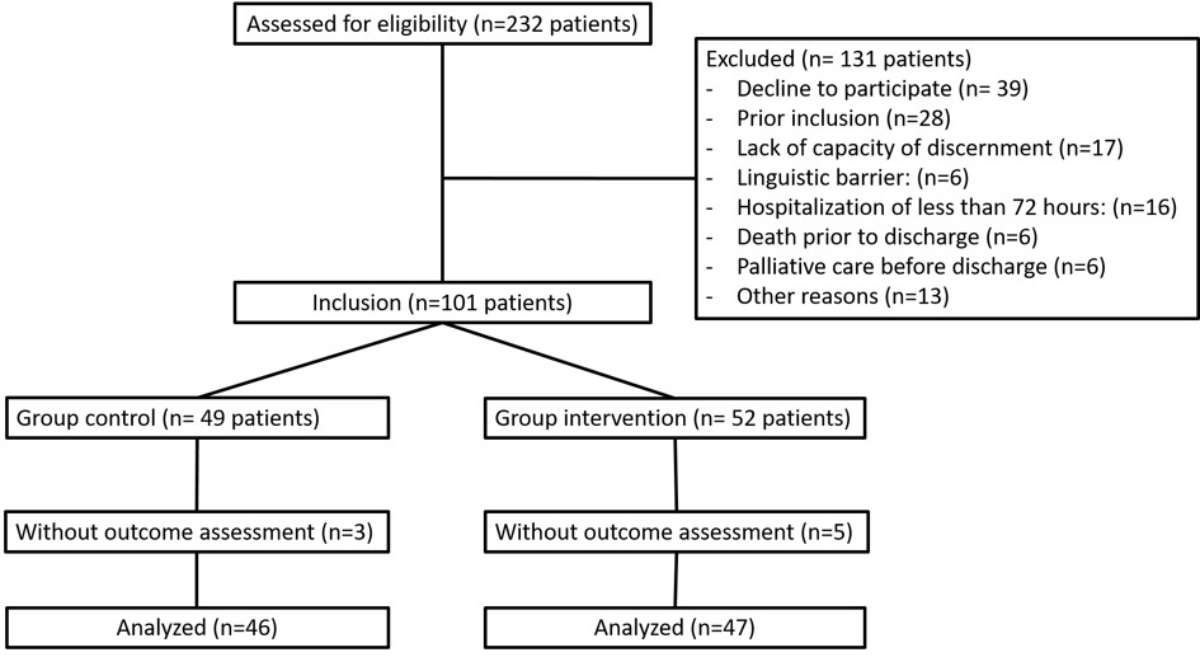
Figure 1CONSORT diagram.
DOI: https://doi.org/https://doi.org/10.57187/s.3394
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and has become the third leading cause of death worldwide [1]. Treatment for COPD relies mainly on the administration of inhaled drugs through various inhaler devices [1], with the aim of reducing the symptom burden and preventing exacerbations. However, the improper use of inhalers or the use of unsuitable devices can limit the effectiveness of these treatments [1]. Unfortunately, inhaler handling errors are unacceptably frequent and do not appear to have improved in recent years, despite efforts to promote therapeutic education and to simplify inhalers [2, 3]. Handling errors are associated with decreased symptom control, reduced quality of life, increased exacerbations, and increased healthcare system use [4–6]. In addition to their proper handling, some devices, such as dry powder inhalers (DPI), require sufficient peak inspiratory flow (PIF) for optimal effectiveness [7]. Insufficient PIF is common in individuals suffering from COPD, both inpatients with COPD exacerbations and stable outpatients [8–10], and is associated with worse disease outcomes, including increased numbers of COPD exacerbations and hospital admissions [11–13].
Each type of inhaler has both advantages and limitations [4], and it is important to select an inhaler adapted to the specific characteristics of a patient [14]. However, healthcare professionals often have limited knowledge of the features of different inhalers and the factors to consider when selecting an inhaler [15, 16]. Although different algorithms to help clinicians select an adapted inhaler exist, their effectiveness has not been validated in prospective trials. These algorithms summarise the elements to consider when selecting an inhaler [17]. Some studies have shown that inpatient therapeutic education programs during hospitalization can reduce the number of exacerbations [18, 19], but the misuse of inhalers during hospitalization remains an important and neglected problem. Furthermore, to our knowledge, no studies have jointly assessed handling errors and insufficient PIF in a hospital setting.
In the current Misused Inhaler and Insufficient Peak Inspiratory Flow (MIPIF) study, we assessed the proportion of misused inhalers at hospital discharge before and after the implementation of an in-hospital intervention among hospitalised patients with COPD.
This monocentric, non-randomised intervention study recruited patients from the internal medicine department of the Hospital of Fribourg (HFR), Fribourg, Switzerland. A control group was recruited between 1 March and 30 June 2022 and an intervention group between 1 August and 15 December 2022. The study was approved by the Ethics Commission of the Canton of Vaud (project number: BASEC 2021-02527), and all participants provided written consent. The results are reported in accordance with the TREND statement [20].
All patients with COPD admitted to the HFR general internal medicine department and who were using an inhaler at home were assessed for eligibility. Exclusion criteria were an inability to complete the initial assessment due to a language barrier or physical or cognitive problems, hospitalization for less than 72 hours, and previous inclusion in the study.
Participants in the control group received standard care. Assessment of inhalation technique or PIF, therapeutic education, or a specialist opinion was provided if deemed necessary by the physician. The intervention consisted of three elements introduced after recruitment of the control group: (a) a systematic standardised assessment of inhalation technique and PIF upon hospital admission, (b) the provision of a written guide to help physicians select an appropriate inhaler, and (c) therapeutic education to correct errors in inhalation technique or to instruct patients when a new inhaler was prescribed. At admission, a physiotherapist assessed inhalation technique using checklists specific to each inhaler and reported the presence of critical errors (see figure S1 in the appendix). In accordance with the literature, a critical error was defined as an action or an inaction that would have a detrimental effect on the delivery of the drug to the distal airways [3]. In the case of critical errors, the patient benefited from a teach-to-goal intervention focused on the specific gaps identified in the initial assessment. The teaching was repeated until the inhalation technique was performed without critical errors [18]. We considered a specific inhaler not to be teachable if the patient remained unable to use the inhaler without critical error after three repetitions of the instructions.
Peak inspiratory flow (PIF) was assessed with the In-Check DIAL G16® (Alliance Tech Medical, Inc., Hillsborough, NC, USA), a handheld device that simulates the variable internal resistances of the different inhalers [21]. PIF was measured at the resistance of the device used by the patient, and the highest value of three inspiratory manoeuvres was recorded [7, 21, 22]. Each physiotherapist received specific training in the use of the checklist and In-Check DIAL G16. The assessment was completed within 72 hours of admission to the internal medicine department, and the results were transmitted to the patient’s physician.
We developed a written guide to help physicians select an appropriate inhaler (see figure S2 in the appendix) [14, 17]. The guide was distributed to all physicians in the internal medicine department, who underwent a 15-min training session regarding its correct use. After the initial assessment, physicians in charge of a patient were free to choose how to adapt the inhaler. If considered necessary, a specialist opinion could be requested to help adjust the treatment. If a new inhaler was prescribed, a physiotherapist provided teach-to-goal therapeutic education. Written material describing the essential steps for optimal inhalation technique was also provided to the patient.
The primary outcome was the proportion of misused inhalers, defined as an inhaler used with at least one critical error or with insufficient PIF, at discharge from the internal medicine department. The assessment was performed by a physiotherapist not involved in the patient’s care. Only three physiotherapists performed this assessment, which consisted of the direct observation of inhalation technique, the measurement of PIF using the In-Check DIAL G16® , and teaching to correct errors. The methods for evaluating the primary outcome corresponded to the initial evaluation methods described above. As nebulised aerosols do not require a minimal PIF for optimal use and are administered by healthcare staff during hospitalization, they were considered to be used correctly.
Secondary outcomes were the proportions of inhalers used with a critical error, used with insufficient PIF, not teachable, and unsuitable. An inhaler was considered unsuitable if the patient was unable to generate sufficient PIF for optimal use or was unable to use the inhaler without critical errors after three teaching sessions. Additional secondary outcomes included the proportion of patients with at least one misused inhaler at discharge, the proportion of misused inhalers by inhaler type, and the length of the hospital stay.
Based on the literature, we estimated that at least 60% of inhalers used by hospitalised COPD patients would be misused and that the intervention would result in an absolute reduction in misused inhalers of 30% [23]. Considering each patient to use one inhaler, we had to include 42 participants in each group to show a difference with a statistical power of 80% and a significance criterion of 5%. To allow for intra-patient correlation (cluster = patient), a clustered sandwich estimator was specified to calculate the standard errors. The difference between the two groups was expressed as absolute risk reduction (ARR) with the confidence interval (CI) estimated from the statistical model mentioned above [24]. A sensitivity analysis of the primary outcome was performed using a logistic regression model to adjust for factors that could affect the outcome and for differences in baseline characteristics between the two groups [8, 14]. The variables included in the adjustment model were sex, age, the presence of a COPD exacerbation at the time of hospitalization, cognitive impairment, visual impairment, and rheumatological diseases affecting dexterity.
To assess whether the effect of the intervention varied according to baseline characteristics, the proportions of misused inhalers were compared among the following prespecified subgroups: sex (male versus female), age (<65 years versus ≥65 years), admission for an acute COPD exacerbation (yes versus no), admission for a respiratory problem (yes versus no), and length of hospitalization (<7 days versus ≥7 days). An interaction was tested in the logistic regression model to determine whether the treatment effect was consistent among subgroups [25].
Secondary outcomes evaluating inhalers were evaluated using a logistic regression model to take the cluster analysis into account. The results were expressed as ARR, with the CIs accounting for intra-patient correlation. Notably, the 95% CIs and p-values were not adjusted for multiple testing and should not be used to infer a definitive treatment effect.
Data were collected and analysed according to the predefined statistical analysis plan without deviation from the protocol. Demographic data on admission were collected by a research nurse and data on inhaler assessment by a physiotherapist. Study data were collected and managed using REDCap electronic data capture tools hosted by the University of Fribourg [26]. Statistical analyses were conducted by the authors. Patients with missing outcomes were excluded from the analysis, and, due to a low number of missing data, no imputation was performed. Continuous variables and categorical values were compared with Student’s t-tests and χ2 tests, respectively. The level of statistical significance was defined as a two-sided p-value <0.05. All analyses were performed using STATA, version 17 (StataCorp, Inc.).
The informed consent forms and study protocol were approved by the Ethics Commission of the Canton of Vaud (project number: BASEC 2021-02527). All patients provided written informed consent prior to their inclusion in the study.
During the study period, 232 patients underwent screening, and 101 were recruited into the study. Three patients in the control group and five in the intervention group did not receive the final assessment, resulting in 46 patients in the control group and 47 in the intervention group (figure 1).

Figure 1CONSORT diagram.
The demographic and medical characteristics of the patients at baseline are shown in table 1. Mean age was 70 years (SD 11 years), 56 patients (60.2%) were men, and 57 patients (62%) were hospitalised for a COPD exacerbation. Baseline characteristics were well balanced between groups except for a higher proportion of rheumatological disorders in the control group than the intervention group (8.7% vs 0%) (table 1).
Table 1Baseline patient characteristics. Means were compared using two-sample t-tests. Categorical variables were compared using χ2 tests.
| Characteristics | Control (n = 46) | Intervention (n = 47) | p-value | |
| Age, years – mean (SD) | 69.7 ± 11.6 | 71.3 ± 10.1 | 0.47 | |
| Female – n (%) | 20 (43.5) | 17 (36.2) | 0.47 | |
| Male – n (%) | 26 (56.5) | 30 (63.8) | 0.47 | |
| Comorbidities* – n (%) | Obesity | 11 (28.3) | 13 (23.4) | 0.59 |
| Cardiovascular disease | 15 (32.6) | 16 (34.8) | 0.88 | |
| Heart failure | 17 (37.0) | 16 (34.0) | 0.77 | |
| Cognitive impairment | 4 (8.7) | 1 (2.1) | 0.16 | |
| Visual impairment | 5 (10.9) | 1 (2.1) | 0.09 | |
| Neurological disease | 1 (2.2) | 3 (6.4) | 0.32 | |
| Rheumatological disease | 4 (8.7) | 0 (0) | 0.04 | |
| Anxiety/depression | 12 (26) | 11 (23.4) | 0.76 | |
| COPD classification* | ||||
| Grade – n (%) | 0.93 | |||
| Grade 1 | 1 (2.2) | 2 (4.3) | ||
| Grade 2 | 12 (26.1) | 15 (31.9) | ||
| Grade 3 | 12 (26.1) | 12 (25.5) | ||
| Grade 4 | 7 (15.2) | 6 (12.8) | ||
| Grade unknown | 14 (30.4) | 12 (25.5) | ||
| Group – n (%) | 0.24 | |||
| Group A | 1 (2.2) | 3 (6.4) | ||
| Group B | 8 (17.4) | 15 (31.9) | ||
| Group E | 24 (52.2) | 20 (40.6) | ||
| Group unknown | 13 (28.3) | 9 (19.2) | ||
| LTOT – n (%) | 17 (37.0) | 13 (27.7) | 0.34 | |
| mMRC – mean (SD) | 3.9 (1.1) | 3.7 (1.1) | 0.33 | |
| CAT score – mean (SD) | 18.9 (7.3) | 16.6 (7.4) | 0.14 | |
| Hospitalization – n (%) | For respiratory complaint | 33 (72) | 34 (72) | 0.95 |
| For COPD exacerbation | 28 (61) | 29 (62) | 0.93 | |
| In intensive care unit | 14 (30) | 11 (23) | 0.45 | |
LTOT: long-term oxygen therapy; mMRC: modified Medical Research Council dyspnea scale; CAT: COPD Assessment Test.
* As reported in the electronic patient record.
At home, patients used an average of 1.9 inhalers (SD 0.9), and this number was similar in the two groups. The types of inhalers used at home in the control and intervention groups were identical, except for pressurised metered-dose inhalers (pMDIs), which were more common in the control group than in the intervention group (37% vs 19%) (table S1 in the appendix).
In the intervention group, 137 inhalers were assessed at admission, of which 63 (46.0%) were nebulisers. At this assessment, there were 55 (40.2%) misused inhalers, 53 (38.7%) due to critical errors and 10 (7.3%) due to insufficient PIF. Furthermore, 13 (9.5%) inhalers were unsuitable.
At discharge, patients used a mean of 2.1 inhalers (SD 1.0) in the control group and 1.7 inhalers (SD 1.0) in the intervention group. Compared with the number of inhalers used at home, the mean number of inhalers at discharge increased in the control group (+0.17, SD 0.82) and decreased in the intervention group (−0.15, SD 0.81). The difference in the mean number of inhalers between admission and discharge between the control and the intervention groups was 0.32 (95% CI 0.01–0.66, p = 0.06) (table S2 in the appendix).
Among the 98 inhalers evaluated in the control group, 60 (61.2%) were misused. Among the 81 inhalers in the intervention group, 17 (21.0%) were misused. Therefore, the intervention reduced the proportion of misused inhalers by 40.2% (95% CI 25.5–55.0; p <0.01) (figure 2 and table 2). The reduction in the proportion of misused inhalers remained similar after adjusting for sex, age category, presence of COPD exacerbation, rheumatological disease, visual acuity impairment, and presence of cognitive impairment (table S3 in the appendix). The benefit of the intervention was similar in the prespecified subgroups, and no heterogeneity of treatment effects was detected (figure 3 and table S4 in the appendix). Given the difference in the proportion of inhalers used at discharge between the control and intervention groups, we conducted a sensitivity analysis to assess the impact of the intervention after adjusting for the type of inhaler used at discharge; this showed a similar effect (ARR 42.25% [95% CI 29.4–61.1]; p <0.01).
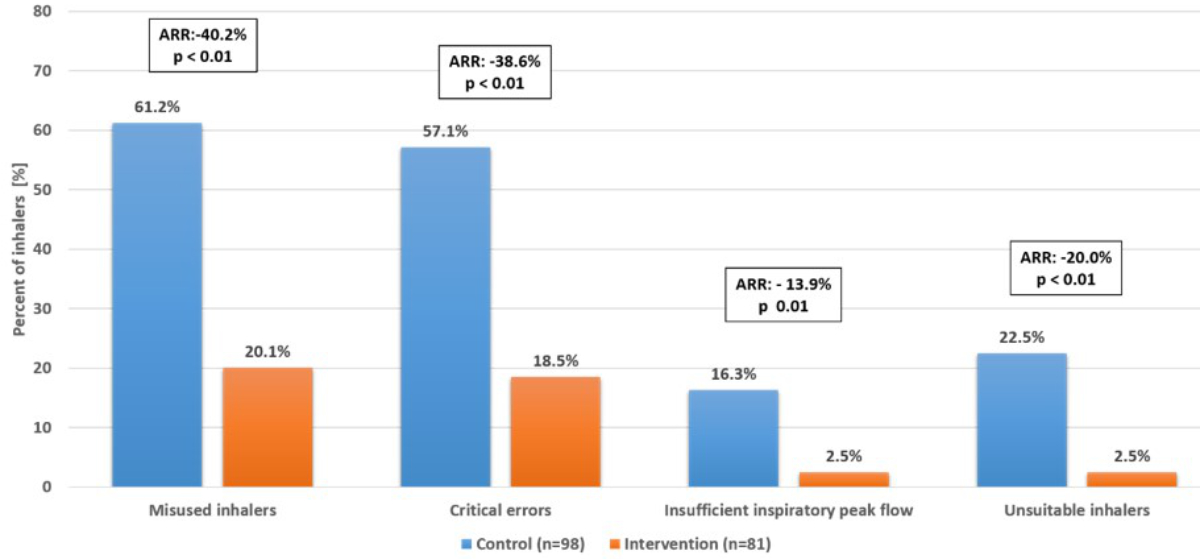
Figure 2Primary and secondary outcomes. Absolute risk reduction of misused inhalers, inhalers used with critical errors, inhalers used with insufficient peak inspiratory flow, and unsuitable inhalers at hospital discharge. Results were adjusted to account for intra-patient correlation; the p-values for secondary outcomes were not adjusted for multiple comparisons.ARR: absolute risk reduction.
Table 2Primary outcome and secondary outcomes by inhaler characteristics. All treatment effects are shown as absolute risk reduction. Results were adjusted to account for intra-patient correlation. The widths of the confidence intervals and p-value for secondary outcomes were not adjusted for multiple comparisons and should not be used for hypothesis testing.
| Inhalers – n (%) | Absolute risk reduction (95% CI) | p-value | |||
| Control (n = 98) | Intervention (n = 81) | ||||
| Primary outcome | Misused inhaler | 60 (61.2) | 17 (21.0) | 40.2 (25.5–55.0) | <0.01 |
| Secondary outcome | Critical error | 56 (57.1) | 15 (18.5) | 38.6 (24.3–52.3) | <0.01 |
| Insufficient PIF | 16 (16.3) | 2 (2.5) | 13.9 (4.2–23.6) | 0.01 | |
| Not teachable | 9 (9.2) | 0 (0) | 9.2 (1.3–17.0) | 0.02 | |
| Unsuitable inhaler | 22 (22.5) | 2 (2.5) | 20.0 (8.2–31.8) | <0.01 | |
PIF: peak inspiratory flow.
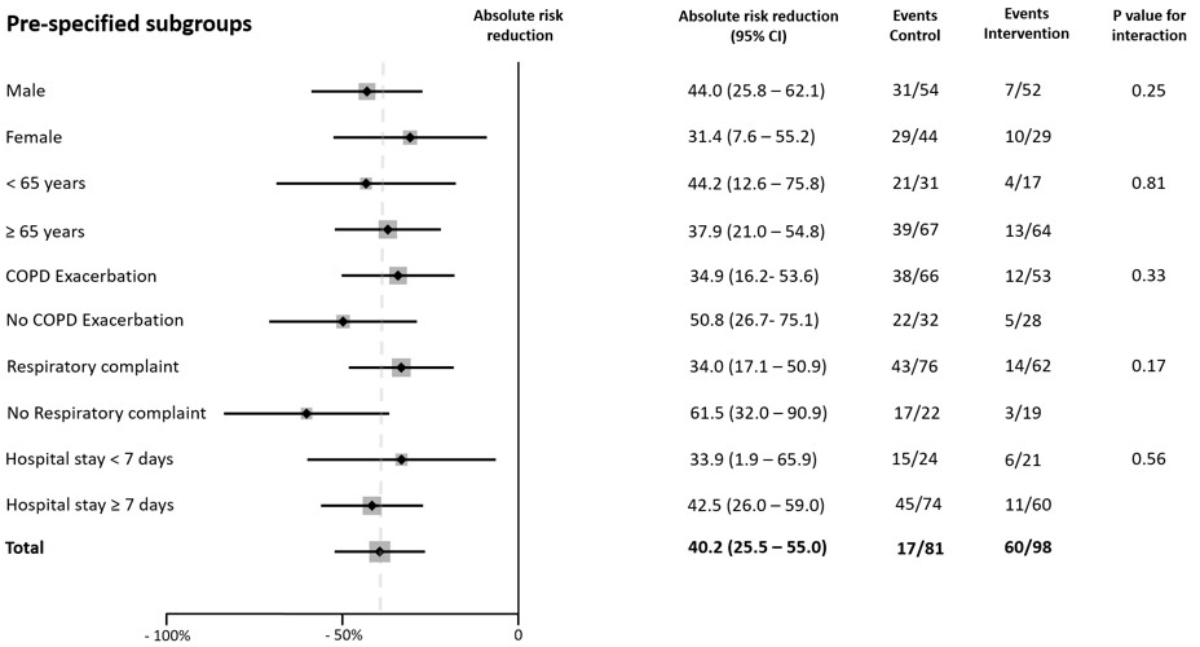
Figure 3Subgroup analysis. Absolute risk reduction (ARR) of misused inhalers at hospital discharge in prespecified subgroups. Results were adjusted to account for intra-patient correlation. The widths of the confidence intervals (CIs) were not adjusted for multiple comparisons, and the reported CIs should not be used for hypothesis testing. The dashed line indicates the ARR in the overall population. ARR: absolute risk reduction; CI: confidence interval.
The intervention reduced the proportion of inhalers used with a critical error by 38.6% (95% CI 24.3–52.3; p <0.01) and those used with an insufficient PIF by 13.9% (95% CI 4.2–23.6; p = 0.01). The proportion of inhalers that were not teachable decreased from 9.2% to 0% (ARR 9.2% [95% CI 0–18.4]; p = 0.02), while the proportion of unsuitable inhalers decreased from 22.5% to 2.5% (AAR 20.0% [95% CI 8.2–31.8]; p <0.01) (figure 2 and table 2).
Thirty-six patients (78.3%) in the control group and 15 (31.9%) in the intervention group had at least one misused inhaler upon discharge from the internal medicine department (ARR 46.3% [95% CI 28.5–64.2]; p <0.01). A reduction in the proportion of misused inhalers was observed for all types of inhalers except pMDIs without a spacer and Breezhaler® inhalers (Novartis, Basel, Switzerland). The proportions of misused pMDIs at discharge were 76.5% in the control group and 80.0% in the intervention group. The proportions of misused Breezhaler® inhalers at discharge were 37.5% in the control group and 28.6% in the intervention group (table S5 in the appendix). Mean length of hospital stay was similar in both groups (control: 9.1, SD 0.7 days; intervention: 10.7, SD 0.9 days [difference: 1.61 days (95% CI −0.67–3.89); p = 0.16]).
Our study showed that an in-hospital intervention consisting of a systematic assessment of inhalation technique and PIF at admission, provision of a written guide to help in selecting an appropriate inhaler, and therapeutic education was associated with a significant reduction in the misuse of inhalers by patients with COPD at hospital discharge. The reduction in misused inhalers was the result of reductions in inhalers used with a critical error and in inhalers used with insufficient PIF. The reduction in misused inhalers was consistent in all prespecified subgroups and after adjustment for potential confounding factors.
The main effect of the intervention tested was the reduction in inhalers used with a critical error. Similar to the published literature, this observation highlights the importance of assessing inhaler technique and providing therapeutic education [4, 5]. However, therapeutic education alone could not solve all problems related to misused inhalers. In the control group, 16% of inhalers were used with insufficient PIF, and 9% of inhalers could not be used adequately, despite sufficient therapeutic education. It is therefore necessary to integrate the measurement of PIF into the evaluation of inhalers and to select an inhaler adapted to a patient’s characteristics. Interestingly, despite the systematic assessment of PIF and the replacement of inhalers used with insufficient PIF, two inhalers in the intervention group were used with insufficient PIF at discharge. Both inhalers were used by patients hospitalised with a COPD exacerbation and with adequate PIF upon admission. Further investigations are necessary to document changes in PIF during a COPD exacerbation to define the best time to measure it during such an exacerbation [13].
The intervention affected the use of all inhalers, with the exception of pMDIs without a spacer and Breezhaler® inhalers. There are several possible reasons for the lack of an effect on pMDIs. Some studies have suggested that pMDIs might be more difficult to learn to use, particularly because of the difficulty in coordinating inhaler activation with inspiration [27–30]. Therapeutic education is also possibly less effective in correcting this type of error. The fact that the intervention was effective when a pMDI was used with a spacer may reinforce this hypothesis. Another explanation is that pMDIs were frequently replaced by nebulizers during hospitalization. Therefore, this type of inhaler was used less frequently, and patients were not trained as often during hospitalization. The lack of effectiveness of our intervention on the proportion of Breezhaler® inhalers misused at discharge is most likely linked to the low proportion of misused Breezhaler® inhalers in the control group (37.5%). A better use of the Breezhaler® compared to that of other inhalers is surprising and has not been reported in the literature [3–5]. The lack of effect of the intervention on pMDI and Breezhaler® use could also be explained by the small sample size.
Despite the evaluation of inhalers and selection of an appropriate inhaler according to a patient’s characteristics and therapeutic education, approximately 20% of inhalers continued to be used with a critical error at hospital discharge. These results contradict those of some studies showing that therapeutic education and the selection of an appropriate inhaler can eliminate critical errors [16, 17]. However, other studies have shown that the selection of a suitable inhaler and therapeutic education do not eliminate errors in inhalation technique [27, 28, 31]. The persistence of these errors is quite surprising given that the inhalers tested at discharge were used correctly at least once during the hospital stay. There are several possible explanations for this persistence of critical errors. First, despite the use of a checklist, the evaluation of inhalation technique by observation remains operator dependent, and subjectivity in evaluation cannot be eliminated. Second, our study included older patients with many comorbidities. Therapeutic education in this particular population has been shown to be less effective [27]. Finally, these results highlight the difficulty of using an inhaler correctly and reinforce the importance of regular therapeutic education. This regular education is especially important, as its benefits have been shown to fade over time [32].
The intervention was also associated with a reduction in the number of inhalers at discharge. The special attention to inhalers during the intervention may have encouraged physicians to review inhaled therapies and thus to discontinue unwarranted treatments. In addition, the written prescribing aid encouraged a reduction in the number of different inhalers used. The reduction in the number of inhalers used was associated with a decrease in the number of errors in inhalation technique, an increase in adherence, and a reduction in costs, which could represent additional advantages of this in-hospital intervention [33, 34]. However, we noted an increase in the use of nebulizers between hospital admission and discharge. The continued use of nebulizers after hospital discharge mainly concerned treatments prescribed as a reserve for patients transferred to rehabilitation or to hospice. The increase in the use of nebulizers therefore seems to be essentially explained by a patient’s destination after hospital discharge.
The intervention offers other advantages. First, it provides support for healthcare professionals in an area for which a lack of knowledge has been well described in the literature [16]. Second, the proposed tools could help healthcare providers to follow the current recommendations, which include regular assessment of inhaler use and selection of an inhaler suited to the patient but do not mention the tools necessary to achieve these goals [1]. Finally, the intervention requires few resources and little expertise and could be integrated into a variety of outpatient and inpatient settings. In addition to the proposed intervention, digital technologies could be useful aids in improving the use of inhalers in future years. Various devices that can assess inhalation technique, PIF, and adherence to treatment are currently on the market [14, 35].
Our study had several strengths. By including all patients with COPD, with or without exacerbation, our study highlighted the importance of assessing inhalation technique and PIF in a large sample of hospitalised COPD patients. Moreover, the effect of our intervention seemed to be consistent across all subgroups studied, including hospitalised patients without COPD exacerbation and without new respiratory problems. The use of validated tools and a small number of assessors allowed a homogeneous evaluation of the outcome. Finally, this intervention will probably improve disease control by reducing the numerous factors causing suboptimal inhaler use, namely, errors in inhaler technique, insufficient PIF, and the prescription of inhalers that could not be used properly despite therapeutic education.
Our study had several limitations. First, it was a monocentric study, so the effectiveness of the intervention should be confirmed in other centres. Second, the study was not randomised. However, assignment to the intervention group according to the date of hospitalization limited the risk of selection bias. Furthermore, baseline demographic characteristics were identical in both groups, and the effects remained similar in the two groups after adjustment for factors that could have influenced the outcome. In addition, due to the short time period over which the study was conducted, it is unlikely that other elements of management changed between the two periods of recruitment. However, the proportions of different types of inhalers used at admission and discharge varied between the two groups, with more pMDIs used in the control group. Since the intervention appears to have been less effective in reducing the suboptimal use of pMDIs, it is possible that these differences may have influenced the estimate of the effect of the intervention. Third, our study did not show a reduction in the length of hospital stay and was not conducted to assess the impact of the intervention on disease control. Nevertheless, other studies have suggested that a reduction in inhalation technique errors and the selection of inhalers used with an adequate PIF result in fewer exacerbations and hospitalizations [19]. Fourth, the diagnosis of COPD was based on information available in a patient’s electronic file, and lung function results were not available for all patients. Therefore, we were not able to confirm that all patients had COPD. However, we recently reported that 87% of patients hospitalised in our department with a diagnosis of COPD had a confirmed obstructive pulmonary disease [36]. Nevertheless, the aim of our study was not to determine whether the prescription of inhalers was justified but to investigate whether the studied intervention improved the use of prescribed inhalers. Fifth, despite the use of standardised tools and a small number of trained assessors not involved in patient management, the assessment of inhalation technique by observation remains somewhat subjective [4]. Finally, several questions remain unanswered. These relate to the effectiveness of the written guide in selecting an inhaler adapted to a patient’s characteristics, the best time to measure PIF during an exacerbation, and the impact of the intervention on the number of subsequent exacerbations and hospitalizations.
Systematic assessment of inhalation technique and PIF, combined with the selection of a suitable inhaler and therapeutic education, was associated with a significant reduction in the proportion of misused inhalers at hospital discharge. Such an in-hospital intervention can significantly reduce the number of misused inhalers at discharge and should be considered for all hospitalised COPD patients.
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
The authors would like to thank all the patients and their families, as well as the team of investigators involved in this study. Statistical data analysis support, under the direction of the authors was provided by Mohamed Faouzi, Division of Biostatistics, Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland. Medical writing support, under the direction of the authors was provided by Rosemary Sudan, Savièse, Switzerland.
Authors' contributions: The study was conceived and designed by GG, PS, TG, PD, DH. GG, PS, and TG oversaw conduct of the study. Data were acquired by GG, PS, and TG. Data were analyzed by GG and interpreted by all authors. All authors read and approved the final version.
This work was funded by a HFR grant (Grant-2201), HFR Fribourg, Fribourg, Switzerland. The funder of the study was not involved in the study design, data collection, data analysis, data interpretation, and writing of the report.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. GOLD. Global strategy for the diagnosis, management, and prevention of chronic lung obstructive disease. 2021.
2.Sanchis J, Gich I, Pedersen S; Aerosol Drug Management Improvement Team (ADMIT). Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest. 2016 Aug;150(2):394–406. 10.1016/j.chest.2016.03.041
3.Chrystyn H, van der Palen J, Sharma R, Barnes N, Delafont B, Mahajan A, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017 Apr;27(1):22. 10.1038/s41533-017-0016-z
4.Usmani OS, Lavorini F, Marshall J, Dunlop WC, Heron L, Farrington E, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018 Jan;19(1):10. 10.1186/s12931-017-0710-y
5.Kocks JW, Chrystyn H, van der Palen J, Thomas M, Yates L, Landis SH, et al. Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. NPJ Prim Care Respir Med. 2018 Nov;28(1):43. 10.1038/s41533-018-0110-x
6.Molimard M, Raherison C, Lignot S, Balestra A, Lamarque S, Chartier A, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017 Feb;49(2):1601794. 10.1183/13993003.01794-2016
7.Ghosh S, Ohar JA, Drummond MB. Peak Inspiratory Flow Rate in Chronic Obstructive Pulmonary Disease: Implications for Dry Powder Inhalers. J Aerosol Med Pulm Drug Deliv. 2017 Dec;30(6):381–7. 10.1089/jamp.2017.1416
8.Mahler DA. The role of inspiratory flow in selection and use of inhaled therapy for patients with chronic obstructive pulmonary disease. Respir Med. 2020 Jan;161:105857. 10.1016/j.rmed.2019.105857
9.Harb HS, Laz NI, Rabea H, Abdelrahim ME. Prevalence and predictors of suboptimal peak inspiratory flow rate in COPD patients. Eur J Pharm Sci. 2020 Apr;147:105298. 10.1016/j.ejps.2020.105298
10.Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of Low Peak Inspiratory Flow Rate at Discharge in Patients Hospitalized for COPD Exacerbation. Chronic Obstr Pulm Dis (Miami). 2017 Jul;4(3):217–24. 10.15326/jcopdf.4.3.2017.0183
11.Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal Inspiratory Flow Rates Are Associated with Chronic Obstructive Pulmonary Disease and All-Cause Readmissions. Ann Am Thorac Soc. 2017 Aug;14(8):1305–11. 10.1513/AnnalsATS.201611-903OC
12.Chen SY, Huang CK, Peng HC, Tsai HC, Huang SY, Yu CJ, et al. Peak-Inspiratory-Flow-Rate Guided Inhalation Therapy Reduce Severe Exacerbation of COPD. Front Pharmacol. 2021 Jun;12:704316. 10.3389/fphar.2021.704316
13.Mahler DA, Halpin DM. Peak Inspiratory Flow as a Predictive Therapeutic Biomarker in COPD. Chest. 2021 Aug;160(2):491–8. 10.1016/j.chest.2021.03.049
14.Renaud Y, Suter P, Grandmaison G. Patient Characteristics to Consider When Selecting an Inhaler for the Treatment of Chronic Obstructive Pulmonary Disease and Available Assessment Methods: A Narrative Review. Respiration. 2023;102(6):416–25. 10.1159/000530277
15.Braman SS, Carlin BW, Hanania NA, Mahler DA, Ohar JA, Pinto-Plata V, et al. Results of a Pulmonologist Survey Regarding Knowledge and Practices With Inhalation Devices for COPD. Respir Care. 2018 Jul;63(7):840–8. 10.4187/respcare.05717
16.Plaza V, Giner J, Rodrigo GJ, Dolovich MB, Sanchis J. Errors in the Use of Inhalers by Health Care Professionals: A Systematic Review. J Allergy Clin Immunol Pract. 2018;6(3):987–95. 10.1016/j.jaip.2017.12.032
17.Halpin DM, Mahler DA. A Systematic Review of Published Algorithms for Selecting an Inhaled Delivery System in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2022 Jul;19(7):1213–20. 10.1513/AnnalsATS.202108-930OC
18.Press VG, Arora VM, Shah LM, Lewis SL, Ivy K, Charbeneau J, et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011 Jun;26(6):635–42. 10.1007/s11606-010-1624-2
19.Capstick TG, Azeez NF, Deakin G, Goddard A, Goddard D, Clifton IJ. Ward based inhaler technique service reduces exacerbations of asthma and COPD. Respir Med. 2021 Oct;187:106583. 10.1016/j.rmed.2021.106583
20.Des Jarlais DC, Lyles C, Crepaz N, Group T; TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004 Mar;94(3):361–6. 10.2105/AJPH.94.3.361
21.Sanders MJ. Guiding Inspiratory Flow: Development of the In-Check DIAL G16, a Tool for Improving Inhaler Technique. Pulm Med. 2017;2017:1495867. 10.1155/2017/1495867
22.Barnes CN, Mahler DA, Ohar JA, Lombardi DA, Crater GD. Peak Inspiratory Flows: Defining Repeatability Limits and a Predictive Equation for Different Inhalers. Chest. 2020 Oct;158(4):1413–9. 10.1016/j.chest.2020.03.072
23.Batterink J, Dahri K, Aulakh A, Rempel C. Evaluation of the use of inhaled medications by hospital inpatients with chronic obstructive pulmonary disease. Can J Hosp Pharm. 2012 Mar;65(2):111–8. 10.4212/cjhp.v65i2.1118
24.Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata J. 2013;13(3):492–509. 10.1177/1536867X1301300304
25.Wang R, Ware JH. Detecting moderator effects using subgroup analyses. Prev Sci. 2013 Apr;14(2):111–20. 10.1007/s11121-011-0221-x
26.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al.; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019 Jul;95:103208. 10.1016/j.jbi.2019.103208
27.Kebede AT, Trapnes E, Lea M, Abrahamsen B, Mathiesen L. Effect of pharmacist-led inhaler technique assessment service on readmissions in hospitalized COPD patients: a randomized, controlled pilot study. BMC Pulm Med. 2022 May;22(1):210. 10.1186/s12890-022-02004-z
28.Hardwell A, Barber V, Hargadon T, McKnight E, Holmes J, Levy ML. Technique training does not improve the ability of most patients to use pressurised metered-dose inhalers (pMDIs). Prim Care Respir J. 2011 Mar;20(1):92–6. 10.4104/pcrj.2010.00088
29.Sulku J, Bröms K, Högman M, Janson C, Lisspers K, Malinovschi A, et al. Critical inhaler technique errors in Swedish patients with COPD: a cross-sectional study analysing video-recorded demonstrations. NPJ Prim Care Respir Med. 2021 Feb;31(1):5. 10.1038/s41533-021-00218-y
30.Takaku Y, Kurashima K, Ohta C, Ishiguro T, Kagiyama N, Yanagisawa T, et al. How many instructions are required to correct inhalation errors in patients with asthma and chronic obstructive pulmonary disease? Respir Med. 2017 Feb;123:110–5. 10.1016/j.rmed.2016.12.012
31.Sulku J, Janson C, Melhus H, Ställberg B, Bröms K, Högman M, et al. Changes in critical inhaler technique errors in inhaled COPD treatment - A one-year follow-up study in Sweden. Respir Med. 2022 Jun;197:106849. 10.1016/j.rmed.2022.106849
32.Klijn SL, Hiligsmann M, Evers SM, Román-Rodríguez M, van der Molen T, van Boven JF. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. NPJ Prim Care Respir Med. 2017 Apr;27(1):24. 10.1038/s41533-017-0022-1
33.Rogliani P, Ora J, Puxeddu E, Matera MG, Cazzola M. Adherence to COPD treatment: myth and reality. Respir Med. 2017 Aug;129:117–23. 10.1016/j.rmed.2017.06.007
34.Levy ML, Dekhuijzen PN, Barnes PJ, Broeders M, Corrigan CJ, Chawes BL, et al. Inhaler technique: facts and fantasies. A view from the Aerosol Drug Management Improvement Team (ADMIT). NPJ Prim Care Respir Med. 2016 Apr;26(1):16017. 10.1038/npjpcrm.2016.17
35.Bosnic-Anticevich S, Bakerly ND, Chrystyn H, Hew M, van der Palen J. Advancing Digital Solutions to Overcome Longstanding Barriers in Asthma and COPD Management. Patient Prefer Adherence. 2023 Jan;17:259–72. 10.2147/PPA.S385857
36.Tschopp J, Dumont P, Hayoz D. True prevalence of COPD and its association with peripheral arterial disease in the internal medicine ward of a tertiary care hospital. Swiss Med Wkly. 2017 Jul;147:w14460.
Table S1Inhalers used by patients at home. Categorical variables were compared using χ2 tests. Means were compared using two-sample t-tests.
| Inhaler used at home | Control (n = 90) | Intervention (n = 88) | p-value |
| Number of inhalers – mean (SD) | 1.96 (0.8) | 1.87 (1.0) | 0.65 |
| pMDIs – n (%) | 19 (21.1) | 9 (10.2) | 0.03 |
| pMDIs + spacer – n (%) | 11 (12.2) | 19 (21.6) | 0.18 |
| BAMDI – n (%) | 0 (0) | 1 (1.1) | 0.33 |
| Respimat® – n (%) | 9 (10) | 12 (13.6) | 0.50 |
| Diskus® – n (%) | 3 (3.3) | 2 (2.3) | 0.63 |
| Turbohaler® – n (%) | 8 (8.9) | 8 (9.1) | 0.96 |
| Handihaler® – n (%) | 8 (8.9) | 8 (9.1) | 0.96 |
| Breezhaler® – n (%) | 8 (8.9) | 9 (10.2) | 0.84 |
| Ellipta® – n (%) | 21 (23.3) | 14 (15.9) | 0.84 |
| Nebulizer – n (%) | 3 (3.3) | 6 (6.8) | 0.50 |
SD: standard deviation; pMDIs: pressurised metered-dose inhalers; BAMDI: breath-actuated metered-dose inhaler.
Table S2Number of inhalers per patient at hospital admission and discharge.
| Total (n = 93) | Control (n = 46) | Intervention (n = 47) | Between-group difference (95% CI) | p-value | |
| Admission – mean (SD) | 1.91 (0.89) | 1.96 (0.79) | 1.87 (0.99) | 0.08 (−0.29–0.45) | 0.65 |
| Discharge – mean (SD) | 1.92 (1.02) | 2.13 (1.02) | 1.72 (0.99) | 0.41 (−0.01–0.82) | 0.05 |
| Difference between admission and discharge – mean (SD) | 0.01 (0.83) | 0.17 (0.82) | −0.15 (0.81) | 0.32 (0.01–0.66) | 0.06 |
SD: standard deviation.
Table S3Primary and secondary outcomes expressed as unadjusted and adjusted odds ratios. Adjusted for sex, age, presence of exacerbation, rheumatological disease, visual impairment, and cognitive impairment. The adjusted and unadjusted analyses accounted for intra-patient correlation.
| Inhaler – n (%) | Unadjusted odds ratio (95% CI) | p-value | Adjusted odds ratio (95% CI) | p-value | ||
| Control (n = 98) | Intervention (n = 81) | |||||
| Misused inhaler | 60 (61.2) | 17 (21.0) | 0.17 (0.08–0.35) | <0.01 | 0.15 (0.07–0.33) | <0.01 |
| Critical error | 56 (57.1) | 15 (18.5) | 0.17 (0.08–0.36) | <0.01 | 0.14 (0.07–0.32) | <0.01 |
| Insufficient PIF | 16 (16.3) | 2 (2.5) | 0.13 (0.03–0.62) | 0.01 | 0.13 (0.02–0.73) | 0.02 |
PIF: peak inspiratory flow.
Table S4Analyses by prespecified subgroup.
| Subgroup | Misused inhaler | Absolute risk reduction (95% CI) | p-value | p-value for interaction | |
| Control n/n total (%) | Intervention n/n total (%) | ||||
| Total | 60/98 (61.2) | 17/81 (21.0) | 40.2 (25.5–55.0) | <0.01 | |
| Female | 29/44 (65.9) | 10/29 (34.5) | 31.4 (7.6–55.2) | 0.01 | 0.25 |
| Male | 31/5 (57.4) | 7/52 (13.5) | 44.0 (25.8–62.1) | <0.01 | |
| Age <65 years | 21/31 (67.7) | 4/17 (23.5) | 44.2 (12.6–75.8) | 0.01 | 0.81 |
| Age ≥65 years | 39/67 (58.2) | 13/64 (20.3) | 37.9 (21.0–54.8) | <0.01 | |
| Absence of exacerbation | 22/32 (68.8) | 5/28 (17.9) | 50.8 (26.7–75.1) | <0.01 | 0.33 |
| Presence of exacerbation | 38/66 (57.6) | 12/53 (22.6) | 34.9 (16.2–53.6) | <0.01 | |
| Absence of respiratory complaint | 17/22 (77.3) | 3/19 (15.8) | 61.5 (32.0–90.9) | <0.01 | 0.17 |
| Presence of respiratory complaint | 43/76 (56.6) | 14/62 (22.6) | 34.0 (17.1–50.9) | <0.01 | |
| Hospital stay <7 days | 15/24 (62.5) | 6/21 (28.6) | 33.9 (1.9–65.9) | 0.04 | 0.56 |
| Hospital stay ≥7 days | 45/74 (60.8) | 11/60 (18.3) | 42.5 (26.0–59.0) | <0.01 | |
A logistic regression model accounting for intra-patient correlation was used to test interactions.
Table S5Outcomes by type of inhaler. The absolute risk reduction and p-value were not calculated for a category with fewer than 10 inhalers.
| Misused inhaler | Absolute risk reduction (95% CI) | p-value | ||
| Control n/n total (%) | Intervention n/n total (%) | |||
| pMDIs | 13/17 (76.5) | 4/5 (80.0) | −3.5 (−47.1–40.1) | 0.87 |
| pMDIs + spacer | 8/9 (88.9) | 5/14 (35.8) | 53.2 (17.5–88.8) | <0.01 |
| BAMDI | 0/0 (0) | 0/1 (0) | – | – |
| Respimat® | 5/9 (55.6) | 3/15 (20) | 35.6 (−3.5–74.6) | 0.07 |
| Diskus® | 2/2 (100) | 0/1 (0) | – | – |
| Ellipta® | 18/23 (78.3) | 1/12 (8.3) | 69.9 (45.7–94.2) | <0.01 |
| Turbohaler® | 5/6 (83.3) | 1/5 (20.0) | 63.3 (15.1–111.6) | 0.01 |
| Handihaler® | 6/8 (75.0) | 1/7 (14.3) | 60.7 (19.7–101.8) | <0.01 |
| Breezhaler® | 3/8 (37.5) | 2/7 (28.6) | 8.93 (−41.6–59.4) | 0.74 |
| Nebulizer | 0/16 (0) | 0/14 (0) | NA | NA |
pMDIs: pressurised metered-dose inhalers; BAMDI: breath-actuated metered-dose inhaler; NA: not applicable, as nebulizers are considered to be used correctly.
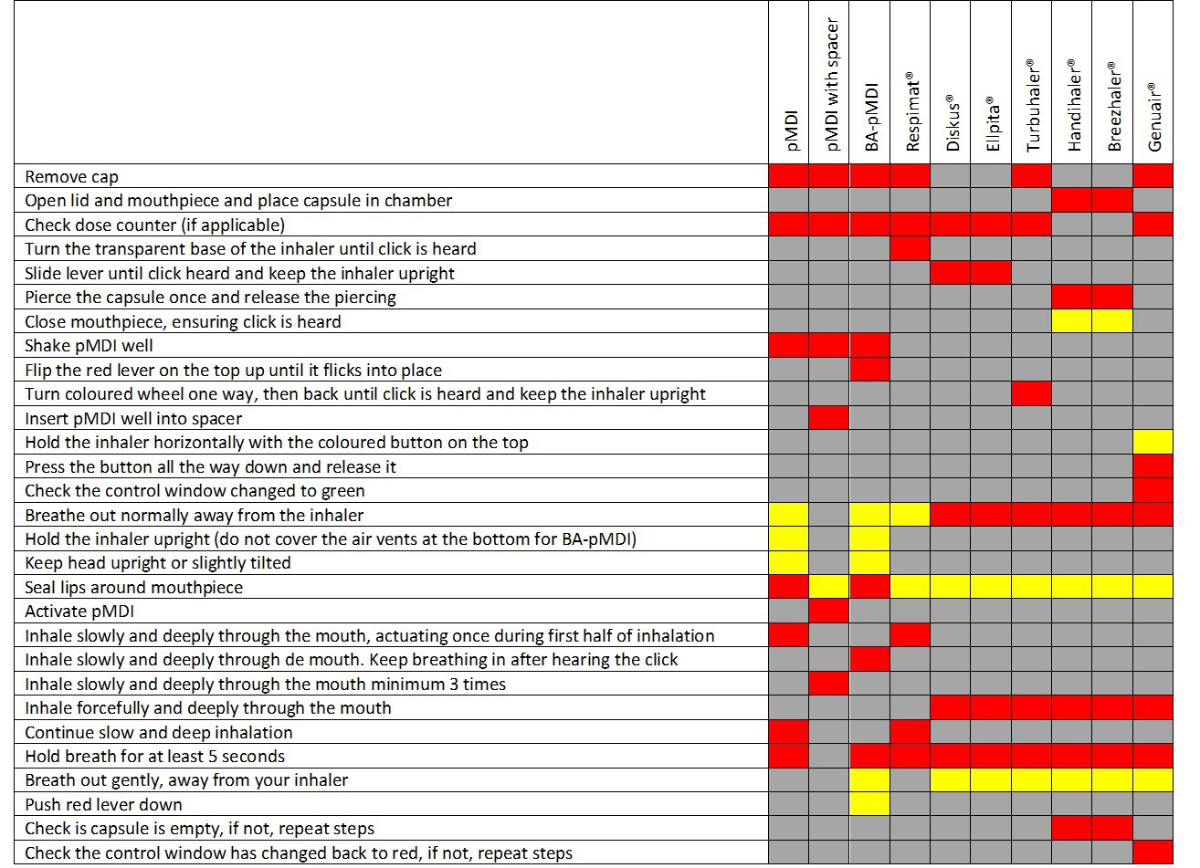
Figure S1Checklist for assessing inhalation technique.
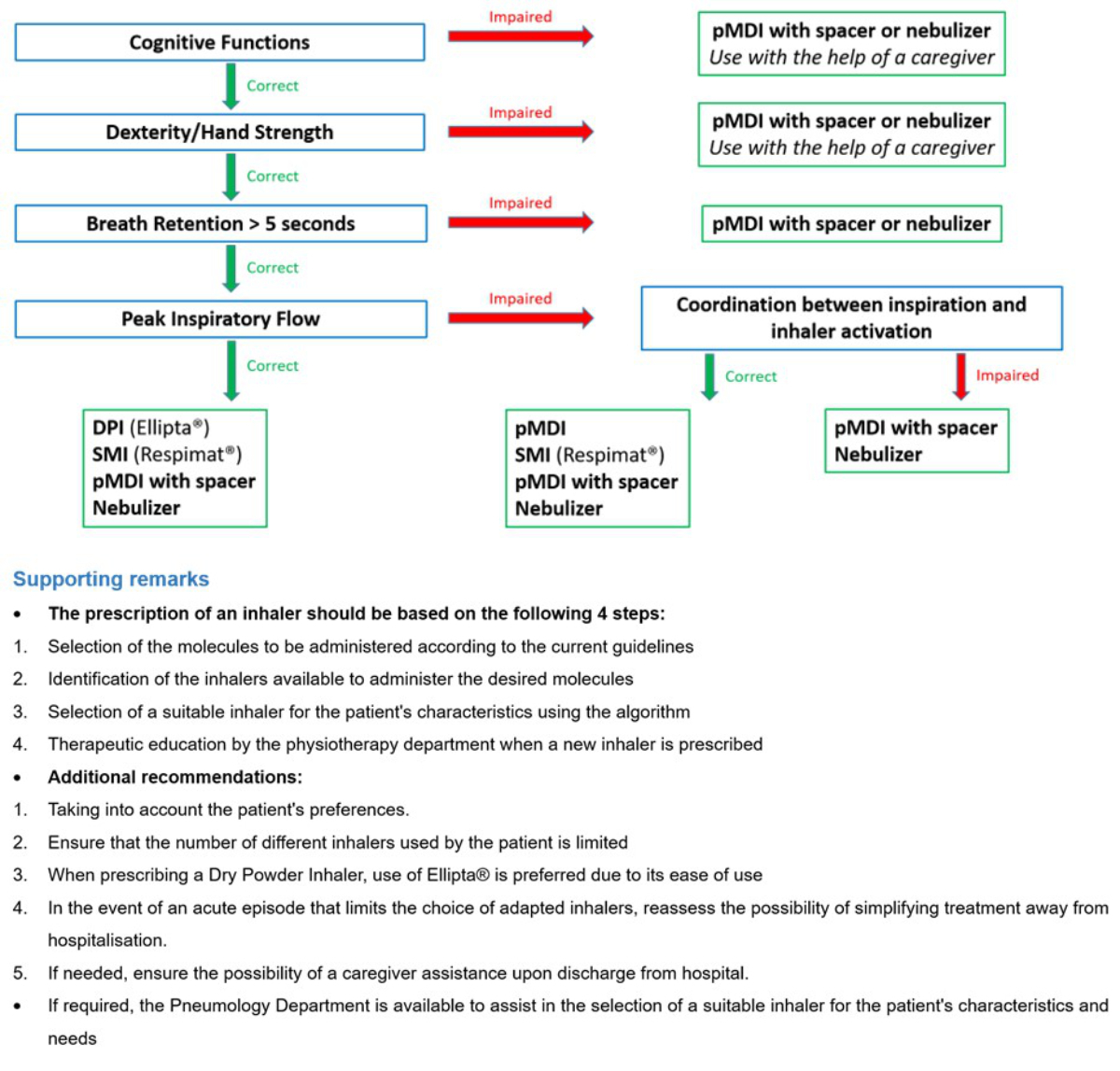
Figure S2Written guide to assist in the selection of an adapted inhaler according to patient characteristics. Patient characteristics are assessed based on demonstration of inhalation technique and measurement of peak inspiratory flow using the In-Check DIAL G16® by the physiotherapy department on admission. DPI: dry powder inhaler; pMDI: pressurized metered-dose inhaler; SMI: soft mist inhaler.