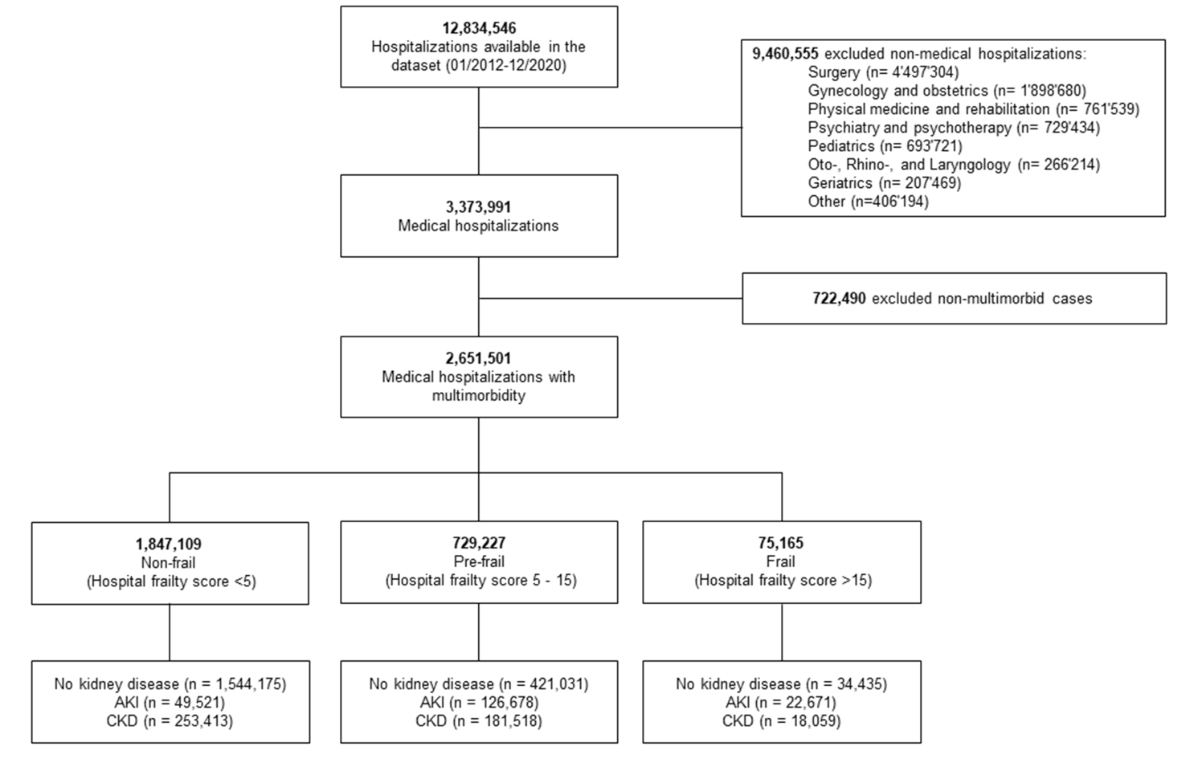
Figure 1Study flowchart.
DOI: https://doi.org/https://doi.org/10.57187/s.3400
Kidney diseases have a major impact on global health and are a clinical challenge for healthcare providers worldwide [1]. In 2019, kidney diseases reached the 10th spot in the top ten causes of death in the global health study [2]. Acute kidney injury (AKI) and chronic kidney disease (CKD) are common in the ageing patient population with multimorbidity presenting to medical wards.
In a previous observational cohort study, we analysed hospitalisations to define associations between multimorbidity (two or more chronic diseases present in the same individual) and in-hospital outcome measures [3, 4]. In a dataset of 2,220,000 records from hospitalisations with a mean age of 68 years, about 80% fulfilled the criteria of multimorbidity with a median of four chronic conditions. While the percentage of hospitalisations with multimorbidity increased over the recent years, it was also associated with a higher likelihood of in-hospital mortality and other outcome parameters [3].
Kidney diseases are caused by many chronic conditions like diabetes or diseases from the cardiovascular spectrum and mirror an additional critical parameter in the complexity of multimorbid patients. However, it is still unclear to what extent the prevalence of a kidney disease is associated with additional resource use in the acute care setting.
In many countries, reimbursement is based on diagnosis-related groups (DRG). While the major part of a case-specific reimbursement is based on the main diagnosis and procedures performed during hospitalisation, additional diagnoses may increase the case mix index and reimbursement. However, it is debated whether this increase in reimbursement is sufficient given the additional multimorbidity-associated complexity in diagnostic and therapeutic work-up.
The aim of this study was to assess whether prevalence of AKI or CKD in medical hospitalisations is associated with additional resource use and a higher risk of mortality when compared to those without kidney disease.
This was a nationwide retrospective observational cohort study in adult hospitalisations (≥18 years) hospitalised for a medical reason in Switzerland between 1 January 2012 and 31 December 2020. Hospitalisation data were obtained from population-based administrative claims data provided by the Federal Statistical Office (Bundesamt für Statistik, Neuchâtel, Switzerland). The database includes all Swiss hospitalisation records from acute care, general and specialty hospitals in Switzerland. Individual-level data on patient demographics, healthcare utilisation, hospital typology, medical diagnoses, diagnostic tests, clinical procedures and in-hospital patient outcomes were provided for all hospitalisations. The data were unidentifiable due to a multiple-step anonymisation procedure. Medical diagnoses were coded using International Classification of Diseases version 10, German Modification (ICD-10-GM) codes. While the coding process in Switzerland is primarily conducted for billing purposes, and the data may be susceptible to information bias, the definition of medical diagnoses is quite reliable. However, SwissDRG codes only one primary diagnosis, with the remaining diagnoses categorised as secondary. The order of these secondary diagnoses may not be specified. The ethical review board of Northwestern Switzerland (Ethikkommission Nordwest- und Zentralschweiz, EKNZ) declared that this study does not fall under the scope of the Human Research Act as data were anonymised before analysis (EKNZ Project-ID: Req-2021-01397). An authorisation from the ethical review board was therefore not required. A study protocol was not prepared for this analysis.
Eligible individuals included adult hospitalisations with underlying multimorbidity hospitalised for a medical condition. Hospitalisations with AKI were identified by applying the ICD-10-GM codes N17.0x, N17.1x, N17.2x, N17.8x, N17.9x and T86.10. AKI stages were not systematically recorded in the dataset before 2017 and were therefore not available before 1 January 2017. Hospitalisations with CKD were identified by applying the ICD-10-GM codes N18.1, N18.2, N18.3, N18.4, N18.5, N18.8x, N18.9, I12.0x, I13.1x, I13.2x, P96.0, T86.11, T86.12, T86.19 and Z94.0. Hospitalisations with codes for AKI and CKD were allocated to the AKI group. Acute-on-chronic kidney disease was categorised as an episode of AKI, based on the hypothesis that AKI may have a more significant effect on patient outcomes and hospital resources than CKD. Hospitalisations in need of dialysis were identified by applying the ICD-10-GM codes T82.4, T85.71, Z49.1, Z49.2 and Z99.2 and by applying the Swiss Operation Classification (CHOP) codes 38.95, 39.27, 39.42, 39.43, 39.95.1, 39.95.2, 39.95.3, 39.95.4, 39.95.I1, 39.95.I2 and 54.98. Hospitalisations in need of temporary haemodiafiltration were not included in the dialysis group. In prior investigations, the use of claims data to identify AKI has been validated against hospital records (with AKI defined as a ≥0.5-mg/dL increment of serum creatinine). It was found that an AKI discharge diagnosis in any position had a positive predictive value of 86% and a specificity of 97% [5]. For CKD, International Classification of Diseases (ICD)-10 based algorithms had a positive predictive value of >80% [6].
This study followed the Strengthening The Reporting of OBservational studies in Epidemiology (STROBE) reporting guideline [7].
Multimorbidity was defined according to the World Health Organization (WHO) as the presence of at least two chronic conditions [8]. We applied the “Chronic Condition Indicator (CCI) for the ICD-10-CM” to distinguish between chronic and acute conditions based on ICD-10 codes. The CCI was developed as part of the Healthcare Cost and Utilization Project, a Federal/State/Industry partnership sponsored by the Agency for Healthcare Research and Quality in the USA [9]. It was designed to facilitate healthcare research using administrative data. It is publicly accessible and undergoes annual updates. For this analysis, the 2019.1 version was used. The CCI divides all ICD-10-CM codes into two categories: chronic or acute. To qualify as chronic, a condition has to last 12 months or longer and meet at least one of the following criteria: placing limitations on self-care, independent living and social interactions, and/or need for ongoing intervention with medical products, services or special equipment [9]. In case of an inconsistency between the ICD-10 codes of the CCI with the American ICD-10 coding standard and the Swiss database records, we chose the most representative code with the highest similarity to merge the ICD-10 codes of the CCI with the ICD-10 codes of the database records.
Frailty was measured using a validated Hospital Frailty Risk Score derived from a broad set of ICD-10 codes, including measures of acuity. The Hospital Frailty Risk Score and its clinical application have been described in detail previously [10]. Using clinically meaningful cut points, we defined the status of non-frail as a score <5, pre-frail as a score of 5–15 and frail as a score >15.
To assess the overall burden of healthcare utilisation among this medical inpatient population with multimorbidity, we defined the following outcomes: Main primary outcomes were all-cause in-hospital mortality, intensive care unit (ICU) treatment, length of hospital stay (LOS) and 30-day hospital readmission. Secondary outcomes encompassed length of ICU stay, need for mechanical ventilation, post-acute care facility discharge and 1-year hospital readmission. All outcome analyses were conducted at discharge record level. There were no missing data for the outcomes of interest.
Descriptive statistics were calculated by the presence of kidney disease for demographic information including age, sex, nationality, level of health care insurance, year of index admission, comorbidities and level of frailty. All baseline data are expressed as mean (standard deviation [SD], measuring the dispersion of a dataset relative to its mean, indicating how spread out the data points are), median (interquartile range [IQR]) or count (%).
Associations between the presence of kidney disease and binary outcomes were estimated using a multivariable logistic regression model adjusted for age, sex, level of healthcare insurance, year of admission, modality of admission (emergency versus planned) and level of hospital care. While LOS was right-skewed, we performed a multivariable generalised linear gamma regression analysis based on log-transformed values. In addition, we excluded hospitalisations with a LOS ≥100 days. Moreover, graphical illustration of odds ratios along increasing numbers of chronic conditions was performed using linear basis spline constructions (B-splines). B-splines are a type of smooth curve-fitting technique used to model and visualise trends across the number of chronic conditions, allowing for flexible data approximation and smoothness by combining several polynomial segments [11]. All analyses were performed within each frailty stratum. We used the Wald test for homogeneity to assess treatment heterogeneity across strata of AKI, CKD and frailty. Stratified analyses by strata of AKI were performed with data after 2016 only. Among hospitalisations for AKI, including those with acute-on-chronic kidney disease, outcomes were categorised solely by AKI stage and not further by CKD stages, as we assume CKD prevalence within the AKI group might be significantly underestimated. All tests were 2-sided, p<0.05 was considered significant and 95% confidence intervals (CIs) were reported for all effect estimates. Our analysis has not been adjusted for multiple testing. All statistical analyses were performed using STATA, version 17.1 (StataCorp LLC).
From 1 January 2012 to 31 December 2020, we identified a total of 2,651,501 medical hospitalisations meeting our inclusion criteria of multimorbidity (figure 1).

Figure 1Study flowchart.
Baseline characteristics stratified by hospitalisations without kidney disease (n = 1,999,641; 75.4%), AKI (n = 198,870; 7.5%) and CKD (n = 452,990; 17.1%) are illustrated in table 1. Baseline characteristics stratified by level of frailty are applicable in the supplemental tables (tables S1 to S3). While hospitalisations without kidney disease had a median age of 71 years (IQR 59–80 years), hospitalisations with AKI or CKD were older with a median age of 78 years (IQR 69–85 years) and 81 years (IQR 73–86 years), respectively. The most prevalent comorbidities across all groups were hypertension (no kidney disease: 49.6%; AKI: 65.7%; CKD: 73.1%), coronary artery disease (no kidney disease: 25.3%; AKI: 29.5%; CKD: 35.9%) and gastrointestinal disorder (no kidney disease: 23.9%; AKI: 36.4%; CKD: 26.9%). Overall, when compared to hospitalisations without kidney disease (2.58 ± 1.61), those with AKI and CKD were sicker with a mean Elixhauser comorbidity index of 4.58 ± 2.10 and 4.51 ± 1.81. A relevant number of hospitalisations had six or more chronic conditions (no kidney disease: 27.7%; AKI: 61.3%; CKD: 66.6%).
Table 1Baseline characteristics among hospitalisations with AKI and CKD hospitalisations.
| Overall | No kidney disease | AKI | CKD | |
| Hospitalisations, n | 2,651,501 | 1,999,641 | 198,870 | 452,990 |
| Patients, n | 1,277,977 | 1,101,521 | 152,840 | 234,893 |
| Demographics | ||||
| Age, median (IQR) [years] | 73 (62–82) | 71 (59–80) | 78 (69–85) | 81 (73–86) |
| Male sex, n (%) | 1,412,459 (53.3) | 1,062,210 (53.1) | 110,326 (55.5) | 239,923 (53.0) |
| Swiss nationality, n (%) | 2,214,964 (83.5) | 1,654,471 (82.7) | 168,364 (84.7) | 392,129 (86.6) |
| Supplementary insurance, n (%) | 624,778 (23.6) | 472,924 (23.7) | 40,068 (20.1) | 111,786 (24.7) |
| Admission data | ||||
| Year of admission, n (%) | ||||
| 2012 | 258,185 (9.7) | 208,566 (10.4) | 7613 (3.8) | 42,006 (9.3) |
| 2013 | 266,840 (10.1) | 215,319 (10.8) | 5939 (3.0) | 45,582 (10.1) |
| 2014 | 278,746 (10.5) | 222,293 (11.1) | 5859 (2.9) | 50,594 (11.2) |
| 2015 | 290,680 (11.0) | 230,631 (11.5) | 6064 (3.0) | 53,985 (11.9) |
| 2016 | 300,982 (11.4) | 236,936 (11.8) | 5829 (2.9) | 58,217 (12.9) |
| 2017 | 305,059 (11.5) | 220,105 (11.0) | 37,897 (19.1) | 47,057 (10.4) |
| 2018 | 313,796 (11.8) | 221,124 (11.1) | 42,474 (21.4) | 50,198 (11.1) |
| 2019 | 323,664 (12.2) | 226,806 (11.3) | 43,526 (21.9) | 53,332 (11.8) |
| 2020 | 313,549 (11.8) | 217,861 (10.9) | 43,669 (22.0) | 52,019 (11.5) |
| Emergency admission, n (%) | 1,922,255 (72.5) | 1,398,651 (69.9) | 173,174 (87.1) | 350,430 (77.4) |
| Admission from home, n (%) | 2,258,473 (85.2) | 1,723,173 (86.2) | 158,344 (79.6) | 376,956 (83.2) |
| Tertiary care hospital: University hospital | 478,318 (18.0) | 367,940 (18.4) | 39,045 (19.6) | 71,333 (15.7) |
| Tertiary care hospital: non-university hospital | 1,566,306 (59.1) | 1,159,366 (58.0) | 124,827 (62.8) | 282,113 (62.3) |
| Secondary care hospital | 606,877 (22.9) | 472,335 (23.6) | 34,998 (17.6) | 99,544 (22.0) |
| Comorbidities, n (%) | ||||
| Hypertension | 1,453,650 (54.8) | 991,661 (49.6) | 130,732 (65.7) | 331,257 (73.1) |
| Obesity | 72,909 (2.7) | 51,225 (2.6) | 6923 (3.5) | 14,761 (3.3) |
| Type 2 diabetes mellitus | 515,830 (19.5) | 318,939 (15.9) | 58,122 (29.2) | 138,769 (30.6) |
| Type 1 diabetes mellitus | 15,840 (0.6) | 11,156 (0.6) | 1369 (0.7) | 3315 (0.7) |
| Dyslipidaemia | 556,385 (21.0) | 412,191 (20.6) | 40,151 (20.2) | 104,043 (23.0) |
| Coronary artery disease | 727,327 (27.4) | 505,823 (25.3) | 58,679 (29.5) | 162,825 (35.9) |
| Myocardial infarction | 158,508 (6.0) | 123,868 (6.2) | 12,206 (6.1) | 22,434 (5.0) |
| Congestive heart failure | 414,812 (15.6) | 216,165 (10.8) | 64,255 (32.3) | 134,392 (29.7) |
| Atrial fibrillation | 545,605 (20.6) | 326,422 (16.3) | 66,831 (33.6) | 152,352 (33.6) |
| Peripheral arterial disease | 143,042 (5.4) | 81,018 (4.1) | 15,011 (7.5) | 47,013 (10.4) |
| Obstructive sleep apnoea syndrome | 91,480 (3.5) | 62,760 (3.1) | 9523 (4.8) | 19,197 (4.2) |
| Cerebrovascular disease | 236,695 (8.9) | 184,097 (9.2) | 13,349 (6.7) | 39,249 (8.7) |
| Ischaemic stroke | 114,915 (4.3) | 95,845 (4.8) | 4524 (2.3) | 14,546 (3.2) |
| Chronic obstructive pulmonary disease | 264,633 (10.0) | 186,241 (9.3) | 24,302 (12.2) | 54,090 (11.9) |
| Gastrointestinal disorder | 672,853 (25.4) | 478,554 (23.9) | 72,302 (36.4) | 121,997 (26.9) |
| Solid cancer | 383,094 (14.4) | 313,986 (15.7) | 25,933 (13.0) | 43,175 (9.5) |
| Haematological malignancy | 89,766 (3.4) | 67,981 (3.4) | 8130 (4.1) | 13,655 (3.0) |
| Musculoskeletal disorder | 584,780 (22.1) | 401,060 (20.1) | 54,954 (27.6) | 128,766 (28.4) |
| Mental disorder | 784,397 (29.6) | 596,705 (29.8) | 68,772 (34.6) | 118,920 (26.3) |
| Alcohol addiction | 181,441 (6.8) | 152,065 (7.6) | 16,065 (8.1) | 13,311 (2.9) |
| Elixhauser comorbidity index, mean (SD) | 3.06 (1.88) | 2.58 (1.61) | 4.58 (2.10) | 4.51 (1.81) |
| Hospital frailty score, n (%) | ||||
| <5 points | 1,847,109 (69.7) | 1,544,175 (77.2) | 49,521 (24.9) | 253,413 (55.9) |
| 5-15 points | 729,227 (27.5) | 421,031 (21.1) | 126,678 (63.7) | 181,518 (40.1) |
| >15 points | 75,165 (2.8) | 34,435 (1.7) | 22,671 (11.4) | 18,059 (4.0) |
| Number of chronic comorbidities, n (%) | ||||
| 2 | 443,338 (16.7) | 415,325 (20.8) | 13,362 (6.7) | 14,651 (3.2) |
| 3 | 451,620 (17.0) | 402,820 (20.1) | 18,202 (9.2) | 30,598 (6.8) |
| 4 | 422,380 (15.9) | 352,964 (17.7) | 21,983 (11.1) | 47,433 (10.5) |
| 5 | 356,980 (13.5) | 274,949 (13.7) | 23,338 (11.7) | 58,693 (13.0) |
| ≥6 | 977,183 (36.9) | 553,583 (27.7) | 121,985 (61.3) | 301,615 (66.6) |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; IQR, interquartile range; SD, standard deviation.
Crude patient outcomes stratified by the presence of AKI and CKD according to level of frailty are illustrated in table 2. In general, the number of events among hospitalisations with AKI and CKD was higher than in those without kidney disease. Moreover, except for hospital readmissions, number of adverse events was also increasing with the severity of frailty. Risk of 30-day and 1-year readmission was highest among non-frail hospitalisations and declined with increasing frailty.
Table 2Crude outcomes among hospitalisations with AKI and CKD hospitalisations.
| Overall | Non-frail | Pre-frail | Frail | |
| No kidney disease | ||||
| Hospitalisation, n | 1,999,641 | 1,544,175 | 421,031 | 34,435 |
| In-hospital mortality, n (%) | 88,523 (4.4) | 56,943 (3.7) | 28,834 (6.8) | 2746 (8.0) |
| ICU stay, n (%) | 209,257 (10.5) | 143,192 (9.3) | 59,682 (14.2) | 6383 (18.5) |
| ICU LOS, median (IQR) [days] | 1.2 (0.8–2.5) | 1.0 (0.8–1.9) | 1.9 (1.0–4.0) | 3.0 (1.3–7.5) |
| Need for intubation among ICU hospitalisations, n (%) | 52,917 (25.3) | 28,100 (19.6) | 21,920 (36.7) | 2897 (45.4) |
| Hospital LOS, median (IQR) [days] | 5.0 (2.0–9.0) | 4.0 (2.0–8.0) | 8.0 (5.0–14.0) | 12.0 (7.0–19.0) |
| Facility discharge, n (%)* | 463,987 (24.3) | 279,249 (18.8) | 164,872 (42.0) | 19,866 (62.7) |
| 30-day all-cause rehospitalisation, n (%)* | 254,024 (13.3) | 207,150 (13.9) | 44,104 (11.2) | 2770 (8.7) |
| 1-year all-cause rehospitalisation, n (%)* | 689,280 (36.1) | 554,842 (37.3) | 126,750 (32.3) | 7688 (24.3) |
| AKI** | ||||
| Hospitalisation, n | 184,755 | 47,083 | 117,119 | 20,553 |
| In-hospital mortality, n (%) | 26,596 (14.4) | 6050 (12.8) | 17,367 (14.8) | 3179 (15.5) |
| ICU stay, n (%) | 40,365 (21.8) | 7531 (16.0) | 26,837 (22.9) | 5997 (29.2) |
| ICU LOS, median (IQR) [days] | 2.8 (1.2–6.1) | 1.8 (0.9–3.5) | 2.8 (1.3–6.2) | 4.7 (2.0–11.6) |
| Need for intubation among ICU hospitalisations, n (%) | 17,735 (43.9) | 2408 (32.0) | 12,137 (45.2) | 3190 (53.2) |
| Hospital LOS, median (IQR) [days] | 9.0 (5.0–15.0) | 7.0 (4.0–11.0) | 10.0 (6.0–16.0) | 14.0 (9.0–24.0) |
| Facility discharge, n (%)* | 59,843 (37.8) | 9822 (23.9) | 39,231 (39.3) | 10,790 (62.1) |
| 30-day all-cause rehospitalisation, n (%)* | 21,737 (13.7) | 6097 (14.9) | 13,662 (13.7) | 1978 (11.4) |
| 1-year all-cause rehospitalisation, n (%)* | 49,101 (31.0) | 14,159 (34.5) | 30,908 (31.0) | 4034 (23.2) |
| CKD** | ||||
| Hospitalisation, n | 395,665 | 222,317 | 158,191 | 15,157 |
| In-hospital mortality, n (%) | 23,376 (5.9) | 9960 (4.5) | 11,899 (7.5) | 1517 (10.0) |
| ICU stay, n (%) | 36,815 (9.3) | 16,741 (7.5) | 17,643 (11.2) | 2431 (16.0) |
| ICU LOS, median (IQR) [days] | 1.6 (0.8–3.0) | 1.1 (0.7–2.0) | 1.8 (1.0–3.8) | 2.8 (1.2–6.4) |
| Need for intubation among ICU hospitalisations, n (%) | 10,225 (27.8) | 3229 (19.3) | 6023 (34.1) | 973 (40.0) |
| Hospital LOS, median (IQR) [days] | 7.0 (4.0–12.0) | 6.0 (3.0–9.0) | 9.0 (5.0–14.0) | 12.0 (7.0–19.0) |
| Facility discharge, n (%)* | 101,691 (27.3) | 39,853 (18.8) | 54,115 (37.0) | 7723 (56.6) |
| 30-day all-cause rehospitalisation, n (%)* | 54,976 (14.8) | 32,416 (15.3) | 20,982 (14.3) | 1578 (11.6) |
| 1-year all-cause rehospitalisation, n (%)* | 163,308 (43.9) | 99,359 (46.8) | 59,766 (40.9) | 4183 (30.7) |
* Patients who died during the hospitalisation were excluded from the calculation regarding facility discharge, 30-day and 1-year all-cause rehospitalisation. ** CKD stages 1 and 2 were not included in the calculation due to the high likelihood of underreporting using administrative data. Calculations refer to more clinically relevant stages (stages 3 to 5, dialysis and unspecified CKD stage). Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay.
More severe AKI stages were associated with a stepwise increase in relative risk of in-hospital mortality, ICU admission, need for intubation and discharge to a post-acute care facility (p for heterogeneity <0.001 for all variables). Similarly, we observed a longer length of ICU stay and hospital stay with more severe AKI stages (p for heterogeneity <0.001 for all variables). In more-frail people, however, the association of AKI and adverse outcome was attenuated. This was most obvious for the association of AKI stage 3 and in-hospital mortality (aOR of 9.92 among non-frail hospitalisations vs 3.52 among frail hospitalisations). Readmission rates did not relevantly change with more severe AKI stages or frailty levels. All results are summarised in table 3.
Table 3Multivariable regression analyses of primary and secondary outcomes among hospitalisations with AKI, according to level of frailty.
| Overall | Non-frail | Pre-frail | Frail | p of heterogeneity | |
| In-hospital mortality: OR (95% CI)* | |||||
| AKI overall | 2.56 (2.52–2.61) | 2.45 (2.36–2.54) | 1.88 (1.83–1.93) | 1.93 (1.80–2.07) | <0.001 |
| AKI, stage 1 | 1.45 (1.41–1.49) | 1.22 (1.15–1.30) | 1.10 (1.06–1.14) | 1.32 (1.21–1.45) | <0.001 |
| AKI, stage 2 | 3.24 (3.13–3.36) | 3.87 (3.58–4.18) | 2.22 (2.12–2.32) | 2.28 (2.06–2.53) | <0.001 |
| AKI, stage 3 | 7.38 (7.15–7.61) | 9.92 (9.26–10.62) | 4.95 (4.76–5.15) | 3.52 (3.19–3.87) | <0.001 |
| AKI, unspecified | 2.17 (2.09–2.25) | 2.23 (2.06–2.40) | 1.61 (1.53–1.69) | 1.66 (1.47–1.86) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| ICU stay: OR (95% CI)* | |||||
| AKI overall | 2.39 (2.36–2.43) | 1.73 (1.67–1.78) | 1.84 (1.80–1.87) | 1.95 (1.84–2.06) | <0.001 |
| AKI, stage 1 | 1.79 (1.75–1.82) | 1.53 (1.47–1.59) | 1.37 (1.33–1.41) | 1.48 (1.38–1.59) | <0.001 |
| AKI, stage 2 | 2.80 (2.72–2.89) | 1.92 (1.78–2.06) | 2.07 (2.00–2.16) | 2.19 (2.01–2.39) | 0.003 |
| AKI, stage 3 | 5.86 (5.70–6.02) | 3.35 (3.13–3.59) | 4.23 (4.08–4.38) | 4.15 (3.82–4.51) | 0.133 |
| AKI, unspecified | 1.43 (1.38–1.48) | 1.32 (1.23–1.42) | 1.10 (1.05–1.15) | 1.01 (0.90–1.12) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| ICU LOS: change in % (95% CI)** | |||||
| AKI overall | 111.71 (106.97–116.55) | 61.00 (55.03–67.19) | 35.91 (32.28–39.64) | 35.15 (26.34–44.57) | <0.001 |
| AKI, stage 1 | 52.36 (47.46– 57.43) | 29.60 (23.00–36.56) | 3.83 (0.07–7.74) | 3.17 (-5.33–12.43) | <0.001 |
| AKI, stage 2 | 104.11 (95.16–113.48) | 71.60 (56.57–88.08) | 31.49 (25.38–37.89) | 21.22 (9.66–34.00) | 0.255 |
| AKI, stage 3 | 211.04 (199.86–222.64) | 152.49 (132.83–173.82) | 83.67 (76.59–91.02) | 81.32 (66.51–97.45) | <0.001 |
| AKI, unspecified | 64.16 (55.08–73.78) | 33.25 (21.80–45.79) | 13.39 (6.59–20.63) | 11.66 (-2.89–28.40) | 0.119 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| Need for intubation: OR (95% CI)* | |||||
| AKI overall | 2.22 (2.16–2.28) | 1.87 (1.75–1.99) | 1.34 (1.29–1.39) | 1.31 (1.19–1.44) | <0.001 |
| AKI, stage 1 | 1.71 (1.64–1.78) | 1.40 (1.28–1.54) | 1.10 (1.04–1.16) | 1.10 (0.96–1.24) | <0.001 |
| AKI, stage 2 | 2.16 (2.05–2.29) | 2.06 (1.78–2.39) | 1.24 (1.16–1.33) | 1.25 (1.08–1.45) | <0.001 |
| AKI, stage 3 | 3.20 (3.06–3.35) | 3.13 (2.76–3.53) | 1.78 (1.68–1.88) | 1.67 (1.47–1.89) | <0.001 |
| AKI, unspecified | 1.97 (1.83–2.11) | 1.87 (1.61–2.17) | 1.26 (1.15–1.38) | 1.12 (0.91–1.37) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| LOS: change in % (95% CI)** | |||||
| AKI overall | 67.13 (66.18–68.08) | 44.91 (43.33–46.51) | 19.00 (18.25–19.75) | 24.62 (22.71–26.55) | 0.001 |
| AKI, stage 1 | 52.07 (50.91–53.24) | 38.61 (36.60–40.65) | 11.34 (10.44–12.24) | 14.95 (12.76–17.18) | <0.001 |
| AKI, stage 2 | 84.41 (82.08–86.78) | 62.31 (57.75–67.01) | 27.29 (25.71–28.90) | 31.87 (28.42–35.42) | 0.591 |
| AKI, stage 3 | 119.41 (116.48–122.39) | 82.12 (76.41–88.01) | 43.00 (41.13–44.89) | 47.91 (43.94–51.99) | <0.001 |
| AKI, unspecified | 48.98 (47.19–50.79) | 29.60 (26.59–32.68) | 10.13 (8.78–11.50) | 17.37 (14.21–20.61) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| Facility discharge: OR (95% CI)* | |||||
| AKI overall | 1.48 (1.46–1.50) | 1.15 (1.12–1.18) | 0.79 (0.77–0.80) | 0.93 (0.89–0.97) | <0.001 |
| AKI, stage 1 | 1.30 (1.28–1.32) | 1.03 (0.99–1.06) | 0.72 (0.70–0.73) | 0.87 (0.82–0.92) | <0.001 |
| AKI, stage 2 | 1.72 (1.68–1.77) | 1.35 (1.26–1.44) | 0.88 (0.85–0.91) | 1.03 (0.95–1.12) | <0.001 |
| AKI, stage 3 | 2.26 (2.19–2.33) | 1.82 (1.69–1.96) | 1.11 (1.07–1.15) | 1.11 (1.02–1.21) | <0.001 |
| AKI, unspecified | 1.35 (1.32–1.39) | 1.11 (1.06–1.18) | 0.72 (0.70–0.74) | 0.85 (0.79–0.92) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| 30-day rehospitalisation: OR (95% CI)* | |||||
| AKI overall | 1.21 (1.19–1.23) | 1.23 (1.19–1.27) | 1.29 (1.26–1.32) | 1.37 (1.27–1.47) | 0.065 |
| AKI, stage 1 | 1.22 (1.19–1.25) | 1.25 (1.20–1.30) | 1.29 (1.25–1.33) | 1.39 (1.27–1.52) | 0.261 |
| AKI, stage 2 | 1.27 (1.22–1.31) | 1.26 (1.17–1.37) | 1.37 (1.30–1.43) | 1.38 (1.22–1.56) | 0.415 |
| AKI, stage 3 | 1.19 (1.14–1.24) | 1.26 (1.15–1.39) | 1.25 (1.19–1.32) | 1.36 (1.20–1.54) | 0.929 |
| AKI, unspecified | 1.15 (1.11–1.20) | 1.14 (1.06–1.21) | 1.26 (1.20–1.32) | 1.31 (1.16–1.49) | 0.108 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| 1-year rehospitalisation: OR (95% CI)* | |||||
| AKI overall | 1.15 (1.13–1.16) | 1.23 (1.20–1.26) | 1.29 (1.27–1.32) | 1.26 (1.19–1.34) | 0.100 |
| AKI, stage 1 | 1.20 (1.18–1.23) | 1.26 (1.22–1.30) | 1.35 (1.32–1.39) | 1.34 (1.25–1.44) | 0.703 |
| AKI, stage 2 | 1.12 (1.08–1.15) | 1.20 (1.13–1.28) | 1.25 (1.21–1.30) | 1.25 (1.13–1.38) | 0.625 |
| AKI, stage 3 | 0.99 (0.96–1.03) | 1.17 (1.08–1.26) | 1.09 (1.04–1.14) | 1.09 (0.98–1.21) | 0.021 |
| AKI, unspecified | 1.14 (1.11–1.17) | 1.20 (1.14–1.27) | 1.31 (1.27–1.36) | 1.25 (1.13–1.38) | 0.217 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
Results for different AKI stages are based on data from 2017–2020 only, as AKI stages were not available (coding issue) in the dataset before 2017. Hospitalisations without kidney diseases served as the reference. Patients who died during the hospitalisation were excluded from the calculations for facility discharge, 30-day and 1-year all-cause rehospitalisation. * OR for binary outcomes calculated by a multivariable logistic model adjusted for age, sex, level of healthcare insurance, year of admission, month of admission, modality of admission (emergency vs planned) and level of hospital care; ** Changes in % for continuous outcomes calculated by a multivariable generalised linear gamma regression based on log-transformed values adjusted for age, sex, level of healthcare insurance, year of admission, month of admission, modality of admission (emergency vs planned) and level of hospital care. Abbreviations: AKI, acute kidney disease; CI, confidence interval; ICU, intensive care unit; LOS, length of stay; OR, odds ratio.
The multivariable regression models stratified by CKD stage (table 4) illustrate a more heterogeneous picture compared to hospitalisations with AKI. In general, more advanced CKD stages were associated with a stepwise increase in relative risk of in-hospital mortality, ICU admission and hospital readmission (p for heterogeneity <0.001, for all variables). In addition, more advanced CKD stages were associated with prolonged LOS and, mainly in individuals in need of dialysis, ICU LOS (p for heterogeneity <0.001, for all variables). The findings for the remaining outcomes did not consistently show a CKD stage-dependent increase in relative risk. More severe levels of frailty did not modify relative risk estimates in a clinically meaningful manner.
Table 4Multivariable regression analyses of primary and secondary outcomes among hospitalisations with CKD, according to level of frailty.
| Overall | Non-frail | Pre-frail | Frail | p of heterogeneity | |
| In-hospital mortality: OR (95% CI) | |||||
| CKD overall | 0.98 (0.96–0.99) | 0.86 (0.84–0.88) | 0.95 (0.93–0.97) | 1.20 (1.12–1.28) | 0.123 |
| CKD, stage 3 | 0.76 (0.74–0.78) | 0.64 (0.62–0.65) | 0.77 (0.75–0.79) | 1.04 (0.96–1.13) | <0.001 |
| CKD, stage 4 | 1.38 (1.34–1.42) | 1.31 (1.25–1.36) | 1.29 (1.23–1.34) | 1.55 (1.37–1.77) | <0.001 |
| CKD, stage 5 | 2.95 (2.80–3.09) | 3.06 (2.86–3.27) | 2.45 (2.27–2.64) | 2.86 (2.20–3.73) | <0.001 |
| Dialysis | 1.55 (1.48–1.61) | 1.14 (1.07–1.22) | 1.49 (1.41–1.58) | 2.94 (2.44–3.55) | <0.001 |
| CKD, unspecified | 1.49 (1.42–1.56) | 1.40 (1.30–1.50) | 1.37 (1.28–1.46) | 1.57 (1.28–1.93) | 0.009 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| ICU stay: OR (95% CI)* | |||||
| CKD overall | 1.01 (1.00–1.02) | 0.90 (0.88–0.91) | 0.95 (0.93–0.96) | 1.11 (1.05–1.17) | <0.001 |
| CKD, stage 3 | 0.91 (0.89–0.92) | 0.83 (0.81–0.85) | 0.85 (0.83– 0.87) | 1.02 (0.96– 1.09) | <0.001 |
| CKD, stage 4 | 0.90 (0.87–0.92) | 0.77 (0.74–0.81) | 0.87 (0.83–0.90) | 1.01 (0.90–1.14) | 0.001 |
| CKD, stage 5 | 1.21 (1.15–1.28) | 1.23 (1.14–1.31) | 0.98 (0.90–1.06) | 1.31 (1.01–1.71) | <0.001 |
| Dialysis | 1.80 (1.75–1.85) | 1.39 (1.34–1.45) | 1.74 (1.67–1.82) | 2.68 (2.27–3.15) | <0.001 |
| CKD, unspecified | 1.26 (1.21–1.32) | 1.20 (1.13–1.27) | 1.09 (1.02–1.16) | 1.19 (1.00–1.42) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | 0.338 | |
| ICU LOS: change in % (95% CI)** | |||||
| CKD overall | 18.72 (16.47–21.01) | 9.25 (6.92–11.62) | -4.57 (-6.86 – -2.21) | 0.13 (-6.78 – 7.54) | <0.001 |
| CKD, stage 3 | 5.99 (3.30–8.74) | 0.21 (-2.65 – 3.15) | -12.33 (-15.11 – -9.45) | -11.09 (-18.83 – -2.61) | <0.001 |
| CKD, stage 4 | 17.06 (11.45–22.95) | 13.45 (6.94–20.35) | -6.99 (-12.19 – -1.49) | -2.08 (-17.55 – 16.30) | <0.001 |
| CKD, stage 5 | -0.72 (-8.96 – 8.26) | -6.65 (-14.78 – 2.26) | -12.48 (-21.88 – -1.96) | -19.03 (-42.80 – 14.60) | 0.002 |
| Dialysis | 68.81 (61.23–76.75) | 44.34 (36.80–52.29) | 24.37 (17.77–31.34) | 61.48 (35.51–92.43) | <0.001 |
| CKD, unspecified | 30.04 (21.37–39.32) | 24.06 (14.77–34.10) | 1.38 (-6.87 – 10.37) | 27.93 (0.20–63.34) | 0.017 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | 0.118 | |
| Need for intubation: OR (95% CI)* | |||||
| CKD overall | 1.19 (1.16–1.22) | 1.03 (0.99–1.07) | 0.99 (0.96–1.03) | 0.95 (0.86–1.05) | <0.001 |
| CKD, stage 3 | 1.11 (1.07–1.15) | 0.94 (0.89–0.99) | 0.97 (0.92–1.01) | 0.96 (0.85–1.08) | <0.001 |
| CKD, stage 4 | 1.26 (1.19–1.34) | 1.19 (1.07–1.32) | 1.01 (0.93–1.10) | 0.92 (0.73–1.16) | <0.001 |
| CKD, stage 5 | 0.83 (0.73–0.93) | 0.70 (0.58–0.84) | 0.77 (0.65–0.91) | 0.84 (0.52–1.34) | 0.604 |
| Dialysis | 1.37 (1.30–1.45) | 1.12 (1.02–1.23) | 1.04 (0.96–1.12) | 1.23 (0.97–1.56) | 0.957 |
| CKD, unspecified | 1.61 (1.48–1.75) | 1.52 (1.33–1.73) | 1.28 (1.13–1.44) | 1.39 (1.01–1.92) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | 0.013 | 0.011 | |
| LOS: change in % (95% CI)** | |||||
| CKD overall | 18.94 (18.52–19.36) | 13.22 (12.70–13.75) | 3.97 (3.49–4.46) | 6.83 (5.35–8.34) | <0.001 |
| CKD, stage 3 | 13.56 (13.06–14.07) | 9.64 (8.99–10.29) | 0.29 (-0.28 – 0.87) | 2.55 (0.84–4.29) | <0.001 |
| CKD, stage 4 | 25.28 (24.23–26.34) | 22.90 (21.48–24.34) | 7.65 (6.57–8.75) | 12.65 (9.20–16.20) | <0.001 |
| CKD, stage 5 | 18.59 (16.52–20.70) | 11.22 (8.70–13.79) | 6.67 (4.28–9.13) | 14.23 (5.39–23.80) | 0.145 |
| Dialysis | 46.43 (44.80–48.08) | 27.76 (25.95–29.59) | 28.35 (26.47–30.26) | 56.46 (47.50–65.97) | <0.001 |
| CKD, unspecified | 26.41 (24.66–28.19) | 20.31 (18.04–22.62) | 7.23 (5.41–9.07) | 15.62 (9.98–21.54) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| Facility discharge: OR (95% CI)* | |||||
| CKD overall | 0.96 (0.95–0.96) | 0.89 (0.88–0.90) | 0.71 (0.70–0.72) | 0.78 (0.75–0.82) | <0.001 |
| CKD, stage 3 | 0.93 (0.93–0.94) | 0.88 (0.86–0.89) | 0.71 (0.70–0.72) | 0.77 (0.74–0.81) | <0.001 |
| CKD, stage 4 | 1.05 (1.04–1.07) | 1.05 (1.02–1.08) | 0.74 (0.73–0.76) | 0.79 (0.72–0.86) | <0.001 |
| CKD, stage 5 | 1.01 (0.97–1.05) | 0.99 (0.93–1.05) | 0.74 (0.70–0.79) | 0.78 (0.62–1.00) | <0.001 |
| Dialysis | 0.80 (0.77–0.82) | 0.74 (0.72–0.77) | 0.58 (0.55–0.60) | 0.67 (0.56–0.80) | <0.001 |
| CKD, unspecified | 1.28 (1.24–1.32) | 1.23 (1.17–1.28) | 0.90 (0.86–0.94) | 1.09 (0.94–1.27) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| 30-day rehospitalisation: OR (95% CI)* | |||||
| CKD overall | 1.26 (1.25–1.28) | 1.21 (1.19–1.22) | 1.44 (1.41–1.46) | 1.42 (1.34–1.52) | <0.001 |
| CKD, stage 3 | 1.18 (1.17–1.20) | 1.13 (1.11–1.15) | 1.34 (1.32–1.37) | 1.40 (1.30–1.51) | <0.001 |
| CKD, stage 4 | 1.39 (1.36–1.43) | 1.33 (1.29–1.37) | 1.60 (1.54–1.65) | 1.58 (1.39–1.81) | <0.001 |
| CKD, stage 5 | 1.65 (1.57–1.72) | 1.55 (1.46–1.64) | 1.95 (1.80–2.10) | 1.70 (1.21–2.39) | <0.001 |
| Dialysis | 1.74 (1.69–1.79) | 1.63 (1.57–1.68) | 2.04 (1.95–2.14) | 2.13 (1.70–2.67) | <0.001 |
| CKD, unspecified | 1.08 (1.03–1.12) | 1.07 (1.01–1.12) | 1.21 (1.13–1.29) | 0.97 (0.75–1.25) | 0.710 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
| 1-year rehospitalisation: OR (95% CI)* | |||||
| CKD overall | 1.45 (1.44–1.46) | 1.48 (1.47–1.49) | 1.53 (1.52–1.55) | 1.39 (1.33–1.45) | <0.001 |
| CKD, stage 3 | 1.36 (1.34–1.37) | 1.36 (1.34–1.38) | 1.47 (1.45–1.50) | 1.38 (1.31–1.46) | 0.102 |
| CKD, stage 4 | 1.55 (1.52–1.58) | 1.63 (1.59–1.67) | 1.61 (1.57–1.66) | 1.56 (1.42–1.71) | <0.001 |
| CKD, stage 5 | 1.94 (1.87–2.01) | 2.09 (1.99–2.19) | 1.86 (1.75–1.98) | 1.58 (1.23–2.03) | <0.001 |
| Dialysis | 2.57 (2.51–2.63) | 2.68 (2.61–2.76) | 2.55 (2.46–2.66) | 1.93 (1.61–2.32) | 0.001 |
| CKD, unspecified | 1.11 (1.08–1.14) | 1.20 (1.16–1.25) | 1.14 (1.09–1.20) | 0.78 (0.66–0.92) | <0.001 |
| p of heterogeneity | <0.001 | <0.001 | <0.001 | <0.001 | |
Hospitalisations without kidney diseases served as the reference. Patients who died during the hospitalisation were excluded from the calculations for facility discharge, 30-day and 1-year all-cause rehospitalisation. * OR for binary outcomes calculated by a multivariable logistic model adjusted for age, sex, level of healthcare insurance, year of admission, month of admission, modality of admission (emergency vs planned) and level of hospital care; ** Changes in % for continuous outcomes calculated by a multivariable generalised linear gamma regression based on log-transformed values adjusted for age, sex, level of healthcare insurance, year of admission, month of admission, modality of admission (emergency vs planned) and level of hospital care. Abbreviations: CI, confidence interval; CKD, chronic kidney disease; ICU, intensive care unit; LOS, length of stay; OR, odds ratio.
We aimed to assess the associations of AKI and CKD with primary outcomes across the continuous spectrum of prevalent chronic conditions, stratified by level of frailty. When compared to hospitalisations without a kidney disease, in those with AKI, we observed a consistently higher relative risk of all primary outcomes along the number of chronic conditions (figure 2). This finding was also consistent for all levels of frailty.
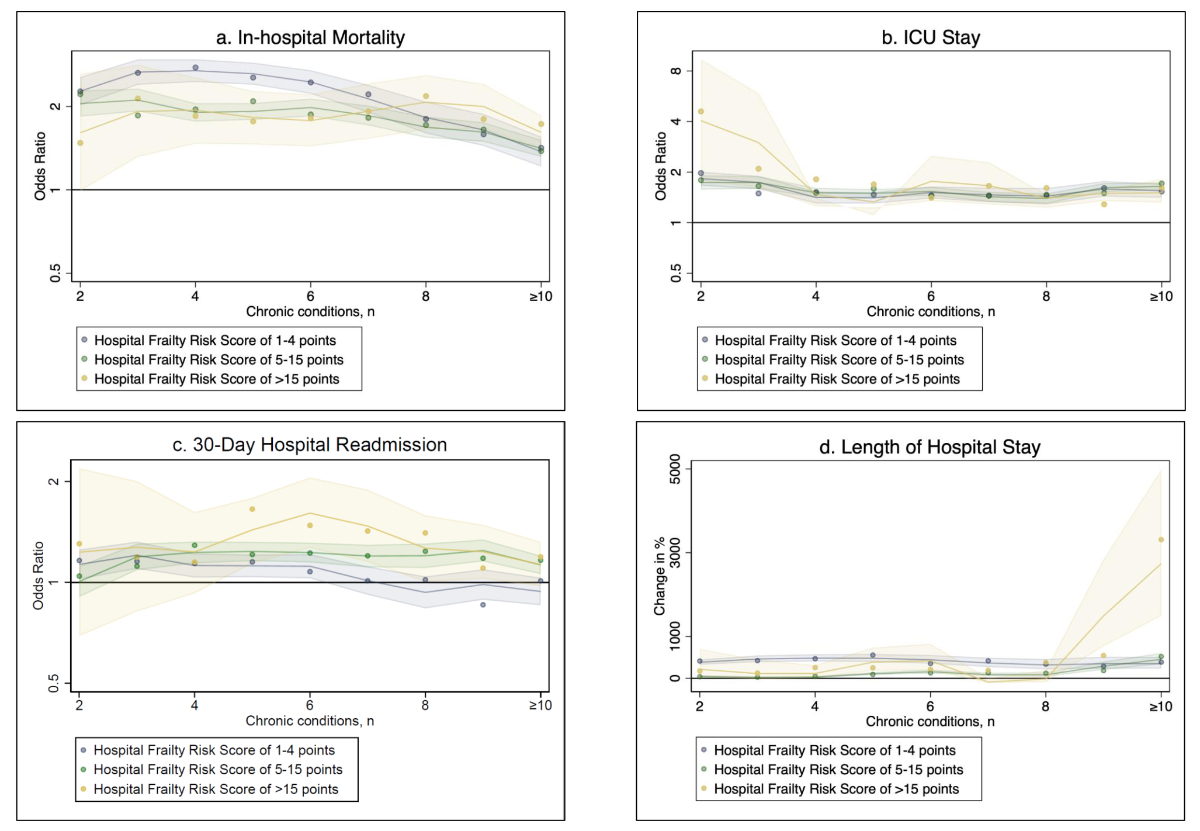
Figure 2B-spline analysis of primary outcomes among hospitalisations with AKI by number of chronic conditions, according to frailty. a) In-hospital mortality, b) ICU stay, c) 30-day hospital readmission and d) length of hospital stay. Shaded regions denote 95% confidence intervals. Abbreviations: ICU, intensive care unit.
Along the number of chronic conditions, in hospitalisations with CKD, the relative risk of in-hospital mortality and ICU stay as well as hospital LOS was similar or even lower as compared to hospitalisations without kidney disease (figure 3). These findings were also consistent for all levels of frailty. Except for non-frail hospitalisations, the relative risk of intubation in pre-frail and frail hospitalisations with CKD was increased along the number of chronic conditions, when compared to those without kidney disease (figure 3).
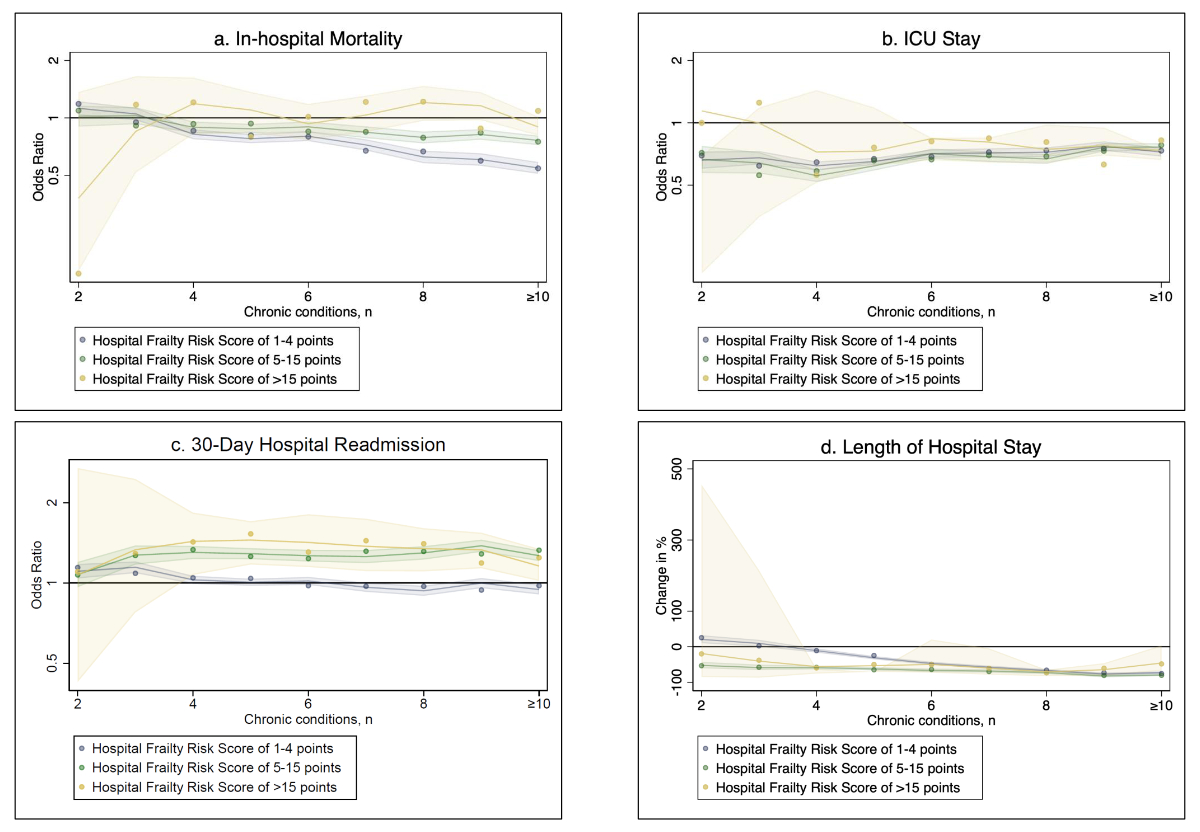
Figure 3B-spline analysis of primary outcomes among hospitalisations with CKD by number of chronic conditions, according to frailty. a) In-hospital mortality, b) ICU stay, c) 30-day hospital readmission and d) length of hospital stay. Shaded regions denote 95% confidence intervals. Abbreviations: ICU, intensive care unit.
We performed a nationwide analysis of medical hospitalisations in Switzerland to explore associations of AKI and CKD with adverse in-hospital outcomes. The main findings of this study include the following aspects: First, estimates show that the number of events among multimorbid hospitalisations with AKI or CKD was higher than in those without kidney disease. Second, adverse outcomes were more common in more severe AKI stages and in more frail people. However, with increasing frailty, the association of AKI and adverse outcomes was attenuated. Third, hospitalisations with increasing severity of CKD had a higher risk of in-hospital mortality, ICU admission, hospital readmission and longer LOS than those without kidney disease. The presence of frailty did not modify this association in a clinically meaningful manner.
After the nationwide implementation of SwissDRG in 2012, in the context of increasing treatment complexity owing to changing demographics and a higher prevalence of multimorbidity and frailty, financial pressure has been growing and hospitals forced to improve the efficiency of their care. In recent years – and further accentuated through the COVID-19 pandemic – many Swiss hospitals have been heavily battling to cope with increasing financial pressure and current standardised reimbursements, as defined by SwissDRG, are no longer cost-covering [12]. While reimbursements are slanted towards the financially most lucrative cause of admission, disease complexity, the presence of chronic diseases and multimorbidity do not relevantly account for additional fees. Although it seems intuitive that underlying chronic conditions, such as kidney diseases, require more in-hospital resources, it is not yet correctly acknowledged by SwissDRG and payers [13]. Thus, this study aimed to assess whether the prevalence of AKI or CKD in medical hospitalisations with multimorbidity is associated with additional in-hospital resource use and a higher risk of mortality when compared to those without kidney disease.
AKI describes a rapid decrease in renal function and is characterised by an increase in serum creatinine [14]. Although the three AKI stages were defined in 2012, only in 2017 was this acknowledged by SwissDRG and become relevant for reimbursement. Although ICD-10 codes for AKI have been reported to have low sensitivity resulting in underreporting of less severe hospitalisations [15–17], in this study we were able to identify a reasonable amount of multimorbid people with AKI. In addition, after the modification by SwissDRG in 2017, the number of people with AKI increased over time from 37,897 hospitalisations in 2017 to 43,669 hospitalisations in 2020. We found that the presence of AKI was associated with a higher use of in-hospital resources and a higher risk of mortality, with increased incidence in more advanced AKI stages. These results are also consistent with previous findings from Europe and United States linking the prevalence of ICD codes for AKI and the higher risk of short-term outcomes and mortality [15, 17, 18]. This association was even more pronounced in a recent study on COVID-19 patients showing a mortality risk of 50% in patients with AKI and 8% in controls without AKI [19].
In our study, the median LOS of all hospitalisations with AKI was 9 days (IQR 5–15 days) vs 5 days (IQR 2–9) in hospitalisations without kidney disease; the longest LOS (nearly 120% longer) was in hospitalisations with AKI stage 3. We did not find relevant differences in LOS between AKI stages 1 and 2, likely due to the retrospective use of ICD-10 codes, underestimating the number of hospitalisations admitted with AKI due to poor recognition of AKI, supporting the notion that AKI is underrecognised and underreported [20].
While – for many outcomes – we found consistent associations across the spectrum of frailty, the attenuation of relative risks in frailer hospitalisations is likely to be driven by a comparable baseline risk of adverse events in the control group, even without kidney disease. Whether the lower probability of hospital readmission among hospitalisations with AKI, as compared to kidney disease negative controls, is linked to closer outpatient monitoring with survival benefit or due to a higher likelihood of out-of-hospital mortality is unclear. However, results from a recent observational study indicate that – based on missing out-of-hospital mortality data and missing data from the outpatient setting – our results might be biased as Schulman et al. found AKI to be associated with higher rates of rehospitalisation (hazard ratio [HR] 1.62; 95% CI 1.60–1.65) [21]. To support the importance of AKI as a reason for higher in-hospital resource use, we found robust estimates across increasing numbers of chronic conditions with similar findings for all frailty levels. This further substantiates the hypothesis that already the presence of an even lower risk stage of AKI (stages 1 and 2) is a relevant cost driver in the setting of complex and multimorbid patients and seems not adequately addressed by current reimbursement. Given the number of hospitalisations with diagnoses of AKI 1 (83,718 hospitalisations; median LOS increased by 52% [to approximately 13.7 days]) and AKI 2 (27,829 hospitalisations; median LOS increased by 84% [to approximately 16.6 days]), and assuming conservative daily hospital costs of CHF 1000, the total hospital reimbursement shortfall would have exceeded CHF 600 million between 2017 and 2020.
CKD is diagnosed by the persistent elevation of urinary albumin excretion (albuminuria), low estimated glomerular filtration rate (eGFR) or other manifestations of kidney damage [22, 23]. According to the loss of renal function, CKD is divided into 7 stages (1 to 5, with additional stratification of stage 3 into 3a and 3b and stage 5 with or without dialysis) [22]. While there was a steep increase in prevalence of AKI codes between 2017 and 2020, hospitalisations with a code of CKD did not rise to the same extent. In detail, the percentage of CKD among the overall hospitalised medical population with multimorbidity rose from 9.5% in 2012 to 12.8% in 2016 and then plateaued (11.5% in 2020). This increase of about 2% is also consistent with previous literature assessing global dimensions of CKD [24]. Associations between CKD stages and adverse in-hospital outcomes were more heterogeneous than in hospitalisations with AKI. In particular, the risk of in-hospital mortality, ICU admission, hospital readmission and a longer LOS was increased in hospitalisations with CKD, with a certain “dose-dependent” effect – meaning that people with more-severe CKD stages were at a higher risk. These findings are comparable to previous studies showing a CKD stage-dependent association of in-hospital outcomes and resource use [25]. Interestingly, the CKD stage-dependent association was no longer observed in hospitalisations with dialysis, even though these hospitalisations carry a high burden of multimorbidity. It could be hypothesised that the decision not to start dialysis in hospitalisations with higher baseline mortality risk might explain this finding [26]. Like in hospitalisations with AKI, the presence of frailty in hospitalisations with CKD did not modify the relative risk estimates in a clinically meaningful manner. However, the absence of effect modification must be interpreted carefully as the development of frailty – a clinically detectable state of decreased physiological reserve and increased vulnerability to stressors and poor clinical outcomes [27, 28] – is also influenced by the presence of CKD by its effect on sarcopenia, mobility, cognitive impairment and exhaustion or through vascular complications [29, 30]. Multimorbidity, polypharmacy and unfavourable behavioural factors add to the complexity and challenges in the management of hospitalisations with kidney disease, which, again, is not yet adequately reimbursed. This is also further evidenced by a previous population-based cohort study of 2.5 million Canadian adults showing that patients seen by nephrologists were consistently more complex [31].
This study has limitations. First, using administrative data is prone to information bias as hospitalisations were selected according to ICD-10 codes with the risk of misclassification and underreporting of diagnoses. Thus, we were not able to evaluate hospitalisations with unrecognised AKI or CKD. Due to this, AKI and CKD, particularly lower stages, may have been underreported in ICD-10 codes, which may underestimate admissions complicated by AKI and CKD [6, 15]. Second, hospitalisations with codes for both AKI and CKD were classified under the AKI group, based on the assumption that AKI was the primary medical condition. To avoid any potential misinterpretation, we conducted a sensitivity analysis that differentiated between hospitalisations for “AKI only” and those involving “acute-on-chronic kidney disease”. This analysis revealed no clinically significant differences in the outcomes for all measured variables between the two groups (data not shown). Third, although we present longitudinal data, for hospitalisations with AKI not all years are represented, and the main analysis of this subpopulation was done with data from 2017 to 2020 only. Fourth, due to administrative restrictions, there was no information on out-of-hospital mortality available. Fifth, the study unit for this investigation was hospitalisations rather than patients. Consequently, hospitalisations could have been included more than once without opportunity for adjustment. Sixth, the non-experimental, observational design of our study limits the ability to draw a firm causal link. Seventh, we did not have information on treatment-level data, including clinical appearance, medication administration, and laboratory data.
This study provides evidence that the presence of kidney disease in hospitalisations with known multimorbidity is associated with additional risk of in-hospital adverse outcomes. Our data show that the presence of AKI entails a clinically relevant higher risk of adverse outcomes as compared to hospitalisations with the same level of frailty but no kidney disease. An accurate and thorough understanding of in-hospital resource utilisation can be of great value, as economic evaluations in the population with kidney disease were often generated using older healthcare resource use estimates or data not derived from real-world evidence.
The availability of data generated or analysed during this study is subject to restrictions for reasons of patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
The study was supported by the Kantonsspital Aarau AG and by a grant of the Fundação Pesquisa e Desenvolvimento Humanitario. The funders had no role in the design or conduct of the study, the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018 Jun;96(6):414–422D.
2. World Health Organization. The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
3. Muller M, Huembelin M, Baechli C, et al. Association of in-hospital multimorbidity with healthcare outcomes in Swiss medical inpatients. Swiss Med Wkly. 2021 Feb;151(506):w20405.
4. Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35(1):75–83.
5. Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol. 2006 Jun;17(6):1688–94.
6. Paik JM, Patorno E, Zhuo M, Bessette LG, York C, Gautam N, et al. Accuracy of identifying diagnosis of moderate to severe chronic kidney disease in administrative claims data. Pharmacoepidemiol Drug Saf. 2022 Apr;31(4):467–75.
7. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007 Oct;335(7624):806–8.
8. WHO. The word health report 2008.
9. Healthcare Cost & Utilization Project. Chronic Condition Indicator (CCI) for ICD-10-CM (beta version). 2018. Available from: https://hcup-us.ahrq.gov/toolssoftware/chronic_icd10/chronic_icd10_archive.jsp
10. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018 May;391(10132):1775–82.
11. Sauerbrei W, Perperoglou A, Schmid M, Abrahamowicz M, Becher H, Binder H, et al. State of the art in selection of variables and functional forms in multivariable analysis-outstanding issues. Diagn Progn Res. 2020;4(1):3.
12. Basel University Hospital. Die Universitätsspitäler der Schweiz warnen vor dem finanziellen Kollaps. 2023;
13. Beck T. Multimorbidität unter SwissDRG: ein Update. Schweizerische Ärztezeitschrift. 25.05.2022 2022;doi:https://doi.org/
14. Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013 May;61(5):649–72.
15. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014 Apr;9(4):682–9.
16. Logan R, Davey P, De Souza N, Baird D, Guthrie B, Bell S. Assessing the accuracy of ICD-10 coding for measuring rates of and mortality from acute kidney injury and the impact of electronic alerts: an observational cohort study. Clin Kidney J. 2020 Dec;13(6):1083–90.
17. Ko S, Venkatesan S, Nand K, Levidiotis V, Nelson C, Janus E. International statistical classification of diseases and related health problems coding underestimates the incidence and prevalence of acute kidney injury and chronic kidney disease in general medical patients. Intern Med J. 2018 Mar;48(3):310–5.
18. Montgomerie C, Spaak J, Evans M, Jacobson SH. Acute Kidney Injury: Clinical Characteristics and Short-Term Outcomes in 1,519 Patients. Kidney Dis (Basel). 2023 Jan;9(1):39–48.
19. Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 2021 Jan;32(1):151–60.
20. Stewart JA. Adding insult to injury: care of patients with acute kidney injury. Br J Hosp Med (Lond). 2009 Jul;70(7):372–3.
21. Schulman IH, Chan K, Der JS, Wilkins KJ, Corns HL, Sayer B, et al. Readmission and Mortality After Hospitalization With Acute Kidney Injury. Am J Kidney Dis. 2023 Jul;82(1):63–74 e1.
22. Andrassy KM. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 2013 Sep;84(3):622–3.
23. Foundation NK. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;(3):1–150.
24. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013 Jul;382(9888):260–72.
25. Liang S, Wang Y, Wang WL, Guo XR, Zhang C, Yang C, et al. Characteristics of hospitalized elderly patients with CKD: a comparison between elderly and non-elderly CKD based on a multicenter cross-sectional study. Int Urol Nephrol. 2023 Jul;56(2):625–33.
26. Schaeffner E. Smoothing transition to dialysis to improve early outcomes after dialysis initiation among old and frail adults-a narrative review. Nephrol Dial Transplant. 2022 Nov;37(12):2307–13.
27. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–63. 10.1093/gerona/59.3.M255
28. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. 10.1093/gerona/56.3.M146
29. Simpson SH, Lin M, Eurich DT. Medication Adherence Affects Risk of New Diabetes Complications: A Cohort Study. Ann Pharmacother. 2016 Sep;50(9):741–6.
30. Hanlon P, Faure I, Corcoran N, Butterly E, Lewsey J, McAllister D, et al. Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: a systematic review and study-level meta-analysis. Lancet Healthy Longev. 2020 Dec;1(3):e106–16.
31. Tonelli M, Wiebe N, Manns BJ, Klarenbach SW, James MT, Ravani P, et al. Comparison of the Complexity of Patients Seen by Different Medical Subspecialists in a Universal Health Care System. JAMA Netw Open. 2018 Nov;1(7):e184852.
Table S1Baseline characteristics stratified by presence of kidney disease among non-frail hospitalisations.
| No kidney disease | AKI | CKD | |
| Hospitalisations, n | 1,544,175 | 49,521 | 253,413 |
| Patients, n | 920,620 | 44,848 | 151,753 |
| Demographics | |||
| Age, median (IQR) [years] | 69 (57–78) | 76 (67–84) | 79 (71–85) |
| Male sex, n (%) | 849,803 (55.0) | 29,872 (60.3) | 144,339 (57.0) |
| Female sex, n (%) | 694,372 (45.0) | 19,649 (39.7) | 109,074 (43.0) |
| Swiss nationality, n (%) | 1,262,958 (81.8) | 41,162 (83.1) | 216,622 (85.5) |
| Other nationality, n (%) | 281,217 (18.2) | 8359 (16.9) | 36,791 (14.5) |
| Supplementary insurance, n (%) | 371,045 (24.0) | 10,489 (21.2) | 64,217 (25.3) |
| Admission data | |||
| Year of admission, n (%) | |||
| 2012 | 167,301 (10.8) | 2653 (5.4) | 25,227 (10.0) |
| 2013 | 168,676 (10.9) | 1580 (3.2) | 26,166 (10.3) |
| 2014 | 172,844 (11.2) | 1521 (3.1) | 28,481 (11.2) |
| 2015 | 176,091 (11.4) | 1384 (2.8) | 29,215 (11.5) |
| 2016 | 179,395 (11.6) | 1211 (2.4) | 29,187 (11.5) |
| 2017 | 171,328 (11.1) | 9784 (19.8) | 27,140 (10.7) |
| 2018 | 170,672 (11.1) | 10,602 (21.4) | 29,139 (11.5) |
| 2019 | 173,752 (11.3) | 10,504 (21.2) | 30,493 (12.0) |
| 2020 | 164,116 (10.6) | 10,282 (20.8) | 28,365 (11.2) |
| Emergency admission, n (%) | 1,016,028 (65.8) | 41,464 (83.7) | 180,053 (71.1) |
| Admission from home, n (%) | 1,367,643 (88.6) | 42,144 (85.1) | 220,975 (87.2) |
| Tertiary care hospital: University hospital | 278,864 (18.1) | 9202 (18.6) | 42,920 (16.9) |
| Tertiary care hospital: non-university hospital | 889,483 (57.6) | 30,688 (62.0) | 155,243 (61.3) |
| Secondary care hospital | 375,828 (24.3) | 9631 (19.4) | 55,250 (21.8) |
| Comorbidities, n (%) | |||
| Hypertension | 745,929 (48.3) | 31,054 (62.7) | 184,210 (72.7) |
| Obesity | 41,292 (2.7) | 1404 (2.8) | 8085 (3.2) |
| Type 2 diabetes mellitus | 239,887 (15.5) | 13,557 (27.4) | 77,739 (30.7) |
| Type 1 diabetes mellitus | 9307 (0.6) | 409 (0.8) | 2080 (0.8) |
| Dyslipidaemia | 335,446 (21.7) | 10,588 (21.4) | 64,364 (25.4) |
| Coronary artery disease | 428,147 (27.7) | 15,568 (31.4) | 99,855 (39.4) |
| Myocardial infarction | 111,210 (7.2) | 3793 (7.7) | 14,210 (5.6) |
| Congestive heart failure | 157,945 (10.2) | 15,874 (32.1) | 74,405 (29.4) |
| Atrial fibrillation | 233,994 (15.2) | 15,277 (30.8) | 81,796 (32.3) |
| Peripheral arterial disease | 64,198 (4.2) | 3061 (6.2) | 27,733 (10.9) |
| Obstructive sleep apnoea syndrome | 49,634 (3.2) | 2196 (4.4) | 11,010 (4.3) |
| Cerebrovascular disease | 77,270 (5.0) | 983 (2.0) | 9816 (3.9) |
| Ischaemic stroke | 40,684 (2.6) | 345 (0.7) | 3619 (1.4) |
| Chronic obstructive pulmonary disease | 140,061 (9.1) | 5331 (10.8) | 29,258 (11.5) |
| Gastrointestinal disorder | 348,419 (22.6) | 15,030 (30.4) | 61,106 (24.1) |
| Solid cancer | 255,989 (16.6) | 7546 (15.2) | 24,867 (9.8) |
| Haematological malignancy | 55,958 (3.6) | 2643 (5.3) | 8569 (3.4) |
| Musculoskeletal disorder | 275,431 (17.8) | 9745 (19.7) | 59,631 (23.5) |
| Mental disorder | 378,659 (24.5) | 7551 (15.2) | 37,020 (14.6) |
| Alcohol addiction | 109,516 (7.1) | 2887 (5.8) | 5638 (2.2) |
| Elixhauser comorbidity index, mean (SD) | 2.35 (1.48) | 3.68 (1.80) | 4.197958 (1.68) |
| Number of chronic comorbidities, n (%) | |||
| 2 | 370,183 (24.0) | 5677 (11.5) | 10,349 (4.1) |
| 3 | 340,295 (22.0) | 6815 (13.8) | 20,882 (8.2) |
| 4 | 282,255 (18.3) | 7278 (14.7) | 31,032 (12.2) |
| 5 | 206,298 (13.4) | 6871 (13.9) | 37,028 (14.6) |
| ≥6 | 345,144 (22.4) | 22,880 (46.2) | 154,122 (60.8) |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; IQR, interquartile range; SD, standard deviation.
Table S2Baseline characteristics stratified by presence of kidney disease among pre-frail hospitalisations.
| No kidney disease | AKI | CKD | |
| Hospitalisations, n | 421,031 | 126,678 | 181,518 |
| Patients, n | 314,819 | 103,911 | 124,812 |
| Demographics | |||
| Age, median (IQR) [years] | 77 (66–84) | 79 (70–85) | 82 (75–87) |
| Male sex, n (%) | 197,446 (46.9) | 69,215 (54.6) | 87,694 (48.3) |
| Female sex, n (%) | 223,585 (53.1) | 57,463 (45.4) | 93,824 (51.7) |
| Swiss nationality, n (%) | 361,537 (85.9) | 107,848 (85.1) | 159,523 (87.9) |
| Other nationality, n (%) | 59,494 (14.1) | 18,830 (14.9) | 21,995 (12.1) |
| Supplementary insurance, n (%) | 94,275 (22.4) | 25,323 (20.0) | 43,336 (23.9) |
| Admission data | |||
| Year of admission, n (%) | |||
| 2012 | 38,835 (9.2) | 4489 (3.5) | 15,591 (8.6) |
| 2013 | 43,706 (10.4) | 3843 (3.0) | 17,972 (9.9) |
| 2014 | 46,175 (11.0) | 3773 (3.0) | 20,205 (11.1) |
| 2015 | 50,453 (12.0) | 3986 (3.1) | 22,540 (12.4) |
| 2016 | 53,164 (12.6) | 3867 (3.1) | 26,090 (14.4) |
| 2017 | 45,058 (10.7) | 24,227 (19.1) | 18,223 (10.0) |
| 2018 | 46,522 (11.0) | 27,083 (21.4) | 19,221 (10.6) |
| 2019 | 48,579 (11.5) | 27,814 (22.0) | 20,680 (11.4) |
| 2020 | 48,539 (11.5) | 27,596 (21.8) | 20,996 (11.6) |
| Emergency admission, n (%) | 353,037 (83.9) | 111,564 (88.1) | 154,596 (85.2) |
| Admission from home, n (%) | 332,375 (78.9) | 100,672 (79.5) | 143,668 (79.1) |
| Tertiary care hospital: University hospital | 80,948 (19.2) | 24,205 (19.1) | 25,251 (13.9) |
| Tertiary care hospital: non-university hospital | 249,559 (59.3) | 80,186 (63.3) | 115,354 (63.5) |
| Secondary care hospital | 90,524 (21.5) | 22,287 (17.6) | 40,913 (22.5) |
| Comorbidities, n (%) | |||
| Hypertension | 225,920 (53.7) | 83,920 (66.2) | 133,538 (73.6) |
| Obesity | 9250 (2.2) | 4524 (3.6) | 6093 (3.4) |
| Type 2 diabetes mellitus | 72,799 (17.3) | 37,712 (29.8) | 55,495 (30.6) |
| Type 1 diabetes mellitus | 1760 (0.4) | 856 (0.7) | 1143 (0.6) |
| Dyslipidaemia | 71,049 (16.9) | 25,095 (19.8) | 36,194 (19.9) |
| Coronary artery disease | 71,667 (17.0) | 36,678 (29.0) | 57,742 (31.8) |
| Myocardial infarction | 11,697 (2.8) | 7067 (5.6) | 7501 (4.1) |
| Congestive heart failure | 53,281 (12.7) | 40,783 (32.2) | 54,884 (30.2) |
| Atrial fibrillation | 83,830 (19.9) | 42,929 (33.9) | 63,633 (35.1) |
| Peripheral arterial disease | 15,444 (3.7) | 9906 (7.8) | 17,602 (9.7) |
| Obstructive sleep apnoea syndrome | 12,246 (2.9) | 6204 (4.9) | 7557 (4.2) |
| Cerebrovascular disease | 93,337 (22.2) | 7610 (6.0) | 23,442 (12.9) |
| Ischaemic stroke | 50,142 (11.9) | 2678 (2.1) | 8982 (4.9) |
| Chronic obstructive pulmonary disease | 43,571 (10.3) | 16,278 (12.8) | 23,024 (12.7) |
| Gastrointestinal disorder | 119,462 (28.4) | 47,379 (37.4) | 54,874 (30.2) |
| Solid cancer | 55,500 (13.2) | 16,311 (12.9) | 17,079 (9.4) |
| Haematological malignancy | 11,522 (2.7) | 4869 (3.8) | 4779 (2.6) |
| Musculoskeletal disorder | 114,337 (27.2) | 36,364 (28.7) | 61,734 (34.0) |
| Mental disorder | 191,962 (45.6) | 45,468 (35.9) | 68,877 (37.9) |
| Alcohol addiction | 39,607 (9.4) | 10,945 (8.6) | 6868 (3.8) |
| Elixhauser comorbidity index, mean (SD) | 3.29 (1.75) | 4.76 (2.05) | 4.87 (1.87) |
| Number of chronic comorbidities, n (%) | |||
| 2 | 44,340 (10.5) | 7355 (5.8) | 4243 (2.3) |
| 3 | 60,366 (14.3) | 10,671 (8.4) | 9462 (5.2) |
| 4 | 67,230 (16.0) | 13,509 (10.7) | 15,715 (8.7) |
| 5 | 64,392 (15.3) | 14,773 (11.7) | 20,481 (11.3) |
| ≥6 | 184,703 (43.9) | 80,370 (63.4) | 131,617 (72.5) |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; IQR, interquartile range; SD, standard deviation.
Table S3Baseline characteristics stratified by presence of kidney disease among frail hospitalisations.
| No kidney disease | AKI | CKD | |
| Hospitalisations, n | 34,435 | 22,671 | 18,059 |
| Patients, n | 30,411 | 20,845 | 16,079 |
| Demographics | |||
| Age, median (IQR) [years] | 81 (74–86) | 81 (74–87) | 84 (78–88) |
| Male sex, n (%) | 14,961 (43.4) | 11,239 (49.6) | 7890 (43.7) |
| Female sex, n (%) | 19,474 (56.6) | 11,432 (50.4) | 10,169 (56.3) |
| Swiss nationality, n (%) | 29,976 (87.1) | 19,354 (85.4) | 15,984 (88.5) |
| Other nationality, n (%) | 4459 (12.9) | 3317 (14.6) | 2075 (11.5) |
| Supplementary insurance, n (%) | 7604 (22.1) | 4256 (18.8) | 4233 (23.4) |
| Admission data | |||
| Year of admission, n (%) | |||
| 2012 | 2430 (7.1) | 471 (2.1) | 1188 (6.6) |
| 2013 | 2937 (8.5) | 516 (2.3) | 1444 (8.0) |
| 2014 | 3274 (9.5) | 565 (2.5) | 1908 (10.6) |
| 2015 | 4087 (11.9) | 694 (3.1) | 2230 (12.3) |
| 2016 | 4377 (12.7) | 751 (3.3) | 2940 (16.3) |
| 2017 | 3719 (10.8) | 3886 (17.1) | 1694 (9.4) |
| 2018 | 3930 (11.4) | 4789 (21.1) | 1838 (10.2) |
| 2019 | 4475 (13.0) | 5208 (23.0) | 2159 (12.0) |
| 2020 | 5206 (15.1) | 5791 (25.5) | 2658 (14.7) |
| Emergency admission, n (%) | 29,586 (85.9) | 20,146 (88.9) | 15,781 (87.4) |
| Admission from home, n (%) | 23,155 (67.2) | 15,528 (68.5) | 12,313 (68.2) |
| Tertiary care hospital: University hospital | 8128 (23.6) | 5638 (24.9) | 3162 (17.5) |
| Tertiary care hospital: non-university hospital | 20,324 (59.0) | 13,953 (61.5) | 11,516 (63.8) |
| Secondary care hospital | 5983 (17.4) | 3080 (13.6) | 3381 (18.7) |
| Comorbidities, n (%) | |||
| Hypertension | 19,812 (57.5) | 15,758 (69.5) | 13,509 (74.8) |
| Obesity | 683 (2.0) | 995 (4.4) | 583 (3.2) |
| Type 2 diabetes mellitus | 6253 (18.2) | 6853 (30.2) | 5535 (30.6) |
| Type 1 diabetes mellitus | 89 (0.3) | 104 (0.5) | 92 (0.5) |
| Dyslipidaemia | 5696 (16.5) | 4468 (19.7) | 3485 (19.3) |
| Coronary artery disease | 6009 (17.5) | 6433 (28.4) | 5228 (28.9) |
| Myocardial infarction | 961 (2.8) | 1346 (5.9) | 723 (4.0) |
| Congestive heart failure | 4939 (14.3) | 7598 (33.5) | 5103 (28.3) |
| Atrial fibrillation | 8598 (25.0) | 8625 (38.0) | 6923 (38.3) |
| Peripheral arterial disease | 1376 (4.0) | 2044 (9.0) | 1678 (9.3) |
| Obstructive sleep apnoea syndrome | 880 (2.6) | 1123 (5.0) | 630 (3.5) |
| Cerebrovascular disease | 13,490 (39.2) | 4756 (21.0) | 5991 (33.2) |
| Ischaemic stroke | 5019 (14.6) | 1501 (6.6) | 1945 (10.8) |
| Chronic obstructive pulmonary disease | 2609 (7.6) | 2693 (11.9) | 1808 (10.0) |
| Gastrointestinal disorder | 10,673 (31.0) | 9893 (43.6) | 6017 (33.3) |
| Solid cancer | 2497 (7.3) | 2076 (9.2) | 1229 (6.8) |
| Haematological malignancy | 501 (1.5) | 618 (2.7) | 307 (1.7) |
| Musculoskeletal disorder | 11,292 (32.8) | 8845 (39.0) | 7401 (41.0) |
| Mental disorder | 26,084 (75.7) | 15,753 (69.5) | 13,023 (72.1) |
| Alcohol addiction | 2942 (8.5) | 2233 (9.8) | 805 (4.5) |
| Elixhauser comorbidity index, mean (SD) | 3.81 (2.05) | 5.53 (2.29) | 5.39 (2.09) |
| Number of chronic comorbidities, n (%) | |||
| 2 | 802 (2.3) | 330 (1.5) | 59 (0.3) |
| 3 | 2159 (6.3) | 716 (3.2) | 254 (1.4) |
| 4 | 3479 (10.1) | 1196 (5.3) | 686 (3.8) |
| 5 | 4259 (12.4) | 1694 (7.5) | 1184 (6.6) |
| ≥6 | 23,736 (68.9) | 18,735 (82.6) | 15,876 (87.9) |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; IQR, interquartile range; SD, standard deviation.