
<0.0001 |
| Female, n (%) |
208 (42) |
112 (43) |
67 (42) |
29 (39) |
0.006 |
| CIT, h |
9.3 (7.6–12.0) |
9.0 (7.1–11.5) |
9.3 (7.8–11.6) |
11.6 (8.1–13.4) |
0.002 |
| DGF, n
(%) |
175 (36) |
74 (29) |
79 (50) |
22 (29) |
0.0002 |
| DCD, n (%) |
51(10) |
20 (8) |
28 (18) |
3 (4) |
0.0008 |
| Cerebrovascular death, n (%) |
288 (59) |
131 (51) |
101 (64) |
56 (75) |
<0.0001 |
| KDRI,
median (IQR) |
2.0 (1.5–2.5) |
1.6 (1.3–1.8) |
2.4 (2.1–1.6) |
3.2 (2.8–3.4) |
<0.0001 |
Results
Recipient
and donor baseline characteristics stratified by donor age
In this
study we investigated 491 patients with a median follow-up of 4.9 years (IQR 2.3–8.3
years) as shown in figure 1. The final study
population consisted of 258 (53%) regular donors (aged <60 years), 158 (32%)
elderly donors (age between 60 and 70 years), and 75 (15%) very old donors (aged
>70 years). We compared recipient and donor baseline characteristics between
the three groups (table 1). At the time of transplantation, recipients of very
old donor grafts were generally older (median age 64 years; IQR 57–69 years) compared
to recipients of elderly
(median 61 years; IQR 54–66 years) and regular (median 55 years; IQR 44–62
years) donor grafts (p = 0.04 and p <0.0001, as well as overall p-value <0.0001; figure
S1). Immunological parameters such as number of transplantations,
presence of pretransplant donor-specific HLA-antibodies, and donor-specific
HLA-antibody classes, differed slightly between the groups (p≤0.04), whereas
HLA-mismatches were not significantly different. Patients with pretransplant donor-specific
HLA-antibodies were younger and (accordingly to risk stratification rules)
received more induction therapy with polyclonal anti T-cell globulin +/- intravenous
immunoglobulins (p-value overall = 0.0001). Baseline immunosuppression did not
differ between the groups (table 1). Pre-emptive transplantations were rare,
and the median time on dialysis before transplantation was 39.4 months (IQR
23.3–65.7 months) and comparable between the groups (table 1).
The median
age of donors did not increase throughout different transplantation periods during
our observation time (figure 2). The incidence of cerebrovascular death was
significantly higher in very old donors (75%) compared to elderly donors (64%)
and regular donors (51%) (overall p-value <0.0001; table 1). Donations
accepted for transplantation after cardiac death were highest in the elderly
donor group (p = 0.0008). Delayed graft function was also highest in patients
who received a kidney from an elderly donor (50%) compared to the other two
groups (both 29%; p = 0.0002). Median cold ischemia time was below 12 hours for
all groups, however cold ischemia time significantly differed between the
groups (p = 0.002; table 1). Donor age is an essential determinant of the KDRI value.
Thus, Kidney Donor Risk Index was lowest in the regular donor group, with a
stepwise increase in Kidney Donor Risk Index from the regular to the elderly
donor group (p <0.0001), and to the very old donor group (p <0.0001; table 1).
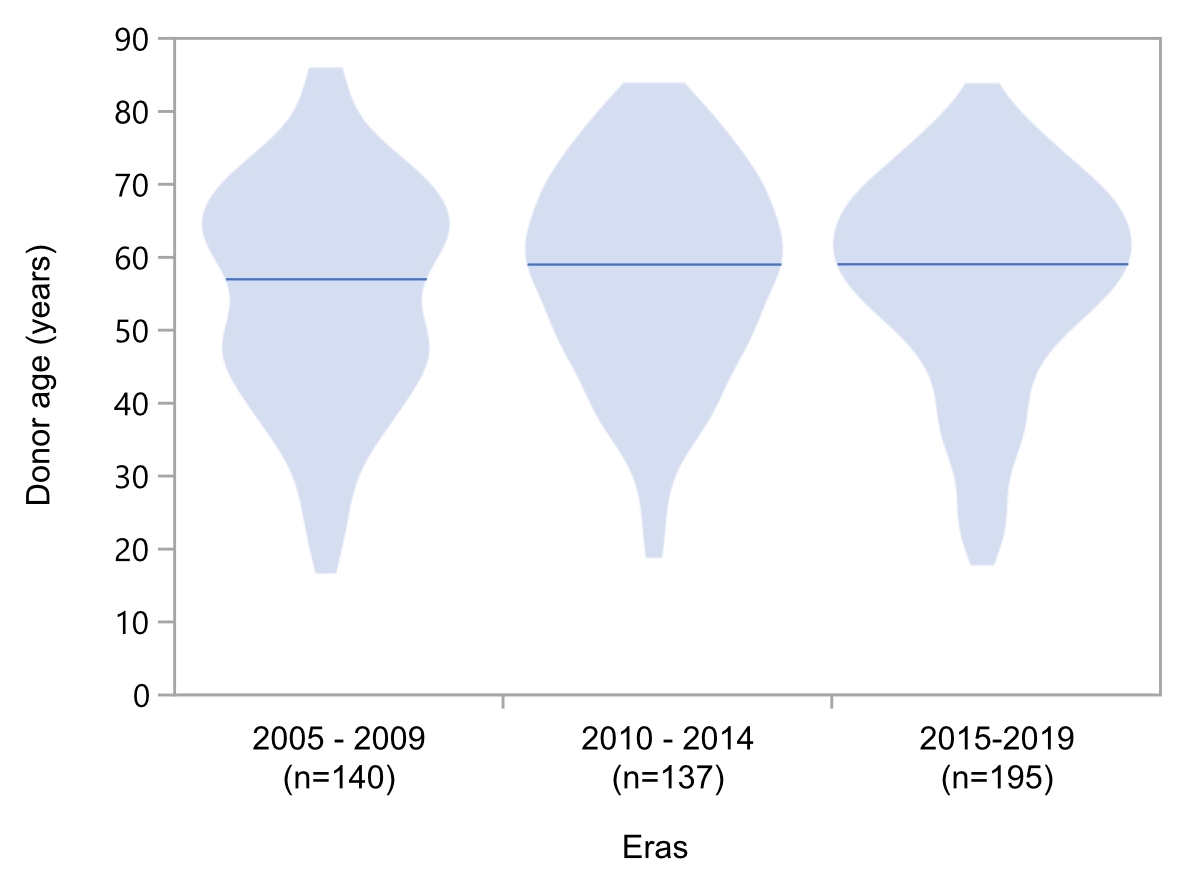
Figure 2Evolution of donor age across different periods
(years 2005–2019). Due to low
numbers, transplantations performed in 2020 (n = 19 as of June 2020) were
excluded from illustrations of donor age by violin plots, however median donor
age during different periods was the same.
Graft
and patient survival of kidney allograft recipients stratified by donor age
During the
observation period, 168 grafts were lost – most of
them due to death with a functioning graft (n = 111). The number of deaths with
a functioning graft did not differ between the groups (p = 0.73). In addition,
we observed n = 57 death-censored graft losses during follow-up. The causes of death,
as well as death-censored graft loss, are depicted in figure 1. Graft loss was proportionally
highest in the very old donor group (n = 11/75), but this did not reach
statistical significance compared to the elderly donor group (14/158), and the regular
donor group (32/258) (p = 0.44; figure 3A).

Figure 3(A) Death-censored graft survival, (B) graft
survival, and (C) patient survival of patients (n = 491) stratified according
to age groups.
Kaplan-Meier
analysis on kidney allograft recipients stratified by donor age demonstrated that
death-censored graft survival, as well as
graft and patient survival, did not differ across the age groups (figure 3A–C).
Specifically, 3-year and 5-year death-censored graft survival in the very old donor
group was 96% and 86%, respectively, and did not significantly differ from the
other two age groups (p = 0.44; figure 3A). As expected, 3-year and 5-year graft survival,
which was mainly triggered by events of death with a
functioning graft, was lower compared to death-censored graft loss, but did not
differ statistically across all age groups (p = 0.71; figure 3B). Specifically, 3-year
and 5-year graft survival in the very old donor group was 84%
and 66%, respectively (figure 3B). In addition, 3-year and 5-year patient
survival in the very old donor group was 87% and 77%, respectively, and did not
differ significantly from the other two age groups (p = 0.87; figure 3C).
Lower
graft function in allograft recipients of elderly and very old donor organs
Graft function was analysed at 12 months and five years posttransplant
by calculating estimated glomerular filtration rate (eGFR) using the Chronic
Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (ml/min/1.73 m2
of body surface). Of the entire study population, 27 patients did not reach the
12-month follow-up visit, either due to early death with a functioning graft (n
= 14) or very early graft loss (n = 13). Therefore, a total of 464 (95%)
patients had eGFR values at 12-month follow-up. Median allograft function was
57 ml/min/1.73 m2 (IQR 44–70 ml/min/1.73 m2) in the regular
donor group, 41 ml/min/1.73 m2 (IQR 31–52 ml/min/1.73 m2) with
the elderly donor group, and 37 ml/min/1.73 m2 (IQR 29–50
ml/min/1.73 m2) in the very old donor group (figure 4).
Statistically, median eGFR values did not differ between the elderly and very
old donor groups (p = 0.53). However, patients with regular donors had significantly
higher median eGFR at their 12-month follow-up visit compared to the elderly and very
old donor groups (both with p-value <0.0001; figure 4). Ultimately, 12
months after transplantation, low graft function – defined as chronic kidney disease
stage 4 or higher (eGFR <30 ml/min/1.73 m2) – was found in 5% (regular
donor group), 21% (elderly donor group), and 28% (very old donor group) of
patients (overall p <0.0001).
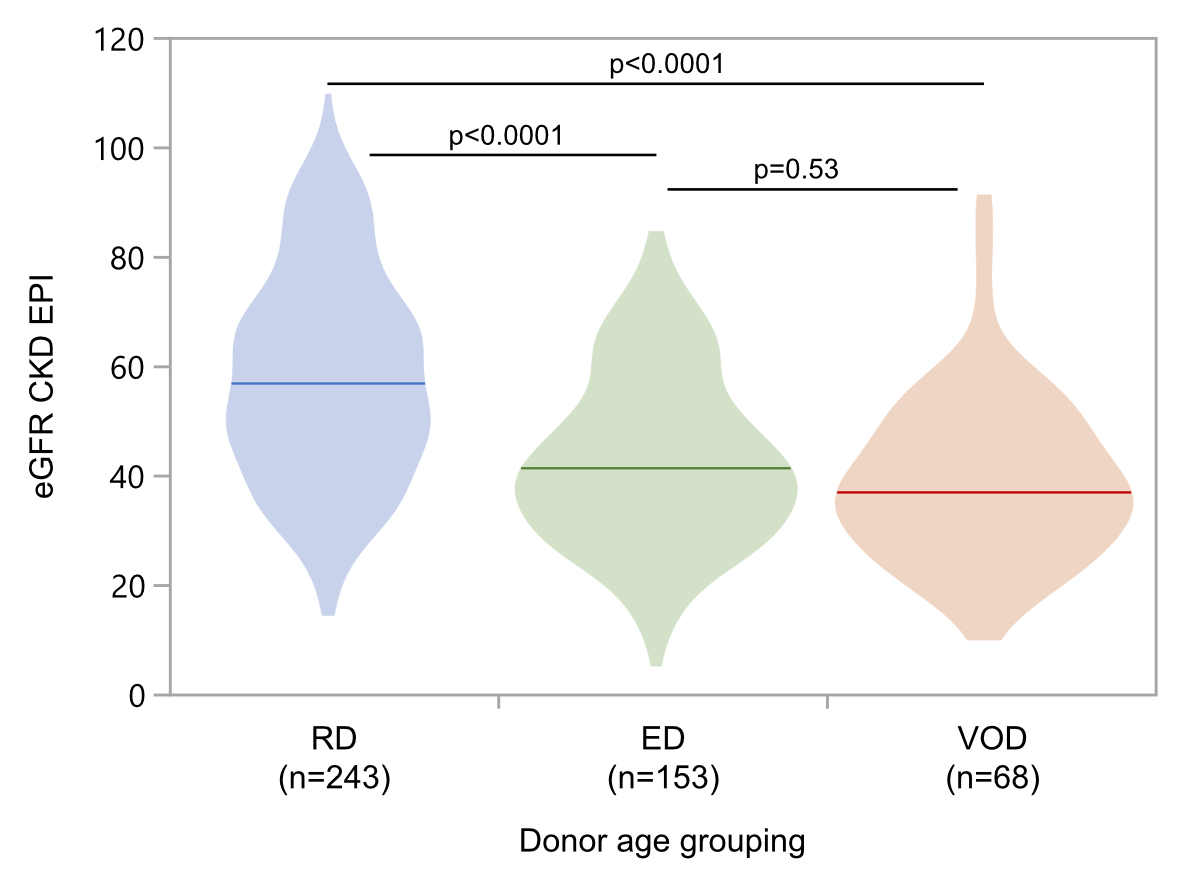
Figure 4Kidneys from elderly
donors (ED; age between 60 and 70 years), and very old donors (VOD; age >70
years) achieved lower eGFR (CKD-EPI) at
12 months posttransplant, compared to regular donor kidneys (RD; age <60
years). eGFR: estimated glomerular
filtration rate (calculated by the CKD-EPI formula; in ml/min/1.73 m2
of body surface); CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration
Early death-censored graft loss (in total n = 13) consisted of n = 6
grafts with primary non-function, n = 2 with ongoing rejection, n = 1 with de
novo glomerulonephritis, and n = 4 with early graft loss due to another
problem. Concerning donor age grouping: there were n = 3 donor organs with primary
non-function in the regular donor group; n = 1 in the elderly donor group, and
n = 2 in the very old donor group. Furthermore, patients with early graft loss were
more likely to have received a kidney allograft from a very old donor than
those without early graft loss (26% vs. 15%), however this did not reach
statistical significance (p = 0.15) – data not shown.
Allograft function did not change over a period of five years posttransplant.
In total, 234 (48%) patients had 5-year follow-up visits and contributed to the
5-year analysis of graft function. Median allograft function was 60
ml/min/1.73 m2 (IQR 43–72 ml/min/1.73 m2) in the regular
donor group, 41 ml/min/1.73 m2 (IQR 29–51 ml/min/1.73 m2) in
the elderly donor group, and 39 ml/min/1.73 m2 (IQR 31–49
ml/min/1.73 m2) in the very old donor group. Median eGFR values did
not differ between the elderly and very old donor groups (p = 1.0), but did
between the regular and elderly donor groups (p <0.0001), as well as between
the regular and very old donor groups (p <0.0001) – data not shown.
Rejection
rates of allograft recipients stratified by donor age
Of the entire study population, 327 (67%) patients had at least one surveillance
biopsy within the first 6 months posttransplant. Furthermore, 280 (57%) patients
had one or more clinically indicated allograft biopsy during the entire
observational period, even though most of the patients (81%) were evaluated for
deteriorating kidney function within the first year posttransplant. During the
entire follow-up period, 153 (31%) patients developed at least one acute
rejection episode, however most of the acute rejection episodes occurred within
the first three years posttransplant (91%) as shown in figure 5A. Time-to-event
analysis demonstrated no difference of occurrence of any acute clinical
rejection event when all three groups, stratified by donor age, were compared with
each other (p = 0.11; figure
5A). Patients who belonged to the regular donor group (n = 89/258) as well as the
elderly donor group (n = 49/158) had numerically more acute clinical rejection
episodes compared to the very old donor group (n = 15/75), however this
difference was statistically significant only between the regular and very old
donor groups (p = 0.03; figure 5A). During
the entire follow-up period, 274 (56%) patients developed at least one acute subclinical
rejection episode. As surveillance biopsies were routinely done at 3 and
6 months posttransplant, figure 5B shows the occurrence of subclinical
rejection episodes only within the first 12 months posttransplant. Again, we found
no statistically significant difference in
occurrence of any subclinical rejection event when all three groups were
compared (p = 0.99; figure 5B).
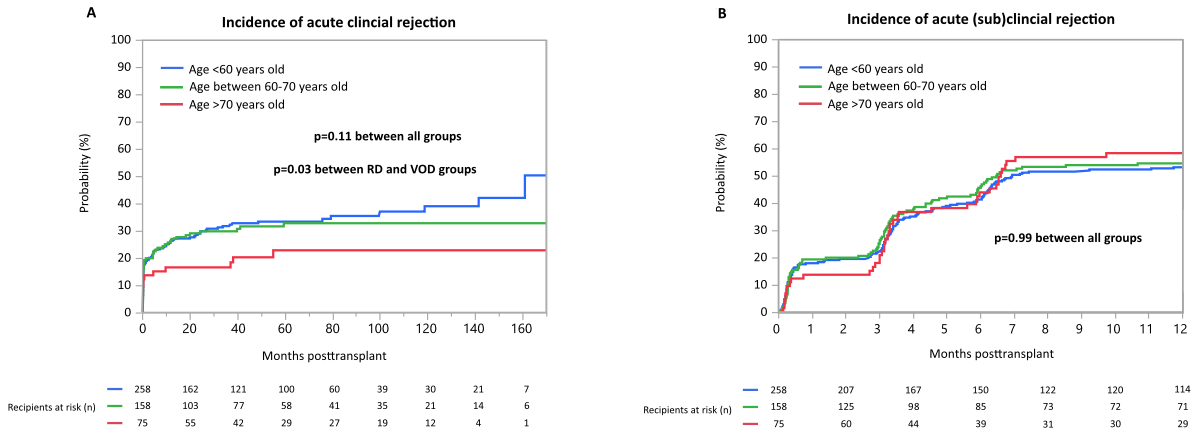
Figure 5Incidence of acute rejection. (A) Incidence of acute clinical rejection
over time, (B) incidence of clinical and subclinical rejection within the first
year post-transplant. Surveillance biopsies were obtained at 3 and 6 months
post-transplant.
(Sub)clinical T-cell-mediated rejection occurred in n = 205 patients (42%),
and (sub)clinical antibody-mediated rejection occurred in n = 98 patients (20%).
Time-to-event analysis demonstrated no difference, either in the occurrence of T-cell-mediated
rejection between the groups stratified by donor age (p = 0.62; supplementary figure
S2A), or in
the occurrence of any antibody-mediated rejection between the groups (p = 0.15; supplementary
figure S2B).
Independent
predictors of graft loss
In a multivariable
Cox proportional hazards analysis – adjusting for potential confounders which were
available at the time of transplantation or the early posttransplant period – donor
age was not a significant
predictor of graft loss (very old donors vs regular donors: Hazard Ratio HR 0.81 [95
confidence
interval CI 0.38–1.89], p-value 0.61; elderly donors vs regular donors: Hazard
Ratio HR 1.49 [95 confidence interval CI 0.77–3.02], p-value 0.24). Presence of
pretransplant donor-specific HLA-antibodies (yes vs no) remained the only
independent predictor within the multivariable model (HR 2.53, 95% CI 1.38–4.54;
p = 0.003) (table 2).
Table 2Independent predictors
of death-censored graft loss (n = 485)*.
| Predictors of graft loss |
Recipients with
death-censored graft loss (n = 51) |
Recipients without graft
loss (n = 434) |
Univariate Cox
proportional analysisHazard ratio (95% CI); p-value |
Multivariable Cox
proportional analysis**Hazard ratio (95% CI); p-value |
| DGF (yes vs no), n (%) |
22 (43) |
153 (35) |
1.57 (0.90–2.73); 0.12 |
1.71
(0.96–3.00); 0.07 |
| CIT,
hours |
9.8 (6.7–13.1) |
9.3 (7.6–11.8) |
1.04 (0.98–1.09); 0.18 |
1.03
(0.97–1.09); 0.29 |
| Donor age group |
<60 y vs >70 y, n |
29/9 |
226/64 |
0.96 (0.45–2.03): 0.91 |
0.81
(0.38–1.89); 0.61 |
| <60 y vs 60–70 y, n |
29/13 |
226/144 |
1.36 (0.70–2.62); 0.36 |
1.49 (0.77–3.02);
0.24 |
| DSA (yes vs no), n (%) |
20 (39) |
73 (17) |
2.52 (1.44–4.43) 0.002 |
2.53
(1.38–4.54); 0.003 |
| HLA-A/B/DR/DQ MM |
5 (3–6) |
5 (4–7) |
0.97 (0.84–1.11); 0.66 |
|
| DCD (yes vs
no), n (%) |
1
(2) |
50 (12) |
0.60
(0.08–4.44); 0.58 |
|
Discussion
The most
striking observation of this study was that transplantations from very old
donors did not show inferior death-censored
graft survival compared to transplantations from elderly donors or regular donors.
We found no difference in early and overall graft loss between the
groups. In addition, organs showing a primary non-function were not exclusively
those harvested from the very old donors. It is important to highlight
that Kaplan-Meier analysis demonstrated remarkable
3- and 5-year death-censored graft survival of transplanted
patients who received grafts from very old donors. As expected, graft survival
− which
was mainly triggered by by death rate with a functional graft
− was
lower than death-censored graft loss, but again did not differ statistically across
age groups. Furthermore, when we adjusted our survival model for possible
confounders, donor age
was not an independent predictor of death-censored graft loss.
Moreover,
we showed a significant relationship between donor age and eGFR at 12 months
posttransplant. Importantly, achievement of eGFR
at 12 months did not differ among donors aged 60 years and older. As expected, patients
with transplantations from donors younger than 60 years had significantly
higher median eGFR at their 12-month follow-up visit, but this had no influence
on mid-term graft and patient outcomes. It is important to put these results into
context and compare them to alternative treatment options; most notably to long-term
dialysis, which has a high annual death rate, especially for older patients [22].
Nevertheless, inferior graft function
might have an impact on quality of life and might therefore reduce the patient benefit
gained from a transplantation. Among other factors affecting graft
outcome, we demonstrated that acute allograft rejection episodes were highest
in patients who belonged to the regular donor group than to the very old donor
group, which was mainly triggered by late acute rejection episodes. Therefore,
we may speculate that the better organ function of kidneys from younger donors,
represented by better eGFR 12 months after transplantation, may be negatively affected
by the occurence of more late acute rejections in long-term. Interestingly,
patient survival was similar across all age groups although recipients of very
old grafts were older at time of transplantation. This may be explained by the
fact that older transplant recipients must be relatively healthy to be accepted
onto the transplant waiting list.
The current
study demonstrated that, at the time of transplantation, there was a strong
relationship between recipient and donor age. Previously, large transplant
centres have established specific old-for-old transplantation programs, where
older donors are allocated to senior candidates on the waiting list [23–25]. While
such an approach seems a
valuable tool for large transplant centres and extensive catchment areas, it is
not feasible for smaller transplant programs like the one in Switzerland. Nevertheless,
in the daily clinical practice of our centre, represented by the current study,
the donor/recipient pairs were well matched by age. Because older kidney
transplant recipients have a shorter life expectancy at the time of transplantation
compared to younger ones, they arguably need less kidney function over time
(i.e., until their end of life). From this perspective, the promising outcomes seen
in kidney allograft recipients with organs harvested from very old donors within
this study, support a centre-specific process of allocating suitable “very old”
donor kidneys to elderly kidney allograft candidates, within the legally
compliant allocation of organs to recipients (allocation is coordinated on a
national level in Switzerland).
Another
interesting observation is that transplantations from very old donors were
performed more frequently without the presence of donor-specific HLA-antibodies.
Although our model selection to explore independent predictors of
death-censored graft loss was based on donor age as a confounder, the
multivariable model identified the presence of donor-specific HLA-antibodies as
the only significant risk factor for death-censored graft loss, which is
consistent with the literature. It is possible that, within the first years
after transplantation, similar rates of rejection-free outcomes in very old
donor transplantations are the result of an overall lower immunological risk
associated with these types of transplants.
Study limitations
Firstly, this
is a single centre study; and although we used an unselected, consecutive
patient population over a long period, the number of patients who received
kidneys from very old donors was rather limited. This might increase the
possibility of a type II error. However, given that we did not find an
association, it can be assumed that the overall effect would be rather low. Secondly,
this is a retrospective analysis of a prospective cohort and therefore it was
not possible to obtain missing data. We did not collect serial sera to screen
for de novo donor-specific HLA-antibodies post-transplant for the entire
study population. Thus, we were not able to include data for the occurrence of de
novo donor-specific HLA-antibodies within the multivariate model. However,
our screening method for donor-specific HLA-antibodies is applicable to all
kidney recipients, independent of donor age, and therefore random absence is
most likely. Thirdly, given the retrospective analysis and the possibility to
decline a transplant offer, a bias by indication may be present. Although we
tried to adjust our statistical model to known confounders, we were limited by
the small sample size and therefore may have residual confounding. Even if this
limits the possibility of finding independent effects of very old donor organs,
the results that carefully selected very old donors show reasonable outcomes are
encouraging.
Conclusion
This study
demonstrates that kidney transplantation from very old donors seems to be a
valid option, taking patient and allograft outcome into account, with
reasonable short- and mid-term outcomes.
Acknowledgements
The authors
thank the staff of the University Hospital Basel Renal Transplant Unit for the
outstanding patient care, and the histocompatibility laboratory for collection
and processing of blood samples for immunological work-up before
transplantation. Furthermore, the authors thank Agim Thaqi from the Federal
Office of Public Health for assisting with extraction of donor derived factors
from the Swiss Organ Allocating System database.
Author
contribution: The individual contribution of each co-author is briefly
summarised as follows: Designed research/study: PHM;
Performed research/study: PHM, KM, DM; Collected data: PHM, SS, CW, PA, MD, JS;
Analysed data: PHM, KM, DM; Wrote paper:
all.
PD Dr. Patricia Hirt-Minkowski
Clinic for Transplantation Immunology and Nephrology
University Hospital Basel
Petersgraben 4
CH-4031 Basel
patricia.hirt-minkowski[at]usb.ch
References
1. Cohen B, Smits JM, Haase B, Persijn G, Vanrenterghem Y, Frei U. Expanding the donor
pool to increase renal transplantation. Nephrol Dial Transplant. 2005 Jan;20(1):34–41.
10.1093/ndt/gfh506
2. Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD—fundamentals
for the practicing nephrologist. Clin J Am Soc Nephrol. 2009 Nov;4(11):1827–31. 10.2215/CJN.02270409
3. Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, et al. Report of the
Crystal City meeting to maximize the use of organs recovered from the cadaver donor.
Am J Transplant. 2002 Sep;2(8):701–11. 10.1034/j.1600-6143.2002.20804.x
4. Tanriover B, Mohan S, Cohen DJ, Radhakrishnan J, Nickolas TL, Stone PW, et al. Kidneys
at higher risk of discard: expanding the role of dual kidney transplantation. Am J
Transplant. 2014 Feb;14(2):404–15. 10.1111/ajt.12553
5. Messina M, Diena D, Dellepiane S, Guzzo G, Lo Sardo L, Fop F, et al. Long-Term Outcomes
and Discard Rate of Kidneys by Decade of Extended Criteria Donor Age. Clin J Am Soc
Nephrol. 2017 Feb;12(2):323–31. 10.2215/CJN.06550616
6. Kuhn C, Lang BM, Lörcher S, Karolin A, Binet I, Beldi G, et al. Outcome of kidney
transplantation from very senior donors in Switzerland - a national cohort study.
Transpl Int. 2021 Apr;34(4):689–99. 10.1111/tri.13836
7. Ojo AO, Hanson JA, Meier-Kriesche HU, Okechukwu CN, Wolfe RA, Leichtman AB, et al. Survival
in recipients of marginal cadaveric donor kidneys compared with other recipients and
wait-listed transplant candidates. J Am Soc Nephrol. 2001 Mar;12(3):589–97. 10.1681/ASN.V123589
8. Aubert O, Kamar N, Vernerey D, Viglietti D, Martinez F, Duong-Van-Huyen JP, et al. Long
term outcomes of transplantation using kidneys from expanded criteria donors: prospective,
population based cohort study. BMJ. 2015 Jul;351:h3557. 10.1136/bmj.h3557
9. Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, et al. Deceased-donor
characteristics and the survival benefit of kidney transplantation. JAMA. 2005 Dec;294(21):2726–33.
10.1001/jama.294.21.2726
10. Chavalitdhamrong D, Gill J, Takemoto S, Madhira BR, Cho YW, Shah T, et al. Patient
and graft outcomes from deceased kidney donors age 70 years and older: an analysis
of the Organ Procurement Transplant Network/United Network of Organ Sharing database.
Transplantation. 2008 Jun;85(11):1573–9. 10.1097/TP.0b013e31817059a1
11. Frei U, Noeldeke J, Machold-Fabrizii V, Arbogast H, Margreiter R, Fricke L, et al. Prospective
age-matching in elderly kidney transplant recipients—a 5-year analysis of the Eurotransplant
Senior Program. Am J Transplant. 2008 Jan;8(1):50–7. 10.1111/j.1600-6143.2007.02014.x
12. Peters-Sengers H, Berger SP, Heemskerk MB, Al Arashi D, Homan van der Heide JJ, Hemke AC,
et al. Stretching the Limits of Renal Transplantation in Elderly Recipients of Grafts
from Elderly Deceased Donors. J Am Soc Nephrol. 2017 Feb;28(2):621–31. 10.1681/ASN.2015080879
13. Querard AH, Le Borgne F, Dion A, Giral M, Mourad G, Garrigue V, et al. Propensity
score-based comparison of the graft failure risk between kidney transplant recipients
of standard and expanded criteria donor grafts: toward increasing the pool of marginal
donors. Am J Transplant. 2018 May;18(5):1151–7. 10.1111/ajt.14651
14. Echterdiek F, Schwenger V, Döhler B, Latus J, Kitterer D, Heemann U, et al. Kidneys
From Elderly Deceased Donors-Is 70 the New 60? Front Immunol. 2019 Nov;10:2701. 10.3389/fimmu.2019.02701
15. Arcos E, Pérez-Sáez MJ, Comas J, Lloveras J, Tort J, Pascual J; Catalan Renal Registry*.
Assessing the Limits in Kidney Transplantation: Use of Extremely Elderly Donors and
Outcomes in Elderly Recipients. Transplantation. 2020 Jan;104(1):176–83. 10.1097/TP.0000000000002748
16. Bielmann D, Hönger G, Lutz D, Mihatsch MJ, Steiger J, Schaub S. Pretransplant risk
assessment in renal allograft recipients using virtual crossmatching. Am J Transplant.
2007 Mar;7(3):626–32. 10.1111/j.1600-6143.2007.01667.x
17. Bächler K, Amico P, Hönger G, Bielmann D, Hopfer H, Mihatsch MJ, et al. Efficacy of
induction therapy with ATG and intravenous immunoglobulins in patients with low-level
donor-specific HLA-antibodies. Am J Transplant. 2010 May;10(5):1254–62. 10.1111/j.1600-6143.2010.03093.x
18. Amico P, Hirt-Minkowski P, Hönger G, Gürke L, Mihatsch MJ, Steiger J, et al. Risk
stratification by the virtual crossmatch: a prospective study in 233 renal transplantations.
Transpl Int. 2011 Jun;24(6):560–9. 10.1111/j.1432-2277.2011.01235.x
19. Wehmeier C, Hönger G, Cun H, Amico P, Hirt-Minkowski P, Georgalis A, et al. Donor
Specificity but Not Broadness of Sensitization Is Associated With Antibody-Mediated
Rejection and Graft Loss in Renal Allograft Recipients. Am J Transplant. 2017 Aug;17(8):2092–102.
10.1111/ajt.14247
20. Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al.; Banff meeting report
writing committee. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated
rejection and antibody-associated arterial lesions. Am J Transplant. 2014 Feb;14(2):272–83.
10.1111/ajt.12590
21. Loupy A, Haas M, Solez K, Racusen L, Glotz D, Seron D, et al. The Banff 2015 Kidney
Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting
Molecular Pathology. Am J Transplant. 2017 Jan;17(1):28–41. 10.1111/ajt.14107
22. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison
of mortality in all patients on dialysis, patients on dialysis awaiting transplantation,
and recipients of a first cadaveric transplant. N Engl J Med. 1999 Dec;341(23):1725–30.
10.1056/NEJM199912023412303
23. Fritsche L, Hörstrup J, Budde K, Reinke P, Giessing M, Tullius S, et al. Old-for-old
kidney allocation allows successful expansion of the donor and recipient pool. Am
J Transplant. 2003 Nov;3(11):1434–9. 10.1046/j.1600-6135.2003.00251.x
24. Giessing M, Budde K, Fritsche L, Slowinski T, Tuerk I, Schoenberger B, et al. “Old-for-old”
cadaveric renal transplantation: surgical findings, perioperative complications and
outcome. Eur Urol. 2003 Dec;44(6):701–8. 10.1016/s0302-2838(03)00380-4 10.1016/S0302-2838(03)00380-4
25. Moers C, Kornmann NS, Leuvenink HG, Ploeg RJ. The influence of deceased donor age
and old-for-old allocation on kidney transplant outcome. Transplantation. 2009 Aug;88(4):542–52.
10.1097/TP.0b013e3181b0fa8b
Appendix: supplementary
figures
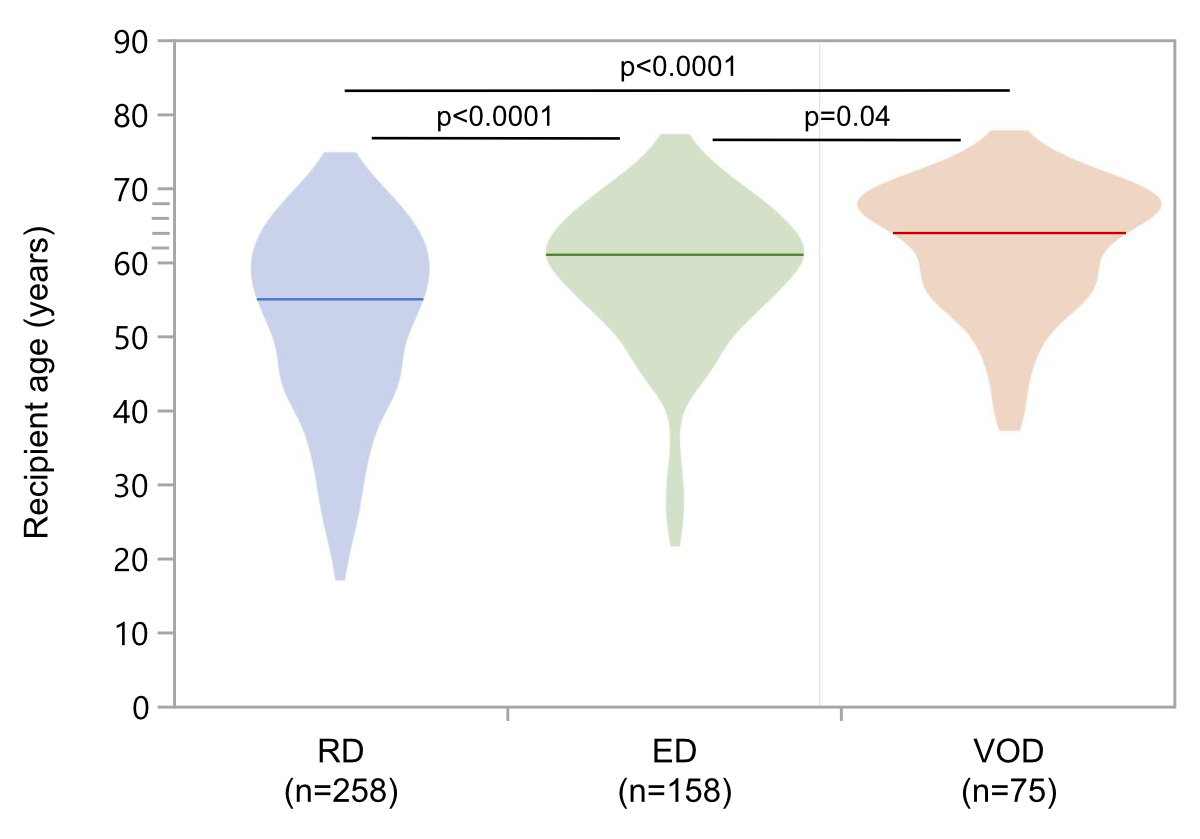
Figure S1At the time of
transplantation, recipients of very old donor grafts were older (VOD; median
age 64 years; IQR 57–69 years) compared to recipients of elderly donor grafts (ED;
median 61 years; IQR 54–66 years) and regular donor grafts (RD; median 55
years; IQR 44–62 years). IQR:
interquartile range
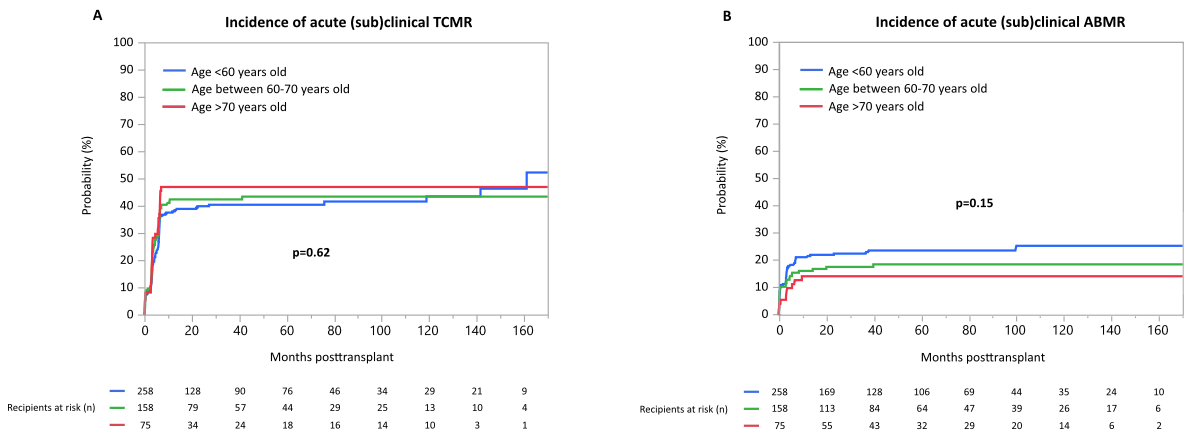
Figure S2(A) Incidence of acute subclinical T-cell-mediated rejection (TCMR), (B)
incidence of acute (subclinical) antibody-mediated rejection (ABMR) during
follow-up.






