Inpatient opioid prescribing patterns and their effect on rehospitalisations: a nested case-control study using data from a Swiss public acute hospital
DOI: https://doi.org/https://doi.org/10.57187/s.3391
Aleksandra Stanisica*,
Dominik Stämpfliab*,
Angela E. Schulthess Lisibachc,
Monika Luttersd,
Andrea M. Burdena
a Institute of Pharmaceutical Sciences, ETH Zurich, Zurich, Switzerland
b Hospital Pharmacy, Kantonsspital Baden, Baden, Switzerland
c Institute
of Primary Health Care (BIHAM), Faculty of Medicine, University of Bern, Bern, Switzerland
d Hospital Pharmacy, Kantonsspital Aarau, Aarau, Switzerland
* These authors
contributed equally to this work.
Summary
AIMS OF THE STUDY: Opioid prescriptions
have increased in Switzerland, even though current guidelines warn of their harms.
If opioids for postoperative analgesia are not tapered before hospital discharge,
patients are at risk of adverse events such as constipation, drowsiness, dependence,
tolerance and withdrawal. The aim of this study was to investigate and quantify
the potential association between opioids prescribed at discharge from hospital
and rehospitalisation.
METHODS: We conducted a nested case-control study using routinely collected
electronic health records from a Swiss public acute hospital. Cases were patients
aged 65 years or older admitted between November 2014 and December 2018, with documented
opioid administration on the day of discharge and rehospitalisation within 18 or
30 days after discharge. Each case was matched to five controls for age, sex, year
of hospitalisation and Charlson Comorbidity Index. We calculated odds ratios for
18-day and 30-day rehospitalisation based on exposure to opioids using a conditional
logistic regression adjusted for potential confounders. Secondary analyses included
stratifications into morphine-equivalent doses of
<50 mg, 50–89 mg and ≥90 mg, and co-prescriptions of gabapentinoids and benzodiazepines.
RESULTS: Of 22,471 included patients, 3144 rehospitalisations were identified,
of which 1698 were 18-day rehospitalisations and 1446 were 30-day rehospitalisations.
Documented opioid administration on the day of discharge was associated with 30-day
rehospitalisation after adjustment for confounders (adjusted odds ratio 1.48; 95%
CI 1.25–1.75, p <0.001), while no difference was observed in the likelihood of
18-day rehospitalisation. The combined prescription of opioids with benzodiazepines
or gabapentinoids and morphine-equivalent doses >50 mg were rare.
CONCLUSIONS: Patients receiving opioids on the day of discharge were 48% more likely
to be readmitted to hospital within 30 days. Clinicians should aim to discontinue
opioids started in hospital before discharge if possible. Patients receiving an
opioid prescription should be educated and monitored as part of opioid stewardship
programmes.
Introduction
Opioids are indicated for moderate or severe
postoperative pain [1], but all patients undergoing
surgery should be considered at risk of developing persistent postoperative opioid
use [2]. Data
from Canada show that about 7% of patients still have opioid prescriptions
seven days after minor surgery, with a 44% increased risk of becoming long-term
opioid users [3]. Interviews with patients with prescription opioid use disorder revealed
that opioid treatment is often initiated in secondary care with little information
about the potential risks of opioid use, and that the treatment is then continued
in primary care without additional consultation [4].
Therefore, if opioids have been started for postoperative pain as part of multimodal
analgesia, they should be weaned before hospital discharge if possible [2] and must have a definite end date [5].
In Switzerland, opioid sales increased by 91%
between the years 2000 and 2019, with a particularly marked increase for oxycodone
[6]. Their use contradicts current treatment guidelines [7, 8], as opioids seem
to be primarily used for pain of non-malignant origin [9]. The increase in sales
in Switzerland has been shown to be accompanied by an increase in the number of
poisoning cases reported to the Swiss Tox Centre [6]. Harm from opioids includes
physical and psychological dependence, tolerance, withdrawal, drowsiness, confusion,
constipation, dry mouth, nausea, vomiting and rehospitalisations [1, 8, 10]. Rehospitalisations are “a return to
the hospital shortly after discharge from a recent hospital stay” and their rates
are used to assess the quality of hospital care [11].
A variety of factors influence the risk of being rehospitalised, including demographic
factors, comorbidities, complexity of hospitalisations, previous hospitalisations
and further social and medical factors [11–13]. These factors can be used in prediction
models to identify patients at risk of rehospitalisation early for preventive measures.
One of these models, the Potentially Avoidable Readmission-Risk Score (PAR-Risk
Score), uses readily available electronic health information to calculate a risk
score. Although an external validation noted a poor performance within the given
dataset overall, 5 of the 12 predictors were still identified to be associated with
rehospitalisation at 30 days. The use of opioids was one of these predictors [14].
The present study aimed to further investigate
and quantify the potential association between opioids prescribed at hospital discharge
and the risk of rehospitalisation, also depending on their dose. This study had
the additional aim of exploring the effect of combining opioids with benzodiazepines
and gabapentinoids on rehospitalisations, as their co-prescription carries an additional
risk of sedation, increased risk of falls, respiratory depression and overdose,
and is considered inappropriate for older patients [8, 15].
Materials and methods
Study design and data source
We conducted a nested case-control study using
routinely collected electronic health records from a Swiss public acute hospital.
The hospital is one of two public hospitals in the canton, each serving one geographical
area. A previously generated dataset of inpatients (surgical and non-surgical) over
the age of 65 and hospitalised for at least 48 hours between November 2014 and December
2018 was used as the base cohort for identifying cases and controls [16]. The dataset,
originally sourced from the hospital’s clinical information system for each patient,
provided detailed information on demographic characteristics, comorbidities including
the Charlson Comorbidity Index, and medication use during hospital stay, and was
used by medical coders for claiming insurance payments [16]. Patients who were in
intensive care for more than 24 hours had to be excluded from dataset generation
because a different clinical information system is used for them.
We report this study according to the RECORD-PE
(REporting of studies Conducted using Observational Routinely collected Data for
PharmacoEpidemiological research) checklist [17]. A study protocol was not previously
published.
Patient selection and outcomes
From the base cohort, eligible patients for
the nested cohort were those who were discharged from the hospital (cohort entry
date). We excluded patients with mental and behavioural disorders caused by opioids
and by multiple substances (ICD-10 F11 and F12), and patients with malignant neoplasms
(ICD-10 C and D). Patients with skin cancer (ICD-10 C43 and C44) were not excluded,
because of the lesser association with pain and opioids [18].
Within this nested cohort, we identified patients
who were rehospitalised 18 and 30 days after discharge (cases). Potential controls
were randomly selected from the nested cohort who were not rehospitalised within
the 18 or 30 days following discharge, respectively.
A visualisation of the study design is shown
in figure 1. [19]
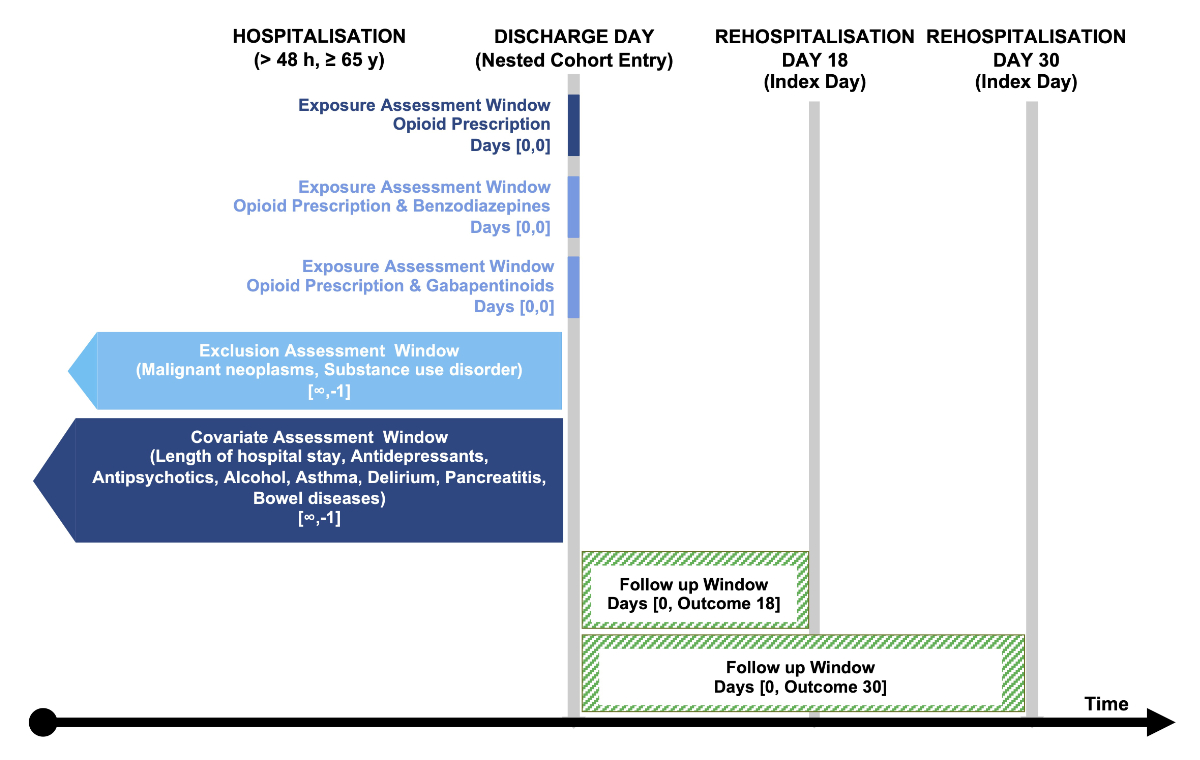
Figure 1Visualisation of the study design depicting nested cohort entry,
index day (18-day or 30-day rehospitalisation), and the assessment windows for the
exposures, inclusion and exclusion criteria, and covariates. Visualisation template
by Schneeweiss et al. [19].
Exposure
The primary exposure of interest was defined
as a documented opioid administration on the cohort entry date (i.e. the last day
of hospitalisation), assuming that patients were discharged with an opioid prescription
as well. Opioids considered were buprenorphine, codeine, dihydrocodeine, fentanyl,
hydromorphone, morphine, methadone, oxycodone, tapentadol, tilidine and tramadol.
Methadone is used by the pain service of this hospital as an additional strong opioid
for opioid-refractory pain and is therefore not an indicator of substitution treatment.
All parenteral, intramuscular and subcutaneous formulations were excluded as these
can only be administered with the assistance of medical personnel and a switch to
oral use would not usually occur on the day of discharge.
In a secondary exposure analysis, we calculated
the daily dose of opioids on the last day of hospitalisation for each patient. The cumulative daily morphine-equivalent
doses (MEDs) were determined by strength of dosing unit, number of units per day
and the morphine conversion factor of the opioid. We used the carefully curated
and published conversion factors by Wertli and colleagues [20]. Morphine-equivalent doses were then stratified into doses of <50 mg,
50–89 mg and ≥90 mg [21].
In an additional
secondary exposure analysis, we examined patients who received a co-prescription
of opioids and benzodiazepines or a co-prescription of opioids and gabapentinoids.
Benzodiazepine and gabapentinoid prescriptions were restricted to oral and rectal
formulations.
As part of the review process, a further secondary
exposure analysis was proposed based on the categorisation of weak and strong opioids.
Opioids considered as weak were codeine, dihydrocodeine and tramadol, including
combination formulations with paracetamol.
Covariates
We assessed covariates potentially associated
with the exposure and rehospitalisation from data collected prior to cohort entry
on the day of discharge, which included age, sex, year of discharge, Charlson Comorbidity
Index, length of stay, department (medicine, surgery), alcohol use disorder, delirium
during hospital stay, asthma, pancreatitis, chronic inflammatory bowel diseases and documented administration
of antidepressants and antipsychotics.
Statistical analysis
One case was matched to five controls on age,
sex, year of discharge and Charlson Comorbidity Index (strata: 0, 1–2, 3–4, ≥5) [19].
Matching was performed separately for 18- and 30-day rehospitalisations. We required
an exact match for sex and year, and allowed a caliper of 5/standard deviation
for age, and a mean standard difference
of 0.2 for the Charlson Comorbidity Index ranges [22]. The matched cases and controls for 18- and 30-day rehospitalisations
were also used for the secondary outcomes.
Descriptive statistics and standardised differences
were used to summarise and compare the patient characteristics of matched cases
and controls, where a standardised difference >0.1 indicates a clinically important
difference [23]. Using the exposure information, we calculated the frequency of
opioid administration by opioid on the last day of hospitalisation. Two continuous
variables were transformed into categorical variables: the Charlson Comorbidity
Index (0; 1–2; 3–4; ≥5) and morphine-equivalent doses (<50 mg; 50–89 mg; ≥90
mg). To normalise the distribution, we performed a logarithmic transformation on
the variable length of stay. Missing entries were interpreted as the absence of
the variable (i.e. not missing at random), as the dataset was also used by medical
coders to claim insurance payments.
To compare the odds of rehospitalisation under
the influence of each exposure, we estimated the odds ratios (OR) and 95% confidence
intervals (CI) using conditional logistic regression. Additionally, our models were
adjusted for potentially confounding variables based on standardised
differences after matching. Model 1 included adjustments for covariates that showed
a standardised difference of >0.1, while model 2 included adjustments for covariates
that showed a standardised difference of ≥0.1 (after rounding to one decimal point).
A sample size calculation was performed to adequately
interpret the results. An a priori sample size calculation (one-sided Fisher’s exact
test) was performed with an alpha of 0.05, a power of 0.8 and an allocation ratio
of 1:5 using previously determined proportions of opioid use in rehospitalised (36.2%)
and non-rehospitalised (26.0%) patients within the same dataset [14, 24]. The sample
size calculation indicated a minimum sample size of 169 opioid-exposed rehospitalisations
and 845 matched controls for statistically correct conclusions.
All
analyses were performed using R statistical software (v 4.2.3) [25]. Matching was performed with the optmatch
package (v 0.10.6) [26]. Standardised differences
were calculated using the stddiff package (v 3.1) [27]. Odds ratios were calculated using the epitools
package (v 0.5.10.1) [28].
Ethics approval
The Ethics Committee of Northwest and Central
Switzerland approved the protocol for the study from which the data were originally
extracted (EKNZ project ID: 2018-01000). The committee also approved the amendment
for the rehospitalisation study. The data were extracted anonymously and informed
consent was not required.
Results
Out of 28,276 inpatient cases, aged 65 years
or older, who were discharged alive between 2014 and 2018, we excluded 5781 cancer
patients and 26 patients with mental and behavioural disorders caused by opioids
and by multiple substance use (with overlapping diagnoses). From the remaining 22,471
patient cases, a total of 3144 rehospitalisations were identified, of which 1698
were rehospitalised within 18 days after discharge and 1446 within 30 days after
discharge. After matching, 8490 controls were assigned to 1698 18-day rehospitalisations
and 7230 controls to 1446 30-day rehospitalisations. A detailed overview of the
number of patients can be found in figure 2.
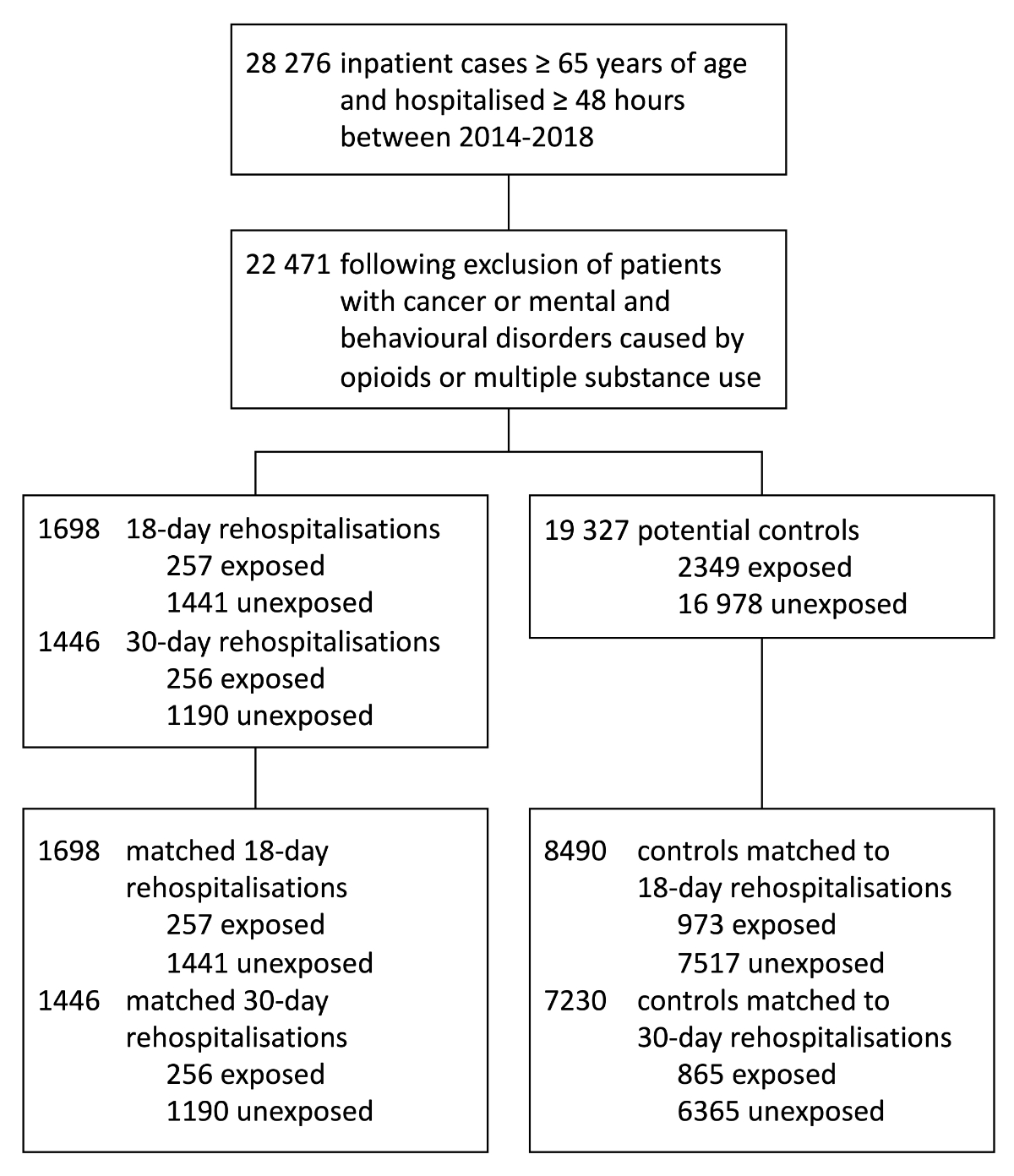
Figure 2Flowchart of included inpatient cases
and 18-day and 30-day rehospitalisations with matched controls and opioid exposure
status on the last day of hospitalisation.
Baseline characteristics of cases and matched
controls for both the 18-day and 30-day outcomes are shown in table 1. Patients’
characteristics were well balanced on all matching criteria.
Table 1Baseline characteristics of matched
cases and controls, stratified by 18-day and 30-day rehospitalisations. Variables
had no missing data.
| Rehospitalisation within 18 days | Rehospitalisation within 30 days |
| Cases (n = 1698) | Controls (n = 8490) | Std. Diff. | Cases (n = 1446) | Controls (n = 7230) | Std. Diff. |
| Age in years, mean ± SD | 78.06 ± 7.36 | 78.06 ± 7.32 | <0.01 | 79.14 ± 7.49 | 79.14 ± 7.45 | <0.01 |
| Male, n (%) | 880 (51.8%) | 4400 (51.8%) | <0.01 | 691 (47.8%) | 3455 (47.8%) | <0.01 |
| Year of discharge, n (%) | | | <0.01 | | | <0.01 |
| 2015 | 382 (22.5%) | 1907 (22.5%) | | 339 (23.4%) | 1680 (23.2%) | |
| 2016 | 409 (24.1%) | 2045 (24.1%) | | 360 (24.9%) | 1818 (25.1%) | |
| 2017 | 442 (26.0%) | 2229 (26.3%) | | 388 (26.8%) | 1944 (26.9%) | |
| 2018 | 465 (27.4%) | 2309 (27.2%) | | 359 (24.8%) | 1788 (24.7%) | |
| Charlson Comorbidity Index, n (%) | | | <0.01 | | | <0.01 |
| 0 | 403 (23.7%) | 2015 (23.7%) | | 307 (21.2%) | 1535 (21.2%) | |
| 1–2 | 838 (49.4%) | 4191 (49.4%) | | 768 (53.1%) | 3840 (53.1%) | |
| 3–4 | 389 (22.9%) | 2013 (23.7%) | | 320 (22.1%) | 1620 (22.4%) | |
| ≥5 | 68 (4.0%) | 271 (3.2%) | | 51 (3.5%) | 235 (3.3%) | |
| Length of stay in days, median (IQR) | 14 (12) | 7 (7) | 0.85 | 8 (8) | 7 (7) | 0.20 |
| Department, n (%) | | | 0.05 | | | 0.03 |
| Medicine | 1031 (60.7%) | 5343 (62.9%) | | 915 (63.3%) | 4661 (64.5%) | |
| Surgery | 667 (39.3%) | 3147 (37.1%) | | 531 (36.7%) | 2569 (35.5%) | |
| Prescriptions, n (%) |
| Opioid | 257 (15.1%) | 973 (11.5%) | 0.11 | 256 (17.7%) | 865 (12.0%) | 0.16 |
| Weak* | 37 (2.2%) | 135 (1.6%) | 0.04 | 34 (2.4%) | 107 (1.5%) | 0.06 |
| Opioid dose, n (%) |
| MED <50 mg | 215 (12.7%) | 846 (10.0%) | 0.09 | 223 (15.4%) | 753 (10.4%) | 0.15 |
| MED 50–89 mg | 12 (0.7%) | 63 (0.7%) | <0.01 | 12 (0.8%) | 57 (0.8%) | 0.01 |
| MED ≥90 mg | 30 (1.8%) | 64 (0.8%) | 0.09 | 21 (1.5%) | 55 (0.8%) | 0.07 |
| Benzodiazepines**, n (%) | 13 (0.8%) | 48 (0.6%) | 0.03 | 13 (0.9%) | 44 (0.6%) | 0.03 |
| Gabapentinoids**, n (%) | 44 (2.6%) | 137 (1.6%) | 0.07 | 38 (2.6%) | 126 (1.7%) | 0.06 |
| Confounders, n (%) |
| Antidepressants | 532 (31.3%) | 2641 (31.1%) | 0.01 | 527 (36.4%) | 2399 (33.2%) | 0.07 |
| Antipsychotics | 462 (27.2%) | 2181 (25.7%) | 0.03 | 448 (31.0%) | 1962 (27.1%) | 0.09 |
| Alcohol use disorder | 46 (2.7%) | 268 (3.2%) | 0.03 | 46 (3.2%) | 201 (2.8%) | 0.02 |
| Delirium | 200 (11.8%) | 761 (9.0%) | 0.09 | 150 (10.4%) | 718 (9.9%) | 0.02 |
| Asthma | 36 (2.1%) | 208 (2.4%) | 0.02 | 42 (2.9%) | 187 (2.6%) | 0.02 |
| Pancreatitis | 21 (1.2%) | 55 (0.6%) | 0.06 | 12 (0.8%) | 52 (0.7%) | 0.01 |
| Chronic inflammatory bowel diseases | 12 (0.7%) | 59 (0.7%) | <0.01 | 16 (1.1%) | 49 (0.7%) | 0.05 |
18-day rehospitalisation
The matched sample for the 18-day rehospitalisation
group consisted of patients with an average age of approximately 78 years, with
51.8% being males. Most patients exhibited a Charlson Comorbidity Index of 1–2,
with a minority having an index of 5 or higher. The median length of stay was significantly
longer for cases compared to matched controls (14 days [interquartile range 9–21]
vs 7 days [4–11]). Analysis of documented opioid administration on the last day
of hospitalisation revealed that 15.1% of patients being readmitted received an
opioid, while only 11.5% of their matched controls did. The majority of opioid administrations
involved morphine-equivalent doses below 50 mg. Co-prescription rates of benzodiazepines
and gabapentinoids with opioids were low.
30-day rehospitalisation
In the matched sample for the 30-day rehospitalisation
group, patients had an average age of approximately 79 years, with 47.8% being males.
Similar to the 18-day rehospitalisation group, most patients had a Charlson Comorbidity
Index of 1–2. The median length of stay was again longer for cases compared to matched
controls (8 days [5–13] vs 7 days [4–11]), although less pronounced than in the
18-day rehospitalisation group. Analysis of opioid administration on the day of
discharge showed a less balanced distribution between cases and controls compared
to the 18-day rehospitalisation group, with 17.7% of rehospitalised patients having
a documented opioid administration on the last day of hospitalisation, compared
to 12.0% of matched controls.
Overall analysis
Aggregated over both rehospitalisation groups,
oxycodone/naloxone was the opioid most commonly administered on the last day of
hospitalisation (37.2%), followed by morphine (14.8%), oxycodone (14.7%) and tramadol
(9.0%) (appendix table S1).
Unadjusted and adjusted ORs and 95% CIs for
both the 18-day and 30-day rehospitalisation groups stratified by exposure are presented
in table 2.
Adjusted ORs and 95% CIs are visualised in figure 3. Prior to adjustment,
documented opioid administration on the last day of hospitalisation was associated
with 18-day rehospitalisation (OR 1.39; 95% CI 1.19–1.61, p = 0.001) and 30-day
rehospitalisation (OR 1.60; 95% CI 1.38–1.88, p = 0.001). After adjusting for the
covariates length of stay, department, gabapentinoids, delirium and pancreatitis
for 18-day rehospitalisation, and length of stay, gabapentinoids, antidepressants,
antipsychotics and chronic inflammatory bowel diseases for 30-day rehospitalisation
in our conditional logistic regression, only 30-day rehospitalisation maintained
its statistically significant association with opioid administration (adjusted
odds ratio [aOR] 1.48; 95% CI 1.25–1.75, p <0.001).
Table 2Unadjusted and adjusted odds
ratios for 18-day and 30-day rehospitalisation stratified by exposure.
Exposures included any documented opioid administration on the last day of
hospitalisation, opioid doses with morphine-equivalent doses, co-prescribed
benzodiazepines and co-prescribed gabapentinoids.
| Number of exposed cases | Number of exposed controls | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI), Model 1* | Adjusted Odds Ratio (95% CI), Model
2** |
| Primary analysis | 18-day rehospitalisation | 257 | 973 | 1.39 (1.19–1.61) | 0.89 (0.75–1.06) | 0.90 (0.74–1.08) |
| 30-day rehospitalisation | 256 | 865 | 1.60 (1.38–1.88) | 1.49 (1.27–1.74) | 1.48 (1.25–1.75) |
| Secondary analysis: Morphine-equivalent
dose (MED) | 18-day rehospitalisation | MED <50 mg | 215 | 846 | 1.32 (1.12–1.55) | 0.89 (0.74–1.07) | 0.90 (0.74–1.09) |
| MED 50–89 mg | 12 | 63 | 0.95 (0.51–1.77) | 0.54 (0.26–1.12) | 0.58 (0.28–1.22) |
| MED ≥90 mg | 30 | 64 | 2.37 (1.53–3.66) | 1.26 (0.78–2.08) | 1.28 (0.76–2.16) |
| 30-day rehospitalisation | MED <50 mg | 223 | 753 | 1.59 (1.35–1.87) | 1.49 (1.26–1.76) | 1.46 (1.23–1.73) |
| MED 50–89 mg | 12 | 57 | 1.05 (0.56–1.97) | 0.93 (0.49–1.74) | 0.81 (0.43–1.54) |
| MED ≥90 mg | 21 | 55 | 1.92 (1.16–3.18) | 1.66 (1.00–2.77) | 1.53 (0.91–2.60) |
| Secondary analysis: Co-prescribed gabapentinoids*** | 18-day rehospitalisation | 44 | 137 | 1.62 (1.15–2.23) | 0.92 (0.62–1.37) | 0.90 (0.60–1.33) |
| 30-day rehospitalisation | 38 | 126 | 1.54 (1.06–2.24) | 1.38 (0.95–2.01) | 1.35 (0.92–1.97) |
| Secondary analysis: Co-prescribed benzodiazepines | 18-day rehospitalisation | 13 | 48 | 1.36 (0.73–2.52) | 1.21 (0.61–2.39) | 1.30 (0.65–2.56) |
| 30-day rehospitalisation | 13 | 44 | 1.48 (0.80–2.76) | 1.38 (0.74–2.57) | 1.29 (0.69–2.42) |
| Secondary analysis: Weak opioids**** | 18-day rehospitalisation | 37 | 135 | 1.38 (0.95–1.99) | 1.37 (0.90–2.09) | 1.46 (0.97–2.18) |
| 30-day rehospitalisation | 34 | 107 | 1.61 (1.09–2.38) | 1.62 (1.10–2.41) | 1.60 (1.08–2.38) |
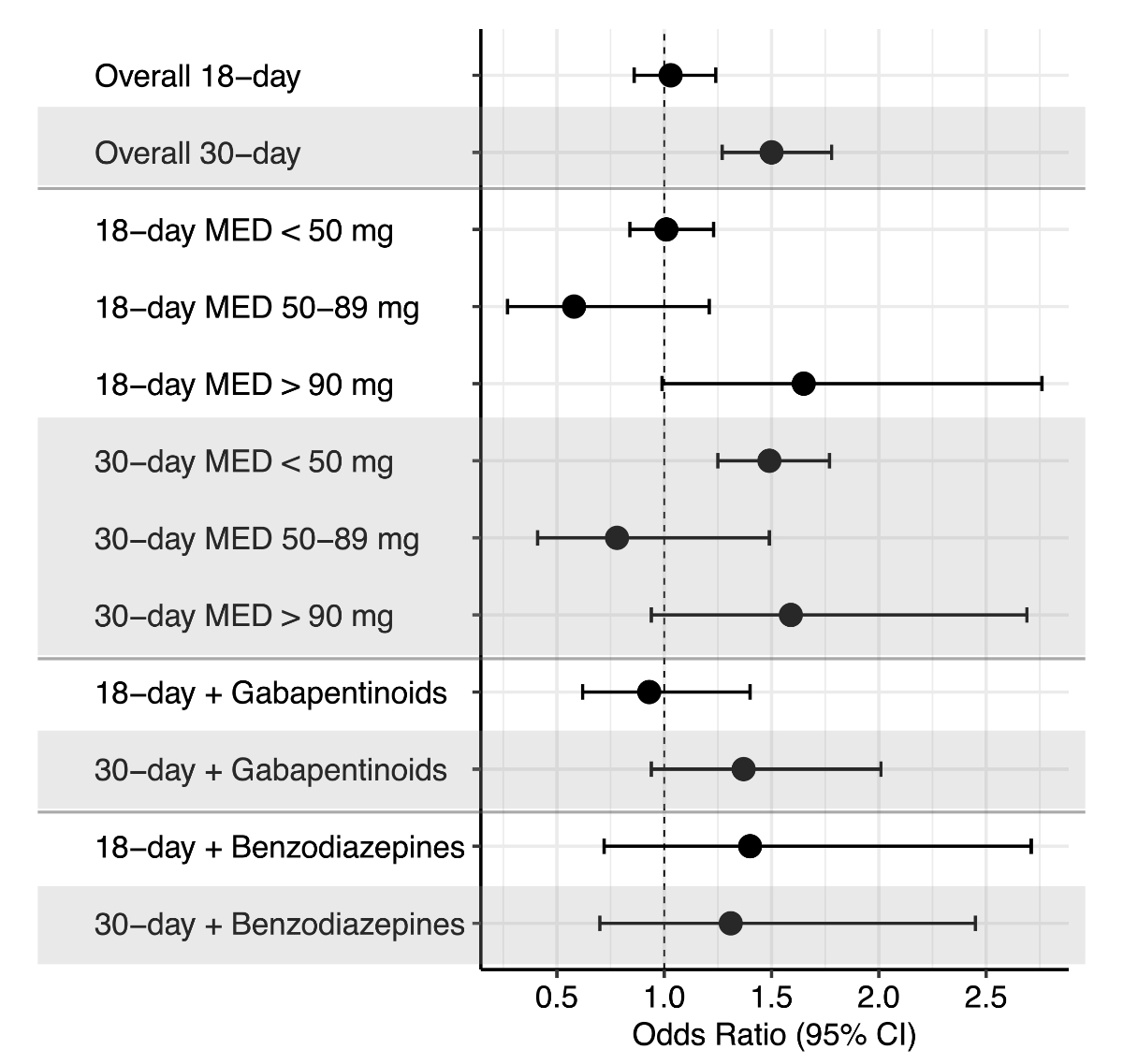
Figure 3Adjusted odds ratios and 95% confidence
intervals (95% CI) for 18-day and 30-day rehospitalisation stratified by exposure. Exposures
included any documented opioid administration on the last day of hospitalisation,
opioid dose as morphine-equivalent dose (MED), co-prescribed benzodiazepines and
co-prescribed gabapentinoids. Adjustment was made for covariates that showed a standardised
difference ≥0.1 (rounded): length of stay, department, gabapentinoids, delirium
and pancreatitis for 18-day rehospitalisation; length of stay, gabapentinoids, antidepressants,
antipsychotics and chronic inflammatory bowel diseases for 30-day rehospitalisation
(Model 2).
Secondary exposure analysis
In the secondary exposure analysis, only morphine-equivalent
doses <50 mg were associated with 30-day rehospitalisation (aOR 1.46; 95% CI
1.23–1.73, p <0.001), while none of the stratified morphine-equivalent doses
were statistically significantly associated with 18-day rehospitalisation after
adjustment (figure 3).
In the additional secondary exposure analysis,
which included co-prescription of opioids and benzodiazepines or co-prescription
of opioids and gabapentinoids, none of the exposures resulted in a statistically
significant association with 18-day or 30-day rehospitalisation (figure 3).
In the suggested further secondary exposure
analysis, which included the categorisation of weak and strong opioids, weak opioids
(codeine, dihydrocodeine, tramadol) were associated with 30-day rehospitalisation
(aOR 1.60; 95% CI 1.08–2.38, p <0.01).
Discussion
In this case-control study of patients aged
65 years and older who were hospitalised for more than 48 hours, patients taking
opioids on the day of discharge were 48% more likely to be rehospitalised within
30 days, while no difference was observed for the likelihood of 18-day rehospitalisation.
When stratified by combined prescription of opioids with benzodiazepines or with
gabapentinoids and morphine-equivalent dose >50 mg, no significant differences
in rehospitalisation were identified; however, the number of events was low and
did not reach the necessary sample size.
Our finding of a 48% increased risk of 30-day
rehospitalisation is consistent with estimates from the external validation of the
PAR-Risk Score, where the prevalence of opioids was 36.2% in rehospitalised patients
and 26.0% in non-rehospitalised patients [14]. Woitok and colleagues, who examined
patterns of prescription opioid use among patients presenting to Swiss emergency
departments, also calculated an increased aOR of 3.57 (95% CI 2.87–4.44, p <0.001)
for the association between opioid use and hospital readmission within 30 days [10].
The higher aOR compared to our results may be explained by the fact that Woitok
and colleagues included less serious adverse effects of opioids leading to an emergency
department visit without hospitalisation and, in addition, patients with neoplasms
prone to hospitalisation. Potentially less serious adverse effects include gastrointestinal
symptoms such as constipation, nausea and vomiting, as well as somnolence, dizziness,
delirium, euphoria, sedation and cholinergic effects such as bradycardia or sweating
[29]; approximately 80% of patients prescribed opioids will experience at least
one adverse effect [30], even with short-term
use [31]. In addition, dizziness and fatigue can lead to more serious outcomes such
as falls, fractures and traffic accidents [31–34]. Herzig and colleagues were able
to show that patients aged 65 years and older with an opioid claim one week after
hospital discharge had a higher incidence of death, healthcare utilisation and any
potential adverse effects, including falls and fractures, compared with a matched
active control group who had only claimed nonsteroidal anti-inflammatory drugs [35].
The association between taking opioids on the
day of discharge and 18-day rehospitalisation lost its statistical significance
after adjusting for the potential confounder length of stay and in a second model
when adjusted for length of stay, department, gabapentinoids, delirium and pancreatitis.
Length of stay is an important variable in predictive models of 30-day rehospitalisation
which may have influenced our results [36, 37].
Although adjusted for and matched on Charlson Comorbidity Score, the difference
in length of stay could be an indication that the cases were more complex patients
overall. Kurteva and colleagues showed that current opioid use was associated with
opioid-related adverse events, with the risk increasing with cumulative exposure:
compared with shorter exposures of 1 to 30 days, longer exposures (60 to 90 days
and >90 days) were associated with a 2-fold increase in the risk of adverse events
[31]. In their analysis of patients with repeated
opioid claims from 2006 to 2014 in Switzerland, Burgstaller and colleagues observed
a clear dose-response relationship between opioid intake and hospitalisation rates
[38]. In particular, hospital admissions were
significantly higher for daily doses above 100 mg, at 54%, compared with 10.7% for
doses below 20 mg. In addition, the duration of opioid treatment showed a steady
increase in the odds with prolonged use, particularly in chronic (>90 days) and
very chronic (>360 days) cases. An analysis of Cochrane reviews that focused
on adverse events associated with opioid use for “medium” (two weeks to two months)
or “long-term” (two months or longer) treatment of chronic non-cancer pain showed
a significantly higher risk of adverse events with opioids than with placebo [39]. In addition, there was a higher rate of withdrawal
from the trials due to adverse events compared to an active comparator. Adverse
events included constipation, dizziness, drowsiness, fatigue, hot flushes, increased
sweating, nausea, pruritus and vomiting.
In our analysis, oxycodone was identified as
the most prescribed opioid. The same prescription pattern was, again, observed by
Woitok and colleagues in patients presenting to Swiss emergency departments [10].
Switzerland has seen a significant increase in oxycodone sales from 2000 to 2019,
with a market share of 12.9%, second only to tramadol [6]. Standardised to the
population, the number of any opioid sales increased by 91.3%, while the rate of
calls for opioid-related poisonings to the Swiss National Poisons Information
Centre also increased by 177%, consequently increasing the risk of rehospitalisation.
A study from the Netherlands, where sales and poisonings increased as well, additionally
showed an increase in the hospital admission rate due to poisoning by (prescription)
opioids [6, 40].
Most patients in our dataset had received opioid
doses of less than 50 mg morphine-equivalent dose, which is also true of the analysis
of patients in Swiss emergency departments by Woitok and colleagues, with a median
morphine-equivalent dose of 30 mg [10]. Usually, an increase in risk with higher
morphine-equivalent dose would be expected [31, 41]. Gomes and colleagues similarly
found an unexpectedly attenuated effect on road trauma in their highest dose category,
which also contradicted otherwise clear dose-response relationships. They discussed
this attenuation with the likelihood of drug diversion and physiological opioid
tolerance in this patient population [34].
However, the most likely explanation for our results is that stratification outside
the >50 mg dose range resulted in sample sizes that were too small to be statistically
significant and hence should be interpreted with utmost caution. The same applies
to the additional secondary analysis, which should have looked at the co-prescription
of gabapentinoids and benzodiazepines. Here, according to a study by Wertli and
colleagues, we would have expected the prevalence of opioids and concomitant benzodiazepines
in chronic non-cancer pain to be around one third [9].
Limitations
Our analysis has important limitations, which
have implications for generalisability and possibly influence the true effect of
opioids on rehospitalisation. In particular, no direct causal relationship can be
inferred between opioid prescriptions at discharge and rehospitalisation, but rather
an association. First, our sample of inpatients over 65 years of age may have been
in poorer health than the general discharged population. Second, as there was no
information on hospital stays of less than 48 hours, it is possible that a proportion
of patients with opioid-related complaints (e.g. constipation, nausea) were not
present in our sample. Therefore, the adverse effects of opioids may have been underestimated.
Third, discharge with an opioid was presumed by a documented use of an opioid on
the day of discharge, excluding patients on parenteral opioids. We were not able
to monitor actual opioid use after discharge. It is therefore possible that the
actual number of opioid users after discharge differs from our figures. Fourth,
the cause of 30-day rehospitalisation and the indication for opioids were unknown. While
administrative 18-day rehospitalisations only included unplanned rehospitalisations,
it is possible that a 30-day rehospitalisation was not related to the initial hospitalisation
or that the indication treated with opioids led to hospitalisation (e.g. exacerbated
pain), leading to an overestimation of the association between opioids and rehospitalisation.
At the same time, the patients could not be followed up, so any hospitalisations
in other institutions could not be identified. Fifth, while we matched patients
based on disease status using the Charlson Comorbidity Index and length of hospital
stay, we cannot rule out the possibility that there are important unmeasured confounders.
For example, socioeconomic status and family support may be important mitigators
of the likelihood of rehospitalisation. Finally, we had a rather small number of
patients in some of the exposure groups, which ultimately limited the interpretability
of some results. By including a sample size calculation, we avoided drawing erroneous
conclusions from these results.
Implications for practice
In light of our findings and those of others
who have shown that opioid use at or after hospital discharge is associated with
an increased likelihood of rehospitalisation, it is recommended that opioid stewardship
programmes be enhanced/implemented to encourage tapering of opioid use during or
immediately after hospitalisation [5, 42].
Opioid stewardship is described as “coordinated interventions designed to improve,
monitor, and evaluate the use of opioids in order to support and protect human health”
[43]. Besides interventions at different levels
and monitoring, a key element is patient education, including information on risks
and adverse effects, and clear instructions on appropriate weaning after discharge
from hospital [2]. While our study cannot
be used to make causal inferences about opioids causing adverse effects leading
to rehospitalisation, our results show that patients receiving opioids are at
risk of rehospitalisation and need to be cared for.
Conclusion
This study found a significant association between
opioid prescription at discharge and 30-day rehospitalisations in patients aged 65 years
or older. Oxycodone was the most prescribed opioid in this dataset from a tertiary
teaching hospital. Clinicians should be aware of the potential adverse effects of
prescribing opioids at discharge and should strive to discontinue opioids started
in hospital before discharge, or give patients and carers clear instructions for
weaning after hospitalisation. Patients should be educated and monitored, potentially
as part of opioid stewardship programmes.
Availability
of data and materials
The dataset analysed and the
unformatted statistical code used are available from the corresponding author upon
request.
Acknowledgments
The authors would like to
thank Patrick E. Beeler for his contributions to the processing of the original
dataset.
Authors’
contributions: Aleksandra Stanisic: conceptualisation,
methodology, formal analysis, writing – original draft. Dominik Stämpfli:
supervision, conceptualisation, methodology, re-analysis, visualisation, writing
– original draft; Angela E. Schulthess Lisibach: data curation, writing – review
and editing. Monika Lutters: data funding, writing – review and editing. Andrea
M. Burden: supervision, conceptualisation, writing – review and editing.
Prof. Andrea Burden, PhD
Institute of Pharmaceutical
Sciences
Department of Chemistry and
Applied Biosciences
Swiss Federal Institute of
Technology, ETH Zurich
Vladimir-Prelog-Weg 4
CH-8093 Zurich
andrea.burden[at]pharma.ethz.ch
References
1. Ari M, Alexander JT, Weyer G. Prescribing Opioids for Pain. JAMA. 2023 May;329(20):1789–90. 10.1001/jama.2023.6539
2. Levy N, Quinlan J, El-Boghdadly K, Fawcett WJ, Agarwal V, Bastable RB, et al. An international multidisciplinary consensus statement on the prevention of opioid-related harm in adult surgical patients. Anaesthesia. 2021 Apr;76(4):520–36. 10.1111/anae.15262
3. Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012 Mar;172(5):425–30. 10.1001/archinternmed.2011.1827
4. Davies LEM, Koster ES, Damen KFM, et al. Patients’ Perspectives on the Development of Prescription Opioid Use Disorder in Patients with Chronic Non-Cancer Pain. Eur Addict Res 2023.29(2):141–9. https://doi.org/10.1159/000529926
5. Levy N, Lord LJ, Lobo DN. UK recommendations on opioid stewardship. BMJ. 2021 Jan;372:m4901. 10.1136/bmj.m4901
6. Hooijman MF, Martinez-De la Torre A, Weiler S, Burden AM. Opioid sales and opioid-related poisonings in Switzerland: A descriptive population-based time-series analysis. Lancet Reg Health Eur. 2022 Jun;20:100437. 10.1016/j.lanepe.2022.100437
7. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—united States, 2016. JAMA. 2016 Apr;315(15):1624–45. 10.1001/jama.2016.1464
8. Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain - United States, 2022. MMWR Recomm Rep. 2022 Nov;71(3):1–95. 10.15585/mmwr.rr7103a1
9. Wertli MM, Held U, Signorell A, Steurer J, Blozik E, Burgstaller JM. Opioid Prescription in Switzerland: Appropriate Comedication use in Cancer and Noncancer Pain. Pain Physician. 2019 Nov;22(6):537–48. 10.36076/ppj/2019.22.537
10. Woitok BK, Büttiker P, Ravioli S, Funk GC, Exadaktylos AK, Lindner G. Patterns of prescription opioid use in Swiss emergency department patients and its association with outcome: a retrospective analysis. BMJ Open. 2020 Sep;10(9):e038079. 10.1136/bmjopen-2020-038079
11. Robinson S, Howie-Esquivel J, Vlahov D. Readmission risk factors after hospital discharge among the elderly. Popul Health Manag. 2012 Dec;15(6):338–51. 10.1089/pop.2011.0095
12. El Morabet N, Uitvlugt EB, van den Bemt BJ, van den Bemt PM, Janssen MJ, Karapinar-Çarkit F. Prevalence and Preventability of Drug-Related Hospital Readmissions: A Systematic Review. J Am Geriatr Soc. 2018 Mar;66(3):602–8. 10.1111/jgs.15244
13. Eggli Y, Lécureux E (2020) Potentiell vermeidbare Rehospitalisationen Akutsomatik. Nationaler Vergleichsbericht, BFS-Daten 2018
14. Higi L, Lisibach A, Beeler PE, Lutters M, Blanc AL, Burden AM, et al. External validation of the PAR-Risk Score to assess potentially avoidable hospital readmission risk in internal medicine patients. PLoS One. 2021 Nov;16(11):e0259864. 10.1371/journal.pone.0259864
15. By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel (2023) American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc n/a: https://doi.org/10.1111/jgs.18372
16. Lisibach A, Gallucci G, Benelli V, et al Evaluation of the association of anticholinergic burden and delirium in older hospitalised patients – A cohort study comparing 19 anticholinergic burden scales. Br J Clin Pharmacol n/a: https://doi.org/10.1111/bcp.15432
17. Langan SM, Schmidt SA, Wing K, Ehrenstein V, Nicholls SG, Filion KB, et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ. 2018 Nov;363:k3532. 10.1136/bmj.k3532
18. American Cancer Society Basal and Squamous Cell Skin Cancer. https://www.cancer.org/cancer/types/basal-and-squamous-cell-skin-cancer.html. Accessed 18 Jul 2023
19. Schneeweiss S, Rassen JA, Brown JS, Rothman KJ, Happe L, Arlett P, et al. Graphical Depiction of Longitudinal Study Designs in Health Care Databases. Ann Intern Med. 2019 Mar;170(6):398–406. 10.7326/M18-3079
20. Wertli MM, Reich O, Signorell A, Burgstaller JM, Steurer J, Held U. Changes over time in prescription practices of pain medications in Switzerland between 2006 and 2013: an analysis of insurance claims. BMC Health Serv Res. 2017 Feb;17(1):167. 10.1186/s12913-017-2086-6
21. Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017 May;189(18):E659–66. 10.1503/cmaj.170363
22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. 10.1016/0021-9681(87)90171-8
23. Mamdani M, Sykora K, Li P, Normand SL, Streiner DL, Austin PC, et al. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005 Apr;330(7497):960–2. 10.1136/bmj.330.7497.960
24. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009 Nov;41(4):1149–60. 10.3758/BRM.41.4.1149
25. R Core Team. (2023) R: A Language and Environment for Statistical Computing. https://www.R-project.org/
26. Hansen BB, Klopfer SO. Optimal Full Matching and Related Designs via Network Flows. J Comput Graph Stat. 2006;15(3):609–27. 10.1198/106186006X137047
27. Du Z, Hao Y (2022) stddiff: Calculate the Standardized Difference for Numeric, Binary and Category Variables
28. Aragon TJ, Fay MP, Wollschlaeger D, Omidpanah A (2020) epitools: Epidemiology Tools
29. Nafziger AN, Barkin RL. Opioid Therapy in Acute and Chronic Pain. J Clin Pharmacol. 2018 Sep;58(9):1111–22. 10.1002/jcph.1276
30. Daoust R, Paquet J, Cournoyer A, Piette É, Morris J, Lessard J, et al. Side effects from opioids used for acute pain after emergency department discharge. Am J Emerg Med. 2020 Apr;38(4):695–701. 10.1016/j.ajem.2019.06.001
31. Kurteva S, Abrahamowicz M, Gomes T, Tamblyn R. Association of Opioid Consumption Profiles After Hospitalization With Risk of Adverse Health Care Events. JAMA Netw Open. 2021 May;4(5):e218782. 10.1001/jamanetworkopen.2021.8782
32. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006 Jul;260(1):76–87. 10.1111/j.1365-2796.2006.01667.x
33. Seppala LJ, Petrovic M, Ryg J, Bahat G, Topinkova E, Szczerbińska K, et al. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Age Ageing. 2021 Jun;50(4):1189–99. 10.1093/ageing/afaa249
34. Gomes T, Redelmeier DA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM. Opioid dose and risk of road trauma in Canada: a population-based study. JAMA Intern Med. 2013 Feb;173(3):196–201. 10.1001/2013.jamainternmed.733
35. Herzig SJ, Anderson TS, Jung Y, Ngo L, Kim DH, McCarthy EP. Relative risks of adverse events among older adults receiving opioids versus NSAIDs after hospital discharge: A nationwide cohort study. PLoS Med. 2021 Sep;18(9):e1003804. 10.1371/journal.pmed.1003804
36. Donzé JD, Williams MV, Robinson EJ, Zimlichman E, Aujesky D, Vasilevskis EE, et al. International Validity of the HOSPITAL Score to Predict 30-Day Potentially Avoidable Hospital Readmissions. JAMA Intern Med. 2016 Apr;176(4):496–502. 10.1001/jamainternmed.2015.8462
37. Blanc AL, Fumeaux T, Stirnemann J, Dupuis Lozeron E, Ourhamoune A, Desmeules J, et al. Development of a predictive score for potentially avoidable hospital readmissions for general internal medicine patients. PLoS One. 2019 Jul;14(7):e0219348. 10.1371/journal.pone.0219348
38. Burgstaller JM, Held U, Signorell A, Blozik E, Steurer J, Wertli MM. Increased risk of adverse events in non-cancer patients with chronic and high-dose opioid use-A health insurance claims analysis. PLoS One. 2020 Sep;15(9):e0238285. 10.1371/journal.pone.0238285
39. Els C, Jackson TD, Kunyk D, Lappi VG, Sonnenberg B, Hagtvedt R, et al. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017 Oct;10(10):CD012509. 10.1002/14651858.CD012509.pub2
40. Kalkman GA, Kramers C, van Dongen RT, van den Brink W, Schellekens A. Trends in use and misuse of opioids in the Netherlands: a retrospective, multi-source database study. Lancet Public Health. 2019 Oct;4(10):e498–505. 10.1016/S2468-2667(19)30128-8
41. Morasco BJ, Yarborough BJ, Smith NX, Dobscha SK, Deyo RA, Perrin NA, et al. Higher Prescription Opioid Dose is Associated With Worse Patient-Reported Pain Outcomes and More Health Care Utilization. J Pain. 2017 Apr;18(4):437–45. 10.1016/j.jpain.2016.12.004
42. Ardeljan LD, Waldfogel JM, Bicket MC, Hunsberger JB, Vecchione TM, Arwood N, et al. Current state of opioid stewardship. Am J Health Syst Pharm. 2020 Apr;77(8):636–43. 10.1093/ajhp/zxaa027
43. Institute for Safe Medication Practices Canada Opioid Stewardship. https://www.ismp-canada.org/opioid_stewardship/. Accessed 17 Jul 2023
Appendix
Table S1Frequency of opioids administered on the last day of hospitalisation to exposed cases and matched controls.
| ATC | Substance(s) | Number (%) |
| N02AA55 | Oxycodone/naloxone | 1206 (37.2%) |
| N02AA01 | Morphine | 479 (14.8%) |
| N02AA05 | Oxycodone | 475 (14.7%) |
| N02AX02 | Tramadol | 293 (9.0%) |
| N02AE01 | Buprenorphine | 275 (8.5%) |
| N07BC02 | Methadone | 172 (5.3%) |
| N02AA03 | Hydromorphone | 120 (3.7%) |
| N02AX06 | Tapentadol | 81 (2.5%) |
| N02AJ13 | Tramadol and paracetamol | 74 (2.3%) |
| N02AB03 | Fentanyl | 46 (1.4%) |
| N02AA59 | Codeine and combinations | 19 (0.5%) |
| N02AA08 | Dihydrocodeine | 2 (0.1%) |


