Feasibility and cost-effectiveness of genetic counselling for all patients with newly
diagnosed ovarian cancer: a single-centre retrospective study
DOI: https://doi.org/https://doi.org/10.57187/s.3386
Saskia Schlootza,
Flurina A. M. Sanerb,
Manuela Rabaglioa,
Sara Imbodenb,
Julian Wampflera
a University Clinic for Medical
Oncology, Inselspital, University Hospital of Bern, University of Bern, Bern, Switzerland
b Department
of Gynaecology and Gynaecologic Oncology, Bern University Hospital, University
of Bern, Bern, Switzerland
Summary
BACKGROUND AND AIMS OF THE STUDY: Due to its importance for
treatment and potential prevention in family members, germline testing for BRCA1/2
in patients with newly diagnosed ovarian cancer is decisive and considered a
standard of care. Maintenance therapy with poly(ADP-ribose) polymerase (PARP) inhibitors
substantially improves progression-free survival in patients with BRCA
mutations and homologous recombination-deficient tumours by inducing synthetic
lethality. In Switzerland, they are licensed only for these patients.
Therefore, it is crucial to test patients early while they are receiving
adjuvant chemotherapy. This study aimed to determine whether genetic
counselling followed by homologous recombination deficiency testing is feasible
for initialising maintenance therapy within eight weeks and cost-effective in
daily practice in Switzerland compared to somatic tumour analysis of all
patients at diagnosis.
METHODS: This single-centre retrospective study included
44 patients with newly diagnosed high-grade serous ovarian cancer of a Federation
of Gynaecology and Obstetrics (FIGO) stage of IIIA-IVB diagnosed between
12/2020 and 12/2022. It collected the outcomes of genetic counselling, germline
testing, and somatic Geneva test for homologous recombination deficiency. Delays
in initiating maintenance therapy, total testing costs per patient, and
progression-free survival were examined to assess feasibility and
cost-effectiveness in clinical practice.
RESULTS: Thirty-seven of 44 patients (84%) with newly diagnosed ovarian cancer
received counselling, of which 34 (77%) were tested for germline BRCA
and other homologous recombination repair gene mutations. Five (15%) BRCA
and three (9%) other homologous recombination deficiency mutations were
identified. Eleven of the remaining 26 patients (42%) had tumours with somatic homologous
recombination deficiency. The mean time to the initiation of maintenance
therapy of 5.2 weeks was not longer than in studies for market authorisation
(SOLO1, PAOLA, and PRIMA). The mean testing costs per patient were 3880 Swiss
Franks (CHF), compared to 5624 CHF if all patients were tested at diagnosis
with the myChoice CDx test (p <0.0001).
CONCLUSION: Using genetic counselling to consent patients with newly diagnosed
ovarian cancer for germline testing fulfils the international gold standard. Subsequent
somatic homologous recombination deficiency analysis complements testing and identifies
more patients who will benefit from PARP inhibitor maintenance therapy.
Contrary to previous health cost model studies, the procedure does not increase
testing costs in the Swiss population and does not delay maintenance therapy.
Therefore, all patients should be offered a primary germline analysis. The
challenge for the future will be to ensure sufficient resources for prompt
genetic counselling and germline testing.
Introduction
High-grade
serous ovarian cancer is often diagnosed at advanced stages and associated with
a high risk of recurrence despite initially high chemosensitivity. For many
years, there has been no improvement in the standard chemotherapy consisting of
carboplatin and paclitaxel [1]. Maintenance therapies with poly(ADP-ribose)
polymerase inhibitors (PARPi), such as olaparib or niraparib, have been
introduced into routine clinical practice [2, 3].
Approximately
15%–20% of all high-grade serous ovarian cancers are associated with a germline
mutation in BRCA1 or BRCA2 and are considered an inherited
disease, also known as hereditary breast and ovarian cancer syndrome [1].
Homologous recombination deficiency (HRD) leads to impaired DNA damage repair
and thereby contributes to ovarian cancer progression. Pathogenic mutations in BRCA1
and BRCA2, regardless of whether they are somatic and acquired upon
tumorigenesis or inherited in the germline, are the leading cause of homologous
recombination deficiency among other gene mutations. Genetic alterations such as
loss of heterozygosity or copy number variations can arise due to homologous
recombination deficiency.
The prevalence
of homologous recombination deficiency in high-grade serous ovarian cancer is
estimated to be around 50% [4]. This proportion is lower in daily practice,
with a recent real-world analysis of 2829 patients finding that 37% of tumours had
a genomic instability score of over 42, implicating homologous recombination deficiency,
and 16% had BRCA
mutations [5]. The gold standard to determine homologous recombination
deficiency is functional tests such as myChoice CDx or Geneva, which have been
validated by the PAOLA trial [6–8].
Maintenance
therapy with a PARPi after adjuvant chemotherapy doubles disease-free survival
rates and enhances clinically meaningful overall and progression-free survival,
especially in patients with BRCA mutations [9]. This effect is also
observed in patients with homologous recombination-deficient ovarian cancer. However,
in patients with homologous recombination-proficient tumours, niraparib maintenance
therapy prolonged progression-free survival by only 2.7 months [10] and olaparib
combined with bevacizumab had no effect [11, 12]. Therefore, the Swiss regulatory
authority licensed maintenance with niraparib or olaparib combined with bevacizumab
only for treating ovarian cancer with homologous recombination deficiency.
Blood testing
patients for germline BRCA mutations (gBRCAmt) is expensive and requires
prior genetic counselling for consent. However, it is the only way to identify
inherited ovarian cancer and BRCA mutations, and it predicts clinical
benefit from PARPi maintenance therapy. Next-generation sequencing (NGS) of
tumour biopsies can detect somatic BRCA mutations (sBRCAmt) and
mutations in other genes that cause homologous recombination deficiency. Like the
functional tests, it can
identify homologous recombination deficiency and predict the clinical benefit of
PARPi maintenance therapy but does not identify inherited cancer predisposition
[1, 8, 13–15].
The
American Society of Clinical Oncology (ASCO) recommends initial genetic
counselling and germline BRCA testing for patients without pathogenic germline
mutations, followed by somatic analysis [16]. This approach is favourable and is
consistent with Swiss law, which requires detailed counselling for germline
testing and somatic analysis that could identify BRCA mutations. It
enables the patient to decline any testing for personal reasons and allows the
detection of gBRCAmt that are not found by somatic testing for technical
reasons. In a Korean cohort of 98 patients with high-grade ovarian cancer, three
(3.1%) carried a gBRCAmt without evidence of a sBRCAmt based on next-generation sequencing
(13% of
BRCAmt) [17]. This approach is time-consuming since germline testing is
strictly regulated by law. It requires an individual request for coverage from
a patient’s health insurance, which can delay testing [18]. In addition, a
health economic model study concluded that germline testing of patients with ovarian
cancer for BRCA mutations followed by somatic tumour-based next-generation sequencing was not cost-efficient
compared to somatic testing at diagnosis, challenging its use from a health
cost perspective [19].
The
first aim of this study was to determine whether providing genetic counselling
and germline analysis to all patients with newly diagnosed ovarian cancer
followed by functional somatic homologous recombination deficiency testing was feasible
in clinical practice,
assessed as initiating maintenance therapy not later than eight weeks after
completion of chemotherapy, as in the SOLO1 market access study; it was nine
weeks in the PAOLA trial and 12 weeks in the PRIMA trial [8–11].
The
second aim of this study was to determine whether this testing approach
increased testing costs in Switzerland compared to a model in which all patients
newly diagnosed with ovarian cancer are provided with a functional homologous recombination
deficiency test
(myChoice CDx and Geneva), followed by genetic counselling for those with positive
tests.
Materials and methods
Bernese testing
approach
In
accordance with Swiss law, regulations on germline diagnostics, and healthcare
insurance policy, in 2021, we implemented a BRCA/homologous
recombination deficiency status testing approach for patients with newly
diagnosed advanced high-grade ovarian cancer at the Inselspital (The University
Hospital of Bern) to fulfil the requirements for prescribing PARPi maintenance
therapy [18]. Patients are referred for genetic counselling by a
multidisciplinary team (MDT). The germline test panel (Twist Bioscience Custom Panel
v3) contains six known homologous recombination genes (BRCA1, BRCA2,
BRIP1, PALB2, RAD51C, and RAD51D) and four mismatch
repair genes (MLH1, MSH2, MSH6, and PMS2). If those
results are negative, an evaluation of tumour homologous recombination
deficiency using the Geneva test is recommended if the patient still fulfils the
clinical criteria for PARPi maintenance therapy [6]. If DNA quality is
insufficient for a Geneva test, a conventional somatic tumour next-generation sequencing
(Illumina
TSO500 panel) was performed to detect sBRCAmt whenever possible. No further
somatic tumour testing is conducted once a patient progresses or stops
responding to platinum-based chemotherapy.
Patients and
treatments
This
study included patients newly diagnosed with high-grade serous ovarian,
fallopian tube, or primary peritoneal cancer between 1 December 2020 and 31 December
2022. Data were censored on 31 March 2023. All patients had at least a Federation
of Gynecology and Obstetrics (FIGO) Stage IIIA. Diagnosis included a diagnostic
laparoscopy with tumour sampling. All patients were planned for surgery
(primary, interval, or delayed debulking) and recommended for (neo-)adjuvant
chemotherapy with six cycles of carboplatin and paclitaxel. Patients with FIGO Stage
III and a BRCAmt were treated for two years of olaparib. Maintenance therapy with
olaparib/bevacizumab was preferred for patients with FIGO Stage IV. Olaparib/bevacizumab
or niraparib was recommended for patients with homologous
recombination-deficient tumours. Maintenance therapy with bevacizumab was
suggested for patients with homologous recombination-proficient tumours of FIGO
Stage III with residual disease or Stage IVA.
Study design
Patients
with newly diagnosed ovarian cancer underwent BRCA and homologous recombination deficiency testing by
the Bernese testing approach (figure 1). We assessed the time from completion of
chemotherapy to initiation of maintenance therapy as the hallmark of clinical
feasibility. The testing approach was considered clinically feasible if
maintenance therapy was initiated no later than eight weeks. The SOLO1, PAOLA,
and PRIMA market access studies started maintenance therapies no later than 8,
9, or 12 weeks, respectively.
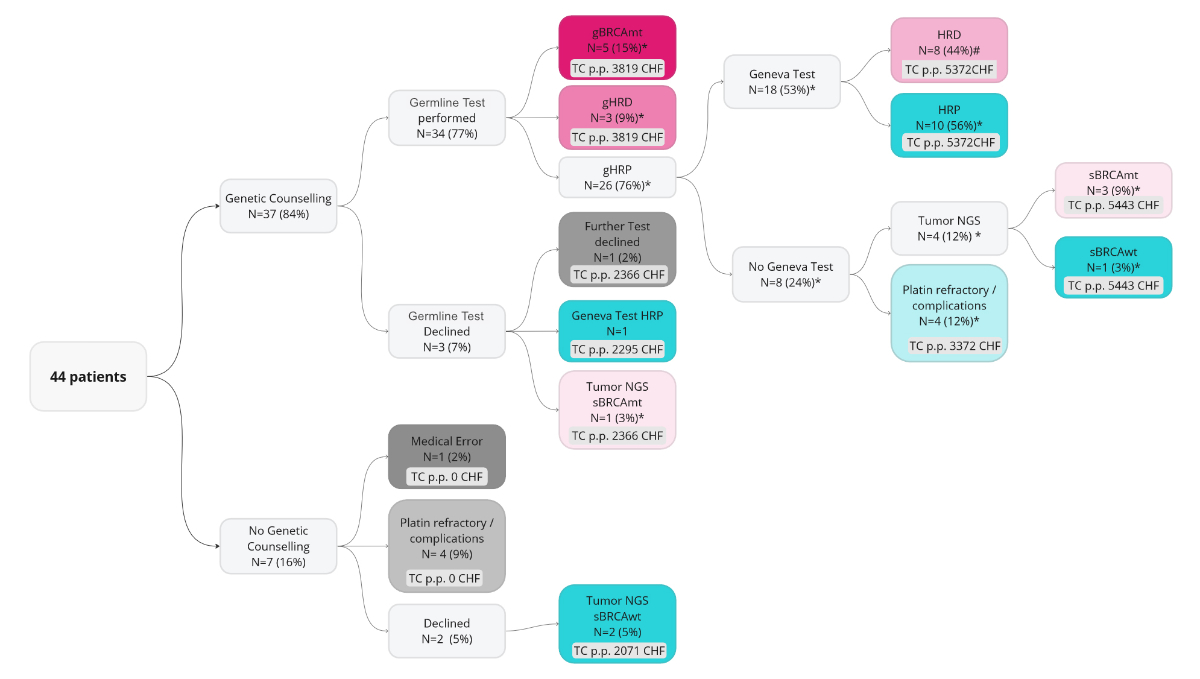
Figure 1The Bernese testing approach – a
real-world testing procedure in clinical practice. * the proportion of all
germline-tested patients; # the proportion of all patients who were germline
BRCA wild type (gBRCAwt) with the Geneva test. NGS: next-generation sequencing.
To assess
cost-effectiveness, we calculated the total testing costs of the entire
population. We compared these costs to a modelled approach in which all
patients are given an initial Geneva HRD or myChoice CDx test at diagnosis, ordered
at the first multidisciplinary team meeting, followed by germline testing for those
with homologous recombination deficiency tumours (figure 2).
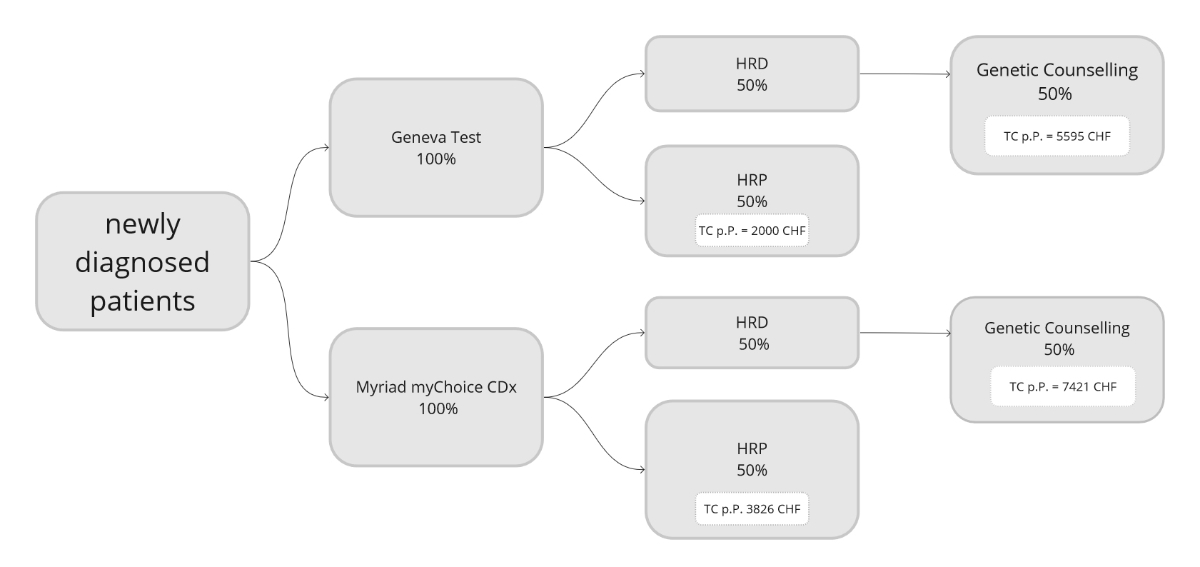
Figure 2The modelled testing costs for the somatic
homologous recombination deficiency (HRD) test first approach.
Ethical considerations
This
study was reviewed and approved by the Local Ethical Committee of the canton of
Bern (KEK Bern: 2023-00071).
Endpoints
We examined
the numbers and proportions of patients with gBRCAmt, sBRCAmt, homologous
recombination-deficient tumours, and homologous recombination-proficient tumours,
and the numbers of patients that did not undergo testing for medical or
personal reasons. We explored the times from diagnosis, defined as the date of the
multidisciplinary team meeting, to genetic counselling, homologous
recombination deficiency analysis, chemotherapy completion, and maintenance therapy
initiation to determine whether our testing approach was feasible in clinical practice.
Disease-free survival (DFS), progression-free survival (PFS), and overall
survival (OS) from diagnosis and the initiation of maintenance therapy were examined.
In addition, total testing and treatment costs per patient were calculated.
Statistical
analyses
All
statistical analyses were performed with GraphPad Prism (version 9.5.1). A 95%
confidence interval (95% CI) was calculated for the mean in all graphs. Mean
costs were compared with an unpaired t-test. Kaplan-Meier survival
functions and corresponding p-values were calculated with the Log-rank
(Mantel–Cox) test. Hazard ratios (HRs) and their 95% CIs were calculated using
the Mantel-Haenszel method.
Costs
The costs were calculated based on the sum
of all costs invoiced to health insurers in the Swiss healthcare system.
Medical and nursing services are billed based on the uniform tariff structure
called TARMED (Tarmed Browser 01.09_BR). Laboratory diagnostics costs were
calculated according to the Swiss list of analyses [20], including genetic
counselling, germline analysis, the Geneva test, and somatic next-generation sequencing.
To determine
the expenses on maintenance therapy, we recorded all regularly scheduled
medical consultations, including nursing services, laboratory analyses, and
medication costs. Total maintenance therapy costs were adjusted to the median
duration of treatment of the ICON7, PRIMA, PAOLA, and SOLO1 studies [10, 11, 21,
22]. The medication prices correspond to the approved official price (table S1
in the appendix).
Results
Patient
characteristics
This
study included 44 patients diagnosed with high-grade serous ovarian cancer and
a median age of 66 (range: 38–88) years (table 1), of which 14 (32%) had FIGO Stage
IV and 30 (68%) had FIGO Stage III. Thirty-eight (86%) patients underwent
debulking surgery and received platinum-based chemotherapy. In addition, 23
patients (52%) had started maintenance therapy, and four (9%) had been planned
for maintenance therapy but had not initiated it at the data cut-off. Moreover,
17 (39%) patients were assigned for follow-up, mainly those with primary
refractory disease or FIGO Stage III homologous recombination-proficient
tumours. Only patients carrying a BRCAmt or with homologous recombination-deficient
tumours started PARPi maintenance. Combined maintenance therapy with olaparib
and bevacizumab was initiated in five patients harbouring a somatic or germline
BRCA mutation and five patients with homologous recombination-deficient tumours
without a BRCA mutation. Olaparib was given as a maintenance therapy to four
patients with either a sBRCAmt or gBRCAmt. Niraparib was only given to patients
with homologous recombination-deficient tumours without a BRCAmt. Bevacizumab
maintenance therapy was only prescribed to patients with homologous-proficient tumours
(table 1).
Table 1Patient characteristics.
| Patient characteristics |
|
| Age at diagnosis (years),
median (range) |
66
(38–88) |
| FIGO Stage at diagnosis, n (%) |
III |
30
(68%) |
| IV |
14
(32%) |
| Surgery, n (%) |
Primary debulking surgery |
16
(36%) |
| Interval debulking surgery |
18
(41%) |
| Delayed debulking surgery |
4
(9%) |
| No debulking surgery
(Laparoscopy only) |
6
(13%) |
| Resection status, n (%) |
R0 |
33
(87%*) |
| R1 |
2
(5%*) |
| R2 |
3
(8%*) |
| N/A (no debulking) |
6
(13%) |
| Systemic treatment, n (%) |
Neoadjuvant chemotherapy |
22
(50%) |
| – Carboplatin/paclitaxel |
9 (20%) |
| – Carboplatin/paclitaxel/bevacizumab |
13 (30%) |
| Adjuvant chemotherapy |
16
(36%) |
| – Carboplatin/paclitaxel |
10 (23%) |
| – Carboplatin/paclitaxel
(including nab-paclitaxel)/bevacizumab |
5 (11%) |
| – Carboplatin monotherapy |
1 (2%) |
| Palliative first-line
chemotherapy |
6
(14%) |
| – Carboplatin/paclitaxel |
3 (7%) |
| – Carboplatin/paclitaxel/bevacizumab |
2 (5%) |
| – Carboplatin monotherapy |
1 (2%) |
| Maintenance therapy |
23
(52%) |
| – Bevacizumab and olaparib |
10 (23%) |
| – Olaparib |
4
(9%) |
| – Niraparib |
3
(7%) |
| – Bevacizumab |
6
(14%) |
| No maintenance therapy |
17
(39%) |
| Not yet started |
4
(9%) |
| Follow-up (months), median
(range) |
13.6
(2.0–27.9) |
Genetic
counselling and homologous recombination deficiency analysis
The
multidisciplinary team recommended genetic counselling to all 44 patients, of which
seven (16%) received no counselling due to medical reasons such as
platinum-refractory disease (n = 2, 5%), defined as progression during
first-line chemotherapy, complications or death (n = 2, 5%), declining the
consultation (n = 2, 5%), or not being offered a consultation (n = 1, 2%); the reason
for this could not be
determined with certainty in the retrospective data analysis. Of the 37 patients (84%)
who received
genetic counselling, three declined germline testing and were offered a Geneva
test (n = 1, homologous recombination-proficient tumour) or somatic next-generation
sequencing (n = 1,
sBRCAmt detected).
Of the
34 patients who underwent germline testing, five (15%) carried a gBRCAmt, and three
(9%) carried a germline mutation in a homologous recombination deficiency-causing
gene. No further testing was recommended for four (12%) of the remaining 26
patients (76%) because they developed platinum-refractory disease, defined as
progression during first-line chemotherapy, or experienced substantial toxicity
that contradicted maintenance therapy. Eighteen patients underwent a Geneva
test, of which eight (44%) were identified as homologous recombination
deficient and 10 (56%) as homologous recombination proficient. The Geneva test
was infeasible for four (12%) patients due to tissue quality, and they were examined
by somatic next-generation sequencing, which identified an sBRCA in three (9%; figures
1, 3, 4 and 5).
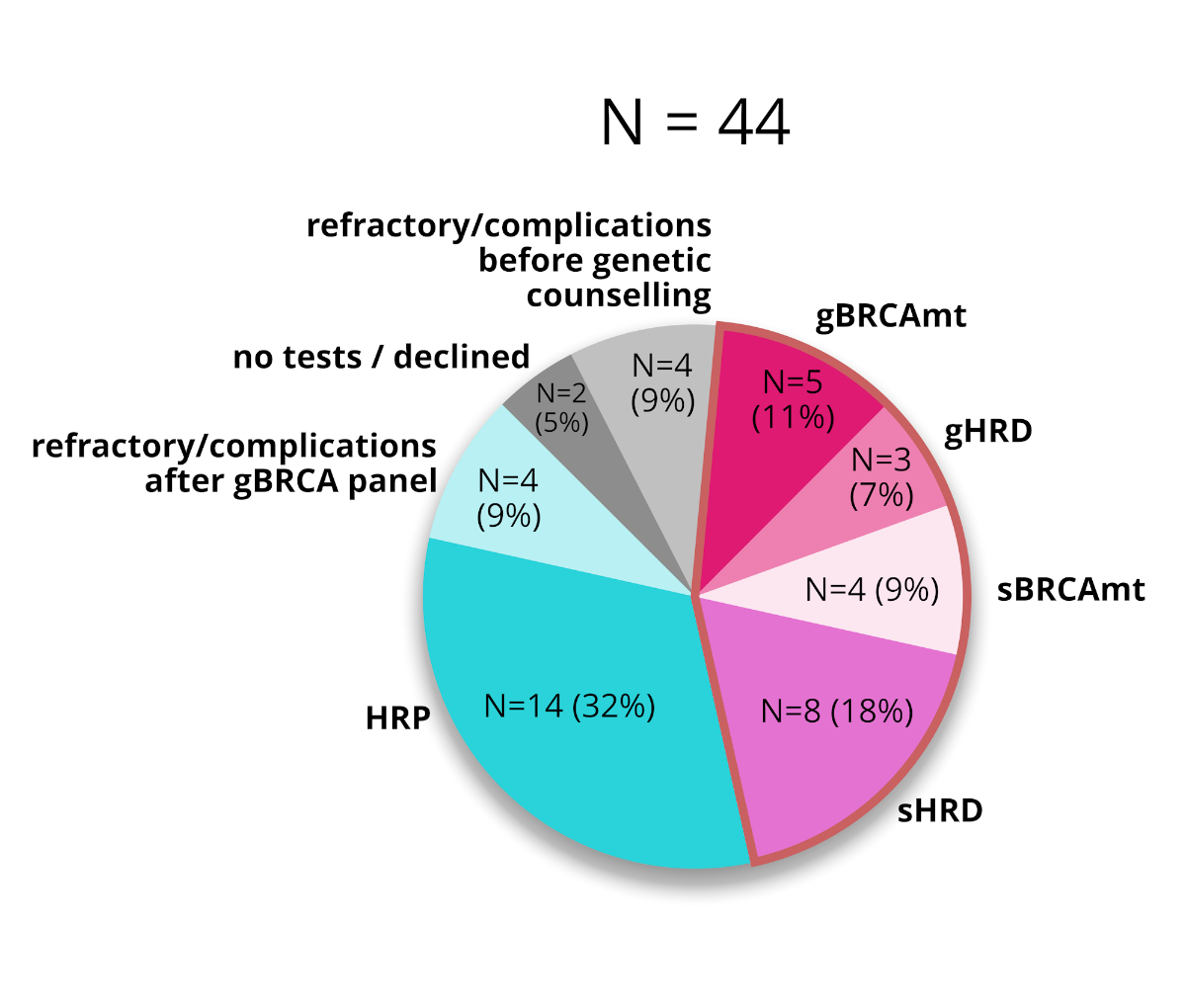
Figure 3The incidences of BRCA mutations (BRCAmt) and
homologous recombination deficiency (HRD) in all patients. HRP: homologous recombination
proficiency.
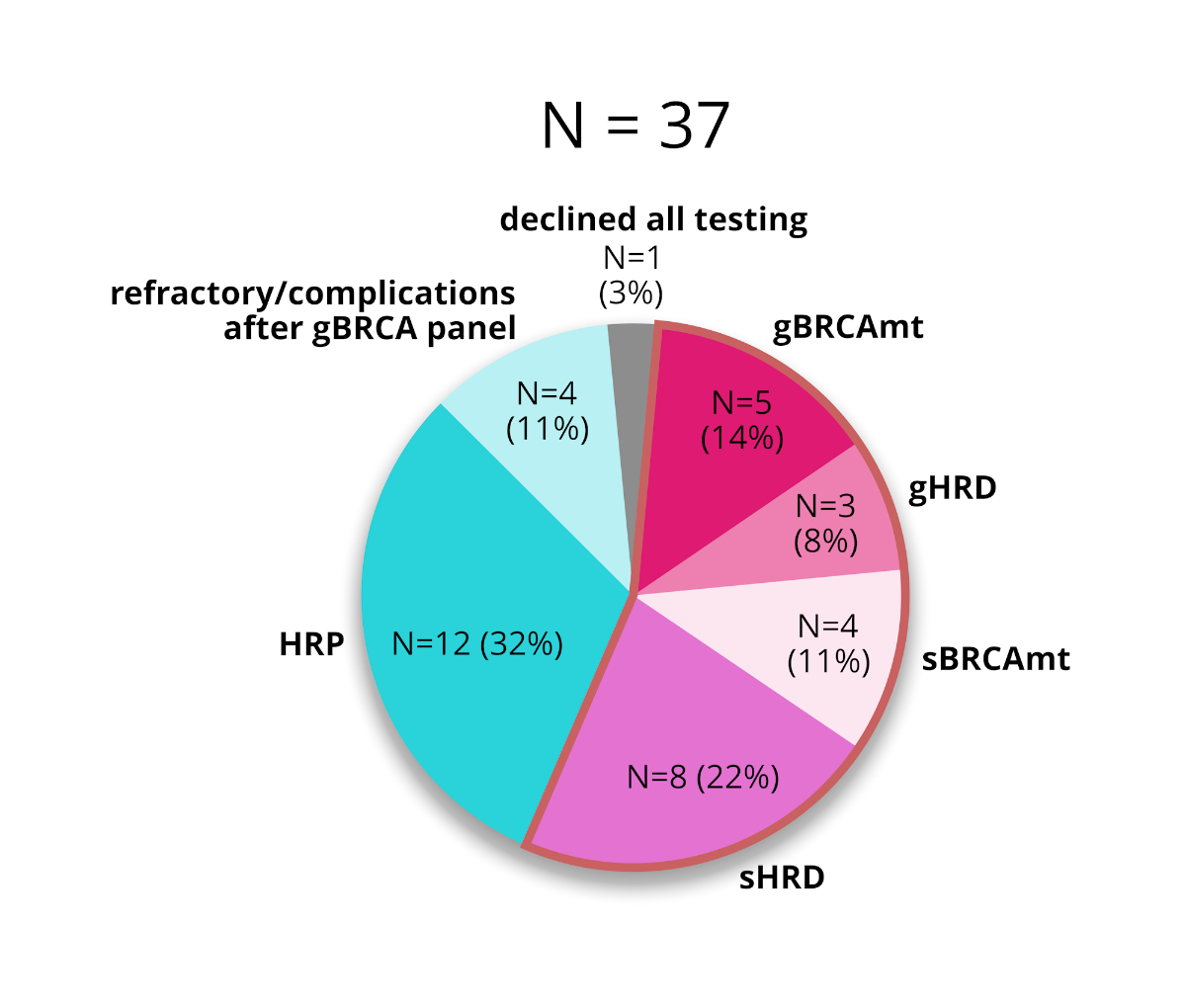
Figure 4The incidences of BRCA mutations (BRCAmt) and
homologous recombination deficiency (HRD) in all patients
who received genetic counselling. HRP: homologous recombination proficiency.
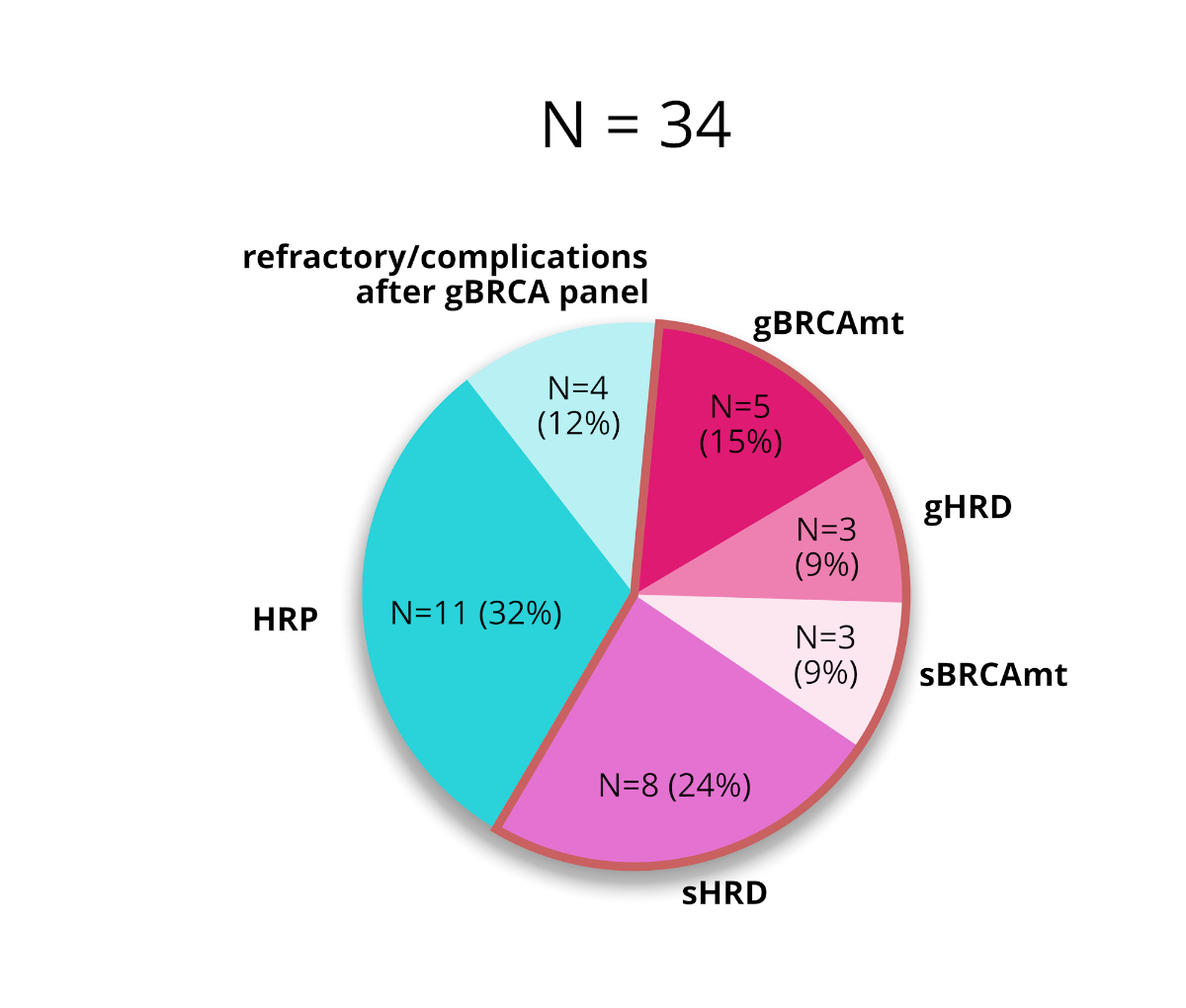
Figure 5The incidences of BRCA mutations (BRCAmt) and
homologous recombination deficiency (HRD) in all patients who received germline
testing. HRP: homologous recombination proficiency.
Feasibility in
clinical practice
Genetic
counselling followed by homologous recombination deficiency testing was
initiated at the first multidisciplinary team meeting. The mean time from this
meeting to the availability of a germline test result was 15.7 weeks, and the
completion of subsequent homologous recombination deficiency testing was 20.2
weeks. The median time from the completion of chemotherapy to the initiation of
maintenance therapy was 5.2 weeks; this estimate includes patients who received
a delayed debulking surgery, defined as surgery after the completion of
chemotherapy, which prolongs the time between the completion of chemotherapy and
the initiation of maintenance. This median was 3.7 weeks when patients with
delayed debulking surgery were excluded (figure 6).
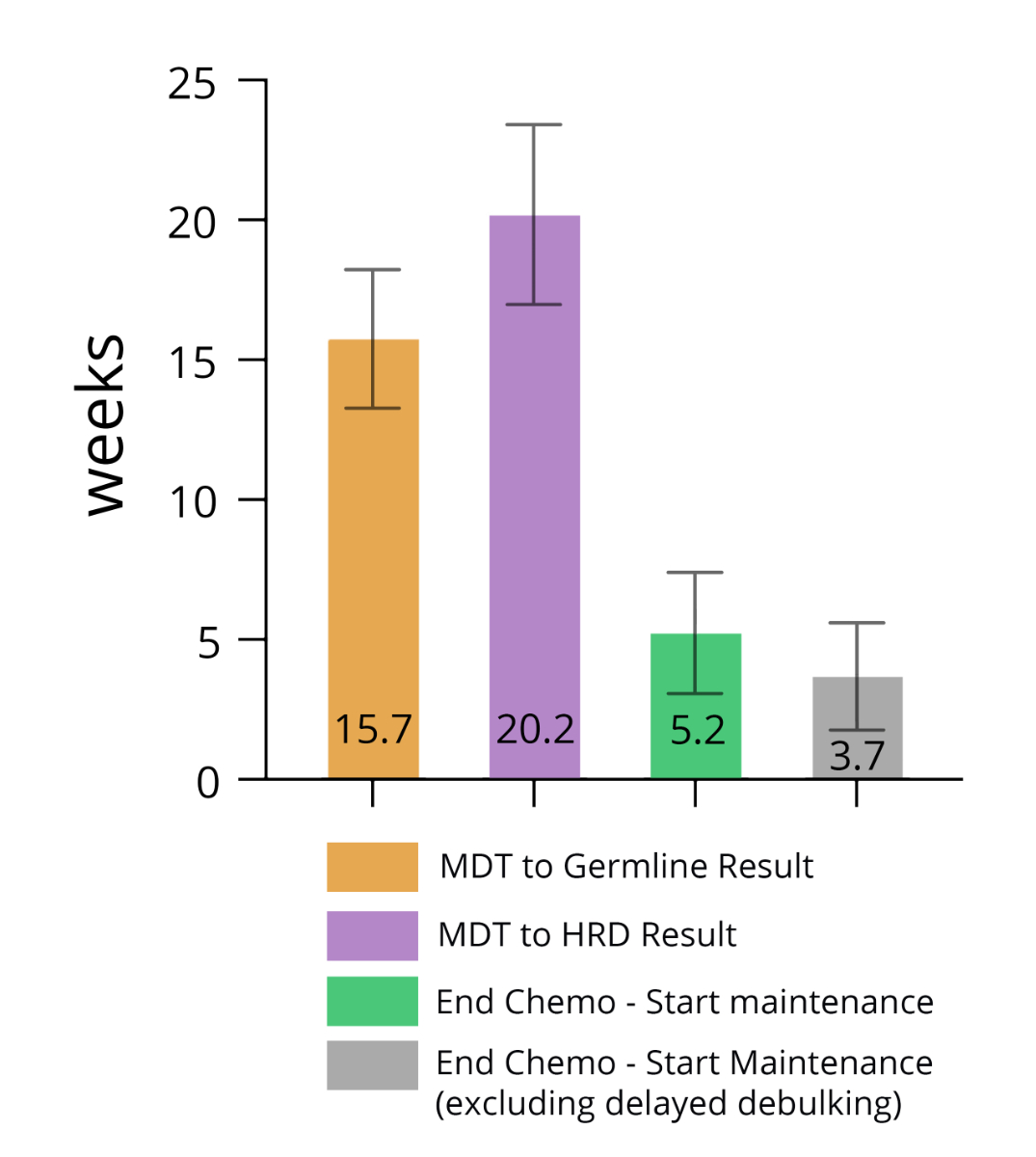
Figure 6The mean time from the multidisciplinary team
(MDT) meeting to the test results and from the completion of chemotherapy to
the initiation of maintenance therapy.
Costs of the Bernese
testing approach
The total
healthcare costs for genetic counselling and homologous recombination
deficiency analysis were summarised (figure 1). Seven patients did not
undergo genetic counselling. Two patients who were not germline tested underwent
tumour next-generation sequencing (2071 CHF per patient [p.p.]). One patient who was
not germline
tested had received prior genetic counselling and later underwent tumour next-generation
sequencing (2366
CHF p.p.). One patient had received genetic counselling but then underwent a Geneva
test instead of a germline test (2295 CHF p.p.). Eight patients carried a gBRCAmt
or another mutation causing homologous recombination deficiency and underwent
no further testing (3819 CHF p.p.). Four patients underwent no further testing after
a non-mutated germline result (3372 CHF p.p). Four patients underwent tumour next-generation
sequencing
and a germline test (5443.6 CHF p.p.). Eighteen patients underwent both a GENEVA
test and a germline analysis (5372 CHF p.p.; figure 1).
The mean
testing cost across the 44 patients was 3880 CHF p.p. If we had used the Myriad
myChoice CDx test instead of the Geneva test, the mean total testing cost would
have been 4669 CHF p.p. Estimates were calculated to compare the cost-effectiveness
of our testing approach to giving a somatic homologous recombination deficiency
test to all patients at diagnosis. If all patients first undergo a Geneva test,
total test costs would be 3798 CHF p.p. If they instead first undergo a Myriad
myChoice CDx test, total testing costs would be 5624 CHF p.p. There was no
statistically significant difference if the Geneva test was used (p = 0.8357).
However, the mean test costs were significantly lower if a myChoice CDx test
was used (p = 0.0467; figure 7).
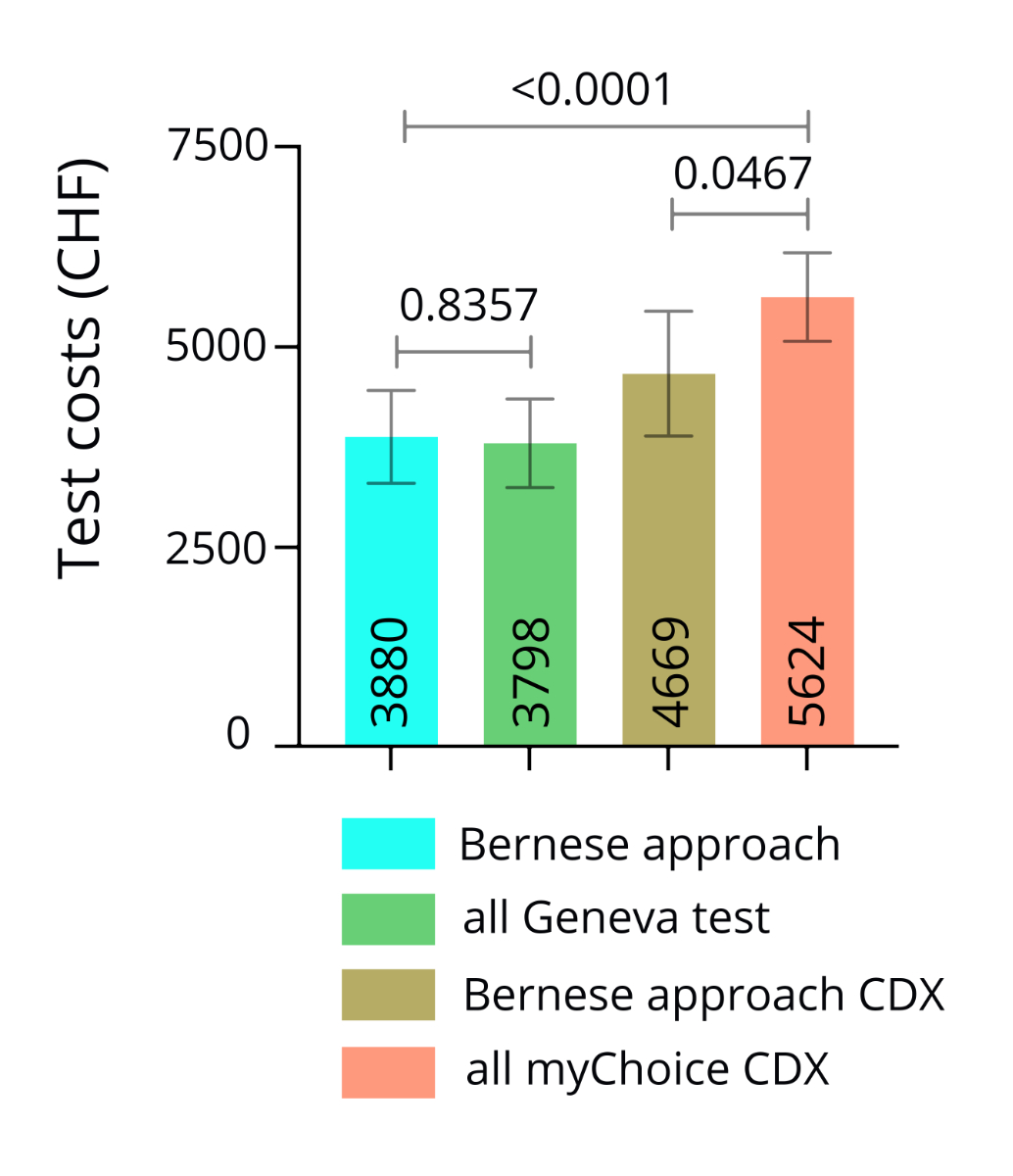
Figure 7The mean testing costs per patient.
Clinical cancer-related
outcomes of the studied cohort
At 12
and 24 months after diagnosis, 84% and 65% were disease-free, respectively (data
maturity was 59% and 16%, respectively; figure 8). The median follow-up time was
13.6 months. Patients with homologous recombination-deficient tumours had a
significantly longer DFS than patients diagnosed with homologous-proficient
tumours (HR = 0.25, 95% CI = 0.07–0.82; p = 0.022; figure 9). One year after
diagnosis, 90% were alive (64% data maturity), and two years after diagnosis, 76%
were alive (data maturity 18%; figure 10). The PFS with maintenance therapy was
significantly longer in patients with homologous recombination-deficient
tumours than in patients with homologous recombination-proficient tumours (HR =
0.09, 95% CI = 0.01–0.81; p = 0.032; figure 11).
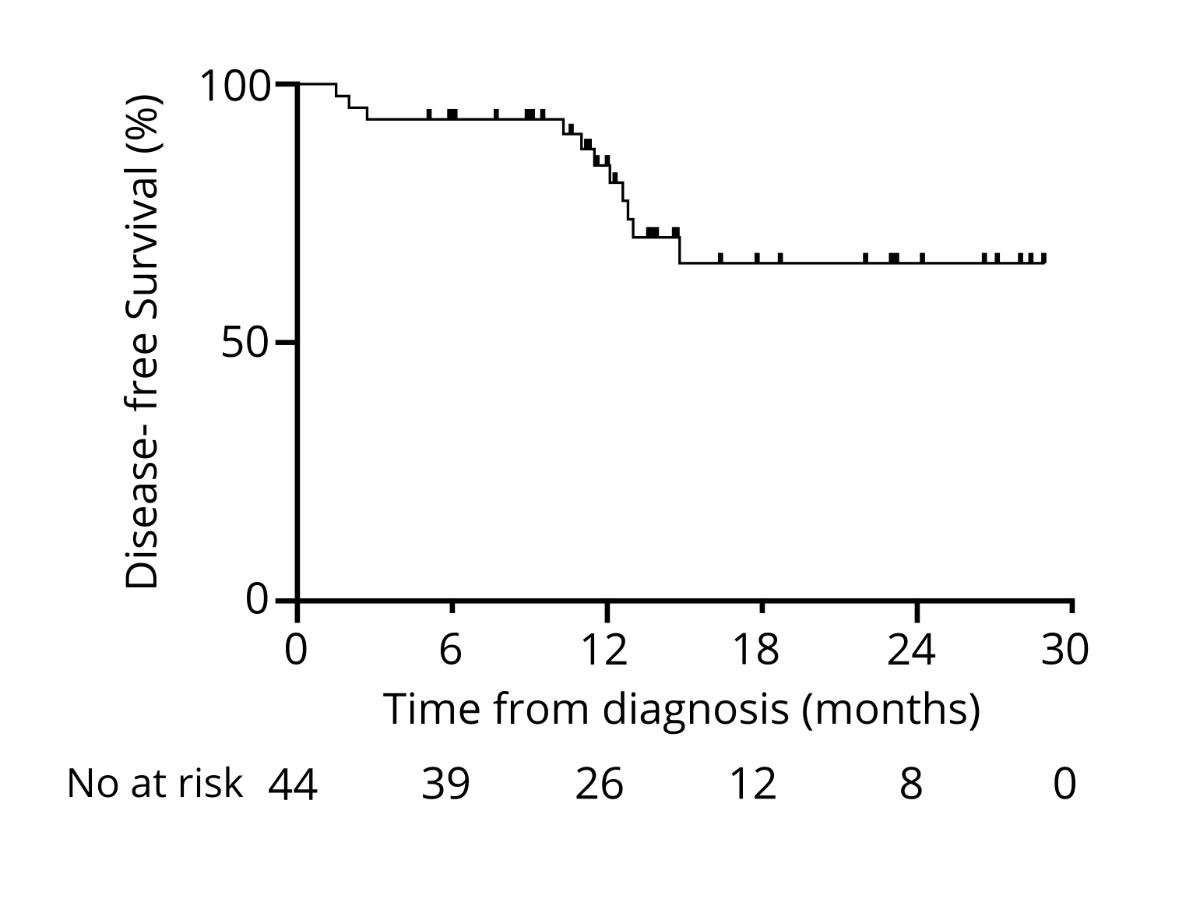
Figure 8Disease-free survival from diagnosis.
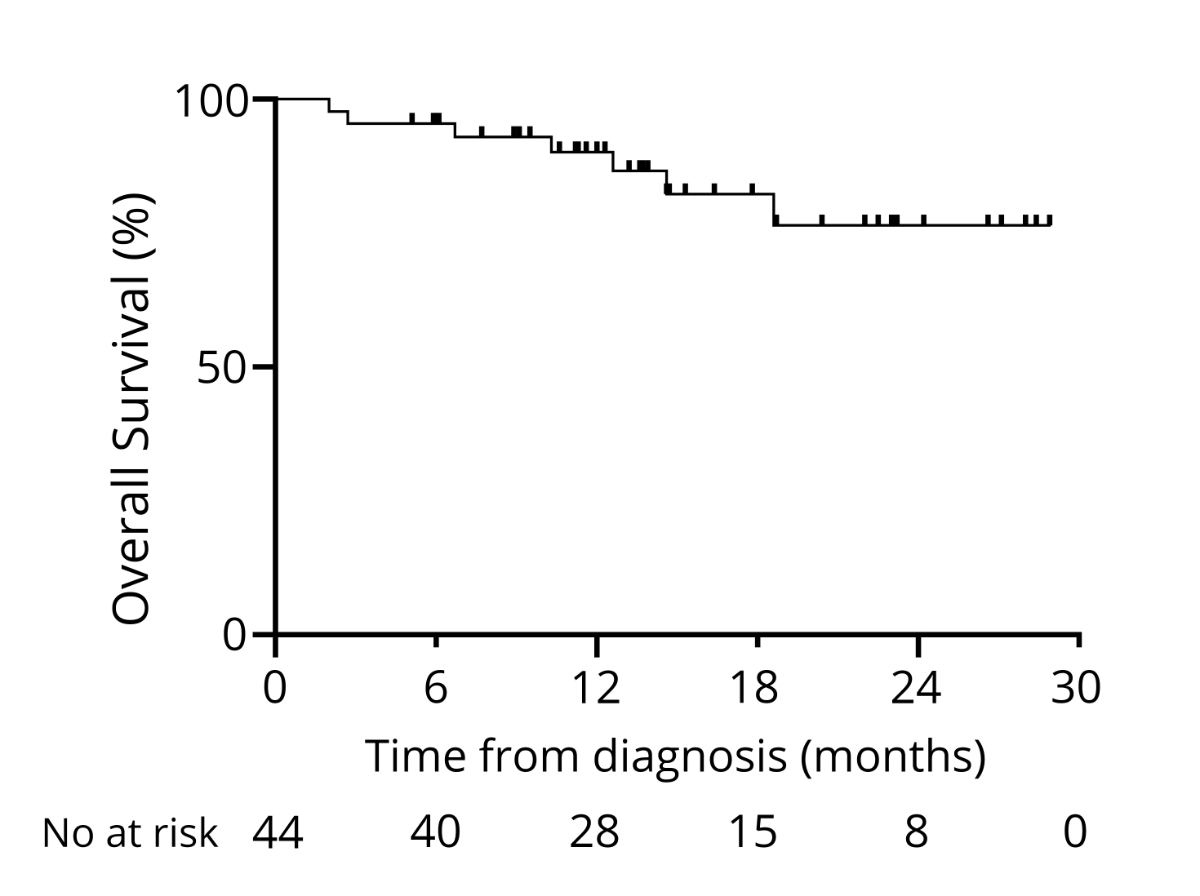
Figure 9Overall
survival from diagnosis.
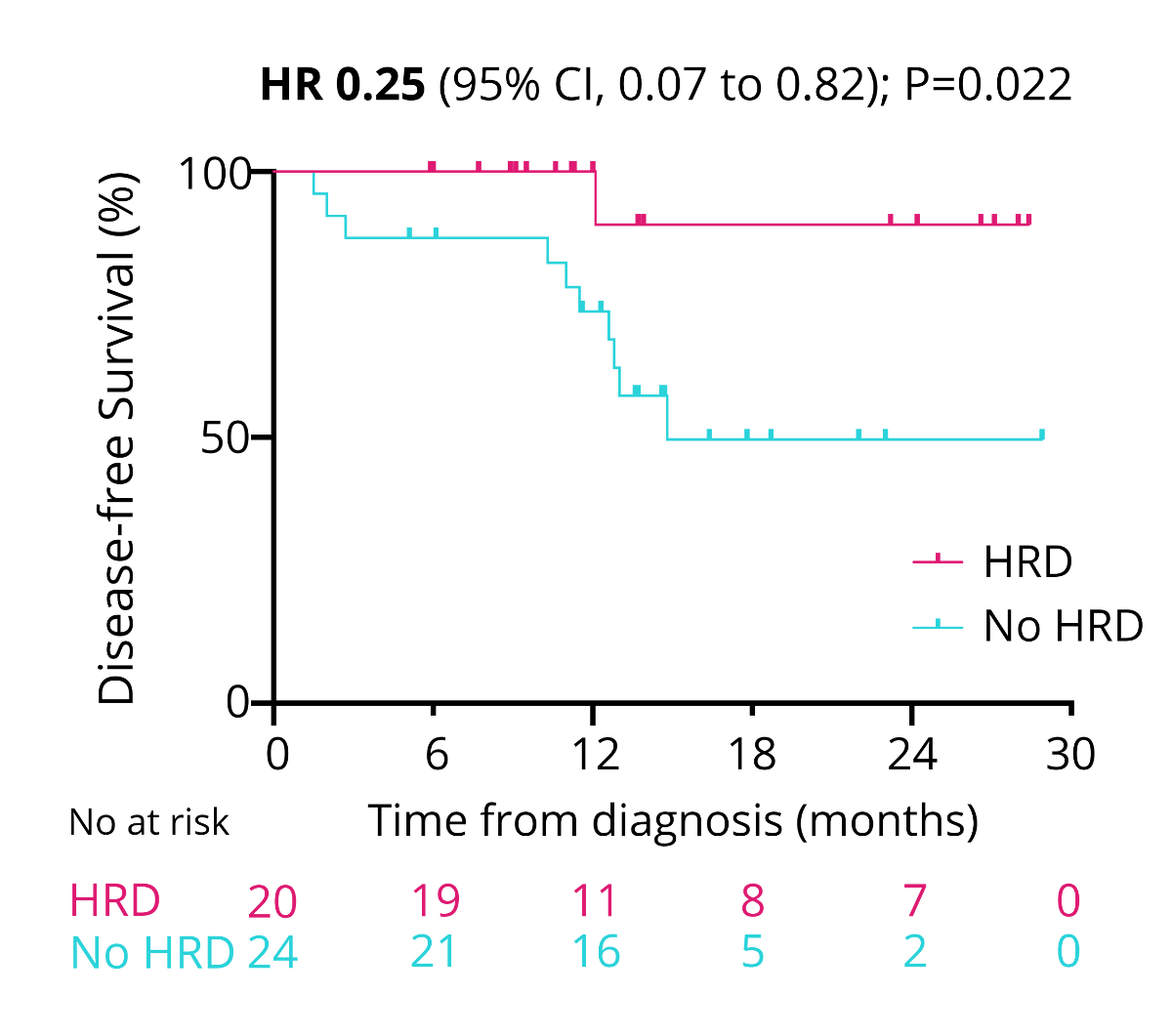
Figure 10Comparison of disease-free survival with homologous recombination deficiency (HRD)
or no homologous recombination deficiency (No HRD).
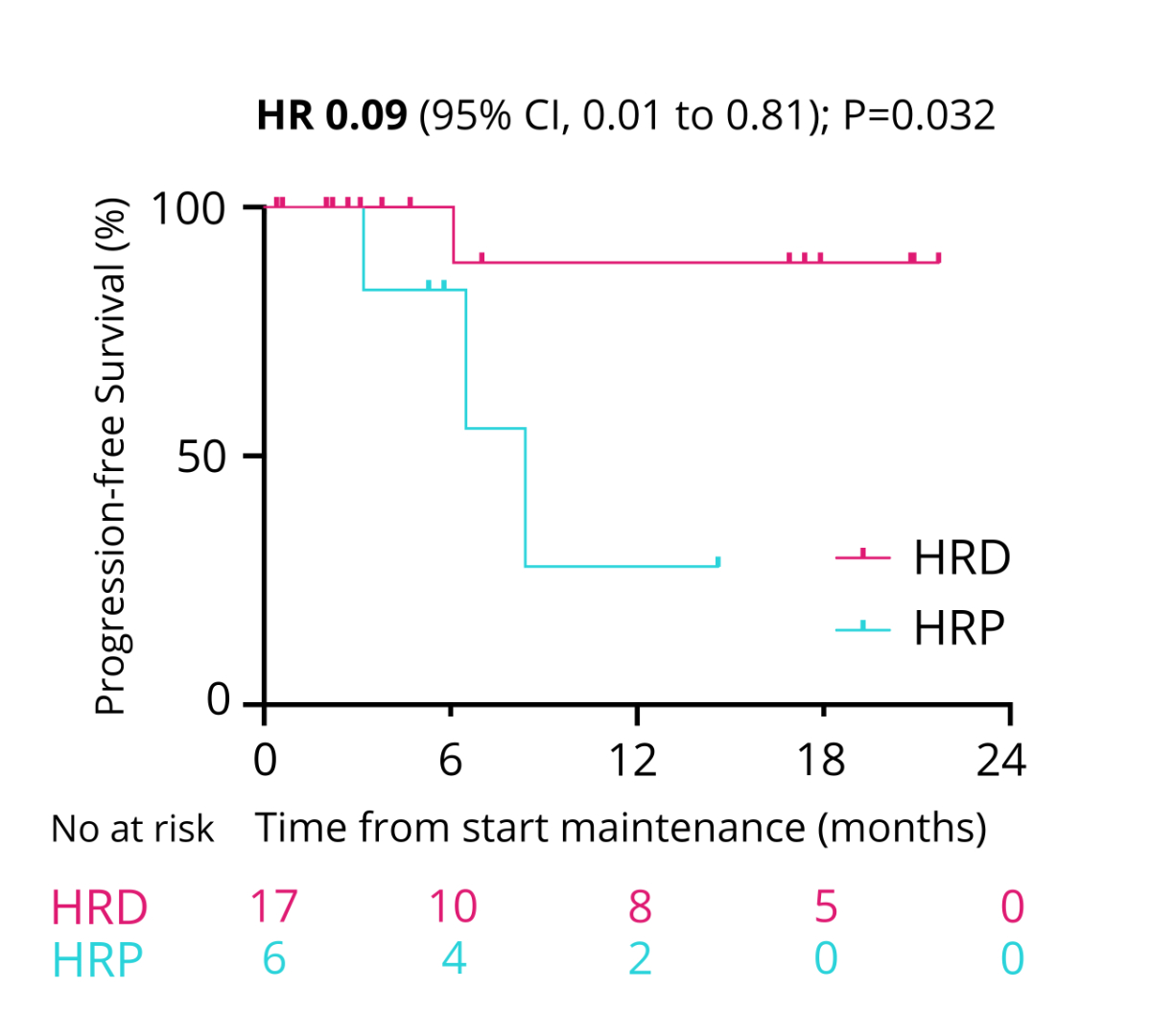
Figure 11Progression-free survival with maintenance therapy.
Total therapy
costs
At our
centre, the resulting total healthcare costs p.p. for maintenance therapy were
133,513 CHF for 24 months of olaparib monotherapy, 126,241 CHF for 24 months of
niraparib monotherapy, 197,576 CHF for combined olaparib (24 months) and
bevacizumab (15 months) therapy, and 38,628 CHF for bevacizumab monotherapy. Adjusted
to the median treatment durations in the SOLO1, PRIMA, PAOLA, and ICON7 studies,
these costs become 133,513 CHF, 58,761 CHF, 164,511 CHF, and 34,769 CHF (table S1).
Discussion
The presented
real-world data demonstrate that a testing approach involving germline analysis
followed by somatic homologous recombination deficiency testing with the Geneva
test for patients with newly diagnosed advanced high-grade serous ovarian
cancer is feasible in clinical practice. It does not lead to clinically
meaningful delays in initiating maintenance therapy. Given the prevention of
unnecessary testing for patients who would not qualify for a PARPi, such as those
with platinum-refractory disease, the total testing costs per patient do not differ
from the algorithm-estimates costs of first testing all patients somatically for
homologous recombination deficiency followed by genetic counselling. Modelling
testing costs with the more expensive myChoice CDx test showed that the Bernese
approach is cost-effective compared to a homologous recombination deficiency
test at diagnosis. Clinical outcomes were not evidently worse than those in
SOLO1, PAOLA, and PRIMA studies. For example, 88% of patients were progression-free
at one year and 74% at two years in the SOLO 1 study. In contrast, these
proportions were 88% and 65% in our cohort with limited follow-up, but considering
that many patients had less favourable prognoses since most did not harbour a
BRCAmt [10, 21]. The Bernese testing approach successfully identified
candidates for PARPi maintenance therapy since DFS and PFS were significantly
longer in those with homologous recombination deficient tumours than those with
non-homologous recombination deficiency tumours.
The
Bernese testing approach follows the current ASCO guidelines [16] and meets the
requirements of Swiss law since homologous recombination deficiency or somatic NGS
testing of unconsented patients is unlawful [18]. Despite the high costs of
genetic counselling and germline analysis, this approach does not increase the testing
costs or is even more cost-effective if the myChoice CDx test is used instead
of the Geneva test. Our real-world data demonstrates that an upfront testing
strategy with a somatic tumour-based test, such as an next-generation sequencing or
homologous recombination deficiency test,
indicated at the first multidisciplinary team does not lower testing costs as a Canadian
model
suggests [19]. Unlike a Dutch study that proposed a reciprocal testing strategy
that combined somatic BRCA testing with somatic next-generation sequencing but did not address homologous
recombination deficiency [23], the Bernese testing approach aims to identify
patients with gBRCAmt and homologous recombination deficient ovarian tumours, which
ensures no patients risk of missing out on gBRCAmt diagnoses [17]. All patients
without gBRCAmt are considered for a Geneva test or, if infeasible, somatic next-generation
sequencing.
The Swiss label for niraparib and olaparib/bevacizumab requires a validated homologous
recombination deficiency test, which can be a Geneva or myChoice CDx test [6].
Despite previous results demonstrating the cost-effectiveness of biomarker-driven
PARPi maintenance, we do not observe this in our cohort since total maintenance
costs would be higher if niraparib were given to all patients with a gBRCAwt [13,
14, 25]. Compared with a prevalence of a genomic instability score of at least 42
of 37% reported in a recent British study on 2829 patients, we found a higher prevalence
of 44%, which could be explained by avoiding testing in patients with primary platinum-refractory
disease [5].
This
study shows for the first time that genetic counselling followed by germline
testing and homologous recombination deficiency analysis for patients without a
gBRCAmt is feasible and cost-effective in clinical practice, provided
sufficient resources for prompt genetic counselling exist. Unlike all other
models, our data account for some patients not being tested for homologous recombination
deficiency for medical
reasons and would never qualify for maintenance therapy [19, 23]. The applied
and suggested testing approach is consistent with current guidelines and permits
an informed-consent-based decision-making process, unlike any routine somatic
tumour next-generation sequencing or homologous recombination deficiency automated
testing approach [1,
8, 16].
To date,
some clinicians and institutes have refrained from consistently conducting
primary germline analysis on patients newly diagnosed with advanced high-grade
serous ovarian cancer due to assumptions that such an approach would lead to
additional costs and delays in initiating maintenance therapy [19, 23].
This study
demonstrates that a germline-first testing approach is both cost-efficient and
feasible in routine clinical practice in Switzerland. Moreover, it should be
routinely offered since it adheres to the international gold standard. It ensures
that patients are well-informed about the potential implications of familial
inherited cancer syndromes, enabling them to make self-determined decisions
regarding the analysis [16]. This approach also maximises the likelihood of
detecting a germline mutation since large BRCA deletions can be missed with
conventional somatic next-generation sequencing panels [17]. It clearly demonstrates
that the requirements
of Swiss law that informed consent be obtained before genetic analysis are
feasible for patients with newly diagnosed ovarian cancer. This testing
approach should be applied to all patients newly diagnosed with ovarian cancer since
it is based on individual informed consent, enables the detection of potentially
homologous recombination deficient tumours, and meets the requirements of the
Swissmedic label for maintenance therapies with olaparib, niraparib, or olaparib
combined with bevacizumab.
As a real-life
retrospective single-centre cohort study with relatively few patients, its
results should be interpreted cautiously. Its conclusions are based on local
tariffs and apply only to the Swiss healthcare system. Therefore, a larger international,
prospective, multicentre, real-world analysis is warranted.
Conclusions
We showed
for the first time that germline testing followed by homologous recombination
deficiency analysis for those without a gBRCAmt is feasible for patients with
newly diagnosed ovarian cancer. This testing approach does not delay the initiation
of maintenance treatment, which was initiated within 5.2 weeks after the completion
of chemotherapy. The pre-test probability of homologous recombination deficiency,
as assessed by the Geneva test, was 44% if patients with primary
platinum-refractory disease were not tested. Finally, we demonstrated that this
test approach does not increase total testing costs per patient. It can even lower
total healthcare costs if homologous recombination deficiency is assessed using
the myChoice CDx test based on Swiss prices.
Acknowledgments
The authors would like to thank all
patients for consenting to the use of their anonymous healthcare data.
Author contributions: Saskia Schlootz: Project
development, data collection and analysis, health care cost calculation, wrote
the manuscript. Flurina A.M. Saner: Project development, data analysis and
editing the final manuscript. Manuela Rabaglio: Project development, data analysis
and editing the final manuscript. Sara Imboden: Project development and editing
the final manuscript. Julian Wampfler: Project development, data collection and
analysis, seeking ethical committee approval, wrote the manuscript.
Dr Julian Wampfler, MD, PhD
Consultant Medical Oncologist
Inselspital, University Hospital of Bern
CH-3010
Bern
julian.wampfler[at]insel.ch
References
1 González-Martín A, Harter P, Leary A, Lorusso D, Miller RE, Pothuri B, et al.; ESMO
Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Newly diagnosed
and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis,
treatment and follow-up. Ann Oncol. 2023 Oct;34(10):833–48. 10.1016/j.annonc.2023.07.011
2 Matulonis U, Herrstedt J, Oza A, Mahner S, Redondo A, Berton D, et al. Long-term safety
and secondary efficacy endpoints in the ENGOT-OV16/NOVA phase III trial of niraparib
in recurrent ovarian cancer. Gynecol Oncol. 2021;162:S24–5. 10.1016/S0090-8258(21)00693-4
3 Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al.; SOLO2/ENGOT-Ov21
investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive
relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis
of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021 May;22(5):620–31.
10.1016/S1470-2045(21)00073-5
4 Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous Recombination
Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov.
2015 Nov;5(11):1137–54. 10.1158/2159-8290.CD-15-0714
5 Morgan RD, Clamp AR, Barnes BM, Timms K, Schlecht H, Yarram-Smith L, et al. Homologous
recombination deficiency in newly diagnosed FIGO stage III/IV high-grade epithelial
ovarian cancer: a multi-national observational study. Int J Gynecol Cancer. 2023 Aug;33(8):1253–9.
10.1136/ijgc-2022-004211
6 Christinat Y, Ho L, Clément S, Genestie C, Sehouli J, Martin AG, et al. 2022-RA-567-ESGO The
Geneva HRD test: clinical validation on 469 samples from the PAOLA-1 trial. Int J
Gynecol Cancer. 2022;32 Suppl 2:A238–9.
7 Heitz F, Ataseven B, Staniczok C, Denkert C, Rhiem K, Hahnen E, et al. Implementing
HRD Testing in Routine Clinical Practice on Patients with Primary High-Grade Advanced
Ovarian Cancer. Cancers (Basel). 2023 Jan;15(3):818. 10.3390/cancers15030818
8 Miller RE, Leary A, Scott CL, Serra V, Lord CJ, Bowtell D, et al. ESMO recommendations
on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor
benefit in ovarian cancer. Ann Oncol. 2020 Dec;31(12):1606–22. 10.1016/j.annonc.2020.08.2102
9 DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim BG, Oaknin A, et al.; SOLO1 Investigators.
Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients With
Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: the SOLO1/GOG 3004 Trial.
J Clin Oncol. 2023 Jan;41(3):609–17. 10.1200/JCO.22.01549
10 González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR,
et al.; PRIMA/ENGOT-OV26/GOG-3012 Investigators. Niraparib in Patients with Newly
Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2019 Dec;381(25):2391–402. 10.1056/NEJMoa1910962
11Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al.;
PAOLA-1 Investigators. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian
Cancer. N Engl J Med. 2019 Dec;381(25):2416–28. 10.1056/NEJMoa1911361
12 Ray-Coquard I, Leary A, Pignata S, Cropet C, González-Martín A, Marth C, et al.; PAOLA-1/ENGOT-ov25
investigators. Olaparib plus bevacizumab first-line maintenance in ovarian cancer:
final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann Oncol. 2023 Aug;34(8):681–92.
10.1016/j.annonc.2023.05.005
13 Gonzalez R, Havrilesky LJ, Myers ER, Secord AA, Dottino JA, Berchuck A, et al. Cost-effectiveness
analysis comparing “PARP inhibitors-for-all” to the biomarker-directed use of PARP
inhibitor maintenance therapy for newly diagnosed advanced stage ovarian cancer. Gynecol
Oncol. 2020 Nov;159(2):483–90. 10.1016/j.ygyno.2020.08.003
14 Penn CA, Wong MS, Walsh CS. Cost-effectiveness of Maintenance Therapy Based on Molecular
Classification Following Treatment of Primary Epithelial Ovarian Cancer in the United
States. JAMA Netw Open. 2020 Dec;3(12):e2028620. 10.1001/jamanetworkopen.2020.28620
15 Sessa C, Balmaña J, Bober SL, Cardoso MJ, Colombo N, Curigliano G, et al.; ESMO Guidelines
Committee. Electronic address: clinicalguidelines@esmo.org. Risk reduction and screening
of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline.
Ann Oncol. 2023 Jan;34(1):33–47. 10.1016/j.annonc.2022.10.004
16 Konstantinopoulos PA, Norquist B, Lacchetti C, Armstrong D, Grisham RN, Goodfellow PJ,
et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline.
J Clin Oncol. 2020 Apr;38(11):1222–45. 10.1200/JCO.19.02960
17 Eoh KJ, Kim HM, Lee JY, Kim S, Kim SW, Kim YT, et al. Mutation landscape of germline
and somatic BRCA1/2 in patients with high-grade serous ovarian cancer. BMC Cancer.
2020 Mar;20(1):204. 10.1186/s12885-020-6693-y
18 Stoll S, Unger S, Azzarello-Burri S, Chappuis P, Graffeo R, Pichert G, et al. Update
Swiss guideline for counselling and testing for predisposition to breast, ovarian,
pancreatic and prostate cancer. Swiss Med Wkly. 2021 Sep;151(3738):w30038. 10.4414/SMW.2021.w30038
19 Kwon JS, Tinker AV, Santos J, Compton K, Sun S, Schrader KA, et al. Germline Testing
and Somatic Tumor Testing for BRCA1/2 Pathogenic Variants in Ovarian Cancer: What Is the Optimal Sequence of Testing? JCO
Precis Oncol. 2022 Oct;6(6):e2200033. 10.1200/PO.22.00033
20 Analysenliste, Krankenpflege-Leistungsverordnung KLV, Anhang 3. In: (EDI) EDdI, editor.
Bundesamt für Gesundheit2023.
21 Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance
Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018 Dec;379(26):2495–505.
10.1056/NEJMoa1810858
22 Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et
al.; ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. N Engl
J Med. 2011 Dec;365(26):2484–96. 10.1056/NEJMoa1103799
23 Witjes VM, Ligtenberg MJ, Vos JR, Braspenning JC, Ausems MG, Mourits MJ, et al. The
most efficient and effective BRCA1/2 testing strategy in epithelial ovarian cancer:
Tumor-First or Germline-First? Gynecol Oncol. 2023 Jul;174:121–8. 10.1016/j.ygyno.2023.04.029
24 González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR,
et al.; PRIMA/ENGOT-OV26/GOG-3012 Investigators. Niraparib in Patients with Newly
Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2019 Dec;381(25):2391–402. 10.1056/NEJMoa1910962
25 Elsea D, Fan L, Mihai A, Moustaid FE, Simmons D, Monberg M, et al. Cost-Effectiveness
Analysis of Olaparib in Combination with Bevacizumab Compared with Bevacizumab Monotherapy
for the First-Line Maintenance Treatment of Homologous Recombination Deficiency-Positive
Advanced Ovarian Cancer. PharmacoEconom Open. 2022 Nov;6(6):811–22. 10.1007/s41669-022-00338-2
Appendix: supplementary table
Table S1Total maintenance healthcare costs, including nursing, laboratory,
consultations, and medications, adjusted by the median duration of maintenance
therapy.
| Maintenance
regimen |
Total costs
for entire maintenance in analogous studies (CHF) |
Total costs
per median treatment duration (CHF) |
| Olaparib |
133,513 |
133,513 |
| Niraparib |
126,241 |
58,761 |
| Olaparib
& bevacizumab |
197,576 |
164,511 |
| Bevacizumab |
38,628 |
34,769 |










