Theory-driven assessment of intentions and behaviours related to mobility of older
inpatients: a survey of patients and healthcare professionals
DOI: https://doi.org/https://doi.org/10.57187/s.3385
Philippe J. Herzoga,
Rose D. L. Herzog-Zibia,
Charlotte Mörib,
Blandine Mooserc,
Carole Elodie Aubertac
a Department
of General Internal Medicine, Inselspital, Bern University Hospital, University
of Bern, Bern, Switzerland
b Institute of Psychology, University
of Bern, Bern, Switzerland
c Institute of Primary Health Care
(BIHAM), University of Bern, Bern, Switzerland
Summary
BACKGROUND: Low mobility of patients during hospitalisation
is associated with adverse outcomes. To successfully change behaviours related to
mobility of older
hospitalised patients, we need to better understand the mechanisms underlying
patient and healthcare professional behaviours. In this study, we thus assessed
patient- and healthcare
professional-reported intentions and behaviours related to mobility of older
patients hospitalised on an acute medical ward, based on a theoretical
framework – the Health Action Process Approach (HAPA) model – and on additional
barriers and facilitators to mobility.
METHODS: We conducted a cross-sectional survey
in April 2022 among patients aged ≥60 years recently hospitalised on an an acute
medical ward of one of three hospitals of different language/cultural regions
of Switzerland, and healthcare professionals (physicians, nurses/nursing
assistants, physiotherapists) working on those wards. The survey assessed the
HAPA model and additional barriers and facilitators to patient mobility at
hospital, as previously identified in the literature. The target behaviour studied
was “to move as much as possible during hospitalisation”
for patients and “to ensure my patients move as much as possible during hospitalisation”
for healthcare
professionals. We conducted hierarchical linear regressions to determine factors
associated with the self-reported intention to perform the behaviour and with
the self-reported behaviour itself.
RESULTS: A total of 142 healthcare professionals
(61 physicians, 59 nurses, 22 physiotherapists) and 200 patients (mean age 74
years) completed the survey. Patients with higher intention to move as much as
possible during hospitalisation scored significantly higher on factual
knowledge, outcome expectancies and risk perception. Healthcare professionals
with higher intention to ensure that their patients move as much as possible
during hospitalisation scored higher on action knowledge, outcome expectancies
and risk perception. The more the patients reported that they moved as much as
possible during hospitalisation, the higher their action knowledge and action
control. The more healthcare professionals reported that they ensure that
patients move as much as possible during hospitalisation, the higher they
scored on factual knowledge, role perception, planning and action control.
CONCLUSIONS: factual and action knowledge,
self-efficacy, outcome expectancies, risk perception, planning and action
control were identified as important drivers of patient- and healthcare
professional-reported intentions and behaviours related to inpatient mobility.
These parameters can be addressed through behaviour-change interventions and
should be considered in future interventions to successfully implement practice
changes, with the goal of improving mobility of older patients during hospitalisation,
and thus the outcomes of this particularly vulnerable population.
Introduction
Low mobility
of patients during an acute hospitalisation has been associated with several
adverse physical, psychological and societal outcomes, particularly in older
adults, including muscle and bone loss, falls, delirium, depression, anxiety,
orthostatic hypotension, prolonged length of hospital stay, functional decline,
institutionalisation and death [1–5]. Furthermore, less than one third of
patients who experience a functional decline during an acute hospitalisation
have recovered one year later, while 40% of them have passed away [6]. While a
significant proportion of patients already have functional limitations at
admission [7, 8], it is crucial to prevent further functional decline during hospitalisation.
Several
interventions have been conducted in the last few decades in an effort to improve
patient mobility during acute hospitalisation, and thus reduce the adverse
consequences of low mobility [9–15]. However, many of them present limitations
making them hardly scalable in clinical practice, so that they have not led to
broad-scale practice changes. For instance, they did not give sufficient consideration
to real-world resources (e.g. staff availability, costs) or did not fully
address possible barriers and facilitators for mobility in hospitals.
To successfully
change behaviours related to mobility of older hospitalised patients, we first need
to better understand the mechanisms underlying behaviours of key stakeholders,
which include patients and healthcare
professionals (HCPs). Several
studies have assessed patient and HCP attitudes or behaviours regarding patient
mobility [16–20], but none assessed the mechanisms of behaviours, a better
understanding of which would contribute to the development and successful implementation
of mobility-fostering interventions. Furthermore, local data in Switzerland are
limited.
Mechanisms
of behaviour can be studied with the Health Action Process Approach (HAPA)
model, one of the most comprehensive health behaviour change models [21, 22].
This theoretical framework suggests that the adoption, initiation and
maintenance of health behaviours is a process consisting of two main phases: (1)
a motivation phase, in which a person develops an intention, and (2) a volition
or action phase, in which the person implements the behaviour. Self-efficacy,
outcome expectancies and risk perception are major drivers of the intention
(motivation phase). Intention, planning and action control are major drivers of
the behaviour (volition phase). The HAPA model has already been applied to
older people and to physical activity [23–25], as well as to HCPs
[26, 27].
In
this study, we thus aimed to identify determinants of behaviours related to mobility
of older medical patients during an acute hospitalisation, as reported by
patients and HCPs (nurses/nursing
assistants, physicians, physiotherapists), based on the HAPA model [21, 22], and
on barriers and facilitators identified in previous studies [18].
Methods
Design and
setting
We conducted
a cross-sectional survey in April 2022 in three hospitals of Switzerland: Bern
University Hospital (Inselspital); Tiefenau Hospital, a small non-university
hospital in Bern; and Fribourg Cantonal Hospital (HFR-Fribourg), a large
non-university hospital. To increase data generalisability, we selected
hospitals of both the German- and French-speaking regions of Switzerland (also
reflecting different cultural aspects) and of different sizes and types
(university and non-university). Exemption from ethical approval was granted by the
Ethics
Committee of the University of Bern, as the study did not fall under the remit
of human research as defined by Swiss regulations (Request number 2021-01383).
Participation was voluntary and participants provided informed consent to
participate. Participants were informed that their data would be analysed
anonymously. The study was performed in accordance with the Declaration of
Helsinki. Findings are reported according to the STROBE reporting standard.
Participants,
sample size and data collection
We
surveyed both patients and healthcare professionals (HCPs), including physicians,
nurses, nursing
assistants and physiotherapists. The proportions of patients and HCPs
from each hospital were chosen to reflect the size of each hospital. We
aimed for a margin of error of 7% with a confidence level of 95%. This level
was chosen because, as a rule of thumb, an acceptable margin of error for a
survey falls between 4% and 8%, and 7% would allow realistic recruitment
numbers with our population sizes.
Patients
Patients
were recruited by telephone by two authors (RH, PH) based on a list of all patients
aged 60 years or older hospitalised in a medical ward of one of the
participating hospitals in the previous year. Patient inclusion criteria were: (a)
age 60 years or older; and (b) hospitalisation in a general internal medicine
ward of one of the three hospitals during the last year. We excluded patients
with cognitive disorders (based on medical records) and those unable to walk
(e.g. wheelchair-bound patients) before hospital admission. Based on a mean discharge
number of 5000 patients yearly (the total for the three hospitals), we
calculated that 189 patients would provide a margin of error of 7% with a confidence
level of 95%. Based on previous experience, we expected that some patients accepting
to participate would not return the survey. We therefore increased the
recruitment target by 20% to account for the expected non-response rate. After
verifying the eligibility criteria of all patients of the list using electronic
health records, two authors (RH, PH) contacted them directly by telephone in
alphabetical order (to avoid any selection bias). A maximum of three attempts were
made to call each patient. During the telephone call, the study was explained
to the patients. Those who agreed to participate received the survey in paper
form by post, together with written information on its goal, a consent form to
sign, as well as a pre-paid envelope to return the survey and the consent form.
Patients who did not return the survey were called back by the same two authors.
There was no financial compensation for participation. Patient answers were
transferred from paper into an electronic file by the senior author (CEA).
Missing data were left empty.
Healthcare professionals
The
only inclusion criterion for HCPs was to be working on a general internal
medicine ward of one of the three selected hospitals. Based on the number of HCPs
working on the medical wards of the three included hospitals (about
500), we calculated that 141 HCPs would provide a margin of error of 7% with a confidence
level of 95%. Since we were more interested in assessing the mechanisms of behaviour
of physician and nursing staff than of physiotherapists (fostering mobility being
a main task of physiotherapists in the studied setting), we planned to include more
physicians and nurses/nursing assistants than physiotherapists. We aimed for
similar proportions of physicians and nurses/nursing assistants, because even
if fostering patient mobility might be perceived more as a nursing task, the physician’s
role might be as important. Nurses (i.e. registered/licensed nurses) and nursing
assistants (i.e. practice nurses) were selected for participation by the heads
of nursing, while physiotherapists and physicians were recruited by e-mail by
the senior author (CEA). HCPs were informed that their participation
was anonymous and voluntary. Those agreeing to participate received a link to answer
the survey on surveymonkey.com (SurveyMonkey®). Since the survey could not be
completed without answering all questions, there were no missing data.
Outcomes
We
assessed two outcomes in two distinct analyses (see in the “Data analysis”
section below), separately for patients and for HCPs: (1) self-reported
intention and (2) self-reported behaviour. Both outcomes were rated on a
5-point Likert scale. The intention was defined as “I intend to move as much as
possible during a future hospitalisation” (for patients) and “I want to ensure
during the next 3 months that my patients move as much as possible during their
hospitalisation” (for HCPs). The behaviour was defined as “I
moved as much as possible during hospitalisation” (for patients, referring to
the last hospitalisation on the medical ward) and “I ensure my patients move as
much as possible during hospitalisation” (for HCPs).
Survey instrument
The
survey questions were based on the HAPA model and on barriers and facilitators
to medical inpatient mobility identified in previous studies [18]. The items
from the HAPA model assessed (referred to as “HAPA variables”) were:
- self-efficacy (belief in ability to perform the behaviour)
- outcome expectancies (expectations of performing the behaviour, beliefs
about the consequences of the behaviour)
- risk perception (subjective assessment
of the risks of not performing the behaviour)
- intention
- planning (consisting
of action planning – when, where and how to perform the behaviour – and coping planning
– anticipating potential barriers to achieving the behaviour)
- action control (self-monitoring
of behaviour implementation)
- behaviour
These items referred to the target behaviours
described in the “Outcomes” section above. The planning variable was not
collected in the patient survey, because the assessed behaviour was in the past.
The items assessed based on barriers and facilitators for mobility at hospital
(referred to as “non-HAPA variables”) were: factual knowledge, action
knowledge, role perception, fear and organisation-environment. The
survey was developed in German and then translated into French by bilingual
members of the research team using a forward-backward translation method [28,
29]. An English version of the questions is provided in tables S1 and S2 in the appendix.
The questions
were rated on a 5-point Likert scale (1 = “disagree”, 2 = “rather disagree”, 3
= “neutral”, 4 = “rather agree”, 5 = “agree”).
In
addition, we collected participant baseline characteristics. For patients, these
included age, sex, educational level, living situation (before and after hospitalisation),
use of walking aids and life-space level according to the University of Alabama
in Birmingham Study of Aging Life-Space Assessment [30]. For HCPs, baseline characteristics
included age, sex, years of work
experience, percentage of work time, graduation year and board certification
(for physicians only). The surveys were tested using a thinking-aloud method
[31] with four patients and six HCPs from all three
hospitals, and the questions were adapted accordingly.
Data analysis
HAPA
and non-HAPA variables were created by calculating the mean of the answers to
the different questions assessing each respective variable (when applicable).
Negatively formulated questions were recoded positively (see tables S1 and S2 in the
appendix) to
allow grouping of the questions. Internal consistency between the different
questions assessing one variable was assessed using Cronbach’s alpha.
We conducted
hierarchical regressions to assess the determinants of intention and behaviour.
Non-HAPA variables were entered in the model first as they are supposed to precede
HAPA variables in the mediation pathway of intention/behaviour (for example,
knowledge leads to outcome expectancies and then to intention). HAPA variables
were then added sequentially based on their proximity to the outcomes: level 1: self-efficacy,
outcome expectancies and risk perception; level 2: intention;
level 3: planning; level 4: action control. Some non-HAPA and HAPA variables
could not be included, because of the nature/content of the questions. For
example, when the behaviour was finished for the patients but ongoing for HCPs. Figure
1 summarises the intention and behaviour
frameworks for patients and HCPs. Thus, the analysis of intention included only two
models
(figures 1A and 1B): (1) model 1
with non-HAPA variables; (2) model 2 with non-HAPA and level 1 HAPA variables.
The analysis of patient behaviour included two models (figure 1C): (1) model 1 with
non-HAPA variables; (2) model 2 with action
control in addition. The analysis of healthcare
professional behaviour included five models (figure 1D): (1) model 1 with non-HAPA
variables; (2) model 2 with
level 1 HAPA variables in addition (i.e. self-efficacy, outcome expectancies
and risk perception); (3) model 3 with the level 2 HAPA variable in addition
(i.e. intention); (4) model 4 with the level 3 HAPA variable in addition (i.e.
planning); (5) model 5 with the level 4 HAPA variable in addition (i.e. action
control). In addition to these unadjusted main models, we conducted sensitivity
analyses adjusting for age and sex.
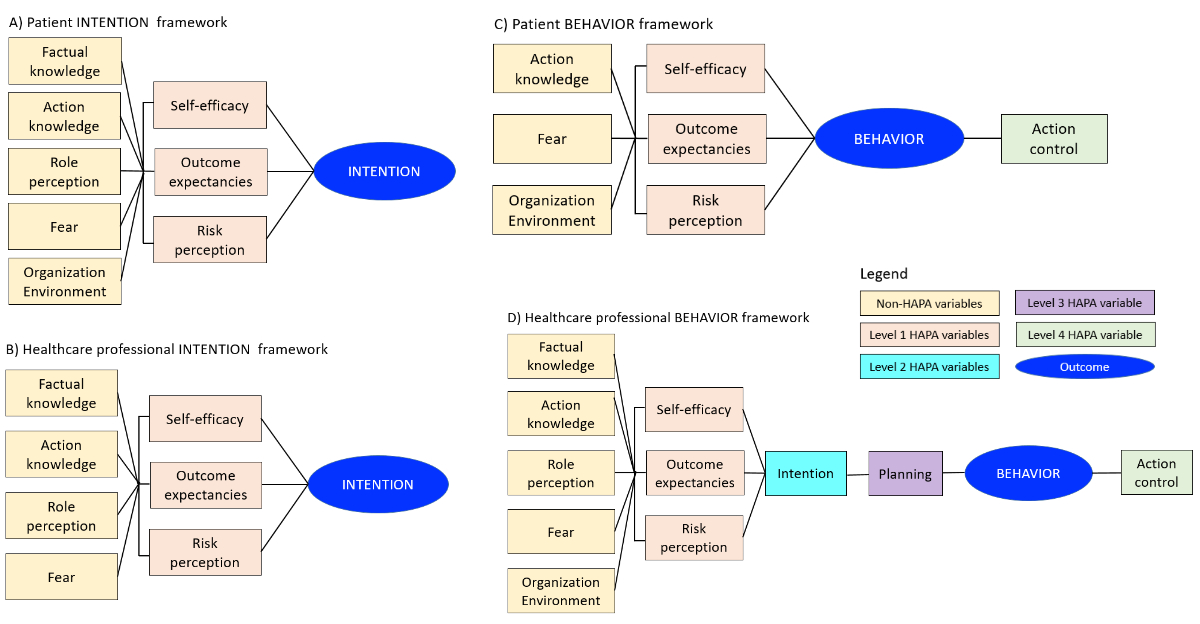
Figure 1Analytical
framework for patient and healthcare professional (HCP) intention and behaviour
hierarchical regression models. In
the different models of the hierarchical regression, the variables were added
consecutively based on theory: non-HAPA variables (yellow boxes: factual
knowledge, action knowledge, role perception, fear, organisation-environment),
HAPA level 1 variables (pink-orange boxes: self-efficacy, outcome expectancies,
risk perception), HAPA level 2 variable (turquoise box: intention), HAPA level
3 variable (purple box: planning) and HAPA level 4 variable (green box: action
control). Not all variables could be included in all frameworks, due to the
nature of the assessment. (A) Patient intention framework; (B) HCP intention framework;
(C) patient behaviour
framework; (D) HCP behaviour framework. HAPA:
Health Action Process Approach.
We
tested model assumptions using residual plots, variance inflation factor to
assess multicollinearity, and the Breusch-Pagan/Cook-Weisberg test for heteroscedasticity.
We used robust standard error when the heteroscedasticity test was significant.
We presented the results as beta coefficients with 95% confidence intervals (95%
CI). We used delta R-squared (R2) to assess the improvement of the
models through the different steps (i.e. how much more of the variance is
explained by the variables added to the model). The significance level was set
at an alpha level of 0.05.
We
performed all analyses using Stata/MP 16.0 (StataCorp LP, College Station,
Texas, USA).
Ethical approval
and consent to participate
The
study was granted a waiver from ethical approval by the Ethics Committee of the
University of Bern, given that it did not fall under the remit of human
research as defined by Swiss regulations. Participation was voluntary and
participants provided informed consent to participate. The protocol was defined
in the grant submission but not registered on a public registry.
Consent
for publication:
Study participants were informed that the results of the study would be
published in peer-reviewed journals.
Results
Descriptive
results
Between
December 2021 and March 2022, we recruited 142 HCPs (61 physicians, 59 nurses/nursing
assistants, 22 physiotherapists) who completed the survey. Among 1017 screened patients,
577 (56.7%) did not meet eligibility criteria and 440 (43.3%) were invited to
participate (figure 2). Of them,
285 (64.8%) initially accepted to participate and 200 (45.5%) finally completed
the survey. Participants’ baseline characteristics are reported in table 1. Patients
had a mean age of 74 years
(standard deviation [SD]: 7.6, range: 60–92) and 74 (37.0%) were female. HCPs had
a mean
age of 32 years (SD: 8.6, range: 19–62) and 107 (75.4%) were female.
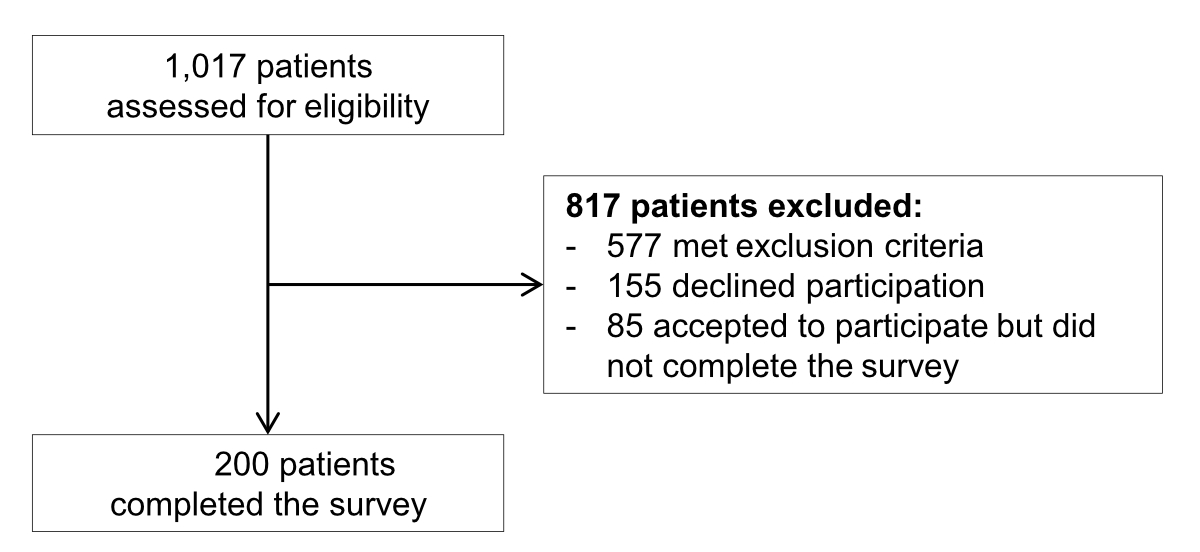
Figure 2Flow chart
of patient recruitment.
Table 1Patient and healthcare professional characteristics and distribution of HAPA
and non-HAPA variables.
| |
Patients
(n = 198) * |
HCPs (n = 142) |
| Characteristic |
| Age
(years), mean (SD) |
74
(7.6) |
32
(8.6) |
| Female,
n (%) |
74
(37.0%) |
107
(75.4%) |
| Hospital, n (%) |
Bern
University Hospital |
86
(43.0%) |
79
(55.6%) |
| Tiefenau
Hospital Bern |
41 (20.5%) |
23 (16.2%) |
| Fribourg
Cantonal Hospital |
71 (35.5%) |
40 (28.2%) |
| Education (maximum level reached), n (%) |
|
|
NA |
| Elementary
school |
28
(14.0%) |
|
| Apprenticeship |
115
(57.5%) |
|
| High
school |
12
(6.0%) |
|
| College |
41
(20.5%) |
|
| Duration
of hospitalisation (days), mean (SD) |
7.3
(5.4) |
NA |
| Life-space
assessment score, mean (SD) ** |
75.7
(34.5) |
NA |
| Working group, n (%) |
|
NA |
|
| Physician |
|
61
(43.0%) |
| Nursing
staff |
|
59
(41.5%) |
| Physiotherapist |
|
22
(15.5%) |
| HAPA variables, mean (SD) *** |
| Intention |
4.3
(0.9) |
4.4
(0.8) |
| Behaviour |
3.9
(1.2) |
3.8
(0.9) |
| Self-efficacy |
3.7
(0.7) |
3.5
(0.8) |
| Outcome
expectancies |
3.9
(0.5) |
4.5
(0.5) |
| Risk
perception |
3.9
(0.8) |
4.4
(0.7) |
| Action
control |
3.9
(1.0) |
3.9
(0.9) |
| Planning |
NA |
3.4
(0.9) |
| Non-HAPA variables, mean (SD) *** |
| Factual
knowledge |
3.6
(0.8) |
4.1
(0.5) |
| Action
knowledge |
4.0
(0.9) |
4.0
(0.9) |
| Role
perception |
3.9
(1.0) |
4.2
(0.8) |
| Fear |
1.8
(1.2) |
2.2
(0.6) |
| Organisation-environment |
3.3
(0.9) |
2.8
(1.0) |
The
distribution of HAPA and non-HAPA variables for patients and HCPs
are reported in table 1. All
mean values of HAPA variables were higher than neutral (neutral corresponding
to 3 on the 5-point Likert scale), ranging for patients from 3.7 (SD: 0.7) for self-efficacy
to 4.3 (SD: 0.9) for intention, and for HCPs from 3.4 (SD: 0.9) for planning to 4.4
(SD: 0.7) for risk perception and 4.4 (SD: 0.8) for intention. Similar results
were obtained for non-HAPA variables, except for organisation-environment for HCPs
(mean:
2.8, SD: 1.0) and for fear (mean: 1.8, SD: 1.2 for patients; mean: 2.2, SD: 0.6
for HCPs).
Correlations and
assessment of model assumptions
Patient
variables that correlated most were self-efficacy and risk perception (Pearson
coefficient: 0.51; table S3 in the appendix).
For HCPs,
the highest correlations were found between self-efficacy and planning (Pearson
coefficient: 0.52), Outcome expectancies and intention (Pearson coefficient:
0.58), and role perception and Behaviour (Pearson coefficient: 0.60; table S4 in the
appendix). The variance
inflation factor was below 2.1 for all variables, making relevant
multicollinearity unlikely. The test for heteroscedasticity was significant for
patient and HCP intention models and the patient behaviour model,
so that we used robust standard errors for those models.
Patient intention
models
The patient
full unadjusted model explained 35.0% of the variance in patient-reported intention
to move as much as possible during hospitalisation (table 2). Factual knowledge, outcome
expectancies and risk perception
were associated with patient-reported intention. Patient-reported intention increased
on average by 0.14 points (95% CI: 0.01–0.26) for each point increase in factual
knowledge, by 0.43 points (95% CI: 0.19–0.67) for each point increase in outcome
expectancies, and by 0.26 points (95% CI: 0.04–0.49) for each point increase in
risk perception. The results were similar in the sensitivity analysis adjusting
for age and sex. Model 2 performed better than model 1, explaining 21.0% more
of the variance (p value for delta R2 <0.001).
Table 2Unadjusted
hierarchical regression models for predicting patient and HCP intention regarding
patient mobility. Results from the
hierarchical regression analysis, presented as beta coefficients with 95%
confidence intervals. 142 patients had data variables for the models.
Significant results are highlighted in bold. The outcome (intention) was
defined as “I intend to move as much as possible during a future hospitalisation”
for the patients and “I want to ensure that my patients move as much as
possible during hospitalisation” for the HCPs. R2
is the amount of the variance explained by the model (0.00 representing 0%,
1.00 representing 100%). The delta R2 (increment between two models)
refers to the amount of the variance additionally explained with the additional
variables. Results were similar in a sensitivity analysis adjusted for age and
sex.
| Patients |
Model 1 |
Model 2 |
| Factual knowledge |
0.18 (0.02; 0.33) |
0.14 (0.01; 0.26) |
| Action knowledge |
0.15 (–0.02; 0.32) |
0.02 (–0.12; 0.16) |
| Role perception |
0.21 (0.07; 0.35) |
0.15 (–0.01; 0.30) |
| Fear |
0.02 (–0.08; 0.12) |
0.02 (–0.07; 0.12) |
| Organisation-environment |
0.00 (–0.11; 0.14) |
–0.02 (–0.13; 0.08) |
| Self-efficacy |
NA |
0.24 (–0.01; 0.50) |
| Outcome expectancies |
NA |
0.43 (0.19; 0.67) |
| Risk perception |
NA |
0.26 (0.04; 0.49) |
| R2
(p value) |
0.14
(<0.001) |
0.35
(<0.001) |
| Delta R2 model 2 – model 1 (p value) |
0.21
(<0.001) |
| Healthcare professionals |
Model 1 |
Model 2 |
| Factual knowledge |
0.38 (0.11; 0.66) |
0.11 (–0.16; 0.38) |
| Action knowledge |
0.21 (0.02; 0.39) |
0.17 (0.03; 0.30) |
| Role perception |
0.20 (0.02; 0.35) |
0.07 (–0.09; 0.24) |
| Fear |
0.14 (–0.08; 0.30) |
0.17 (–0.01; 0.34) |
| Organisation-environment |
0.01 (–0.10; 0.13) |
0.04 (–0.05; 0.14) |
| Self-efficacy |
NA |
0.21 (0.01; 0.40) |
| Outcome expectancies |
NA |
0.41 (0.14; 0.70) |
| Risk perception |
NA |
0.24 (0.11; 0.37) |
| R2
(p value) |
0.30
(<0.001) |
0.51
(<0.001) |
| Delta R2 model 2 – model 1 (p value) |
0.21
(<0.001) |
Healthcare professional
intention models
The HCP full unadjusted model (model 2) explained 50.0% of the variance in
HCP-reported
intention to ensure patients move as much as possible (table 2). Action
knowledge, self-efficacy, Outcome expectancies and risk perception were
associated with HCP-reported intention. HCP-reported intention increased on average
by 0.17 points (95% CI: 0.03–0.30) for each point increase in action knowledge,
by 0.21 points (95% CI: 0.01–0.40) for each point increase in self-efficacy, by
0.42 points (95% CI: 0.14–0.70) for each point increase in outcome expectancies,
and by 0.24 points (95% CI: 0.11–0.37) for each point increase in risk perception.
The results were similar in the sensitivity analysis adjusting for age and sex.
Model 2 was better than model 1, explaining 21.0% more of the variance (p value
for delta R2 <0.001).
Patient behaviour
models
The patient
full unadjusted model (model 2) explained 32.0% of patient-reported behaviour (table
3). Patient-reported mobility increased on average by 0.37 points (95% CI: 0.14–0.60)
for each point increase in action knowledge, and by 0.42 points (95% CI: 0.25–0.60)
for each point increase in action control. The results were similar in the
sensitivity analysis adjusting for age and sex. Model 2 was better than model 1,
explaining 9.0% more of the variance (p value for delta R2 was 0.009).
Table 3Unadjusted hierarchical regression models predicting
patient and HCP behaviour regarding patient mobility. Results from the hierarchical
regression analysis, presented as beta coefficients with 95% confidence
intervals. 138 patients had complete data for models 1–4 and 128 patients for
model 5. Significant results are highlighted in bold. The outcome (behaviour)
was defined as “I moved as much as possible during hospitalisation” for the
patients and “I ensured that my patients moved as much as possible during hospitalisation”
for the HCPs. R2 is the amount of the
variance explained by the model (0.00 representing 0%, 1.00 representing 100%).
The delta R2 (increment between two models) refers to the amount of
the variance additionally explained with the additional variables. Results were
similar in a sensitivity analysis adjusted for age and sex.
| Patients |
Model 1 |
Model 2 |
|
|
|
| Action
knowledge |
0.57
(0.37; 0.77) |
0.37
(0.14; 0.60) |
|
|
|
| Fear |
–0.10
(–0.27; 0.06) |
–0.06
(–0.21; 0.08) |
|
|
|
| Organisation-environment |
–0.07
(–0.26; 0.11) |
–0.11
(–0.32; 0.09) |
|
|
|
| Action
control |
NA |
0.42
(0.25; 0.60) |
|
|
|
| R2 (p value) |
0.23 (<0.001) |
0.32 (<0.001) |
|
|
|
| Delta R2
model 2 – model 1 (p value) |
0.09 (0.009) |
|
|
|
| Healthcare
professionals |
Model 1* |
Model 2* ** |
Model 3** *** |
Model 4 |
Model 5 |
| Factual
knowledge |
0.45
(0.13; 0.76) |
0.40
(0.07; 0.72) |
0.38
(0.0504; 0.70) |
0.33
(0.02; 0.65) |
0.26
(0.00; 0.52) |
| Action
knowledge |
0.12
(–0.04; 0.27) |
0.09
(–0.06; 0.25) |
0.05
(–0.10; 0.22) |
0.06
(–0.09; 0.21) |
0.01
(–0.12; 0.15) |
| Role
perception |
0.48
(0.32; 0.64) |
0.45
(0.28; 0.61) |
0.43
(0.27; 0.59) |
0.40
(0.24; 0.56) |
0.27
(0.13; 0.41) |
| Fear |
–0.02
(–0.22; 0.17) |
–0.01
(–0.19; 0.20) |
–0.03
(–0.23; 0.17) |
–0.03
(–0.22; 0.19) |
0.14
(–0.03; 0.30) |
| Organisation-environment |
0.08
(–0.04; 0.21) |
0.09
(–0.04; 0.21) |
0.08
(–0.04; 0.21) |
0.07
(–0.05; 0.26) |
0.06
(–0.05; 0.16) |
| Self-efficacy |
NA |
0.24
(0.05; 0.43) |
0.19
(0.00; 0.39) |
0.05
(–0.15; 0.26) |
–0.04
(–0.22; 0.14) |
| Outcome
expectancies |
NA |
–0.04
(–0.34; 0.25) |
–0.14
(–0.42; 0.18) |
–0.10
(–0.38; 0.19) |
–0.06
(–0.30; 0.19) |
| Risk
perception |
NA |
0.00
(–0.20; 0.20) |
–0.05
(–0.26; 0.15) |
0.00
(–0.20; 0.20) |
0.03
(–0.14; 0.20) |
| Intention |
NA |
NA |
0.21
(0.00; 0.42) |
0.16
(–0.04; 0.37) |
0.02
(–0.17; 0.21) |
| Planning |
NA |
NA |
NA |
0.25
(0.10; 0.40) |
0.20
(0.07; 0.34) |
| Action
control |
NA |
NA |
NA |
NA |
0.47
(0.34; 0.61) |
| R2 (p value) |
0.45 (<0.001) |
0.47 (<0.001) |
0.49 (<0.001) |
0.53 (<0.001) |
0.65 (<0.001) |
| Delta R2
model 5 – model 1 (p value) |
0.20 (<0.001) |
Healthcare
professional behaviour models
The HCP full unadjusted model (model 5) explained 65.0% of HCP-reported behaviour
(table 3). Factual knowledge (beta
coefficient: 0.26 [95% CI: 0.00–0.52]), role perception (beta coefficient: 0.27
[95% CI: 0.13–0.41]), planning (beta coefficient: 0.20 [95% CI: 0.07–0.34]) and
action control (beta coefficient 0.47 [95% CI: 0.34–0.61]) were associated with
HCP-reported
behaviour. The results were similar in the sensitivity analysis adjusting for
age and sex. The HCP full model was better than model 1,
explaining 20.0% more of the variance (p value for delta R2
<0.001).
Discussion
In
this theory-driven analysis, we assessed potential determinants of patient- and
HCP-reported intentions and behaviours related to mobility of older patients during
an acute hospitalisation in a medical ward. Action knowledge and action control seemed
key
factors of patient behaviour, and factual knowledge, role perception, planning and
action control of HCP behaviour. The several identified
potential drivers of mobility-related intentions and behaviours provide useful
information for the development of future interventions for increasing the mobility
of older hospitalised patients, to ensure that these interventions successfully
lead to behaviour change in clinical practice.
Fear,
which has previously been identified as a barrier to patient mobility [18], was
not significantly associated with patient- and HCP-reported intention or behaviour.
Of
note, fear was rated rather low in this study, so that the findings might not
apply to patients and HCPs with a higher level of fear. While about
one third of older people develop a fear of falling, even without experiencing
a fall [32, 33], and reducing fear is important in general to improve HCP and patient
well-being and HCP work motivation
[34, 35], further study is warranted to determine whether reducing fear of
fall/injury can improve behaviours related to mobility of older hospitalised
patients.
Factual
knowledge was associated with patient-reported intention and HCP-reported behaviour,
and action knowledge with patient-reported behaviour and HCP-reported
intention. Previous interventions to increase mobility have targeted knowledge
in several ways. While some studies focused on factual knowledge (e.g.
education about the importance of patient mobility) [10, 36], other studies
addressed action knowledge as well [11, 13, 37]. Concordant with implementation
science theories, our findings suggest that future interventions should target
not only factual knowledge (i.e. “what to do”), but also action knowledge (i.e.
“how to do it”). This could include information on where, when and how to move,
for example with walking itineraries or exercises, or a goal-setting process
with concrete daily mobility objectives, which was effective in previous
studies [11, 13, 37]. Furthermore, we found an association between perceiving
patient mobility as a part of HCP work tasks (i.e.
role perception) and HCP-reported behaviour. Ensuring that patient mobility is taught
as a main work task in healthcare professional studies and training might help to
improve healthcare
professional role perception and thus how they ensure patients move as
much as possible during their hospitalisation.
Self-efficacy,
outcome expectancies and risk perception were associated with HCP-reported
intention, while planning and action control were associated with their behaviour.
Several possibilities exist to target those aspects that seem important to
improve HCP behaviour related to patient mobility. First, implementing
practical training could help to improve self-efficacy, including
self-confidence. Second, discussions between HCPs about their
outcome expectancies and risk perception could help adjust or correct them,
notably reduce misbeliefs. For example, the misbelief that letting patients lie
for a few days will not significantly impact their outcomes, or that fostering mobility
increases the risk of falls. Finally, developing practical guidelines and
algorithms about patient mobility for HCPs could contribute
to improve planning and action control.
Outcome
expectancies and risk perception were associated with patient-reported
intention, and action control with patient-reported behaviour. These three
parameters seem accessible to change. Discussions between HCPs and patients might
help identify patient expectations and potential
misbeliefs. A common misbelief is that one should rest in bed to recover,
likely related to hospital set-up and organisation (beds available all day
long, bedside visits, …) [18]. While the latter cannot be easily modified, it
is nevertheless quite feasible to address patient misbeliefs about outcome
expectancies and risk perception. Including their relatives in the process might
also be important in preventing them from spreading these misbeliefs. Finally, action
control might be improved by providing patients with tools to set and monitor
goals, such as a mobility diary, and by discussing these goals and progress
regularly with the patients. In the future, interactive technology measuring mobility
(e.g. smartphone apps, smart watches), might also help elderly patients monitor
their mobility and progress.
Limitations and
strengths
This
study has several strengths. First, we conducted the survey with both patients
and HCPs and
included the main healthcare
professional categories involved in patient mobility at hospital (nurses/nursing
assistants, physicians, physiotherapists). Second, the sample was large enough
to provide a 7% margin of error with a confidence level of 95%. Third, we
assessed and analysed not only variables identified in previous studies on
patient mobility, but also variables of the HAPA model, allowing a
theory-driven assessment of mobility behaviours. Fourth, we conducted the
survey in three hospitals of different sizes and cultural/language regions,
increasing result generalisability. Fifth, we studied a broad patient
population, not limiting the study to specific health conditions.
We
must acknowledge several limitations. First, only patients and HCPs who agreed
to answer the survey were included, so that the results might not be generalisable
to all patients and HCPs. This
is, however, a limitation of any such study. Second, recruitment by the heads
of nursing might have introduced a selection bias. To reduce this risk, they
were asked to provide a sample of HCPs of various ages, years of experience and
qualification degrees. Furthermore, selection was conducted by several
different people, because there is one head of nursing on each ward (and not
only one in each hospital), which might have helped reduce selection bias. Third,
the subsamples of HCPs were too small to assess for differences across professions
(physicians, nurses/nursing assistants, physiotherapists). Nevertheless, the majority
of our sample were physicians and nurses/nursing assistants, whose behaviour change
is most likely to help modify practices, and are thus most important to
assess. In the setting we studied, fostering mobility is indeed considered a
main task of physiotherapists, but not of physicians and nurses/nursing
assistants. However, this might be different in other settings. Fourth,
patients who were wheelchair-bound before hospitalisation and those with
cognitive impairment were excluded. While it is important that wheelchair-bound patients
continue to be able to transfer themselves for example from wheelchair to bed, such
patients most frequently are already hardly moving
independently before hospitalisation, and likely represent a different patient
collective that should be studied separately. Whereas mobility is very
important for cognitively impaired patients, asking them to answer questions
about a past hospitalisation would likely not have yielded reliable answers.
Fifth, the study used a cross-sectional design with, for patients, self-reporting,
which does not rule out an information bias, without repeat measurements and with
assessment of past behaviour. This did not allow us to assess the HAPA model
completely, nor to study a temporal sequence, nor to assess intraindividual
correlations, nor to measure patient mobility objectively. However, while mobility
can be measured objectively, most other variables that we assessed cannot (e.g.
the intention to move). Of note, patient perception of mobility during hospitalisation
might also have been different if it had been studied during hospitalisation. Sixth,
several items that could confound or mediate the associations (such as
functional ability, use of a walking aid or hospitalisation diagnosis) were not
collected. However, we were interested in studying mechanisms of intention and behaviour
that can be targeted through an intervention, and thus did not focus on or
adjust for specific health conditions or functional ability, which would have
limited result generalisability. Finally, the study was conducted in
Switzerland only, so results might not be generalisable to other countries with
different healthcare systems.
Conclusion and
clinical implications
This
study assessing determinants of patient- and HCP-reported intentions and behaviours
identified several
potential drivers of patient and HCP behaviour
related to mobility of older hospitalised medical patients, which can be
addressed through specific interventions. The findings of this study can inform the
development of behaviour change interventions to help successfully implement
practice modifications, in order to improve mobility of older patients hospitalised
on an acute medical ward and, in turn, their outcomes.
Availability of
data and material
The
datasets analysed during the current study and the codes used for analysis are
available from the corresponding author upon reasonable request. No specific
library or package was used for the analyses.
Acknowledgments
We thank all participants to the survey for
contributing to this research. We thank Professor Jennifer Inauen for
contributing to creating the questionnaire to assess the HAPA model.
Authors’ contribution: Conception and
design of the study: CA, CM. Data collection: PH, RH, CM, CA. Data analysis and
interpretation: CM, CA. Manuscript drafting: CA. Revising the manuscript
critically for important intellectual content: PH, RH, CM, BM. Approval of the
version of the manuscript to be published: All authors.
Carole Elodie Aubert,
MD, MSc
Inselspital
Bern University
Hospital
Anna-von-Krauchthal
Weg 7
CH-3010 Bern
caroleelodie.aubert[at]insel.ch
References
1. Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized
older patients. J Am Geriatr Soc. 2004 Aug;52(8):1263–70. doi: https://doi.org/10.1111/j.1532-5415.2004.52354.x
2. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility
during hospitalization of older adults. J Am Geriatr Soc. 2009 Sep;57(9):1660–5. doi: https://doi.org/10.1111/j.1532-5415.2009.02393.x
3. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993 Feb;118(3):219–23.
doi: https://doi.org/10.7326/0003-4819-118-3-199302010-00011
4. Markey DW, Brown RJ. An interdisciplinary approach to addressing patient activity
and mobility in the medical-surgical patient. J Nurs Care Qual. 2002 Jul;16(4):1–12.
doi: https://doi.org/10.1097/00001786-200207000-00002
5. Kalisch BJ, Lee S, Dabney BW. Outcomes of inpatient mobilization: a literature review.
J Clin Nurs. 2014 Jun;23(11-12):1486–501. doi: https://doi.org/10.1111/jocn.12315
6. Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, et al. Recovery
of activities of daily living in older adults after hospitalization for acute medical
illness. J Am Geriatr Soc. 2008 Dec;56(12):2171–9. doi: https://doi.org/10.1111/j.1532-5415.2008.02023.x
7. Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss
of independence in activities of daily living in older adults hospitalized with medical
illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003 Apr;51(4):451–8.
doi: https://doi.org/10.1046/j.1532-5415.2003.51152.x
8. D’Onofrio A, Büla C, Rubli E, Butrogno F, Morin D. Functional trajectories of older
patients admitted to an Acute Care Unit for Elders. Int J Older People Nurs. 2018 Mar;13(1):e12164.
doi: https://doi.org/10.1111/opn.12164
9. Hamilton AC, Lee N, Stilphen M, Hu B, Schramm S, Frost F, et al. Increasing Mobility
via In-hospital Ambulation Protocol Delivered by Mobility Technicians: A Pilot Randomized
Controlled Trial. J Hosp Med. 2019 May;14(5):272–7. doi: https://doi.org/10.12788/jhm.3153
10. Hastings SN, Sloane R, Morey MC, Pavon JM, Hoenig H. Assisted early mobility for hospitalized
older veterans: preliminary data from the STRIDE program. J Am Geriatr Soc. 2014 Nov;62(11):2180–4.
doi: https://doi.org/10.1111/jgs.13095
11. Brown CJ, Foley KT, Lowman JD Jr, MacLennan PA, Razjouyan J, Najafi B, et al. Comparison
of Posthospitalization Function and Community Mobility in Hospital Mobility Program
and Usual Care Patients: A Randomized Clinical Trial. JAMA Intern Med. 2016 Jul;176(7):921–7.
doi: https://doi.org/10.1001/jamainternmed.2016.1870
12. Raymond MJ, Jeffs KJ, Winter A, Soh SE, Hunter P, Holland AE. The effects of a high-intensity
functional exercise group on clinical outcomes in hospitalised older adults: an assessor-blinded,
randomised-controlled trial. Age Ageing. 2017 Mar;46(2):208–13. doi: https://doi.org/10.1093/ageing/afw215
13. Teodoro CR, Breault K, Garvey C, Klick C, O’Brien J, Purdue T, et al. STEP-UP: Study
of the Effectiveness of a Patient Ambulation Protocol. Medsurg Nurs. 2016;25(2):111–6.
14. Wood W, Tschannen D, Trotsky A, Grunawalt J, Adams D, Chang R, et al. A mobility program
for an inpatient acute care medical unit. Am J Nurs. 2014 Oct;114(10):34–40. doi: https://doi.org/10.1097/01.NAJ.0000454850.14395.eb
15. Taylor NF, Harding KE, Dennett AM, Febrey S, Warmoth K, Hall AJ, et al. Behaviour
change interventions to increase physical activity in hospitalised patients: a systematic
review, meta-analysis and meta-regression. Age Ageing. 2022 Jan;51(1):afab154. doi: https://doi.org/10.1093/ageing/afab154
16. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized
general medicine patients: survey development and results. Am J Phys Med Rehabil.
2015 Apr;94(4):304–12. doi: https://doi.org/10.1097/PHM.0000000000000185
17. So C, Pierluissi E. Attitudes and expectations regarding exercise in the hospital
of hospitalized older adults: a qualitative study. J Am Geriatr Soc. 2012 Apr;60(4):713–8.
doi: https://doi.org/10.1111/j.1532-5415.2012.03900.x
18. Mani H, Möri C, Mattmann M, Liechti F, Inauen J, Aujesky D, et al. Barriers and facilitators
to mobility of patients hospitalised on an acute medical ward: a systematic review.
Age Ageing. 2022 Jul;51(7):afac159. doi: https://doi.org/10.1093/ageing/afac159
19. van Dijk-Huisman HC, Raeven-Eijkenboom PH, Magdelijns FJ, Sieben JM, de Bie RA, Lenssen AF.
Barriers and enablers to physical activity behaviour in older adults during hospital
stay: a qualitative study guided by the theoretical domains framework. BMC Geriatr.
2022 Apr;22(1):314. doi: https://doi.org/10.1186/s12877-022-02887-x
20. King B, Bodden J, Steege L, Brown CJ. Older adults experiences with ambulation during
a hospital stay: A qualitative study. Geriatr Nurs. 2021;42(1):225–32. doi: https://doi.org/10.1016/j.gerinurse.2020.08.005
21. Schwarzer R. Modeling health behavior change: how to predict and modify the adoption
and maintenance of health behaviors. Appl Psychol. 2008;57(1):1–29. doi: https://doi.org/10.1111/j.1464-0597.2007.00325.x
22. Schwarzer R. Health action process approach (HAPA) as a theoretical framework to understand
behavior change. Actual Psicol. 2016;30(121):119–30. doi: https://doi.org/10.15517/ap.v30i121.23458
23. Bierbauer W, Inauen J, Schaefer S, Kleemeyer MM, Lüscher J, König C, et al. Health
Behavior Change in Older Adults: Testing the Health Action Process Approach at the
Inter- and Intraindividual Level. Appl Psychol Health Well-Being. 2017 Nov;9(3):324–48.
doi: https://doi.org/10.1111/aphw.12094
24. Caudroit J, Stephan Y, Le Scanff C. Social cognitive determinants of physical activity
among retired older individuals: an application of the health action process approach.
Br J Health Psychol. 2011 May;16(Pt 2):404–17. doi: https://doi.org/10.1348/135910710X518324
25. Renner B, Spivak Y, Kwon S, Schwarzer R. Does age make a difference? Predicting physical
activity of South Koreans. Psychol Aging. 2007 Sep;22(3):482–93. doi: https://doi.org/10.1037/0882-7974.22.3.482
26. Derksen C, Keller FM, Lippke S. Obstetric Healthcare Workers’ Adherence to Hand Hygiene
Recommendations during the COVID-19 Pandemic: Observations and Social-Cognitive Determinants.
Appl Psychol Health Well-Being. 2020 Dec;12(4):1286–305. doi: https://doi.org/10.1111/aphw.12240
27. Lutze B, Chaberny IF, Graf K, Krauth C, Lange K, Schwadtke L, et al. Intensive care
physicians’ and nurses’ perception that hand hygiene prevents pathogen transmission:
belief strength and associations with other cognitive factors. J Health Psychol. 2017 Jan;22(1):89–100.
doi: https://doi.org/10.1177/1359105315595123
28. Tsang S, Royse CF, Terkawi AS. Guidelines for developing, translating, and validating
a questionnaire in perioperative and pain medicine. Saudi J Anaesth. 2017 May;11(5 Suppl
1):S80–9. doi: https://doi.org/10.4103/sja.SJA_203_17
29. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality
of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993 Dec;46(12):1417–32.
doi: https://doi.org/10.1016/0895-4356(93)90142-N
30. Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling
older adults. J Am Geriatr Soc. 2003 Nov;51(11):1610–4. doi: https://doi.org/10.1046/j.1532-5415.2003.51512.x
31. Ericsson KA, Simon HA. Thinking-Aloud Processes. Protocol Analysis: Verbal Reports
as Data. Cambridge (MA): MIT Press; 1993. doi: https://doi.org/10.7551/mitpress/5657.001.0001
32. Vellas BJ, Wayne SJ, Romero LJ, Baumgartner RN, Garry PJ. Fear of falling and restriction
of mobility in elderly fallers. Age Ageing. 1997 May;26(3):189–93. doi: https://doi.org/10.1093/ageing/26.3.189
33. Austin N, Devine A, Dick I, Prince R, Bruce D. Fear of falling in older women: a longitudinal
study of incidence, persistence, and predictors. J Am Geriatr Soc. 2007 Oct;55(10):1598–603.
doi: https://doi.org/10.1111/j.1532-5415.2007.01317.x
34. Veenstra GL, Dabekaussen KF, Molleman E, Heineman E, Welker GA. Health care professionals’
motivation, their behaviors, and the quality of hospital care: A mixed-methods systematic
review. Health Care Manage Rev. 2022 Apr-Jun;47(2):155–67. doi: https://doi.org/10.1097/HMR.0000000000000284
35. Andah E, Essang B, Friend C, Greenley S, Harvey K, Spears M, et al. Understanding
the impact of professional motivation on the workforce crisis in medicine: a rapid
review. BJGP Open. 2021 Apr;5(2):BJGPO.2021.0005. doi: https://doi.org/10.3399/BJGPO.2021.0005
36. Moreno NA, de Aquino BG, Garcia IF, Tavares LS, Costa LF, Giacomassi IW, et al. Physiotherapist
advice to older inpatients about the importance of staying physically active during
hospitalisation reduces sedentary time, increases daily steps and preserves mobility:
a randomised trial. J Physiother. 2019 Oct;65(4):208–14. doi: https://doi.org/10.1016/j.jphys.2019.08.006
37. Liu B, Moore JE, Almaawiy U, Chan WH, Khan S, Ewusie J, et al.; MOVE ON Collaboration.
Outcomes of Mobilisation of Vulnerable Elders in Ontario (MOVE ON): a multisite interrupted
time series evaluation of an implementation intervention to increase patient mobilisation.
Age Ageing. 2018 Jan;47(1):112–9. doi: https://doi.org/10.1093/ageing/afx128
Appendix
The appendix is available in the pdf version of the article at https://doi.org/10.57187/s.3385.

