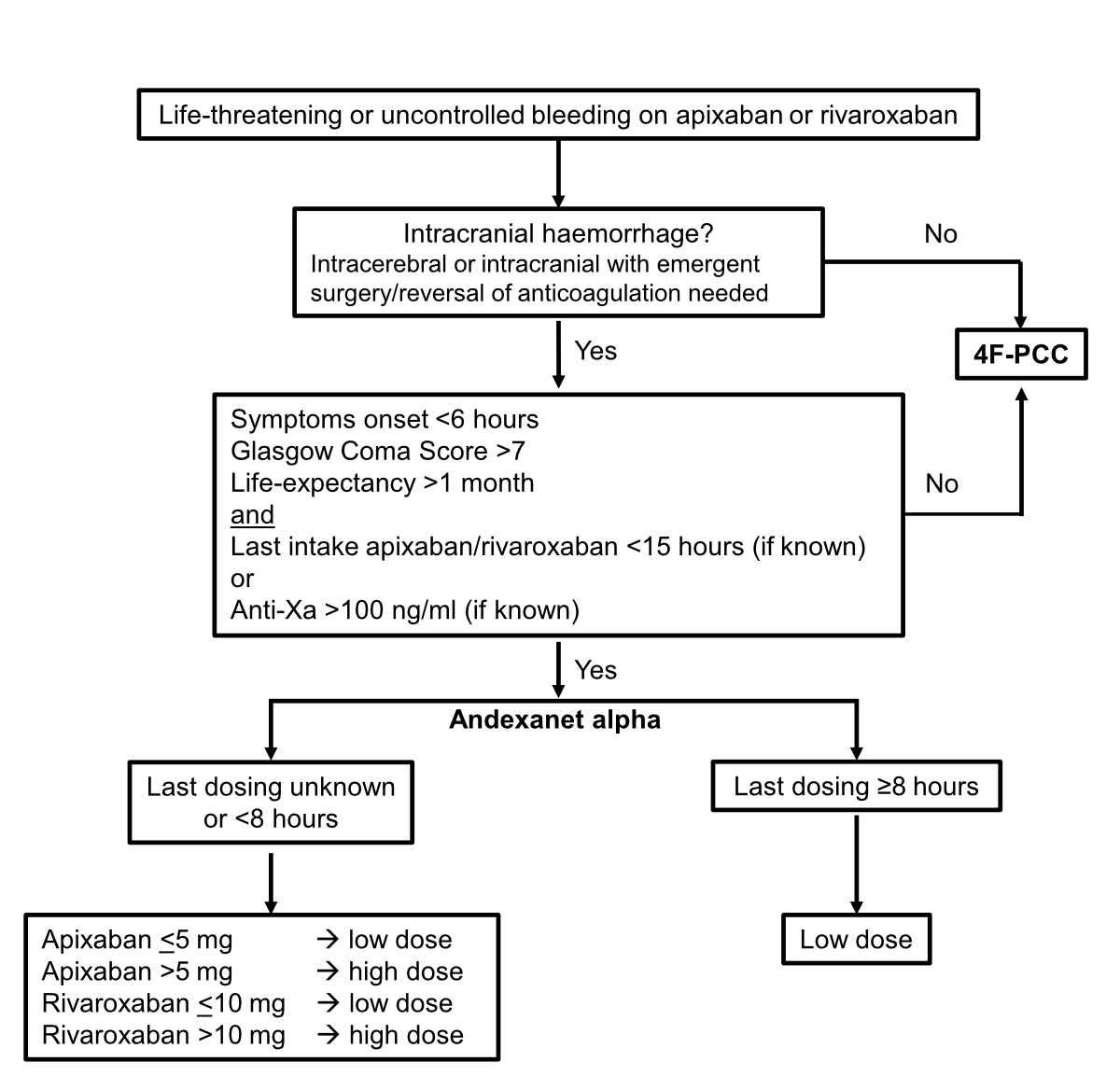
Figure 1Algorithm to guide andexanet alfa administration. 4F-PCC, four-factor prothrombin complex concentrate (e.g. Beriplex®, Octaplex® and Prothromplex®).
DOI: https://doi.org/https://doi.org/10.57187/smw.2023.40113
Anticoagulants are essential in preventing and treating thrombosis. Unfortunately, their use is accompanied by an enhanced risk of bleeding. The risk of major bleeding has been reduced by half with direct oral anticoagulants (DOACs) since their introduction compared with that with vitamin K antagonists. This means that, for example, the annual rate of major bleeding and intracranial haemorrhage is still 2–3% and 0.3–0.5% in patients with atrial fibrillation receiving DOACs, respectively [1].
Among DOACs, oral direct factor Xa inhibitors [apixaban (Eliquis®), edoxaban (Lixiana®) and rivaroxaban (Xarelto®)] are increasingly becoming commonly used for oral anticoagulation [2]. They have a rapid onset of action and significant anticoagulant activity. This activity can be expected as early as within the first 4 hours of administration and may last up to 48 hours or more in some patients (e.g. patients with impaired renal function) [3, 4]. Importantly, the in-hospital mortality rate is nearly 30% in patients with spontaneous intracranial haemorrhage receiving factor Xa inhibitors, with a higher risk of mortality in patients on factor Xa inhibitors than in those without anticoagulation [5].
Acute major bleeding events associated with the use of factor Xa inhibitors may be challenging to manage. Four-factor prothrombin complex concentrates (4F-PCCs; e.g. Beriplex®, Octaplex® and Prothromplex®) are plasma-derived concentrates that contain factors II, VII, IX and X. The haemostatic efficacy of 4F-PCCs has been studied in two prospective cohorts; these drugs yielded good effects in 69% and 65% of patients, respectively [7, 8]. Andexanet alfa (OndexxyaTM, AstraZeneca AG) is a specially designed recombinant version of human factor Xa that acts as a decoy receptor to reverse the effects of factor Xa inhibitors in cases of bleeding [9]. It was registered for use in Switzerland on 2 December 2020 [10, 11]. Current evidence is based on an open-label, single-arm, observational phase IIIb/IV study (ANNEXA-4 study) that enrolled 479 patients with major bleeding (69% of them had intracranial haemorrhage) and prior use of a factor Xa inhibitor (apixaban, rivaroxaban, edoxaban or enoxaparin) [12, 13]. Notably, the efficacy of andexanet alfa was evaluated in patients with baseline anti-Xa activity of at least 75 ng/ml, and around 95% of the population had an anti-Xa activity above 100 ng/ml [12, 13]. The ANNEXA-4 study showed a clinically good to excellent haemostasis with the infusion of andexanet alfa in 80% of cases of haemorrhage and a reduction in anti-Xa activity of more than 90% during infusion in the DOAC-treated groups and 75% in the enoxaparin-treated group. However, in the ANNEXA-4 study, andexanet alfa-mediated decrease in anti-Xa activity seemed to be modestly predictive of a better haemostatic efficacy only in patients with intracranial haemorrhage [12, 13]. The post-hoc ANNEXA-4 sub-study focused on haemostatic efficacy and anti-factor Xa reversal in patients with intracranial haemorrhage and showed that andexanet alfa improved haemostasis and reduced anti-factor Xa activity [14]. In an indirect comparative study employing ANNEXA-4 study data and a synthetic control arm of 4F-PCC-treated patients with intracranial haemorrhage, andexanet alfa was associated with a greater likelihood of achieving effective haemostasis (86% versus 68%, odds ratio = 2.7, confidence interval = 1.2–6.4) [15]. In a retrospective study analyzing the use of the health-system guideline, a comparable haemostatic efficacy was observed in patients receiving andexanet alfa and 4F-PCC, and a greater incidence of thromboembolic events was noted in patients receiving andexanet alfa than in those receiving 4F-PCC [16]. Another small retrospective study showed a comparable haemostatic efficacy and no difference in thromboembolic events between andexanet alfa and 4F-PCC in patients with intracranial haemorrhage [17]. In another study, andexanet alfa was associated with a lower rate of haematoma expansion in patients with atraumatic factor Xa inhibitor-related intracranial haemorrhage, but without translating into significantly improved clinical outcomes [18]. Finally, a recent meta-analysis showed a similar efficacy between 4F-PCC and andexanet alfa in patients with life-threatening bleeding, with a particularly high thrombotic rate with andexanet alfa [19]. Indeed, in the ANNEXA-4 study, 10% of patients had a thromboembolic complication, and 16% died [13]. Therefore, the mortality rate among the patients in the ANNEXA-4 trial is lower than that among some cohorts of patients with factor Xa inhibitor-associated major bleeding [7, 8, 20, 21].
Taken together, the available data point to a clinical benefit of andexanet alfa that might be restricted to patients with intracranial haemorrhage and raise concerns about thromboembolic events. It is worth noting that from June 2023, the ANNEXA-I trial (NCT03661528), a post-marketing phase IV trial comparing the efficacy and safety of andexanet alfa in patients on oral FXa inhibitor therapy, including apixaban and rivaroxaban, with acute intracranial haemorrhage, versus usual care (including 4F-PCC), has been stopped after the interim analysis of the first 450 patients [67]. The decision was based on fulfilment of pre-specified stopping criteria of superior haemostatic efficacy, the ability to limit the expansion of life-threatening bleeding in the brain, compared with usual care. The final data are currently being analysed and publication is still expected in 2023.
Along with clinical study data, it must be recognized that compared with andexanet alfa, 4F-PCC provides a major cost advantage in Switzerland. At the time of manuscript submission, the ex-factory price for andexanet alfa (“Preisliste” from AstraZeneca, January 2023) is 49,881 CHF for the low dose and 89,785.80 CHF for the high dose. Conversely, the compendium prices for 4F-PCC (5,000 U, high dosing based on 100-kg weight) are 3,058 CHF for Beriplex®, 3,181 CHF for Octaplex® and 3,330 CHF for Prothromplex® [10]. Moreover, the cost of andexanet alfa is currently not reimbursed by health insurance companies or through Swiss Diagnosis Related Groups with supplement billing.
Most guidelines (table 1) advise the use of specific antidotes, when available, to reverse the effects of DOACs. If specific reversal agents are not available, guidelines suggest using nonspecific agents instead, including 4F-PCCs. However, for patients with major bleeding during DOAC therapy for venous thromboembolism, the 2018 ASH guidelines recommend the use of either andexanet alfa or 4F-PCCs in addition to holding DOACs or holding DOACs alone. No recommendation is made regarding the use of one agent over the other owing to the lack of comparative studies. Finally, for patients who require long-term or indefinite anticoagulation and who survive major bleeding, the 2018 ASH guidelines suggest resuming DOACs within 90 days rather than discontinuing them indefinitely [22]. In line with this recommendation, it is essential to assess patients’ indication for anticoagulation and the underlying thrombotic risk to anticipate the timing and dose of thromboprophylaxis as well as full anticoagulation resumption following anticoagulation reversal.
Table 1Guidelines on the use of andexanet alfa in the management of bleeding.
| Name of the guideline | Apixaban/rivaroxaban | Edoxaban |
| American College of Gastroenterology-Canadian Association of Gastroenterology Clinical Practice Guideline 2022 [56] | Suggested: No routine administration of andexanet alfa or PCC for acute gastrointestinal bleeding. However, andexanet alfa could be envisaged for life-threatening gastrointestinal bleeding in hospitalized patients who have been under apixaban or rivaroxaban treatment in the last 24 hours. | − |
| American College of Cardiology 2020 [57] | Consider: activated charcoal | Consider: activated charcoal |
| 1st line: andexanet alfa | 1st line: andexanet alfa (off-label); | |
| 2nd line: 4F-PCC, aPCC | 2nd line: 4F-PCC, aPCC | |
| Anticoagulation Forum 2019 [6] | Suggested: andexanet alfa | Suggested: andexanet alfa (off-label) or 4F-PCC |
| If andexanet alfa is not available: 4F-PCC | ||
| American College of Emergency Physicians 2019 [58] | Recommended: andexanet alfa | − |
| Alternative: PCC | ||
| American Heart Association/American College of Cardiology/Heart Rhythm Society 2019 [59] | “Can be useful”: andexanet alfa | − |
| European Stroke Organisation 2019 [60] | 1st line: andexanet alfa | 1st line:PCC |
| 2nd line: 4F-PCC | ||
| National Comprehensive Cancer Network 2019 [61] | Consider: activated charcoal | Consider: activated charcoal |
| Recommended: andexanet alfa | Recommended: aPCC, 4F-PCC, rFVIIa, 3F-PCC | |
| Alternatives: aPCC, 4F-PCC, rFVIIa, 3F-PCC | ||
| American College of Chest Physicians 2018 [62] | Management framework: DOAC-specific reversal agent | − |
| If DOAC-specific reversal agent is not available: PCC | ||
| American Society of Hematology 2018 [22] | Suggested: andexanet alfa or 4F-PCC | − |
| European Heart Rhythm Association 2018 [63] | Recommended: andexanet alfa | − |
| Alternative: 4F-PCC, aPCC | ||
| International Society for Thrombosis and Haemostasis 2016 [64] | Agents under investigation: andexanet alfa, ciraparantag | − |
| Neurocritical Care Society/Society of Critical Care Medicine 2016 [65] | Consider: activated charcoal | − |
| Suggested: 4F-PCC, aPCC | ||
| Alternative: rFVIIa | ||
| American Heart Association/American Stroke Association 2015 [66] | Consider: FEIBA, PCC, rFVIIa, activated charcoal | − |
aPCC = activated prothrombin complex concentrate; DOAC = direct oral anticoagulant; 3F-PCC = three-factor prothrombin complex concentrate; 4F-PCC = four-factor prothrombin complex concentrate; FEIBA = factor VIII bypassing agent; PCC = prothrombin complex concentrate; rFVIIa = recombinant activated factor VII
Antithrombotic therapy can be reinstated as soon as medically indicated, after treatment of the bleeding episode and removal of its cause, if patients’ clinical condition is stable and if proper haemostasis has been achieved.
Although in cases of major bleeding, additional measures aside from supportive management might be required and include possible combination of specific and nonspecific reversal agents, the current guidelines do not provide recommendations regarding these combinations. Notably, however, the peri-/intraoperative use of andexanet alfa is off-label.
The Working Party of Hemostasis (WPH) of the Swiss Society of Hematology (SGH-SSH) offers a guideline for the utilization of andexanet alfa in the management of bleeding in patients on factor Xa inhibitors based on current available evidence, international guidelines and consensus opinions of experts. The paper integrates reversal anticoagulation with factor Xa inhibitors in the management of life-threatening or uncontrolled bleeding, including intracranial haemorrhage, allowing direct implementation. In addition, the Working Party of Hemostasis of the Swiss Society of Hematology comments on the monitoring of the effect of andexanet alfa and the limitations created by this anticoagulation reversal approach for resumption of anticoagulation in the follow-up of a major bleeding event.
The major complication of DOACs is serious or life-threatening bleeding, which may require rapid reversal of anticoagulation. Because DOACs have a short half-life, anticoagulation reversal is generally not necessary in patients with non-life-threatening bleeding. Equally, reversal of DOACs is generally not warranted in patients who are not bleeding and who require non-urgent invasive procedures. However, urgent invasive procedures in non-bleeding patients on DOACs may prompt anticoagulation reversal [23, 24].
Indicated for adult patients treated with a direct factor Xa inhibitor (apixaban or rivaroxaban) when reversal of anticoagulation is needed owing to life-threatening or uncontrolled bleeding.
Andexanet alfa is currently subject to additional monitoring to allow rapid identification of new safety information. Therefore, new suspected or serious adverse reactions must be reported.
Indicated for patients treated with apixaban or rivaroxaban when reversal of anticoagulation is needed owing to life-threatening or uncontrolled bleeding.
Andexanet alfa is a recombinant variant of the human factor Xa protein that is catalytically inactive owing to a mutation of the active-site S419A and a deletion of the membrane-binding gamma-carboxyglutamic acid domain. However, andexanet alfa keeps the structural similarity to endogenous factor Xa to be bound by factor Xa-inhibiting drugs, such as direct factor Xa inhibitors and antithrombin-dependent anticoagulants [9], but is unable to assemble into the prothrombinase complex and cleave prothrombin to generate thrombin. Andexanet alfa binds to apixaban, betrixaban, edoxaban and rivaroxaban with an affinity that corresponds to that of endogenous factor Xa (i.e. 0.5–1.5 nmol/l) [9]. Consequently, andexanet alfa scavenges factor Xa inhibitors, reversing the anticoagulant effects of factor Xa inhibitors and restoring the activity of endogenous factor Xa.
The distribution of andexanet alfa is 5.3 ± 2.6 l, approximately equivalent to the blood volume [10]. There are currently no data on the metabolism of andexanet alfa. Its clearance rate is 4.4 ± 1.2 L/hour with low renal elimination. The elimination half-life ranges from 4 to 7 hours [10, 25, 26]. According to knowledge of native factor Xa kinetics, andexanet alfa is likely to be rapidly degraded in the plasma by endogenous proteases, compatible with its relatively short effective half-life. Biliary and/or faecal excretion of therapeutic proteins is not a known route of protein elimination. Consequently, it is not considered necessary to adjust the dose in patients with hepatic insufficiency. According to available pharmacokinetic data, andexanet alfa has either limited or no renal clearance and hence would not require dose adjustment in patients with renal insufficiency [10, 11, 25, 26]. The effective half-life of andexanet alfa is 30–60 minutes [27]. Andexanet alfa is administered as a bolus followed by an infusion to sustain anticoagulation reversal until the drug is cleared from the circulation [10].
Andexanet alfa does not alter the effects of non-factor Xa-based inhibitors (e.g. dabigatran) and is currently not approved by Swissmedic, the EMA and the FDA for reversal of the anticoagulant effect of edoxaban, low-molecular-weight heparin and fondaparinux owing to the current lack of data [10, 11, 25, 26]. Nevertheless, recent publications suggest the effectiveness of andexanet alfa for edoxaban reversal as well [13, 28].
Monitoring of therapy should be based mainly on clinical parameters indicating adequate response (e.g. achieving haemostasis), lack of efficacy (e.g. rebleeding) and adverse events (e.g. thromboembolic episodes). Monitoring of andexanet alfa therapy should not be based on anti-factor Xa activity, as commercially available anti-factor Xa activity assays are not appropriate for measuring anti-factor Xa activity after andexanet alfa administration because they show falsely elevated levels of anti-factor Xa activity, resulting in significant underestimation of andexanet alfa reversal activity [29, 30]. This is due to the high sample dilution in the early step of commercial anti-Xa activity tests, causing the andexanet alfa–factor Xa inhibitor binding/unbinding equilibrium to shift towards the unbound state. This increases the amount of factor Xa inhibitors in the free or unbound state and thereby in the factor Xa assay. A modified commercially available anti-Xa activity test has been developed to minimize the sample dilution effect [9, 31]. This test has been used in all studies implying the use of andexanet alfa [9, 12, 31].
The development of thrombotic events is a major concern for patients with anticoagulant-related bleeding who are at an increased risk not just owing to anticoagulant withdrawal and reversal but also because of haemostasis activation, invasive procedure or critical illness. However, the rate of thrombosis is comparable among patients with anticoagulant (including VKA and DOAC)-related major bleeding treated with fresh frozen plasma, 4F-PCC, idarucizumab or andexanet alfa (4–10%) [24].
Thrombotic episodes have been reported after andexanet alfa therapy [12, 19]. Patients on factor Xa inhibitors have underlying medical conditions that predispose them to thrombotic events. Factor Xa inhibitor reversal exposes these patients to the thrombotic risk of their pre-existing disease. In addition, an independent prothrombotic effect of andexanet alfa, such as tissue factor pathway inhibitor (TFPI) inhibition within 10–20 hours following andexanet alfa administration, cannot be excluded [10–12, 25, 26, 31, 32]. The duration of this effect in bleeding patients is unknown.
In healthy volunteers, dose-dependent transient increases in the coagulation markers F1+2, thrombin-antithrombin complexes and D-dimer as well as thrombin generation have been observed after andexanet alfa administration. This points to an activation of the coagulation system, which may be related to the observation of a concomitant TFPI inhibition [10–12, 25, 26, 31, 32]. Therefore, biological parameters such as anti-factor Xa activity, measurement of thrombin generation or markers of thrombosis may not be good indicators for evaluating both efficacy and thrombotic risk [30].
No thrombotic events have been reported in healthy volunteers [31]. In the ANNEXA-4 study, coagulation activation markers were not measured [12]. Monitoring for signs and symptoms of thrombosis is therefore highly advised in patients on andexanet alfa. Resumption of anticoagulant therapy should be considered as soon as possible after reversal therapy to reduce the occurrence of a thrombotic event. In addition, the relationship between laboratory markers of thrombosis and the development of clinical thrombosis is uncertain [33].
Andexanet alfa should be used in combination with standard supportive haemostatic measures based on medical needs [10, 11, 25, 26], including endoscopy, angiography or surgery. Ultimately, definitive haemostatic intervention should be employed to stop bleeding [24].
The safety of andexanet alfa has not been evaluated in patients who have previously received 4F-PCCs, recombinant factor VIIa (rFVIIa) or blood products within 7 days prior to the bleeding event, as these patients have been excluded from clinical trials [12]. Concomitant treatment with other procoagulant factors (3F- or 4F-PCC, activated PCC or rFVIIa) should be avoided owing to the current lack of data regarding the combination of these agents [10, 11, 25, 26]. However, if clinically necessary (i.e. unresolved major bleeding), the use of these agents after the end of andexanet alfa infusion may be considered owing to the relatively short half-life [10]. Fresh frozen plasma, platelet concentrate and tranexamic acid should be used if appropriate, in line with local guidelines on massive transfusion.
Andexanet alfa also binds to antithrombin-dependent factor Xa anticoagulants, such as unfractionated heparin, by binding to heparin-activated antithrombin [34, 35]. Consequently, andexanet alfa administration before interventions under heparin anticoagulation can promote heparin resistance or unresponsiveness, particularly in cardiovascular surgery [36–38]. Therefore, it is recommended not to use andexanet alfa prior to heparinization, especially in patients needing emergent cardiac surgery with cardiopulmonary bypass [39]. As a result of this interaction, the effect of neither heparin nor andexanet alfa can be monitored, since routine coagulation tests do not provide reliable results and cannot be used for monitoring. If patients are on andexanet alfa and require systemic anticoagulation for an urgent procedure, clinicians may need to consider alternative agents [26] (i.e. bivalirudin if andexanet alfa is given before cardiopulmonary bypass surgery) [39] or argatroban [37]. This topic has been approached only in case reports [36–38] and is now critically discussed in the field of cardiovascular surgery [40–42]. Therefore, prospective studies are needed before an update of actual recommendations [39].
The duration of heparin neutralization by andexanet alfa has not been studied. Further, the use of andexanet alfa as an antidote to heparin or low-molecular-weight heparin has not been evaluated and is currently not recommended.
The ANNEXA-4 study lacks a comparator group. Therefore, the findings are observational, and correlations could be confounded particularly with selection bias [12, 13]. The recommendations against the use of andexanet alfa in patients with life-threatening bleeds other than intracranial haemorrhage were based on a meta-analysis [43] and small retrospective studies [16, 17, 44] because these studies did not demonstrate an advantage of prescribing andexanet alfa over 4F-PCC in this indication. Conversely, the use of 4F-PCC during bleeding in the context of anticoagulation with DOACs is based on few clinical data, and animal and in vitro or ex vivo studies as well as a lack of alternative treatment options [45–53].
Andexanet alfa is administered in accordance with the algorithm shown in figure 1.

Figure 1Algorithm to guide andexanet alfa administration. 4F-PCC, four-factor prothrombin complex concentrate (e.g. Beriplex®, Octaplex® and Prothromplex®).
The andexanet alfa dosage is determined in accordance with the data shown in table 2.
Table 2Modality of administration of andexanet alfa [31].
| Dose | Initial intravenous bolus | Follow-up intravenous infusion |
| Low (5 vials of 200 mg) | 400 mg at a target rate of 30 mg/minute for ~15 minutes | 4 mg/minute over 120 minutes (480 mg) |
| High (9 vials of 200 mg) | 800 mg at a target rate of 30 mg/minute for up to ~30 minutes | 8 mg/minute over 120 minutes (960 mg) |
The WPH/SGH-SSH recommendations integrate multiple guidelines regarding the use of andexanet alfa in the management of bleeding in patients on factor Xa inhibitors. These recommendations propose that andexanet alfa is potentially suitable for the management of intracranial haemorrhage with apixaban or rivaroxaban when reversal of anticoagulation is needed. However, the WPH/SGH-SSH does not recommend the administration of andexanet alfa over 4F-PCC for other life-threatening or uncontrolled bleeding because of the current lack of evidence. Finally, the WPH/SGH-SSH provides guidance on andexanet alfa administration, highlighting indications and risks.
The WPH/SGH-SSH recommendations may be in contrast to other statements of different societies. The limitations of the WPH/SGH-SSH recommendations are inherent to the substantially low level of evidence that currently exists for most aspects of the use of andexanet alfa for reversal of anticoagulation with factor Xa inhibitors. Moreover, the cost of andexanet alfa, which is relatively high, is currently not reimbursed by health insurance companies or through Swiss Diagnosis Related Groups with supplement billing [55]. The superiority of andexanet alfa to 4F-PCC in terms of haemostasis is currently not established. Nevertheless, the interim analysis of the ANNEXA-I study showed superior haemostatic efficacy and an ability to limit the expansion of a potentially fatal cerebral haemorrhage compared with usual treatment [67]. This new information supports our current recommendation to administer andexanet alfa to patients with acute intracranial haemorrhage. Publication of the full analysis of the study is still awaited.
In conclusion, the WPH/SGH-SSH hopes that the recommendations will guide Swiss clinicians in managing patients with life-threatening or uncontrolled bleeding under anticoagulation with apixaban or rivaroxaban until more data are available.
The WPH/SGH-SSH include (alphabetical order): Lorenzo Alberio (Service and Central Laboratory of Hematology, Lausanne University Hospital, Lausanne, Switzerland), Anne Angelillo-Scherrer (Department of Hematology and Central Hematology Laboratory, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland), Lars Asmis (Center for perioperative Thrombosis and Hemostasis, Zurich, Switzerland), Eugenia Biguzzi (Clinic of Hematology, Oncology Institute of Southern Switzerland, Ente Ospedaliero Cantonale, Bellinzona, Switzerland), Alessandro Casini (Division of Angiology and Hemostasis, Geneva University Hospital, Geneva, Switzerland), Pierre Fontana (Division of Angiology and Hemostasis, Geneva University Hospital, Geneva, Switzerland), Elena Galfetti (Clinic of Hematology, Oncology Institute of Southern Switzerland, Ente Ospedaliero Cantonale, Bellinzona, Switzerland), Bernhard Gerber (Clinic of Hematology, Oncology Institute of Southern Switzerland, Ente Ospedaliero Cantonale, Bellinzona, Switzerland), Lukas Graf (Cantonal Hospital of St Gallen, St Gallen, Switzerland), Johanna A. Kremer Hovinga (Department of Hematology and Central Hematology Laboratory, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland), Wolfgang Korte (Cantonal Hospital of St Gallen, St Gallen, Switzerland), Maria Martinez (Division of Hematology, Basel University Hospital, Basel, Switzerland), Jan-Dirk Studt (University Clinic of Hematology, Zurich University Hospital, Zurich, Switzerland), Alice Trinchero (University Clinic of Hematology, Zurich University Hospital, Zurich, Switzerland), Walter A. Wuillemin (Division of Hematology and Central Hematology Laboratory, Cantonal Hospital of Lucerne, Lucerne, Switzerland).
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflict of interest related to the content of this manuscript was disclosed.
1. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014 Mar;383(9921):955–62. 10.1016/S0140-6736(13)62343-0
2. Chen A, Stecker E, A Warden B. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J Am Heart Assoc. 2020 Jul;9(13):e017559. 10.1161/JAHA.120.017559
3. Bortman LV, Mitchell F, Naveiro S, Pérez Morales J, Gonzalez CD, Di Girolamo G, et al. Direct Oral Anticoagulants: An Updated Systematic Review of Their Clinical Pharmacology and Clinical Effectiveness and Safety in Patients With Nonvalvular Atrial Fibrillation. J Clin Pharmacol. 2023 Apr;63(4):383–96. 10.1002/jcph.2184
4. Bertaggia-Calderara D, Kröll D, Gerschheimer C, Nicolas N, Nett P, Stirnimann G, et al. Effect of Rivaroxaban on thrombin generation in vivo. A study in obese patients. Int J Lab Hematol. 2018 Feb;40(1):e11–4. 10.1111/ijlh.12767
5. Xian Y, Zhang S, Inohara T, Grau-Sepulveda M, Matsouaka RA, Peterson ED, et al.; Clinical Characteristics and Outcomes Associated With Oral Anticoagulant Use Among Patients Hospitalized With Intracerebral Hemorrhage. Clinical Characteristics and Outcomes Associated With Oral Anticoagulant Use Among Patients Hospitalized With Intracerebral Hemorrhage. JAMA Netw Open. 2021 Feb;4(2):e2037438. 10.1001/jamanetworkopen.2020.37438
6. Cuker A, Burnett A, Triller D, Crowther M, Ansell J, Van Cott EM, et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol. 2019 Jun;94(6):697–709. 10.1002/ajh.25475
7. Majeed A, Ågren A, Holmström M, Bruzelius M, Chaireti R, Odeberg J, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017 Oct;130(15):1706–12. 10.1182/blood-2017-05-782060
8. Schulman S, Gross PL, Ritchie B, Nahirniak S, Lin Y, Lieberman L, et al.; Study Investigators. Prothrombin Complex Concentrate for Major Bleeding on Factor Xa Inhibitors: A Prospective Cohort Study. Thromb Haemost. 2018 May;118(5):842–51. 10.1055/s-0038-1636541
9. Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013 Apr;19(4):446–51. 10.1038/nm.3102
12. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, et al.; ANNEXA-4 Investigators. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. 2019 Apr;380(14):1326–35. 10.1056/NEJMoa1814051
13. Milling TJ Jr, Middeldorp S, Xu L, Koch B, Demchuk A, Eikelboom JW, et al.; ANNEXA-4 Investigators. Final Study Report of Andexanet Alfa for Major Bleeding With Factor Xa Inhibitors. Circulation. 2023 Mar;147(13):1026–38. 10.1161/CIRCULATIONAHA.121.057844
14. Demchuk AM, Yue P, Zotova E, Nakamya J, Xu L, Milling TJ Jr, et al.; ANNEXA-4 Investigators. Hemostatic Efficacy and Anti-FXa (Factor Xa) Reversal With Andexanet Alfa in Intracranial Hemorrhage: ANNEXA-4 Substudy. Stroke. 2021 Jun;52(6):2096–105. 10.1161/STROKEAHA.120.030565
15. Costa OS, Connolly SJ, Sharma M, Beyer-Westendorf J, Christoph MJ, Lovelace B, et al. Andexanet alfa versus four-factor prothrombin complex concentrate for the reversal of apixaban- or rivaroxaban-associated intracranial hemorrhage: a propensity score-overlap weighted analysis. Crit Care. 2022 Jun;26(1):180. 10.1186/s13054-022-04043-8
16. Schmidt LE, Hinton MS, Martin ND. Real-World Reversal of Factor Xa Inhibition in the Setting of Major Life-Threatening Bleeding or Urgent Surgery. J Pharm Pract. 2022 Sep;•••:8971900221125516. 10.1177/08971900221125516
17. Lipski M, Pasciolla S, Wojcik K, Jankowitz B, Igneri LA. Comparison of 4-factor prothrombin complex concentrate and andexanet alfa for reversal of apixaban and rivaroxaban in the setting of intracranial hemorrhage. J Thromb Thrombolysis. 2023 Apr;55(3):519–26. 10.1007/s11239-022-02752-z
18. Huttner HB, Gerner ST, Kuramatsu JB, Connolly SJ, Beyer-Westendorf J, Demchuk AM, et al.; Hematoma Expansion and Clinical Outcomes in Patients With Factor-Xa Inhibitor-Related Atraumatic Intracerebral Hemorrhage Treated Within the ANNEXA-4 Trial Versus Real-World Usual Care. Hematoma Expansion and Clinical Outcomes in Patients With Factor-Xa Inhibitor-Related Atraumatic Intracerebral Hemorrhage Treated Within the ANNEXA-4 Trial Versus Real-World Usual Care. Stroke. 2022 Feb;53(2):532–43. 10.1161/STROKEAHA.121.034572
19. Gómez-Outes A, Alcubilla P, Calvo-Rojas G, Terleira-Fernández AI, Suárez-Gea ML, Lecumberri R, et al. Meta-Analysis of Reversal Agents for Severe Bleeding Associated With Direct Oral Anticoagulants. J Am Coll Cardiol. 2021 Jun;77(24):2987–3001. 10.1016/j.jacc.2021.04.061
20. Panos NG, Cook AM, John S, Jones GM, Kelly H, Choi RK, et al.; Neurocritical Care Society (NCS) Pharmacy Study Group. Factor Xa Inhibitor-Related Intracranial Hemorrhage: Results From a Multicenter, Observational Cohort Receiving Prothrombin Complex Concentrates. Circulation. 2020 May;141(21):1681–9. 10.1161/CIRCULATIONAHA.120.045769
21. Milling TJ Jr, Clark CL, Feronti C, Song SS, Torbati SS, Fermann GJ, et al. Management of Factor Xa inhibitor-associated life-threatening major hemorrhage: A retrospective multi-center analysis. Am J Emerg Med. 2018 Mar;36(3):396–402. 10.1016/j.ajem.2017.08.042
22. Witt DM, Nieuwlaat R, Clark NP, Ansell J, Holbrook A, Skov J, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018 Nov;2(22):3257–91. 10.1182/bloodadvances.2018024893
23. Milling TJ, Pollack CV. A review of guidelines on anticoagulation reversal across different clinical scenarios - Is there a general consensus? Am J Emerg Med. 2020 Sep;38(9):1890–903. 10.1016/j.ajem.2020.05.086
24. Salter B, Crowther M. A Historical Perspective on the Reversal of Anticoagulants. Semin Thromb Hemost. 2022 Nov;48(8):955–70. 10.1055/s-0042-1753485
25. https://www.ema.europa.eu/en/medicines/human/EPAR/ondexxya
26. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/andexxa
27. Kaatz S, Bhansali H, Gibbs J, Lavender R, Mahan CE, Paje DG. Reversing factor Xa inhibitors - clinical utility of andexanet alfa. J Blood Med. 2017 Sep;8:141–9. 10.2147/JBM.S121550
28. Benz AP, Xu L, Eikelboom JW, Middeldorp S, Milling TJ Jr, Crowther M, et al.; ANNEXA-4 Investigators. Andexanet Alfa for Specific Anticoagulation Reversal in Patients with Acute Bleeding during Treatment with Edoxaban. Thromb Haemost. 2022 Jun;122(6):998–1005. 10.1055/s-0041-1740180
29. European Medicines Agency. Direct Health Care Professional Communication (DHCP): Ondexxya (Andexanet Alfa): Commercial Anti-FXa Activity Assays Are Unsuitable for Measuring AntiFXa Activity Following Administration of Andexanet Alfa. Accessed October 18, 2020 at: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/direct-healthcare-professional-communications
30. Douxfils J, Adcock DM, Bates SM, Favaloro EJ, Gouin-Thibault I, Guillermo C, et al. 2021 Update of the International Council for Standardization in Haematology Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb Haemost. 2021 Aug;121(8):1008–20. 10.1055/a-1450-8178
31. Siegal DM, Curnutte JT, Connolly SJ, Lu G, Conley PB, Wiens BL, et al.; Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. 2015 Dec;373(25):2413–24. 10.1056/NEJMoa1510991
32. Lu G, Lin JP, Curnutte JT, Conley PB. Effect of Andexanet-TFPI Interaction on in Vitro Thrombin Formation and Coagulation Markers in the TF-Pathway. Blood. 2017;130 Suppl_1:629. 10.1182/blood.V130.Suppl_1.629.629
33. Cushman M, Barnes GD, Creager MA, Diaz JA, Henke PK, Machlus KR, et al.; American Heart Association Council on Peripheral Vascular Disease; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and the International Society on Thrombosis and Haemostasis. Venous Thromboembolism Research Priorities: A Scientific Statement From the American Heart Association and the International Society on Thrombosis and Haemostasis. Circulation. 2020 Aug;142(6):e85–94. 10.1161/CIR.0000000000000818
34. Kalathottukaren MT, Creagh AL, Abbina S, Lu G, Karbarz MJ, Pandey A, et al. Comparison of reversal activity and mechanism of action of UHRA, andexanet, and PER977 on heparin and oral FXa inhibitors. Blood Adv. 2018 Aug;2(16):2104–14. 10.1182/bloodadvances.2016003616
35. Siddiqui F, Tafur A, Bontekoe E, Iqbal O, Jeske W, Mehrotra S, et al. Assay-Based Differentiation in the Neutralization Profile of Unfractionated Heparin, Enoxaparin, and Fondaparinux by Andexanet Alfa. Clin Appl Thromb Hemost. 2020;26:1076029619895120. 10.1177/1076029619895120
36. Apostel HJ, Winckers K, Bidar E, Schreiber JU; Successful Antithrombin Administration in Andexanet Alfa-Associated Heparin Resistance. Successful Antithrombin Administration in Andexanet Alfa-Associated Heparin Resistance. J Cardiothorac Vasc Anesth. 2021 Mar;35(3):904–7. 10.1053/j.jvca.2020.10.042
37. Eche IM, Elsamadisi P, Wex N, Wyers MC, Brat GA, Cunningham K, et al. Intraoperative Unfractionated Heparin Unresponsiveness during Endovascular Repair of a Ruptured Abdominal Aortic Aneurysm following Administration of Andexanet Alfa for the Reversal of Rivaroxaban. Pharmacotherapy. 2019 Aug;39(8):861–5. 10.1002/phar.2306
38. Watson CJ, Zettervall SL, Hall MM, Ganetsky M; Difficult Intraoperative Heparinization Following Andexanet Alfa Administration. Difficult Intraoperative Heparinization Following Andexanet Alfa Administration. Clin Pract Cases Emerg Med. 2019 Oct;3(4):390–4. 10.5811/cpcem.2019.9.43650
39. Navas-Blanco JR, Martini A, Fabbro M 2nd. Expert Consensus Decision Pathway of the American College of Cardiology on Management of Bleeding in Patients With Oral Anticoagulants: A Review of the 2020 Update for Perioperative Physicians. J Cardiothorac Vasc Anesth. 2021 Aug;35(8):2471–9. 10.1053/j.jvca.2021.02.024
40. Erdoes G, Birschmann I, Nagler M, Koster A. Andexanet Alfa-Induced Heparin Resistance: When Anticoagulation Really Remains Reversed. J Cardiothorac Vasc Anesth. 2021 Mar;35(3):908–9. 10.1053/j.jvca.2020.11.052
41. Levy JH, Connors JM. Andexanet Alfa Use in Cardiac Surgical Patients: A Xa Inhibitor and Heparin Reversal Agent. J Cardiothorac Vasc Anesth. 2021 Jan;35(1):265–6. 10.1053/j.jvca.2020.09.091
42. Levy JH, Welsby I. Andexanet Alfa Use in Patients Requiring Cardiopulmonary Bypass: quo Vadis? A A Pract. 2019 Dec;13(12):477. 10.1213/XAA.0000000000001115
43. Nederpelt CJ, Naar L, Krijnen P, le Cessie S, Kaafarani HM, Huisman MV, et al. Andexanet Alfa or Prothrombin Complex Concentrate for Factor Xa Inhibitor Reversal in Acute Major Bleeding: A Systematic Review and Meta-Analysis. Crit Care Med. 2021 Oct;49(10):e1025–36. 10.1097/CCM.0000000000005059
44. Nederpelt CJ, Naar L, Sylvester KW, Barra ME, Roberts RJ, Velmahos GC, et al. Evaluation of oral factor Xa inhibitor-associated extracranial bleeding reversal with andexanet alfa. J Thromb Haemost. 2020 Oct;18(10):2532–41. 10.1111/jth.15031
45. Dinkelaar J, Molenaar PJ, Ninivaggi M, de Laat B, Brinkman HJ, Leyte A. In vitro assessment, using thrombin generation, of the applicability of prothrombin complex concentrate as an antidote for Rivaroxaban. J Thromb Haemost. 2013 Jun;11(6):1111–8. 10.1111/jth.12236
46. Ghosh S, Krege W, Doerr B, Mischnik M, Pragst I, Dickneite G, et al. Evaluation of the prothrombotic potential of four-factor prothrombin complex concentrate (4F-PCC) in animal models. PLoS One. 2021 Oct;16(10):e0258192. 10.1371/journal.pone.0258192
47. Godier A, Miclot A, Le Bonniec B, Durand M, Fischer AM, Emmerich J, et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology. 2012 Jan;116(1):94–102. 10.1097/ALN.0b013e318238c036
48. Herzog E, Kaspereit F, Krege W, Doerr B, Mueller-Cohrs J, Pragst I, et al. Effective reversal of edoxaban-associated bleeding with four-factor prothrombin complex concentrate in a rabbit model of acute hemorrhage. Anesthesiology. 2015 Feb;122(2):387–98. 10.1097/ALN.0000000000000520
49. Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012 Aug;108(2):217–24. 10.1160/TH12-03-0179
50. Perzborn E, Gruber A, Tinel H, Marzec UM, Buetehorn U, Buchmueller A, et al. Reversal of rivaroxaban anticoagulation by haemostatic agents in rats and primates. Thromb Haemost. 2013 Jul;110(1):162–72.
51. Tao J, Bukanova EN, Akhtar S. Safety of 4-factor prothrombin complex concentrate (4F-PCC) for emergent reversal of factor Xa inhibitors. J Intensive Care. 2018 Jun;6(1):34. 10.1186/s40560-018-0303-y
52. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011 Oct;124(14):1573–9. 10.1161/CIRCULATIONAHA.111.029017
53. Brinkman HJ, Zuurveld M, Meijers JC. In vitro reversal of direct factor Xa inhibitors: direct comparison of andexanet alfa and prothrombin complex concentrates Cofact and Beriplex/Kcentra. Res Pract Thromb Haemost. 2022 Aug;6(5):e12775. 10.1002/rth2.12775
54. Kaserer A, Kiavialaitis GE, Braun J, Schedler A, Stein P, Rössler J, et al. Impact of rivaroxaban plasma concentration on perioperative red blood cell loss. Transfusion. 2020 Jan;60(1):197–205. 10.1111/trf.15560
55. Frontera JA, Bhatt P, Lalchan R, Yaghi S, Ahuja T, Papadopoulos J, et al. Cost comparison of andexanet versus prothrombin complex concentrates for direct factor Xa inhibitor reversal after hemorrhage. J Thromb Thrombolysis. 2020 Jan;49(1):121–31. 10.1007/s11239-019-01973-z
56. Abraham NS, Barkun AN, Sauer BG, Douketis J, Laine L, Noseworthy PA, et al. American College of Gastroenterology-Canadian Association of Gastroenterology Clinical Practice Guideline: Management of Anticoagulants and Antiplatelets During Acute Gastrointestinal Bleeding and the Periendoscopic Period. Am J Gastroenterol. 2022 Apr;117(4):542–58. 10.14309/ajg.0000000000001627
57. Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020 Aug;76(5):594–622. 10.1016/j.jacc.2020.04.053
58. Baugh CW, Levine M, Cornutt D, Wilson JW, Kwun R, Mahan CE, et al. Anticoagulant Reversal Strategies in the Emergency Department Setting: Recommendations of a Multidisciplinary Expert Panel. Ann Emerg Med. 2020 Oct;76(4):470–85. 10.1016/j.annemergmed.2019.09.001
59. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019 Jul;140(2):e125–51. 10.1161/CIR.0000000000000665
60. Christensen H, Cordonnier C, Kõrv J, Lal A, Ovesen C, Purrucker JC, et al.; European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage. European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage. Eur Stroke J. 2019 Dec;4(4):294–306. 10.1177/2396987319849763
61. www.nccn.org/professionals/physician_gls/pdf/vte.pdf.
62. Lip GY, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018 Nov;154(5):1121–201. 10.1016/j.chest.2018.07.040
63. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al.; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018 Apr;39(16):1330–93. 10.1093/eurheartj/ehy136
64. Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI; Subcommittee on Control of Anticoagulation. When and how to use antidotes for the reversal of direct oral anticoagulants: guidance from the SSC of the ISTH. J Thromb Haemost. 2016 Mar;14(3):623–7. 10.1111/jth.13227
65. Frontera JA, Lewin JJ 3rd, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, et al. Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. 2016 Feb;24(1):6–46. 10.1007/s12028-015-0222-x
66. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015 Jul;46(7):2032–60. 10.1161/STR.0000000000000069